Publication P The Institution of Engineering and Technology (IET) Reprinted by permission of The Institution of Engineering and Technology.
|
|
|
- Cuthbert Hill
- 6 years ago
- Views:
Transcription
1 Publication P6 Robert Tenno and Antti Pohjoranta Exact controls for the superconformal via fill process. In: Proceedings of the UKACC International Conference on Control Manchester, UK. 2 4 September pages The Institution of Engineering and Technology IET) Reprinted by permission of The Institution of Engineering and Technology.
2 EXACT CONTROLS FOR THE SUPERCONFORMAL VIA FILL PROCESS Robert Tenno,1 Antti Pohjoranta Helsinki University of Technology TKK), Control Engineering, P.O. Box 5500, Espoo, Finland Abstract: This paper reports a means for stabilizing the microvia ll ratio on a desired level, using the total plating time and the system galvanostat setpoint current density as optimal controls. Both control variables are solved as functions of the process state as well as selected manufacturer preference variables that are typical for the via ll technology applied in multilayered printed circuit board production. The optimal controls are obtained as a system of two equations and solved numerically with the gradient descent method. Results of the numerical analysis are presented and discussed. Keywords: via ll control, distributed parameters systems control Figure 1. A microvia cross-section SEMphotograph. The letters indicate: B = y B, V = y V,S= H. 1. INTRODUCTION The microvia ll process is an electrochemical process in which metallic copper interconnects are made between adjacent layers of multilayered printed circuit boards MLBs) by rst drilling holes on an individual board layer and then lling these holes with copper electrodeposition. 1 robert.tenno@tkk. Superconformal deposit growth at the microvia domain is obtained by adding specic growth inhibiting suppressor) and enhancing accelerator) chemicals, whose concentrations must be well balanced Andricacos et al., 1998). In practice, these are regulated at constant levels, while the plating time and plating current density are operational parameters, adjusted to obtain a good ll result. A common measure for the via ll success is the via ll ratio, r r = y V τ) 100%. 1) H + y B τ) In 1) τ is the plating time s), y is the deposit thickness m) measured vertically in the via bottom center point y V ) and on the level board surface y B ). H is the thickness of the dielectric board substrate m). These are illustrated in Fig. 1. The subscripts V and B are used also further in the text to denote the corresponding locations. In MLB manufacturing, a common requirement is r 80% denoted R=80% from here on). Also a thin deposit on the level board is a good ll characteristic as well as a short ll time, which directly increases productivity of a plating bath,
3 Figure 2. The control system architecture. but, which also as a disadvantage increases the risk of deposit failures caused by too high plating current density. The mentioned requirements are accounted for in the following cost function J J τ =R H + y B τ)) y V τ)] 2 + k 1 y 2 Bτ)+k 2 τ 2 2) In 2) k 1 is the weight on aiming to obtain a thin deposit on the level board surface and k 2 is the weight on compromising mass-production against risk of deposit failures. The weights are chosen by the manufacturer thus called the production preferences). Minimization of 2) includes two problems: i) the deposit growth control problem and ii) the process stopping control problem. In manual control, these are solved by a process operator. A system developed for automatic via ll control is shown in Fig. 2; the controller minimizes the cost function 2) with respect to a PDE system developed for the via ll process. The optimal controls plating time and current density) are evaluated upon the chosen target ll ratio, the control preferences and the included species' concentrations. The optimal current density is limited with a hard restriction or soft limit, to prevent failures caused by II) ion depletion inside the via. The optimal current density, on which limits are applied is given to the system galvanostat as current density setpoint to be realized by adjusting the voltage between the plating bath electrodes appropriately. The optimal cell voltage and the optimal plating time yield the desired ll ratio at the process output. These ideas along with their mathematical formulation are discussed further. Thus far the related literature is mainly focused on experimental studies Dow et al., 2003), Lefebvre et al., 2003) aiming for bottom-up ll through a good choice additives. In microchip manufacturing the process is extensively studied and modelled Moat et al., 2005), Vereecken et al., 2005), Akolkar and Landau, 2004), Gabrielli et al., 2005), Wheeler et al., 2003) while similar studies for microvias have been reported only few. The via ll control literature is focused on process monitoring and practical realization aspects Dow et al., 2006), Dow and Liu, 2006) except for Tenno and Pohjoranta, 2007), where a simple control method is developed. The control model applied here bases on the curvature enhanced accelerator accumulation eects Moat et al., 2007) documented as a predictive model for the microvia ll process in Tenno and Pohjoranta, 2008), Pohjoranta and Tenno, 2007). Although many similar models have been proposed Moat et al., 2005), Vereecken et al., 2005), Akolkar and Landau, 2004), Gabrielli et al., 2005), Wheeler et al., 2003), these are for microchip technology with ca. hundred times smaller vias and signicantly shorter plating times, which make application of such models in the microvia ll processes unfeasible. 2. PROBLEM FORMULATION The control problem is to choose the optimal stopping time to minimize the cost function in 2) with respect to deposit growth on the level board y B and inside the via y V. The process is modelled with nite element model, in a 2D, Cartesian changing geometry system, applying the arbitrary Lagrange-Eulerian ALE) method, as documented thoroughly in Tenno and Pohjoranta, 2008), Pohjoranta and Tenno, 2007). The required deposit thickness value changes are obtained as in 3)-4). dy B = y B 0) = 0 dy V = y V 0) = H y y X dx + Y ]B dy y y X dx + Y ]V dy 3) 4) The dierentials y/ X, y/ Y ) in 3)-4) are components of the deformation gradient, essential in the ALE method. The spatial deformation eld i.e. the displacements) is a velocity eld solved from the Laplace equation in a vector form) 2 u = 0, where u m/s) is the 2D velocity vector, u =u, v] T, the X-directional velocity being u = dx dt and the Y- directional velocity v = dy dt. The model as well as the process behind the control system, where the mentioned variables are obtained from, are documented thoroughly in the cited references and thus, for brevity, only a brief walk through the relevant equations and terms is
4 given in this proceedings article. For clarity, widespanning equations are collected in Tbl. 1). 2.1 The process model equations u n on the cathode is determined by the cathode current density i c A/m 2 ), obtained from 7) with 8) in Tbl. 1. Here ρ =2Fρ /M C/m 3 ), where M = kg/mol, ρ = 8960 kg/m 3 and F = C/mol. n is the cathode surface normal vector. i 0 is the II)/s) exchange current density A/m 2 ), K =77.85 V 1, α i are apparent transfer coecients subscript a for anodic, c for cathodic), E is the driving potential for electrode reaction V), k A is the ratio between the anode and cathode areas, μ i are the proportional coverage offree adsorption sites. c is the II) ion concentration mol/m 3 ), c 0 is 1000 mol/m3 and c lim 3.5 mol/m 3. i c is controlled by a galvanostat that sets E upon a chosen i B c = 9). However, the current density is limited by mass transfer, a limit approximated here as i lim =2Fc b D /δ, where c b is c in the electrolyte bulk, D is II) diusivity m 2 /s) and δ the thickness of the diusion layer m). The target current density is admissible if k g i lim, k g < 1. The condition assures the upper limit current density time-variable) inside the via, given in 10). In the bulk solution, mass transfer of species is modelled with regular mass transfer equations diusion and Nernst-Planck w/o electroneutrality) 11) and on the cathode surface with surface mass balance equations 12). T = L, where L is a projection operator L = I nn T, I being the identity tensor. dψ dt = T inc1 ψ), with ψ0) = 0 and T inc 2000 s. ki c is the the consumption reaction rate coecient for species i m/s), D i is diusivity m 2 /s) and Γ 0 i is the maximum surface coverage mol/m 2 ). Di s is the surface diusivity for i m 2 /s), ki a the adsorption rate m 3 /mol/s), ki d the desorption rate s 1 ) and c i the additive i's concentration mol/m 3 ). In 11), the electric potential φ satises σ φ = 0, with a set voltage on the anodic boundary and i c on the cathodic boundary. σ is the electric conductivity ofthe electrolyte, S/m. 2.2 The controls The optimal current density should be limited before applied as a setpoint for a galvanostat by either ofthe following modications: 1) A hard restriction based on a constant mass transfer limit approximation i set =min,k g i B ) lim 5) 2) A soft limit, approximated by using a c measurement on the via bottom c V ) i set = c V c V + cb /100 min,k g i B ) lim 6) In 5) and 6) is the optimal current density without limitation A/m 2 ), i set is the setpoint for galvanostat with limitation A/m 2 ) and c V is the concentration ofii) ions in the via bottom, mol/m 3 ). The former current density i set in 5)) is recalled further as the hard-limited current and the latter one in 6)) as the soft-limited current density. The unrestricted current density will be found as the optimal control in Section OPTIMAL CONTROLS The target current density and plating time are xed constants for a galvanostat. In practice, they are found experimentally for each MLB product; here they are found from the model using either the exhaustive search or a faster iterative method explained below. The deposition thickness on the level board and in via is expressed with the applied controls by using Faraday's law y B τ,i)= i ρ τ, y V τ,i)= i τ ϕt)dt. 18) ρ 0 In 18) i and τ are the applied controls, the subscript indices from,τ target being omitted from here on to shorten the further formulae. ϕt) is the via ll function on the level board and on the via bottom V and B, respectively) 15). Minimizing the cost function 2) with respect to the growth processes 18) gives the optimal controls in the following semi-explicit form 19)- 21). iτ) =ρwτ), 19) with the auxiliary function 20) τr zτ) wτ) = HR τr zτ)) 2 + k 1 τ, 20) where zτ) is a solution ofthe equation 21) dz = ϕt), z0) = 0. 21) dt The plating time satises the nonlinear equation 16). The solution of19)-21) can be found iteratively using a simple gradient descent GD) method 17).
5 Table 1. List of wide-spanning equations. a i c = 2i 0 a 0 ) αa1+αc) μ a =1, μ c =1 θ Supp, i V c = 1 θ V itarget Supp c V 1 θsupp B c B i V lim = kgib lim 1 θ V Supp 1 θ B Supp c V c B μ c k A μ a a a 0 μ c ) = c c 0 c V + c lim c B + clim αc sinh K c + c lim c 0 + clim αcαa E α a + α c ) ) ) ) 1+αc } αa ) ) c V + c lim αc } αa c B + clim 7) 8) 9) 10) θ Supp θ Acc c Supp = D Supp 2 c Acc c Supp, = D Acc 2 c Acc + KD Acc c Acc φ) c = D c )+K D c φ) 11) + D s Acc 2 T θ Acc + u T T θ Acc + θ Acc L u = k a Acc c Acc1 θ Acc ) k d Acc ψθ Acc + D s Supp 2 T θ Supp + u T T θ Supp + θ Supp L u = k a Supp c Supp1 θ Supp θ Acc ) ψk d Supp θ Supp 12) D Supp c Supp t, x c,y c)=ksupp c c Supp +Γ 0 Supp ka Supp c Suppψ1 θ Supp θ Acc ) 13) D Acc c Acc t, x c,y c)=kacc c c Acc +Γ 0 Acc ka Acc c Accψ1 θ Acc ) 14) ϕt) = 1 θ V Supp c V c B 1 θsupp B τ R R ϕτ)) + k 1 + λ n+1 = λ n 2 ρ ) ) c V + c lim αc } αa c B + clim ) k2 wτ) 2 R ϕτ)) ] gτ n,i n) fτ n,i n) zτ) HR wτ) ] 15) ] =0 16), n =0, ) In 17) λ = τ i ] T is the optimal control, similarly as in 18), limited with the hard 5) or soft limit 6). The other functions are standard components of the GD algorithm, i.e. functions 19) and 16) designated here as f and g along with their derivatives, all approximated upon the iterated controls τ = τ n, i = i n as given below. fτ n,i n )=Rτ n z n )RH + w n τ n ) w k z n ] + k 1 w n τn 2 gτ n,i n )=i n R ϕ n )RH + w n τ n ) w n z n ] + τ n ρ k 2 + k 1 wn 2 ) 4. NUMERICAL ANALYSIS RESULTS In this section, the optimal controls are discussed and experimented along with hard and soft limits. The target ll ratio was chosen R = 96%. Above a gradient descent based method was given to nd the optimal controls. An exhaustive search method to complete the same is simpler but much more time consuming than the GD method. An interesting result provided by the exhaustive search, however, is the possibility to visualize the cost function 1) in the neighborhood of the optimal control values. The visualization shows, that the cost function has an "optimum valley", which has a rather level bottom. The GD method nds the valley bottom quickly 5-10 iterations) but in order to locate the exact optimum, several 15-20) more iterations are required to proceed along the valley bottom. If the exact optimum is sought for, an exhaustive search may be feasible. Both methods are applied in this section. 4.1 Optimal controls restricted with hard limits The ll ratio obtained in simulations depends on plating time as well as applied target current density as shown in Fig. 3a). The target end-time ll ratio of 96% was reached with all examined plating times. Naturally, the target was reached faster if a larger current density was applied. In Fig. 3c) the colors indicate levels of equal cost function value and the four markers show four dierent optimal controls. The eect of production preferences is evident. For example, if k 1 = 10 7, k 2 = Case 2 in Tbl. 2) then the optimal controls are τ target = 4220 s and
6 r %] r %] A/m 2 ] A/m 2 ] a) End-time Fill ratio as function hard-limited applied current density, with various plating times. b) End-point ll ratio as function soft-limited applied current density, with various plating times. τ target s] τ target s] A/m 2 ] A/m 2 ] c) The "optimum valley" of control cost, in terms of plating time and hard-limited applied current density. The marked optimal controls are those listed in Table 2. d) The "optimum valley" of control cost, in terms of plating time and soft-limited applied current density. The marked optimal controls are those listed in Table 3. Table 2. Optimal controls with hardlimited current. Case Preferences τ target Marker n:o k 1 k 2 s) A/m 2 ) = 184 A/m 2, correspondingly denoted in the cost function behavior plot in Fig. 3c) with a square marker. Other points marked in the cost function "optimum valley" are optimal controls corresponding to other preferences k 1 and k 2 as listed in Tbl. 2). If k 1 =10 7, k 2 =0case 1 in Table 2), then a relatively low current density of 173 A/m 2 and a long plating time of 4480 s are optimal. If k 1 =0, k 2 = the situation is opposite and i, τ) = 252 A/m 2, 3620 s). If k 1 = k 2 =0, the optimal controls 211 A/m 2 and 3860 sec are not well dened as we will be seen later. The cost function shape is the same for all preferences but the extremum point depends on the chosen preferences. 4.2 Optimal controls restricted with soft limits Similar results as for hard-limited control current density can be found for the soft-limited control current density. The desired ll ratio of 96% is achieved for all controls as shown in Fig. 3b). Table 3. Optimal controls with softlimited current. Case Preferences τ target Marker n:o k 1 k 2 s) A/m 2 ) Because the soft limitation 6) yields lower current densities than the hard limit, 5) the cost function and its "valley bottom" Fig. 3d)) is slightly shifted to the direction of higher current densities and longer plaiting time, compared to that with hard limits Fig. 3c)). Generally, also the optimal controls are only slightly shifted but for k 1 = k 2 =0case 3) the shift is large, probably due to computational instability created by the, in this case, ill-posed cost function.
7 Generally, the soft limit increases optimal current density and decreases plating time compared to the hard restriction, the end-time ll ratio remaining nearly the same. It is known that the microvia ll process behavior is strongly dependent on plating bath conditions. Therefore also optimal controls depend on process variables such as all the species' bulk solution concentrations, which should thus be either stabilized at a chosen setpoint or if not, the optimal controls should be periodically recalculated. Verication of the reported model against practical, empirical data is given in Pohjoranta and Tenno, 2007), Tenno and Pohjoranta, 2008) where also a simulation of how the bulk solution II) ions', suppressor and accelerator additives' concentration aects the ll process output is given. It is shown that the model is adequate in respect to the measured data. 5. CONCLUSION Though the via ll process along with its model are complex, optimal selections for plating time and system galvanostat setpoint current density can be found. These are controls, coupled with a nonlinear relationship and whose exact location in the time-current plane depend on the production preferences, specically how important is a thin thickness of deposit on the level board and how important is a short plating period compared to the risk of deposition failures caused by too high plating current density. Without predened preferences the controls are not well posed; a desired end-time ll ratio alone does not properly dene the optimal controls. The performed simulations convince that by running the process with optimally, the target ll ratio is reached at a predicted optimal plating time for each of chosen production preferences. A hard restriction and soft limit can both be used to prevent II) ion depletion inside the via, as was tested in the performed experiments. The soft limit moves the increases optimal current density and shortens optimal plating time compared to a hard restriction on current density. The optimal controls depend on the process state and on species' concentrations, whereby these should either be regulated at chosen levels or if not, the optimal controls should be periodically recalculated. REFERENCES Akolkar, R. and U. Landau 2004). A timedependent transport-kinetics model for additive interactions in copper interconnect metallization ), C702C711. ISSN Andricacos, P. C., C. Uzoh, J. O. Dukovic, J. Horkans and H. Deligianni 1998). Damascene copper electroplating for chip interconnections. 425), Dow, W.-P. and C.-W. Liu 2006). Evaluating the lling performance of a copper plating formula using a simple galvanostat method. 1533), C190C194. ISSN Dow, W-P., H-S. Huang and Z. Lin 2003). Interactions between brightener and chloride ions on copper electroplating for laser-drilled via-hole lling. 69), C134C136. ISSN Dow, W.-P., M.-Y. Yen, C.-W. Chou, C.-W. Liu, W.-H. Yang and C.-H. Chen 2006). Practical monitoring of lling performance in a copper plating bath. 98), C134C137. ISSN Gabrielli, C., P. Mocoteguy, H. Perrot, D. Nieto- Sanz and A. Zdunek 2005). A model for copper deposition in the damascene process. 5111), ISSN Lefebvre, M., G. Allardyce, M. Seita, H. Tsuchida, M. Kusaka and S. Hayashi 2003). Copper electroplating technology for microvia lling. Circuit World 292), 914. ISSN Moat, T. P., D. Wheeler, M. D. Edelstein and D. Josell 2005). Superconformal lm growth: Mechanism and quantication. 491), ISSN Moat, T.P., D. Wheeler, S.-K. Kim and D. Josell 2007). rvature enhanced adsorbate coverage mechanism for bottom-up superlling and bump control in damascene processing. 531), Pohjoranta, A. J. and R. Tenno 2007). A method for microvia ll process modelling in a cu-cu-electrode system with additives ), D502D509. Tenno, R. and A. J. Pohjoranta 2008). An ale model for prediction and control of the microvia ll process with two additives. 1555), Tenno, R. and A.J. Pohjoranta 2007). Microvia ll ratio control. IEEE International Symposium on Industrial Electronics 2007, Vigo, Spain. Vereecken, P. M., R. A. Binstead, H. Deligiani and P. C. Andricacos 2005). The chemistry of additives in damascene copper plating. 491), 318. ISSN Wheeler, D., D. Josell and T.P. Moat 2003). Modeling superconformal electrodeposition using the level set method. 1505), C302 C310. ISSN
Copper damascene electrodeposition and additives
 Journal of Electroanalytical Chemistry 559 (2003) 137/142 www.elsevier.com/locate/jelechem Copper damascene electrodeposition and additives K. Kondo *, N. Yamakawa, Z. Tanaka, K. Hayashi Department of
Journal of Electroanalytical Chemistry 559 (2003) 137/142 www.elsevier.com/locate/jelechem Copper damascene electrodeposition and additives K. Kondo *, N. Yamakawa, Z. Tanaka, K. Hayashi Department of
A model for copper deposition in the damascene process
 Electrochimica Acta 51 (2006) 1462 1472 A model for copper deposition in the damascene process C. Gabrielli a,,p.moçotéguy a, H. Perrot a, D. Nieto-Sanz b, A. Zdunek c a UPR 15 CNRS, LISE, University P.
Electrochimica Acta 51 (2006) 1462 1472 A model for copper deposition in the damascene process C. Gabrielli a,,p.moçotéguy a, H. Perrot a, D. Nieto-Sanz b, A. Zdunek c a UPR 15 CNRS, LISE, University P.
An Empirical Relation between the Plating Process and Accelerator Coverage in Cu Superfilling
 Cu Superfilling Process 1603 http://dx.doi.org/10.5012/bkcs.2012.33.5.1603 An Empirical Relation between the Plating Process and Accelerator Coverage in Cu Superfilling Sung Ki Cho, Myung Jun Kim, Hyo-Chol
Cu Superfilling Process 1603 http://dx.doi.org/10.5012/bkcs.2012.33.5.1603 An Empirical Relation between the Plating Process and Accelerator Coverage in Cu Superfilling Sung Ki Cho, Myung Jun Kim, Hyo-Chol
Influence of Convection-Dependent Adsorption of Additives on Microvia Filling by Copper Electroplating
 0013-4651/2005/152 6 /C425/10/$7.00 The Electrochemical Society, Inc. Influence of Convection-Dependent Adsorption of Additives on Microvia Filling by Copper Electroplating Wei-Ping Dow,*,z Her-Shu Huang,
0013-4651/2005/152 6 /C425/10/$7.00 The Electrochemical Society, Inc. Influence of Convection-Dependent Adsorption of Additives on Microvia Filling by Copper Electroplating Wei-Ping Dow,*,z Her-Shu Huang,
18.3 Electrolysis. Dr. Fred Omega Garces. Chemistry 201. Driving a non-spontaneous Oxidation-Reduction Reaction. Miramar College.
 18.3 Electrolysis Driving a non-spontaneous Oxidation-Reduction Reaction Dr. Fred Omega Garces Chemistry 201 Miramar College 1 Electrolysis Voltaic Vs. Electrolytic Cells Voltaic Cell Energy is released
18.3 Electrolysis Driving a non-spontaneous Oxidation-Reduction Reaction Dr. Fred Omega Garces Chemistry 201 Miramar College 1 Electrolysis Voltaic Vs. Electrolytic Cells Voltaic Cell Energy is released
Role of additives for copper damascene electrodeposition experimental study on inhibition and acceleration effects
 Chemistry Physical & Theoretical Chemistry fields Okayama University Year 2003 Role of additives for copper damascene electrodeposition experimental study on inhibition and acceleration effects Kazuo Kondo
Chemistry Physical & Theoretical Chemistry fields Okayama University Year 2003 Role of additives for copper damascene electrodeposition experimental study on inhibition and acceleration effects Kazuo Kondo
Influence of Molecular Weight of Polyethylene Glycol on Microvia Filling by Copper Electroplating
 0013-4651/2005/152 11 /C769/7/$7.00 The Electrochemical Society, Inc. Influence of Molecular Weight of Polyethylene Glycol on Microvia Filling by Copper Electroplating Wei-Ping Dow,*,z Ming-Yao Yen, Wen-Bing
0013-4651/2005/152 11 /C769/7/$7.00 The Electrochemical Society, Inc. Influence of Molecular Weight of Polyethylene Glycol on Microvia Filling by Copper Electroplating Wei-Ping Dow,*,z Ming-Yao Yen, Wen-Bing
Solution of the Two-Dimensional Steady State Heat Conduction using the Finite Volume Method
 Ninth International Conference on Computational Fluid Dynamics (ICCFD9), Istanbul, Turkey, July 11-15, 2016 ICCFD9-0113 Solution of the Two-Dimensional Steady State Heat Conduction using the Finite Volume
Ninth International Conference on Computational Fluid Dynamics (ICCFD9), Istanbul, Turkey, July 11-15, 2016 ICCFD9-0113 Solution of the Two-Dimensional Steady State Heat Conduction using the Finite Volume
Sung Ki Cho and Jae Jeong Kim*
 Korean Chem. Eng. Res., 54(1), 108-113 (2016) http://dx.doi.org/10.9713/kcer.2016.54.1.108 PISSN 0304-128X, EISSN 2233-9558 A Study on the Deposit Uniformity and Profile of Cu Electroplated in Miniaturized,
Korean Chem. Eng. Res., 54(1), 108-113 (2016) http://dx.doi.org/10.9713/kcer.2016.54.1.108 PISSN 0304-128X, EISSN 2233-9558 A Study on the Deposit Uniformity and Profile of Cu Electroplated in Miniaturized,
A Performance Simulation Tool for Bipolar Pulsed PCB Plating
 Abstract A Performance Simulation Tool for Bipolar Pulsed PCB Plating Gert Nelissen; Bart Van den Bossche, Luc Wanten ElSyCa N.V., Kranenberg 6, 1731 Zellik, Belgium Presented to IPC in Anaheim, CA 2005
Abstract A Performance Simulation Tool for Bipolar Pulsed PCB Plating Gert Nelissen; Bart Van den Bossche, Luc Wanten ElSyCa N.V., Kranenberg 6, 1731 Zellik, Belgium Presented to IPC in Anaheim, CA 2005
Chemistry 1011 TOPIC TEXT REFERENCE. Electrochemistry. Masterton and Hurley Chapter 18. Chemistry 1011 Slot 5 1
 Chemistry 1011 TOPIC Electrochemistry TEXT REFERENCE Masterton and Hurley Chapter 18 Chemistry 1011 Slot 5 1 18.5 Electrolytic Cells YOU ARE EXPECTED TO BE ABLE TO: Construct a labelled diagram to show
Chemistry 1011 TOPIC Electrochemistry TEXT REFERENCE Masterton and Hurley Chapter 18 Chemistry 1011 Slot 5 1 18.5 Electrolytic Cells YOU ARE EXPECTED TO BE ABLE TO: Construct a labelled diagram to show
Electrochemistry. Goal: Understand basic electrochemical reactions. Half Cell Reactions Nernst Equation Pourbaix Diagrams.
 Electrochemistry Goal: Understand basic electrochemical reactions Concepts: Electrochemical Cell Half Cell Reactions Nernst Equation Pourbaix Diagrams Homework: Applications Battery potential calculation
Electrochemistry Goal: Understand basic electrochemical reactions Concepts: Electrochemical Cell Half Cell Reactions Nernst Equation Pourbaix Diagrams Homework: Applications Battery potential calculation
Basic overall reaction for hydrogen powering
 Fuel Cell Basics Basic overall reaction for hydrogen powering 2H 2 + O 2 2H 2 O Hydrogen produces electrons, protons, heat and water PEMFC Anode reaction: H 2 2H + + 2e Cathode reaction: (½)O 2 + 2H +
Fuel Cell Basics Basic overall reaction for hydrogen powering 2H 2 + O 2 2H 2 O Hydrogen produces electrons, protons, heat and water PEMFC Anode reaction: H 2 2H + + 2e Cathode reaction: (½)O 2 + 2H +
Electrodeposition of nanomaterials. W. Schwarzacher H. H. Wills Physics Laboratory, University of Bristol
 Electrodeposition of nanomaterials W. Schwarzacher H. H. Wills Physics Laboratory, University of Bristol Report Documentation Page Form Approved OMB No. 0704-0188 Public reporting burden for the collection
Electrodeposition of nanomaterials W. Schwarzacher H. H. Wills Physics Laboratory, University of Bristol Report Documentation Page Form Approved OMB No. 0704-0188 Public reporting burden for the collection
Modeling of the 3D Electrode Growth in Electroplating
 Modeling of the 3D Electrode Growth in Electroplating Marius PURCAR, Calin MUNTEANU, Alexandru AVRAM, Vasile TOPA Technical University of Cluj-Napoca, Baritiu Street 26-28, 400027 Cluj-Napoca, Romania;
Modeling of the 3D Electrode Growth in Electroplating Marius PURCAR, Calin MUNTEANU, Alexandru AVRAM, Vasile TOPA Technical University of Cluj-Napoca, Baritiu Street 26-28, 400027 Cluj-Napoca, Romania;
FUNCTION OF ADDITIVES IN COPPER ELECTRODEPOSITION FOR SEMICONDUCTOR DEVICES METALLIZATION. James David Adolf. For the degree of Master of Science
 FUNCTION OF ADDITIVES IN COPPER ELECTRODEPOSITION FOR SEMICONDUCTOR DEVICES METALLIZATION by James David Adolf Submitted in partial fulfillment of the requirements For the degree of Master of Science Dissertation
FUNCTION OF ADDITIVES IN COPPER ELECTRODEPOSITION FOR SEMICONDUCTOR DEVICES METALLIZATION by James David Adolf Submitted in partial fulfillment of the requirements For the degree of Master of Science Dissertation
NONLINEAR FEEDBACK CONTROL OF A COUPLED KINETIC MONTE CARLO-FINITE DIFFERENCE CODE
 NONLINEAR FEEDBACK CONTROL OF A COUPLED KINETIC MONTE CARLO-FINITE DIFFERENCE CODE Effendi Rusli, Timothy O. Drews, David L. Ma, Richard C. Alkire, Richard D. Braatz University of Illinois at Urbana-Champaign
NONLINEAR FEEDBACK CONTROL OF A COUPLED KINETIC MONTE CARLO-FINITE DIFFERENCE CODE Effendi Rusli, Timothy O. Drews, David L. Ma, Richard C. Alkire, Richard D. Braatz University of Illinois at Urbana-Champaign
Lecture 14. Electrolysis.
 Lecture 14 Electrolysis: Electrosynthesis and Electroplating. 95 Electrolysis. Redox reactions in which the change in Gibbs energy G is positive do not occur spontaneously. However they can be driven via
Lecture 14 Electrolysis: Electrosynthesis and Electroplating. 95 Electrolysis. Redox reactions in which the change in Gibbs energy G is positive do not occur spontaneously. However they can be driven via
MODELLING OF FLEXIBLE MECHANICAL SYSTEMS THROUGH APPROXIMATED EIGENFUNCTIONS L. Menini A. Tornambe L. Zaccarian Dip. Informatica, Sistemi e Produzione
 MODELLING OF FLEXIBLE MECHANICAL SYSTEMS THROUGH APPROXIMATED EIGENFUNCTIONS L. Menini A. Tornambe L. Zaccarian Dip. Informatica, Sistemi e Produzione, Univ. di Roma Tor Vergata, via di Tor Vergata 11,
MODELLING OF FLEXIBLE MECHANICAL SYSTEMS THROUGH APPROXIMATED EIGENFUNCTIONS L. Menini A. Tornambe L. Zaccarian Dip. Informatica, Sistemi e Produzione, Univ. di Roma Tor Vergata, via di Tor Vergata 11,
Mass transfer by migration & diffusion (Ch. 4)
 Mass transfer by migration & diffusion (Ch. 4) Mass transfer equation Migration Mixed migration & diffusion near an electrode Mass transfer during electrolysis Effect of excess electrolyte Diffusion Microscopic
Mass transfer by migration & diffusion (Ch. 4) Mass transfer equation Migration Mixed migration & diffusion near an electrode Mass transfer during electrolysis Effect of excess electrolyte Diffusion Microscopic
Tertiary Current Distributions on the Wafer in a Plating Cell
 Tertiary Current Distributions on the Wafer in a Plating Cell Lizhu Tong 1 1 Kesoku Engineering System Co., Ltd. 1-9-5 Uchikanda, Chiyoda-ku, Tokyo 101-0047, Japan, tong@kesco.co.jp Abstract: The tertiary
Tertiary Current Distributions on the Wafer in a Plating Cell Lizhu Tong 1 1 Kesoku Engineering System Co., Ltd. 1-9-5 Uchikanda, Chiyoda-ku, Tokyo 101-0047, Japan, tong@kesco.co.jp Abstract: The tertiary
Topics in Boundary Element
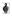 Topics in Boundary Element Research Edited by C. A. Brebbia Volume 7 Electrical Engineering Applications With 186 Figures and 11 Tables Springer-Verlag Berlin Heidelberg New York London Paris Tokyo Hong
Topics in Boundary Element Research Edited by C. A. Brebbia Volume 7 Electrical Engineering Applications With 186 Figures and 11 Tables Springer-Verlag Berlin Heidelberg New York London Paris Tokyo Hong
A Boundary Condition for Porous Electrodes
 Electrochemical Solid-State Letters, 7 9 A59-A63 004 0013-4651/004/79/A59/5/$7.00 The Electrochemical Society, Inc. A Boundary Condition for Porous Electrodes Venkat R. Subramanian, a, *,z Deepak Tapriyal,
Electrochemical Solid-State Letters, 7 9 A59-A63 004 0013-4651/004/79/A59/5/$7.00 The Electrochemical Society, Inc. A Boundary Condition for Porous Electrodes Venkat R. Subramanian, a, *,z Deepak Tapriyal,
SCIENCES & TECHNOLOGY
 Pertanika J. Sci. & Technol. 22 (2): 645-655 (2014) SCIENCES & TECHNOLOGY Journal homepage: http://www.pertanika.upm.edu.my/ Numerical Modelling of Molten Carbonate Fuel Cell: Effects of Gas Flow Direction
Pertanika J. Sci. & Technol. 22 (2): 645-655 (2014) SCIENCES & TECHNOLOGY Journal homepage: http://www.pertanika.upm.edu.my/ Numerical Modelling of Molten Carbonate Fuel Cell: Effects of Gas Flow Direction
Electrolysis. Electrolysis is the process of using electrical energy to break a compound apart or to reduced an metal ion to an element.
 Electrolysis Electrolysis is the process of using electrical energy to break a compound apart or to reduced an metal ion to an element. Electrolysis is done in an electrolytic cell. Electrolytic cells
Electrolysis Electrolysis is the process of using electrical energy to break a compound apart or to reduced an metal ion to an element. Electrolysis is done in an electrolytic cell. Electrolytic cells
Chemistry 2000 Lecture 15: Electrochemistry
 Chemistry 2000 Lecture 15: Electrochemistry Marc R. Roussel February 21, 2018 Marc R. Roussel Chemistry 2000 Lecture 15: Electrochemistry February 21, 2018 1 / 33 Electrochemical cells Electrochemical
Chemistry 2000 Lecture 15: Electrochemistry Marc R. Roussel February 21, 2018 Marc R. Roussel Chemistry 2000 Lecture 15: Electrochemistry February 21, 2018 1 / 33 Electrochemical cells Electrochemical
Short Communication Through-Hole Filling by Copper Electroplating Using Sodium Thiazolinyl-Dithiopropane Sulfonate as the Single Additive
 Int. J. Electrochem. Sci., 7 (2012) 10644-10651 International Journal of ELECTROCHEMICAL SCIENCE www.electrochemsci.org Short Communication Through-Hole Filling by Copper Electroplating Using Sodium Thiazolinyl-Dithiopropane
Int. J. Electrochem. Sci., 7 (2012) 10644-10651 International Journal of ELECTROCHEMICAL SCIENCE www.electrochemsci.org Short Communication Through-Hole Filling by Copper Electroplating Using Sodium Thiazolinyl-Dithiopropane
Chapter 24. Electrogravimetry and Coulometry
 Chapter 24 Electrogravimetry and Coulometry Dynamic Electrochemical Methods of analysis Electrolysis Electrogravimetric and Coulometric Methods For a cell to do any useful work or for an electrolysis to
Chapter 24 Electrogravimetry and Coulometry Dynamic Electrochemical Methods of analysis Electrolysis Electrogravimetric and Coulometric Methods For a cell to do any useful work or for an electrolysis to
Advanced Analytical Chemistry Lecture 12. Chem 4631
 Advanced Analytical Chemistry Lecture 12 Chem 4631 What is a fuel cell? An electro-chemical energy conversion device A factory that takes fuel as input and produces electricity as output. O 2 (g) H 2 (g)
Advanced Analytical Chemistry Lecture 12 Chem 4631 What is a fuel cell? An electro-chemical energy conversion device A factory that takes fuel as input and produces electricity as output. O 2 (g) H 2 (g)
CRACK-TIP DRIVING FORCE The model evaluates the eect of inhomogeneities by nding the dierence between the J-integral on two contours - one close to th
 ICF 100244OR Inhomogeneity eects on crack growth N. K. Simha 1,F.D.Fischer 2 &O.Kolednik 3 1 Department ofmechanical Engineering, University of Miami, P.O. Box 248294, Coral Gables, FL 33124-0624, USA
ICF 100244OR Inhomogeneity eects on crack growth N. K. Simha 1,F.D.Fischer 2 &O.Kolednik 3 1 Department ofmechanical Engineering, University of Miami, P.O. Box 248294, Coral Gables, FL 33124-0624, USA
Heat and Mass Transfer over Cooled Horizontal Tubes 333 x-component of the velocity: y2 u = g sin x y : (4) r 2 The y-component of the velocity eld is
 Scientia Iranica, Vol., No. 4, pp 332{338 c Sharif University of Technology, October 2004 Vapor Absorption into Liquid Films Flowing over a Column of Cooled Horizontal Tubes B. Farhanieh and F. Babadi
Scientia Iranica, Vol., No. 4, pp 332{338 c Sharif University of Technology, October 2004 Vapor Absorption into Liquid Films Flowing over a Column of Cooled Horizontal Tubes B. Farhanieh and F. Babadi
Electrochemical Cells
 Electrochemistry Electrochemical Cells The Voltaic Cell Electrochemical Cell = device that generates electricity through redox rxns 1 Voltaic (Galvanic) Cell An electrochemical cell that produces an electrical
Electrochemistry Electrochemical Cells The Voltaic Cell Electrochemical Cell = device that generates electricity through redox rxns 1 Voltaic (Galvanic) Cell An electrochemical cell that produces an electrical
Electrochemical Cells: Virtual Lab
 Electrochemical Cells: Virtual Lab Electrochemical cells involve the transfer of electrons from one species to another. In these chemical systems, the species that loses electrons is said to be oxidized
Electrochemical Cells: Virtual Lab Electrochemical cells involve the transfer of electrons from one species to another. In these chemical systems, the species that loses electrons is said to be oxidized
Electrochemistry. The study of the interchange of chemical and electrical energy.
 Electrochemistry The study of the interchange of chemical and electrical energy. Oxidation-reduction (redox) reaction: involves a transfer of electrons from the reducing agent to the oxidizing agent. oxidation:
Electrochemistry The study of the interchange of chemical and electrical energy. Oxidation-reduction (redox) reaction: involves a transfer of electrons from the reducing agent to the oxidizing agent. oxidation:
Electrochemistry. Electrochemical Process. The Galvanic Cell or Voltaic Cell
 Electrochemistry Electrochemical Process The conversion of chemical energy into electrical energy and the conversion of electrical energy into chemical energy are electrochemical process. Recall that an
Electrochemistry Electrochemical Process The conversion of chemical energy into electrical energy and the conversion of electrical energy into chemical energy are electrochemical process. Recall that an
Solutions for Assignment-6
 Solutions for Assignment-6 Q1. What is the aim of thin film deposition? [1] (a) To maintain surface uniformity (b) To reduce the amount (or mass) of light absorbing materials (c) To decrease the weight
Solutions for Assignment-6 Q1. What is the aim of thin film deposition? [1] (a) To maintain surface uniformity (b) To reduce the amount (or mass) of light absorbing materials (c) To decrease the weight
Chapter 18 Electrochemistry. Electrochemical Cells
 Chapter 18 Electrochemistry Chapter 18 1 Electrochemical Cells Electrochemical Cells are of two basic types: Galvanic Cells a spontaneous chemical reaction generates an electric current Electrolytic Cells
Chapter 18 Electrochemistry Chapter 18 1 Electrochemical Cells Electrochemical Cells are of two basic types: Galvanic Cells a spontaneous chemical reaction generates an electric current Electrolytic Cells
Basic overall reaction for hydrogen powering
 Fuel Cell Basics Basic overall reaction for hydrogen powering 2H 2 + O 2 2H 2 O Hydrogen produces electrons, protons, heat and water PEMFC Anode reaction: H 2 2H + + 2e Cathode reaction: (½)O 2 + 2H +
Fuel Cell Basics Basic overall reaction for hydrogen powering 2H 2 + O 2 2H 2 O Hydrogen produces electrons, protons, heat and water PEMFC Anode reaction: H 2 2H + + 2e Cathode reaction: (½)O 2 + 2H +
Zn+2 (aq) + Cu (s) Oxidation: An atom, ion, or molecule releases electrons and is oxidized. The oxidation number of the atom oxidized increases.
 Oxidation-Reduction Page 1 The transfer of an electron from one compound to another results in the oxidation of the electron donor and the reduction of the electron acceptor. Loss of electrons (oxidation)
Oxidation-Reduction Page 1 The transfer of an electron from one compound to another results in the oxidation of the electron donor and the reduction of the electron acceptor. Loss of electrons (oxidation)
Mechanism of SPS acceleration in a PEG containing copper plating bath
 University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Spring 2012 Mechanism of SPS acceleration in a PEG containing copper plating bath
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Spring 2012 Mechanism of SPS acceleration in a PEG containing copper plating bath
On Mean Curvature Diusion in Nonlinear Image Filtering. Adel I. El-Fallah and Gary E. Ford. University of California, Davis. Davis, CA
 On Mean Curvature Diusion in Nonlinear Image Filtering Adel I. El-Fallah and Gary E. Ford CIPIC, Center for Image Processing and Integrated Computing University of California, Davis Davis, CA 95616 Abstract
On Mean Curvature Diusion in Nonlinear Image Filtering Adel I. El-Fallah and Gary E. Ford CIPIC, Center for Image Processing and Integrated Computing University of California, Davis Davis, CA 95616 Abstract
IV. Transport Phenomena. Lecture 23: Ion Concentration Polarization
 IV. Transport Phenomena Lecture 23: Ion Concentration Polarization MIT Student (and MZB) Ion concentration polarization in electrolytes refers to the additional voltage drop (or internal resistance ) across
IV. Transport Phenomena Lecture 23: Ion Concentration Polarization MIT Student (and MZB) Ion concentration polarization in electrolytes refers to the additional voltage drop (or internal resistance ) across
Design of Decentralised PI Controller using Model Reference Adaptive Control for Quadruple Tank Process
 Design of Decentralised PI Controller using Model Reference Adaptive Control for Quadruple Tank Process D.Angeline Vijula #, Dr.N.Devarajan * # Electronics and Instrumentation Engineering Sri Ramakrishna
Design of Decentralised PI Controller using Model Reference Adaptive Control for Quadruple Tank Process D.Angeline Vijula #, Dr.N.Devarajan * # Electronics and Instrumentation Engineering Sri Ramakrishna
ELECTROCHEMISTRY. these are systems involving oxidation or reduction there are several types METALS IN CONTACT WITH SOLUTIONS OF THEIR IONS
 Electrochemistry 1 ELECTROCHEMISTRY REDOX Reduction gain of electrons Cu 2+ (aq) + 2e > Cu(s) Oxidation removal of electrons Zn(s) > Zn 2+ (aq) + 2e HALF CELLS these are systems involving oxidation or
Electrochemistry 1 ELECTROCHEMISTRY REDOX Reduction gain of electrons Cu 2+ (aq) + 2e > Cu(s) Oxidation removal of electrons Zn(s) > Zn 2+ (aq) + 2e HALF CELLS these are systems involving oxidation or
ELECTROCHEMISTRY. these are systems involving oxidation or reduction there are several types METALS IN CONTACT WITH SOLUTIONS OF THEIR IONS
 Electrochemistry 1 ELECTROCHEMISTRY REDOX Reduction gain of electrons Cu 2+ (aq) + 2e > Cu(s) Oxidation removal of electrons Zn(s) > Zn 2+ (aq) + 2e HALF CELLS these are systems involving oxidation or
Electrochemistry 1 ELECTROCHEMISTRY REDOX Reduction gain of electrons Cu 2+ (aq) + 2e > Cu(s) Oxidation removal of electrons Zn(s) > Zn 2+ (aq) + 2e HALF CELLS these are systems involving oxidation or
Monte Carlo Simulation of the Electrodeposition of Copper
 C406 0013-4651/2002/149 8 /C406/7/$7.00 The Electrochemical Society, Inc. Monte Carlo Simulation of the Electrodeposition of Copper II. Acid Sulfate Solution with Blocking Additive Timothy J. Pricer, a,
C406 0013-4651/2002/149 8 /C406/7/$7.00 The Electrochemical Society, Inc. Monte Carlo Simulation of the Electrodeposition of Copper II. Acid Sulfate Solution with Blocking Additive Timothy J. Pricer, a,
General Chemistry 1412 Spring 2008 Instructor: Dr. Shawn Amorde Website:
 General Chemistry 1412 Spring 2008 Instructor: Dr. Shawn Amorde Website: www.austincc.edu/samorde Email: samorde@austincc.edu Lecture Notes Chapter 21 (21.1-21.25) Suggested Problems () Outline 1. Introduction
General Chemistry 1412 Spring 2008 Instructor: Dr. Shawn Amorde Website: www.austincc.edu/samorde Email: samorde@austincc.edu Lecture Notes Chapter 21 (21.1-21.25) Suggested Problems () Outline 1. Introduction
A modication of the lm. model. Heikki Haario. Department of Mathematics. University of Helsinki. Hallituskatu 15, Helsinki.
 A modication of the lm model Heikki Haario Department of Mathematics University of Helsinki Hallituskatu 15, 00100 Helsinki Finland e-mail: hhaario@convex.csc.i Thomas I. Seidman Department of Mathematics
A modication of the lm model Heikki Haario Department of Mathematics University of Helsinki Hallituskatu 15, 00100 Helsinki Finland e-mail: hhaario@convex.csc.i Thomas I. Seidman Department of Mathematics
J.TAUSCH AND J.WHITE 1. Capacitance Extraction of 3-D Conductor Systems in. Dielectric Media with high Permittivity Ratios
 JTAUSCH AND JWHITE Capacitance Extraction of -D Conductor Systems in Dielectric Media with high Permittivity Ratios Johannes Tausch and Jacob White Abstract The recent development of fast algorithms for
JTAUSCH AND JWHITE Capacitance Extraction of -D Conductor Systems in Dielectric Media with high Permittivity Ratios Johannes Tausch and Jacob White Abstract The recent development of fast algorithms for
Explicit approximations to estimate the perturbative diusivity in the presence of convectivity and damping (Part 1): Semi-innite slab approximations
 Explicit approximations to estimate the perturbative diusivity in the presence of convectivity and damping (Part 1): Semi-innite slab approximations M. van Berkel 1,2,3,4, H.J. Zwart 5,6, G.M.D. Hogeweij
Explicit approximations to estimate the perturbative diusivity in the presence of convectivity and damping (Part 1): Semi-innite slab approximations M. van Berkel 1,2,3,4, H.J. Zwart 5,6, G.M.D. Hogeweij
A Tertiary Current Distribution Model with Complex Additive Chemistry and Turbulent Flow
 A Tertiary Current Distribution Model with Complex Additive Chemistry and Turbulent Flow L. A. Gochberg a and J.-C. Sheu b a Novellus Systems, Inc., San Jose, CA, b CFD Research, Corp., Huntsville, AL
A Tertiary Current Distribution Model with Complex Additive Chemistry and Turbulent Flow L. A. Gochberg a and J.-C. Sheu b a Novellus Systems, Inc., San Jose, CA, b CFD Research, Corp., Huntsville, AL
Copper electrodeposition in presence and absence of EDTA using reticulated vitreous carbon electrode
 Copper electrodeposition in presence and absence of EDTA using reticulated vitreous carbon electrode P. H. Britto a, L. A. M. Ruotolo a,b a Department of Chemical Engineering, Federal University of São
Copper electrodeposition in presence and absence of EDTA using reticulated vitreous carbon electrode P. H. Britto a, L. A. M. Ruotolo a,b a Department of Chemical Engineering, Federal University of São
IE 5531: Engineering Optimization I
 IE 5531: Engineering Optimization I Lecture 14: Unconstrained optimization Prof. John Gunnar Carlsson October 27, 2010 Prof. John Gunnar Carlsson IE 5531: Engineering Optimization I October 27, 2010 1
IE 5531: Engineering Optimization I Lecture 14: Unconstrained optimization Prof. John Gunnar Carlsson October 27, 2010 Prof. John Gunnar Carlsson IE 5531: Engineering Optimization I October 27, 2010 1
CH 223 Friday Sept. 08, 2017 L14B
 CH 223 Friday Sept. 08, 2017 L14B Previously: Relationships between E cell, K, and ΔG Concentration and cell potential Nernst equation for non-standard conditions: E cell = E 0 cell - 0.0592 n log Q at
CH 223 Friday Sept. 08, 2017 L14B Previously: Relationships between E cell, K, and ΔG Concentration and cell potential Nernst equation for non-standard conditions: E cell = E 0 cell - 0.0592 n log Q at
in order to insure that the Liouville equation for f(?; t) is still valid. These equations of motion will give rise to a distribution function f(?; t)
 G25.2651: Statistical Mechanics Notes for Lecture 21 Consider Hamilton's equations in the form I. CLASSICAL LINEAR RESPONSE THEORY _q i = @H @p i _p i =? @H @q i We noted early in the course that an ensemble
G25.2651: Statistical Mechanics Notes for Lecture 21 Consider Hamilton's equations in the form I. CLASSICAL LINEAR RESPONSE THEORY _q i = @H @p i _p i =? @H @q i We noted early in the course that an ensemble
Fernando O. Raineri. Office Hours: MWF 9:30-10:30 AM Room 519 Tue. 3:00-5:00 CLC (lobby).
 Fernando O. Raineri Office Hours: MWF 9:30-10:30 AM Room 519 Tue. 3:00-5:00 CLC (lobby). P1) What is the reduction potential of the hydrogen electrode g bar H O aq Pt(s) H,1 2 3 when the aqueous solution
Fernando O. Raineri Office Hours: MWF 9:30-10:30 AM Room 519 Tue. 3:00-5:00 CLC (lobby). P1) What is the reduction potential of the hydrogen electrode g bar H O aq Pt(s) H,1 2 3 when the aqueous solution
Prof. Mario L. Ferrari
 Sustainable Energy Mod.1: Fuel Cells & Distributed Generation Systems Dr. Ing. Mario L. Ferrari Thermochemical Power Group (TPG) - DiMSET University of Genoa, Italy Lesson II Lesson II: fuel cells (electrochemistry)
Sustainable Energy Mod.1: Fuel Cells & Distributed Generation Systems Dr. Ing. Mario L. Ferrari Thermochemical Power Group (TPG) - DiMSET University of Genoa, Italy Lesson II Lesson II: fuel cells (electrochemistry)
CHM 213 (INORGANIC CHEMISTRY): Applications of Standard Reduction Potentials. Compiled by. Dr. A.O. Oladebeye
 CHM 213 (INORGANIC CHEMISTRY): Applications of Standard Reduction Potentials Compiled by Dr. A.O. Oladebeye Department of Chemistry University of Medical Sciences, Ondo, Nigeria Electrochemical Cell Electrochemical
CHM 213 (INORGANIC CHEMISTRY): Applications of Standard Reduction Potentials Compiled by Dr. A.O. Oladebeye Department of Chemistry University of Medical Sciences, Ondo, Nigeria Electrochemical Cell Electrochemical
MODELING AND EXPERIMENTAL VALIDATION OF ELECTROPLATING DEPOSIT DISTRIBUTIONS IN COPPER SULFATE SOLUTIONS. Mark Robert Robison
 MODELING AND EXPERIMENTAL VALIDATION OF ELECTROPLATING DEPOSIT DISTRIBUTIONS IN COPPER SULFATE SOLUTIONS by Mark Robert Robison A thesis submitted to the faculty of The University of Utah in partial fulfillment
MODELING AND EXPERIMENTAL VALIDATION OF ELECTROPLATING DEPOSIT DISTRIBUTIONS IN COPPER SULFATE SOLUTIONS by Mark Robert Robison A thesis submitted to the faculty of The University of Utah in partial fulfillment
Electrochemistry Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry
 2012 Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry Electricity from Chemistry Many chemical reactions involve the transfer of electrons between atoms or ions electron transfer
2012 Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry Electricity from Chemistry Many chemical reactions involve the transfer of electrons between atoms or ions electron transfer
Lecture 29: Kinetics of Oxidation of Metals: Part 2: Wagner Parabolic
 Lecture 9: Kinetics of Oxidat of Metals: Part : Wagner Parabolic Mod Today s topics Oxidat of metals: controlled by both the ic diffus (carried by M + and O - ) and the ectronic diffus (carried by e -
Lecture 9: Kinetics of Oxidat of Metals: Part : Wagner Parabolic Mod Today s topics Oxidat of metals: controlled by both the ic diffus (carried by M + and O - ) and the ectronic diffus (carried by e -
Mathematical Modeling of Electrodeposition
 Mathematical Modeling of Electrodeposition Ralph E. White * and Venkat R. Subramanian Center for Electrochemical Engineering Department of Chemical Engineering, University of South Carolina Columbia, SC
Mathematical Modeling of Electrodeposition Ralph E. White * and Venkat R. Subramanian Center for Electrochemical Engineering Department of Chemical Engineering, University of South Carolina Columbia, SC
Chpt 20: Electrochemistry
 Cell Potential and Free Energy When both reactants and products are in their standard states, and under constant pressure and temperature conditions where DG o = nfe o DG o is the standard free energy
Cell Potential and Free Energy When both reactants and products are in their standard states, and under constant pressure and temperature conditions where DG o = nfe o DG o is the standard free energy
Superfilling and the Curvature Enhanced Accelerator Coverage Mechanism
 Superfilling and the Curvature Enhanced Accelerator Coverage Mechanism by T. P. Moffat, D. Wheeler, and D. Josell State-of-the-art manufacturing of semiconductor devices involves the electrodeposition
Superfilling and the Curvature Enhanced Accelerator Coverage Mechanism by T. P. Moffat, D. Wheeler, and D. Josell State-of-the-art manufacturing of semiconductor devices involves the electrodeposition
The Application of a Numerical Model of a Proton Exchange Membrane Fuel Cell to the Estimations of Some Cell Parameters
 The Application of a Numerical Model of a Proton Exchange Membrane Fuel Cell to the Estimations of Some Cell Parameters Ágnes Havasi 1, Róbert Horváth 2 and Tamás Szabó 3 1 Eötvös Loránd University, Pázmány
The Application of a Numerical Model of a Proton Exchange Membrane Fuel Cell to the Estimations of Some Cell Parameters Ágnes Havasi 1, Róbert Horváth 2 and Tamás Szabó 3 1 Eötvös Loránd University, Pázmány
Mathematical Modeling All Solid State Batteries
 Katharina Becker-Steinberger, Stefan Funken, Manuel Landsdorfer, Karsten Urban Institute of Numerical Mathematics Konstanz, 04.03.2010 Mathematical Modeling All Solid State Batteries Page 1/31 Mathematical
Katharina Becker-Steinberger, Stefan Funken, Manuel Landsdorfer, Karsten Urban Institute of Numerical Mathematics Konstanz, 04.03.2010 Mathematical Modeling All Solid State Batteries Page 1/31 Mathematical
John P.F.Sum and Peter K.S.Tam. Hong Kong Polytechnic University, Hung Hom, Kowloon.
 Note on the Maxnet Dynamics John P.F.Sum and Peter K.S.Tam Department of Electronic Engineering, Hong Kong Polytechnic University, Hung Hom, Kowloon. April 7, 996 Abstract A simple method is presented
Note on the Maxnet Dynamics John P.F.Sum and Peter K.S.Tam Department of Electronic Engineering, Hong Kong Polytechnic University, Hung Hom, Kowloon. April 7, 996 Abstract A simple method is presented
Spurious Chaotic Solutions of Dierential. Equations. Sigitas Keras. September Department of Applied Mathematics and Theoretical Physics
 UNIVERSITY OF CAMBRIDGE Numerical Analysis Reports Spurious Chaotic Solutions of Dierential Equations Sigitas Keras DAMTP 994/NA6 September 994 Department of Applied Mathematics and Theoretical Physics
UNIVERSITY OF CAMBRIDGE Numerical Analysis Reports Spurious Chaotic Solutions of Dierential Equations Sigitas Keras DAMTP 994/NA6 September 994 Department of Applied Mathematics and Theoretical Physics
Multilayer Ceramic Chip Capacitors
 SELECTION OF CERAMIC CHIP CAPACITORS JARO Multilayer Ceramic Chip Capacitors offer the most complete range of characteristics and configurations available in the industry. We suggest your selection of
SELECTION OF CERAMIC CHIP CAPACITORS JARO Multilayer Ceramic Chip Capacitors offer the most complete range of characteristics and configurations available in the industry. We suggest your selection of
Electron Transfer Reactions
 ELECTROCHEMISTRY 1 Electron Transfer Reactions 2 Electron transfer reactions are oxidation- reduction or redox reactions. Results in the generation of an electric current (electricity) or be caused by
ELECTROCHEMISTRY 1 Electron Transfer Reactions 2 Electron transfer reactions are oxidation- reduction or redox reactions. Results in the generation of an electric current (electricity) or be caused by
MAT 22B - Lecture Notes
 MAT 22B - Lecture Notes 2 September 2015 Systems of ODEs This is some really cool stu. Systems of ODEs are a natural and powerful way to model how multiple dierent interrelated quantities change through
MAT 22B - Lecture Notes 2 September 2015 Systems of ODEs This is some really cool stu. Systems of ODEs are a natural and powerful way to model how multiple dierent interrelated quantities change through
NRAO CHEMICAL LAB REPORT NO. 2 THEORY OF ELECTRODEPOSITED METALS. J. Lichtenberger
 NRAO CHEMICAL LAB REPORT NO. 2 THEORY OF ELECTRODEPOSITED METALS J. Lichtenberger National Radio Astronomy Observatory Charlottesville, Virginia November 1975 ABSTRACT A brief introduction to the theoretical
NRAO CHEMICAL LAB REPORT NO. 2 THEORY OF ELECTRODEPOSITED METALS J. Lichtenberger National Radio Astronomy Observatory Charlottesville, Virginia November 1975 ABSTRACT A brief introduction to the theoretical
CSCI1950V Project 4 : Smoothed Particle Hydrodynamics
 CSCI1950V Project 4 : Smoothed Particle Hydrodynamics Due Date : Midnight, Friday March 23 1 Background For this project you will implement a uid simulation using Smoothed Particle Hydrodynamics (SPH).
CSCI1950V Project 4 : Smoothed Particle Hydrodynamics Due Date : Midnight, Friday March 23 1 Background For this project you will implement a uid simulation using Smoothed Particle Hydrodynamics (SPH).
Ch 18 Electrochemistry OIL-RIG Reactions
 Ch 18 Electrochemistry OIL-RIG Reactions Alessandro Volta s Invention Modified by Dr. Cheng-Yu Lai Daily Electrochemistry Appliactions Electrochemistry: The area of chemistry that examines the transformations
Ch 18 Electrochemistry OIL-RIG Reactions Alessandro Volta s Invention Modified by Dr. Cheng-Yu Lai Daily Electrochemistry Appliactions Electrochemistry: The area of chemistry that examines the transformations
Programming, numerics and optimization
 Programming, numerics and optimization Lecture C-3: Unconstrained optimization II Łukasz Jankowski ljank@ippt.pan.pl Institute of Fundamental Technological Research Room 4.32, Phone +22.8261281 ext. 428
Programming, numerics and optimization Lecture C-3: Unconstrained optimization II Łukasz Jankowski ljank@ippt.pan.pl Institute of Fundamental Technological Research Room 4.32, Phone +22.8261281 ext. 428
Pulsed Current and Potential Response of Acid Copper System with Additives and the Double Layer Effect
 Journal of The Electrochemical Society, 149 5 C229-C236 2002 0013-4651/2002/149 5 /C229/8/$7.00 The Electrochemical Society, Inc. Pulsed Current and Potential Response of Acid Copper System with Additives
Journal of The Electrochemical Society, 149 5 C229-C236 2002 0013-4651/2002/149 5 /C229/8/$7.00 The Electrochemical Society, Inc. Pulsed Current and Potential Response of Acid Copper System with Additives
A Precise Model of TSV Parasitic Capacitance Considering Temperature for 3D IC DENG Quan ZHANG Min-Xuan ZHAO Zhen-Yu LI Peng
 International Conference on Automation, Mechanical Control and Computational Engineering (AMCCE 2015) A Precise Model of TSV Parasitic Capacitance Considering Temperature for 3D IC DENG Quan ZHANG Min-Xuan
International Conference on Automation, Mechanical Control and Computational Engineering (AMCCE 2015) A Precise Model of TSV Parasitic Capacitance Considering Temperature for 3D IC DENG Quan ZHANG Min-Xuan
Electrochemical reaction
 Electrochemical reaction electrochemistry electrochem. reaction mechanism electrode potential Faradays law electrode reaction kinetics 1 Electrochemistry in industry Chlor-Alkali galvano industry production
Electrochemical reaction electrochemistry electrochem. reaction mechanism electrode potential Faradays law electrode reaction kinetics 1 Electrochemistry in industry Chlor-Alkali galvano industry production
Electrolysis Active Learning During Class Activity Tom Greenbowe Department of Chemistry & Biochemistry University of Oregon Eugene, Oregon
 Electrolysis Active Learning During Class Activity Tom Greenbowe Department of Chemistry & Biochemistry University of Oregon Eugene, Oregon Electrolytic cells the use of electrical energy to drive thermodynamically
Electrolysis Active Learning During Class Activity Tom Greenbowe Department of Chemistry & Biochemistry University of Oregon Eugene, Oregon Electrolytic cells the use of electrical energy to drive thermodynamically
V. Electrostatics Lecture 24: Diffuse Charge in Electrolytes
 V. Electrostatics Lecture 24: Diffuse Charge in Electrolytes MIT Student 1. Poisson-Nernst-Planck Equations The Nernst-Planck Equation is a conservation of mass equation that describes the influence of
V. Electrostatics Lecture 24: Diffuse Charge in Electrolytes MIT Student 1. Poisson-Nernst-Planck Equations The Nernst-Planck Equation is a conservation of mass equation that describes the influence of
AP Chemistry: Electrochemistry Multiple Choice Answers
 AP Chemistry: Electrochemistry Multiple Choice Answers 14. Questions 14-17 The spontaneous reaction that occurs when the cell in the picture operates is as follows: 2Ag + + Cd (s) à 2 Ag (s) + Cd 2+ (A)
AP Chemistry: Electrochemistry Multiple Choice Answers 14. Questions 14-17 The spontaneous reaction that occurs when the cell in the picture operates is as follows: 2Ag + + Cd (s) à 2 Ag (s) + Cd 2+ (A)
Electroplating/ Electrodeposition
 Electroplating/ Electrodeposition Wei Yan ABC s of Electrochemistry 03/22/2012 OUTLINE Introduction Electroplating Setup Importance of Electrodeposition Electrochemistry Fundamentals Factors affecting
Electroplating/ Electrodeposition Wei Yan ABC s of Electrochemistry 03/22/2012 OUTLINE Introduction Electroplating Setup Importance of Electrodeposition Electrochemistry Fundamentals Factors affecting
AP CHEMISTRY NOTES 12-1 ELECTROCHEMISTRY: ELECTROCHEMICAL CELLS
 AP CHEMISTRY NOTES 12-1 ELECTROCHEMISTRY: ELECTROCHEMICAL CELLS Review: OXIDATION-REDUCTION REACTIONS the changes that occur when electrons are transferred between reactants (also known as a redox reaction)
AP CHEMISTRY NOTES 12-1 ELECTROCHEMISTRY: ELECTROCHEMICAL CELLS Review: OXIDATION-REDUCTION REACTIONS the changes that occur when electrons are transferred between reactants (also known as a redox reaction)
Nernst voltage loss in oxyhydrogen fuel cells
 Nernst voltage loss in oxyhydrogen fuel cells Jinzhe Lyu (Division for Experimental Physics, School of Nuclear Science & Engineering, National Research Tomsk Polytechnic University, Lenina Ave. 43, Tomsk,
Nernst voltage loss in oxyhydrogen fuel cells Jinzhe Lyu (Division for Experimental Physics, School of Nuclear Science & Engineering, National Research Tomsk Polytechnic University, Lenina Ave. 43, Tomsk,
Chapter 3 Electrochemical methods of Analysis-Potentiometry
 Chapter 3 Electrochemical methods of Analysis-Potentiometry Electroanalytical chemistry Contents Introduction Galvanic and electrolytic cells Salt bridge Electrode potential and cell potential Indicator
Chapter 3 Electrochemical methods of Analysis-Potentiometry Electroanalytical chemistry Contents Introduction Galvanic and electrolytic cells Salt bridge Electrode potential and cell potential Indicator
Spectral transforms. Contents. ARPEGE-Climat Version 5.1. September Introduction 2
 Spectral transforms ARPEGE-Climat Version 5.1 September 2008 Contents 1 Introduction 2 2 Spectral representation 2 2.1 Spherical harmonics....................... 2 2.2 Collocation grid.........................
Spectral transforms ARPEGE-Climat Version 5.1 September 2008 Contents 1 Introduction 2 2 Spectral representation 2 2.1 Spherical harmonics....................... 2 2.2 Collocation grid.........................
quantitative information on the error caused by using the solution of the linear problem to describe the response of the elastic material on a corner
 Quantitative Justication of Linearization in Nonlinear Hencky Material Problems 1 Weimin Han and Hong-ci Huang 3 Abstract. The classical linear elasticity theory is based on the assumption that the size
Quantitative Justication of Linearization in Nonlinear Hencky Material Problems 1 Weimin Han and Hong-ci Huang 3 Abstract. The classical linear elasticity theory is based on the assumption that the size
electrodeposition is a special case of electrolysis where the result is deposition of solid material on an electrode surface.
 Electrochemical Methods Electrochemical Deposition is known as electrodeposition - see CHEM* 1050 - electrolysis electrodeposition is a special case of electrolysis where the result is deposition of solid
Electrochemical Methods Electrochemical Deposition is known as electrodeposition - see CHEM* 1050 - electrolysis electrodeposition is a special case of electrolysis where the result is deposition of solid
Copyright 2018 Dan Dill 1
 when the ions each are 1 M, Zn is consumed. This means 1. 1 2. 1 3. 1 4. 1. More information needed Lecture 24 CH102 A1 (MWF 9:0 am) Monday, March 26, 2018 Cell voltage,, and electrical energy Calculating
when the ions each are 1 M, Zn is consumed. This means 1. 1 2. 1 3. 1 4. 1. More information needed Lecture 24 CH102 A1 (MWF 9:0 am) Monday, March 26, 2018 Cell voltage,, and electrical energy Calculating
Chapter 20. Electrochemistry
 Chapter 20. Electrochemistry 20.1 Oxidation-Reduction Reactions Oxidation-reduction reactions = chemical reactions in which the oxidation state of one or more substance changes (redox reactions). Recall:
Chapter 20. Electrochemistry 20.1 Oxidation-Reduction Reactions Oxidation-reduction reactions = chemical reactions in which the oxidation state of one or more substance changes (redox reactions). Recall:
Effect of Additives on Shape Evolution during Electrodeposition
 0013-4651/2008/155 3 /D223/11/$23.00 The Electrochemical Society Effect of Additives on Shape Evolution during Electrodeposition III. Trench Infill for On-Chip Interconnects Yan Qin, a, * Xiaohai Li, a,
0013-4651/2008/155 3 /D223/11/$23.00 The Electrochemical Society Effect of Additives on Shape Evolution during Electrodeposition III. Trench Infill for On-Chip Interconnects Yan Qin, a, * Xiaohai Li, a,
Rening the submesh strategy in the two-level nite element method: application to the advection diusion equation
 INTERNATIONAL JOURNAL FOR NUMERICAL METHODS IN FLUIDS Int. J. Numer. Meth. Fluids 2002; 39:161 187 (DOI: 10.1002/d.219) Rening the submesh strategy in the two-level nite element method: application to
INTERNATIONAL JOURNAL FOR NUMERICAL METHODS IN FLUIDS Int. J. Numer. Meth. Fluids 2002; 39:161 187 (DOI: 10.1002/d.219) Rening the submesh strategy in the two-level nite element method: application to
MAT 22B - Lecture Notes
 MAT 22B - Lecture Notes 4 September 205 Solving Systems of ODE Last time we talked a bit about how systems of ODE arise and why they are nice for visualization. Now we'll talk about the basics of how to
MAT 22B - Lecture Notes 4 September 205 Solving Systems of ODE Last time we talked a bit about how systems of ODE arise and why they are nice for visualization. Now we'll talk about the basics of how to
Outline Introduction: Problem Description Diculties Algebraic Structure: Algebraic Varieties Rank Decient Toeplitz Matrices Constructing Lower Rank St
 Structured Lower Rank Approximation by Moody T. Chu (NCSU) joint with Robert E. Funderlic (NCSU) and Robert J. Plemmons (Wake Forest) March 5, 1998 Outline Introduction: Problem Description Diculties Algebraic
Structured Lower Rank Approximation by Moody T. Chu (NCSU) joint with Robert E. Funderlic (NCSU) and Robert J. Plemmons (Wake Forest) March 5, 1998 Outline Introduction: Problem Description Diculties Algebraic
= w 2. w 1. B j. A j. C + j1j2
 Local Minima and Plateaus in Multilayer Neural Networks Kenji Fukumizu and Shun-ichi Amari Brain Science Institute, RIKEN Hirosawa 2-, Wako, Saitama 35-098, Japan E-mail: ffuku, amarig@brain.riken.go.jp
Local Minima and Plateaus in Multilayer Neural Networks Kenji Fukumizu and Shun-ichi Amari Brain Science Institute, RIKEN Hirosawa 2-, Wako, Saitama 35-098, Japan E-mail: ffuku, amarig@brain.riken.go.jp
Tutorials : Corrosion Part 1: Theory and basics
 Tutorials : Corrosion Part 1: Theory and basics Outline A. Definition and effects of corrosion B. General thermodynamics and kinetics in electrochemistry C. Thermodynamics and kinetics in corrosion 2 2/21
Tutorials : Corrosion Part 1: Theory and basics Outline A. Definition and effects of corrosion B. General thermodynamics and kinetics in electrochemistry C. Thermodynamics and kinetics in corrosion 2 2/21
Supplementary information
 1 2 Supplementary information 3 4 5 6 Supplementary Figure 1 7 8 Supplementary Figure 1 ǀ Characterization of the lysozyme fibrils by atomic force microscopy 9 (AFM) and scanning electron microscopy (SEM).
1 2 Supplementary information 3 4 5 6 Supplementary Figure 1 7 8 Supplementary Figure 1 ǀ Characterization of the lysozyme fibrils by atomic force microscopy 9 (AFM) and scanning electron microscopy (SEM).
4. Higher Order Linear DEs
 4. Higher Order Linear DEs Department of Mathematics & Statistics ASU Outline of Chapter 4 1 General Theory of nth Order Linear Equations 2 Homogeneous Equations with Constant Coecients 3 The Method of
4. Higher Order Linear DEs Department of Mathematics & Statistics ASU Outline of Chapter 4 1 General Theory of nth Order Linear Equations 2 Homogeneous Equations with Constant Coecients 3 The Method of
Review. Chapter 17 Electrochemistry. Outline. Voltaic Cells. Electrochemistry. Mnemonic
 Review William L Masterton Cecile N. Hurley Edward J. Neth cengage.com/chemistry/masterton Chapter 17 Electrochemistry Oxidation Loss of electrons Occurs at electrode called the anode Reduction Gain of
Review William L Masterton Cecile N. Hurley Edward J. Neth cengage.com/chemistry/masterton Chapter 17 Electrochemistry Oxidation Loss of electrons Occurs at electrode called the anode Reduction Gain of
Recovering Copulae from Conditional Quantiles
 Wolfgang K. Härdle Chen Huang Alexander Ristig Ladislaus von Bortkiewicz Chair of Statistics C.A.S.E. Center for Applied Statistics and Economics HumboldtUniversität zu Berlin http://lvb.wiwi.hu-berlin.de
Wolfgang K. Härdle Chen Huang Alexander Ristig Ladislaus von Bortkiewicz Chair of Statistics C.A.S.E. Center for Applied Statistics and Economics HumboldtUniversität zu Berlin http://lvb.wiwi.hu-berlin.de
