CHAPTER 4 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS
|
|
|
- Easter Reeves
- 6 years ago
- Views:
Transcription
1 4-1 CHAPTER 4 IMPERFECTIONS IN SOLIDS PROBLEM SOLUTIONS Vacancies and Self-Interstitials 4.1 In order to compute the fraction of atom sites that are vacant in copper at 1357 K, we must employ Equation 4.1. As stated in the problem, Q v = 0.90 ev/atom. Thus, N v N = exp Q v = exp kt 0.90 ev /atom ( ev/atom- K) (1357 K) = 4.56 x 10-4
2 4-36 Dislocations Linear Defects 4.6 The Burgers vector and dislocation line are perpendicular for edge dislocations, parallel for screw dislocations, and neither perpendicular nor parallel for mixed dislocations.
3 In the drawing below is shown the atoms on the (100) face of an FCC unit cell; the interstitial site is at the center of the edge. The diameter of an atom that will just fit into this site (r) is just the difference between that unit cell edge length (a) and the radii of the two host atoms that are located on either side of the site (R); that is r = a R However, for FCC a is related to R according to Equation 3.1 as a = R ; therefore, solving for r from the above equation gives r = a R A (100) face of a BCC unit cell is shown below. = R R = 0.41R
4 4-6 The interstitial atom that just fits into this interstitial site is shown by the small circle. It is situated in the plane of this (100) face, midway between the two vertical unit cell edges, and one quarter of the distance between the bottom and top cell edges. From the right triangle that is defined by the three arrows we may write a + a 4 = (R + r) However, from Equation 3.3, a = 4R, and, therefore, making this substitution, the above equation takes the form 3 4R 3 + 4R 4 3 = R +Rr + r After rearrangement the following quadratic equation results: r + Rr 0.667R = 0 And upon solving for r: r = (R) ± (R) (4)(1)( 0.667R )
5 4-7 = R ±.58R And, finally r(+) = r( ) = R +.58R = 0.91R R.58R =.91R Of course, only the r(+) root is possible, and, therefore, r = 0.91R. BCC. Thus, for a host atom of radius R, the size of an interstitial site for FCC is approximately 1.4 times that for
6 4-47 DESIGN PROBLEMS Specification of Composition 4.D1 This problem calls for us to compute the concentration of lithium (in wt%) that, when added to aluminum, will yield a density of.47 g/cm 3. Solution of this problem requires the use of Equation 4.10a, which takes the form ρ ave = 100 C Li ρ Li C Li ρ Al inasmuch as C Li + C Al = 100. According to the table inside the front cover, the respective densities of Li and Al are and.71 g/cm 3. Upon solving for C Li from the above equation, we get C Li = 100 ρ Li (ρ Al ρ ave ) ρ ave (ρ Al ρ Li ) = (100)(0.534 g /cm3 )(.71 g /cm 3.47 g /cm 3 ) (.47 g /cm 3 )(.71 g /cm g /cm 3 ) =.38 wt%
7 5-3 Diffusion Mechanisms 5.3 (a) With vacancy diffusion, atomic motion is from one lattice site to an adjacent vacancy. Selfdiffusion and the diffusion of substitutional impurities proceed via this mechanism. On the other hand, atomic motion is from interstitial site to adjacent interstitial site for the interstitial diffusion mechanism. (b) Interstitial diffusion is normally more rapid than vacancy diffusion because: (1) interstitial atoms, being smaller, are more mobile; and () the probability of an empty adjacent interstitial site is greater than for a vacancy adjacent to a host (or substitutional impurity) atom.
8 (a) The driving force is that which compels a reaction to occur. (b) The driving force for steady-state diffusion is the concentration gradient.
9 This problem calls for computation of the diffusion coefficient for a steady-state diffusion situation. Let us first convert the carbon concentrations from weight percent to kilograms carbon per meter cubed using Equation 4.9a. For wt% C C C " = C C ρ C C C + C Fe ρ Fe 10 3 = g /cm g /cm kg C/m 3 Similarly, for wt% C C C " = g /cm g /cm = kg C/m 3 Now, using a rearranged form of Equation 5.3 x D = J A x B C A C B = ( kg/m 10 -s) 3 m 1.18 kg /m kg /m 3 =.3 x m /s
10 This problem calls for an estimate of the time necessary to achieve a carbon concentration of 0.35 wt% at a point 6.0 mm from the surface. From Equation 5.6b, x Dt = constant But since the temperature is constant, so also is D constant, and or x t = constant x 1 = x t 1 t Thus, (.0 mm) 15 h = (6.0 mm) t from which t = 135 h
N = N A Pb A Pb. = ln N Q v kt. = kt ln v N
 5. Calculate the energy for vacancy formation in silver, given that the equilibrium number of vacancies at 800 C (1073 K) is 3.6 10 3 m 3. The atomic weight and density (at 800 C) for silver are, respectively,
5. Calculate the energy for vacancy formation in silver, given that the equilibrium number of vacancies at 800 C (1073 K) is 3.6 10 3 m 3. The atomic weight and density (at 800 C) for silver are, respectively,
DIFFUSION IN SOLIDS. IE-114 Materials Science and General Chemistry Lecture-5
 DIFFUSION IN SOLIDS IE-114 Materials Science and General Chemistry Lecture-5 Diffusion The mechanism by which matter is transported through matter. It is related to internal atomic movement. Atomic movement;
DIFFUSION IN SOLIDS IE-114 Materials Science and General Chemistry Lecture-5 Diffusion The mechanism by which matter is transported through matter. It is related to internal atomic movement. Atomic movement;
Introduction To Materials Science FOR ENGINEERS, Ch. 5. Diffusion. MSE 201 Callister Chapter 5
 Diffusion MSE 201 Callister Chapter 5 1 Goals: Diffusion - how do atoms move through solids? Fundamental concepts and language Diffusion mechanisms Vacancy diffusion Interstitial diffusion Impurities Diffusion
Diffusion MSE 201 Callister Chapter 5 1 Goals: Diffusion - how do atoms move through solids? Fundamental concepts and language Diffusion mechanisms Vacancy diffusion Interstitial diffusion Impurities Diffusion
ME 254 MATERIALS ENGINEERING 1 st Semester 1430/ st Med-Term Exam (1.5 hrs)
 ME 254 MATERIALS ENGINEERING 1 st Semester 1430/1431 1 st Med-Term Exam (1.5 hrs) قسم الهندسة الميكانيكية Question 1 a) Classify the materials based on their properties and performance, give some examples.
ME 254 MATERIALS ENGINEERING 1 st Semester 1430/1431 1 st Med-Term Exam (1.5 hrs) قسم الهندسة الميكانيكية Question 1 a) Classify the materials based on their properties and performance, give some examples.
MSE 360 Exam 1 Spring Points Total
 MSE 360 Exam 1 Spring 011 105 Points otal Name(print) ID number No notes, books, or information stored in calculator memories may be used. he NCSU academic integrity policies apply to this exam. As such,
MSE 360 Exam 1 Spring 011 105 Points otal Name(print) ID number No notes, books, or information stored in calculator memories may be used. he NCSU academic integrity policies apply to this exam. As such,
water adding dye partial mixing homogenization time
 iffusion iffusion is a process of mass transport that involves the movement of one atomic species into another. It occurs by ranom atomic jumps from one position to another an takes place in the gaseous,
iffusion iffusion is a process of mass transport that involves the movement of one atomic species into another. It occurs by ranom atomic jumps from one position to another an takes place in the gaseous,
plane in a cubic unit cell. Clearly label the axes. (b) Draw two directions in the ( 112)
 Midterm Examination - Thursday, February 5, 8:00 9:5 AM Place all answers in a 8.5" x " Bluebook Allowed:, double-sided 8.5" x " page of notes must return with exam. Required: Picture ID when returning
Midterm Examination - Thursday, February 5, 8:00 9:5 AM Place all answers in a 8.5" x " Bluebook Allowed:, double-sided 8.5" x " page of notes must return with exam. Required: Picture ID when returning
Class 29: Reciprocal Space 3: Ewald sphere, Simple Cubic, FCC and BCC in Reciprocal Space
 Class 29: Reciprocal Space 3: Ewald sphere, Simple Cubic, FCC and BCC in Reciprocal Space We have seen that diffraction occurs when, in reciprocal space, Let us now plot this information. Let us designate
Class 29: Reciprocal Space 3: Ewald sphere, Simple Cubic, FCC and BCC in Reciprocal Space We have seen that diffraction occurs when, in reciprocal space, Let us now plot this information. Let us designate
Kinetics. Rate of change in response to thermodynamic forces
 Kinetics Rate of change in response to thermodynamic forces Deviation from local equilibrium continuous change T heat flow temperature changes µ atom flow composition changes Deviation from global equilibrium
Kinetics Rate of change in response to thermodynamic forces Deviation from local equilibrium continuous change T heat flow temperature changes µ atom flow composition changes Deviation from global equilibrium
Experiment 7: Understanding Crystal Structures
 Experiment 7: Understanding Crystal Structures To do well in this laboratory experiment you need to be familiar with the concepts of lattice, crystal structure, unit cell, coordination number, the different
Experiment 7: Understanding Crystal Structures To do well in this laboratory experiment you need to be familiar with the concepts of lattice, crystal structure, unit cell, coordination number, the different
CRYSTAL STRUCTURES WITH CUBIC UNIT CELLS
 CRYSTAL STRUCTURES WITH CUBIC UNIT CELLS Crystalline solids are a three dimensional collection of individual atoms, ions, or whole molecules organized in repeating patterns. These atoms, ions, or molecules
CRYSTAL STRUCTURES WITH CUBIC UNIT CELLS Crystalline solids are a three dimensional collection of individual atoms, ions, or whole molecules organized in repeating patterns. These atoms, ions, or molecules
Extrinsic Point Defects: Impurities
 Extrinsic Point Defects: Impurities Substitutional and interstitial impurities Sol solutions, solubility limit Entropy of ing, eal solution model Enthalpy of ing, quasi-chemical model Ideal and regular
Extrinsic Point Defects: Impurities Substitutional and interstitial impurities Sol solutions, solubility limit Entropy of ing, eal solution model Enthalpy of ing, quasi-chemical model Ideal and regular
Diffusion. Diffusion. Diffusion in Solid Materials
 Atoms movements in materials Diffusion Movement of atoms in solids, liquids and gases is very important Eamples: Hardening steel, chrome-plating, gas reactions, Si wafers.. etc. We will study: Atomic mechanisms
Atoms movements in materials Diffusion Movement of atoms in solids, liquids and gases is very important Eamples: Hardening steel, chrome-plating, gas reactions, Si wafers.. etc. We will study: Atomic mechanisms
CHAPTER 3 THE STRUCTURE OF CRYSTALLINE SOLIDS PROBLEM SOLUTIONS
 CHAPTER THE STRUCTURE OF CRYSTALLINE SOLIDS PROBLEM SOLUTIONS Fundamental Concepts.1 What is the difference between atomic structure and crystal structure? Atomic structure relates to the number of protons
CHAPTER THE STRUCTURE OF CRYSTALLINE SOLIDS PROBLEM SOLUTIONS Fundamental Concepts.1 What is the difference between atomic structure and crystal structure? Atomic structure relates to the number of protons
Steady-state diffusion is diffusion in which the concentration of the diffusing atoms at
 Chapter 7 What is steady state diffusion? Steady-state diffusion is diffusion in which the concentration of the diffusing atoms at any point, x, and hence the concentration gradient at x, in the solid,
Chapter 7 What is steady state diffusion? Steady-state diffusion is diffusion in which the concentration of the diffusing atoms at any point, x, and hence the concentration gradient at x, in the solid,
Materials for Civil and Construction Engineers CHAPTER 2. Nature of Materials
 Materials for Civil and Construction Engineers CHAPTER 2 Nature of Materials Bonds 1. Primary Bond: forms when atoms interchange or share electrons in order to fill the outer (valence) shells like noble
Materials for Civil and Construction Engineers CHAPTER 2 Nature of Materials Bonds 1. Primary Bond: forms when atoms interchange or share electrons in order to fill the outer (valence) shells like noble
ISSUES TO ADDRESS...
 Chapter 12: Electrical Properties School of Mechanical Engineering Choi, Hae-Jin Materials Science - Prof. Choi, Hae-Jin Chapter 12-1 ISSUES TO ADDRESS... How are electrical conductance and resistance
Chapter 12: Electrical Properties School of Mechanical Engineering Choi, Hae-Jin Materials Science - Prof. Choi, Hae-Jin Chapter 12-1 ISSUES TO ADDRESS... How are electrical conductance and resistance
n i exp E g 2kT lnn i E g 2kT
 HOMEWORK #10 12.19 For intrinsic semiconductors, the intrinsic carrier concentration n i depends on temperature as follows: n i exp E g 2kT (28.35a) or taking natural logarithms, lnn i E g 2kT (12.35b)
HOMEWORK #10 12.19 For intrinsic semiconductors, the intrinsic carrier concentration n i depends on temperature as follows: n i exp E g 2kT (28.35a) or taking natural logarithms, lnn i E g 2kT (12.35b)
Interfacial Defects. Grain Size Determination
 Interfacial Defects 4.27 For an FCC single crystal, would you expect the surface energy for a (00) plane to be greater or less than that for a () plane? Why? (Note: You may want to consult the solution
Interfacial Defects 4.27 For an FCC single crystal, would you expect the surface energy for a (00) plane to be greater or less than that for a () plane? Why? (Note: You may want to consult the solution
4. Interpenetrating simple cubic
 2 1. The correct structure t of CsClCl crystal is 1. Simple cubic 2. Body centered cubic 3. Face centered cubic 4. Interpenetrating simple cubic If corner as well as the particle at the center are same
2 1. The correct structure t of CsClCl crystal is 1. Simple cubic 2. Body centered cubic 3. Face centered cubic 4. Interpenetrating simple cubic If corner as well as the particle at the center are same
Appendix 4. Appendix 4A Heat Capacity of Ideal Gases
 Appendix 4 W-143 Appendix 4A Heat Capacity of Ideal Gases We can determine the heat capacity from the energy content of materials as a function of temperature. The simplest material to model is an ideal
Appendix 4 W-143 Appendix 4A Heat Capacity of Ideal Gases We can determine the heat capacity from the energy content of materials as a function of temperature. The simplest material to model is an ideal
3.3 Show for the body-centered cubic crystal structure that the unit cell edge length a and the atomic radius R are related through a =4R/ 3.
 EGR 1 Materials Science, HW, CH :, 6, 7, 11, 0,, 0a-c,. Show for the body-centered cubic crystal structure that the unit cell edge length a and the atomic radius R are related through a =4R/. Consider
EGR 1 Materials Science, HW, CH :, 6, 7, 11, 0,, 0a-c,. Show for the body-centered cubic crystal structure that the unit cell edge length a and the atomic radius R are related through a =4R/. Consider
Defects in Crystals. Image borrowed from who got it from Davis & Reynolds 1996.
 Defects in Crystals Image borrowed from http://comp.uark.edu/~pjansma/geol3513_25_defmechs1_04.ppt who got it from Davis & Reynolds 1996. It's easy to think of real crystals as having these ideal structures.
Defects in Crystals Image borrowed from http://comp.uark.edu/~pjansma/geol3513_25_defmechs1_04.ppt who got it from Davis & Reynolds 1996. It's easy to think of real crystals as having these ideal structures.
HW #3. Concentration of nitrogen within the surface (at the location in question), C B = 2 kg/m3
 HW #3 Problem 5.7 a. To Find: The distance from the high-pressure side at which the concentration of nitrogen in steel equals 2.0 kg/m 3 under conditions of steady-state diffusion. b. Given: D = 6 10-11
HW #3 Problem 5.7 a. To Find: The distance from the high-pressure side at which the concentration of nitrogen in steel equals 2.0 kg/m 3 under conditions of steady-state diffusion. b. Given: D = 6 10-11
Unit 1 Part 1: Significant Figures and Scientific Notation. Objective understand significant figures and their rules. Be able to use scientific
 Unit 1 Part 1: Significant Figures and Scientific Notation. Objective understand significant figures and their rules. Be able to use scientific notation in calculations. Significant figures - consist of
Unit 1 Part 1: Significant Figures and Scientific Notation. Objective understand significant figures and their rules. Be able to use scientific notation in calculations. Significant figures - consist of
Module 16. Diffusion in solids II. Lecture 16. Diffusion in solids II
 Module 16 Diffusion in solids II Lecture 16 Diffusion in solids II 1 NPTEL Phase II : IIT Kharagpur : Prof. R. N. Ghosh, Dept of Metallurgical and Materials Engineering Keywords: Micro mechanisms of diffusion,
Module 16 Diffusion in solids II Lecture 16 Diffusion in solids II 1 NPTEL Phase II : IIT Kharagpur : Prof. R. N. Ghosh, Dept of Metallurgical and Materials Engineering Keywords: Micro mechanisms of diffusion,
CHAPTER 12 STRUCTURES AND PROPERTIES OF CERAMICS PROBLEM SOLUTIONS
 CHAPTER 12 STRUCTURES AND PROPERTIES OF CERAMICS PROBLEM SOLUTIONS Crystal Structures 12.1 For a ceramic compound, what are the two characteristics of the component ions that determine the crystal structure?
CHAPTER 12 STRUCTURES AND PROPERTIES OF CERAMICS PROBLEM SOLUTIONS Crystal Structures 12.1 For a ceramic compound, what are the two characteristics of the component ions that determine the crystal structure?
Materials and Devices in Electrical Engineering
 Examination WS 02/03 Materials and Devices in Electrical Engineering Monday 17 th of March, 9:00 11:00, International Department, SR. 203 Notice 1. It is allowed to use any kind of aids (books, scripts,
Examination WS 02/03 Materials and Devices in Electrical Engineering Monday 17 th of March, 9:00 11:00, International Department, SR. 203 Notice 1. It is allowed to use any kind of aids (books, scripts,
Thermally Activated Processes
 General Description of Activated Process: 1 Diffusion: 2 Diffusion: Temperature Plays a significant role in diffusion Temperature is not the driving force. Remember: DRIVING FORCE GRADIENT of a FIELD VARIALE
General Description of Activated Process: 1 Diffusion: 2 Diffusion: Temperature Plays a significant role in diffusion Temperature is not the driving force. Remember: DRIVING FORCE GRADIENT of a FIELD VARIALE
Materials and Devices in Electrical Engineering
 Solution for Examination WS 0/0 Materials and Devices in Electrical Engineering Monday 17 th of March, 9:00 11:00, International Department, SR. 0 Notice 1. It is allowed to use any kind of aids (books,
Solution for Examination WS 0/0 Materials and Devices in Electrical Engineering Monday 17 th of March, 9:00 11:00, International Department, SR. 0 Notice 1. It is allowed to use any kind of aids (books,
Bulk Structures of Crystals
 Bulk Structures of Crystals 7 crystal systems can be further subdivided into 32 crystal classes... see Simon Garrett, "Introduction to Surface Analysis CEM924": http://www.cem.msu.edu/~cem924sg/lecturenotes.html
Bulk Structures of Crystals 7 crystal systems can be further subdivided into 32 crystal classes... see Simon Garrett, "Introduction to Surface Analysis CEM924": http://www.cem.msu.edu/~cem924sg/lecturenotes.html
Introduction to Engineering Materials ENGR2000. Dr.Coates
 Introduction to Engineering Materials ENGR2000 Chapter 18: Electrical Properties Dr.Coates 18.2 Ohm s Law V = IR where R is the resistance of the material, V is the voltage and I is the current. l R A
Introduction to Engineering Materials ENGR2000 Chapter 18: Electrical Properties Dr.Coates 18.2 Ohm s Law V = IR where R is the resistance of the material, V is the voltage and I is the current. l R A
Topics to discuss...
 MME 467: Ceramics for Advanced Applications Lecture 18 Defects in Ceramics 2 Ref: Barsoum, Fundamentals of Ceramics, Ch6, McGraw-Hill, 2000 Prof. A. K. M. B. Rashid Department of MME, BUET, Dhaka Topics
MME 467: Ceramics for Advanced Applications Lecture 18 Defects in Ceramics 2 Ref: Barsoum, Fundamentals of Ceramics, Ch6, McGraw-Hill, 2000 Prof. A. K. M. B. Rashid Department of MME, BUET, Dhaka Topics
Diffusion. Diffusion = the spontaneous intermingling of the particles of two or more substances as a result of random thermal motion
 Diffusion Diffusion = the spontaneous intermingling of the particles of two or more substances as a result of random thermal motion Fick s First Law Γ ΔN AΔt Γ = flux ΔN = number of particles crossing
Diffusion Diffusion = the spontaneous intermingling of the particles of two or more substances as a result of random thermal motion Fick s First Law Γ ΔN AΔt Γ = flux ΔN = number of particles crossing
Physics 212 Midterm 2 Form A
 1. A wire contains a steady current of 2 A. The charge that passes a cross section in 2 s is: A. 3.2 10-19 C B. 6.4 10-19 C C. 1 C D. 2 C E. 4 C 2. In a Physics 212 lab, Jane measures the current versus
1. A wire contains a steady current of 2 A. The charge that passes a cross section in 2 s is: A. 3.2 10-19 C B. 6.4 10-19 C C. 1 C D. 2 C E. 4 C 2. In a Physics 212 lab, Jane measures the current versus
1 8 =1 8 8 =1 6 =3. Unit cell Atoms at corner Atoms at faces Atoms at centre. Total no. of atoms per unit cell. bcc. fcc
 Q. No. Amorphous substances show () Short and long range order (2) Short range order (3) Long range order (4) Have no sharp M.P. Option and 3 are correct Option 2 2 and 3 are correct Option 3 3 and 4 are
Q. No. Amorphous substances show () Short and long range order (2) Short range order (3) Long range order (4) Have no sharp M.P. Option and 3 are correct Option 2 2 and 3 are correct Option 3 3 and 4 are
Section 7: Diffusion. Jaeger Chapter 4. EE143 Ali Javey
 Section 7: Diffusion Jaeger Chapter 4 Surface Diffusion: Dopant Sources (a) Gas Source: AsH 3, PH 3, B 2 H 6 (b) Solid Source BN Si BN Si (c) Spin-on-glass SiO 2 +dopant oxide (d) Liquid Source. Fick s
Section 7: Diffusion Jaeger Chapter 4 Surface Diffusion: Dopant Sources (a) Gas Source: AsH 3, PH 3, B 2 H 6 (b) Solid Source BN Si BN Si (c) Spin-on-glass SiO 2 +dopant oxide (d) Liquid Source. Fick s
Physics 2220 Fall 2010 George Williams SECOND MIDTERM - REVIEW PROBLEMS
 Physics 0 Fall 010 George Williams SECOND MIDTERM - REVIEW PROBLEMS The last four problems are from last years second midterm. Solutions are available on the class web site.. There are no solutions for,
Physics 0 Fall 010 George Williams SECOND MIDTERM - REVIEW PROBLEMS The last four problems are from last years second midterm. Solutions are available on the class web site.. There are no solutions for,
Chapter 2 Atoms, chemical bonding, material structure, and physical properties Homework Solutions
 Chapter 2 Atoms, chemical bonding, material structure, and physical properties Homework Solutions Concept questions 1. The Pauli exclusion principle says that no two electrons that occupy the same space
Chapter 2 Atoms, chemical bonding, material structure, and physical properties Homework Solutions Concept questions 1. The Pauli exclusion principle says that no two electrons that occupy the same space
Would you be breaking the speed limit in a 40 mi/h zone if you were traveling at 60 km/h?
 Lesson Starter Would you be breaking the speed limit in a 40 mi/h zone if you were traveling at 60 km/h? one kilometer = 0.62 miles 60 km/h = 37.2 mi/h You would not be speeding! km/h and mi/h measure
Lesson Starter Would you be breaking the speed limit in a 40 mi/h zone if you were traveling at 60 km/h? one kilometer = 0.62 miles 60 km/h = 37.2 mi/h You would not be speeding! km/h and mi/h measure
Dopant Diffusion. (1) Predeposition dopant gas. (2) Drive-in Turn off dopant gas. dose control. Doped Si region
 Dopant Diffusion (1) Predeposition dopant gas dose control SiO Si SiO Doped Si region () Drive-in Turn off dopant gas or seal surface with oxide profile control (junction depth; concentration) SiO SiO
Dopant Diffusion (1) Predeposition dopant gas dose control SiO Si SiO Doped Si region () Drive-in Turn off dopant gas or seal surface with oxide profile control (junction depth; concentration) SiO SiO
Defects. Defects. Kap. 3 States of aggregation. Perfect Crystal
 Kap. 3 States of aggregation Defects Perfect Crystal A A perfect crystal with every atom in the correct position does not exist. Only a hypothetical situation at 0 K Crystals are like people: it is the
Kap. 3 States of aggregation Defects Perfect Crystal A A perfect crystal with every atom in the correct position does not exist. Only a hypothetical situation at 0 K Crystals are like people: it is the
Atomic Arrangement. Primer in Materials Spring
 Atomic Arrangement Primer in Materials Spring 2017 30.4.2017 1 Levels of atomic arrangements No order In gases, for example the atoms have no order, they are randomly distributed filling the volume to
Atomic Arrangement Primer in Materials Spring 2017 30.4.2017 1 Levels of atomic arrangements No order In gases, for example the atoms have no order, they are randomly distributed filling the volume to
Massachusetts Institute of Technology Department of Materials Science and Engineering
 Massachusetts Institute of Technology Department of Materials Science and Engineering 3.05 Thermodynamics and Kinetics of Materials Fall 003 November 7, 003 We are looking at the incorporation of Al 3
Massachusetts Institute of Technology Department of Materials Science and Engineering 3.05 Thermodynamics and Kinetics of Materials Fall 003 November 7, 003 We are looking at the incorporation of Al 3
Investigations of the effects of 7 TeV proton beams on LHC collimator materials and other materials to be used in the LHC
 Russian Research Center Kurchatov Institute Investigations of the effects of 7 ev proton beams on LHC collimator materials and other materials to be used in the LHC A.I.Ryazanov Aims of Investigations:
Russian Research Center Kurchatov Institute Investigations of the effects of 7 ev proton beams on LHC collimator materials and other materials to be used in the LHC A.I.Ryazanov Aims of Investigations:
Lecture 1: Atomic Diffusion
 Part IB Materials Science & Metallurgy H. K. D. H. Bhadeshia Course A, Metals and Alloys Lecture 1: Atomic Diffusion Mass transport in a gas or liquid generally involves the flow of fluid (e.g. convection
Part IB Materials Science & Metallurgy H. K. D. H. Bhadeshia Course A, Metals and Alloys Lecture 1: Atomic Diffusion Mass transport in a gas or liquid generally involves the flow of fluid (e.g. convection
Math. Model Exam (1) Mid-year. Fifth primary. Question 1: Question 2: Answer the following: a = (to the nearest )
 Model Exam (1) Question 1: Answer the following: a- 65.3814 + 63.4027 = (to the nearest ) b- 53.27 2.1 = (to the nearest tenth) c- (3.425 + 1.07) 2.8 = (to the nearest hundredth) d- 9.568 9 = (to the nearest
Model Exam (1) Question 1: Answer the following: a- 65.3814 + 63.4027 = (to the nearest ) b- 53.27 2.1 = (to the nearest tenth) c- (3.425 + 1.07) 2.8 = (to the nearest hundredth) d- 9.568 9 = (to the nearest
Remember the purpose of this reading assignment is to prepare you for class. Reading for familiarity not mastery is expected.
 Remember the purpose of this reading assignment is to prepare you for class. Reading for familiarity not mastery is expected. After completing this reading assignment and reviewing the intro video you
Remember the purpose of this reading assignment is to prepare you for class. Reading for familiarity not mastery is expected. After completing this reading assignment and reviewing the intro video you
collisions of electrons. In semiconductor, in certain temperature ranges the conductivity increases rapidly by increasing temperature
 1.9. Temperature Dependence of Semiconductor Conductivity Such dependence is one most important in semiconductor. In metals, Conductivity decreases by increasing temperature due to greater frequency of
1.9. Temperature Dependence of Semiconductor Conductivity Such dependence is one most important in semiconductor. In metals, Conductivity decreases by increasing temperature due to greater frequency of
Figure 1: A 3-D ball-and-spring model of the tin wire. Figure 2: The top view and side view of a simple cubic lattice.
 Question (5) Bond stiffness of solid tin. A certain tin wire (white tin, as opposed to gray tin) has a length of.5 m and a square rectangular cross section that is ( mm x mm). With a tension of 200 N applied
Question (5) Bond stiffness of solid tin. A certain tin wire (white tin, as opposed to gray tin) has a length of.5 m and a square rectangular cross section that is ( mm x mm). With a tension of 200 N applied
Theory of Electromigration
 Theory of Electromigration Electromigration is the transport of material in a conductor under the influence of an applied electric field. All conductors are susceptible to electromigration, therefore it
Theory of Electromigration Electromigration is the transport of material in a conductor under the influence of an applied electric field. All conductors are susceptible to electromigration, therefore it
CHAPTER 2. NATURE OF MATERIALS
 Materials for Civil and Construction Engineers 4th Edition Mamlouk SOLUTIONS MANUAL Full clear download (no formatting errors) at: https://testbankreal.com/download/materials-for-civiland-construction-engineers-4th-edition-mamlouksolutions-manual/
Materials for Civil and Construction Engineers 4th Edition Mamlouk SOLUTIONS MANUAL Full clear download (no formatting errors) at: https://testbankreal.com/download/materials-for-civiland-construction-engineers-4th-edition-mamlouksolutions-manual/
INTRODUCTION TO THE DEFECT STATE IN MATERIALS
 INTRODUCTION TO THE DEFECT STATE IN MATERIALS DEFECTS, DEFECTS, DEFECTS CAN T LIVE WITH THEM!!! CAN T LIVE WITHOUT THEM!!! INTRODUCTION TO DEFECT STATE IN MATERIALS DEFECTS, DEFECTS, DEFECTS Perfect crystals
INTRODUCTION TO THE DEFECT STATE IN MATERIALS DEFECTS, DEFECTS, DEFECTS CAN T LIVE WITH THEM!!! CAN T LIVE WITHOUT THEM!!! INTRODUCTION TO DEFECT STATE IN MATERIALS DEFECTS, DEFECTS, DEFECTS Perfect crystals
Condensed Matter Physics Prof. G. Rangarajan Department of Physics Indian Institute of Technology, Madras
 (Refer Slide Time: 00:11) Condensed Matter Physics Prof. G. Rangarajan Department of Physics Indian Institute of Technology, Madras Diffraction Methods for Crystal Structure Worked Examples Next, we have
(Refer Slide Time: 00:11) Condensed Matter Physics Prof. G. Rangarajan Department of Physics Indian Institute of Technology, Madras Diffraction Methods for Crystal Structure Worked Examples Next, we have
Nearly Free Electron Gas model - I
 Nearly Free Electron Gas model - I Contents 1 Free electron gas model summary 1 2 Electron effective mass 3 2.1 FEG model for sodium...................... 4 3 Nearly free electron model 5 3.1 Primitive
Nearly Free Electron Gas model - I Contents 1 Free electron gas model summary 1 2 Electron effective mass 3 2.1 FEG model for sodium...................... 4 3 Nearly free electron model 5 3.1 Primitive
Phy 100 s Lab - Measurement techniques for mass, size and density. Name Course & Sec. Lab Partner
 Phy 100 s Lab - techniques for mass, size and density. Name Course & Sec Lab Partner Date 1. You should have a metal block and a metal cylinder both made of the same material. If you are unsure if the
Phy 100 s Lab - techniques for mass, size and density. Name Course & Sec Lab Partner Date 1. You should have a metal block and a metal cylinder both made of the same material. If you are unsure if the
CHAPTER 12 ELECTRICAL PROPERTIES PROBLEM SOLUTIONS
 Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for testing or instructional purposes only to students enrolled in courses for which the textbook has
Excerpts from this work may be reproduced by instructors for distribution on a not-for-profit basis for testing or instructional purposes only to students enrolled in courses for which the textbook has
(D) Blv/R Counterclockwise
 1. There is a counterclockwise current I in a circular loop of wire situated in an external magnetic field directed out of the page as shown above. The effect of the forces that act on this current is
1. There is a counterclockwise current I in a circular loop of wire situated in an external magnetic field directed out of the page as shown above. The effect of the forces that act on this current is
ECE 142: Electronic Circuits Lecture 3: Semiconductors
 Faculty of Engineering ECE 142: Electronic Circuits Lecture 3: Semiconductors Agenda Intrinsic Semiconductors Extrinsic Semiconductors N-type P-type Carrier Transport Drift Diffusion Semiconductors A semiconductor
Faculty of Engineering ECE 142: Electronic Circuits Lecture 3: Semiconductors Agenda Intrinsic Semiconductors Extrinsic Semiconductors N-type P-type Carrier Transport Drift Diffusion Semiconductors A semiconductor
Numerical modeling of hydrogen/deuterium absorption in transition-metal alloys
 Numerical modeling of hydrogen/deuterium absorption in transition-metal alloys Olga Dmitriyeva, Rick Cantwell, Matt McConnell Coolescence LLC Boulder, Colorado, U.S.A. 1 QuantumEspresso Electronic structure
Numerical modeling of hydrogen/deuterium absorption in transition-metal alloys Olga Dmitriyeva, Rick Cantwell, Matt McConnell Coolescence LLC Boulder, Colorado, U.S.A. 1 QuantumEspresso Electronic structure
Modified Maxwell-Garnett equation for the effective transport coefficients in inhomogeneous media
 J. Phys. A: Math. Gen. 3 (998) 7227 7234. Printed in the UK PII: S0305-4470(98)93976-2 Modified Maxwell-Garnett equation for the effective transport coefficients in inhomogeneous media Juris Robert Kalnin
J. Phys. A: Math. Gen. 3 (998) 7227 7234. Printed in the UK PII: S0305-4470(98)93976-2 Modified Maxwell-Garnett equation for the effective transport coefficients in inhomogeneous media Juris Robert Kalnin
Atomic Arrangement. Primer Materials For Science Teaching Spring
 Atomic Arrangement Primer Materials For Science Teaching Spring 2016 31.3.2015 Levels of atomic arrangements No order In gases, for example the atoms have no order, they are randomly distributed filling
Atomic Arrangement Primer Materials For Science Teaching Spring 2016 31.3.2015 Levels of atomic arrangements No order In gases, for example the atoms have no order, they are randomly distributed filling
Introduction to Engineering Materials ENGR2000 Chapter 12: Structures and Properties of Ceramics. Dr. Coates
 Introduction to Engineering Materials ENGR2000 Chapter 12: Structures and Properties of Ceramics Dr. Coates 12.1 Introduction Ceramics Compounds between metallic & non-metallic elements Predominantly ionic
Introduction to Engineering Materials ENGR2000 Chapter 12: Structures and Properties of Ceramics Dr. Coates 12.1 Introduction Ceramics Compounds between metallic & non-metallic elements Predominantly ionic
Chapter 5: Diffusion (2)
 Chapter 5: Diffusin () ISSUES TO ADDRESS... Nn-steady state diffusin and Fick s nd Law Hw des diffusin depend n structure? Chapter 5-1 Class Eercise (1) Put a sugar cube inside a cup f pure water, rughly
Chapter 5: Diffusin () ISSUES TO ADDRESS... Nn-steady state diffusin and Fick s nd Law Hw des diffusin depend n structure? Chapter 5-1 Class Eercise (1) Put a sugar cube inside a cup f pure water, rughly
PLEASE DO NOT WRITE ON THIS EXAM!!
 Chemistry First Semester Exam 2015 Multiple Choice Identify the choice that best completes the statement or answers the question. Record the answer on both the clicker and this test. Use this graph to
Chemistry First Semester Exam 2015 Multiple Choice Identify the choice that best completes the statement or answers the question. Record the answer on both the clicker and this test. Use this graph to
Introduction into defect studies. in ceramic materials(iii) Structure, Defects and Defect Chemistry. Z. Wang. January 18, 2002
 Introduction into defect studies in ceramic materials(iii) Structure, Defects and Defect Chemistry Z. Wang January 18, 2002 1. Mass, Charge and Site Balance The Schottky reactions for NaCl and MgO, respectively,
Introduction into defect studies in ceramic materials(iii) Structure, Defects and Defect Chemistry Z. Wang January 18, 2002 1. Mass, Charge and Site Balance The Schottky reactions for NaCl and MgO, respectively,
Carriers Concentration in Semiconductors - V. Prof.P. Ravindran, Department of Physics, Central University of Tamil Nadu, India
 Carriers Concentration in Semiconductors - V 1 Prof.P. Ravindran, Department of Physics, Central University of Tamil Nadu, India http://folk.uio.no/ravi/semi2013 Motion and Recombination of Electrons and
Carriers Concentration in Semiconductors - V 1 Prof.P. Ravindran, Department of Physics, Central University of Tamil Nadu, India http://folk.uio.no/ravi/semi2013 Motion and Recombination of Electrons and
Exam 2 Solutions. = /10 = / = /m 3, where the factor of
 PHY049 Fall 007 Prof. Yasu Takano Prof. Paul Avery Oct. 17, 007 Exam Solutions 1. (WebAssign 6.6) A current of 1.5 A flows in a copper wire with radius 1.5 mm. If the current is uniform, what is the electron
PHY049 Fall 007 Prof. Yasu Takano Prof. Paul Avery Oct. 17, 007 Exam Solutions 1. (WebAssign 6.6) A current of 1.5 A flows in a copper wire with radius 1.5 mm. If the current is uniform, what is the electron
Write your name here:
 MSE 102, Fall 2013 Final Exam Write your name here: Instructions: Answer all questions to the best of your abilities. Be sure to write legibly and state your answers clearly. The point values for each
MSE 102, Fall 2013 Final Exam Write your name here: Instructions: Answer all questions to the best of your abilities. Be sure to write legibly and state your answers clearly. The point values for each
Chemistry 801: Nanostructured Materials and Interfaces
 Chemistry 801: Nanostructured Materials and Interfaces Problem set 1: Answer key 1. For a perspective on surface-to-volume ratio, consider a sphere whose radius is 1 cm and determine its surface area and
Chemistry 801: Nanostructured Materials and Interfaces Problem set 1: Answer key 1. For a perspective on surface-to-volume ratio, consider a sphere whose radius is 1 cm and determine its surface area and
MECH6661 lectures 6/1 Dr. M. Medraj Mech. Eng. Dept. - Concordia University. Fe3C (cementite)
 Outline Solid solution Gibbs free energy of binary solutions Ideal solution Chemical potential of an ideal solution Regular solutions Activity of a component Real solutions Equilibrium in heterogeneous
Outline Solid solution Gibbs free energy of binary solutions Ideal solution Chemical potential of an ideal solution Regular solutions Activity of a component Real solutions Equilibrium in heterogeneous
On my honor, I have neither given nor received unauthorized aid on this examination.
 Instructor(s): Kumar/Stewart PHYSIS DEPRTMENT PHY 05 Exam 1 September 7, 011 Name (print, last first): Signature: On my honor, I have neither given nor received unauthorized aid on this examination. YOUR
Instructor(s): Kumar/Stewart PHYSIS DEPRTMENT PHY 05 Exam 1 September 7, 011 Name (print, last first): Signature: On my honor, I have neither given nor received unauthorized aid on this examination. YOUR
Circle Theorems. Angles at the circumference are equal. The angle in a semi-circle is x The angle at the centre. Cyclic Quadrilateral
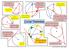 The angle in a semi-circle is 90 0 Angles at the circumference are equal. A B They must come from the same arc. Look out for a diameter. 2x Cyclic Quadrilateral Opposite angles add up to 180 0 A They must
The angle in a semi-circle is 90 0 Angles at the circumference are equal. A B They must come from the same arc. Look out for a diameter. 2x Cyclic Quadrilateral Opposite angles add up to 180 0 A They must
CHEMISTRY 448/548 Winter 2009
 CHEMISTRY 448/548 Winter 2009 Assignment #1 (50 pts) - Due Jan 16 th. SHOW WORKING FOR FULL CREDIT. ALL DRAWINGS MUST BE NEAT AND ACCURATE. SLOPPY WORK WILL BE PENALIZED! 1. (5 pts) In class we discussed
CHEMISTRY 448/548 Winter 2009 Assignment #1 (50 pts) - Due Jan 16 th. SHOW WORKING FOR FULL CREDIT. ALL DRAWINGS MUST BE NEAT AND ACCURATE. SLOPPY WORK WILL BE PENALIZED! 1. (5 pts) In class we discussed
763333A SOLDID STATE PHYSICS Exercise 1 Spring 2013
 763333A SOLDID STATE PHYSICS Exercise 1 Spring 2013 1. Fcc as a Bravais lattice Show that the fcc structure is a Bravais lattice. For this choose appropriate a 1, a 2 and a 3 so that the expression r =
763333A SOLDID STATE PHYSICS Exercise 1 Spring 2013 1. Fcc as a Bravais lattice Show that the fcc structure is a Bravais lattice. For this choose appropriate a 1, a 2 and a 3 so that the expression r =
Hussein Ayedh. PhD Studet Department of Physics
 Hussein Ayedh PhD Studet Department of Physics OUTLINE Introduction Semiconductors Basics DLTS Theory DLTS Requirements Example Summary Introduction Energetically "deep trapping levels in semiconductor
Hussein Ayedh PhD Studet Department of Physics OUTLINE Introduction Semiconductors Basics DLTS Theory DLTS Requirements Example Summary Introduction Energetically "deep trapping levels in semiconductor
Name Date Class CHAPTER ASSESSMENT. a. Refers to how close a series of measurements are to one another
 Data Analysis Reviewing Vocabulary Match each term in Column A with its definition in Column B. d f j h e b i g c a Column A 1. base unit 2. derived unit 3. graph 4. scientific notation 5. accuracy 6.
Data Analysis Reviewing Vocabulary Match each term in Column A with its definition in Column B. d f j h e b i g c a Column A 1. base unit 2. derived unit 3. graph 4. scientific notation 5. accuracy 6.
Problem Solving Section 1
 Problem Solving Section 1 Problem 1: Copper has a mass density ρ m 8.95 gmcm 3 and an electrical resistivity ρ 1.55 10 8 ohm m at room temperature. Calculate, (a). The concentration of the conduction electrons.
Problem Solving Section 1 Problem 1: Copper has a mass density ρ m 8.95 gmcm 3 and an electrical resistivity ρ 1.55 10 8 ohm m at room temperature. Calculate, (a). The concentration of the conduction electrons.
R measurements (resistivity, magnetoresistance, Hall). Makariy A. Tanatar
 R measurements (resistivity, magnetoresistance, Hall). 590B Makariy A. Tanatar April 18, 2014 Resistivity Typical resistivity temperature dependence: metals, semiconductors Magnetic scattering Resistivities
R measurements (resistivity, magnetoresistance, Hall). 590B Makariy A. Tanatar April 18, 2014 Resistivity Typical resistivity temperature dependence: metals, semiconductors Magnetic scattering Resistivities
Quick Questions. 1. Two charges of +1 µc each are separated by 1 cm. What is the force between them?
 92 3.10 Quick Questions 3.10 Quick Questions 1. Two charges of +1 µc each are separated by 1 cm. What is the force between them? 0.89 N 90 N 173 N 15 N 2. The electric field inside an isolated conductor
92 3.10 Quick Questions 3.10 Quick Questions 1. Two charges of +1 µc each are separated by 1 cm. What is the force between them? 0.89 N 90 N 173 N 15 N 2. The electric field inside an isolated conductor
Materials and Devices in Electrical Engineering
 Examination WS 01/02 Materials and Devices in Electrical Engineering Monday 11 th of March, 9:00 to 11:00, SR 203, International Department building It is allowed to use any kind of media (books, scripts,
Examination WS 01/02 Materials and Devices in Electrical Engineering Monday 11 th of March, 9:00 to 11:00, SR 203, International Department building It is allowed to use any kind of media (books, scripts,
EXAM TWO PART ONE CHM 451 (INORGANIC CHEMISTRY) DR. MATTSON 1 NOVEMBER 2012
 EXAM TWO PART ONE CHM 451 (INORGANIC CHEMISTRY) DR. MATTSON 1 NOVEMBER 2012 NAME: Instructions: This exam has two parts. In Part One, only a pencil and a non-programmable calculator may be used. When you
EXAM TWO PART ONE CHM 451 (INORGANIC CHEMISTRY) DR. MATTSON 1 NOVEMBER 2012 NAME: Instructions: This exam has two parts. In Part One, only a pencil and a non-programmable calculator may be used. When you
DIFFUSION - Chapter 7
 DIFFUSION - Chapter 7 Doping profiles determine many short-channel characteristics in MOS devices. Resistance impacts drive current. Scaling implies all lateral and vertical dimensions scale by the same
DIFFUSION - Chapter 7 Doping profiles determine many short-channel characteristics in MOS devices. Resistance impacts drive current. Scaling implies all lateral and vertical dimensions scale by the same
4. An electron moving in the positive x direction experiences a magnetic force in the positive z direction. If B x
 Magnetic Fields 3. A particle (q = 4.0 µc, m = 5.0 mg) moves in a uniform magnetic field with a velocity having a magnitude of 2.0 km/s and a direction that is 50 away from that of the magnetic field.
Magnetic Fields 3. A particle (q = 4.0 µc, m = 5.0 mg) moves in a uniform magnetic field with a velocity having a magnitude of 2.0 km/s and a direction that is 50 away from that of the magnetic field.
Answer all the questions
 SECTION A ( 38 marks) Answer all the questions 1 The following information refer to the set A and set B. Set A = { -3, -2, 2, 3 } Set B = { 4, 9 } The relations between set A and set B is defined by the
SECTION A ( 38 marks) Answer all the questions 1 The following information refer to the set A and set B. Set A = { -3, -2, 2, 3 } Set B = { 4, 9 } The relations between set A and set B is defined by the
VERY SHORT ANSWER TYPE QUESTIONS (1 Mark)
 UNIT I 10 Chemistry-XII THE SOLID STATE VERY SHORT ANSWER TYPE QUESTIONS (1 Mark) Q. 1. What do you mean by paramagnetic substance? Ans. Weakly attracted by magnetic eld and these substances are made of
UNIT I 10 Chemistry-XII THE SOLID STATE VERY SHORT ANSWER TYPE QUESTIONS (1 Mark) Q. 1. What do you mean by paramagnetic substance? Ans. Weakly attracted by magnetic eld and these substances are made of
R A M A K R I S H N A M I S S I O N V I D Y A M A N D I R A (Residential Autonomous College under University of Calcutta)
 R A M A K R I S N A M I S S I N V I D Y A M A N D I R A (Residential Autonomous College under University of Calcutta) P.G. FIRST YEAR M.A./M.SC. FIRST SEMESTER (July December), 2012 Mid-Semester Examination,
R A M A K R I S N A M I S S I N V I D Y A M A N D I R A (Residential Autonomous College under University of Calcutta) P.G. FIRST YEAR M.A./M.SC. FIRST SEMESTER (July December), 2012 Mid-Semester Examination,
PH575 Spring Lecture #19 Semiconductors: electrical & optical properties: Kittel Ch. 8 pp ; Ch. 20
 PH575 Spring 2014 Lecture #19 Semiconductors: electrical & optical properties: Kittel Ch. 8 pp. 205-214; Ch. 20 Simplified diagram of the filling of electronic band structure in various types of material,
PH575 Spring 2014 Lecture #19 Semiconductors: electrical & optical properties: Kittel Ch. 8 pp. 205-214; Ch. 20 Simplified diagram of the filling of electronic band structure in various types of material,
Physics 2135 Exam 3 April 18, 2017
 Physics 2135 Exam 3 April 18, 2017 Exam Total / 200 Printed Name: Rec. Sec. Letter: Solutions for problems 6 to 10 must start from official starting equations. Show your work to receive credit for your
Physics 2135 Exam 3 April 18, 2017 Exam Total / 200 Printed Name: Rec. Sec. Letter: Solutions for problems 6 to 10 must start from official starting equations. Show your work to receive credit for your
Bravais Lattice + Basis = Crystal Structure
 Bravais Lattice + Basis = Crystal Structure A crystal structure is obtained when identical copies of a basis are located at all of the points of a Bravais lattice. Consider the structure of Cr, a I-cubic
Bravais Lattice + Basis = Crystal Structure A crystal structure is obtained when identical copies of a basis are located at all of the points of a Bravais lattice. Consider the structure of Cr, a I-cubic
Chemistry 1. Worksheet 2. Units and Unit Conversions. 1 MathTutorDVD.com
 Chemistry 1 Worksheet 2 Units and Unit Conversions 1 1) Give the abbreviation for the following units. a. kilogram b. meter c. gram d. second e. Kelvin f. Celsius 2) List what each of the following units
Chemistry 1 Worksheet 2 Units and Unit Conversions 1 1) Give the abbreviation for the following units. a. kilogram b. meter c. gram d. second e. Kelvin f. Celsius 2) List what each of the following units
Due to the quantum nature of electrons, one energy state can be occupied only by one electron.
 In crystalline solids, not all values of the electron energy are possible. The allowed intervals of energy are called allowed bands (shown as blue and chess-board blue). The forbidden intervals are called
In crystalline solids, not all values of the electron energy are possible. The allowed intervals of energy are called allowed bands (shown as blue and chess-board blue). The forbidden intervals are called
The samples used in these calculations were arranged as perfect diamond crystal of
 Chapter 5 Results 5.1 Hydrogen Diffusion 5.1.1 Computational Details The samples used in these calculations were arranged as perfect diamond crystal of a2 2 2 unit cells, i.e. 64 carbon atoms. The effect
Chapter 5 Results 5.1 Hydrogen Diffusion 5.1.1 Computational Details The samples used in these calculations were arranged as perfect diamond crystal of a2 2 2 unit cells, i.e. 64 carbon atoms. The effect
CHEMISTRY. The correlation between structure and properties helps in discovering new solid materials with desired properties
 CHEMISTRY 1 The correlation between structure and properties helps in discovering new solid materials with desired properties like high temperature superconductors, magnetic materials, biodegradable polymers
CHEMISTRY 1 The correlation between structure and properties helps in discovering new solid materials with desired properties like high temperature superconductors, magnetic materials, biodegradable polymers
vand v 3. Find the area of a parallelogram that has the given vectors as adjacent sides.
 Name: Date: 1. Given the vectors u and v, find u vand v v. u= 8,6,2, v = 6, 3, 4 u v v v 2. Given the vectors u nd v, find the cross product and determine whether it is orthogonal to both u and v. u= 1,8,
Name: Date: 1. Given the vectors u and v, find u vand v v. u= 8,6,2, v = 6, 3, 4 u v v v 2. Given the vectors u nd v, find the cross product and determine whether it is orthogonal to both u and v. u= 1,8,
FORM 5 PHYSICS TIME: 2 Hours
 DIRECTORATE FOR QUALITY AND STANDARDS IN EDUCATION Department for Curriculum Management and elearning Track 3 Educational Assessment Unit Annual Examinations for Secondary Schools 2013 FORM 5 PHYSICS TIME:
DIRECTORATE FOR QUALITY AND STANDARDS IN EDUCATION Department for Curriculum Management and elearning Track 3 Educational Assessment Unit Annual Examinations for Secondary Schools 2013 FORM 5 PHYSICS TIME:
Diffusion in multicomponent solids. Anton Van der Ven Department of Materials Science and Engineering University of Michigan Ann Arbor, MI
 Diffusion in multicomponent solids nton Van der Ven Department of Materials Science and Engineering University of Michigan nn rbor, MI Coarse graining time Diffusion in a crystal Two levels of time coarse
Diffusion in multicomponent solids nton Van der Ven Department of Materials Science and Engineering University of Michigan nn rbor, MI Coarse graining time Diffusion in a crystal Two levels of time coarse
EMA5001 Lecture 2 Interstitial Diffusion & Fick s 1 st Law. Prof. Zhe Cheng Mechanical & Materials Engineering Florida International University
 EMA500 Lecture Interstitial Diffusion & Fick s st Law Prof. Zhe Cheng Mechanical & Materials Engineering Florida International University Substitutional Diffusion Different possibilities Exchange Mechanis
EMA500 Lecture Interstitial Diffusion & Fick s st Law Prof. Zhe Cheng Mechanical & Materials Engineering Florida International University Substitutional Diffusion Different possibilities Exchange Mechanis
16EC401 BASIC ELECTRONIC DEVICES UNIT I PN JUNCTION DIODE. Energy Band Diagram of Conductor, Insulator and Semiconductor:
 16EC401 BASIC ELECTRONIC DEVICES UNIT I PN JUNCTION DIODE Energy bands in Intrinsic and Extrinsic silicon: Energy Band Diagram of Conductor, Insulator and Semiconductor: 1 2 Carrier transport: Any motion
16EC401 BASIC ELECTRONIC DEVICES UNIT I PN JUNCTION DIODE Energy bands in Intrinsic and Extrinsic silicon: Energy Band Diagram of Conductor, Insulator and Semiconductor: 1 2 Carrier transport: Any motion
ATOMISTIC MODELING OF DIFFUSION IN ALUMINUM
 ATOMISTIC MODELING OF DIFFUSION IN ALUMINUM S. GRABOWSKI, K. KADAU and P. ENTEL Theoretische Physik, Gerhard-Mercator-Universität Duisburg, 47048 Duisburg, Germany (Received...) Abstract We present molecular-dynamics
ATOMISTIC MODELING OF DIFFUSION IN ALUMINUM S. GRABOWSKI, K. KADAU and P. ENTEL Theoretische Physik, Gerhard-Mercator-Universität Duisburg, 47048 Duisburg, Germany (Received...) Abstract We present molecular-dynamics
