REDUCTION FOR MICHAELIS-MENTEN-HENRI KINETICS IN THE PRESENCE OF DIFFUSION
|
|
|
- Cordelia Rich
- 6 years ago
- Views:
Transcription
1 26 International Conference in Honor of Jacqueline Fleckinger. Electronic Journal of Differential Equations, Conference 6, 27, pp.?? ISSN: URL: or ftp ejde.math.txstate.edu (login: ftp) REDUCTION FOR MICHAELIS-MENTEN-HENRI KINETICS IN THE PRESENCE OF DIFFUSION LEONID V. KALACHEV, HANS G. KAPER, TASSO J. KAPER, NIKOLA POPOVIĆ, AND ANTONIOS ZAGARIS Dedicated to Jacqueline Fleckinger on her 65-th birthday Abstract. The Michaelis-Menten-Henri (MMH) mechanism is one of the paradigm reaction mechanisms in biology and chemistry. In its simplest form, it involves a substrate that reacts (reversibly) with an enzyme, forming a complex which is transformed (irreversibly) into a product and the enzyme. Given these basic kinetics, a dimension reduction has traditionally been achieved in two steps, by using conservation relations to reduce the number of species and by exploiting the inherent fast-slow structure of the resulting equations. In the present article, we investigate how the dynamics change if the species are additionally allowed to diffuse. We study the two extreme regimes of large diffusivities and of small diffusivities, as well as an intermediate regime in which the time scale of diffusion is comparable to that of the fast reaction kinetics. We show that reduction is possible in each of these regimes, with the nature of the reduction being regime dependent. Our analysis relies on the classical method of matched asymptotic expansions to derive approximations for the solutions that are uniformly valid in space and time.. Introduction One of the paradigm reaction mechanisms in biology and chemistry often referred to as the Michaelis-Menten-Henri (MMH) mechanism involves a substrate (S) that reacts (reversibly) with an enzyme (E) to form a complex (C) which, in turn, is transformed (irreversibly) into a product (P ) and the enzyme [, 6]. The reaction mechanism is represented symbolically by S + E k C k2 P + E, (.) k where k, k, and k 2 are rate constants. The MMH mechanism models the kinetics of many fundamental reactions. Examples from biochemistry include those discussed in [4, 6, 7, 8, 7, 2, 22, 23, 24, 26, 2 Mathematics Subject Classification. 35K57, 35B4, 92C45, 4A6. Key words and phrases. Michaelis-Menten-Henri mechanism; diffusion; dimension reduction; matched asymptotics. c 27 Texas State University - San Marcos. Published??, 27.
2 2 L. KALACHEV, H. KAPER, T. KAPER, N. POPOVIĆ, A. ZAGARIS EJDE/CONF/6 3], and examples involving nutrient uptake in cells and heterogeneous catalytic reactions are analyzed in [5, Chapter 7.] and [2], respectively. The MMH mechanism is also presented as a prototypical mechanism exhibiting fast and slow dynamics and, hence, the potential for dimension reduction in numerous textbooks, see, e.g., [3, 5, 9, 2]... MMH Kinetics. Equations governing the kinetics of (.) may be derived from the law of mass action, ds d t = k SE + k C, (.2a) de d t = k SE + (k + k 2 )C, (.2b) dc d t = k SE (k + k 2 )C, (.2c) dp d t = k 2C, (.2d) where S, E, C, and P denote the concentrations of substrate, enzyme, complex, and product, respectively. The initial conditions are S() = S, E() = Ē, C() =, and P () =. Dimension reduction in (.2) is traditionally achieved in two steps. The first step uses the pair of conservation relations that exist for the mechanism (.). In particular, the total concentration of enzyme (free and bound in complex) is constant; that is, E(t) + C(t) = Ē for all t >. In addition, S(t) + C(t) + P (t) = S for all t >. Therefore, there is a decrease from four variables to two, and the governing equations (.2) reduce to ds d t = kēs + (k S + k )C, (.3a) dc d t = kēs (k S + k + k 2 )C. (.3b) The second reduction step exploits the separation of time scales. In particular, Ē S. Hence, there is a small, positive, dimensionless parameter, ε = Ē S, (.4) and the nondimensionalized equations are naturally formulated as a fast-slow system, where The dimensionless parameters are ṡ = s + (s + κ λ)c, εċ = s (s + κ)c, (.5a) (.5b) t = k Ē t, s = S S, e = Ē E, and c = C Ē. (.6) κ = k + k 2 k S and λ = k 2 k S. (.7) During a short O(ε) initial transient period, the variable c is fast and rises rapidly to its maximum value, while the variable s is slow and remains essentially constant. Subsequently, the evolution of c is slaved to that of s, and both c and s evolve
3 EJDE/CONF/6 REDUCTION FOR MICHAELIS-MENTEN-HENRI KINETICS 3 slowly toward their equilibrium value, zero. This slaving is often referred to as reduced kinetics. From the point of view of dynamical systems, the system (.5) has an asymptotically stable, invariant, slow manifold. During the transient, the concentrations relax to the slow manifold, decaying exponentially toward it. Subsequently, on the O() time scale, the reaction kinetics play out near this manifold. Since the slow manifold is only one-dimensional, a reduction is achieved. To leading order, the slow manifold is given by c = s s + κ, (.8) and the reduced system dynamics on it are governed by a single equation, ds dt = λs s + κ. This leading-order slow manifold is obtained directly from (.5b) with ε = and is referred to as the quasi-steady state approximation [, 3, 5, 9, 22, 25] in chemistry and as the critical manifold in mathematics. Higher-order corrections to the critical manifold, which is sufficiently accurate for many applications, may be calculated using geometric singular perturbation theory, see for example [, 2]. We emphasize that the critical manifold is only approximately invariant under the dynamics of (.5); the exact slow manifold is invariant. Additional studies of the accuracy of the quasi-steady state approximation are given in [, 9, 5, 2, 2, 25, 27]. Remark.. For all sufficiently small, positive ε, there is a family of slow manifolds, all of which are exponentially (O(e k/ε ) for some k > ) close to each other, i.e., the asymptotic expansions of these slow manifolds are the same to all powers of ε, see [, 2], for example. For convenience, we will sometimes refer to the (rather than to a ) slow manifold..2. MMH Kinetics with Diffusion. Given the effectiveness of the two reduction steps in the kinetics problem (.2), one is naturally led to ask what happens when the species are simultaneously permitted to diffuse, and whether any similar reduction can be achieved. The conservation relations used in the first reduction step of the kinetics analysis do not generalize. However, there is still a separation of time scales in the reaction kinetics, and the process of diffusion introduces one (or more) additional time scale(s). Therefore, one expects that dimension reduction may still be achieved by exploiting the separation of time scales, and the purpose of this article is to investigate this possibility. The problem with diffusion is governed by the evolution of the concentrations of substrate, enzyme, and complex in time and space. The concentration of product can be found by quadrature as a function of these other concentrations, since the second reaction in (.) is irreversible. The governing equations in one space dimension are S t = k 2 S SE + k C + D S x 2, (.9a) E t = k 2 E SE + (k + k 2 )C + D E x 2, (.9b) C t = k 2 C SE (k + k 2 )C + D C x 2, (.9c)
4 4 L. KALACHEV, H. KAPER, T. KAPER, N. POPOVIĆ, A. ZAGARIS EJDE/CONF/6 with x [, l], subject to no-flux (Neumann) boundary conditions S = E = C = (.) x x=,l x x=,l x x=,l and initial conditions S(, x) = S i ( x), E(, x) = E i ( x), and C(, x) =. (.) Here, l > is the O() size (length) of the reactor, D S, D E, and D C denote the diffusivities of S, E, and C, respectively, and S i and E i are given, smooth functions describing the initial spatial profiles of substrate and enzyme, respectively. We nondimensionalize (.9) (.) as follows. The nondimensional spatial variable is x = x/l. Time and the species concentrations are nondimensionalized as in (.6), but now S and Ē denote the spatial averages S = l l S i ( x) d x and Ē = l l E i ( x) d x. The nondimensional parameters are again given by (.7), and the diffusivities are scaled as δ = D S k l 2Ē, a = D E, and b = D C. (.2) D S D S Thus, we obtain the equations s t = se + (κ λ)c + δ 2 s (.3a) e t = ε (se κc) + aδ 2 e (.3b) c t = ε (se κc) + bδ 2 c x 2 (.3c) on the unit interval, subject to the Neumann boundary conditions s = e = c =, x x=, x x=, x x=, (.4) and the initial conditions s(, x) = s i (x), e(, x) = e i (x), and c(, x) =. (.5) Here, s i = S i / S and e i = E i /Ē. We assume < ε and that a and b are O(). In vector notation, equations (.3) may be written as where u t = ε F ε(u) + δd 2 u (.6) εse + ε(κ λ)c s F ε (u) = (se κc) u = e, (.7) se κc c and D = diag(, a, b). Moreover, we use [F ε (u)] k to denote the kth-order terms in the Taylor expansion of F ε with respect to ε. Given a formal asymptotic expansion u(, x, ε) = k= u k(, x)ε k of the solution of (.6), [F ε (u)] k will generically be a function of u,..., u k.
5 EJDE/CONF/6 REDUCTION FOR MICHAELIS-MENTEN-HENRI KINETICS 5.3. Summary of Main Results. The impact of diffusion depends on the time scales associated with the species diffusivities relative to those of the reaction kinetics. We examine a spectrum of species diffusivities here: (i) Large diffusivities, δ = O(/ε 2 ): the diffusive time scale is shorter than both the fast and the slow kinetics time scales; (ii) Moderately large diffusivities, δ = O(/ε): the diffusive time scale is comparable to the time scale of the fast kinetics; and (iii) Small diffusivities, δ = O(ε): the diffusive time scale is longer than both kinetics time scales. Our principal findings are that reduction is possible in all regimes under consideration. In regime (i), diffusion effectively homogenizes the concentrations of all three species on the super-fast (τ = t/ε 2 ) time scale. Then, the dynamics on the fast (η = t/ε) and slow (t) time scales are given by the classical MMH kinetics mechanism, with the fast reactions occurring on the fast scale and the reduced kinetics taking place on the slow time scale. We treat this regime primarily to introduce the method we use throughout. In regime (ii), the species undergo both diffusion and the fast reaction on the fast (η = t/ε) time scale. In particular, the substrate concentration satisfies the homogeneous heat equation to leading order; hence, it homogenizes exponentially in time. The enzyme and complex concentrations satisfy nonautonomous, linear reaction-diffusion equations to leading order, and they also homogenize exponentially in time, approaching points on the classical critical manifold. Then, on the slow (t) time scale, the solution is essentially spatially homogeneous. The concentration of substrate evolves according to the classical reduced equation, while the enzyme and complex concentrations are constrained to lie on the critical manifold, to leading order. Most significantly, these leading-order results are independent of the diffusivities of the enzyme and complex, even when these diffusivities are unequal. In regime (iii), the MMH reaction kinetics take place at every point in the domain effectively decoupled from the kinetics at any other point. On the fast (η = t/ε) time scale, enzyme and substrate bind to form complex with the amount of complex at each point x depending on the initial enzyme concentration e i (x), while on the slow (t) time scale the substrate and complex concentrations slowly approach equilibrium in an x-dependent manner. We label these dynamics as pointwise fast kinetics and pointwise slow, reduced kinetics, respectively. Also, on the slow time scale, the enzyme concentration returns essentially to the initial enzyme profile. Then, on asymptotically large or super-slow (ζ = εt) time scales, the enzyme profile homogenizes. The observed dynamics and the time scales in these regimes are summarized in Table. We use matched asymptotic expansions in time in a straightforward manner in each of the regimes identified above. (Equivalent results could, for example, be obtained via the so-called boundary function method [29].) Moreover, we present numerical simulations in every regime to further illustrate our analysis.
6 6 L. KALACHEV, H. KAPER, T. KAPER, N. POPOVIĆ, A. ZAGARIS EJDE/CONF/6 Regime δ Dynamics Time scale (i) /ε 2 homogenization of s, e, c super-fast τ = t/ε 2 fast kinetics fast η = t/ε slow reduced kinetics slow t (ii) /ε homogenization & fast kinetics fast η = t/ε slow reduced kinetics slow t (iii) ε pointwise fast kinetics fast η = t/ε pointwise slow reduced kinetics slow t homogenization of e super-slow ζ = εt Table. Summary of the observed dynamics and the time scales of (.3) in regimes (i) (iii). Remark.2. The regime of moderately small diffusivities, δ = O(), will be analyzed in a separate article. In this regime, the diffusive time scale is comparable to that of the slow kinetics. Preliminary results suggest that the fast dynamics are similar to those in regime (iii), but without the concentrations being homogenized, while the slow dynamics are governed by a reaction-diffusion equation for the concentration of substrate, with the concentrations of enzyme and complex slaved to it. Remark.3. The MMH mechanism in the presence of diffusion is analyzed here as a prototype problem. The methods we employ may be used for other mechanisms with one or more kinetics time scales. Remark.4. The analysis may also be extended to problems in which the domain length l is not O(). For example, the analysis of regime (i) also applies to problems in which l is small and the diffusivities are not large. In that case, it is natural to scale the spatial variable as x = ε x. With this scaling, the diffusion terms in (.3) are of the form δ 2 s x 2 = δ 2 s ε 2 x 2. Hence, diffusion dominates again, even if the actual diffusion coefficients are δ = O(). Remark.5. The influence of diffusion in the MMH mechanism has also been studied in [3]. Specifically, the reduced kinetics model (.5) is considered and a diffusion term is added for the substrate only, with O() diffusion coefficient. Via an inertial manifold approach, it is shown that this system may be reduced to a single reaction-diffusion equation for s, in which the diffusivity has a concentrationdependent correction at O(ε). The fast transients for this model are also calculated, and extensions are given for general systems with fast-slow kinetics in which the slow species also diffuse. Remark.6. It has been shown in [27] that the effective small parameter in the MMH mechanism is ε = Ē/( S +K M ), where K M = (k +k 2 )/k is the Michaelis- Menten constant. Hence, there is a wider range of physical parameters for which one has a separation of time scales. Our method can be applied to the equations with this small parameter as well; however, here we use the traditional scaling, since it is still the one that is most commonly used.
7 EJDE/CONF/6 REDUCTION FOR MICHAELIS-MENTEN-HENRI KINETICS 7 This article is organized as follows. The regimes (i) (iii) are analyzed in Sections 2 4, respectively. In Section 5, the results of the preceding sections are discussed, and the theoretical results are further illustrated using numerical simulations. In Appendix A, it is shown via a Turing analysis that the homogeneous attractor of (.3) is linearly stable, irrespective of the magnitudes of the diffusion coefficients. Appendix B contains a technical result relating to Section Large Diffusivities In this section, we consider the regime in which the diffusivities of all species are large, δ = O(/ε 2 ); for convenience, we choose δ = /ε 2 in (.3). Here, the time scale on which diffusion acts is much shorter than that of the fast kinetics. There is a very short transient period, O(ε 2 ) in duration, in which the initially heterogeneous species concentrations, given by (.5), homogenize and during which essentially no reaction takes place. After this short transient, the problem reduces to the wellunderstood, classical problem of pure kinetics for the homogeneous solution, see, e.g., [5]. We treat this regime in some detail to introduce the method employed in this article in an elementary context. After introduction of δ = /ε 2, equations (.3) become ε 2 s t = ε2 se + ε 2 (κ λ)c + 2 s ε 2 e t = ε(se κc) + a 2 e ε 2 c t = ε(se κc) + b 2 c x 2. Equivalently, in vector form, (2.a) (2.b) (2.c) ε 2 u t = εf ε(u) + D 2 u (2.2) where F is defined in (.7). 2.. Homogenization: The Super-Fast (Inner) Time Scale. To study the initial transient period, we let τ = t/ε 2 denote the super-fast (inner) time and write û(τ, x, ε) = u(t, x, ε). The governing equations become û τ = εf ε(û) + D 2 û (2.3) with initial and boundary conditions û(, x, ε) = u i (x) and û =. x x=, (2.4) We consider (2.3) and (2.4) over an O()-interval of τ time, starting at τ =. Asymptotically, as ε +, the solution can be expressed using the Ansatz û(τ, x, ε) = û (τ, x) + εû (τ, x) + O(ε 2 ). Hence, we expand both sides of (2.3) in powers of ε to obtain a recursive sequence of differential equations for û k, k =, 2,.... At O(), û satisfies the homogeneous heat equation, O() : L τ û =, (2.5)
8 8 L. KALACHEV, H. KAPER, T. KAPER, N. POPOVIĆ, A. ZAGARIS EJDE/CONF/6 where L τ = / τ D 2 / subject to Neumann boundary conditions. The solution is û (τ, x) = û k e D(kπ)2τ cos (kπx). (2.6) k= Here, the coefficients û k are the Fourier coefficients of the initial distribution u i (x) with respect to {cos (kπx)} k, and they are constant during the fast transients on the τ time scale. Asymptotically, as τ, we find û (τ, x) û = u i (x) dx =, (2.7) where we used (.5). Therefore, asymptotically, the effect of diffusion in (2.) is to smear out the initial distributions of the reactants until they are effectively uniformly distributed over the entire spatial domain. Remark 2.. It will suffice to consider the leading-order fast solution (2.6) to accomplish matching to lowest order in the next subsection. To that end, it is useful to write (2.6) as where d = min{, a, b} and where we used (2.7). û (τ, x) = (,, ) T + O(e dπ2τ ), (2.8) 2.2. Fast Kinetics: The Fast Time Scale. The fast kinetics take place on the fast η = t/ε time scale, during which the system dynamics are given by ε ũ η = εf ε(ũ) + D 2 ũ (2.9) subject to Neumann boundary conditions. Here, ũ = ũ(η, x, ε), and we assume ũ(η, x, ε) = ũ (η, x) + εũ (η, x) + O(ε 2 ). Then, expanding (2.9) in powers of ε and rearranging the resulting equations, we find O() : D 2 ũ =, (2.a) x2 O(ε) : D 2 ũ x 2 = ũ η + [F ε(ũ)], (2.b) subject to Neumann boundary conditions. It will suffice to consider the dynamics to this order to obtain a uniform leading-order approximation to the solution of the original system (2.). Integrating (2.a) and taking into account the boundary conditions, we conclude that ũ (η, x) = ũ (η), (2.) i.e., that ũ is independent of x. Similarly, it follows from (2.b) that D 2 ũ x 2 dx = D ũ = = x x= ( dũ dη + [F ε(ũ)] ) dx. (2.2)
9 EJDE/CONF/6 REDUCTION FOR MICHAELIS-MENTEN-HENRI KINETICS 9 Since the integrand in the right member of (2.2) is independent of x, we see that the dynamics of ũ are governed by the ordinary differential equation which we write out componentwise, dũ dη = [F ε(ũ)], d s dη =, dẽ dη = ( s ẽ κ c ), d c dη = s ẽ κ c. (2.3a) (2.3b) (2.3c) These equations are the same as one finds to leading order in the classical MMH kinetics problem; hence, they can be solved explicitly: s (η) s () and ẽ (η) + c (η) = ẽ () + c () for all η. The initial conditions for (2.3) are determined by matching with the leading-order equations on the super-fast (τ) scale; notably, we require that lim τ û (τ, x) = lim η + ũ (η, x). Now, by (2.8), Hence, we have In turn, it follows that and, hence, ũ (η, x) = (, lim τ û(τ, x) = (,, ) T. (2.4) s (η) and ẽ (η) = c (η). (2.5) d c dη = ( + κ) c, with c () =, ( κ + e (+κ)η ), + κ ( e (+κ)η )) T. (2.6) + κ Therefore, on the fast (η) scale, the species concentrations are essentially homogeneous, and the fast chemistry occurs, with the binding of enzyme and substrate to form complex Slow Reduced Kinetics: The Slow (Outer) Time Scale. The slow, reduced kinetics take place on the slow (t) time scale. The dynamics are governed by the original system, (2.2), subject to Neumann boundary conditions. We expand u(t, x, ε) = u (t, x) + εu (t, x) + ε 2 u 2 (t, x) + O(ε 3 ) and equate coefficients of equal powers of ε to obtain O() : D 2 u =, (2.7a) x2 O(ε) : D 2 u x 2 = [F ε(u)], (2.7b) O(ε 2 ) : D 2 u 2 x 2 = u t + [F ε(u)]. (2.7c) Applying the same type of solvability argument used in Section 2.2, we conclude from (2.7a) that u (t, x) = u (t). Similarly, the solvability for (2.7b) implies
10 L. KALACHEV, H. KAPER, T. KAPER, N. POPOVIĆ, A. ZAGARIS EJDE/CONF/6 u (t, x) = u (t), since the right member of (2.7b) is independent of x and, hence, [F ε (u)] =. In turn, this yields s (t)e (t) κc (t) =. (2.8) Next, by writing out (2.7c) componentwise and by applying a solvability argument similar to the one used in (2.2), we find ds dt = s e + (κ λ)c, de dt = (s e + s e κc ), dc dt = s e + s e κc. (2.9a) (2.9b) (2.9c) In turn, equation (2.9a) may be simplified using (2.8) to obtain ds /dt = λc. In addition, (2.9b) and (2.9c) imply e (t) + c (t) = e () + c () for all t, (2.2) where the constant is to be determined by matching with the equations on the fast (η) scale: lim η ũ (η) = lim t + u (t). From (2.6), we find Hence, ( lim ũ(η) κ =, η + κ, ) T. (2.2) + κ e (t) + c (t) =. (2.22) Finally, we combine (2.8) and (2.22) to obtain the critical manifold from the classical kinetics problem with ε =, c (t) = s (t) s (t) + κ and e κ (t) = s (t) + κ. (2.23) Moreover, we see that the reduced equation for s (t) on this critical manifold is ds dt = λ s s + κ with s () =, (2.24) just as is the case for the pure MMH kinetics problem, see for example [5]. The solution of (2.24) is known implicitly, s (t) + κ ln s (t) = λt +. (2.25) Finally, the rate of approach toward the slow manifold is determined by the dynamics on the fast (η) scale, cf. (2.6) The Uniformly Valid Leading-Order Approximation. In this regime of large diffusivities, the leading-order approximation, uniformly valid in time and space, to the solution of (.3) is obtained by combining the expressions for û, ũ, and u (recall (2.6), (2.6), (2.23), and (2.25)) and subtracting their respective
11 EJDE/CONF/6 REDUCTION FOR MICHAELIS-MENTEN-HENRI KINETICS common parts (recall (2.4) and (2.2)). We find s(t, x) = s (t) + O ( e π2 δt ) + O(ε), κ e(t, x) = s (t) + κ + e (+κ) + κ c(t, x) = s (t) s (t) + κ e (+κ) + κ δt δt (2.26a) + O ( e bπ2 δt ) + O(ε), (2.26b) + O ( e bπ2 δt ) + O(ε), (2.26c) where s is defined by (2.25), and we recall that δ = /ε 2, i.e., δ is asymptotically larger than the rate constant corresponding to the faster of the two kinetics scales. The physical interpretation of (2.26) is that, during a short initial time interval of O(ε 2 ), diffusion effectively homogenizes the species concentrations. Thereafter, the concentrations of all three species are essentially uniform and independent of the fine structure of the initial distributions, and they evolve as in the classical MMH kinetics problem. On the fast time scale, enzyme rapidly binds to form complex, while in the phase space the concentrations quickly approach the slow manifold. Then, on the slow (outer) time scale, one observes the reduced kinetics; the concentrations evolve toward equilibrium along the slow manifold, with the concentrations of enzyme and complex being slaved to that of the substrate. In the limit as δ, the expressions in (2.26) agree with the results for the chemical kinetics problem considered for example in Lin and Segel [5, Equations (4) and (5)]. Moreover, the algebraic corrections at O(ε) and upwards are independent of x, and they are governed by ordinary differential equations in t. These are obtained from solvability conditions, as is shown for u, for example, in the following subsection Higher-Order Corrections. On the slow time scale t, the O(ε)-corrections to the leading-order solution are characterized by the O(ε 3 )-terms in (2.2), O(ε 3 ) : D 2 u 3 x 2 = u t + [F ε(u)] 2. (2.27) Equation (2.7c) yields that u 2 (t, x) = u 2 (t); hence, application of the solvability condition to (2.27) implies ds dt = s e s e + (κ λ)c, de dt = (s e 2 + s e + s 2 e κc 2 ), dc dt = s e 2 + s e + s 2 e κc 2. Therefore, e (t) + c (t) = e () + c () =, since matching with the next-order approximation on the super-fast and fast scales shows that this constant is zero. Hence, e = c ; moreover, recalling that e = c, we see that ds dt = ( c )s + (κ λ + s )c, which is precisely [5, Equation (25a)]. One may proceed in a similar manner to obtain the asymptotics of u to any order, as well as the O(ε) and higher-order corrections to the slow manifold, as in the classical MMH kinetics problem.
12 2 L. KALACHEV, H. KAPER, T. KAPER, N. POPOVIĆ, A. ZAGARIS EJDE/CONF/6 We observe that one may also calculate the higher-order corrections to the leading-order solution on the super-fast time scale. At O(ε), system (2.3) yields ŝ τ = 2 ŝ ê τ = (ŝ ê κĉ ) + a 2 ê ĉ τ = ŝ ê κĉ + b 2 ĉ x 2. Hence, substituting the leading-order solution (2.6), one sees that the constant terms, corresponding to k =, lead to linearly growing and decaying terms in ê and ĉ. These secular terms render the expansion invalid as an approximation on time scales of τ = O(/ε). Therefore, one needs to use the multiple scales method (or, alternatively, the boundary function method), as follows. We let û = û (η, τ, x) = û (η) + û k e D(kπ)2τ cos(kπx), so that û varies on the fast scale, while for k, û k remains constant, as before. Therefore, the equations at O(ε) are now k= ŝ τ + ŝ η = 2 ŝ ê τ + ê η = (ŝ ê κĉ ) + a 2 ê ĉ τ + ĉ η = ŝ ê κĉ + b 2 ĉ x 2. Solvability (or elimination of the secular terms ) implies that one should choose the η-dependence in û so that ŝ η =, ê η = (ŝ ê κĉ ), ĉ η = ŝ ê κĉ. By this choice, the solution of the system for û is bounded. Also, we observe that these equations are exactly the same as equations (2.3) for ũ, as expected. For an exposition of the method of multiple scales, see [4], for example. 3. Moderately Large Diffusivities In this section, we examine the regime of moderately large diffusivities, δ = O(/ε); for convenience, we take δ = /ε in (.3). Here, the diffusive time scale is of the same order of magnitude as that of the fast kinetics. We show that, on the fast time scale, s satisfies the homogeneous heat equation to leading order and hence approaches one exponentially in time. At the same time, to leading order, e and c satisfy inhomogeneous, linear reaction-diffusion equations, and they approach constant values exponentially in time. Moreover, the homogeneous values of e and s are, to leading order, precisely those values
13 EJDE/CONF/6 REDUCTION FOR MICHAELIS-MENTEN-HENRI KINETICS 3 corresponding to the point on the slow kinetics manifold, which is to be expected. The rate constant for the exponential convergence in time to this homogeneous state is one order of magnitude smaller than in regime (i), see Section 2.. On the long time scale, s is the reaction progress variable. It satisfies the classical slow reduced MMH kinetics equation. At the same time, the homogeneous enzyme and complex concentrations are slaved to that of s and lie on the critical manifold to leading order. Most significantly, the leading order dynamics in this regime turn out to be independent of the diffusivities a and b. The equations in this regime are ε s t = εse + ε(κ λ)c + 2 s ε e t = (se κc) + a 2 e ε c t = se κc + b 2 c x 2. Equivalently, in vector form, they are given by ε u t = F ε(u) + D 2 u x 2. (3.a) (3.b) (3.c) 3.. Homogenization and Fast Kinetics: The Fast (Inner) Time Scale. Recall that û(η, x, ε) = u(t, x, ε) on the fast time scale given by η = t/ε. Then, the governing equations become ŝ η = εŝê + ε(κ λ)ĉ + 2 ŝ ê η = (ŝê κĉ) + a 2 ê ĉ η = ŝê κĉ + b 2 ĉ (3.2a) (3.2b) (3.2c) subject to the usual initial conditions and Neumann boundary conditions. Making the Ansatz û(η, x, ε) = û (η, x) + εû (η, x) + O(ε 2 ), we find to lowest order that ŝ η = 2 ŝ (3.3a) ê η = (ŝ ê κĉ ) + a 2 ê ĉ η = ŝ ê κĉ + b 2 ĉ x 2. (3.3b) (3.3c) Hence, ŝ satisfies the homogeneous heat equation with Neumann boundary conditions and with initial data given by ŝ (, x) = s i (x), which implies that ŝ (η, x) = ŝ k e (kπ)2η cos (kπx), (3.4) where ŝ = and ŝ k = 2 k= ŝ (, x) cos (kπx) dx = 2 s i (x) cos (kπx) dx for k.
14 4 L. KALACHEV, H. KAPER, T. KAPER, N. POPOVIĆ, A. ZAGARIS EJDE/CONF/6 Next, we find the corresponding expressions for ê and ĉ. Equations (3.3b) and (3.3c) are linear in ê and ĉ, with some of the coefficients depending on η and x through s. Hence, due to the Neumann boundary conditions on ê and ĉ, one can write ê (η, x) = ê k (η) cos (kπx) and ĉ (η, x) = ĉ k (η) cos (kπx) (3.5) k= for some sets of coefficients {ê k (η)} and {ĉ k (η)}. Substituting (3.4) and (3.5) into (3.3b) and (3.3c), making use of the identity cos (mπx) cos (nπx) = ( ) cos ((m + n)πx) + cos ((m n)πx) 2 as well as of ŝ =, and collecting coefficients of like cosines, we find that ê k and ĉ k must satisfy the following infinite system of linear ordinary differential equations: dê dη = (ê κĉ ) F, (3.6a) dĉ dη = ê κĉ + F (3.6b) for the zeroth Fourier mode, and dê ( k dη = + a(kπ) 2 + ) 4(kπ)2 t 2ŝ(2k)e ê k + κĉ k F k, (3.7a) dĉ k dη = ( κ + b(kπ) 2) ( ĉ k + + ) 4(kπ)2 t 2ŝ(2k)e ê k + F k (3.7b) for the kth Fourier mode, k. Here, F k is defined via F (η) = ŝ m e (mπ)2ηê m (η), 2 m F k (η) = ( ŝ (m+k) e (m+k)2 π 2η ) + ŝ m k e (m k)2 π 2 η ê m (η), k. 2 m m k In particular, (3.6) yields that ê (η) + ĉ (η) is constant in this regime. Then, solving (3.6) and taking into account the identities ê () = ĉ () = ê (, x) dx = ĉ (, x) dx = k= e i (x) dx =, c i (x) dx = as well as the fact that ê k and ĉ k must be bounded in η, we find ê (η) = κ min{π + 2 O(e,+κ}η ) and ĉ (η) = min{π + 2 O(e,+κ}η ). + κ + κ (3.9) Similar expressions can be derived for ê k and ĉ k with k ; in particular, one can show that ê k, ĉ k = O(e dπ2η ), where we recall d = min{, a, b}. Hence, we conclude that for η large, û (η, x) = (, κ + κ, ) T + O(e min{dπ 2,+κ}η ). (3.) + κ
15 EJDE/CONF/6 REDUCTION FOR MICHAELIS-MENTEN-HENRI KINETICS 5 See Appendix B for details Reduced Kinetics: The Slow (Outer) Time Scale. On the slow (outer) time scale, the system dynamics are naturally described by (3.) in the original time t. To lowest order, we find = 2 s = (s e κc ) + a 2 e (3.a) (3.b) = s e κc + b 2 c (3.c) where we again make the Ansatz u(t, x, ε) = u (t, x) + εu (t, x) + O(ε 2 ). Integrating (3.a) with respect to x and making use of the Neumann boundary conditions, we conclude immediately that s (t, x) s (t). Similarly, it follows from either (3.b) or (3.c) that s (t) e (t, x) dx κ c (t, x) dx =. (3.2) To obtain explicit formulae for e and c, we rescale x via x = x/ a, and observe that the ratio α = a b is the relevant parameter, rather than a and b separately. We rewrite (3.b) and (3.c) as a four-dimensional linear system with t-dependent coefficients, e = f, f = s (t)e κc, c = d, d = α ( s (t)e κc ), where the prime denotes (partial) differentiation with respect to x. The general solution of (3.3) is given by (3.3a) (3.3b) (3.3c) (3.3d) e (t, x) = κγ (t) + κγ 2 (t) x + γ 3 (t)e s(t)+ακ x γ 4 (t)e s (t)+ακ x, (3.4a) c (t, x) = s (t)γ (t) + s (t)γ 2 (t) x αγ 3 (t)e s(t)+ακ x + αγ 4 (t)e s (t)+ακ x, (3.4b) where f = e and d = c can be found from (3.4), and γ,..., γ 4 are t-dependent constants of integration. Making use of the Neumann boundary conditions on e and c in (3.4), one sees that γ 2, γ 3, and γ 4 must be identically zero. Hence, the only solution (3.4) that satisfies the boundary conditions is given by (e, c )(t) = γ (t) ( κ, s (t) ), (3.5) where γ (t) is as yet undetermined, and it follows that (3.2) reduces to s (t)e (t) κc (t) =. (3.6) Summarizing, we see that e and c are spatially uniform on the slow time scale and that the constraint (3.6) is satisfied at all times.
16 6 L. KALACHEV, H. KAPER, T. KAPER, N. POPOVIĆ, A. ZAGARIS EJDE/CONF/6 To describe the dynamics of u on the t time scale, we consider the O(ε)-terms in (3.), ds dt = s e + (κ λ)c + 2 s (3.7a) de dt = (s e + s e κc ) + a 2 e (3.7b) dc dt = s e + s e κc + b 2 c x 2. (3.7c) First, an integration of (3.7a) over x [, ] in combination with (3.6) yields ds dt = λc. (3.8) To find the corresponding dynamics of e and c, we add (3.7b) and (3.7c) and integrate the resulting expression with respect to x, which gives de dt + dc dt =. Hence, e (t) + c (t) = e () + c () for all t. Finally, taking into account that lim t + u(t, x) must equal we obtain lim η û(η, x) = (, e (t) + c (t) =. κ + κ, ) T, + κ Combining this identity with (3.5) and solving the resulting equation for γ, we find γ (t) = (s (t) + κ) and therefore e (t) = κ s (t) + κ and c (t) = s (t) s (t) + κ, (3.9) see (2.23). Finally, substitution of (3.9) into (3.8) yields the governing equation for s, ds dt = λ s s + κ with s () =, (3.2) see also (2.24). This approximation is independent of the values of a and b, and hence it coincides with the one in Section 2. The unequal diffusivities of e and c do not influence the leading-order asymptotics of (3.) The Uniformly Valid Leading-Order Approximation. In this regime, δ is of the same size as the rate constant corresponding to the faster of the two kinetics time scales, i.e., δ is moderately large. Therefore, given the expressions for û and u in (3.) and (3.9), respectively, with s (t) given by (3.2), the uniformly valid leading-order approximation for u is s(t, x) = s (t) + O(e min{π2,+κ}δt ) + O(ε), (3.2a) κ min{aπ e(t, x) = + O(e ) + O(ε), s (t) + κ (3.2b) c(t, x) = s (t) min{bπ + O(e ) + O(ε), s (t) + κ (3.2c)
17 EJDE/CONF/6 REDUCTION FOR MICHAELIS-MENTEN-HENRI KINETICS 7 where δ = /ε. Again, we emphasize that this approximation is independent of the values of a and b. Correspondingly, the leading-order dynamics may be interpreted physically in the same manner as that found in the regime of large diffusivities (Section 2.4), although of course the error terms here are more significant Higher-Order Corrections. Similar reasoning as used in Section 3.2 can be applied to determine the asymptotics of u n, for any n. In particular, one can show that u n remains spatially uniform for all times t, u n = u n (t), and that the state of the system at time t is determined by the set of equations ds n (t) = [F,ε (u(t))] n, dt (3.22a) e n (t) + c n (t) =, (3.22b) s (t)e n (t) κc n (t) = ċ n + r n (t). (3.22c) Here, the overdot denotes differentiation with respect to time, F,ε (u) = se + (κ λ)c, and the term r n = [F 2,ε (u)] n + s e n κc n, with F 2,ε (u) = se + κc, is a function of u,..., u n, s n, and e exclusively. The proof is by induction. First, at O(ε n ), (3.a) reads ds n (t) dt = [F,ε (u)] n (t) + 2 s n (t, x) x 2. Now, ds n /dt = [F,ε (u)] n by the induction hypothesis, and thus 2 s n / x 2 =. Using the boundary conditions, then, we find that s n (t, x) = s n (t). Next, at O(ε n ), (3.b) and (3.c) yield ė n r n = (s e n κc n ) + a 2 e n ċ n + r n = s e n κc n + b 2 c n Here again, we rescale x via x = x/ a and rewrite these equations as a fourdimensional, inhomogeneous, linear system with t-dependent coefficients, e n = f n, f n = s e n κc n + ė n r n, c n = d n, d n = α(s e n κc n + ė n r n ), (3.24a) (3.24b) (3.24c) (3.24d) where the prime denotes (partial) differentiation with respect to x and we have used that ė n (t) + ċ n (t) = by the induction hypothesis.
18 8 L. KALACHEV, H. KAPER, T. KAPER, N. POPOVIĆ, A. ZAGARIS EJDE/CONF/6 The general solution of (3.24) is given by e n (t, x) = κγ (t) + κγ 2 (t) x + γ 3 (t)e s(t)+ακ x γ 4 (t)e s (t)+ακ x + ėn (t) r n (t)( cosh( s (t) + ακ x) ) (3.25a), s (t) + ακ c n (t, x) = s (t)γ (t) + s (t)γ 2 (t) x αγ 3 (t)e s(t)+ακ x + αγ 4 (t)e s (t)+ακ x αėn (t) r n (t)( cosh( s (t) + ακ x) ), s (t) + ακ (3.25b) where f n = e n and d n = c n can be found from (3.25) and γ,..., γ 4 are t-dependent constants of integration. Making use of the Neumann boundary conditions on e n and c n, one sees that γ 2, γ 3, and γ 4 are such that e n (t, x) = e n (t) = κγ (t) ėn (t) r n (t), s (t) + ακ (3.26a) c n (t, x) = c n (t) = s (t)γ (t) + α ėn (t) r n (t). s (t) + ακ (3.26b) In summary, these formulas show that u n = u n (t). Next, by integrating the O(ε n+ )-terms in (3.a) and using the boundary conditions, we obtain (ds n/dt) dx = [F,ε(u)] n dx. To evaluate these integrals, we observe that s n, and thus also ds n /dt, is a function of time only. Moreover, [F,ε (u)] n is a function of u,..., u n and thus also a function of time only. Therefore, ds n /dt = [F,ε (u)] n, as desired. Finally, at O(ε n+ ), (3.b) and (3.c) yield ė n = [F 2,ε (u)] n + a 2 e n+ ċ n = [F 2,ε (u)] n + b 2 c n+ x 2. Integrating both members of these equations over the spatial domain [, ], using the boundary conditions and recalling that e n and c n are functions of time only, we obtain the identity ė n (t) + ċ n (t) =. Recalling also that e(t, x) + c(t, x) = and that e (t) + c (t) =, we conclude that e n (t) + c n (t) =, as desired. Therefore, combining (3.26) with the identity ė n (t) + ċ n (t) =, we obtain (3.22), and the proof is complete. Remark 3.. Equations (3.22) show that the system dynamics are governed solely by the chemical kinetics together with the conservation law e(t) + c(t) =, to within all algebraic orders in ε. Thus, all of the results that are valid for the usual, nondiffusive MMH kinetics (such as the existence of a slow invariant manifold with an asymptotic expansion in powers of ε [2]) also apply to this case. 4. Small Diffusivities In this section, we consider the regime in which the species diffusivities are small, δ = ε, so that the time scale on which diffusion acts is much longer than that of the slow kinetics. The solution in this regime depends on the fine structure of the initial distributions, as well as on the local distributions of the species. We will show that, on
19 EJDE/CONF/6 REDUCTION FOR MICHAELIS-MENTEN-HENRI KINETICS 9 the fast and slow time scales, the effects of diffusion may be neglected to a fairly good approximation (to within O(ε 2 )) and that the kinetics are largely decoupled at each point x in space. In particular, for each fixed x, the kinetics follow the classical MMH kinetics. Complex is formed on the fast time scale, with the species concentrations rapidly approaching an x-dependent point on the slow manifold. We label the dynamics on the fast time scale as pointwise fast kinetics. Then, the reaction proceeds on the slow time scale, with the concentrations of substrate and complex evolving along the slow manifold to zero, pointwise in x. Also, the enzyme concentration evolves to leading order toward the initial profile e i (x), which still depends on x. We label the dynamics on the slow time scale as pointwise slow reduced kinetics. Finally, on the super-slow time scale, diffusion homogenizes the enzyme concentration profile. Equations (.3) with δ = ε are ε s t = εse + ε(κ λ)c + ε2 2 s ε e t = (se κc) + aε2 2 e ε c t = se κc + bε2 2 c x 2. In vector notation, (4.) is given by (4.a) (4.b) (4.c) ε u t = F ε(u) + ε 2 D 2 u x 2. (4.2) One factor of ε in (4.a) is redundant; however, we retain it for consistency of notation. 4.. Pointwise Fast Kinetics: The Fast (Inner) Time Scale. On the fast (η = t/ε) time scale, the full governing equations are given by û η = F ε(û) + ε 2 D 2 û (4.3) subject to the usual initial and boundary conditions, see Section. Again, we expand û(η, x, ε) = û (η, x) + εû (η, x) + O(ε 2 ). To lowest order, (4.3) implies û O() : η = [F ε(û)], (4.4) subject to Neumann boundary conditions and the initial condition û (, x) = u i (x). (4.5) Solving the system (4.4) and (4.5) componentwise, we find ŝ (η, x) = ŝ (, x); hence, ŝ (η, x) s i (x) for all η. Moreover, so that ê η + ĉ η =, ê (η, x) + ĉ (η, x) = ê (, x) + ĉ (, x) e i (x) for all η. (4.6) Now, given that ŝ = s i and ê = e i ĉ, we see from (4.4) that ĉ η = (s i + κ)ĉ + s i e i with ĉ (, x) =,
20 2 L. KALACHEV, H. KAPER, T. KAPER, N. POPOVIĆ, A. ZAGARIS EJDE/CONF/6 which has the solution In summary, we have û (η, x) = ( s i (x), e i (x) s i (x) + κ ĉ (η, x) = s i(x)e i (x) s i (x) + κ ( e (s i(x)+κ)η ). ( κ + si (x)e (si(x)+κ)η), s i(x)e i (x)( e (s )) T i(x)+κ)η. s i (x) + κ (4.7) The physical interpretation of these results is that, on the fast time scale, diffusion has no effect on the concentration amplitudes to O() and O(ε); it only influences the amplitudes at O(ε 2 ). Instead, on the fast time scale, the kinetics have center stage and are essentially independent at each point x. In particular, pointwise in x and to leading order in ε, ŝ remains unaltered, and ê and ĉ are constrained to satisfy the linear conservation law (4.6), while the concentrations evolve to the appropriate (x-dependent) point on the leading-order slow manifold as η, which is exactly dictated by the chemical kinetics Pointwise Slow Reduced Kinetics: The Slow Time Scale. The slow time scale is given by the original time scale t itself; hence, with a slight abuse of notation to ensure consistency with Section 2, we replace u by ũ = ũ(t, x, ε) in (4.2) and make the Ansatz ũ(t, x, ε) = ũ (t, x) + εũ (t, x) + O(ε 2 ). After expanding with respect to ε and rearranging, we have O() : [F ε (ũ)] =, (4.8a) ũ O(ε) : = [F ε (ũ)], (4.8b) t with Neumann boundary conditions on ũ and ũ and with initial conditions to be determined by matching with the equations on the fast time scale. Writing (4.8a) componentwise, we see that s ẽ = κ c, which we can substitute into (4.8b) to obtain s t = λ c. Also, we deduce from (4.8b) that ẽ (t, x) + c (t, x) = ẽ () + c () must hold. Imposing the matching condition lim η û (η, x) = lim t + ũ (t, x) and taking into consideration that lim û(η, x) = η ( s i (x), κe i (x) s i (x) + κ, s ) T i(x)e i (x), (4.9) s i (x) + κ we conclude that ẽ (t, x) + c (t, x) e i (x). Therefore, recalling (4.8a), we obtain ẽ (t, x) = where s (t, x) solves κe i(x) s (t, x) + κ and c (t, x) = s (t, x)e i (x) s (t, x) + κ, (4.) s t = λ e i(x) s + κ s with s (, x) = s i (x). (4.) This reduced equation for s (t, x) is of the same form as (2.24), the equation for s (t) in the large diffusivities regime; however, the interpretations differ. Here,
21 EJDE/CONF/6 REDUCTION FOR MICHAELIS-MENTEN-HENRI KINETICS 2 s (t, x) depends on x through e i (x), whereas s (t) is independent of x. Moreover, we recall that s may be found only implicitly, as was shown for s (t) in (2.25). Therefore, we conclude that, at each point x in the domain, s is the natural reaction progress variable and that the enzyme and complex concentrations are given as functions of it, as in the chemical kinetics problem. However, we emphasize that, in this small-diffusivity regime, the sum of ẽ and c depends on x and is equal to the local initial enzyme profile e i (x), not uniformly equal to one, as in the classical kinetics problem. This means, among other things, that for certain spatial profiles e i (x), the dynamic evolution of ũ takes place outside the unit cube, i.e., outside the region that is feasible for the classical kinetics problem Homogenization of Enzyme: The Super-Slow (Outer) Time Scale. Due to the fact that δ = ε in this regime, diffusion acts on the super-slow (ζ = εt) time scale, and we express the governing equations as ε 2 u ζ = F ε(u) + ε 2 D 2 u (4.2) subject to Neumann boundary conditions. We make the Ansatz u(ζ, x, ε) = u (ζ, x) + εu (ζ, x) + ε 2 u 2 (ζ, x) + O(ε 3 ), substitute it into (4.2), expand, and rearrange the resulting equations to obtain O() : [F ε (u)] =, (4.3a) O(ε) : [F ε (u)] =, (4.3b) O(ε 2 ) : L ζ u = [F ε (u)] 2, (4.3c) where L ζ = / ζ D 2 / supplemented by Neumann boundary conditions. First, observe that (4.3a) gives s e = κc, as on the slow (t) scale. Then, (4.3b) shows that λc =, which implies c (since λ by definition). Moreover, we see from that same equation (4.3b) that which we use in (4.3c) to obtain s e + s e = κc, s ζ 2 s x 2 = λc. Similarly, to find e, we note that (4.3c) and the identity c derived above imply L ζ c = ; hence, Therefore, L ζ e = and e (ζ, x) = s e 2 + s e + s 2 e = κc 2. e k e a(kπ)2ζ cos (kπx), k= with the constant coefficients e k to be determined by matching with the dynamics on the t scale. Since matching requires lim t ũ (t, x) = lim ζ + u (ζ, x) and since lim ũ(t, x) = (, e i (x), ) T t (4.4)
22 22 L. KALACHEV, H. KAPER, T. KAPER, N. POPOVIĆ, A. ZAGARIS EJDE/CONF/6 by (4.) and (4.), it follows that e solves the homogeneous heat equation with nonzero initial conditions and Neumann boundary conditions. Hence, the enzyme concentration will generically be nonzero. More precisely, since e = e i (x) dx =, it follows that e (ζ, x) = + O(e aπ2ζ ). All reactions have to leading order been completed when diffusion sets in, i.e., there is only enzyme left to diffuse until a spatially homogeneous distribution of e has been reached. Finally, given e (x) and (4.3a), we conclude that s must hold. Hence, to summarize, we have where again d = min{, a, b}. u (ζ, x) = (,, ) T + O(e dπ2ζ ), (4.5) 4.4. The Uniformly Valid Leading-Order Approximation. We combine the expressions for û, ũ, and u, which are the leading-order approximations to the solution u of (4.2) on the fast, slow, and super-slow time scales, respectively, into one uniformly valid, leading-order approximation for u. Given (4.7), (4.) and (4.), and (4.5), as well as (4.9) and (4.4), a straightforward calculation shows s(t, x) = s (t, x) + O(e π2 δt ) + O(ε), e(t, x) = s (t, x)e i (x) s (t, x) + κ + s i(x)e i (x) s i (x) + κ e (si(x)+κ)t/δ + O(e aπ 2 δt ) + O(ε), (4.6a) (4.6b) c(t, x) = s (t, x)e i (x) s (t, x) + κ s i(x)e i (x) s i (x) + κ e (si(x)+κ)t/δ + O(e bπ 2 δt ) + O(ε), (4.6c) where s is defined by (4.). Here, we also recall that δ = ε, i.e., δ is asymptotically smaller than the smaller of the two kinetics rate constants Higher-Order Corrections. In this regime, the higher-order algebraic corrections u j, j, may be found explicitly. The O(ε)-terms in (4.3) are û O(ε) : η = [F ε(û)]. (4.7) In particular, the equation for the first component, ŝ, is ŝ η = ŝ ê + (κ λ)ĉ = s i(x)e i (x) s i (x) + κ ( λ + e (si(x)+κ)η( s i (x) + κ λ )), (4.8) where we used (4.7). Hence, there will be a secular term in ŝ that grows linearly in η, due to the η-independent (first) term in (4.8). A remedy is at hand using the method of multiple scales. In particular, let the terms in the expansion of û depend not only on the fast time η, but also on the slow time t and on a sequence of successively longer time scales as needed, depending on the order of the truncation of the asymptotic expansion. We start with the O()-term, A (t, x)ŝ (η, x), in the expansion for ŝ. As in Section 4., it is again the case that ŝ = s i (x), independently of η, and that (4.6) holds. Moreover, the leading-order terms for the complex and enzyme concentrations may be found as
23 EJDE/CONF/6 REDUCTION FOR MICHAELIS-MENTEN-HENRI KINETICS 23 in Section 4.. For example, to leading order, the complex concentration is given by A (t, x)s i (x)e i (x)( e (A ) (t,x)s i(x)+κ)η. A (t, x)s i (x) + κ Substituting these solutions into (4.3) and collecting the O(ε)-terms, we find s i (x) A t ŝ + A η = s i(x)e i (x)a ( λ + ( A s i (x) + κ λ ) e (Asi(x)+κ)η). A s i (x) + κ (4.9) Therefore, if we choose A (t, x) such that the η-independent term vanishes from the right hand side of (4.9), then we obtain a regular (bounded) solution for ŝ. Specifically, we choose the dependence of A (t, x) on t so that A t = λ e i(x)a A s i (x) + κ. (4.2) This requirement is equivalent to applying a solvability condition to equation (4.9), which forces the bad terms to drop out. The resulting equation for ŝ may be integrated directly to obtain a bounded function. Moreover, as one expects, the equation (4.2) for A (t, x) is precisely equation (4.), which was obtained from the leading-order analysis on the slow time scale, with s (t, x) replaced by A (t, x)s i (x). Higher-order terms ŝ j, j, may be found in an analogous manner. The corresponding amplitude functions A i (for i =,..., j) depend on t, εt,..., ε j t, as well as on x, and are determined by solvability conditions, just as A (t, x) was determined by the solvability condition on the equation for ŝ above. Of course, the corrections to the complex and enzyme concentrations need to be determined simultaneously using the multiple scales Ansatz. 5. Interpretation and Conclusions In the present article, we have studied the effects of diffusion on the classical MMH reaction mechanism. We have investigated the two extreme regimes of large diffusivities and of small diffusivities, as well as an intermediate regime of moderately large diffusivities. A brief summary of the observed dynamics and time scales in each regime was given in Table. Here, we summarize our findings in more detail and illustrate the analytical results with direct numerical simulations. Regime (i): large diffusivities, δ = /ε 2. There is a brief initial transient period of O(ε 2 ) during which the concentrations become spatially homogeneous, with values equal to the spatial averages of the initial concentration profiles. This period is followed by another short, O(ε)-period during which the fast kinetics act. During this second period, the homogeneous concentrations everywhere in the domain are brought into the regime where the MMH reduction holds. Then, for large times, the dynamics are exactly the same as those found in the pure kinetics problem, as described above. The leading-order asymptotics, uniformly valid in time and space, are given in (2.26). The results of a typical simulation of (.3) for this regime are shown in Figure. In particular, the super-fast initial transient appears to last for essentially only one timestep, as is evident from Figures (a) and (b).
REDUCTION FOR MICHAELIS-MENTEN-HENRI KINETICS IN THE PRESENCE OF DIFFUSION
 26 International Conference in Honor of Jacqueline Fleckinger. Electronic Journal of Differential Equations, Conference 6, 27, pp. 55 84. ISSN: 72-669. URL: http://ejde.math.txstate.edu or http://ejde.math.unt.edu
26 International Conference in Honor of Jacqueline Fleckinger. Electronic Journal of Differential Equations, Conference 6, 27, pp. 55 84. ISSN: 72-669. URL: http://ejde.math.txstate.edu or http://ejde.math.unt.edu
Front Speeds, Cut-Offs, and Desingularization: A Brief Case Study
 Contemporary Mathematics Front Speeds, Cut-Offs, and Desingularization: A Brief Case Study Nikola Popović Abstract. The study of propagation phenomena in reaction-diffusion systems is a central topic in
Contemporary Mathematics Front Speeds, Cut-Offs, and Desingularization: A Brief Case Study Nikola Popović Abstract. The study of propagation phenomena in reaction-diffusion systems is a central topic in
Singular perturbation theory
 Singular perturbation theory Marc R Roussel October 19, 2005 1 Introduction When we apply the steady-state approximation (SSA) in chemical kinetics, we typically argue that some of the intermediates are
Singular perturbation theory Marc R Roussel October 19, 2005 1 Introduction When we apply the steady-state approximation (SSA) in chemical kinetics, we typically argue that some of the intermediates are
Notes for Expansions/Series and Differential Equations
 Notes for Expansions/Series and Differential Equations In the last discussion, we considered perturbation methods for constructing solutions/roots of algebraic equations. Three types of problems were illustrated
Notes for Expansions/Series and Differential Equations In the last discussion, we considered perturbation methods for constructing solutions/roots of algebraic equations. Three types of problems were illustrated
Mathematical Model of Catalytic Chemical Reactor. Sintayehu Agegnehu Matintu* School of Mathematical and Statistical Sciences, Hawassa University,
 Mathematical Model of Catalytic Chemical Reactor Sintayehu Agegnehu Matintu* School of Mathematical and Statistical Sciences, Hawassa University, P.O.Box 05, Hawassa, Ethiopia Abstract This study concerns
Mathematical Model of Catalytic Chemical Reactor Sintayehu Agegnehu Matintu* School of Mathematical and Statistical Sciences, Hawassa University, P.O.Box 05, Hawassa, Ethiopia Abstract This study concerns
THE CRITICAL WAVE SPEED FOR THE FISHER-KOLMOGOROV-PETROWSKII-PISCOUNOV EQUATION WITH CUT-OFF
 THE CRITICAL WAVE SPEED FOR THE FISHER-KOLMOGOROV-PETROWSKII-PISCOUNOV EQUATION WITH CUT-OFF FREDDY DUMORTIER, NIKOLA POPOVIĆ, AND TASSO J. KAPER Abstract. The Fisher-Kolmogorov-Petrowskii-Piscounov (FKPP)
THE CRITICAL WAVE SPEED FOR THE FISHER-KOLMOGOROV-PETROWSKII-PISCOUNOV EQUATION WITH CUT-OFF FREDDY DUMORTIER, NIKOLA POPOVIĆ, AND TASSO J. KAPER Abstract. The Fisher-Kolmogorov-Petrowskii-Piscounov (FKPP)
Homework Exercises and Solutions for Mathematics Fall 2016
 Homework Exercises and Solutions for Mathematics 677 - Fall 216 Remark: Solutions may include maple files or matlab files. Assignment 1: (due Sept. 27, 216 1. In the real world trimolecular reactions are
Homework Exercises and Solutions for Mathematics 677 - Fall 216 Remark: Solutions may include maple files or matlab files. Assignment 1: (due Sept. 27, 216 1. In the real world trimolecular reactions are
6.1 Enzyme Kinetics: Basic Enzyme Reaction
 6. Reaction Kinetics 6.1 Enzyme Kinetics: Basic Enzyme Reaction Biochemical reactions are continually taking place in all living organisms and most of them involve proteins called enzymes, which act as
6. Reaction Kinetics 6.1 Enzyme Kinetics: Basic Enzyme Reaction Biochemical reactions are continually taking place in all living organisms and most of them involve proteins called enzymes, which act as
Enzyme Kinetics. Jonathan Gent and Douglas Saucedo May 24, 2002
 Enzyme Kinetics Jonathan Gent and Douglas Saucedo May 24, 2002 Abstract This paper consists of a mathematical derivation of the Michaelis-Menten equation, which models the rate of reaction of certain enzymatic
Enzyme Kinetics Jonathan Gent and Douglas Saucedo May 24, 2002 Abstract This paper consists of a mathematical derivation of the Michaelis-Menten equation, which models the rate of reaction of certain enzymatic
2 Matched asymptotic expansions.
 2 Matched asymptotic expansions. In this section we develop a technique used in solving problems which exhibit boundarylayer phenomena. The technique is known as method of matched asymptotic expansions
2 Matched asymptotic expansions. In this section we develop a technique used in solving problems which exhibit boundarylayer phenomena. The technique is known as method of matched asymptotic expansions
Exercise Set 4. D s n ds + + V. s dv = V. After using Stokes theorem, the surface integral becomes
 Exercise Set Exercise - (a) Let s consider a test volum in the pellet. The substract enters the pellet by diffusion and some is created and disappears due to the chemical reaction. The two contribute to
Exercise Set Exercise - (a) Let s consider a test volum in the pellet. The substract enters the pellet by diffusion and some is created and disappears due to the chemical reaction. The two contribute to
A SHORT PROOF OF INCREASED PARABOLIC REGULARITY
 Electronic Journal of Differential Equations, Vol. 15 15, No. 5, pp. 1 9. ISSN: 17-6691. URL: http://ejde.math.txstate.edu or http://ejde.math.unt.edu ftp ejde.math.txstate.edu A SHORT PROOF OF INCREASED
Electronic Journal of Differential Equations, Vol. 15 15, No. 5, pp. 1 9. ISSN: 17-6691. URL: http://ejde.math.txstate.edu or http://ejde.math.unt.edu ftp ejde.math.txstate.edu A SHORT PROOF OF INCREASED
Lecture 4: Numerical solution of ordinary differential equations
 Lecture 4: Numerical solution of ordinary differential equations Department of Mathematics, ETH Zürich General explicit one-step method: Consistency; Stability; Convergence. High-order methods: Taylor
Lecture 4: Numerical solution of ordinary differential equations Department of Mathematics, ETH Zürich General explicit one-step method: Consistency; Stability; Convergence. High-order methods: Taylor
Putzer s Algorithm. Norman Lebovitz. September 8, 2016
 Putzer s Algorithm Norman Lebovitz September 8, 2016 1 Putzer s algorithm The differential equation dx = Ax, (1) dt where A is an n n matrix of constants, possesses the fundamental matrix solution exp(at),
Putzer s Algorithm Norman Lebovitz September 8, 2016 1 Putzer s algorithm The differential equation dx = Ax, (1) dt where A is an n n matrix of constants, possesses the fundamental matrix solution exp(at),
1 Separation of Variables
 Jim ambers ENERGY 281 Spring Quarter 27-8 ecture 2 Notes 1 Separation of Variables In the previous lecture, we learned how to derive a PDE that describes fluid flow. Now, we will learn a number of analytical
Jim ambers ENERGY 281 Spring Quarter 27-8 ecture 2 Notes 1 Separation of Variables In the previous lecture, we learned how to derive a PDE that describes fluid flow. Now, we will learn a number of analytical
FAST AND HETEROCLINIC SOLUTIONS FOR A SECOND ORDER ODE
 5-Oujda International Conference on Nonlinear Analysis. Electronic Journal of Differential Equations, Conference 14, 6, pp. 119 14. ISSN: 17-6691. URL: http://ejde.math.txstate.edu or http://ejde.math.unt.edu
5-Oujda International Conference on Nonlinear Analysis. Electronic Journal of Differential Equations, Conference 14, 6, pp. 119 14. ISSN: 17-6691. URL: http://ejde.math.txstate.edu or http://ejde.math.unt.edu
arxiv: v1 [math.ap] 13 Jul 2017
![arxiv: v1 [math.ap] 13 Jul 2017 arxiv: v1 [math.ap] 13 Jul 2017](/thumbs/83/88180062.jpg) arxiv:1707.04043v1 [math.ap] 13 Jul 2017 Quasi-steady state reduction for the Michaelis-Menten reaction-diffusion system Martin Frank, Christian Lax, Sebastian Walcher, Olaf Wittich March 28, 2018 Abstract
arxiv:1707.04043v1 [math.ap] 13 Jul 2017 Quasi-steady state reduction for the Michaelis-Menten reaction-diffusion system Martin Frank, Christian Lax, Sebastian Walcher, Olaf Wittich March 28, 2018 Abstract
Gas solid reaction with porosity change
 Nonlinear Differential Equations, Electron. J. Diff. Eqns., Conf. 5, 2, pp. 247 252 http://ejde.math.swt.edu or http://ejde.math.unt.edu ftp ejde.math.swt.edu or ejde.math.unt.edu (login: ftp) Gas solid
Nonlinear Differential Equations, Electron. J. Diff. Eqns., Conf. 5, 2, pp. 247 252 http://ejde.math.swt.edu or http://ejde.math.unt.edu ftp ejde.math.swt.edu or ejde.math.unt.edu (login: ftp) Gas solid
Class Meeting # 2: The Diffusion (aka Heat) Equation
 MATH 8.52 COURSE NOTES - CLASS MEETING # 2 8.52 Introduction to PDEs, Fall 20 Professor: Jared Speck Class Meeting # 2: The Diffusion (aka Heat) Equation The heat equation for a function u(, x (.0.). Introduction
MATH 8.52 COURSE NOTES - CLASS MEETING # 2 8.52 Introduction to PDEs, Fall 20 Professor: Jared Speck Class Meeting # 2: The Diffusion (aka Heat) Equation The heat equation for a function u(, x (.0.). Introduction
A First Course on Kinetics and Reaction Engineering. Class 9 on Unit 9
 A First Course on Kinetics and Reaction Engineering Class 9 on Unit 9 Part I - Chemical Reactions Part II - Chemical Reaction Kinetics Where We re Going A. Rate Expressions - 4. Reaction Rates and Temperature
A First Course on Kinetics and Reaction Engineering Class 9 on Unit 9 Part I - Chemical Reactions Part II - Chemical Reaction Kinetics Where We re Going A. Rate Expressions - 4. Reaction Rates and Temperature
We denote the space of distributions on Ω by D ( Ω) 2.
 Sep. 1 0, 008 Distributions Distributions are generalized functions. Some familiarity with the theory of distributions helps understanding of various function spaces which play important roles in the study
Sep. 1 0, 008 Distributions Distributions are generalized functions. Some familiarity with the theory of distributions helps understanding of various function spaces which play important roles in the study
Damped Harmonic Oscillator
 Damped Harmonic Oscillator Wednesday, 23 October 213 A simple harmonic oscillator subject to linear damping may oscillate with exponential decay, or it may decay biexponentially without oscillating, or
Damped Harmonic Oscillator Wednesday, 23 October 213 A simple harmonic oscillator subject to linear damping may oscillate with exponential decay, or it may decay biexponentially without oscillating, or
Application of the perturbation iteration method to boundary layer type problems
 DOI 10.1186/s40064-016-1859-4 RESEARCH Open Access Application of the perturbation iteration method to boundary layer type problems Mehmet Pakdemirli * *Correspondence: mpak@cbu.edu.tr Applied Mathematics
DOI 10.1186/s40064-016-1859-4 RESEARCH Open Access Application of the perturbation iteration method to boundary layer type problems Mehmet Pakdemirli * *Correspondence: mpak@cbu.edu.tr Applied Mathematics
Notes on Fourier Series and Integrals Fourier Series
 Notes on Fourier Series and Integrals Fourier Series et f(x) be a piecewise linear function on [, ] (This means that f(x) may possess a finite number of finite discontinuities on the interval). Then f(x)
Notes on Fourier Series and Integrals Fourier Series et f(x) be a piecewise linear function on [, ] (This means that f(x) may possess a finite number of finite discontinuities on the interval). Then f(x)
Regulation of metabolism
 Regulation of metabolism So far in this course we have assumed that the metabolic system is in steady state For the rest of the course, we will abandon this assumption, and look at techniques for analyzing
Regulation of metabolism So far in this course we have assumed that the metabolic system is in steady state For the rest of the course, we will abandon this assumption, and look at techniques for analyzing
Introduction LECTURE 1
 LECTURE 1 Introduction The source of all great mathematics is the special case, the concrete example. It is frequent in mathematics that every instance of a concept of seemingly great generality is in
LECTURE 1 Introduction The source of all great mathematics is the special case, the concrete example. It is frequent in mathematics that every instance of a concept of seemingly great generality is in
1 Solutions in cylindrical coordinates: Bessel functions
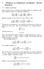 1 Solutions in cylindrical coordinates: Bessel functions 1.1 Bessel functions Bessel functions arise as solutions of potential problems in cylindrical coordinates. Laplace s equation in cylindrical coordinates
1 Solutions in cylindrical coordinates: Bessel functions 1.1 Bessel functions Bessel functions arise as solutions of potential problems in cylindrical coordinates. Laplace s equation in cylindrical coordinates
1. Introduction to Chemical Kinetics
 1. Introduction to Chemical Kinetics objectives of chemical kinetics 1) Determine empirical rate laws H 2 + I 2 2HI How does the concentration of H 2, I 2, and HI change with time? 2) Determine the mechanism
1. Introduction to Chemical Kinetics objectives of chemical kinetics 1) Determine empirical rate laws H 2 + I 2 2HI How does the concentration of H 2, I 2, and HI change with time? 2) Determine the mechanism
Supporting Information. Methods. Equations for four regimes
 Supporting Information A Methods All analytical expressions were obtained starting from quation 3, the tqssa approximation of the cycle, the derivation of which is discussed in Appendix C. The full mass
Supporting Information A Methods All analytical expressions were obtained starting from quation 3, the tqssa approximation of the cycle, the derivation of which is discussed in Appendix C. The full mass
Perturbation theory for anharmonic oscillations
 Perturbation theory for anharmonic oscillations Lecture notes by Sergei Winitzki June 12, 2006 Contents 1 Introduction 1 2 What is perturbation theory 1 21 A first example 1 22 Limits of applicability
Perturbation theory for anharmonic oscillations Lecture notes by Sergei Winitzki June 12, 2006 Contents 1 Introduction 1 2 What is perturbation theory 1 21 A first example 1 22 Limits of applicability
Physics 342 Lecture 22. The Hydrogen Atom. Lecture 22. Physics 342 Quantum Mechanics I
 Physics 342 Lecture 22 The Hydrogen Atom Lecture 22 Physics 342 Quantum Mechanics I Friday, March 28th, 2008 We now begin our discussion of the Hydrogen atom. Operationally, this is just another choice
Physics 342 Lecture 22 The Hydrogen Atom Lecture 22 Physics 342 Quantum Mechanics I Friday, March 28th, 2008 We now begin our discussion of the Hydrogen atom. Operationally, this is just another choice
Stochastic Volatility and Correction to the Heat Equation
 Stochastic Volatility and Correction to the Heat Equation Jean-Pierre Fouque, George Papanicolaou and Ronnie Sircar Abstract. From a probabilist s point of view the Twentieth Century has been a century
Stochastic Volatility and Correction to the Heat Equation Jean-Pierre Fouque, George Papanicolaou and Ronnie Sircar Abstract. From a probabilist s point of view the Twentieth Century has been a century
Fourier Series and Integrals
 Fourier Series and Integrals Fourier Series et f(x) beapiece-wiselinearfunctionon[, ] (Thismeansthatf(x) maypossessa finite number of finite discontinuities on the interval). Then f(x) canbeexpandedina
Fourier Series and Integrals Fourier Series et f(x) beapiece-wiselinearfunctionon[, ] (Thismeansthatf(x) maypossessa finite number of finite discontinuities on the interval). Then f(x) canbeexpandedina
Answers to Problem Set Number MIT (Fall 2005).
 Answers to Problem Set Number 6. 18.305 MIT (Fall 2005). D. Margetis and R. Rosales (MIT, Math. Dept., Cambridge, MA 02139). December 12, 2005. Course TA: Nikos Savva, MIT, Dept. of Mathematics, Cambridge,
Answers to Problem Set Number 6. 18.305 MIT (Fall 2005). D. Margetis and R. Rosales (MIT, Math. Dept., Cambridge, MA 02139). December 12, 2005. Course TA: Nikos Savva, MIT, Dept. of Mathematics, Cambridge,
Chemical Kinetics. Topic 7
 Chemical Kinetics Topic 7 Corrosion of Titanic wrec Casón shipwrec 2Fe(s) + 3/2O 2 (g) + H 2 O --> Fe 2 O 3.H 2 O(s) 2Na(s) + 2H 2 O --> 2NaOH(aq) + H 2 (g) Two examples of the time needed for a chemical
Chemical Kinetics Topic 7 Corrosion of Titanic wrec Casón shipwrec 2Fe(s) + 3/2O 2 (g) + H 2 O --> Fe 2 O 3.H 2 O(s) 2Na(s) + 2H 2 O --> 2NaOH(aq) + H 2 (g) Two examples of the time needed for a chemical
CHAPTER I INTRODUCTION
 CHAPTER I INTRODUCTION Preliminaries. Perturbation theory is the study of the effects of small disturbances in the mathematical model of a physical system. The model could be expressed as an algebraic
CHAPTER I INTRODUCTION Preliminaries. Perturbation theory is the study of the effects of small disturbances in the mathematical model of a physical system. The model could be expressed as an algebraic
Disturbance Attenuation for a Class of Nonlinear Systems by Output Feedback
 Disturbance Attenuation for a Class of Nonlinear Systems by Output Feedback Wei in Chunjiang Qian and Xianqing Huang Submitted to Systems & Control etters /5/ Abstract This paper studies the problem of
Disturbance Attenuation for a Class of Nonlinear Systems by Output Feedback Wei in Chunjiang Qian and Xianqing Huang Submitted to Systems & Control etters /5/ Abstract This paper studies the problem of
Minkowski spacetime. Pham A. Quang. Abstract: In this talk we review the Minkowski spacetime which is the spacetime of Special Relativity.
 Minkowski spacetime Pham A. Quang Abstract: In this talk we review the Minkowski spacetime which is the spacetime of Special Relativity. Contents 1 Introduction 1 2 Minkowski spacetime 2 3 Lorentz transformations
Minkowski spacetime Pham A. Quang Abstract: In this talk we review the Minkowski spacetime which is the spacetime of Special Relativity. Contents 1 Introduction 1 2 Minkowski spacetime 2 3 Lorentz transformations
Last Update: March 1 2, 201 0
 M ath 2 0 1 E S 1 W inter 2 0 1 0 Last Update: March 1 2, 201 0 S eries S olutions of Differential Equations Disclaimer: This lecture note tries to provide an alternative approach to the material in Sections
M ath 2 0 1 E S 1 W inter 2 0 1 0 Last Update: March 1 2, 201 0 S eries S olutions of Differential Equations Disclaimer: This lecture note tries to provide an alternative approach to the material in Sections
The Stability and Dynamics of a Spike in the One-Dimensional Keller-Segel model
 Preprint 1 The Stability and Dynamics of a Spike in the One-Dimensional Keller-Segel model K. KANG, T. KOLOKOLNIKOV and. J. WARD Kyungkeun Kang, Department of athematics, University of British Columbia,
Preprint 1 The Stability and Dynamics of a Spike in the One-Dimensional Keller-Segel model K. KANG, T. KOLOKOLNIKOV and. J. WARD Kyungkeun Kang, Department of athematics, University of British Columbia,
Problem set 3: Solutions Math 207B, Winter Suppose that u(x) is a non-zero solution of the eigenvalue problem. (u ) 2 dx, u 2 dx.
 Problem set 3: Solutions Math 27B, Winter 216 1. Suppose that u(x) is a non-zero solution of the eigenvalue problem u = λu < x < 1, u() =, u(1) =. Show that λ = (u ) 2 dx u2 dx. Deduce that every eigenvalue
Problem set 3: Solutions Math 27B, Winter 216 1. Suppose that u(x) is a non-zero solution of the eigenvalue problem u = λu < x < 1, u() =, u(1) =. Show that λ = (u ) 2 dx u2 dx. Deduce that every eigenvalue
Program for the rest of the course
 Program for the rest of the course 16.4 Enzyme kinetics 17.4 Metabolic Control Analysis 19.4. Exercise session 5 23.4. Metabolic Control Analysis, cont. 24.4 Recap 27.4 Exercise session 6 etabolic Modelling
Program for the rest of the course 16.4 Enzyme kinetics 17.4 Metabolic Control Analysis 19.4. Exercise session 5 23.4. Metabolic Control Analysis, cont. 24.4 Recap 27.4 Exercise session 6 etabolic Modelling
Practice Problems For Test 3
 Practice Problems For Test 3 Power Series Preliminary Material. Find the interval of convergence of the following. Be sure to determine the convergence at the endpoints. (a) ( ) k (x ) k (x 3) k= k (b)
Practice Problems For Test 3 Power Series Preliminary Material. Find the interval of convergence of the following. Be sure to determine the convergence at the endpoints. (a) ( ) k (x ) k (x 3) k= k (b)
Chapter Four Gelfond s Solution of Hilbert s Seventh Problem (Revised January 2, 2011)
 Chapter Four Gelfond s Solution of Hilbert s Seventh Problem (Revised January 2, 2011) Before we consider Gelfond s, and then Schneider s, complete solutions to Hilbert s seventh problem let s look back
Chapter Four Gelfond s Solution of Hilbert s Seventh Problem (Revised January 2, 2011) Before we consider Gelfond s, and then Schneider s, complete solutions to Hilbert s seventh problem let s look back
IV. Transport Phenomena Lecture 18: Forced Convection in Fuel Cells II
 IV. Transport Phenomena Lecture 18: Forced Convection in Fuel Cells II MIT Student (and MZB) As discussed in the previous lecture, we are interested in forcing fluid to flow in a fuel cell in order to
IV. Transport Phenomena Lecture 18: Forced Convection in Fuel Cells II MIT Student (and MZB) As discussed in the previous lecture, we are interested in forcing fluid to flow in a fuel cell in order to
HIGHER ORDER MULTI-TERM TIME-FRACTIONAL PARTIAL DIFFERENTIAL EQUATIONS INVOLVING CAPUTO-FABRIZIO DERIVATIVE
 Electronic Journal of Differential Equations, Vol. 27 27), No. 243, pp.. ISSN: 72-669. URL: http://ejde.math.txstate.edu or http://ejde.math.unt.edu HIGHER ORDER MULTI-TERM TIME-FRACTIONAL PARTIAL DIFFERENTIAL
Electronic Journal of Differential Equations, Vol. 27 27), No. 243, pp.. ISSN: 72-669. URL: http://ejde.math.txstate.edu or http://ejde.math.unt.edu HIGHER ORDER MULTI-TERM TIME-FRACTIONAL PARTIAL DIFFERENTIAL
Chapter 18. Remarks on partial differential equations
 Chapter 8. Remarks on partial differential equations If we try to analyze heat flow or vibration in a continuous system such as a building or an airplane, we arrive at a kind of infinite system of ordinary
Chapter 8. Remarks on partial differential equations If we try to analyze heat flow or vibration in a continuous system such as a building or an airplane, we arrive at a kind of infinite system of ordinary
Lecture 6 Asymptotic Structure for Four-Step Premixed Stoichiometric Methane Flames
 Lecture 6 Asymptotic Structure for Four-Step Premixed Stoichiometric Methane Flames 6.-1 Previous lecture: Asymptotic description of premixed flames based on an assumed one-step reaction. basic understanding
Lecture 6 Asymptotic Structure for Four-Step Premixed Stoichiometric Methane Flames 6.-1 Previous lecture: Asymptotic description of premixed flames based on an assumed one-step reaction. basic understanding
Often, in this class, we will analyze a closed-loop feedback control system, and end up with an equation of the form
 ME 32, Spring 25, UC Berkeley, A. Packard 55 7 Review of SLODEs Throughout this section, if y denotes a function (of time, say), then y [k or y (k) denotes the k th derivative of the function y, y [k =
ME 32, Spring 25, UC Berkeley, A. Packard 55 7 Review of SLODEs Throughout this section, if y denotes a function (of time, say), then y [k or y (k) denotes the k th derivative of the function y, y [k =
MATH 131P: PRACTICE FINAL SOLUTIONS DECEMBER 12, 2012
 MATH 3P: PRACTICE FINAL SOLUTIONS DECEMBER, This is a closed ook, closed notes, no calculators/computers exam. There are 6 prolems. Write your solutions to Prolems -3 in lue ook #, and your solutions to
MATH 3P: PRACTICE FINAL SOLUTIONS DECEMBER, This is a closed ook, closed notes, no calculators/computers exam. There are 6 prolems. Write your solutions to Prolems -3 in lue ook #, and your solutions to
MIT (Spring 2014)
 18.311 MIT (Spring 014) Rodolfo R. Rosales May 6, 014. Problem Set # 08. Due: Last day of lectures. IMPORTANT: Turn in the regular and the special problems stapled in two SEPARATE packages. Print your
18.311 MIT (Spring 014) Rodolfo R. Rosales May 6, 014. Problem Set # 08. Due: Last day of lectures. IMPORTANT: Turn in the regular and the special problems stapled in two SEPARATE packages. Print your
The One-Dimensional Heat Equation
 The One-Dimensional Heat Equation R. C. Trinity University Partial Differential Equations February 24, 2015 Introduction The heat equation Goal: Model heat (thermal energy) flow in a one-dimensional object
The One-Dimensional Heat Equation R. C. Trinity University Partial Differential Equations February 24, 2015 Introduction The heat equation Goal: Model heat (thermal energy) flow in a one-dimensional object
n v molecules will pass per unit time through the area from left to
 3 iscosity and Heat Conduction in Gas Dynamics Equations of One-Dimensional Gas Flow The dissipative processes - viscosity (internal friction) and heat conduction - are connected with existence of molecular
3 iscosity and Heat Conduction in Gas Dynamics Equations of One-Dimensional Gas Flow The dissipative processes - viscosity (internal friction) and heat conduction - are connected with existence of molecular
Part II => PROTEINS and ENZYMES. 2.7 Enzyme Kinetics 2.7a Chemical Kinetics 2.7b Enzyme Inhibition
 Part II => PROTEINS and ENZYMES 2.7 Enzyme Kinetics 2.7a Chemical Kinetics 2.7b Enzyme Inhibition Section 2.7a: Chemical Kinetics Synopsis 2.7a - Chemical kinetics (or reaction kinetics) is the study of
Part II => PROTEINS and ENZYMES 2.7 Enzyme Kinetics 2.7a Chemical Kinetics 2.7b Enzyme Inhibition Section 2.7a: Chemical Kinetics Synopsis 2.7a - Chemical kinetics (or reaction kinetics) is the study of
NONSTANDARD NUMERICAL METHODS FOR A CLASS OF PREDATOR-PREY MODELS WITH PREDATOR INTERFERENCE
 Sixth Mississippi State Conference on Differential Equations and Computational Simulations, Electronic Journal of Differential Equations, Conference 15 (2007), pp. 67 75. ISSN: 1072-6691. URL: http://ejde.math.txstate.edu
Sixth Mississippi State Conference on Differential Equations and Computational Simulations, Electronic Journal of Differential Equations, Conference 15 (2007), pp. 67 75. ISSN: 1072-6691. URL: http://ejde.math.txstate.edu
Linear Theory of Evolution to an Unstable State
 Chapter 2 Linear Theory of Evolution to an Unstable State c 2012 by William Klein, Harvey Gould, and Jan Tobochnik 1 October 2012 2.1 Introduction The simple theory of nucleation that we introduced in
Chapter 2 Linear Theory of Evolution to an Unstable State c 2012 by William Klein, Harvey Gould, and Jan Tobochnik 1 October 2012 2.1 Introduction The simple theory of nucleation that we introduced in
ON THE SOLUTION OF DIFFERENTIAL EQUATIONS WITH DELAYED AND ADVANCED ARGUMENTS
 2003 Colloquium on Differential Equations and Applications, Maracaibo, Venezuela. Electronic Journal of Differential Equations, Conference 13, 2005, pp. 57 63. ISSN: 1072-6691. URL: http://ejde.math.txstate.edu
2003 Colloquium on Differential Equations and Applications, Maracaibo, Venezuela. Electronic Journal of Differential Equations, Conference 13, 2005, pp. 57 63. ISSN: 1072-6691. URL: http://ejde.math.txstate.edu
ASYMPTOTIC METHODS IN MECHANICS
 ASYMPTOTIC METHODS IN MECHANICS by I. Argatov and G. Mishuris Aberystwyth University 2011 Contents Introduction............................... 4 1 Asymptotic expansions 5 1.1 Introduction to asymptotic
ASYMPTOTIC METHODS IN MECHANICS by I. Argatov and G. Mishuris Aberystwyth University 2011 Contents Introduction............................... 4 1 Asymptotic expansions 5 1.1 Introduction to asymptotic
Exact and approximate solutions for a century-old problem: A general treatment of Henri-Michaelis-Menten enzyme kinetics
 Exact and approximate solutions for a century-old problem: A general treatment of Henri-Michaelis-Menten enzyme kinetics Mário N. Berberan-Santos a) Centro de Química-Física Molecular and IN - Institute
Exact and approximate solutions for a century-old problem: A general treatment of Henri-Michaelis-Menten enzyme kinetics Mário N. Berberan-Santos a) Centro de Química-Física Molecular and IN - Institute
Transport by convection. Coupling convection-diffusion
 Transport by convection. Coupling convection-diffusion 24 mars 2017 1 When can we neglect diffusion? When the Peclet number is not very small we cannot ignore the convection term in the transport equation.
Transport by convection. Coupling convection-diffusion 24 mars 2017 1 When can we neglect diffusion? When the Peclet number is not very small we cannot ignore the convection term in the transport equation.
Last Update: April 7, 201 0
 M ath E S W inter Last Update: April 7, Introduction to Partial Differential Equations Disclaimer: his lecture note tries to provide an alternative approach to the material in Sections.. 5 in the textbook.
M ath E S W inter Last Update: April 7, Introduction to Partial Differential Equations Disclaimer: his lecture note tries to provide an alternative approach to the material in Sections.. 5 in the textbook.
Extending the quasi-steady state approximation by changing variables
 6 Extending the quasi-steady state approximation by changing variables José A.M. Borghans 1, Rob J. de Boer 1 & Lee A. Segel 2 1 Theoretical Biology, Utrecht University, Utrecht, The Netherlands. 2 Department
6 Extending the quasi-steady state approximation by changing variables José A.M. Borghans 1, Rob J. de Boer 1 & Lee A. Segel 2 1 Theoretical Biology, Utrecht University, Utrecht, The Netherlands. 2 Department
Stability of Feedback Solutions for Infinite Horizon Noncooperative Differential Games
 Stability of Feedback Solutions for Infinite Horizon Noncooperative Differential Games Alberto Bressan ) and Khai T. Nguyen ) *) Department of Mathematics, Penn State University **) Department of Mathematics,
Stability of Feedback Solutions for Infinite Horizon Noncooperative Differential Games Alberto Bressan ) and Khai T. Nguyen ) *) Department of Mathematics, Penn State University **) Department of Mathematics,
Generalized eigenvector - Wikipedia, the free encyclopedia
 1 of 30 18/03/2013 20:00 Generalized eigenvector From Wikipedia, the free encyclopedia In linear algebra, for a matrix A, there may not always exist a full set of linearly independent eigenvectors that
1 of 30 18/03/2013 20:00 Generalized eigenvector From Wikipedia, the free encyclopedia In linear algebra, for a matrix A, there may not always exist a full set of linearly independent eigenvectors that
13 PDEs on spatially bounded domains: initial boundary value problems (IBVPs)
 13 PDEs on spatially bounded domains: initial boundary value problems (IBVPs) A prototypical problem we will discuss in detail is the 1D diffusion equation u t = Du xx < x < l, t > finite-length rod u(x,
13 PDEs on spatially bounded domains: initial boundary value problems (IBVPs) A prototypical problem we will discuss in detail is the 1D diffusion equation u t = Du xx < x < l, t > finite-length rod u(x,
Advanced Mathematical Methods for Scientists and Engineers I
 Carl M. Bender Steven A. Orszag Advanced Mathematical Methods for Scientists and Engineers I Asymptotic Methods and Perturbation Theory With 148 Figures Springer CONTENTS! Preface xiii PART I FUNDAMENTALS
Carl M. Bender Steven A. Orszag Advanced Mathematical Methods for Scientists and Engineers I Asymptotic Methods and Perturbation Theory With 148 Figures Springer CONTENTS! Preface xiii PART I FUNDAMENTALS
Lecture 11: Non-Equilibrium Statistical Physics
 Massachusetts Institute of Technology MITES 2018 Physics III Lecture 11: Non-Equilibrium Statistical Physics In the previous notes, we studied how systems in thermal equilibrium can change in time even
Massachusetts Institute of Technology MITES 2018 Physics III Lecture 11: Non-Equilibrium Statistical Physics In the previous notes, we studied how systems in thermal equilibrium can change in time even
4. Complex Oscillations
 4. Complex Oscillations The most common use of complex numbers in physics is for analyzing oscillations and waves. We will illustrate this with a simple but crucially important model, the damped harmonic
4. Complex Oscillations The most common use of complex numbers in physics is for analyzing oscillations and waves. We will illustrate this with a simple but crucially important model, the damped harmonic
The method of lines (MOL) for the diffusion equation
 Chapter 1 The method of lines (MOL) for the diffusion equation The method of lines refers to an approximation of one or more partial differential equations with ordinary differential equations in just
Chapter 1 The method of lines (MOL) for the diffusion equation The method of lines refers to an approximation of one or more partial differential equations with ordinary differential equations in just
Rational derivation of the Boussinesq approximation
 Rational derivation of the Boussinesq approximation Kiyoshi Maruyama Department of Earth and Ocean Sciences, National Defense Academy, Yokosuka, Kanagawa 239-8686, Japan February 22, 2019 Abstract This
Rational derivation of the Boussinesq approximation Kiyoshi Maruyama Department of Earth and Ocean Sciences, National Defense Academy, Yokosuka, Kanagawa 239-8686, Japan February 22, 2019 Abstract This
Physics 250 Green s functions for ordinary differential equations
 Physics 25 Green s functions for ordinary differential equations Peter Young November 25, 27 Homogeneous Equations We have already discussed second order linear homogeneous differential equations, which
Physics 25 Green s functions for ordinary differential equations Peter Young November 25, 27 Homogeneous Equations We have already discussed second order linear homogeneous differential equations, which
RIGOROUS ASYMPTOTIC EXPANSIONS FOR LAGERSTROM S MODEL EQUATION A GEOMETRIC APPROACH
 RIGOROUS ASYMPTOTIC EXPANSIONS FOR LAGERSTROM S MODEL EQUATION A GEOMETRIC APPROACH NIKOLA POPOVIĆ AND PETER SZMOLYAN Abstract. The present work is a continuation of the geometric singular perturbation
RIGOROUS ASYMPTOTIC EXPANSIONS FOR LAGERSTROM S MODEL EQUATION A GEOMETRIC APPROACH NIKOLA POPOVIĆ AND PETER SZMOLYAN Abstract. The present work is a continuation of the geometric singular perturbation
Introduction to multiscale modeling and simulation. Explicit methods for ODEs : forward Euler. y n+1 = y n + tf(y n ) dy dt = f(y), y(0) = y 0
 Introduction to multiscale modeling and simulation Lecture 5 Numerical methods for ODEs, SDEs and PDEs The need for multiscale methods Two generic frameworks for multiscale computation Explicit methods
Introduction to multiscale modeling and simulation Lecture 5 Numerical methods for ODEs, SDEs and PDEs The need for multiscale methods Two generic frameworks for multiscale computation Explicit methods
Practice Problems For Test 3
 Practice Problems For Test 3 Power Series Preliminary Material. Find the interval of convergence of the following. Be sure to determine the convergence at the endpoints. (a) ( ) k (x ) k (x 3) k= k (b)
Practice Problems For Test 3 Power Series Preliminary Material. Find the interval of convergence of the following. Be sure to determine the convergence at the endpoints. (a) ( ) k (x ) k (x 3) k= k (b)
Lecture 2 Supplementary Notes: Derivation of the Phase Equation
 Lecture 2 Supplementary Notes: Derivation of the Phase Equation Michael Cross, 25 Derivation from Amplitude Equation Near threshold the phase reduces to the phase of the complex amplitude, and the phase
Lecture 2 Supplementary Notes: Derivation of the Phase Equation Michael Cross, 25 Derivation from Amplitude Equation Near threshold the phase reduces to the phase of the complex amplitude, and the phase
1 The Observability Canonical Form
 NONLINEAR OBSERVERS AND SEPARATION PRINCIPLE 1 The Observability Canonical Form In this Chapter we discuss the design of observers for nonlinear systems modelled by equations of the form ẋ = f(x, u) (1)
NONLINEAR OBSERVERS AND SEPARATION PRINCIPLE 1 The Observability Canonical Form In this Chapter we discuss the design of observers for nonlinear systems modelled by equations of the form ẋ = f(x, u) (1)
ON A NESTED BOUNDARY-LAYER PROBLEM. X. Liang. R. Wong. Dedicated to Professor Philippe G. Ciarlet on the occasion of his 70th birthday
 COMMUNICATIONS ON doi:.3934/cpaa.9.8.49 PURE AND APPLIED ANALYSIS Volume 8, Number, January 9 pp. 49 433 ON A NESTED BOUNDARY-LAYER PROBLEM X. Liang Department of Mathematics, Massachusetts Institute of
COMMUNICATIONS ON doi:.3934/cpaa.9.8.49 PURE AND APPLIED ANALYSIS Volume 8, Number, January 9 pp. 49 433 ON A NESTED BOUNDARY-LAYER PROBLEM X. Liang Department of Mathematics, Massachusetts Institute of
A Study of the Van der Pol Equation
 A Study of the Van der Pol Equation Kai Zhe Tan, s1465711 September 16, 2016 Abstract The Van der Pol equation is famous for modelling biological systems as well as being a good model to study its multiple
A Study of the Van der Pol Equation Kai Zhe Tan, s1465711 September 16, 2016 Abstract The Van der Pol equation is famous for modelling biological systems as well as being a good model to study its multiple
Vectors. January 13, 2013
 Vectors January 13, 2013 The simplest tensors are scalars, which are the measurable quantities of a theory, left invariant by symmetry transformations. By far the most common non-scalars are the vectors,
Vectors January 13, 2013 The simplest tensors are scalars, which are the measurable quantities of a theory, left invariant by symmetry transformations. By far the most common non-scalars are the vectors,
Math 345 Intro to Math Biology Lecture 19: Models of Molecular Events and Biochemistry
 Math 345 Intro to Math Biology Lecture 19: Models of Molecular Events and Biochemistry Junping Shi College of William and Mary, USA Molecular biology and Biochemical kinetics Molecular biology is one of
Math 345 Intro to Math Biology Lecture 19: Models of Molecular Events and Biochemistry Junping Shi College of William and Mary, USA Molecular biology and Biochemical kinetics Molecular biology is one of
Mathematical modeling of critical conditions in the thermal explosion problem
 Mathematical modeling of critical conditions in the thermal explosion problem G. N. Gorelov and V. A. Sobolev Samara State University, Russia Abstract The paper is devoted to the thermal explosion problem
Mathematical modeling of critical conditions in the thermal explosion problem G. N. Gorelov and V. A. Sobolev Samara State University, Russia Abstract The paper is devoted to the thermal explosion problem
with a proper choice of the potential U(r). Clearly, we should include the potential of the ions, U ion (r):.
 The Hartree Equations So far we have ignored the effects of electron-electron (e-e) interactions by working in the independent electron approximation. In this lecture, we shall discuss how this effect
The Hartree Equations So far we have ignored the effects of electron-electron (e-e) interactions by working in the independent electron approximation. In this lecture, we shall discuss how this effect
Finite Differences for Differential Equations 28 PART II. Finite Difference Methods for Differential Equations
 Finite Differences for Differential Equations 28 PART II Finite Difference Methods for Differential Equations Finite Differences for Differential Equations 29 BOUNDARY VALUE PROBLEMS (I) Solving a TWO
Finite Differences for Differential Equations 28 PART II Finite Difference Methods for Differential Equations Finite Differences for Differential Equations 29 BOUNDARY VALUE PROBLEMS (I) Solving a TWO
A COUNTEREXAMPLE TO AN ENDPOINT BILINEAR STRICHARTZ INEQUALITY TERENCE TAO. t L x (R R2 ) f L 2 x (R2 )
 Electronic Journal of Differential Equations, Vol. 2006(2006), No. 5, pp. 6. ISSN: 072-669. URL: http://ejde.math.txstate.edu or http://ejde.math.unt.edu ftp ejde.math.txstate.edu (login: ftp) A COUNTEREXAMPLE
Electronic Journal of Differential Equations, Vol. 2006(2006), No. 5, pp. 6. ISSN: 072-669. URL: http://ejde.math.txstate.edu or http://ejde.math.unt.edu ftp ejde.math.txstate.edu (login: ftp) A COUNTEREXAMPLE
MATH 205C: STATIONARY PHASE LEMMA
 MATH 205C: STATIONARY PHASE LEMMA For ω, consider an integral of the form I(ω) = e iωf(x) u(x) dx, where u Cc (R n ) complex valued, with support in a compact set K, and f C (R n ) real valued. Thus, I(ω)
MATH 205C: STATIONARY PHASE LEMMA For ω, consider an integral of the form I(ω) = e iωf(x) u(x) dx, where u Cc (R n ) complex valued, with support in a compact set K, and f C (R n ) real valued. Thus, I(ω)
TOPIC 6: Chemical kinetics
 TOPIC 6: Chemical kinetics Reaction rates Reaction rate laws Integrated reaction rate laws Reaction mechanism Kinetic theories Arrhenius law Catalysis Enzimatic catalysis Fuente: Cedre http://loincognito.-iles.wordpress.com/202/04/titanic-
TOPIC 6: Chemical kinetics Reaction rates Reaction rate laws Integrated reaction rate laws Reaction mechanism Kinetic theories Arrhenius law Catalysis Enzimatic catalysis Fuente: Cedre http://loincognito.-iles.wordpress.com/202/04/titanic-
Theoretical Models for Chemical Kinetics
 Theoretical Models for Chemical Kinetics Thus far we have calculated rate laws, rate constants, reaction orders, etc. based on observations of macroscopic properties, but what is happening at the molecular
Theoretical Models for Chemical Kinetics Thus far we have calculated rate laws, rate constants, reaction orders, etc. based on observations of macroscopic properties, but what is happening at the molecular
A. One-Substrate Reactions (1) Kinetic concepts
 A. One-Substrate Reactions (1) Kinetic concepts (2) Kinetic analysis (a) Briggs-Haldane steady-state treatment (b) Michaelis constant (K m ) (c) Specificity constant (3) Graphical analysis (4) Practical
A. One-Substrate Reactions (1) Kinetic concepts (2) Kinetic analysis (a) Briggs-Haldane steady-state treatment (b) Michaelis constant (K m ) (c) Specificity constant (3) Graphical analysis (4) Practical
Boundary layer flows The logarithmic law of the wall Mixing length model for turbulent viscosity
 Boundary layer flows The logarithmic law of the wall Mixing length model for turbulent viscosity Tobias Knopp D 23. November 28 Reynolds averaged Navier-Stokes equations Consider the RANS equations with
Boundary layer flows The logarithmic law of the wall Mixing length model for turbulent viscosity Tobias Knopp D 23. November 28 Reynolds averaged Navier-Stokes equations Consider the RANS equations with
PHYSICS 715 COURSE NOTES WEEK 1
 PHYSICS 715 COURSE NOTES WEEK 1 1 Thermodynamics 1.1 Introduction When we start to study physics, we learn about particle motion. First one particle, then two. It is dismaying to learn that the motion
PHYSICS 715 COURSE NOTES WEEK 1 1 Thermodynamics 1.1 Introduction When we start to study physics, we learn about particle motion. First one particle, then two. It is dismaying to learn that the motion
An Introduction to Numerical Methods for Differential Equations. Janet Peterson
 An Introduction to Numerical Methods for Differential Equations Janet Peterson Fall 2015 2 Chapter 1 Introduction Differential equations arise in many disciplines such as engineering, mathematics, sciences
An Introduction to Numerical Methods for Differential Equations Janet Peterson Fall 2015 2 Chapter 1 Introduction Differential equations arise in many disciplines such as engineering, mathematics, sciences
Simple kinetics of enzyme action
 Simple kinetics of enzyme action It is established that enzymes form a bound complex to their reactants (i.e. substrates) during the course of their catalysis and prior to the release of products. This
Simple kinetics of enzyme action It is established that enzymes form a bound complex to their reactants (i.e. substrates) during the course of their catalysis and prior to the release of products. This
Quantitative Understanding in Biology Module IV: ODEs Lecture I: Introduction to ODEs
 Quantitative Understanding in Biology Module IV: ODEs Lecture I: Introduction to ODEs Ordinary Differential Equations We began our exploration of dynamic systems with a look at linear difference equations.
Quantitative Understanding in Biology Module IV: ODEs Lecture I: Introduction to ODEs Ordinary Differential Equations We began our exploration of dynamic systems with a look at linear difference equations.
A LOWER BOUND ON BLOWUP RATES FOR THE 3D INCOMPRESSIBLE EULER EQUATION AND A SINGLE EXPONENTIAL BEALE-KATO-MAJDA ESTIMATE. 1.
 A LOWER BOUND ON BLOWUP RATES FOR THE 3D INCOMPRESSIBLE EULER EQUATION AND A SINGLE EXPONENTIAL BEALE-KATO-MAJDA ESTIMATE THOMAS CHEN AND NATAŠA PAVLOVIĆ Abstract. We prove a Beale-Kato-Majda criterion
A LOWER BOUND ON BLOWUP RATES FOR THE 3D INCOMPRESSIBLE EULER EQUATION AND A SINGLE EXPONENTIAL BEALE-KATO-MAJDA ESTIMATE THOMAS CHEN AND NATAŠA PAVLOVIĆ Abstract. We prove a Beale-Kato-Majda criterion
Consider a volume Ω enclosing a mass M and bounded by a surface δω. d dt. q n ds. The Work done by the body on the surroundings is.
 The Energy Balance Consider a volume Ω enclosing a mass M and bounded by a surface δω. δω At a point x, the density is ρ, the local velocity is v, and the local Energy density is U. U v The rate of change
The Energy Balance Consider a volume Ω enclosing a mass M and bounded by a surface δω. δω At a point x, the density is ρ, the local velocity is v, and the local Energy density is U. U v The rate of change
Rigorous asymptotic expansions for Lagerstrom s model equation a geometric approach
 Rigorous asymptotic expansions for Lagerstrom s model equation a geometric approach Nikola Popović Peter Szmolyan July 7, 2004 Abstract The present work is a continuation of the geometric singular perturbation
Rigorous asymptotic expansions for Lagerstrom s model equation a geometric approach Nikola Popović Peter Szmolyan July 7, 2004 Abstract The present work is a continuation of the geometric singular perturbation
Diffusion influence on Michaelis Menten kinetics
 JOURNAL OF CHEMICAL PHYSICS VOLUME 5, NUMBER 3 5 JULY 200 Diffusion influence on Michaelis Menten kinetics Hyojoon Kim, Mino Yang, Myung-Un Choi, and Kook Joe Shin a) School of Chemistry, Seoul National
JOURNAL OF CHEMICAL PHYSICS VOLUME 5, NUMBER 3 5 JULY 200 Diffusion influence on Michaelis Menten kinetics Hyojoon Kim, Mino Yang, Myung-Un Choi, and Kook Joe Shin a) School of Chemistry, Seoul National
EXISTENCE AND APPROXIMATION OF SOLUTIONS OF SECOND ORDER NONLINEAR NEUMANN PROBLEMS
 Electronic Journal of Differential Equations, Vol. 2005(2005), No. 03, pp. 1 10. ISSN: 1072-6691. URL: http://ejde.math.txstate.edu or http://ejde.math.unt.edu ftp ejde.math.txstate.edu (login: ftp) EXISTENCE
Electronic Journal of Differential Equations, Vol. 2005(2005), No. 03, pp. 1 10. ISSN: 1072-6691. URL: http://ejde.math.txstate.edu or http://ejde.math.unt.edu ftp ejde.math.txstate.edu (login: ftp) EXISTENCE
Generalized Solovev equilibrium with sheared flow of arbitrary direction and stability consideration
 Generalized Solovev equilibrium with sheared flow of arbitrary direction and stability consideration D.A. Kaltsas and G.N. Throumoulopoulos Department of Physics, University of Ioannina, GR 451 10 Ioannina,
Generalized Solovev equilibrium with sheared flow of arbitrary direction and stability consideration D.A. Kaltsas and G.N. Throumoulopoulos Department of Physics, University of Ioannina, GR 451 10 Ioannina,
Problem Set Number 01, MIT (Winter-Spring 2018)
 Problem Set Number 01, 18.306 MIT (Winter-Spring 2018) Rodolfo R. Rosales (MIT, Math. Dept., room 2-337, Cambridge, MA 02139) February 28, 2018 Due Monday March 12, 2018. Turn it in (by 3PM) at the Math.
Problem Set Number 01, 18.306 MIT (Winter-Spring 2018) Rodolfo R. Rosales (MIT, Math. Dept., room 2-337, Cambridge, MA 02139) February 28, 2018 Due Monday March 12, 2018. Turn it in (by 3PM) at the Math.
