Entropy ISSN
|
|
|
- Merryl Riley
- 6 years ago
- Views:
Transcription
1 Entropy 2005 Entropy ISSN Short Note Light at ambient temperature in violation of the Kelvin-Planck Statement of the Second Law? Thomas V. Prevenslik 3F Mountain View, Discovery Bay, Hong Kong. Received: Abstract: The Kelvin-Planck statement of the second law of thermodynamics precludes the extraction of work from a single thermal reservoir. Very early Carnot showed the efficiency of a heat engine vanishes if operated between reservoirs at the same temperature, or that work cannot be extracted from a single reservoir. Recent attempts to extract work from a single reservoir have relied on exotic quantum Carnot and photon steam engines, but require microwave energy to raise the efficiency of the work extracted above the classical Carnot limit. But exotic quantum engines need not be invoked to extract work from a single reservoir provided a heat engine is devise d that produces light as the temperature is lowered to absolute zero. Since means may be devised for the light to lift a weight, and since the temperature approaches absolute zero, work is extracted from a single reservoir in violation of the Kelvin-Planck statement. Rather than performing work, the process finds utility by producing light from the environment at ambient temperature suggesting the Kelvin-Planck statement may need to be refined. Keywords: Second law, Kelvin-Planck statement, suppressed IR,, frequency up-conversion Introduction Traditional statements of the second law [1] include the impossibility of extracting work W from a single reservoir. In the Kelvin-Planck statement,
2 It is impossible to construct a device that operates in a cycle and produces no other effect than the production of work W and exchange of heat with a single reservoir. But the Kelvin-Planck statement is but one of more than half dozen [2] common statements of the second law dating back to Carnot 180 years ago. In 1824, Carnot showed the efficiency η of a classical engine (CE) operating between a high TH and low TC temperature reservoirs, TC η = 1 (1) T For the CE supplied by heat from a reservoir and rejecting heat to the same reservoir, the temperatures T H = T C and η= 0, and therefore consistent with the Kelvin-Planck statement the conversion of heat from a single reservoir into work is not possible [3]. However, this limitation may be avoided by a CE converting heat from a reservoir at high temperature T H reservoir and rejecting heat to a reservoir having T C at absolute zero ha ving an efficiency η = 1. Recently, work is proposed [3] extracted from a single reservoir using a quantum engine (E). The working fluid in the E is photons that produce radiation pressure in an optical having mirror ends. One mirror functions as the face of a piston while the other is fixed in thermal contact with a reservoir at temperature T C. Atoms flowing through the optical at temperature T H form the other thermal reservoir that exchanges heat with the photons. If the atoms flowing in the optical are 2-state systems that emit and absorb radiation at the same frequency, then the efficiency of the E and CE are the same. But if the atoms are 3-state systems, the lower states may be made coherent by providing microwave energy in the optical. The E efficiency η is, TC η = η nε cosφ (2) T where, ηis the CE efficiency, φ is the phase difference between the lowest atom energy states, ε is a small number that characterizes the magnitude of the quantum coherence, and n is the average number of thermal photons in the mode of length L, 1 n = (3) hc exp 1 λkth where, λ = 2L. By selecting the microwave energy to produce a phase difference φ = π, Equation (2) shows that even for a single reservoir where T H = T C the E efficiency η exceeds the CE efficienc y η. Although the E improves the CE efficiency, the improvement is not significant. Typically, the E efficiency may improve the CE by a few percent, e.g., for η ~ 0.3 the η ~ An alternate approach is to devise a process that uses a CE to extract work from a single reservoir by spontaneously lowering the temperature T C of the low temperature reservoir to absolute zero. One such process for improving the efficiency of a CE is to extract work W from a single reservoir by producing vacuum ultraviolet (VUV) radiation in the suppression of IR radiation from the surface atoms in a quantum electrodynamics () shown in Figure 1. H H 2
3 Tamb Heat Engine W VUV Kelvin-Planck Surface Atoms at absolute zero Light at Ambient Figure 1 Kelvin-Planck Statement and Light at Ambient In order for the process to have utility, the VUV radiation is used to excite a fluorophore in the surface to produce visible light. In Figure 1, work W from a single reservoir in the Kelvin-Planck statement is contrasted with the production of VUV radiation in a from a single reservoir comprising the environment at ambient temperature. Since the VUV by some means can be devised to lift a weight, the VUV is equivalent to work W. Here, the surface atoms serve as the medium in the CE producing visible light. Since there is no difference between the production of VUV and work W from a single reservoir, it may appear that the VUV radiation from the ambient reservoir is precluded by the Kelvin-Planck statement of the second law. But this is not true. Analysis The production of VUV by the suppression of IR radiation leaves the surface atoms without kt energy, and therefore the temperature of the atoms spontaneously tends to absolute zero. Consistent with the second law, the surface atoms at absolute zero recover ambient temperature as heat from the ambient spontaneously flows into the surface atoms shown in Figures 2 and 3. Ambient VUV Surface Atoms Absolute zero Figure 2 Light at Ambient 3
4 Surface Atoms hυ (a) Ambient = 0 (b) Suppression of IR radiation from Surface Atoms VUV Photon emission < 0 (c) Surface atoms at Absolute zero > 0 T = 0 (d) Ambient Recovery = 0 Figure 3 Light at Ambient Initially, the of radius R is assumed formed in a solid or liquid continuum as shown in Figure 3(a). The has resonant wavelength λ = 4R. By selecting the radius R ~ 0.05 microns, the is resonant in the VUV having wavelength λ ~ 0.2 microns. The temperatures are ambient everywhere, and therefore the atoms in the surface tend to emit IR radiation. But long wavelength IR radiation is suppressed in the VUV resonant as shown in Figure 3(b). To conserve the loss of IR energy, the EM energy is gained at the resonant frequency of the, as all lower frequencies are inadmissible. Photons at VUV frequencies are produced, as depicted by the emission of photon hυ. The ambient reservoir loses heat < 0. Once the thermal kt energy of the surface atoms is depleted, the VUV radiation vanishes and the temperature of the surface atoms tends to absolute zero as shown in Figure 3(c). But heat flows spontaneously by conduction from the ambient surroundings at temperature Tamb into the surface atoms at absolute zero. The surface atoms gain heat > 0. The VUV radiation finds utility by exciting a fluorophore provided in the surface of the to produce a continuous source of VIS light. Recovery of ambient temperature by surface atoms through conductive heat flow from the surroundings is only required to be faster than the lifetime of the fluorophore. After ambient temperature is fully recovered, the heat = 0. Since Figure 3(d) is equivalent to Figure 3(a), the thermal kt energy of the surface atoms is recovered, and the cycle is repeated to produce steady light at ambient temperature. Conclusion Work in the form of VIS light is spontaneously extracted by a CE from a single reservoir comprising the environment at ambient temperature by suppressing the IR radiation from atoms in the surface of a resonant in the VUV. Exotic E are not required. Although consistent with the second law in that heat can only flow from the ambient to absolute zero, the Kelvin-Planck statement may need to be refined to accommodate the suppression of IR radiation in a. 4
5 References 1. Duncan, T. L., Semura, J. S. The Deep Physics Behind the Second Law : Information and Energy as Independent Forms of Bookkepping. Entropy, 2004, 6, Nikulov, A., Sheehan, D. The Second Law Mystique, Entropy, 6, Scully, M. O., Zubairy, M. S., Agarwal, G. S., and Walther, H. Extracting Work from a Single Heat Bath via Vanishing uantum Coherence, Science (2003) by MDPI ( Reproduction for noncommercial purposes permitted. 5
Photon steam engines. Work can be extracted from a single heat bath at the boundary between classical and quantum thermodynamics
 Photon steam engines Work can be extracted from a single heat bath at the boundary between classical and quantum thermodynamics A quantum Carnot engine. Hot atoms flow from a heat bath at temperature T
Photon steam engines Work can be extracted from a single heat bath at the boundary between classical and quantum thermodynamics A quantum Carnot engine. Hot atoms flow from a heat bath at temperature T
ULTRASONIC IONIZATION SPECTROSCOPY ABSTRACT
 ULTRASONIC IONIZATION SPECTROSCOPY Thomas Prevenslik Consultant 15 H, Greenwood Court, Discovery Bay, Hong Kong E-mail: micronenergy@yahoo.com Christopher Beling, Chor Keung Cheung Department of Physics
ULTRASONIC IONIZATION SPECTROSCOPY Thomas Prevenslik Consultant 15 H, Greenwood Court, Discovery Bay, Hong Kong E-mail: micronenergy@yahoo.com Christopher Beling, Chor Keung Cheung Department of Physics
Blackbody Radiation in Microscopic Gaps
 Asian J. Energy Environ., Vol. 5, Issue 2, (2004), pp. 8-97 Blackbody Radiation in Microscopic Gaps 14 B, Brilliance Court Discovery Bay, Hong Kong Email: bibliotec2002@yahoo.com (Received : 1 January
Asian J. Energy Environ., Vol. 5, Issue 2, (2004), pp. 8-97 Blackbody Radiation in Microscopic Gaps 14 B, Brilliance Court Discovery Bay, Hong Kong Email: bibliotec2002@yahoo.com (Received : 1 January
Wavelength λ Velocity v. Electric Field Strength Amplitude A. Time t or Distance x time for 1 λ to pass fixed point. # of λ passing per s ν= 1 p
 Introduction to Spectroscopy (Chapter 6) Electromagnetic radiation (wave) description: Wavelength λ Velocity v Electric Field Strength 0 Amplitude A Time t or Distance x Period p Frequency ν time for 1
Introduction to Spectroscopy (Chapter 6) Electromagnetic radiation (wave) description: Wavelength λ Velocity v Electric Field Strength 0 Amplitude A Time t or Distance x Period p Frequency ν time for 1
Proceedings of the ASME th Micro/Nanoscale Heat & Mass Transfer International Conference MNHMT2013 December 11-14, 2013, Hong Kong, China
 Proceedings of the ASME 2013 4th Micro/Nanoscale Heat & Mass Transfer International Conference MNHMT2013 December 11-14, 2013, Hong Kong, China MNHMT2013-22030 THE FOURIER LAW AT MACRO AND NANOSCALES Thomas
Proceedings of the ASME 2013 4th Micro/Nanoscale Heat & Mass Transfer International Conference MNHMT2013 December 11-14, 2013, Hong Kong, China MNHMT2013-22030 THE FOURIER LAW AT MACRO AND NANOSCALES Thomas
October 18, 2011 Carnot cycle - 1
 Carnot Cycle In 1824, Sadi Carnot (1796-1832) published a short book, eflections on the Motive Power of Fire (The book is now free online You should try it out) To construct an engine, Carnot noted, at
Carnot Cycle In 1824, Sadi Carnot (1796-1832) published a short book, eflections on the Motive Power of Fire (The book is now free online You should try it out) To construct an engine, Carnot noted, at
Write the electron configuration for Chromium (Cr):
 Write the electron configuration for Chromium (Cr): Energy level Aufbau Principle Atomic orbital Quantum Hund s Rule Atomic number Electron Configuration Whole number Pauli Exlcusion Principle Quantum
Write the electron configuration for Chromium (Cr): Energy level Aufbau Principle Atomic orbital Quantum Hund s Rule Atomic number Electron Configuration Whole number Pauli Exlcusion Principle Quantum
The Kelvin-Planck statement of the second law
 The Kelvin-Planck statement of the second law It is impossible for any device that operates on a cycle to receive heat from a single reservoir and produce a net amount of work Q W E =ΔE net net net, mass
The Kelvin-Planck statement of the second law It is impossible for any device that operates on a cycle to receive heat from a single reservoir and produce a net amount of work Q W E =ΔE net net net, mass
ATS150 Global Climate Change Spring 2019 Candidate Questions for Exam #1
 1. How old is the Earth? About how long ago did it form? 2. What are the two most common gases in the atmosphere? What percentage of the atmosphere s molecules are made of each gas? 3. About what fraction
1. How old is the Earth? About how long ago did it form? 2. What are the two most common gases in the atmosphere? What percentage of the atmosphere s molecules are made of each gas? 3. About what fraction
Atomic Spectroscopy and Energy Levels
 Activity 18 Atomic Spectroscopy and Energy Levels Why? The emission of light by the hydrogen atom and other atoms played a key role in helping scientists to understand the electronic structure of atoms.
Activity 18 Atomic Spectroscopy and Energy Levels Why? The emission of light by the hydrogen atom and other atoms played a key role in helping scientists to understand the electronic structure of atoms.
22. Lasers. Stimulated Emission: Gain. Population Inversion. Rate equation analysis. Two-level, three-level, and four-level systems
 . Lasers Stimulated Emission: Gain Population Inversion Rate equation analysis Two-level, three-level, and four-level systems What is a laser? LASER: Light Amplification by Stimulated Emission of Radiation
. Lasers Stimulated Emission: Gain Population Inversion Rate equation analysis Two-level, three-level, and four-level systems What is a laser? LASER: Light Amplification by Stimulated Emission of Radiation
Correlated Emission Laser, Quenching Of Spontaneous Noise and Coupled Pendulum Analogy
 RESEARCH INVENTY: International Journal of Engineering and Science ISBN: 2319-6483, ISSN: 2278-4721, Vol. 2, Issue 1 (January 2013), PP 11-15 www.researchinventy.com Correlated Emission Laser, Quenching
RESEARCH INVENTY: International Journal of Engineering and Science ISBN: 2319-6483, ISSN: 2278-4721, Vol. 2, Issue 1 (January 2013), PP 11-15 www.researchinventy.com Correlated Emission Laser, Quenching
Bohr. Electronic Structure. Spectroscope. Spectroscope
 Bohr Electronic Structure Bohr proposed that the atom has only certain allowable energy states. Spectroscope Using a device called a it was found that gaseous elements emitted electromagnetic radiation
Bohr Electronic Structure Bohr proposed that the atom has only certain allowable energy states. Spectroscope Using a device called a it was found that gaseous elements emitted electromagnetic radiation
Reversibility, Irreversibility and Carnot cycle. Irreversible Processes. Reversible Processes. Carnot Cycle
 Reversibility, Irreversibility and Carnot cycle The second law of thermodynamics distinguishes between reversible and irreversible processes. If a process can proceed in either direction without violating
Reversibility, Irreversibility and Carnot cycle The second law of thermodynamics distinguishes between reversible and irreversible processes. If a process can proceed in either direction without violating
The energy of the emitted light (photons) is given by the difference in energy between the initial and final states of hydrogen atom.
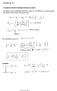 Lecture 20-21 Page 1 Lectures 20-21 Transitions between hydrogen stationary states The energy of the emitted light (photons) is given by the difference in energy between the initial and final states of
Lecture 20-21 Page 1 Lectures 20-21 Transitions between hydrogen stationary states The energy of the emitted light (photons) is given by the difference in energy between the initial and final states of
R O Y G B V. Spin States. Outer Shell Electrons. Molecular Rotations. Inner Shell Electrons. Molecular Vibrations. Nuclear Transitions
 Spin States Molecular Rotations Molecular Vibrations Outer Shell Electrons Inner Shell Electrons Nuclear Transitions NMR EPR Microwave Absorption Spectroscopy Infrared Absorption Spectroscopy UV-vis Absorption,
Spin States Molecular Rotations Molecular Vibrations Outer Shell Electrons Inner Shell Electrons Nuclear Transitions NMR EPR Microwave Absorption Spectroscopy Infrared Absorption Spectroscopy UV-vis Absorption,
Chapter 13. Phys 322 Lecture 34. Modern optics
 Chapter 13 Phys 3 Lecture 34 Modern optics Blackbodies and Lasers* Blackbodies Stimulated Emission Gain and Inversion The Laser Four-level System Threshold Some lasers Pump Fast decay Laser Fast decay
Chapter 13 Phys 3 Lecture 34 Modern optics Blackbodies and Lasers* Blackbodies Stimulated Emission Gain and Inversion The Laser Four-level System Threshold Some lasers Pump Fast decay Laser Fast decay
OPTICAL GAIN AND LASERS
 OPTICAL GAIN AND LASERS 01-02-1 BY DAVID ROCKWELL DIRECTOR, RESEARCH & DEVELOPMENT fsona COMMUNICATIONS MARCH 6, 2001 OUTLINE 01-02-2 I. DEFINITIONS, BASIC CONCEPTS II. III. IV. OPTICAL GAIN AND ABSORPTION
OPTICAL GAIN AND LASERS 01-02-1 BY DAVID ROCKWELL DIRECTOR, RESEARCH & DEVELOPMENT fsona COMMUNICATIONS MARCH 6, 2001 OUTLINE 01-02-2 I. DEFINITIONS, BASIC CONCEPTS II. III. IV. OPTICAL GAIN AND ABSORPTION
NOTES: 5.3 Light and Atomic Spectra (more Quantum Mechanics!)
 NOTES: 5.3 Light and Atomic Spectra (more Quantum Mechanics!) Light WAVE or PARTICLE? Electromagnetic Radiation Electromagnetic radiation includes: -radio waves -microwaves -infrared waves -visible light
NOTES: 5.3 Light and Atomic Spectra (more Quantum Mechanics!) Light WAVE or PARTICLE? Electromagnetic Radiation Electromagnetic radiation includes: -radio waves -microwaves -infrared waves -visible light
What Makes a Laser Light Amplification by Stimulated Emission of Radiation Main Requirements of the Laser Laser Gain Medium (provides the light
 What Makes a Laser Light Amplification by Stimulated Emission of Radiation Main Requirements of the Laser Laser Gain Medium (provides the light amplification) Optical Resonator Cavity (greatly increase
What Makes a Laser Light Amplification by Stimulated Emission of Radiation Main Requirements of the Laser Laser Gain Medium (provides the light amplification) Optical Resonator Cavity (greatly increase
T s change via collisions at boundary (not mechanical interaction)
 Lecture 14 Interaction of 2 systems at different temperatures Irreversible processes: 2nd Law of Thermodynamics Chapter 19: Heat Engines and Refrigerators Thermal interactions T s change via collisions
Lecture 14 Interaction of 2 systems at different temperatures Irreversible processes: 2nd Law of Thermodynamics Chapter 19: Heat Engines and Refrigerators Thermal interactions T s change via collisions
External (differential) quantum efficiency Number of additional photons emitted / number of additional electrons injected
 Semiconductor Lasers Comparison with LEDs The light emitted by a laser is generally more directional, more intense and has a narrower frequency distribution than light from an LED. The external efficiency
Semiconductor Lasers Comparison with LEDs The light emitted by a laser is generally more directional, more intense and has a narrower frequency distribution than light from an LED. The external efficiency
1) Introduction 2) Photo electric effect 3) Dual nature of matter 4) Bohr s atom model 5) LASERS
 1) Introduction 2) Photo electric effect 3) Dual nature of matter 4) Bohr s atom model 5) LASERS 1. Introduction Types of electron emission, Dunnington s method, different types of spectra, Fraunhoffer
1) Introduction 2) Photo electric effect 3) Dual nature of matter 4) Bohr s atom model 5) LASERS 1. Introduction Types of electron emission, Dunnington s method, different types of spectra, Fraunhoffer
Einstein s Approach to Planck s Law
 Supplement -A Einstein s Approach to Planck s Law In 97 Albert Einstein wrote a remarkable paper in which he used classical statistical mechanics and elements of the old Bohr theory to derive the Planck
Supplement -A Einstein s Approach to Planck s Law In 97 Albert Einstein wrote a remarkable paper in which he used classical statistical mechanics and elements of the old Bohr theory to derive the Planck
Theory of optically thin emission line spectroscopy
 Theory of optically thin emission line spectroscopy 1 Important definitions In general the spectrum of a source consists of a continuum and several line components. Processes which give raise to the continuous
Theory of optically thin emission line spectroscopy 1 Important definitions In general the spectrum of a source consists of a continuum and several line components. Processes which give raise to the continuous
Optical Spectroscopy 1 1. Absorption spectroscopy (UV/vis)
 Optical Spectroscopy 1 1. Absorption spectroscopy (UV/vis) 2 2. Circular dichroism (optical activity) CD / ORD 3 3. Fluorescence spectroscopy and energy transfer Electromagnetic Spectrum Electronic Molecular
Optical Spectroscopy 1 1. Absorption spectroscopy (UV/vis) 2 2. Circular dichroism (optical activity) CD / ORD 3 3. Fluorescence spectroscopy and energy transfer Electromagnetic Spectrum Electronic Molecular
Chapter 5 Electrons In Atoms
 Chapter 5 Electrons In Atoms 5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms 5.3 Atomic Emission Spectra and the Quantum Mechanical Model 1 Copyright Pearson Education, Inc., or its affiliates.
Chapter 5 Electrons In Atoms 5.1 Revising the Atomic Model 5.2 Electron Arrangement in Atoms 5.3 Atomic Emission Spectra and the Quantum Mechanical Model 1 Copyright Pearson Education, Inc., or its affiliates.
CARNOT CYCLE = T = S ( U,V )
 hermodynamics CANO CYCE Do not trouble students with history In 1824, Sadi Carnot (1796-1832) published a short book, eflections on the Motive Power of Fire (he book is now free online You should try it
hermodynamics CANO CYCE Do not trouble students with history In 1824, Sadi Carnot (1796-1832) published a short book, eflections on the Motive Power of Fire (he book is now free online You should try it
Do Now: Bohr Diagram, Lewis Structures, Valence Electrons 1. What is the maximum number of electrons you can fit in each shell?
 Chemistry Ms. Ye Name Date Block Do Now: Bohr Diagram, Lewis Structures, Valence Electrons 1. What is the maximum number of electrons you can fit in each shell? 1 st shell 2 nd shell 3 rd shell 4 th shell
Chemistry Ms. Ye Name Date Block Do Now: Bohr Diagram, Lewis Structures, Valence Electrons 1. What is the maximum number of electrons you can fit in each shell? 1 st shell 2 nd shell 3 rd shell 4 th shell
Physics and the Quantum Mechanical Model
 chemistry 1 of 38 Mechanical Model Neon advertising signs are formed from glass tubes bent in various shapes. An electric current passing through the gas in each glass tube makes the gas glow with its
chemistry 1 of 38 Mechanical Model Neon advertising signs are formed from glass tubes bent in various shapes. An electric current passing through the gas in each glass tube makes the gas glow with its
Reversible Processes. Furthermore, there must be no friction (i.e. mechanical energy loss) or turbulence i.e. it must be infinitely slow.
 Reversible Processes A reversible thermodynamic process is one in which the universe (i.e. the system and its surroundings) can be returned to their initial conditions. Because heat only flows spontaneously
Reversible Processes A reversible thermodynamic process is one in which the universe (i.e. the system and its surroundings) can be returned to their initial conditions. Because heat only flows spontaneously
Classical Approach to 2 nd Law for CM
 Classical Approach to 2 nd aw for CM Start with observations about the ability to build devices (thermodynamic cycles) Clausius Statement of 2 nd aw concerns cycles that cause heat transfer from low temperature
Classical Approach to 2 nd aw for CM Start with observations about the ability to build devices (thermodynamic cycles) Clausius Statement of 2 nd aw concerns cycles that cause heat transfer from low temperature
Skoog Chapter 6 Introduction to Spectrometric Methods
 Skoog Chapter 6 Introduction to Spectrometric Methods General Properties of Electromagnetic Radiation (EM) Wave Properties of EM Quantum Mechanical Properties of EM Quantitative Aspects of Spectrochemical
Skoog Chapter 6 Introduction to Spectrometric Methods General Properties of Electromagnetic Radiation (EM) Wave Properties of EM Quantum Mechanical Properties of EM Quantitative Aspects of Spectrochemical
Information in Radio Waves
 Summative Assessment: Natural Sources of Radio Performance expectation: Develop and use a model of two objects interacting through electric or magnetic fields to illustrate the forces between objects and
Summative Assessment: Natural Sources of Radio Performance expectation: Develop and use a model of two objects interacting through electric or magnetic fields to illustrate the forces between objects and
Chapter 1. From Classical to Quantum Mechanics
 Chapter 1. From Classical to Quantum Mechanics Classical Mechanics (Newton): It describes the motion of a classical particle (discrete object). dp F ma, p = m = dt dx m dt F: force (N) a: acceleration
Chapter 1. From Classical to Quantum Mechanics Classical Mechanics (Newton): It describes the motion of a classical particle (discrete object). dp F ma, p = m = dt dx m dt F: force (N) a: acceleration
5.3. Physics and the Quantum Mechanical Model
 Chemistry 5-3 Physics and the Quantum Mechanical Model Neon advertising signs are formed from glass tubes bent in various shapes. An electric current passing through the gas in each glass tube makes the
Chemistry 5-3 Physics and the Quantum Mechanical Model Neon advertising signs are formed from glass tubes bent in various shapes. An electric current passing through the gas in each glass tube makes the
Electromagnetic Radiation. Physical Principles of Remote Sensing
 Electromagnetic Radiation Physical Principles of Remote Sensing Outline for 4/3/2003 Properties of electromagnetic radiation The electromagnetic spectrum Spectral emissivity Radiant temperature vs. kinematic
Electromagnetic Radiation Physical Principles of Remote Sensing Outline for 4/3/2003 Properties of electromagnetic radiation The electromagnetic spectrum Spectral emissivity Radiant temperature vs. kinematic
Observation of Atomic Spectra
 Observation of Atomic Spectra Introduction In this experiment you will observe and measure the wavelengths of different colors of light emitted by atoms. You will first observe light emitted from excited
Observation of Atomic Spectra Introduction In this experiment you will observe and measure the wavelengths of different colors of light emitted by atoms. You will first observe light emitted from excited
aka Light Properties of Light are simultaneously
 Today Interaction of Light with Matter Thermal Radiation Kirchhoff s Laws aka Light Properties of Light are simultaneously wave-like AND particle-like Sometimes it behaves like ripples on a pond (waves).
Today Interaction of Light with Matter Thermal Radiation Kirchhoff s Laws aka Light Properties of Light are simultaneously wave-like AND particle-like Sometimes it behaves like ripples on a pond (waves).
QM all started with - - The Spectrum of Blackbody Radiation
 QM all started with - - The Spectrum of Blackbody Radiation Thermal Radiation: Any object, not at zero temperature, emits electromagnetic called thermal. When we measure the intensity of a real object,
QM all started with - - The Spectrum of Blackbody Radiation Thermal Radiation: Any object, not at zero temperature, emits electromagnetic called thermal. When we measure the intensity of a real object,
Chapter 7 Atomic Structure -1 Quantum Model of Atom. Dr. Sapna Gupta
 Chapter 7 Atomic Structure -1 Quantum Model of Atom Dr. Sapna Gupta The Electromagnetic Spectrum The electromagnetic spectrum includes many different types of radiation which travel in waves. Visible light
Chapter 7 Atomic Structure -1 Quantum Model of Atom Dr. Sapna Gupta The Electromagnetic Spectrum The electromagnetic spectrum includes many different types of radiation which travel in waves. Visible light
Chapter 39. Particles Behaving as Waves
 Chapter 39 Particles Behaving as Waves 39.1 Electron Waves Light has a dual nature. Light exhibits both wave and particle characteristics. Louis de Broglie postulated in 1924 that if nature is symmetric,
Chapter 39 Particles Behaving as Waves 39.1 Electron Waves Light has a dual nature. Light exhibits both wave and particle characteristics. Louis de Broglie postulated in 1924 that if nature is symmetric,
Quantum Optomechanical Heat Engine
 Quantum Optomechanical Heat Engine ~Ando-Lab Seminar on Sep. 2nd~ Kentaro Komori 1 Optomechanics Thermodynamics 2 Contents Ø What is the heat engine? Ø Hamiltonian and polaritons Ø Otto cycle Ø Work and
Quantum Optomechanical Heat Engine ~Ando-Lab Seminar on Sep. 2nd~ Kentaro Komori 1 Optomechanics Thermodynamics 2 Contents Ø What is the heat engine? Ø Hamiltonian and polaritons Ø Otto cycle Ø Work and
Thermal Noise Driven Heat Engines
 The important thing is not to stop questioning. uriosity has its own reason for existing. (Albert Einstein) Thermal Noise Driven Heat Engines L.B. Kish Department of Electrical and omputer Engineering,
The important thing is not to stop questioning. uriosity has its own reason for existing. (Albert Einstein) Thermal Noise Driven Heat Engines L.B. Kish Department of Electrical and omputer Engineering,
Applied Thermodynamics. Gas Power Cycles
 Applied Thermodynamics Gas Power Cycles By: Mohd Yusof Taib Faculty of Mechanical Engineering myusof@ump.edu.my Chapter Description Aims To identify and recognized ideal thermodynamics cycle. To analyze
Applied Thermodynamics Gas Power Cycles By: Mohd Yusof Taib Faculty of Mechanical Engineering myusof@ump.edu.my Chapter Description Aims To identify and recognized ideal thermodynamics cycle. To analyze
Higher -o-o-o- Past Paper questions o-o-o- 3.4 Spectra
 Higher -o-o-o- Past Paper questions 1991-2010 -o-o-o- 3.4 Spectra 1992 Q37 The diagram below shows the energy levels for the hydrogen atom. (a) Between which two energy levels would an electron transition
Higher -o-o-o- Past Paper questions 1991-2010 -o-o-o- 3.4 Spectra 1992 Q37 The diagram below shows the energy levels for the hydrogen atom. (a) Between which two energy levels would an electron transition
What are Lasers? Light Amplification by Stimulated Emission of Radiation LASER Light emitted at very narrow wavelength bands (monochromatic) Light
 What are Lasers? What are Lasers? Light Amplification by Stimulated Emission of Radiation LASER Light emitted at very narrow wavelength bands (monochromatic) Light emitted in a directed beam Light is coherenent
What are Lasers? What are Lasers? Light Amplification by Stimulated Emission of Radiation LASER Light emitted at very narrow wavelength bands (monochromatic) Light emitted in a directed beam Light is coherenent
ATMO/OPTI 656b Spring 2009
 Nomenclature and Definition of Radiation Quantities The various Radiation Quantities are defined in Table 2-1. Keeping them straight is difficult and the meanings may vary from textbook to textbook. I
Nomenclature and Definition of Radiation Quantities The various Radiation Quantities are defined in Table 2-1. Keeping them straight is difficult and the meanings may vary from textbook to textbook. I
Radiation in the Earth's Atmosphere. Part 1: Absorption and Emission by Atmospheric Gases
 Radiation in the Earth's Atmosphere Part 1: Absorption and Emission by Atmospheric Gases Electromagnetic Waves Electromagnetic waves are transversal. Electric and magnetic fields are perpendicular. In
Radiation in the Earth's Atmosphere Part 1: Absorption and Emission by Atmospheric Gases Electromagnetic Waves Electromagnetic waves are transversal. Electric and magnetic fields are perpendicular. In
Thermodynamics and Atomic Physics II
 Thermodynamics and Atomic Physics II 1. Heat from a source at 550 K is added to the working fluid of an engine operating at a steady rate. The temperature of the surroundings is 300 K. The efficiency of
Thermodynamics and Atomic Physics II 1. Heat from a source at 550 K is added to the working fluid of an engine operating at a steady rate. The temperature of the surroundings is 300 K. The efficiency of
8 Wavefunctions - Schrödinger s Equation
 8 Wavefunctions - Schrödinger s Equation So far we have considered only free particles - i.e. particles whose energy consists entirely of its kinetic energy. In general, however, a particle moves under
8 Wavefunctions - Schrödinger s Equation So far we have considered only free particles - i.e. particles whose energy consists entirely of its kinetic energy. In general, however, a particle moves under
Basic thermodynamics. heat to the high temperature reservoir.
 Consider a heat engine that is operating in a cyclic process takes heat (QH) from a high temperature reservoir & converts completely into work (W), violating the Kelvin Planck statement. Let the work W,
Consider a heat engine that is operating in a cyclic process takes heat (QH) from a high temperature reservoir & converts completely into work (W), violating the Kelvin Planck statement. Let the work W,
Laser Types Two main types depending on time operation Continuous Wave (CW) Pulsed operation Pulsed is easier, CW more useful
 What Makes a Laser Light Amplification by Stimulated Emission of Radiation Main Requirements of the Laser Laser Gain Medium (provides the light amplification) Optical Resonator Cavity (greatly increase
What Makes a Laser Light Amplification by Stimulated Emission of Radiation Main Requirements of the Laser Laser Gain Medium (provides the light amplification) Optical Resonator Cavity (greatly increase
Laserphysik. Prof. Yong Lei & Dr. Yang Xu. Fachgebiet Angewandte Nanophysik, Institut für Physik
 Laserphysik Prof. Yong Lei & Dr. Yang Xu Fachgebiet Angewandte Nanophysik, Institut für Physik Contact: yong.lei@tu-ilmenau.de; yang.xu@tu-ilmenau.de Office: Heisenbergbau V 202, Unterpörlitzer Straße
Laserphysik Prof. Yong Lei & Dr. Yang Xu Fachgebiet Angewandte Nanophysik, Institut für Physik Contact: yong.lei@tu-ilmenau.de; yang.xu@tu-ilmenau.de Office: Heisenbergbau V 202, Unterpörlitzer Straße
Chapters 31 Atomic Physics
 Chapters 31 Atomic Physics 1 Overview of Chapter 31 Early Models of the Atom The Spectrum of Atomic Hydrogen Bohr s Model of the Hydrogen Atom de Broglie Waves and the Bohr Model The Quantum Mechanical
Chapters 31 Atomic Physics 1 Overview of Chapter 31 Early Models of the Atom The Spectrum of Atomic Hydrogen Bohr s Model of the Hydrogen Atom de Broglie Waves and the Bohr Model The Quantum Mechanical
Flow of Fluids and Solids at the Nanoscale
 Proceedings of the 2 nd International Conference on Heat Transfer and Fluid Flow Barcelona, Spain July 20-21, 2015 Paper No. XXX (The number assigned by the OpenConf System) Flow of Fluids and Solids at
Proceedings of the 2 nd International Conference on Heat Transfer and Fluid Flow Barcelona, Spain July 20-21, 2015 Paper No. XXX (The number assigned by the OpenConf System) Flow of Fluids and Solids at
Chapter 16 The Second Law of Thermodynamics
 Chapter 16 The Second Law of Thermodynamics To examine the directions of thermodynamic processes. To study heat engines. To understand internal combustion engines and refrigerators. To learn and apply
Chapter 16 The Second Law of Thermodynamics To examine the directions of thermodynamic processes. To study heat engines. To understand internal combustion engines and refrigerators. To learn and apply
Cavity QED induced colloidal attraction?
 Cavity QED induced colloidal attraction? T. V. Prevenslik 14B, Brilliance Court, Discovery Bay, Hong Kong Abstract - The paradox of why like-charge macroions having weak van der Waals attraction condense
Cavity QED induced colloidal attraction? T. V. Prevenslik 14B, Brilliance Court, Discovery Bay, Hong Kong Abstract - The paradox of why like-charge macroions having weak van der Waals attraction condense
Physics 9e/Cutnell. correlated to the. College Board AP Physics 2 Course Objectives
 correlated to the College Board AP Physics 2 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring Understanding 1.A:
correlated to the College Board AP Physics 2 Course Objectives Big Idea 1: Objects and systems have properties such as mass and charge. Systems may have internal structure. Enduring Understanding 1.A:
MODERN OPTICS. P47 Optics: Unit 9
 MODERN OPTICS P47 Optics: Unit 9 Course Outline Unit 1: Electromagnetic Waves Unit 2: Interaction with Matter Unit 3: Geometric Optics Unit 4: Superposition of Waves Unit 5: Polarization Unit 6: Interference
MODERN OPTICS P47 Optics: Unit 9 Course Outline Unit 1: Electromagnetic Waves Unit 2: Interaction with Matter Unit 3: Geometric Optics Unit 4: Superposition of Waves Unit 5: Polarization Unit 6: Interference
Photons as Working Body of Solar Engines
 17 Photons as Working Body of Solar Engines V.I. Laptev 1 and H. Khlyap 2 1 Russian New University, 2 Kaiserslautern University, 1 Russian Federation 2 Germany 1. Introduction Models of solar cells are
17 Photons as Working Body of Solar Engines V.I. Laptev 1 and H. Khlyap 2 1 Russian New University, 2 Kaiserslautern University, 1 Russian Federation 2 Germany 1. Introduction Models of solar cells are
Radiation processes and mechanisms in astrophysics I. R Subrahmanyan Notes on ATA lectures at UWA, Perth 18 May 2009
 Radiation processes and mechanisms in astrophysics I R Subrahmanyan Notes on ATA lectures at UWA, Perth 18 May 009 Light of the night sky We learn of the universe around us from EM radiation, neutrinos,
Radiation processes and mechanisms in astrophysics I R Subrahmanyan Notes on ATA lectures at UWA, Perth 18 May 009 Light of the night sky We learn of the universe around us from EM radiation, neutrinos,
Lasers PH 645/ OSE 645/ EE 613 Summer 2010 Section 1: T/Th 2:45-4:45 PM Engineering Building 240
 Lasers PH 645/ OSE 645/ EE 613 Summer 2010 Section 1: T/Th 2:45-4:45 PM Engineering Building 240 John D. Williams, Ph.D. Department of Electrical and Computer Engineering 406 Optics Building - UAHuntsville,
Lasers PH 645/ OSE 645/ EE 613 Summer 2010 Section 1: T/Th 2:45-4:45 PM Engineering Building 240 John D. Williams, Ph.D. Department of Electrical and Computer Engineering 406 Optics Building - UAHuntsville,
CHAPTER 3 The Experimental Basis of Quantum Theory
 CHAPTER 3 The Experimental Basis of Quantum Theory 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Discovery of the X Ray and the Electron Determination of Electron Charge Line Spectra Quantization As far as I can
CHAPTER 3 The Experimental Basis of Quantum Theory 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Discovery of the X Ray and the Electron Determination of Electron Charge Line Spectra Quantization As far as I can
10/4/2011. Tells you the number of protons
 Atomic Structure The arrangement of the subatomic particles within the atom determines the chemical properties of the elements How they interact with one another The types of ions and structures that they
Atomic Structure The arrangement of the subatomic particles within the atom determines the chemical properties of the elements How they interact with one another The types of ions and structures that they
I understand the relationship between energy and a quanta I understand the difference between an electron s ground state and an electron s excited
 NCCS 1.1.2 & 1.1.3 I understand the relationship between energy and a quanta I understand the difference between an electron s ground state and an electron s excited state I will describe how an electron
NCCS 1.1.2 & 1.1.3 I understand the relationship between energy and a quanta I understand the difference between an electron s ground state and an electron s excited state I will describe how an electron
What the Einstein Relations Tell Us
 What the Einstein Relations Tell Us 1. The rate of spontaneous emission A21 is proportional to υ 3. At higher frequencies A21 >> B(υ) and all emission is spontaneous. A 21 = 8π hν3 c 3 B(ν) 2. Although
What the Einstein Relations Tell Us 1. The rate of spontaneous emission A21 is proportional to υ 3. At higher frequencies A21 >> B(υ) and all emission is spontaneous. A 21 = 8π hν3 c 3 B(ν) 2. Although
NEURON SYNAPSE BY QUANTUM MECHANICS
 NEURON SYNAPSE BY QUANTUM MECHANICS THOMAS PREVENSLIK QED Radiations Discovery Bay, Hong Kong Email: qedradiation@gmail.com ABSTRACT Mainstream theory of neurons is based on chemical signaling by neurotransmitters
NEURON SYNAPSE BY QUANTUM MECHANICS THOMAS PREVENSLIK QED Radiations Discovery Bay, Hong Kong Email: qedradiation@gmail.com ABSTRACT Mainstream theory of neurons is based on chemical signaling by neurotransmitters
Chemistry 304B, Spring 1999 Lecture 5 1. UV Spectroscopy:
 Chemistry 304B, Spring 1999 Lecture 5 1 Ultraviolet spectroscopy; UV Spectroscopy: Infrared spectroscopy; Nuclear magnetic resonance spectroscopy General basis of spectroscopy: Shine light at a collection
Chemistry 304B, Spring 1999 Lecture 5 1 Ultraviolet spectroscopy; UV Spectroscopy: Infrared spectroscopy; Nuclear magnetic resonance spectroscopy General basis of spectroscopy: Shine light at a collection
Wavelength (λ)- Frequency (ν)- Which of the following has a higher frequency?
 Name: Unit 5- Light and Energy Electromagnetic Spectrum Notes Electromagnetic radiation is a form of energy that emits wave-like behavior as it travels through space. Amplitude (a)- Wavelength (λ)- Which
Name: Unit 5- Light and Energy Electromagnetic Spectrum Notes Electromagnetic radiation is a form of energy that emits wave-like behavior as it travels through space. Amplitude (a)- Wavelength (λ)- Which
SECOND LAW OF THERMODYNAMICS
 SECOND LAW OF THERMODYNAMICS 2 ND Law of Thermodynamics Puts a limitation on the conversion of some forms of energy Determines the scope of an energy conversion and if an energy conversion is possible
SECOND LAW OF THERMODYNAMICS 2 ND Law of Thermodynamics Puts a limitation on the conversion of some forms of energy Determines the scope of an energy conversion and if an energy conversion is possible
Are absorption and spontaneous or stimulated emission inverse processes? The answer is subtle!
 Applied Physics B (9) 5:5 https://doi.org/7/s34-9-733-z Are absorption and spontaneous or stimulated emission inverse processes? The answer is subtle! Markus Pollnau Received: October 8 / Accepted: 4 January
Applied Physics B (9) 5:5 https://doi.org/7/s34-9-733-z Are absorption and spontaneous or stimulated emission inverse processes? The answer is subtle! Markus Pollnau Received: October 8 / Accepted: 4 January
Kinetic Theory continued
 Chapter 12 Kinetic Theory continued 12.4 Kinetic Theory of Gases The particles are in constant, random motion, colliding with each other and with the walls of the container. Each collision changes the
Chapter 12 Kinetic Theory continued 12.4 Kinetic Theory of Gases The particles are in constant, random motion, colliding with each other and with the walls of the container. Each collision changes the
Engineering Thermodynamics. Chapter 5. The Second Law of Thermodynamics
 5.1 Introduction Chapter 5 The Second aw of Thermodynamics The second law of thermodynamics states that processes occur in a certain direction, not in just any direction. Physical processes in nature can
5.1 Introduction Chapter 5 The Second aw of Thermodynamics The second law of thermodynamics states that processes occur in a certain direction, not in just any direction. Physical processes in nature can
Electromagnetic Radiation
 Electromagnetic Radiation aka Light Properties of Light are simultaneously wave-like AND particle-like Sometimes it behaves like ripples on a pond (waves). Sometimes it behaves like billiard balls (particles).
Electromagnetic Radiation aka Light Properties of Light are simultaneously wave-like AND particle-like Sometimes it behaves like ripples on a pond (waves). Sometimes it behaves like billiard balls (particles).
de = j ν dvdωdtdν. (1)
 Transfer Equation and Blackbodies Initial questions: There are sources in the centers of some galaxies that are extraordinarily bright in microwaves. What s going on? The brightest galaxies in the universe
Transfer Equation and Blackbodies Initial questions: There are sources in the centers of some galaxies that are extraordinarily bright in microwaves. What s going on? The brightest galaxies in the universe
Example of a Plane Wave LECTURE 22
 Example of a Plane Wave http://www.acs.psu.edu/drussell/demos/evanescentwaves/plane-x.gif LECTURE 22 EM wave Intensity I, pressure P, energy density u av from chapter 30 Light: wave or particle? 1 Electromagnetic
Example of a Plane Wave http://www.acs.psu.edu/drussell/demos/evanescentwaves/plane-x.gif LECTURE 22 EM wave Intensity I, pressure P, energy density u av from chapter 30 Light: wave or particle? 1 Electromagnetic
Lecture 21: Introducing the Second Law, Irreversibilities
 ME 200 Thermodynamics I Spring 2016 Lecture 21: Introducing the Second Law, Irreversibilities Yong Li Shanghai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Chuan Road Shanghai,
ME 200 Thermodynamics I Spring 2016 Lecture 21: Introducing the Second Law, Irreversibilities Yong Li Shanghai Jiao Tong University Institute of Refrigeration and Cryogenics 800 Dong Chuan Road Shanghai,
INTRODUCTION Radiation differs from conduction and convection in that it does not require the presence of a material medium to take place.
 RADIATION INTRODUCTION Radiation differs from conduction and convection in that it does not require the presence of a material medium to take place. Radiation: The energy emitted by matter in the form
RADIATION INTRODUCTION Radiation differs from conduction and convection in that it does not require the presence of a material medium to take place. Radiation: The energy emitted by matter in the form
Kinetic Theory continued
 Chapter 12 Kinetic Theory continued 12.4 Kinetic Theory of Gases The particles are in constant, random motion, colliding with each other and with the walls of the container. Each collision changes the
Chapter 12 Kinetic Theory continued 12.4 Kinetic Theory of Gases The particles are in constant, random motion, colliding with each other and with the walls of the container. Each collision changes the
Sources of radiation
 Sources of radiation Most important type of radiation is blackbody radiation. This is radiation that is in thermal equilibrium with matter at some temperature T. Lab source of blackbody radiation: hot
Sources of radiation Most important type of radiation is blackbody radiation. This is radiation that is in thermal equilibrium with matter at some temperature T. Lab source of blackbody radiation: hot
Classical Black Holes Are Hot. Erik Curiel
 Classical Black Holes Are Hot Erik Curiel Munich Center For Mathematical Philosophy Ludwig-Maximilians-Universität and Black Hole Initiative Harvard University erik@strangebeautiful.com Erik Curiel (MCMP;
Classical Black Holes Are Hot Erik Curiel Munich Center For Mathematical Philosophy Ludwig-Maximilians-Universität and Black Hole Initiative Harvard University erik@strangebeautiful.com Erik Curiel (MCMP;
Preliminary Examination - Day 2 August 16, 2013
 UNL - Department of Physics and Astronomy Preliminary Examination - Day August 16, 13 This test covers the topics of Quantum Mechanics (Topic 1) and Thermodynamics and Statistical Mechanics (Topic ). Each
UNL - Department of Physics and Astronomy Preliminary Examination - Day August 16, 13 This test covers the topics of Quantum Mechanics (Topic 1) and Thermodynamics and Statistical Mechanics (Topic ). Each
X Rays must be viewed from space used for detecting exotic objects such as neutron stars and black holes also observing the Sun.
 6/25 How do we get information from the telescope? 1. Galileo drew pictures. 2. With the invention of photography, we began taking pictures of the view in the telescope. With telescopes that would rotate
6/25 How do we get information from the telescope? 1. Galileo drew pictures. 2. With the invention of photography, we began taking pictures of the view in the telescope. With telescopes that would rotate
CHEM6416 Theory of Molecular Spectroscopy 2013Jan Spectroscopy frequency dependence of the interaction of light with matter
 CHEM6416 Theory of Molecular Spectroscopy 2013Jan22 1 1. Spectroscopy frequency dependence of the interaction of light with matter 1.1. Absorption (excitation), emission, diffraction, scattering, refraction
CHEM6416 Theory of Molecular Spectroscopy 2013Jan22 1 1. Spectroscopy frequency dependence of the interaction of light with matter 1.1. Absorption (excitation), emission, diffraction, scattering, refraction
PHYS 172: Modern Mechanics Fall 2009
 PHYS 172: Modern Mechanics Fall 2009 Lecture 14 Energy Quantization Read 7.1 7.9 Reading Question: Ch. 7, Secs 1-5 A simple model for the hydrogen atom treats the electron as a particle in circular orbit
PHYS 172: Modern Mechanics Fall 2009 Lecture 14 Energy Quantization Read 7.1 7.9 Reading Question: Ch. 7, Secs 1-5 A simple model for the hydrogen atom treats the electron as a particle in circular orbit
Modern Physics. Unit 6: Hydrogen Atom - Radiation Lecture 6.5: Optical Absorption. Ron Reifenberger Professor of Physics Purdue University
 Modern Physics Unit 6: Hydrogen tom - Radiation Lecture 6.5: Optical bsorption Ron Reifenberger Professor of Physics Purdue University 1 We now have a simple quantum model for how light is emitted. How
Modern Physics Unit 6: Hydrogen tom - Radiation Lecture 6.5: Optical bsorption Ron Reifenberger Professor of Physics Purdue University 1 We now have a simple quantum model for how light is emitted. How
Lecture 6 - spectroscopy
 Lecture 6 - spectroscopy 1 Light Electromagnetic radiation can be thought of as either a wave or as a particle (particle/wave duality). For scattering of light by particles, air, and surfaces, wave theory
Lecture 6 - spectroscopy 1 Light Electromagnetic radiation can be thought of as either a wave or as a particle (particle/wave duality). For scattering of light by particles, air, and surfaces, wave theory
Classification following properties of the system in Intensive and Extensive
 Unit I Classification following properties of the system in Intensive and Extensive Extensive : mass, weight, volume, potential energy, Kinetic energy, Internal energy, entropy, exergy, energy, magnetization
Unit I Classification following properties of the system in Intensive and Extensive Extensive : mass, weight, volume, potential energy, Kinetic energy, Internal energy, entropy, exergy, energy, magnetization
Chapter 7: Quantum Statistics
 Part II: Applications SDSMT, Physics 2013 Fall 1 Introduction Photons, E.M. Radiation 2 Blackbody Radiation The Ultraviolet Catastrophe 3 Thermal Quantities of Photon System Total Energy Entropy 4 Radiation
Part II: Applications SDSMT, Physics 2013 Fall 1 Introduction Photons, E.M. Radiation 2 Blackbody Radiation The Ultraviolet Catastrophe 3 Thermal Quantities of Photon System Total Energy Entropy 4 Radiation
C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 6 THE PERIODIC TABLE & ATOMIC STRUCTURE INSTR : FİLİZ ALSHANABLEH
 C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 6 THE PERIODIC TABLE & ATOMIC STRUCTURE 0 1 INSTR : FİLİZ ALSHANABLEH CHAPTER 6 THE PERIODIC TABLE & ATOMIC STRUCTURE The Electromagnetic Spectrum The Wave
C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 6 THE PERIODIC TABLE & ATOMIC STRUCTURE 0 1 INSTR : FİLİZ ALSHANABLEH CHAPTER 6 THE PERIODIC TABLE & ATOMIC STRUCTURE The Electromagnetic Spectrum The Wave
Chapter 3. Electromagnetic Theory, Photons. and Light. Lecture 7
 Lecture 7 Chapter 3 Electromagnetic Theory, Photons. and Light Sources of light Emission of light by atoms The electromagnetic spectrum see supplementary material posted on the course website Electric
Lecture 7 Chapter 3 Electromagnetic Theory, Photons. and Light Sources of light Emission of light by atoms The electromagnetic spectrum see supplementary material posted on the course website Electric
TOPIC # 6 The RADIATION LAWS
 TOPIC # 6 The RADIATION LAWS More KEYS to unlocking the topics of: The GREENHOUSE EFFECT, GLOBAL WARMING & OZONE DEPLETION! Topic #6 pp 33-38 OBJECTIVES FOR TODAY S CLASS: To understand the essentials
TOPIC # 6 The RADIATION LAWS More KEYS to unlocking the topics of: The GREENHOUSE EFFECT, GLOBAL WARMING & OZONE DEPLETION! Topic #6 pp 33-38 OBJECTIVES FOR TODAY S CLASS: To understand the essentials
A system of two lenses is achromatic when the separation between them is
 L e c t u r e 1 5 1 Eyepieces Single eye lens in a telescope / microscope produces spherical and chromatic aberrations. The field of view is also narrow. The eye lens is replaced by a system of lenses
L e c t u r e 1 5 1 Eyepieces Single eye lens in a telescope / microscope produces spherical and chromatic aberrations. The field of view is also narrow. The eye lens is replaced by a system of lenses
UNIT : QUANTUM THEORY AND THE ATOM
 Name St.No. Date(YY/MM/DD) / / Section UNIT 102-10: QUANTUM THEORY AND THE ATOM OBJECTIVES Atomic Spectra for Hydrogen, Mercury and Neon. 1. To observe various atomic spectra with a diffraction grating
Name St.No. Date(YY/MM/DD) / / Section UNIT 102-10: QUANTUM THEORY AND THE ATOM OBJECTIVES Atomic Spectra for Hydrogen, Mercury and Neon. 1. To observe various atomic spectra with a diffraction grating
ME 476 Solar Energy UNIT TWO THERMAL RADIATION
 ME 476 Solar Energy UNIT TWO THERMAL RADIATION Unit Outline 2 Electromagnetic radiation Thermal radiation Blackbody radiation Radiation emitted from a real surface Irradiance Kirchhoff s Law Diffuse and
ME 476 Solar Energy UNIT TWO THERMAL RADIATION Unit Outline 2 Electromagnetic radiation Thermal radiation Blackbody radiation Radiation emitted from a real surface Irradiance Kirchhoff s Law Diffuse and
Introduction. Electromagnetic Waves. Electromagnetic Waves
 Introduction Much of the information we know about electrons comes from studies of interactions of light and matter. In the early 1900 s, scientists discovered that light has properties of both a wave
Introduction Much of the information we know about electrons comes from studies of interactions of light and matter. In the early 1900 s, scientists discovered that light has properties of both a wave
A. 24 B. 27 C. 30 D. 32 E. 33. A. It is impossible to tell from the information given. B. 294 mm C. 122 mm D. 10 mm E. 60 mm A. 1 H B. C. D. 19 F " E.
 CHEMISTRY 110 EXAM 1 Sept. 24, 2012 FORM A 1. A microwave oven uses 2.45! 10 9 Hz electromagnetic waves to heat food. What is the wavelength of this radiation in mm? A. It is impossible to tell from the
CHEMISTRY 110 EXAM 1 Sept. 24, 2012 FORM A 1. A microwave oven uses 2.45! 10 9 Hz electromagnetic waves to heat food. What is the wavelength of this radiation in mm? A. It is impossible to tell from the
Reflection = EM strikes a boundary between two media differing in η and bounces back
 Reflection = EM strikes a boundary between two media differing in η and bounces back Incident ray θ 1 θ 2 Reflected ray Medium 1 (air) η = 1.00 Medium 2 (glass) η = 1.50 Specular reflection = situation
Reflection = EM strikes a boundary between two media differing in η and bounces back Incident ray θ 1 θ 2 Reflected ray Medium 1 (air) η = 1.00 Medium 2 (glass) η = 1.50 Specular reflection = situation
Recall: The Importance of Light
 Key Concepts: Lecture 19: Light Light: wave-like behavior Light: particle-like behavior Light: Interaction with matter - Kirchoff s Laws The Wave Nature of Electro-Magnetic Radiation Visible light is just
Key Concepts: Lecture 19: Light Light: wave-like behavior Light: particle-like behavior Light: Interaction with matter - Kirchoff s Laws The Wave Nature of Electro-Magnetic Radiation Visible light is just
