Notes on Ewald summation techniques
|
|
|
- William Dorsey
- 6 years ago
- Views:
Transcription
1 February 3, 011 Notes on Ewald summation techniques Adapted from similar presentation in PHY 71 he total electrostatic potential energy of interaction between point charges {q i } at the positions {r i } is given by W = 1 4πε 0 i<j q i q j r i r j = 1 q i q j r i r j. (1) In this expression, the first form explicitly counts all pairs, while the second form counts all interactions and divides by to compensate for double counting. For a finite system of charges, this expression can be evaluated directly, however, for a large or infinite system, the expression 1 does not converge and numerical tricks must be used to evaluate the energy. In fact, for the infinite system, one has an infinite amount of charge and the energy of interaction is undefined. If the system is neutral, it is possible to define a meaningful interaction energy by use of an Ewald transformation. he basic idea of the Ewald approach is as follows. he error function erf(x) and its complement erfc(x) are defined as: erf(x) = x e t dt and erfc(x) = 1 erf(x) = e t dt. () π π 0 x Ewald noted that 1 r = erf( 1 ηr) + erfc( 1 ηr). (3) r r In this expression, the first term goes to a constant ( η/π) as r 0, but has a long range tail as r. he second term has a singular behavior as r 0, but vanishes exponentially as r. his is illustrated in the plot below where η was chosen to be η = 4.
2 10 9 erf(x)/x (1-erf(x))/x 1/x hus, Ewald s idea is to replace a single divergent summation with two convergent summations. he first summation has a convergent summation in the form of its Fourier transform and the second has a convergent direct summation. hus the calculation of the electrostatic energy would be evaluated using: W = 1 q i q j r i r j = 1 q i q j erf( 1 η ri r j ) + r i r j q i q j erfc( 1 η ri r j ). r i r j (4) For an appropriate choice of the parameter η, the second summation in Eq. 4 converges quickly and can be evaluated directly. he first term in the summation of Eq. 4 must be transformed into Fourier space. In order to described these summations explicitly, we assume that we have a periodic lattice so that every ion can be located by r i = τ α + a location τ α within a unit cell and a periodic translation vector, In this way, the summation becomes: 1 = N (5) ij αβ, where N denotes the number of unit cells in the system. Since we have a periodic system, N is infinite, but the energy per unit cell W/N is well defined. he other identity that we 1 Note that for any two lattice translations i and j, i j = k, where k is also a lattice translation.
3 must use is that a sum over lattice translations may be transformed into an equivalent sum over reciprocal lattice translations according to the identity: δ 3 (r ) = 1 e i r, (6) Ω where Ω denotes the unit cell volume. he proof of this relation is given in the appendix. he first term of Eq. 4 thus becomes q i q j erf( 1 η ri r j )) = N q α q β r i r j αβ erf( 1 η τα τ β + ) τ α τ β + α q α η, (7) π where the last term in Eq. 7 comes from subtracting out the self-interaction (i = j) term from the complete lattice sum. Using the short hand notation, τ αβ τ α τ β, the lattice sum can be evaluated: his becomes, 1 Ω erf( 1 η ταβ + ) τ αβ + = d 3 re i r erf( 1 η ταβ + r ) τ αβ + r d 3 r = 4π Ω δ 3 (r ) erf( 1 η ταβ + r ). (8) τ αβ + r 0 e i τ αβ e /η + 1 where the last term, which is infinite, comes from the = 0 contribution. 1 η 0 du, (9) If the last term of Eq. 9 cannot be eliminated, it is clear that the electrostatic energy is infinite. he term can be eliminated if and only if the system is neutral. hus, it is only meaningful to calculate the electrostatic energy of a neutral periodic system. If the actual system has a net charge of Q α q α, we could calculate a meaningful energy if we add to the actual charge density, a compensating uniform density charge density of Q/Ω. aking all of the terms into account, we find the final Ewald expression to be: u 3 W N = αβ q α q β 4π Ω e i τ αβ e /η 0 η π δ αβ + erfc( 1 η ταβ + ) 4πQ τ αβ + Ωη, (10) where the in the summation over lattice translations indicates that all self-interaction terms should be omitted. Appendix I comments on lattice vectors and reciprocal lattice vectors In this discussion, will assume we have a 3-dimensional periodic system. It can be easily generalized to 1- or - dimensional systems. In general, a translation vector can be described a linear combination of the three primitive translation vectors 1,, and 3 : = n n + n 3 3, (11)
4 where {n 1, n, n 3 } are integers. Note that the unit cell volume Ω can be expressed in terms of the primitive translation vectors according to: Ω = 1 ( 3 ). (1) he reciprocal lattice vectors can generally be written as a linear combination of the three primitive reciprocal lattice vectors 1,, and 3 : = m m + m 3 3, (13) where {m 1, m, m 3 } are integers. he primitive reciprocal lattice vectors are determined from the primitive translation vectors according to the identities: i j = πδ ij. (14) Note that the volume of the primitive reciprocal lattice is given by 1 ( 3 ) = (π)3 Ω. (15) Some examples of this are given below. Proof of Eq. 6 Consider the geometric series +M k= M e ik( 1 r) = sin ( (M + 1 ) 1 r ) sin( 1 r/). (16) he behavior of the right hand side of Eq. (16) is that it is small in magnitude very except when the denominator vanishes. his occurs whenever 1 r/ = n 1 π, where n 1 represents any integer. If we take the limit M, we find that the function represents the behavior of a sum of delta functions: sin ( (M + 1 lim ) 1 r ) = π δ( 1 (r n 1 1 ). (17) M sin( 1 r/) n 1 he summation over all lattice translations n 1 1 is due to the fact that sin( 1 r/) = 0 whenever r = n 1 1. Carrying out the geometric summations in the right hand side of Eq. 6 for all three reciprocal lattice vectors and taking the limit as in Eq. 17, e i r = (π) 3 δ( 1 (r n 1 1 ))δ( (r n ))δ( 3 (r n 3 3 )). (18) n 1,n,n 3 Finally, the right hand side of Eq. 18 can be simplified by eliminating the reciprocal lattice vectors from the δ functions: e i r = which is consistent with Eq. 6. (π) 3 1 ( 3 ) δ 3 (r ) = Ωδ 3 (r ), (19)
5 Appendix II examples In these examples, denote the length of the unit cell by a. CsCl structure here are two kinds of sites τ Cs = 0 and τ Cl = a (ˆx + ŷ + ẑ). 1 = aˆx = aŷ 3 = aẑ. (0) 1 = π a ˆx = π a ŷ 3 = π a ẑ. (1) In these terms, Eq. 10 can be broken into two summations: W N = q ( 1 + ), () where and 1 4π Ω 0 erfc( η 0 (1 ei τ Cl )e /η η π (3) erfc( η τ Cl + ). (4) τ Cl + In this expression, the sum over α and β has a total of 4 contributions pairs of identical contributions. For Cs-Cs or Cl-Cl interactions, τ αβ = 0 and q α = q β resulting in repulsive contributions. For Cs-Cl or Cl-Cs interations, τ αβ = τ Cl and q α = q β resulting in attractive contributions. his expression can be evaluated using Maple, which gives the result W N = q ( 4.071). (5) a NaCl structure here are two kinds of sites τ Na = 0 and τ Cl = a ˆx. he primitive lattice has the translation vectors: and the reciprocal vectors: 1 = a (ˆx + ŷ) = a (ŷ + ẑ) 3 = a (ˆx + ẑ), (6) 1 = π a (ˆx + ŷ ẑ) = π a ( ˆx + ŷ + ẑ) 3 = π (ˆx ŷ + ẑ). (7) a
6 Note that there are many materials that have this structure, including MgO in which Mg has a charge of +e and O has a charge of -e. Zinc-blende structure his structure has the same bravais lattice as NaCl, but the basis vectors are τ Zn = 0 and τ S = a (ˆx + ŷ + ẑ). In this case Zn has charge of +e and S has a charge of -e. 4
7 Figure 1: Diagram of CsCl structure, indicating translation vectors and atomic site vectors τ. τ
Series. Definition. a 1 + a 2 + a 3 + is called an infinite series or just series. Denoted by. n=1
 Definition a 1 + a 2 + a 3 + is called an infinite series or just series. Denoted by a n, or a n. Chapter 11: Sequences and, Section 11.2 24 / 40 Given a series a n. The partial sum is the sum of the first
Definition a 1 + a 2 + a 3 + is called an infinite series or just series. Denoted by a n, or a n. Chapter 11: Sequences and, Section 11.2 24 / 40 Given a series a n. The partial sum is the sum of the first
E12 UNDERSTANDING CRYSTAL STRUCTURES
 E1 UNDERSTANDING CRYSTAL STRUCTURES 1 Introduction In this experiment, the structures of many elements and compounds are rationalized using simple packing models. The pre-work revises and extends the material
E1 UNDERSTANDING CRYSTAL STRUCTURES 1 Introduction In this experiment, the structures of many elements and compounds are rationalized using simple packing models. The pre-work revises and extends the material
Neighbor Tables Long-Range Potentials
 Neighbor Tables Long-Range Potentials Today we learn how we can handle long range potentials. Neighbor tables Long-range potential Ewald sums MSE485/PHY466/CSE485 1 Periodic distances Minimum Image Convention:
Neighbor Tables Long-Range Potentials Today we learn how we can handle long range potentials. Neighbor tables Long-range potential Ewald sums MSE485/PHY466/CSE485 1 Periodic distances Minimum Image Convention:
Non-bonded interactions
 speeding up the number-crunching continued Marcus Elstner and Tomáš Kubař December 3, 2013 why care? number of individual pair-wise interactions bonded interactions proportional to N: O(N) non-bonded interactions
speeding up the number-crunching continued Marcus Elstner and Tomáš Kubař December 3, 2013 why care? number of individual pair-wise interactions bonded interactions proportional to N: O(N) non-bonded interactions
3a 2. a 1 = 3a. a 2 = 3a
 Physics 195 / Applied Physics 195 Assignment #4 Professor: Donhee Ham Teaching Fellows: Brendan Deveney and Laura Adams Date: Oct. 6, 017 Due: 1:45pm + 10 min grace period, Oct. 13, 017 at the dropbox
Physics 195 / Applied Physics 195 Assignment #4 Professor: Donhee Ham Teaching Fellows: Brendan Deveney and Laura Adams Date: Oct. 6, 017 Due: 1:45pm + 10 min grace period, Oct. 13, 017 at the dropbox
PERIODIC SYSTEMS. Chapter LATTICES
 Chapter 3 PERIODIC SYSTEMS For most solids, the atomic arrangement of maximum stability (and therefore the equilibrium atomic configuration at zero temperature) is that of a perfect crystal, defined as
Chapter 3 PERIODIC SYSTEMS For most solids, the atomic arrangement of maximum stability (and therefore the equilibrium atomic configuration at zero temperature) is that of a perfect crystal, defined as
Review of Power Series
 Review of Power Series MATH 365 Ordinary Differential Equations J. Robert Buchanan Department of Mathematics Fall 2018 Introduction In addition to the techniques we have studied so far, we may use power
Review of Power Series MATH 365 Ordinary Differential Equations J. Robert Buchanan Department of Mathematics Fall 2018 Introduction In addition to the techniques we have studied so far, we may use power
Lattice energy of ionic solids
 1 Lattice energy of ionic solids Interatomic Forces Solids are aggregates of atoms, ions or molecules. The bonding between these particles may be understood in terms of forces that play between them. Attractive
1 Lattice energy of ionic solids Interatomic Forces Solids are aggregates of atoms, ions or molecules. The bonding between these particles may be understood in terms of forces that play between them. Attractive
The Helmholtz theorem at last!
 Problem. The Helmholtz theorem at last! Recall in class the Helmholtz theorem that says that if if E =0 then E can be written as E = φ () if B =0 then B can be written as B = A (2) (a) Let n be a unit
Problem. The Helmholtz theorem at last! Recall in class the Helmholtz theorem that says that if if E =0 then E can be written as E = φ () if B =0 then B can be written as B = A (2) (a) Let n be a unit
MP464: Solid State Physics Problem Sheet
 MP464: Solid State Physics Problem Sheet 1 Write down primitive lattice vectors for the -dimensional rectangular lattice, with sides a and b in the x and y-directions respectively, and a face-centred rectangular
MP464: Solid State Physics Problem Sheet 1 Write down primitive lattice vectors for the -dimensional rectangular lattice, with sides a and b in the x and y-directions respectively, and a face-centred rectangular
Keble College - Hilary 2012 Section VI: Condensed matter physics Tutorial 2 - Lattices and scattering
 Tomi Johnson Keble College - Hilary 2012 Section VI: Condensed matter physics Tutorial 2 - Lattices and scattering Please leave your work in the Clarendon laboratory s J pigeon hole by 5pm on Monday of
Tomi Johnson Keble College - Hilary 2012 Section VI: Condensed matter physics Tutorial 2 - Lattices and scattering Please leave your work in the Clarendon laboratory s J pigeon hole by 5pm on Monday of
Condensed Matter Physics April, 8, LUMS School of Science and Engineering
 Condensed Matter Physics April, 8, 0 LUMS School of Science and Engineering PH-33 Solution of assignment 5 April, 8, 0 Interplanar separation Answer: To prove that the reciprocal lattice vector G = h b
Condensed Matter Physics April, 8, 0 LUMS School of Science and Engineering PH-33 Solution of assignment 5 April, 8, 0 Interplanar separation Answer: To prove that the reciprocal lattice vector G = h b
Reciprocal Lattice. Let A(r) be some function featuring discrete translation symmetry:
 Reciprocal Lattice From Quantum Mechanics we know that symmetries have kinematic implications. Each symmetry predetermines its specific quantum numbers and leads to certain constraints (conservation laws/selection
Reciprocal Lattice From Quantum Mechanics we know that symmetries have kinematic implications. Each symmetry predetermines its specific quantum numbers and leads to certain constraints (conservation laws/selection
N-body simulations. Phys 750 Lecture 10
 N-body simulations Phys 750 Lecture 10 N-body simulations Forward integration in time of strongly coupled classical particles or (rigid-body composite objects) dissipation Brownian motion Very general
N-body simulations Phys 750 Lecture 10 N-body simulations Forward integration in time of strongly coupled classical particles or (rigid-body composite objects) dissipation Brownian motion Very general
Non-bonded interactions
 speeding up the number-crunching Marcus Elstner and Tomáš Kubař May 8, 2015 why care? key to understand biomolecular structure and function binding of a ligand efficiency of a reaction color of a chromophore
speeding up the number-crunching Marcus Elstner and Tomáš Kubař May 8, 2015 why care? key to understand biomolecular structure and function binding of a ligand efficiency of a reaction color of a chromophore
Solution to Exercise 2
 Department of physics, NTNU TFY Mesoscopic Physics Spring Solution to xercise Question Apart from an adjustable constant, the nearest neighbour nn) tight binding TB) band structure for the D triangular
Department of physics, NTNU TFY Mesoscopic Physics Spring Solution to xercise Question Apart from an adjustable constant, the nearest neighbour nn) tight binding TB) band structure for the D triangular
Atomic Arrangement. Primer in Materials Spring
 Atomic Arrangement Primer in Materials Spring 2017 30.4.2017 1 Levels of atomic arrangements No order In gases, for example the atoms have no order, they are randomly distributed filling the volume to
Atomic Arrangement Primer in Materials Spring 2017 30.4.2017 1 Levels of atomic arrangements No order In gases, for example the atoms have no order, they are randomly distributed filling the volume to
Size-dependent physical properties of nanostructures: Introduction
 Size-dependent physical properties of nanostructures: Introduction can protect you... Five times stronger than steel, this shiny beast is also twice as strong as any bullet stopping or bomb protection
Size-dependent physical properties of nanostructures: Introduction can protect you... Five times stronger than steel, this shiny beast is also twice as strong as any bullet stopping or bomb protection
Ionic Bonding. Chem
 Whereas the term covalent implies sharing of electrons between atoms, the term ionic indicates that electrons are taken from one atom by another. The nature of ionic bonding is very different than that
Whereas the term covalent implies sharing of electrons between atoms, the term ionic indicates that electrons are taken from one atom by another. The nature of ionic bonding is very different than that
Report Form for Experiment 6: Solid State Structures
 Report Form for Experiment 6: Solid State Structures Note: Many of these questions will not make sense if you are not reading the accompanying lab handout. Station 1. Simple Cubic Lattice 1. How many unit
Report Form for Experiment 6: Solid State Structures Note: Many of these questions will not make sense if you are not reading the accompanying lab handout. Station 1. Simple Cubic Lattice 1. How many unit
II crystal structure
 II crstal structure 2-1 basic concept > Crstal structure = lattice structure + basis > Lattice point: positions (points) in the structure which are identical. > Lattice translation vector > Lattice plane
II crstal structure 2-1 basic concept > Crstal structure = lattice structure + basis > Lattice point: positions (points) in the structure which are identical. > Lattice translation vector > Lattice plane
2. Diffraction as a means to determine crystal structure
 2. Diffraction as a means to determine crystal structure Recall de Broglie matter waves: He atoms: [E (ev)] 1/2 = 0.14 / (Å) E 1Å = 0.0196 ev Neutrons: [E (ev)] 1/2 = 0.28 / (Å) E 1Å = 0.0784 ev Electrons:
2. Diffraction as a means to determine crystal structure Recall de Broglie matter waves: He atoms: [E (ev)] 1/2 = 0.14 / (Å) E 1Å = 0.0196 ev Neutrons: [E (ev)] 1/2 = 0.28 / (Å) E 1Å = 0.0784 ev Electrons:
Taylor Series and Series Convergence (Online)
 7in 0in Felder c02_online.te V3 - February 9, 205 9:5 A.M. Page CHAPTER 2 Taylor Series and Series Convergence (Online) 2.8 Asymptotic Epansions In introductory calculus classes the statement this series
7in 0in Felder c02_online.te V3 - February 9, 205 9:5 A.M. Page CHAPTER 2 Taylor Series and Series Convergence (Online) 2.8 Asymptotic Epansions In introductory calculus classes the statement this series
1 + lim. n n+1. f(x) = x + 1, x 1. and we check that f is increasing, instead. Using the quotient rule, we easily find that. 1 (x + 1) 1 x (x + 1) 2 =
 Chapter 5 Sequences and series 5. Sequences Definition 5. (Sequence). A sequence is a function which is defined on the set N of natural numbers. Since such a function is uniquely determined by its values
Chapter 5 Sequences and series 5. Sequences Definition 5. (Sequence). A sequence is a function which is defined on the set N of natural numbers. Since such a function is uniquely determined by its values
2. Diffraction as a means to determine crystal structure
 Page 1 of 22 2. Diffraction as a means to determine crystal structure Recall de Broglie matter waves: 2 p h E = where p = 2m λ h 1 E = ( ) 2m λ hc E = hυ = ( photons) λ ( matter wave) He atoms: [E (ev)]
Page 1 of 22 2. Diffraction as a means to determine crystal structure Recall de Broglie matter waves: 2 p h E = where p = 2m λ h 1 E = ( ) 2m λ hc E = hυ = ( photons) λ ( matter wave) He atoms: [E (ev)]
d 1 µ 2 Θ = 0. (4.1) consider first the case of m = 0 where there is no azimuthal dependence on the angle φ.
 4 Legendre Functions In order to investigate the solutions of Legendre s differential equation d ( µ ) dθ ] ] + l(l + ) m dµ dµ µ Θ = 0. (4.) consider first the case of m = 0 where there is no azimuthal
4 Legendre Functions In order to investigate the solutions of Legendre s differential equation d ( µ ) dθ ] ] + l(l + ) m dµ dµ µ Θ = 0. (4.) consider first the case of m = 0 where there is no azimuthal
Ceramic Bonding. CaF 2 : large SiC: small
 Recall ceramic bonding: - Mixed ionic and covalent. - % ionic character ( f ) increases with difference in electronegativity Large vs small ionic bond character: Ceramic Bonding CaF : large SiC: small
Recall ceramic bonding: - Mixed ionic and covalent. - % ionic character ( f ) increases with difference in electronegativity Large vs small ionic bond character: Ceramic Bonding CaF : large SiC: small
Introduction to Series and Sequences Math 121 Calculus II Spring 2015
 Introduction to Series and Sequences Math Calculus II Spring 05 The goal. The main purpose of our study of series and sequences is to understand power series. A power series is like a polynomial of infinite
Introduction to Series and Sequences Math Calculus II Spring 05 The goal. The main purpose of our study of series and sequences is to understand power series. A power series is like a polynomial of infinite
Appendix 2A Indices of Planes and Directions in Hexagonal Crystals
 Appendix 2 Appendix 2A Indices of Planes and Directions in Hexagonal Crystals Figure 2A.1a shows a hexagonal unit cell defined by two equal unit cell lattice parameters a 1 and a 2 in the basal plane,
Appendix 2 Appendix 2A Indices of Planes and Directions in Hexagonal Crystals Figure 2A.1a shows a hexagonal unit cell defined by two equal unit cell lattice parameters a 1 and a 2 in the basal plane,
Errata 1. p. 5 The third line from the end should read one of the four rows... not one of the three rows.
 Errata 1 Front inside cover: e 2 /4πɛ 0 should be 1.44 10 7 ev-cm. h/e 2 should be 25800 Ω. p. 5 The third line from the end should read one of the four rows... not one of the three rows. p. 8 The eigenstate
Errata 1 Front inside cover: e 2 /4πɛ 0 should be 1.44 10 7 ev-cm. h/e 2 should be 25800 Ω. p. 5 The third line from the end should read one of the four rows... not one of the three rows. p. 8 The eigenstate
Notes on Fourier Series and Integrals Fourier Series
 Notes on Fourier Series and Integrals Fourier Series et f(x) be a piecewise linear function on [, ] (This means that f(x) may possess a finite number of finite discontinuities on the interval). Then f(x)
Notes on Fourier Series and Integrals Fourier Series et f(x) be a piecewise linear function on [, ] (This means that f(x) may possess a finite number of finite discontinuities on the interval). Then f(x)
Assignment 4. u n+1 n(n + 1) i(i + 1) = n n (n + 1)(n + 2) n(n + 2) + 1 = (n + 1)(n + 2) 2 n + 1. u n (n + 1)(n + 2) n(n + 1) = n
 Assignment 4 Arfken 5..2 We have the sum Note that the first 4 partial sums are n n(n + ) s 2, s 2 2 3, s 3 3 4, s 4 4 5 so we guess that s n n/(n + ). Proving this by induction, we see it is true for
Assignment 4 Arfken 5..2 We have the sum Note that the first 4 partial sums are n n(n + ) s 2, s 2 2 3, s 3 3 4, s 4 4 5 so we guess that s n n/(n + ). Proving this by induction, we see it is true for
Power series and Taylor series
 Power series and Taylor series D. DeTurck University of Pennsylvania March 29, 2018 D. DeTurck Math 104 002 2018A: Series 1 / 42 Series First... a review of what we have done so far: 1 We examined series
Power series and Taylor series D. DeTurck University of Pennsylvania March 29, 2018 D. DeTurck Math 104 002 2018A: Series 1 / 42 Series First... a review of what we have done so far: 1 We examined series
Bravais Lattice + Basis = Crystal Structure
 Bravais Lattice + Basis = Crystal Structure A crystal structure is obtained when identical copies of a basis are located at all of the points of a Bravais lattice. Consider the structure of Cr, a I-cubic
Bravais Lattice + Basis = Crystal Structure A crystal structure is obtained when identical copies of a basis are located at all of the points of a Bravais lattice. Consider the structure of Cr, a I-cubic
This ODE arises in many physical systems that we shall investigate. + ( + 1)u = 0. (λ + s)x λ + s + ( + 1) a λ. (s + 1)(s + 2) a 0
 Legendre equation This ODE arises in many physical systems that we shall investigate We choose We then have Substitution gives ( x 2 ) d 2 u du 2x 2 dx dx + ( + )u u x s a λ x λ a du dx λ a λ (λ + s)x
Legendre equation This ODE arises in many physical systems that we shall investigate We choose We then have Substitution gives ( x 2 ) d 2 u du 2x 2 dx dx + ( + )u u x s a λ x λ a du dx λ a λ (λ + s)x
Nearly Free Electron Gas model - I
 Nearly Free Electron Gas model - I Contents 1 Free electron gas model summary 1 2 Electron effective mass 3 2.1 FEG model for sodium...................... 4 3 Nearly free electron model 5 3.1 Primitive
Nearly Free Electron Gas model - I Contents 1 Free electron gas model summary 1 2 Electron effective mass 3 2.1 FEG model for sodium...................... 4 3 Nearly free electron model 5 3.1 Primitive
Solid State Physics 460- Lecture 5 Diffraction and the Reciprocal Lattice Continued (Kittel Ch. 2)
 Solid State Physics 460- Lecture 5 Diffraction and the Reciprocal Lattice Continued (Kittel Ch. 2) Ewald Construction 2θ k out k in G Physics 460 F 2006 Lect 5 1 Recall from previous lectures Definition
Solid State Physics 460- Lecture 5 Diffraction and the Reciprocal Lattice Continued (Kittel Ch. 2) Ewald Construction 2θ k out k in G Physics 460 F 2006 Lect 5 1 Recall from previous lectures Definition
Integrals in Electrostatic Problems
 PHYS 119 Integrals in Electrostatic Problems Josh McKenney University of North Carolina at Chapel Hill (Dated: January 6, 2016) 1 FIG. 1. Three positive charges positioned at equal distances around an
PHYS 119 Integrals in Electrostatic Problems Josh McKenney University of North Carolina at Chapel Hill (Dated: January 6, 2016) 1 FIG. 1. Three positive charges positioned at equal distances around an
Topic 7 Notes Jeremy Orloff
 Topic 7 Notes Jeremy Orloff 7 Taylor and Laurent series 7. Introduction We originally defined an analytic function as one where the derivative, defined as a limit of ratios, existed. We went on to prove
Topic 7 Notes Jeremy Orloff 7 Taylor and Laurent series 7. Introduction We originally defined an analytic function as one where the derivative, defined as a limit of ratios, existed. We went on to prove
Polynomial Approximations and Power Series
 Polynomial Approximations and Power Series June 24, 206 Tangent Lines One of the first uses of the derivatives is the determination of the tangent as a linear approximation of a differentiable function
Polynomial Approximations and Power Series June 24, 206 Tangent Lines One of the first uses of the derivatives is the determination of the tangent as a linear approximation of a differentiable function
Properties of substances are largely dependent on the bonds holding the material together.
 Basics of Chemical Bonding AP Chemistry Lecture Outline Properties of substances are largely dependent on the bonds holding the material together. Basics of Bonding A chemical bond occurs when atoms or
Basics of Chemical Bonding AP Chemistry Lecture Outline Properties of substances are largely dependent on the bonds holding the material together. Basics of Bonding A chemical bond occurs when atoms or
1 Solutions in cylindrical coordinates: Bessel functions
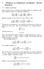 1 Solutions in cylindrical coordinates: Bessel functions 1.1 Bessel functions Bessel functions arise as solutions of potential problems in cylindrical coordinates. Laplace s equation in cylindrical coordinates
1 Solutions in cylindrical coordinates: Bessel functions 1.1 Bessel functions Bessel functions arise as solutions of potential problems in cylindrical coordinates. Laplace s equation in cylindrical coordinates
Notes: Most of the material presented in this chapter is taken from Jackson, Chap. 2, 3, and 4, and Di Bartolo, Chap. 2. 2π nx i a. ( ) = G n.
 Chapter. Electrostatic II Notes: Most of the material presented in this chapter is taken from Jackson, Chap.,, and 4, and Di Bartolo, Chap... Mathematical Considerations.. The Fourier series and the Fourier
Chapter. Electrostatic II Notes: Most of the material presented in this chapter is taken from Jackson, Chap.,, and 4, and Di Bartolo, Chap... Mathematical Considerations.. The Fourier series and the Fourier
Phys 412 Solid State Physics. Lecturer: Réka Albert
 Phys 412 Solid State Physics Lecturer: Réka Albert What is a solid? A material that keeps its shape Can be deformed by stress Returns to original shape if it is not strained too much Solid structure
Phys 412 Solid State Physics Lecturer: Réka Albert What is a solid? A material that keeps its shape Can be deformed by stress Returns to original shape if it is not strained too much Solid structure
Atomic Arrangement. Primer Materials For Science Teaching Spring
 Atomic Arrangement Primer Materials For Science Teaching Spring 2016 31.3.2015 Levels of atomic arrangements No order In gases, for example the atoms have no order, they are randomly distributed filling
Atomic Arrangement Primer Materials For Science Teaching Spring 2016 31.3.2015 Levels of atomic arrangements No order In gases, for example the atoms have no order, they are randomly distributed filling
Failures and successes of the free electron model
 Failures and successes of the free electron model Temperature dependence on the electric conduc1vity j e = E = ne2 m electron gas electron- phonon coupling Drude s model predicts that σ is independent
Failures and successes of the free electron model Temperature dependence on the electric conduc1vity j e = E = ne2 m electron gas electron- phonon coupling Drude s model predicts that σ is independent
Complex Series. Chapter 20
 hapter 20 omplex Series As in the real case, representation of functions by infinite series of simpler functions is an endeavor worthy of our serious consideration. We start with an examination of the
hapter 20 omplex Series As in the real case, representation of functions by infinite series of simpler functions is an endeavor worthy of our serious consideration. We start with an examination of the
Geometry of Crystal Lattice
 0 Geometry of Crystal Lattice 0.1 Translational Symmetry The crystalline state of substances is different from other states (gaseous, liquid, amorphous) in that the atoms are in an ordered and symmetrical
0 Geometry of Crystal Lattice 0.1 Translational Symmetry The crystalline state of substances is different from other states (gaseous, liquid, amorphous) in that the atoms are in an ordered and symmetrical
Crystal Structure Determination II
 Crystal Structure Determination II Dr. Falak Sher Pakistan Institute of Engineering and Applied Sciences 09/10/2010 Diffraction Intensities The integrated intensity, I (hkl) (peak area) of each powder
Crystal Structure Determination II Dr. Falak Sher Pakistan Institute of Engineering and Applied Sciences 09/10/2010 Diffraction Intensities The integrated intensity, I (hkl) (peak area) of each powder
Convergence of sequences and series
 Convergence of sequences and series A sequence f is a map from N the positive integers to a set. We often write the map outputs as f n rather than f(n). Often we just list the outputs in order and leave
Convergence of sequences and series A sequence f is a map from N the positive integers to a set. We often write the map outputs as f n rather than f(n). Often we just list the outputs in order and leave
Tutorial on the smooth particle-mesh Ewald algorithm
 Tutorial on the smooth particle-mesh Ewald algorithm These notes explain the smooth particle-mesh Ewald algorithm (SPME) in detail. The accompanying library libpme6 is a fully-featured implementation of
Tutorial on the smooth particle-mesh Ewald algorithm These notes explain the smooth particle-mesh Ewald algorithm (SPME) in detail. The accompanying library libpme6 is a fully-featured implementation of
Chap 3 Scattering and structures
 Chap 3 Scattering and structures Dept of Phys M.C. Chang Von Laue was struck in 1912 by the intuition that X-ray might scatter off crystals in the way that ordinary light scatters off a diffraction grating.
Chap 3 Scattering and structures Dept of Phys M.C. Chang Von Laue was struck in 1912 by the intuition that X-ray might scatter off crystals in the way that ordinary light scatters off a diffraction grating.
Mathematics 22: Lecture 19
 Mathematics 22: Lecture 19 Legendre s Equation Dan Sloughter Furman University February 5, 2008 Dan Sloughter (Furman University) Mathematics 22: Lecture 19 February 5, 2008 1 / 11 Example: Legendre s
Mathematics 22: Lecture 19 Legendre s Equation Dan Sloughter Furman University February 5, 2008 Dan Sloughter (Furman University) Mathematics 22: Lecture 19 February 5, 2008 1 / 11 Example: Legendre s
In particular, if A is a square matrix and λ is one of its eigenvalues, then we can find a non-zero column vector X with
 Appendix: Matrix Estimates and the Perron-Frobenius Theorem. This Appendix will first present some well known estimates. For any m n matrix A = [a ij ] over the real or complex numbers, it will be convenient
Appendix: Matrix Estimates and the Perron-Frobenius Theorem. This Appendix will first present some well known estimates. For any m n matrix A = [a ij ] over the real or complex numbers, it will be convenient
Midterm I Results. Mean: 35.5 (out of 100 pts) Median: 33 Mode: 25 Max: 104 Min: 2 SD: 18. Compare to: 2013 Mean: 59% 2014 Mean: 51%??
 Midterm I Results Mean: 35.5 (out of 100 pts) Median: 33 Mode: 25 Max: 104 Min: 2 SD: 18 Compare to: 2013 Mean: 59% 2014 Mean: 51%?? Crystal Thermodynamics and Electronic Structure Chapter 7 Monday, October
Midterm I Results Mean: 35.5 (out of 100 pts) Median: 33 Mode: 25 Max: 104 Min: 2 SD: 18 Compare to: 2013 Mean: 59% 2014 Mean: 51%?? Crystal Thermodynamics and Electronic Structure Chapter 7 Monday, October
80 Wyner PreCalculus Spring 2017
 80 Wyner PreCalculus Spring 2017 CHAPTER NINE: DERIVATIVES Review May 16 Test May 23 Calculus begins with the study of rates of change, called derivatives. For example, the derivative of velocity is acceleration
80 Wyner PreCalculus Spring 2017 CHAPTER NINE: DERIVATIVES Review May 16 Test May 23 Calculus begins with the study of rates of change, called derivatives. For example, the derivative of velocity is acceleration
Part IB. Further Analysis. Year
 Year 2004 2003 2002 2001 10 2004 2/I/4E Let τ be the topology on N consisting of the empty set and all sets X N such that N \ X is finite. Let σ be the usual topology on R, and let ρ be the topology on
Year 2004 2003 2002 2001 10 2004 2/I/4E Let τ be the topology on N consisting of the empty set and all sets X N such that N \ X is finite. Let σ be the usual topology on R, and let ρ be the topology on
... 3, , = a (1) 3 3 a 2 = a (2) The reciprocal lattice vectors are defined by the condition a b = 2πδ ij, which gives
 PHZ646: Fall 013 Problem set # 4: Crystal Structure due Monday, 10/14 at the time of the class Instructor: D. L. Maslov maslov@phys.ufl.edu 39-0513 Rm. 114 Office hours: TR 3 pm-4 pm Please help your instructor
PHZ646: Fall 013 Problem set # 4: Crystal Structure due Monday, 10/14 at the time of the class Instructor: D. L. Maslov maslov@phys.ufl.edu 39-0513 Rm. 114 Office hours: TR 3 pm-4 pm Please help your instructor
Mathematics 324 Riemann Zeta Function August 5, 2005
 Mathematics 324 Riemann Zeta Function August 5, 25 In this note we give an introduction to the Riemann zeta function, which connects the ideas of real analysis with the arithmetic of the integers. Define
Mathematics 324 Riemann Zeta Function August 5, 25 In this note we give an introduction to the Riemann zeta function, which connects the ideas of real analysis with the arithmetic of the integers. Define
CHEM6085: Density Functional Theory
 Lecture 11 CHEM6085: Density Functional Theory DFT for periodic crystalline solids C.-K. Skylaris 1 Electron in a one-dimensional periodic box (in atomic units) Schrödinger equation Energy eigenvalues
Lecture 11 CHEM6085: Density Functional Theory DFT for periodic crystalline solids C.-K. Skylaris 1 Electron in a one-dimensional periodic box (in atomic units) Schrödinger equation Energy eigenvalues
ENGIN 211, Engineering Math. Fourier Series and Transform
 ENGIN 11, Engineering Math Fourier Series and ransform 1 Periodic Functions and Harmonics f(t) Period: a a+ t Frequency: f = 1 Angular velocity (or angular frequency): ω = ππ = π Such a periodic function
ENGIN 11, Engineering Math Fourier Series and ransform 1 Periodic Functions and Harmonics f(t) Period: a a+ t Frequency: f = 1 Angular velocity (or angular frequency): ω = ππ = π Such a periodic function
FORMULA SHEET FOR QUIZ 2 Exam Date: November 8, 2017
 MASSACHUSETTS INSTITUTE OF TECHNOLOGY Physics Department Physics 8.07: Electromagnetism II November 5, 207 Prof. Alan Guth FORMULA SHEET FOR QUIZ 2 Exam Date: November 8, 207 A few items below are marked
MASSACHUSETTS INSTITUTE OF TECHNOLOGY Physics Department Physics 8.07: Electromagnetism II November 5, 207 Prof. Alan Guth FORMULA SHEET FOR QUIZ 2 Exam Date: November 8, 207 A few items below are marked
Katznelson Problems. Prakash Balachandran Duke University. June 19, 2009
 Katznelson Problems Prakash Balachandran Duke University June 9, 9 Chapter. Compute the Fourier coefficients of the following functions (defined by their values on [ π, π)): f(t) { t < t π (t) g(t) { t
Katznelson Problems Prakash Balachandran Duke University June 9, 9 Chapter. Compute the Fourier coefficients of the following functions (defined by their values on [ π, π)): f(t) { t < t π (t) g(t) { t
Legendre s Equation. PHYS Southern Illinois University. October 13, 2016
 PHYS 500 - Southern Illinois University October 13, 2016 PHYS 500 - Southern Illinois University Legendre s Equation October 13, 2016 1 / 10 The Laplacian in Spherical Coordinates The Laplacian is given
PHYS 500 - Southern Illinois University October 13, 2016 PHYS 500 - Southern Illinois University Legendre s Equation October 13, 2016 1 / 10 The Laplacian in Spherical Coordinates The Laplacian is given
Coarse-Grained Models!
 Coarse-Grained Models! Large and complex molecules (e.g. long polymers) can not be simulated on the all-atom level! Requires coarse-graining of the model! Coarse-grained models are usually also particles
Coarse-Grained Models! Large and complex molecules (e.g. long polymers) can not be simulated on the all-atom level! Requires coarse-graining of the model! Coarse-grained models are usually also particles
A Brief Outline of Math 355
 A Brief Outline of Math 355 Lecture 1 The geometry of linear equations; elimination with matrices A system of m linear equations with n unknowns can be thought of geometrically as m hyperplanes intersecting
A Brief Outline of Math 355 Lecture 1 The geometry of linear equations; elimination with matrices A system of m linear equations with n unknowns can be thought of geometrically as m hyperplanes intersecting
MTS 105 LECTURE 5: SEQUENCE AND SERIES 1.0 SEQUENCE
 MTS 105 LECTURE 5: SEQUENCE AND SERIES 1.0 SEQUENCE A sequence is an endless succession of numbers placed in a certain order so that there is a first number, a second and so on. Consider, for example,
MTS 105 LECTURE 5: SEQUENCE AND SERIES 1.0 SEQUENCE A sequence is an endless succession of numbers placed in a certain order so that there is a first number, a second and so on. Consider, for example,
Electrons in a periodic potential
 Chapter 3 Electrons in a periodic potential 3.1 Bloch s theorem. We consider in this chapter electrons under the influence of a static, periodic potential V (x), i.e. such that it fulfills V (x) = V (x
Chapter 3 Electrons in a periodic potential 3.1 Bloch s theorem. We consider in this chapter electrons under the influence of a static, periodic potential V (x), i.e. such that it fulfills V (x) = V (x
ζ (s) = s 1 s {u} [u] ζ (s) = s 0 u 1+sdu, {u} Note how the integral runs from 0 and not 1.
![ζ (s) = s 1 s {u} [u] ζ (s) = s 0 u 1+sdu, {u} Note how the integral runs from 0 and not 1. ζ (s) = s 1 s {u} [u] ζ (s) = s 0 u 1+sdu, {u} Note how the integral runs from 0 and not 1.](/thumbs/94/118787137.jpg) Problem Sheet 3. From Theorem 3. we have ζ (s) = + s s {u} u+sdu, (45) valid for Res > 0. i) Deduce that for Res >. [u] ζ (s) = s u +sdu ote the integral contains [u] in place of {u}. ii) Deduce that for
Problem Sheet 3. From Theorem 3. we have ζ (s) = + s s {u} u+sdu, (45) valid for Res > 0. i) Deduce that for Res >. [u] ζ (s) = s u +sdu ote the integral contains [u] in place of {u}. ii) Deduce that for
Infinite Series. 1 Introduction. 2 General discussion on convergence
 Infinite Series 1 Introduction I will only cover a few topics in this lecture, choosing to discuss those which I have used over the years. The text covers substantially more material and is available for
Infinite Series 1 Introduction I will only cover a few topics in this lecture, choosing to discuss those which I have used over the years. The text covers substantially more material and is available for
Green s Function for Problems with Layers
 Chapter 9 Green s Function for Problems with Layers 9. Continuity conditions The medium comprises two or more straight or curved layers. The properties of the medium are constant within each layer; they
Chapter 9 Green s Function for Problems with Layers 9. Continuity conditions The medium comprises two or more straight or curved layers. The properties of the medium are constant within each layer; they
4.2 Elastic and inelastic neutron scattering
 4.2 ELASTIC AD IELASTIC EUTRO SCATTERIG 73 4.2 Elastic and inelastic neutron scattering If the scattering system is assumed to be in thermal equilibrium at temperature T, the average over initial states
4.2 ELASTIC AD IELASTIC EUTRO SCATTERIG 73 4.2 Elastic and inelastic neutron scattering If the scattering system is assumed to be in thermal equilibrium at temperature T, the average over initial states
Solutions to Homework 2
 Solutions to Homewor Due Tuesday, July 6,. Chapter. Problem solution. If the series for ln+z and ln z both converge, +z then we can find the series for ln z by term-by-term subtraction of the two series:
Solutions to Homewor Due Tuesday, July 6,. Chapter. Problem solution. If the series for ln+z and ln z both converge, +z then we can find the series for ln z by term-by-term subtraction of the two series:
PHY 396 K. Solutions for problems 1 and 2 of set #5.
 PHY 396 K. Solutions for problems 1 and of set #5. Problem 1a: The conjugacy relations  k,  k,, Ê k, Ê k, follow from hermiticity of the Âx and Êx quantum fields and from the third eq. 6 for the polarization
PHY 396 K. Solutions for problems 1 and of set #5. Problem 1a: The conjugacy relations  k,  k,, Ê k, Ê k, follow from hermiticity of the Âx and Êx quantum fields and from the third eq. 6 for the polarization
Generalized sources (Sect. 6.5). The Dirac delta generalized function. Definition Consider the sequence of functions for n 1, Remarks:
 Generalized sources (Sect. 6.5). The Dirac delta generalized function. Definition Consider the sequence of functions for n, d n, t < δ n (t) = n, t 3 d3 d n, t > n. d t The Dirac delta generalized function
Generalized sources (Sect. 6.5). The Dirac delta generalized function. Definition Consider the sequence of functions for n, d n, t < δ n (t) = n, t 3 d3 d n, t > n. d t The Dirac delta generalized function
Chemical Bonding -- Lewis Theory (Chapter 9)
 Chemical Bonding -- Lewis Theory (Chapter 9) Ionic Bonding 1. Ionic Bond Electrostatic attraction of positive (cation) and negative (anion) ions Neutral Atoms e - transfer (IE and EA) cation + anion Ionic
Chemical Bonding -- Lewis Theory (Chapter 9) Ionic Bonding 1. Ionic Bond Electrostatic attraction of positive (cation) and negative (anion) ions Neutral Atoms e - transfer (IE and EA) cation + anion Ionic
LECTURE 10: REVIEW OF POWER SERIES. 1. Motivation
 LECTURE 10: REVIEW OF POWER SERIES By definition, a power series centered at x 0 is a series of the form where a 0, a 1,... and x 0 are constants. For convenience, we shall mostly be concerned with the
LECTURE 10: REVIEW OF POWER SERIES By definition, a power series centered at x 0 is a series of the form where a 0, a 1,... and x 0 are constants. For convenience, we shall mostly be concerned with the
Section 9.8. First let s get some practice with determining the interval of convergence of power series.
 First let s get some practice with determining the interval of convergence of power series. First let s get some practice with determining the interval of convergence of power series. Example (1) Determine
First let s get some practice with determining the interval of convergence of power series. First let s get some practice with determining the interval of convergence of power series. Example (1) Determine
The Prime Number Theorem
 The Prime Number Theorem We study the distribution of primes via the function π(x) = the number of primes x 6 5 4 3 2 2 3 4 5 6 7 8 9 0 2 3 4 5 2 It s easier to draw this way: π(x) = the number of primes
The Prime Number Theorem We study the distribution of primes via the function π(x) = the number of primes x 6 5 4 3 2 2 3 4 5 6 7 8 9 0 2 3 4 5 2 It s easier to draw this way: π(x) = the number of primes
Math review. Math review
 Math review 1 Math review 3 1 series approximations 3 Taylor s Theorem 3 Binomial approximation 3 sin(x), for x in radians and x close to zero 4 cos(x), for x in radians and x close to zero 5 2 some geometry
Math review 1 Math review 3 1 series approximations 3 Taylor s Theorem 3 Binomial approximation 3 sin(x), for x in radians and x close to zero 4 cos(x), for x in radians and x close to zero 5 2 some geometry
12.1 Arithmetic Progression Geometric Progression General things about sequences
 ENGR11 Engineering Mathematics Lecture Notes SMS, Victoria University of Wellington Week Five. 1.1 Arithmetic Progression An arithmetic progression is a sequence where each term is found by adding a fixed
ENGR11 Engineering Mathematics Lecture Notes SMS, Victoria University of Wellington Week Five. 1.1 Arithmetic Progression An arithmetic progression is a sequence where each term is found by adding a fixed
Math Homework 2
 Math 73 Homework Due: September 8, 6 Suppose that f is holomorphic in a region Ω, ie an open connected set Prove that in any of the following cases (a) R(f) is constant; (b) I(f) is constant; (c) f is
Math 73 Homework Due: September 8, 6 Suppose that f is holomorphic in a region Ω, ie an open connected set Prove that in any of the following cases (a) R(f) is constant; (b) I(f) is constant; (c) f is
Physics 221A Fall 1996 Notes 16 Bloch s Theorem and Band Structure in One Dimension
 Physics 221A Fall 1996 Notes 16 Bloch s Theorem and Band Structure in One Dimension In these notes we examine Bloch s theorem and band structure in problems with periodic potentials, as a part of our survey
Physics 221A Fall 1996 Notes 16 Bloch s Theorem and Band Structure in One Dimension In these notes we examine Bloch s theorem and band structure in problems with periodic potentials, as a part of our survey
UNIT I SOLID STATE PHYSICS
 UNIT I SOLID STATE PHYSICS CHAPTER 1 CRYSTAL STRUCTURE 1.1 INTRODUCTION When two atoms are brought together, two kinds of forces: attraction and repulsion come into play. The force of attraction increases
UNIT I SOLID STATE PHYSICS CHAPTER 1 CRYSTAL STRUCTURE 1.1 INTRODUCTION When two atoms are brought together, two kinds of forces: attraction and repulsion come into play. The force of attraction increases
Sums and Products. a i = a 1. i=1. a i = a i a n. n 1
 Sums and Products -27-209 In this section, I ll review the notation for sums and products Addition and multiplication are binary operations: They operate on two numbers at a time If you want to add or
Sums and Products -27-209 In this section, I ll review the notation for sums and products Addition and multiplication are binary operations: They operate on two numbers at a time If you want to add or
Notes for Expansions/Series and Differential Equations
 Notes for Expansions/Series and Differential Equations In the last discussion, we considered perturbation methods for constructing solutions/roots of algebraic equations. Three types of problems were illustrated
Notes for Expansions/Series and Differential Equations In the last discussion, we considered perturbation methods for constructing solutions/roots of algebraic equations. Three types of problems were illustrated
Chapter 2. X-ray X. Diffraction and Reciprocal Lattice. Scattering from Lattices
 Chapter. X-ray X Diffraction and Reciprocal Lattice Diffraction of waves by crystals Reciprocal Lattice Diffraction of X-rays Powder diffraction Single crystal X-ray diffraction Scattering from Lattices
Chapter. X-ray X Diffraction and Reciprocal Lattice Diffraction of waves by crystals Reciprocal Lattice Diffraction of X-rays Powder diffraction Single crystal X-ray diffraction Scattering from Lattices
Chapter 6 INORGANIC THERMODYNAMICS. Exercises
 Chapter 6 INORGANIC THERMODYNAMICS Exercises 6. (a) A reaction that occurs without external help, or a reaction for which G is negative. (b) A measure of disorder. (c) The enthalpy change when a mole of
Chapter 6 INORGANIC THERMODYNAMICS Exercises 6. (a) A reaction that occurs without external help, or a reaction for which G is negative. (b) A measure of disorder. (c) The enthalpy change when a mole of
Physics of Condensed Matter I
 Physics of Condensed Matter I 1100-4INZ`PC Solid State 1 Faculty of Physics UW Jacek.Szczytko@fuw.edu.pl Chemical bonding and molecules Born Oppenheimer approximation Max Born (1882-1970) Jacob R. Oppenheimer
Physics of Condensed Matter I 1100-4INZ`PC Solid State 1 Faculty of Physics UW Jacek.Szczytko@fuw.edu.pl Chemical bonding and molecules Born Oppenheimer approximation Max Born (1882-1970) Jacob R. Oppenheimer
Computer simulation methods (2) Dr. Vania Calandrini
 Computer simulation methods (2) Dr. Vania Calandrini in the previous lecture: time average versus ensemble average MC versus MD simulations equipartition theorem (=> computing T) virial theorem (=> computing
Computer simulation methods (2) Dr. Vania Calandrini in the previous lecture: time average versus ensemble average MC versus MD simulations equipartition theorem (=> computing T) virial theorem (=> computing
Chapter 8. Bonding : General Concepts Chemical Bondings
 Chapter 8. onding : General Concepts Chemical ondings create Diversity in the Universe Why and how do they make chemical bonds? and what do they make? Types of Chemical onds Chemical bonds: orces that
Chapter 8. onding : General Concepts Chemical ondings create Diversity in the Universe Why and how do they make chemical bonds? and what do they make? Types of Chemical onds Chemical bonds: orces that
Using Molecular Dynamics to Compute Properties CHEM 430
 Using Molecular Dynamics to Compute Properties CHEM 43 Heat Capacity and Energy Fluctuations Running an MD Simulation Equilibration Phase Before data-collection and results can be analyzed the system
Using Molecular Dynamics to Compute Properties CHEM 43 Heat Capacity and Energy Fluctuations Running an MD Simulation Equilibration Phase Before data-collection and results can be analyzed the system
Statistical Mechanics and Combinatorics : Lecture IV
 Statistical Mechanics and Combinatorics : Lecture IV Dimer Model Local Statistics We ve already discussed the partition function Z for dimer coverings in a graph G which can be computed by Kasteleyn matrix
Statistical Mechanics and Combinatorics : Lecture IV Dimer Model Local Statistics We ve already discussed the partition function Z for dimer coverings in a graph G which can be computed by Kasteleyn matrix
MP464: Solid State Physics Problem Sheet
 MP464: Solid State Physics Problem Sheet 1) Write down primitive lattice vectors for the -dimensional rectangular lattice, with sides a and b in the x and y-directions respectively, and a face-centred
MP464: Solid State Physics Problem Sheet 1) Write down primitive lattice vectors for the -dimensional rectangular lattice, with sides a and b in the x and y-directions respectively, and a face-centred
Power series solutions for 2nd order linear ODE s (not necessarily with constant coefficients) a n z n. n=0
 Lecture 22 Power series solutions for 2nd order linear ODE s (not necessarily with constant coefficients) Recall a few facts about power series: a n z n This series in z is centered at z 0. Here z can
Lecture 22 Power series solutions for 2nd order linear ODE s (not necessarily with constant coefficients) Recall a few facts about power series: a n z n This series in z is centered at z 0. Here z can
Review (2) Calculus II (201-nyb-05/05,06) Winter 2019
 Review () Calculus II (-nyb-5/5,6) Winter 9 Note. You should also review the integrals eercises on the first review sheet. Eercise. Evaluate each of the following integrals. a. sin 3 ( )cos ( csc 3 (log)cot
Review () Calculus II (-nyb-5/5,6) Winter 9 Note. You should also review the integrals eercises on the first review sheet. Eercise. Evaluate each of the following integrals. a. sin 3 ( )cos ( csc 3 (log)cot
Fourier transforms, Generalised functions and Greens functions
 Fourier transforms, Generalised functions and Greens functions T. Johnson 2015-01-23 Electromagnetic Processes In Dispersive Media, Lecture 2 - T. Johnson 1 Motivation A big part of this course concerns
Fourier transforms, Generalised functions and Greens functions T. Johnson 2015-01-23 Electromagnetic Processes In Dispersive Media, Lecture 2 - T. Johnson 1 Motivation A big part of this course concerns
Metallic and Ionic Structures and Bonding
 Metallic and Ionic Structures and Bonding Ionic compounds are formed between elements having an electronegativity difference of about 2.0 or greater. Simple ionic compounds are characterized by high melting
Metallic and Ionic Structures and Bonding Ionic compounds are formed between elements having an electronegativity difference of about 2.0 or greater. Simple ionic compounds are characterized by high melting
5. Orthogonal matrices
 L Vandenberghe EE133A (Spring 2017) 5 Orthogonal matrices matrices with orthonormal columns orthogonal matrices tall matrices with orthonormal columns complex matrices with orthonormal columns 5-1 Orthonormal
L Vandenberghe EE133A (Spring 2017) 5 Orthogonal matrices matrices with orthonormal columns orthogonal matrices tall matrices with orthonormal columns complex matrices with orthonormal columns 5-1 Orthonormal
13 Maximum Modulus Principle
 3 Maximum Modulus Principle Theorem 3. (maximum modulus principle). If f is non-constant and analytic on an open connected set Ω, then there is no point z 0 Ω such that f(z) f(z 0 ) for all z Ω. Remark
3 Maximum Modulus Principle Theorem 3. (maximum modulus principle). If f is non-constant and analytic on an open connected set Ω, then there is no point z 0 Ω such that f(z) f(z 0 ) for all z Ω. Remark
