CHAPTER 14: HEAT AND HEAT TRANSFER METHODS
|
|
|
- Amice Short
- 6 years ago
- Views:
Transcription
1 College Physics Student s Manual Chapter CHAPTER : HEAT AND HEAT TRANSFER METHODS. TEMPERATURE CHANGE AND HEAT CAPACITY. On a hot day, the temperature of an 80,000- L swimming pool increases by.0 C. What is the net heat transfer during this heating? Ignore any complications, such as loss of by evaporation. The heat input is given by mcδt, where the specific heat of is c 86 /kg C. The mass is given by m ρ V (.00 and the temperature change is ΔT mcδt (8.00 m kg/m (80,000L L.0 C. Therefore, kg(86/kg C(.0 C.0 kg, 8 9. Following vigorous exercise, the body temperature of an kg person is 0.0 C. At what rate in watts must the person transfer thermal energy to reduce the body temperature to 7.0 C in 0.0 min, assuming the body continues to produce energy watt joule/second orw /s at the rate of W? ( First, calculate how much heat must be dissipated: mchuman bodyδt (80.0kg(00 /kg C(0 C - 7 C 8.0 Then, since power is heat divided by time, we can get the power required to produce the calculated amount of heat in 0.0 minutes:
2 College Physics Student s Manual Chapter P t (0 min(60 s/min cooling W. Now, since the body continues to produce heat at a rate of W, we need to add that to the required cooling power: Prequired Pcooling + Pbody 67 W + W 67 W.. PHASE CHANGE AND LATENT HEAT. A bag containing 0 C is much more effective in absorbing energy than one containing the same amount of 0 C. (a How much heat transfer is necessary to raise the temperature of kg of from 0 C to 0.0 C? (b How much heat transfer is required to first melt kg of 0 C and then raise its temperature? (c Explain how your answer supports the contention that the is more effective. (a Use mcδt, since there is no phase change involved in heating the : mcδt (0.800 kg(86 /kg C(0.0 C.00 (b To determine the heat required, we must melt the, using ml, and then add the heat required to raise the temperature of melted using mcδt, so that mlf + mcδt (0.800 kg( /kg (c The is much more effective in absorbing heat because it first must be melted, which requires a lot of energy, then it gains the heat that the also would. The first.67 of heat is used to melt the, then it absorbs the.00 of heat that the absorbs. f 6
3 College Physics Student s Manual Chapter 9. How many grams of coffee must evaporate from 0 g of coffee in a 0- g glass cup to cool the coffee from 9.0 C to.0 C? You may assume the coffee has the same thermal properties as and that the average heat of vaporization is 0 k/kg (60 cal/g. (You may neglect the change in mass of the coffee as it cools, which will give you an answer that is slightly larger than correct. The heat gained in evaporating the coffee equals the heat leaving the coffee and glass to lower its temperature, so that ML m c ΔT + m cgδt, where M is the mass of coffee that evaporates. Solving for the evaporated coffee gives: v c c g M ΔT ( mccc + mgc L (9.0 C.0 C 60 cal/g v g [(0g(.00 cal/g C + (0g(0.0 cal/g C ].0 g Not that we did the problem in calories and grams, since the latent heat was given in those units, and the result we wanted was in grams. We could have done the problem in standard units, and then converted back to grams to get the same answer.. If you pour 0.00 kg of 0.0 C onto a.0- kg block of (which is initially at.0 C, what is the final temperature? You may assume that the cools so rapidly that effects of the surroundings are negligible. The heat gained by the equals the heat lost by the. Since we do not know the final state of the / combination, we first need to compare the heat needed to raise the to 0 C and the heat available from the. First, we need to calculate how much heat would be required to raise the temperature of the to 0 C : mcδt (.0 kg(090 /kg C( C.76 Now, we need to calculate how much heat is given off to lower the to mcδt (0.00kg(86/kg C(0.0 C C : Since this is less than the heat required to heat the, we need to calculate how 7
4 College Physics Student s Manual Chapter much heat is given off to convert the to : mlf (0.00 kg( /kg.0 Thus, the total amount of heat given off to turn the to at C : Since >, we have determined that the final state of the / is at some temperature below 0 C. Now, we need to calculate the final temperature. We set the heat lost from the equal to the heat gained by the, where we now know that the final state is at T < 0 C: f m c ΔT + m L + m c ΔT m c ΔT lost by gained by, or 0 0 f 0?? Substituting for the change in temperatures (being careful that Δ T is always positive and simplifying gives m c (0 C + L + ( c (0 T ] m c [ T ( C]. [ f f f Solving for the final temperature gives T f m [ c (0 C + Lf ] m ( m + m c c ( C and so finally, T f (0.00 kg[(86 /kg C(0 C + (0.00 kg +.0 kg(090 /kg C (.0kg(090 /kg C( C (0.00 kg +.0 kg(090 /kg C. C /kg]. CONDUCTION. A man consumes 000 kcal of food in one day, converting most of it to maintain body temperature. If he loses half this energy by evaporating (through breathing and sweating, how many kilograms of evaporate? 8
5 College Physics Student s Manual Chapter Use mlv, where 000 kcal and Lv(7 C 80 kcal/kg, ml 0 kcal 80 kcal/kg v(7 C m Lv(7 C.9 kg 8. Compare the rate of heat conduction through a.0- cm- thick wall that has an area of.0 m and a thermal conductivity tw that of glass wool with the rate of heat conduction through a window that is 0.70 cm thick and that has an area of assuming the same temperature difference across each..00 m, ka( T T Use the rate of heat transfer by conduction,, and take the ratio of t d the wall to the window. The temperature difference for the wall and the window will be the same: ( / t ( / t wall window k k wall window A A wall d window window d wall ( 0.0 /s m C(.0 m (0.70 m (0.8 /s m C(.00 m (.0 m wall : window, or :window : wall So windows conduct more heat than walls. This should seem reasonable, since in the winter the windows feel colder than the walls..6 CONVECTION 0. One winter day, the climate control system of a large university classroom building malfunctions. As a result, 00 m of excess cold air is brought in each minute. At what rate in kilowatts must heat transfer occur to warm this air by.0 C (that is, to bring the air to room temperature? 9
6 College Physics Student s Manual Chapter Use mcδt in combination with the equations mcδt ρvcδt P t t t 7.7 W 77. kw. P and m ρv to get: t 7/kg C.0 C (.9 kg/m ( 00 m ( ( 60.0 s.7 RADIATION 8. (a Calculate the rate of heat transfer by radiation from a car radiator at C into a 0.0 C environment, if the radiator has an emissivity of 0.70 and a.0 - m surface area. (b Is this a significant fraction of the heat transfer by an automobile engine? To answer this, assume a horsepower of 00 hp (. kw and the efficiency of automobile engines as %. (a Using the Stefan- Boltzmann law of radiation, making sure to convert the temperatures into units of Kelvin, the rate of heat transfer is: t σ ea( T T (.67 8 /s m K (0.70(.0 m [( K ( 8 K ] W (b Assuming an automobile engine is 00 horsepower and the efficiency of a gasoline 00 horsepower engine is %, the engine consumes 800 horsepower in order % to generate the 00 horsepower. Therefore, 800 horsepower is lost due to hp heating. Since hp 76 W, the radiator transfers W 0.78 hp 76 W from radiation, which is not a significant fraction because the heat is primarily transferred from the radiator by other means.
7 College Physics Student s Manual Chapter 6. (a A shirtless rider under a circus tent feels the heat radiating from the sunlit portion of the tent. Calculate the temperature of the tent canvas based on the following information: The shirtless rider s skin temperature is.0 C and has an emissivity of The exposed area of skin is 0.00 m. He receives radiation at the rate of 0.0 W half what you would calculate if the entire region behind him was hot. The rest of the surroundings are at.0 C. (b Discuss how this situation would change if the sunlit side of the tent was nearly pure white and if the rider was covered by a white tunic. (a Use the Stefan- Boltzmann law of radiation: t T A σe ( T T / t + T σe( A/, / ( / t + T σea / (0.0 W 8 (.67 /s m K (0.970(0.00 m.6 K 8. C + (07 K (b A pure white object reflects more of the radiant energy that hits it, so the white tent would prevent more of the sunlight from heating up the inside of the tent, and the white tunic would prevent that radiant energy inside the tent from heating the rider. Therefore, with a white tent, the temperature would be lower than 8. C, and the rate of radiant heat transferred to the rider would be less than 0.0 W. / 70. Integrated Concepts (a Suppose you start a workout on a Stairmaster, producing power at the same rate as climbing 6 stairs per minute. Assuming your mass is 76.0 kg and your efficiency is 0.0%, how long will it take for your body temperature to rise.00º C if all other forms of heat transfer in and out of your body are balanced? (b Is this consistent with your experience in getting warm while exercising?
8 College Physics Student s Manual Chapter (a You produce power at a rate of 68 W, and since you are 0% efficient, you must produced 68 W have generated: P generated P W. efficiency 0.0 If only 68 W of power was useful, the power available to heat the body is P W 68 W.70 W. wasted mcδt Now, Pwasted, so that t t mcδt (76.0 kg(00 /kg C(.00 C t P.7 W wasted 97.s (b This says that it takes about a minute and a half to generate enough heat to raise the temperature of your body by.00 C, which seems quite reasonable. Generally, within five minutes of working out on a Stairmaster, you definitely feel warm and probably are sweating to keep your body from overheating. 76. Unreasonable Results (a What is the temperature increase of an 80.0 kg person who consumes 00 kcal of food in one day with 9.0% of the energy transferred as heat to the body? (b What is unreasonable about this result? (c Which premise or assumption is responsible? (0.90(00 kcal (a mcδt, so thatδt 6 C. mc (80.0 kg(0.8 kcal/kg C This says that the temperature of the person is 7 C + 6 C 7 C! (b Any temperature increase greater than about C would be unreasonably large. 7 C 6 F. In this case the final temperature of the person would rise to ( (c The assumption that the person retains 9% of the energy as body heat is unreasonable. Most of the food consumed on a day is converted to body heat, losing energy by sweating and breathing, etc.
AP PHYSICS 2 WHS-CH-14 Heat Show all your work, equations used, and box in your answers! 1 108kg
 AP PHYSICS 2 WHS-CH-4 Heat Show all your work, equations used, and box in your answers! James Prescott Joule (88 889) James Prescott Joule studied the nature of heat, and discovered its relationship to
AP PHYSICS 2 WHS-CH-4 Heat Show all your work, equations used, and box in your answers! James Prescott Joule (88 889) James Prescott Joule studied the nature of heat, and discovered its relationship to
Chapter 12 Solutions. Q Reason: We ll use Equation Q = McΔT and solve for M. We are given. In each case we want to solve for.
 Chapter 12 Solutions Q12.12. Reason: Assume the gas is an ideal gas, and use the ideal gas law pv = nrt. Since the number of moles doesn t change and R is a constant, then Equation 12.14 gives In each
Chapter 12 Solutions Q12.12. Reason: Assume the gas is an ideal gas, and use the ideal gas law pv = nrt. Since the number of moles doesn t change and R is a constant, then Equation 12.14 gives In each
Lecture 23. Specific Heat and Phase Changes
 Lecture 23 Specific Heat and Phase Changes Today s Topics: Heat and Temperature Change Specific heat Heat and Phase Change Latent heat Heat and Temperature Change Heat is energy that flows from a higher-temperature
Lecture 23 Specific Heat and Phase Changes Today s Topics: Heat and Temperature Change Specific heat Heat and Phase Change Latent heat Heat and Temperature Change Heat is energy that flows from a higher-temperature
PHYSICS 231 INTRODUCTORY PHYSICS I
 PHYSICS 231 INTRODUCTORY PHYSICS I Lecture 17 Heat: Q = Energy transferred due to microscopic contact Recap - Heat Transfer Heat can: Change temperature Q = mc!t c = specific heat For water: c= 1.0 cal/(g
PHYSICS 231 INTRODUCTORY PHYSICS I Lecture 17 Heat: Q = Energy transferred due to microscopic contact Recap - Heat Transfer Heat can: Change temperature Q = mc!t c = specific heat For water: c= 1.0 cal/(g
Physics Mechanics
 1 Physics 170 - Mechanics Lecture 35 Heat 2 Definition and Units of Heat Heat is a form of energy, and therefore is measured in joules. There are other units of heat, the most common one is the kilocalorie:
1 Physics 170 - Mechanics Lecture 35 Heat 2 Definition and Units of Heat Heat is a form of energy, and therefore is measured in joules. There are other units of heat, the most common one is the kilocalorie:
Chapter 11. Energy in Thermal Processes
 Chapter 11 Energy in Thermal Processes Energy Transfer When two objects of different temperatures are placed in thermal contact, the temperature of the warmer decreases and the temperature of the cooler
Chapter 11 Energy in Thermal Processes Energy Transfer When two objects of different temperatures are placed in thermal contact, the temperature of the warmer decreases and the temperature of the cooler
Chapter 11 Thermal Transport
 Chapter 11 Thermal Transport GOALS When you have mastered the contents of this chapter, you will be able to achieve the following goals: Definitions Define the following terms, and use them in an operational
Chapter 11 Thermal Transport GOALS When you have mastered the contents of this chapter, you will be able to achieve the following goals: Definitions Define the following terms, and use them in an operational
Thermodynamics - Heat Transfer June 04, 2013
 THERMODYNAMICS - Heat and Heat Transfer: Heat (Q) is a form of Energy that is transferred between an object and another object or its surrounding environment due to a difference in Temperature. Heat is
THERMODYNAMICS - Heat and Heat Transfer: Heat (Q) is a form of Energy that is transferred between an object and another object or its surrounding environment due to a difference in Temperature. Heat is
0 o K is called absolute zero. Water Freezes: 273 o K Water Boils: 373 o K
 Part I Notes Temperature and Heat The terms at the right all mean the same thing. The heat energy of a substance is the sum of the kinetic and potential energies of all of the atoms and molecules in the
Part I Notes Temperature and Heat The terms at the right all mean the same thing. The heat energy of a substance is the sum of the kinetic and potential energies of all of the atoms and molecules in the
Chapters 17 &19 Temperature, Thermal Expansion and The Ideal Gas Law
 Chapters 17 &19 Temperature, Thermal Expansion and The Ideal Gas Law Units of Chapter 17 & 19 Temperature and the Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion Heat and Mechanical Work
Chapters 17 &19 Temperature, Thermal Expansion and The Ideal Gas Law Units of Chapter 17 & 19 Temperature and the Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion Heat and Mechanical Work
Chapter 10 Temperature and Heat
 Chapter 10 Temperature and Heat Thermodynamics deals with 1. Temperature. 2. The transfer and transformation of energy. 3. The relationship between macroscopic properties and microscopic dynamics. Temperature
Chapter 10 Temperature and Heat Thermodynamics deals with 1. Temperature. 2. The transfer and transformation of energy. 3. The relationship between macroscopic properties and microscopic dynamics. Temperature
2012 Thermodynamics Division C
 Team: Team Number: Team Member Names: 1. 2. Instructions: Answer all questions on the test paper. If you need more room, you may attach extra paper. The test is worth a total of 50 points. Show all work
Team: Team Number: Team Member Names: 1. 2. Instructions: Answer all questions on the test paper. If you need more room, you may attach extra paper. The test is worth a total of 50 points. Show all work
Chapter 12. Temperature and Heat. continued
 Chapter 12 Temperature and Heat continued 12.3 The Ideal Gas Law THE IDEAL GAS LAW The absolute pressure of an ideal gas is directly proportional to the Kelvin temperature and the number of moles (n) of
Chapter 12 Temperature and Heat continued 12.3 The Ideal Gas Law THE IDEAL GAS LAW The absolute pressure of an ideal gas is directly proportional to the Kelvin temperature and the number of moles (n) of
Kinetic Theory continued
 Chapter 12 Kinetic Theory continued 12.4 Kinetic Theory of Gases The particles are in constant, random motion, colliding with each other and with the walls of the container. Each collision changes the
Chapter 12 Kinetic Theory continued 12.4 Kinetic Theory of Gases The particles are in constant, random motion, colliding with each other and with the walls of the container. Each collision changes the
SPECIFIC HEAT CAPACITY AND HEAT OF FUSION
 SPECIFIC HEAT CAPACITY AND HEAT OF FUSION Apparatus on each table: Thermometer, metal cube, complete calorimeter, outer calorimeter can (aluminum only), balance, 4 styrofoam cups, graduated container,
SPECIFIC HEAT CAPACITY AND HEAT OF FUSION Apparatus on each table: Thermometer, metal cube, complete calorimeter, outer calorimeter can (aluminum only), balance, 4 styrofoam cups, graduated container,
Energy in Thermal Processes. Heat and Internal Energy
 Energy in Thermal Processes Heat and Internal Energy Internal energy U: associated with the microscopic components of a system: kinetic and potential energies. The larger the number of internal degrees
Energy in Thermal Processes Heat and Internal Energy Internal energy U: associated with the microscopic components of a system: kinetic and potential energies. The larger the number of internal degrees
Physics 231. Topic 13: Heat. Alex Brown Dec 1, MSU Physics 231 Fall
 Physics 231 Topic 13: Heat Alex Brown Dec 1, 2015 MSU Physics 231 Fall 2015 1 8 th 10 pm correction for 3 rd exam 9 th 10 pm attitude survey (1% for participation) 10 th 10 pm concept test timed (50 min))
Physics 231 Topic 13: Heat Alex Brown Dec 1, 2015 MSU Physics 231 Fall 2015 1 8 th 10 pm correction for 3 rd exam 9 th 10 pm attitude survey (1% for participation) 10 th 10 pm concept test timed (50 min))
PHYS102 Previous Exam Problems. Temperature, Heat & The First Law of Thermodynamics
 PHYS102 Previous Exam Problems CHAPTER 18 Temperature, Heat & The First Law of Thermodynamics Equilibrium & temperature scales Thermal expansion Exchange of heat First law of thermodynamics Heat conduction
PHYS102 Previous Exam Problems CHAPTER 18 Temperature, Heat & The First Law of Thermodynamics Equilibrium & temperature scales Thermal expansion Exchange of heat First law of thermodynamics Heat conduction
Kinetic Theory continued
 Chapter 12 Kinetic Theory continued 12.4 Kinetic Theory of Gases The particles are in constant, random motion, colliding with each other and with the walls of the container. Each collision changes the
Chapter 12 Kinetic Theory continued 12.4 Kinetic Theory of Gases The particles are in constant, random motion, colliding with each other and with the walls of the container. Each collision changes the
Physics 111. Lecture 36 (Walker: ) Heat Capacity & Specific Heat Heat Transfer. May 1, Quiz (Chaps. 14 & 16) on Wed.
 Physics 111 Lecture 36 (Walker: 16.4-6) Heat Capacity & Specific Heat Heat Transfer May 1, 2009 Quiz (Chaps. 14 & 16) on Wed. May 6 Lecture 36 1/26 Heat Capacity (C) The heat capacity C of an object is
Physics 111 Lecture 36 (Walker: 16.4-6) Heat Capacity & Specific Heat Heat Transfer May 1, 2009 Quiz (Chaps. 14 & 16) on Wed. May 6 Lecture 36 1/26 Heat Capacity (C) The heat capacity C of an object is
Chapter 1 Heating Processes
 Chapter 1 Heating Processes Section 1.1 Heat and temperature Worked example: Try yourself 1.1.1 CALCULATING THE CHANGE IN INTERNAL ENERGY A student places a heating element and a paddle wheel apparatus
Chapter 1 Heating Processes Section 1.1 Heat and temperature Worked example: Try yourself 1.1.1 CALCULATING THE CHANGE IN INTERNAL ENERGY A student places a heating element and a paddle wheel apparatus
ConcepTest PowerPoints
 ConcepTest PowerPoints Chapter 14 Physics: Principles with Applications, 6 th edition Giancoli 2005 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for
ConcepTest PowerPoints Chapter 14 Physics: Principles with Applications, 6 th edition Giancoli 2005 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for
4.1. Physics Module Form 4 Chapter 4 - Heat GCKL UNDERSTANDING THERMAL EQUILIBRIUM. What is thermal equilibrium?
 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. ( Heat, Temperature ) is a form of energy that flows from a hot body to a cold body. 2. The SI unit for ( heat, temperature) is Joule,
4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. ( Heat, Temperature ) is a form of energy that flows from a hot body to a cold body. 2. The SI unit for ( heat, temperature) is Joule,
Chapter Notes: Temperature, Energy and Thermal Properties of Materials Mr. Kiledjian
 Chapter 10-11 Notes: Temperature, Energy and Thermal Properties of Materials Mr. Kiledjian 1) Temperature 2) Expansion of Matter 3) Ideal Gas Law 4) Kinetic Theory of Gases 5) Energy, Heat transfer and
Chapter 10-11 Notes: Temperature, Energy and Thermal Properties of Materials Mr. Kiledjian 1) Temperature 2) Expansion of Matter 3) Ideal Gas Law 4) Kinetic Theory of Gases 5) Energy, Heat transfer and
Chapter 14 Heat. Lecture PowerPoints. Chapter 14 Physics: Principles with Applications, 7 th edition Giancoli
 Lecture PowerPoints Chapter 14 Physics: Principles with Applications, 7 th edition Giancoli This work is protected by United States copyright laws and is provided solely for the use of instructors in teaching
Lecture PowerPoints Chapter 14 Physics: Principles with Applications, 7 th edition Giancoli This work is protected by United States copyright laws and is provided solely for the use of instructors in teaching
Chapter 14 Temperature and Heat
 Chapter 14 Temperature and Heat To understand temperature and temperature scales. To describe thermal expansion and its applications. To explore and solve problems involving heat, phase changes and calorimetry.
Chapter 14 Temperature and Heat To understand temperature and temperature scales. To describe thermal expansion and its applications. To explore and solve problems involving heat, phase changes and calorimetry.
Preview. Heat Section 1. Section 1 Temperature and Thermal Equilibrium. Section 2 Defining Heat. Section 3 Changes in Temperature and Phase
 Heat Section 1 Preview Section 1 Temperature and Thermal Equilibrium Section 2 Defining Heat Section 3 Changes in Temperature and Phase Heat Section 1 TEKS The student is expected to: 6E describe how the
Heat Section 1 Preview Section 1 Temperature and Thermal Equilibrium Section 2 Defining Heat Section 3 Changes in Temperature and Phase Heat Section 1 TEKS The student is expected to: 6E describe how the
Chapter 11. Energy in Thermal Processes
 Chapter 11 Energy in Thermal Processes Energy Transfer When two objects of different temperatures are placed in thermal contact, the temperature of the warmer decreases and the temperature of the cooler
Chapter 11 Energy in Thermal Processes Energy Transfer When two objects of different temperatures are placed in thermal contact, the temperature of the warmer decreases and the temperature of the cooler
Heat Transfer. Phys101 Lectures 33, 34. Key points: Heat as Energy Transfer Specific Heat Heat Transfer: Conduction, Convection, Radiation.
 Phys101 Lectures 33, 34 Heat Transfer Key points: Heat as Energy Transfer Specific Heat Heat Transfer: Conduction, Convection, Radiation. Ref: 14-1,2,3,4,6,7,8. Page 1 Heat as Energy Transfer We often
Phys101 Lectures 33, 34 Heat Transfer Key points: Heat as Energy Transfer Specific Heat Heat Transfer: Conduction, Convection, Radiation. Ref: 14-1,2,3,4,6,7,8. Page 1 Heat as Energy Transfer We often
Page 1 SPH3U. Heat. What is Heat? Thermal Physics. Waterloo Collegiate Institute. Some Definitions. Still More Heat
 SPH3U Thermal Physics electrons and holes in semiconductors An Introductory ourse in Thermodynamics converting energy into work magnetism thin films and surface chemistry thermal radiation (global warming)
SPH3U Thermal Physics electrons and holes in semiconductors An Introductory ourse in Thermodynamics converting energy into work magnetism thin films and surface chemistry thermal radiation (global warming)
Chapter 11. Energy in Thermal Processes
 Chapter 11 Energy in Thermal Processes Vocabulary, 3 Kinds of Energy Internal Energy U Energy of a system due to microscopic motion and inter-molucular forces Work W -F x -P V is work done by expansion
Chapter 11 Energy in Thermal Processes Vocabulary, 3 Kinds of Energy Internal Energy U Energy of a system due to microscopic motion and inter-molucular forces Work W -F x -P V is work done by expansion
4.1. Physics Module Form 4 Chapter 4 - Heat GCKL UNDERSTANDING THERMAL EQUILIBRIUM. What is thermal equilibrium?
 Physics Module Form 4 Chapter 4 - Heat GCKL 2010 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. (, Temperature ) is a form of energy that flows from a hot body to a cold body.
Physics Module Form 4 Chapter 4 - Heat GCKL 2010 4.1 4 UNDERSTANDING THERMAL EQUILIBRIUM What is thermal equilibrium? 1. (, Temperature ) is a form of energy that flows from a hot body to a cold body.
Chapter 11. Energy in Thermal Processes
 Chapter 11 Energy in Thermal Processes Vocabulary, 3 Kinds of Energy Internal Energy U = Energy of microscopic motion and intermolucular forces Work W = -F x = -P V is work done by compression (next chapter)
Chapter 11 Energy in Thermal Processes Vocabulary, 3 Kinds of Energy Internal Energy U = Energy of microscopic motion and intermolucular forces Work W = -F x = -P V is work done by compression (next chapter)
Energy, Temperature, & Heat. Energy, Temperature, & Heat. Temperature Scales 1/17/11
 Energy, Temperature, & Heat Energy is the ability to do work (push, pull, lift) on some form of matter. Chapter 2 Potential energy is the potential for work (mass x gravity x height) Kinetic energy is
Energy, Temperature, & Heat Energy is the ability to do work (push, pull, lift) on some form of matter. Chapter 2 Potential energy is the potential for work (mass x gravity x height) Kinetic energy is
Chapter 5: Thermochemistry. Problems: , , 5.100, 5.106, 5.108, , 5.121, 5.126
 Chapter 5: Thermochemistry Problems: 5.1-5.95, 5.97-98, 5.100, 5.106, 5.108, 5.118-5.119, 5.121, 5.126 Energy: Basic Concepts and Definitions energy: capacity to do work or to produce heat thermodynamics:
Chapter 5: Thermochemistry Problems: 5.1-5.95, 5.97-98, 5.100, 5.106, 5.108, 5.118-5.119, 5.121, 5.126 Energy: Basic Concepts and Definitions energy: capacity to do work or to produce heat thermodynamics:
Thermal Conductivity, k
 Homework # 85 Specific Heats at 20 C and 1 atm (Constant Pressure) Substance Specific Heat, c Substance Specific Heat, c kcal/kg C J/kg C kcal/kg C J/kg C Solids Aluminum 0.22 900 Brass 0.090 377 Copper
Homework # 85 Specific Heats at 20 C and 1 atm (Constant Pressure) Substance Specific Heat, c Substance Specific Heat, c kcal/kg C J/kg C kcal/kg C J/kg C Solids Aluminum 0.22 900 Brass 0.090 377 Copper
7. (2) Of these elements, which has the greatest number of atoms in a mole? a. hydrogen (H) b. oxygen (O) c. iron (Fe) d. gold (Au) e. all tie.
 General Physics I Exam 5 - Chs. 13,14,15 - Heat, Kinetic Theory, Thermodynamics Dec. 14, 2010 Name Rec. Instr. Rec. Time For full credit, make your work clear to the grader. Show formulas used, essential
General Physics I Exam 5 - Chs. 13,14,15 - Heat, Kinetic Theory, Thermodynamics Dec. 14, 2010 Name Rec. Instr. Rec. Time For full credit, make your work clear to the grader. Show formulas used, essential
EDULABZ INTERNATIONAL. Heat ASSIGNMENT
 Heat ASSIGNMENT 1. Fill in the blank spaces by choosing the correct words from the list given below : List : substance, thermal capacity, mass, latent, heat, cold, constant, water, J C 1, fusion, hot.
Heat ASSIGNMENT 1. Fill in the blank spaces by choosing the correct words from the list given below : List : substance, thermal capacity, mass, latent, heat, cold, constant, water, J C 1, fusion, hot.
The First Law of Thermodynamics *
 OpenStax-CNX module: m42232 1 The First Law of Thermodynamics * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 Abstract Dene the rst law
OpenStax-CNX module: m42232 1 The First Law of Thermodynamics * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 Abstract Dene the rst law
Section 2. Energy Fundamentals
 Section 2 Energy Fundamentals 1 Energy Fundamentals Open and Closed Systems First Law of Thermodynamics Second Law of Thermodynamics Examples of heat engines and efficiency Heat Transfer Conduction, Convection,
Section 2 Energy Fundamentals 1 Energy Fundamentals Open and Closed Systems First Law of Thermodynamics Second Law of Thermodynamics Examples of heat engines and efficiency Heat Transfer Conduction, Convection,
Chapter 1: 20, 23, 35, 41, 68, 71, 76, 77, 80, 85, 90, 101, 103 and 104.
 Chapter 1: 0, 3, 35, 1, 68, 71, 76, 77, 80, 85, 90, 101, 103 and 10. 1-0 The filament of a 150 W incandescent lamp is 5 cm long and has a diameter of 0.5 mm. The heat flux on the surface of the filament,
Chapter 1: 0, 3, 35, 1, 68, 71, 76, 77, 80, 85, 90, 101, 103 and 10. 1-0 The filament of a 150 W incandescent lamp is 5 cm long and has a diameter of 0.5 mm. The heat flux on the surface of the filament,
Each of the following 50 questions are worth 2 points each. Answer a = true b = false
 PHY 262 Exam 3-----preview----- 8/7/2012 NAME This exam is closed book and closed notes, but open calculator. You have about 80 minutes to complete the exam (~ 9:30 10:50). The actual exam will be 50 questions
PHY 262 Exam 3-----preview----- 8/7/2012 NAME This exam is closed book and closed notes, but open calculator. You have about 80 minutes to complete the exam (~ 9:30 10:50). The actual exam will be 50 questions
1. Thermal energy is transferred through the glass windows of a house mainly by. D. radiation and convection. (1)
 1. Thermal energy is transferred through the glass windows of a house mainly by A. conduction. B. radiation. C. conduction and convection. D. radiation and convection. 2. The specific latent heat of vaporization
1. Thermal energy is transferred through the glass windows of a house mainly by A. conduction. B. radiation. C. conduction and convection. D. radiation and convection. 2. The specific latent heat of vaporization
Chapter 10 Test Form B
 Chapter 10 Test Form A 1. B 2. A 3. A 4. B 5. D 6. B 7. B 8. A 9. A 10. A 11. B 12. D 13. A 14. C 15. No, heat and cold do not flow between objects. Energy transferred between objects changes the temperature
Chapter 10 Test Form A 1. B 2. A 3. A 4. B 5. D 6. B 7. B 8. A 9. A 10. A 11. B 12. D 13. A 14. C 15. No, heat and cold do not flow between objects. Energy transferred between objects changes the temperature
Exam 2--PHYS 151--Spring 2017
 Name: Exam 2--PHYS 151--Spring 2017 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Consider this diagram of a human heart. Which section is the right
Name: Exam 2--PHYS 151--Spring 2017 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Consider this diagram of a human heart. Which section is the right
Chapter 18 Temperature, Heat, and the First Law of Thermodynamics. Thermodynamics and Statistical Physics
 Chapter 18 Temperature, Heat, and the First Law of Thermodynamics Thermodynamics and Statistical Physics Key contents: Temperature scales Thermal expansion Temperature and heat, specific heat Heat and
Chapter 18 Temperature, Heat, and the First Law of Thermodynamics Thermodynamics and Statistical Physics Key contents: Temperature scales Thermal expansion Temperature and heat, specific heat Heat and
!U = Q " P!V. Q = mc!t. Vocabulary, 3 Kinds of Energy. Chapter 11. Energy in Thermal Processes. Example Temperature and Specific Heat
 Vocabulary, 3 Kinds of Energy Chapter 11 Energy in Thermal Processes Internal Energy U = Energy of microscopic motion and intermolucular forces Work W = -F!x = -P!V is work done by compression (next chapter)
Vocabulary, 3 Kinds of Energy Chapter 11 Energy in Thermal Processes Internal Energy U = Energy of microscopic motion and intermolucular forces Work W = -F!x = -P!V is work done by compression (next chapter)
Physics 101: Lecture 25 Heat
 Final Physics 101: Lecture 25 Heat Today s lecture will cover Textbook Chapter 14.1-14.5 Physics 101: Lecture 25, Pg 1 Internal Energy Energy of all molecules including Random motion of individual molecules»
Final Physics 101: Lecture 25 Heat Today s lecture will cover Textbook Chapter 14.1-14.5 Physics 101: Lecture 25, Pg 1 Internal Energy Energy of all molecules including Random motion of individual molecules»
Lecture PowerPoints. Chapter 14 Physics: Principles with Applications, 6 th edition Giancoli
 Lecture PowerPoints Chapter 14 Physics: Principles with Applications, 6 th edition Giancoli 2005 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for
Lecture PowerPoints Chapter 14 Physics: Principles with Applications, 6 th edition Giancoli 2005 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for
Chapter 2 Energy and Matter 2.1 Energy
 Chapter 2 Energy and Matter 2.1 Energy 1 Energy Energy makes objects move. makes things stop. is needed to do work. 2 Work Work is done when you climb. you lift a bag of groceries. you ride a bicycle.
Chapter 2 Energy and Matter 2.1 Energy 1 Energy Energy makes objects move. makes things stop. is needed to do work. 2 Work Work is done when you climb. you lift a bag of groceries. you ride a bicycle.
General Physics (PHY 2130)
 General Physics (PHY 2130) Lecture 34 Heat Heat transfer Conduction Convection Radiation http://www.physics.wayne.edu/~apetrov/phy2130/ Lightning Review Last lecture: 1. Thermal physics Heat. Specific
General Physics (PHY 2130) Lecture 34 Heat Heat transfer Conduction Convection Radiation http://www.physics.wayne.edu/~apetrov/phy2130/ Lightning Review Last lecture: 1. Thermal physics Heat. Specific
(in m^3) 4.A 1.62 B 2.35 C 3.41 D pt 5.A B C pt
 Mark Reeves - Physics 21 Spring 2012 1 An automobile driver fills his 17.1-L steel gasoline tank in the cool of the morning when the temperature of the tank and the gasoline is 15.0 C and the pressure
Mark Reeves - Physics 21 Spring 2012 1 An automobile driver fills his 17.1-L steel gasoline tank in the cool of the morning when the temperature of the tank and the gasoline is 15.0 C and the pressure
Chapter 11. Important to distinguish between them. They are not interchangeable. They mean very different things when used in physics Internal Energy
 Chapter 11 Energy in Thermal Processes Energy Transfer When two objects of different temperatures are placed in thermal contact, the temperature of the warmer decreases and the temperature of the cooler
Chapter 11 Energy in Thermal Processes Energy Transfer When two objects of different temperatures are placed in thermal contact, the temperature of the warmer decreases and the temperature of the cooler
A) 3.1 m/s B) 9.9 m/s C) 14 m/s D) 17 m/s E) 31 m/s
 1. A large tank, open at the top, is filled with water to a depth of 15 m. A spout located 10.0 m above the bottom of the tank is then opened as shown in the drawing. With what speed will water emerge
1. A large tank, open at the top, is filled with water to a depth of 15 m. A spout located 10.0 m above the bottom of the tank is then opened as shown in the drawing. With what speed will water emerge
TEMPERATURE. 8. Temperature and Heat 1
 TEMPERATURE Heat is the energy that is transferred between objects because of a temperature difference Terms such as transfer of heat or heat flow from object A to object B simply means that the total
TEMPERATURE Heat is the energy that is transferred between objects because of a temperature difference Terms such as transfer of heat or heat flow from object A to object B simply means that the total
40P (2 x 60 x 60) = 2.5 x 10 6 (4200)(5) P = 1.82 x 10 5 W
 NAME : F.3C ( ) Marks: /50 Form 3 Physics Assessment on Heat Time allowed: 45 minutes Section A (34 marks) 1. An indoor swimming pool containing 2.5 x 10 6 kg of water uses 40 identical heaters to maintain
NAME : F.3C ( ) Marks: /50 Form 3 Physics Assessment on Heat Time allowed: 45 minutes Section A (34 marks) 1. An indoor swimming pool containing 2.5 x 10 6 kg of water uses 40 identical heaters to maintain
3.3 Phase Changes 88 A NATURAL APPROACH TO CHEMISTRY. Section 3.3 Phase Changes
 Section 3.3 Phase Changes 3.3 Phase Changes Solid, liquid and gas During a phase change, a substance rearranges the order of its particles (atoms or molecules). Examples of phase change include melting
Section 3.3 Phase Changes 3.3 Phase Changes Solid, liquid and gas During a phase change, a substance rearranges the order of its particles (atoms or molecules). Examples of phase change include melting
Temp vs. Heat. Absolute Temperature Scales. Common Temperature Scales. Thermal Energy. Heat and Temperature are not the same!!
 Thermal Energy Heat and Temperature are not the same!! Cold is the absence of heat, not an energy Same concept as light/dark Cold can t come in, heat flows out Heat flows from High Temp Low Temp Temp vs.
Thermal Energy Heat and Temperature are not the same!! Cold is the absence of heat, not an energy Same concept as light/dark Cold can t come in, heat flows out Heat flows from High Temp Low Temp Temp vs.
Lecture 22. Temperature and Heat
 Lecture 22 Temperature and Heat Today s Topics: 0 th Law of Thermodynamics Temperature Scales Thermometers Thermal Expansion Heat, Internal Energy and Work Heat Transfer Temperature and the Zeroth Law
Lecture 22 Temperature and Heat Today s Topics: 0 th Law of Thermodynamics Temperature Scales Thermometers Thermal Expansion Heat, Internal Energy and Work Heat Transfer Temperature and the Zeroth Law
Thermodynamics. Thermodynamics is the study of the collective properties of a system containing many bodies (typically of order 10 23!
 Thermodynamics Thermodynamics is the study of the collective properties of a system containing many bodies (typically of order 10 23!) Chapter18 Thermodynamics Thermodynamics is the study of the thermal
Thermodynamics Thermodynamics is the study of the collective properties of a system containing many bodies (typically of order 10 23!) Chapter18 Thermodynamics Thermodynamics is the study of the thermal
Thermochemistry. The study of energy changes that occur during chemical reactions and changes in state.
 Energy Thermochemistry The study of energy changes that occur during chemical reactions and changes in state. The Nature of Energy Energy - the ability to do work or produce heat Energy is stored in the
Energy Thermochemistry The study of energy changes that occur during chemical reactions and changes in state. The Nature of Energy Energy - the ability to do work or produce heat Energy is stored in the
2. If the volume of a container holding a gas is reduced, what will happen to the presure within the container?
 1. Which gas law states that the volume of a fixed mass of a gas is directly proportional to its Kelvin temperature if the pressure is kept constant? A. Boyle s law B. Charles law C. Dalton s law D. Gay-Lussac
1. Which gas law states that the volume of a fixed mass of a gas is directly proportional to its Kelvin temperature if the pressure is kept constant? A. Boyle s law B. Charles law C. Dalton s law D. Gay-Lussac
Core Concepts. PowerPoint Lectures to accompany Physical Science, 8e. Chapter 4 Heat and Temperature. New Symbols for this Chapter 2/14/2011
 PowerPoint Lectures to accompany Physical Science, 8e Chapter 4 Heat and Temperature Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. New Symbols for this Chapter
PowerPoint Lectures to accompany Physical Science, 8e Chapter 4 Heat and Temperature Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. New Symbols for this Chapter
S6. (a) State what is meant by an ideal gas...
 IB PHYSICS Name: DEVIL PHYSICS Period: Date: BADDEST CLASS ON CAMPUS TSOKOS CHAPTER 3 TEST REVIEW S1. Thermal energy is transferred through the glass windows of a house mainly by A. conduction. B. radiation.
IB PHYSICS Name: DEVIL PHYSICS Period: Date: BADDEST CLASS ON CAMPUS TSOKOS CHAPTER 3 TEST REVIEW S1. Thermal energy is transferred through the glass windows of a house mainly by A. conduction. B. radiation.
Thermal Energy. Practice Quiz Solutions
 Thermal Energy Practice Quiz Solutions What is thermal energy? What is thermal energy? Thermal energy is the energy that comes from heat. This heat is generated by the movement of tiny particles within
Thermal Energy Practice Quiz Solutions What is thermal energy? What is thermal energy? Thermal energy is the energy that comes from heat. This heat is generated by the movement of tiny particles within
Questions and Problems
 668 Chapter 15 Thermodynamics II Q = 0 and so U = 2W. For process a S b we have U 7 0 (the internal energy increases because the temperature increases) and W 7 0 (the gas expands), so Q = U + W is positive
668 Chapter 15 Thermodynamics II Q = 0 and so U = 2W. For process a S b we have U 7 0 (the internal energy increases because the temperature increases) and W 7 0 (the gas expands), so Q = U + W is positive
Figure 1.1. Relation between Celsius and Fahrenheit scales. From Figure 1.1. (1.1)
 CHAPTER I ELEMENTS OF APPLIED THERMODYNAMICS 1.1. INTRODUCTION. The Air Conditioning systems extract heat from some closed location and deliver it to other places. To better understanding the principles
CHAPTER I ELEMENTS OF APPLIED THERMODYNAMICS 1.1. INTRODUCTION. The Air Conditioning systems extract heat from some closed location and deliver it to other places. To better understanding the principles
THERMAL PROPERTIES OF MATTER
 CHP # 8 HERMA PROPERIES OF MAER Q.1 Differentiate between heat and temperature? (Ans) Heat It can be defined as "the sum of kinetic energy of the molecules present in a substance is called heat". Heat
CHP # 8 HERMA PROPERIES OF MAER Q.1 Differentiate between heat and temperature? (Ans) Heat It can be defined as "the sum of kinetic energy of the molecules present in a substance is called heat". Heat
NO MID-TERM. Thermal Energy. Mathematica. CPR news. How energy can enter or leave a system. Power 25/03/11. in this course. Heat and Temperature
 NO MID-TERM Thermal Energy Heat and Temperature in this course. Clickers Channel D Mathematica Optional (but very powerful) software for doing maths and substituting numbers into equations. Also good mathematical
NO MID-TERM Thermal Energy Heat and Temperature in this course. Clickers Channel D Mathematica Optional (but very powerful) software for doing maths and substituting numbers into equations. Also good mathematical
Agenda. Chapter 10, Problem 26. All matter is made of atoms. Atomic Structure 4/8/14. What is the structure of matter? Atomic Terminology
 Agenda Today: HW Quiz, Thermal physics (i.e., heat) Thursday: Finish thermal physics, atomic structure (lots of review from chemistry!) Chapter 10, Problem 26 A boy reaches out of a window and tosses a
Agenda Today: HW Quiz, Thermal physics (i.e., heat) Thursday: Finish thermal physics, atomic structure (lots of review from chemistry!) Chapter 10, Problem 26 A boy reaches out of a window and tosses a
Topic 5: Energetics. Heat & Calorimetry. Thursday, March 22, 2012
 Topic 5: Energetics Heat & Calorimetry 1 Heat is energy that is transferred from one object to another due to a difference in temperature Temperature is a measure of the average kinetic energy of a body
Topic 5: Energetics Heat & Calorimetry 1 Heat is energy that is transferred from one object to another due to a difference in temperature Temperature is a measure of the average kinetic energy of a body
LECTURE 09: Heat transfer mechanisms
 LECTURE 09: Heat transfer mechanisms Select LEARNING OBJECTIVES: Understand there are three mechanisms by which thermal energy can be transferred as heat. Understand that all objects, everywhere, are constantly
LECTURE 09: Heat transfer mechanisms Select LEARNING OBJECTIVES: Understand there are three mechanisms by which thermal energy can be transferred as heat. Understand that all objects, everywhere, are constantly
5) The amount of heat needed to raise the temperature of 1 gram of a substance by 1 C is called: Page Ref: 69
 Homework #2 Due 9/19/14 1) If the maximum temperature for a particular day is 26 C and the minimum temperature is 14 C, what would the daily mean temperature be? (Page Ref: 66) 2) How is the annual mean
Homework #2 Due 9/19/14 1) If the maximum temperature for a particular day is 26 C and the minimum temperature is 14 C, what would the daily mean temperature be? (Page Ref: 66) 2) How is the annual mean
Name Partner. Thermal Physics. Part I: Heat of Vaporization of Nitrogen. Introduction:
 Name Partner Thermal Physics Part I: Heat of Vaporization of Nitrogen Introduction: The heat of vaporization of a liquid, L v, is the energy required to vaporize (boil) a unit mass of substance. Thus if
Name Partner Thermal Physics Part I: Heat of Vaporization of Nitrogen Introduction: The heat of vaporization of a liquid, L v, is the energy required to vaporize (boil) a unit mass of substance. Thus if
What Do You Think? Investigate GOALS. Part A: Freezing Water
 Activity 5 Freezing Water GOALS In this activity you will: Determine the freezing point of water. Show graphically what happens to the temperature as water is cooled to freezing and while it is freezing.
Activity 5 Freezing Water GOALS In this activity you will: Determine the freezing point of water. Show graphically what happens to the temperature as water is cooled to freezing and while it is freezing.
Review: Heat, Temperature, Heat Transfer and Specific Heat Capacity
 Name: Block: Date: IP 614 Review: Heat, Temperature, Heat Transfer and Specific Heat Capacity All these questions are real MCAS questions! 1. In a copper wire, a temperature increase is the result of which
Name: Block: Date: IP 614 Review: Heat, Temperature, Heat Transfer and Specific Heat Capacity All these questions are real MCAS questions! 1. In a copper wire, a temperature increase is the result of which
Temperature of body can be increased by doing work on it. Here W = E mgh = E internal
 Heat (C19.1-6, 10) Temperature (T) is measure of average KE of all molecules Internal energy (or Thermal Energy) is sum of total energy of all molecules. Heat is transfer of IE from one body to another.
Heat (C19.1-6, 10) Temperature (T) is measure of average KE of all molecules Internal energy (or Thermal Energy) is sum of total energy of all molecules. Heat is transfer of IE from one body to another.
UNIT 1 - FORCE TEMPERATURE IN THERMAL SYSTEMS ACTIVITY LESSON DESCRIPTION SCORE/POINTS
 NAME PERIOD UNIT 1 - FORCE TEMPERATURE IN THERMAL SYSTEMS ACTIVITY LESSON DESCRIPTION SCORE/POINTS 1. TX PP. 64-76 /46 2. WS READING GUIDE CONCEPT APPLICATION /21 3. MS MATH PRACTICE (Heat formula) /20
NAME PERIOD UNIT 1 - FORCE TEMPERATURE IN THERMAL SYSTEMS ACTIVITY LESSON DESCRIPTION SCORE/POINTS 1. TX PP. 64-76 /46 2. WS READING GUIDE CONCEPT APPLICATION /21 3. MS MATH PRACTICE (Heat formula) /20
Thermal Effects. IGCSE Physics
 Thermal Effects IGCSE Physics Starter What is the difference between heat and temperature? What unit is thermal energy measured in? And what does it depend on? In which direction does heat flow? Heat (Thermal
Thermal Effects IGCSE Physics Starter What is the difference between heat and temperature? What unit is thermal energy measured in? And what does it depend on? In which direction does heat flow? Heat (Thermal
By Marek Tuliszka D.Sc. Department of Biophysics Poznań University of Medical Sciences
 By Marek Tuliszka D.Sc. Department of Biophysics Poznań University of Medical Sciences ! CHEMICAL WORK: Secretion of hydrochloric acid (HCl) by the stomach and sodium bicarbonate (NaHCO 3 ) by the pancreas.
By Marek Tuliszka D.Sc. Department of Biophysics Poznań University of Medical Sciences ! CHEMICAL WORK: Secretion of hydrochloric acid (HCl) by the stomach and sodium bicarbonate (NaHCO 3 ) by the pancreas.
1. How much heat was needed to raise the bullet to its final temperature?
 Name: Date: Use the following to answer question 1: A 0.0500-kg lead bullet of volume 5.00 10 6 m 3 at 20.0 C hits a block that is made of an ideal thermal insulator and comes to rest at its center. At
Name: Date: Use the following to answer question 1: A 0.0500-kg lead bullet of volume 5.00 10 6 m 3 at 20.0 C hits a block that is made of an ideal thermal insulator and comes to rest at its center. At
Heat gained by soda = Heat lost by watermelon Qsoda = Qwatermelon
 PHYS1114 SAMPLE EXAM 5 SOLUTIONS Spring 2013 Professor Kenny L. Tapp 1. Dermatologists often remove small precancerous skin lesions by freezing them quickly with liquid nitrogen, which has a temperature
PHYS1114 SAMPLE EXAM 5 SOLUTIONS Spring 2013 Professor Kenny L. Tapp 1. Dermatologists often remove small precancerous skin lesions by freezing them quickly with liquid nitrogen, which has a temperature
Section 3.5 Thermal Comfort and Heat Stress
 Section 3.5 Thermal Comfort and Heat Stress Table 3.6 Metabolic rate as a function of physical activity for a 70 kg adult man (abstracted from ASHRAE, 1997). activity metabolic rate (W) metabolic rate
Section 3.5 Thermal Comfort and Heat Stress Table 3.6 Metabolic rate as a function of physical activity for a 70 kg adult man (abstracted from ASHRAE, 1997). activity metabolic rate (W) metabolic rate
A). Yes. B). No. Q15 Is it possible for a solid metal ball to float in mercury?
 Q15 Is it possible for a solid metal ball to float in mercury? A). Yes. B). No. The upward force is the weight of liquid displaced and the downward force is the weight of the ball. If the density of the
Q15 Is it possible for a solid metal ball to float in mercury? A). Yes. B). No. The upward force is the weight of liquid displaced and the downward force is the weight of the ball. If the density of the
UNIVERSITY COLLEGE LONDON. University of London EXAMINATION FOR INTERNAL STUDENTS. For The Following Qualifications:-
 UNIVERSITY COLLEGE LONDON University of London EXAMINATION FOR INTERNAL STUDENTS For The Following Qualifications:- B.Sc. M.Sci. Physics 1B28: Thermal Physics COURSE CODE : PHYSIB28 UNIT VALUE : 0.50 DATE
UNIVERSITY COLLEGE LONDON University of London EXAMINATION FOR INTERNAL STUDENTS For The Following Qualifications:- B.Sc. M.Sci. Physics 1B28: Thermal Physics COURSE CODE : PHYSIB28 UNIT VALUE : 0.50 DATE
CALORIEMETRY. Similar to the other forms of the energy, The S.I unit of heat is joule. joule is represented as J.
 CALORIEMETRY CALORIMETRY Heat is the kinetic energy due to random motion of the molecules of a substance is called heat energy. Heat is a an invisible energy, that causes in us the sensation of hotness
CALORIEMETRY CALORIMETRY Heat is the kinetic energy due to random motion of the molecules of a substance is called heat energy. Heat is a an invisible energy, that causes in us the sensation of hotness
Phy 212: General Physics II
 Phy 212: General Physics II Chapter 18: Temperature, Heat & the 1 st Law of Thermodynamics Lecture Notes What is Temperature? 1. Temperature (T) is a measure of how hot or cold something is 2. Temperature
Phy 212: General Physics II Chapter 18: Temperature, Heat & the 1 st Law of Thermodynamics Lecture Notes What is Temperature? 1. Temperature (T) is a measure of how hot or cold something is 2. Temperature
Chapter 3. Basic Principles. Contents
 Chapter 3. Basic Principles Contents 3.1 Introduction 3.2 Heat 3.3 Sensible Heat 3.4 Latent Heat 3.5 Evaporative Cooling 3.6 Convection 3.7 Transport 3.8 Energy Transfer Mediums 3.9 Radiation 3.10 Greenhouse
Chapter 3. Basic Principles Contents 3.1 Introduction 3.2 Heat 3.3 Sensible Heat 3.4 Latent Heat 3.5 Evaporative Cooling 3.6 Convection 3.7 Transport 3.8 Energy Transfer Mediums 3.9 Radiation 3.10 Greenhouse
14 HEAT AND HEAT TRANSFER METHODS
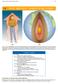 Chapter 14 Heat and Heat Transfer Methods 513 14 HEAT AND HEAT TRANSFER METHODS Figure 14.1 (a) The chilling effect of a clear breezy night is produced by the wind and by radiative heat transfer to cold
Chapter 14 Heat and Heat Transfer Methods 513 14 HEAT AND HEAT TRANSFER METHODS Figure 14.1 (a) The chilling effect of a clear breezy night is produced by the wind and by radiative heat transfer to cold
For more info visit
 Heat:- Heat is the agent which produces in us the sensation of warmth and makes bodies hot. It is form of energy. The part of thermal energy which flows from one body to the other due to temperature difference
Heat:- Heat is the agent which produces in us the sensation of warmth and makes bodies hot. It is form of energy. The part of thermal energy which flows from one body to the other due to temperature difference
PHYSICS 149: Lecture 26
 PHYSICS 149: Lecture 26 Chapter 14: Heat 14.1 Internal Energy 14.2 Heat 14.3 Heat Capacity and Specific Heat 14.5 Phase Transitions 14.6 Thermal Conduction 14.7 Thermal Convection 14.8 Thermal Radiation
PHYSICS 149: Lecture 26 Chapter 14: Heat 14.1 Internal Energy 14.2 Heat 14.3 Heat Capacity and Specific Heat 14.5 Phase Transitions 14.6 Thermal Conduction 14.7 Thermal Convection 14.8 Thermal Radiation
Temperature and Its Measurement
 Temperature and Its Measurement When the physical properties are no longer changing, the objects are said to be in thermal equilibrium. Two or more objects in thermal equilibrium have the same temperature.
Temperature and Its Measurement When the physical properties are no longer changing, the objects are said to be in thermal equilibrium. Two or more objects in thermal equilibrium have the same temperature.
Temperature and Thermometers. Temperature is a measure of how hot or cold something is. Most materials expand when heated.
 Heat Energy Temperature and Thermometers Temperature is a measure of how hot or cold something is. Most materials expand when heated. Thermometers are instruments designed to measure temperature. In order
Heat Energy Temperature and Thermometers Temperature is a measure of how hot or cold something is. Most materials expand when heated. Thermometers are instruments designed to measure temperature. In order
Physics 221, March 22
 Physics 221, March 22 Key Concepts: Temperature and pressure Heat Regulating heat flow Thermal properties of matter Temperature The average kinetic energy of the random motion of the molecules of a substance
Physics 221, March 22 Key Concepts: Temperature and pressure Heat Regulating heat flow Thermal properties of matter Temperature The average kinetic energy of the random motion of the molecules of a substance
LECTURE 9 LATENT HEAT & PHASE CHANGE. Lecture Instructor: Kazumi Tolich
 LECTURE 9 LATENT HEAT & PHASE CHANGE Lecture Instructor: Kazumi Tolich Lecture 9 2! Reading chapter 17-5 to 17-6.! Latent heats " Latent heat of fusion " Latent heat of vaporization " Latent heat of sublimation!
LECTURE 9 LATENT HEAT & PHASE CHANGE Lecture Instructor: Kazumi Tolich Lecture 9 2! Reading chapter 17-5 to 17-6.! Latent heats " Latent heat of fusion " Latent heat of vaporization " Latent heat of sublimation!
The triple points of neon and carbon dioxide are K and K respectively. Express these temperatures on the Celsius and Fahrenheit scales.
 Question 11.1: The triple points of neon and carbon dioxide are 24.57 K and 216.55 K respectively. Express these temperatures on the Celsius and Fahrenheit scales. Kelvin and Celsius scales are related
Question 11.1: The triple points of neon and carbon dioxide are 24.57 K and 216.55 K respectively. Express these temperatures on the Celsius and Fahrenheit scales. Kelvin and Celsius scales are related
(Heat capacity c is also called specific heat) this means that the heat capacity number c for water is 1 calorie/gram-k.
 Lecture 23: Ideal Gas Law and The First Law of Thermodynamics 1 (REVIEW) Chapter 17: Heat Transfer Origin of the calorie unit A few hundred years ago when people were investigating heat and temperature
Lecture 23: Ideal Gas Law and The First Law of Thermodynamics 1 (REVIEW) Chapter 17: Heat Transfer Origin of the calorie unit A few hundred years ago when people were investigating heat and temperature
Topic 3: Thermal physics 3.1 Thermal concepts
 Understandings: Molecular theory of solids, liquids and gases Temperature and absolute temperature Internal energy Specific heat capacity Phase change Specific latent heat Applications and skills: Describing
Understandings: Molecular theory of solids, liquids and gases Temperature and absolute temperature Internal energy Specific heat capacity Phase change Specific latent heat Applications and skills: Describing
Quiz C&J page 316 (top), Check Your Understanding #6:... use a straw
 Quiz on Chapter 11 Quiz 9 1. C&J page 316 (top), Check Your Understanding #6:... use a straw Quiz 9 1. C&J page 316 (top), Check Your Understanding #6:... use a straw 2. What volume of helium has the same
Quiz on Chapter 11 Quiz 9 1. C&J page 316 (top), Check Your Understanding #6:... use a straw Quiz 9 1. C&J page 316 (top), Check Your Understanding #6:... use a straw 2. What volume of helium has the same
MATTER IN OUR SURROUNDINGS
 CLASS 9 MATTER IN OUR SURROUNDINGS Matter: Anything That occupies space and has mass. Matter is made up of particles. Particles of matter are very small or tiny. Characteristics of particles of matter
CLASS 9 MATTER IN OUR SURROUNDINGS Matter: Anything That occupies space and has mass. Matter is made up of particles. Particles of matter are very small or tiny. Characteristics of particles of matter
