Analysis of Biochemical Equilibria Relevant to the Immune Response: Finding the Dissociation Constants
|
|
|
- Blaze Parker
- 5 years ago
- Views:
Transcription
1 Bull Math Biol 202) 74:7 206 DOI 0.007/s ORIGINAL ARTICLE Analysis of Biochemical Equilibria Relevant to the Immune Response: Fining the Dissociation Constants L.J. Cummings R. Perez-Castillejos E.T. Mack Receive: 5 May 20 / Accepte: 5 January 202 / Publishe online: 2 February 202 Society for Mathematical Biology 202 Abstract This paper analyzes the biochemical equilibria between bivalent receptors, homo-bifunctional ligans, monovalent inhibitors, an their complexes. Such reaction schemes arise in the immune response, where immunoglobulins bivalent receptors) bin to pathogens or allergens. The equilibria may be escribe by an infinite system of algebraic equations, which accounts for complexes of arbitrary size n n being the number of receptors present in the complex). The system can be reuce to just 3 algebraic equations for the concentrations of free unboun) receptor, free ligan an free inhibitor. Concentrations of all other complexes can be written explicitly in terms of these variables. We analyze how concentrations of key experimentallymeasurable) quantities vary with system parameters. Such measure quantities can furnish important information about issociation constants in the system, which are ifficult to obtain by other means. We provie analytical expressions an suggest specific experiments that coul be use to etermine the issociation constants. Keywors Antiboy Aggregation Bivalent ligan Immune system Introuction In the immune response, multivalent antiboies an antigens or pathogens) bin together an form noncovalent complexes, which may be large. The efficacy of the L.J. Cummings ) Department of Mathematical Sciences, New Jersey Institute of Technology, University Heights, Newark, NJ 0702, USA lina.cummings@njit.eu R. Perez-Castillejos Department of Biomeical Engineering, New Jersey Institute of Technology, University Heights, Newark, NJ 0702, USA E.T. Mack BP Biofuels Global Technology Center, 4955 Directors Place, San Diego, CA 922, USA
2 72 L.J. Cummings et al. response of the immune system towar a pathogen is often relate to the number of antiboies that bin to a pathogen Murphy et al. 2008). This immune response can be particularly profoun in an allergic reaction Golstein 988), where the antigen is an allergen such as pollen; an the allergic response is a useful an much-stuie moel system for the immune response. In this paper, we follow a moel propose by Mack et al. 20), which itself generalizes one presente in a classic paper by Dembo an Golstein 978). We consier a system containing ientical symmetric bivalent receptors antiboies, or immunoglobulins), bivalent ligans antigens; pathogens or allergens), an monovalent ligans. Bivalent ligans can crosslink two or more bivalent receptors, forming aggregates or oligomers) comprising multiple ligans an receptors. The monovalent ligans act to inhibit the immune response, since while a site on a bivalent receptor is occupie by a monovalent ligan, no crosslinking reaction can occur. The original Dembo an Golstein moel Dembo an Golstein 978) for the ynamic equilibrium between complexes in this system mae several key assumptions to facilitate analytical progress, among them: ) bivalent ligan is present in the mixture in great excess, so that the amount of free unboun) ligan present may be assume to be the same as the al amount of ligan in the original mixture; an 2) the affinities of the monovalent an singly-boun bivalent ligans for the bining sites of bivalent receptors are ientical. Both of these conitions are relaxe in our moel. The Dembo an Golstein moel has also been generalize in several ifferent irections by these an other authors, to account for heterogeneous populations of bivalent receptors Golstein an Wofsy 980), for asymmetrical bivalent ligans Wofsy 980), anforcooperativityinthebining WofsyanGolstein987). More recently, Posner et al. 995a, 995b) stuie a time-epenent moel that preicts the concentrations of bivalent receptors an ligans in a mixture as functions of time, an Barisas 2003) propose a moel similar to that of Dembo an Golstein that escribes a population of bivalent receptors, an ligans with valences 2. Other approaches to moeling such complex multivalent systems inclue so-calle rulebase moeling ; see, e.g., Colvin et al. 2009), Faeer et al. 2005), an Hlavacek et al. 2006). Using the original moel of Dembo an Golstein, Golstein 988) reporte an exact result for the al concentration of bivalent ligan that maximizes the concentration of complexes containing two or more bivalent receptors. Such an explicit preiction is very useful, as an experiment may then be carrie out an compare with the moel preiction, enabling estimates to be mae of various issociation or bining constants. Mack et al. 20) carrie out a similar analysis for their extene moel to reuce the system to three algebraic equations, which are reaily solve numerically. Here, we consier the same extene system, but focus principally on specific asympic limits in which analytical progress can be mae. We analyze how concentrations of key experimentally-measurable) quantities vary as a function of the system parameters. Determining such functional relationships allows us to extract the values of system parameters such as the issociation constants, which are ifficult to measure irectly from experimentally-measurable ata Henrickson et al. 2002; Hlavacek et al. 999; Posner et al. 2002; Sklar et al. 2002). Such information is useful as it provies a means of quantifying the response of various antiboies to given
3 Analysis of Biochemical Equilibria Relevant to the Immune Response 73 pathogens. In contrast to previous approaches base on numerical calculations e.g., Mack et al. 20), we fin analytical expressions that can be use to etermine system parameters irectly from experimental ata see also Mack et al. 2008). We expect the simple analytical expressions presente here to be of great interest to experimentalists who are unfamiliar with numerical techniques. Specific experiments are suggeste that will allow the experimentalist to use the analytical formulae to etermine issociation constants for the system. In orer to facilitate the use of the paper by nonmathematicians, each section of mathematical analysis is followe by a separate subsection ientifie by the title Determining the issociation constants), suggesting how the results might be use to etermine certain of the issociation constants in the laboratory. Aitionally, two tables summarize the results of this paper: Table lists the efinitions an Table 2 the main analytical expressions etermine in this stuy. 2 Reaction Scheme an Mathematical Moel Following Mack et al. 20), we consier a moel system in which ientical symmetric bivalent receptors, ientical symmetric bivalent ligans, an ientical monovalent ligans inhibitor molecules) are present. The various possible complexes that may form in such a system, an the ynamic) equilibria between them, are etaile in Scheme of that paper, which appears in aapte form here as Fig.. Iniviual equilibria are epicte in Fig. as pairs of arrows pointing in opposite irections:. Each of the two bining sites of a bivalent receptor epicte in Fig. as ) can noncovalently bin to either one monovalent ligan epicte as ) or to one of the two bining moieties of a bivalent ligan epicte as ). The uppermost block of Fig. shows the complete set of seven possible receptor-ligan complexes that can form with one bivalent receptor: the unboun free) receptor ientifie in Fig. as Z ); the bivalent receptor boun to one of the bining sites of one bivalent ligan A ); the bivalent receptor boun to one of the bining sites of two bivalent ligans simultaneously B ); the bivalent receptor boun to the two bining sites of one bivalent ligan C ); the bivalent receptor boun to one monovalent ligan D ); the bivalent receptor boun to two monovalent ligans E ); an the bivalent receptor boun to one of the bining sites of one bivalent ligan an to one monovalent ligan simultaneously F ). The subscript inicates that these molecular complexes contain only one bivalent receptor. The central block in Fig. shows the complexes containing exactly two bivalent receptors: accoringly, the subscript for the complexes in this block is 2. In general, the seven complexes with n bivalent receptors are escribe as A n, B n, C n, D n, E n, an F n bottom block of Fig. ). The etaile escription of the equilibria of the system stuie here can be foun elsewhere Mack et al. 20). Briefly, the equilibria between all the molecular species in this system are escribe through the prouct of stoichiometric factors erive from the ratio of the number of bining moieties to the available number of bining sites) an four issociation constants Connors 987; Mack et al. 20). The monovalent issociation constant K mono relates the concentration of free unboun) monovalent ligan ientifie as I in Fig. ); the concentration of bivalent receptors either free or boun) with at least one available bining site A n, D n, Z n in Fig. );
4 74 L.J. Cummings et al. Fig. Summary of all possible ynamic equilibria between symmetric bivalent ligans ), symmetric bivalent receptors ), an monovalent ligans ), aapte from Mack et al. 20) an the concentration of the complex after the monovalent ligan is boun to the available receptor site F n, E n, D n, respectively, for the preceing list). The intermolecular issociation constant K inter relates the concentration of free unboun) bivalent ligan ientifie as L in Fig. ); the concentration of bivalent receptors either free or boun) with at least one available bining site A n, D n, Z n in Fig. ); an the concentration of the resulting complex when the bivalent ligan is boun to the available receptor site B n, F n, A n, respectively, for the preceing list). The
5 Analysis of Biochemical Equilibria Relevant to the Immune Response 75 intramolecular issociation constant K intra relates the concentration of complexes exhibiting a bivalent ligan with one free moiety, the other being boun to a complex presenting a bivalent receptor with an unoccupie bining site A n in Fig. ); an the concentration of circular or cyclic complexes with all bining moieties of bivalent ligans an all bining sites of bivalent receptors boun to each other C n in Fig. ). Finally, the cooperativity parameter α escribes the tenency of complexes with n ) bivalent receptors to form complexes with n receptors. Equations ) 6) escribe each of the equilibria in the bottom block of Fig. as a function of the issociation constants introuce above. In this manuscript, the presence of an asterisk ) enotes that a quantity is imensional we nonimensionalize below an rop the ); in all cases, the imensions are those of concentration, i.e., moles per volume. The overbrace in the equations enotes the cyclic complex, where a chain of alternating ligan-receptor pairs is joine up into a ring intramolecular boning) by a single bivalent ligan, which joins the two en receptors of the chain together. In our moel, the intramolecular issociation constant may epen on the size n of the cyclic complex forme: K,n intra. This size-epenence is another important ifference between our moel an that of Dembo an Golstein 978). We consier two cases to encompass intramolecular bining: i) The first case escribes the possibility that the bivalent ligan is too short to span the istance between the two bining sites of a bivalent receptor, which ultimately prevents the formation of mono-receptor cyclic complexes C ). Mathematically, this case is escribe with K intra, = while all K,n> intra are equal. ii) The secon case is that of bivalent ligans long enough to bin the two sites of one bivalent receptor simultaneously. Mathematically, this case has all K,n intra equal. The equilibrium between free bivalent receptors Z ) an complexes of two bivalent receptors with two available bining sites Z2 ) is escribe through the cooperativity parameter α as Z2 = α/k inter )Z A = 4α/K inter ) 2 )Z )2 L the secon equality on substitution for A = 4/K inter )Z L ). The general situation is summarize by the set of equations below, with the equilibrium between Zn an Z n given by 7): [ ] = 4 K inter [ ] = [ {}}{ ] = K inter 2nK intra,n [ ] 2 = K mono [ ] = 2K mono [ ] = 2 K inter [ ] [ ], A n = 4 K inter [ ] [ ], B n = [ ], C n = [ ] [ ], D n = 2 K inter 2nK intra,n K mono [ ] [ ], E n = 2K mono [ ] [ ], F n = 2 K inter Z n L, ) A n L, 2) A n, 3) Z n I, 4) D n I, 5) D n L, 6)
6 76 L.J. Cummings et al. [ ] 4α = K inter Zn = 4α ) 2 ) n [ ] n [ ] n, ) n L ) 7) n ) Z n. K inter ) 2 This system of equilibria is augmente by mass conservation conitions, which state that the al amount of receptor R ), ligan L ), an inhibitor I ) in the system must be conserve. Accounting for the amounts in complexes of all sizes, these conitions are R = n Zn + A n + B n + C n + D n + E n + F n ), 8) n= L = L + n )Z n + na n + n + )B n + nc n + n )D n n= + n )En + nf n ), 9) I + D n + 2En + F n ). 0) n= Normalizing all concentrations with K inter,) 7) become A n = 4Z n L, ) B n = A n L, 2) C n = where K mono = K mono /K inter, Kn intra imensionless moel), A n = A n /K inter 2nK intra n A n, 3) D n = 2 K mono Z ni, 4) E n = 2K mono D ni, 5) F n = 2D n L, 6) Z n = 4αLZ ) n Z, 7) = K,n intra/k inter we rop subscripts in the an similarly for all new unstarre quantities introuce. The mass conservation equations 8) 0) are unchange except that s are roppe as quantities become imensionless, an we can simplify by replacing 9) with 8) minus 9)). In imensionless form then, 8) 0) are equivalent to R = nz n + A n + B n + C n + D n + E n + F n ), 8) n= R L = L + I = I + Z n B n + D n + E n ), 9) n= D n + 2E n + F n ). 20) n=
7 Analysis of Biochemical Equilibria Relevant to the Immune Response 77 The complete mathematical moel of the equilibria shown in Fig. consists of ) 20). In general, we must solve this system of 0 equations for quantities A n, B n, C n, D n, E n, F n, Z n, Z, L, I, given the al amounts of receptor, ligan, an inhibitor, R, L, I ae to the system initially, an the values of the normalize issociation constants Kn intra, K mono. We simplify matters by noting that we may substitute for Z n from 7) into) an 4), an then for A n from ) into 2) an 3), an finally for D n from 4) into5) an 6). This sequence of operations gives expressions for all quantities A n F n, an Z n, in terms of Z, L an I. Moreover, on substitution of these expressions into the conservation equations 8) 20), we are able to compute the require infinite sums explicitly, assuming 4αLZ < this conition, which can be checke a posteriori, is mathematically necessary for convergence of the infinite sums; failure to satisfy this conition means that the sums o not converge, an woul point physically to some kin of bifurcation in the system, where all the mass ens up in extremely large complexes). We have Z n = Z 4αLZ ) k Z =, 4αLZ 0 A n = 4L Z n = 4LZ, 4αLZ B n = L A n = 4L2 Z, 4αLZ D n = 2I K mono I E n = 2K mono Z n = D n = 2IZ K mono 4αLZ ), I 2 Z K mono ) 2 4αLZ ), 4ILZ F n = 2L D n = K mono 4αLZ ) we o not require C n, though this can be evaluate). For the receptor conservation law 8), we also nee to evaluate nz n an similar quantities, for which we note, writing x = 4αLZ, that nz n = Z nx n = Z x Hence, ) x n Z nz n = 4αLZ ) 2, 0 4LZ na n = 4L nz n = 4αLZ ) 2, = Z x) ) = Z x x) 2.
8 78 L.J. Cummings et al. 4L 2 Z nb n = L na n = 4αLZ ) 2. For nc n,wehave nc n = /2) A n /Kn intra ), an as state, we wish to consier the possibility that the issociation constants Kn intra vary with n. For general n-epenence, we cannot compute the sum explicitly, but we consier the two special state cases of relevance: Case i), where K intra = an all other Kn intra are equal short bivalent ligan); an Case ii), where all the Kn intra, incluing K intra, are equal long bivalent ligan). In case i), the cyclic complex with just one receptor an one inhibitor,, is not forme for example, if the ligan is too short to span the two sites of a single bivalent receptor), but all other cyclic complexes are equally likely to form. We have ropping the subscript n from Kn intra henceforth) nc n = 2K intra A n = ) 4LZ 2K intra 4LZ 4αLZ an We also have = nc n = 2K intra 2 8αL 2 Z 2 K intra 4αLZ ) nd n = 2I K mono I ne n = 2K mono A n = nz n = nd n = case i) ), 2LZ K intra 4αLZ ) 2IZ K mono 4αLZ ) 2, case ii) ). I 2 Z K mono ) 2 4αLZ ) 2, 4ILZ nf n = 2L nd n = K mono 4αLZ ) 2. Substitution of these expressions into the conservation equations 8) 20) then reuces the whole system to three algebraic equations for Z, L an I, which after simplification take the form: [ Z R = 4αLZ ) 2 + I ) 2 ] + 2L + 8αL2 Z Kmono K intra 4αLZ ) in case i), 2) [ Z R = 4αLZ ) 2 + I ) 2 + 2L + 2L ] Kmono K intra 4αLZ ) in case ii), 22)
9 Analysis of Biochemical Equilibria Relevant to the Immune Response 79 Z R = L L + 4αLZ ) 2IZ I = I + K mono 4αLZ ) + I K ) + 2L mono ) + I + 2L Kmono where 23) an 24) apply in both cases i) an ii). + I 2L Kmono ), 23), 24) 3 Preictive Analysis We want to use our moel to extract information such as the values of issociation constants for the system. One approach is the following: A quantity that the experimentalist can etermine in any given experiment is the concentration of complexes of size n 2 by which we mean, complexes containing at least two receptor molecules) in the mixture. Consier a titration experiment in which ligan is slowly ae to known fixe) amounts of receptor an inhibitor. If we monitor the concentration of complexes as a function of ligan concentration methos for oing this are iscusse by, for example, Henrickson et al. 2002, Hlavacek et al. 999, Posner et al. 2002, Sklar et al. 2002, an references therein), then we shoul be able to compare this concentration profile, or at least key features of it, with preictions from our moel. Easy features to compare coul be the value of the ligan concentration at which the concentration of complexes is maximize an/or the value of this maximum concentration), or we might measure the initial graient of the titration concentration curve, an compare this with preictions from the moel. In our notation, the relative concentration of complexes of size n 2, which we call,is = R Z + A + B + C + D + E + F ) ), 25) R so that in case i) = Z + I ) 2 + 2L, 26) R Kmono while in case ii) = Z [ + I ) 2 + 2L + 2L R Kmono K intra ]. 27) Although in principle any require information a turning-point, or a graient of f at a given point) may be compute numerically for given values of the system parameters, etermining such information analytically, in terms of arbitrary system parameter values, using 2) 24), is very ifficult. In the following sections, we iscuss certain simplifie cases in which analytical progress may be mae, an the preictive powers of the reuce moels.
10 80 L.J. Cummings et al. 4 Case Without Monovalent Inhibitor The main simplification of relevance is when no inhibitor monovalent ligan) is present. In this case, 24) reuces to an ientity, an 2) or22)) an 23) simplify: [ ] Z R = 4αLZ ) 2 + 2L) 2 + 8αL2 Z K intra 4αLZ ) in case i), or 28) [ Z R = 4αLZ ) 2 + 2L) 2 + 2L ] K intra 4αLZ ) in case ii), an 29) R = L L + Z 4L 2 ) 4αLZ ). 30) Solving 30)forZ, L + R L Z = 4 α)l 2 3) + 4αLR L ) an substituting in 28) or29) gives a sixth-orer polynomial equation for the free ligan concentration L in case i), an a quartic in case ii). Neither have general analytical solution, but special cases may be solve exactly. 4. Cooperativity α =. Case ii), Long Bivalent Ligan Setting α = lowers by one) the egree of the polynomial satisfie by L, so that case ii) reuces to a cubic for L, with analytical solution. Case i) is governe by a quintic an is still intractable in general. So, we first consier case ii) with α = which, as we shall see, provies insight into the short bivalent ligan case i). In case ii) α = ), L satisfies 4 + 2K intra L ) L 3 2L 2 2R + 2L + K intra 4L R ) 2 2L + 4R ) )) + L 2L 2R K intra 4L R ) 2 2L + 4R ) )) + K intra L = 0 32) or, introucing convenient shorthan notation for the coefficients in 32) consiering R an K intra fixe but allowing L to vary) gl,l ) := b 0 L )L 3 + b L )L 2 + b 2 L )L + b 3 L ) = 0. 33) The cubic 33) has three solutions, given in terms of the following parameters: Q = 3b 2b 0 b 2 9b0 2, R= 9b 0b b 2 27b0 2b 3 2b 3 54b0 3, D = Q 3 + R 2, S = R + D ) /3 ) /3,, T = R D with ST = Q. The solutions are
11 Analysis of Biochemical Equilibria Relevant to the Immune Response 8 L L ) = S + T b, 34) 3b 0 L 2 L ) = 2 S + T) b + i 3 S T), 35) 3b 0 2 L 3 L ) = 2 S + T) b i 3 S T), 36) 3b 0 2 an here the iscriminant D 0 always, which leas to real an in general, istinct) roots S an T are complex conjugates). Hence, LL ) is given by the relevant root real, positive an, to guarantee convergence of the infinite sums, such that 0 < 4LZ < ), with Z then given by Z = L + R L 37) + 4LR L ) from 3). It is not ifficult to check that there is always exactly one relevant solution for L: although two solutions are positive an, therefore, possible caniates, one of these always leas to 4LZ > an so is unacceptable. The relevant solution is the smaller positive root though which of the three expressions 34), 35), or 36)thisis given by varies epening on the values of the parameters, as we shall see explicitly below). A special case of the system 32), 37) arises when L = R + /2. When this happens, the cubic equation 32) has a simple factorization: 2L) 2 L + K intra) + K intra R + /2) ) = 0, an the only relevant root is the repeate one, L = /2. This situation correspons to a maximum value with respect to L ) of the quantity efine in 27), as we shall now see. With L = R + /2 an L = /2, the solution 37) forz is unefine, an careful local analysis is require to etermine its value. This we o by examining the cubic equation 32) when L = R + /2 + ɛ for ɛ, an etermining the appropriate root L, correct to orer ɛ. Forɛ of either sign but small), we have L = 2 + ɛl + O ɛ 2), Z = L + Oɛ), 38) 2 + L ) where L = [ + + 8λK intra ) /2], λ= + 2K intra + 2R ). 39) 2λ Further asympic analysis enables us to etermine higher-orer corrections to Z an L. Substituting these asympic expressions for Z an L into our expression 27) setting I = 0) for, we fin that has no orer-ɛ contribution, an that its orer ɛ 2 correction term is strictly negative that is, consiere as a function of L, it has a local maximum at the value L = R + /2. The value of Mathematically, L increasing through the value R + /2 correspons to the crossing over of the two positive roots mentione above: the smaller root increases through the value /2 an then ceases to be relevant, while the larger root ecreases through /2 an becomes the relevant one. Moreover, at this value of L, examination of 37) givesz = /2 an unphysical result) except in the special case L = /2. Thus for L = R + /2 we always require L = /2.
12 82 L.J. Cummings et al. at this local maximum is foun by setting L = R + /2, I = 0, L = /2, an Z = L )/2 + L )) in 27), with L givenby39). This proceure gives after simplification),max = + 4Kintra )[2λ + 8λK intra ] 2K intra R [2λ λK intra ] with λ as given in 39) above. 40) 4.. Determining the Dissociation Constants If the value of the cooperativity parameter α is close to unity, then these results can be use to etermine the issociation constants K inter, K intra,asfollows.note that, since the moel is mae imensionless by scaling concentrations with the unknown K inter, we must here work with imensional quantities that the experimentalist woul know. Carry out a titration with no inhibitor, an a known concentration of receptor, R. At regular points throughout the experiment, measure the concentration C of complexes of size n 2 which, in the notation above, is given by C = R e.g. Henrickson et al. 2002; Hlavacek et al. 999; Posner et al. 2002; Sklar et al. 2002). Determine, as closely as possible perhaps using a curve-fit to extrapolate if only a few ata points are taken) the value of ae ligan, L, at which C is maximize, an the corresponing maximal value, Cmax = R f,max.we know, from the analysis above that the maximum is obtaine when L = R + /2 or, in imensional terms, when L = R + K inter. 2 Hence, from the recore maximizing value of L, we obtain K inter. Next, we know that at the maximum, 40) hols. With K inter now etermine, we know all quantities in 40) except for K intra R = R /K inter, an,max = Cmax /R ). Hence, 40) is a nonlinear equation to be solve for Kintra. After rearrangement, this reuces to a cubic, one root of which is the physically-relevant one it is possible that ambiguity will arise at this stage). Finally, then K intra = K inter K intra. 4.2 Cooperativity α =. Case i), Short Bivalent Ligan Remarkably, although case i) is governe by a ifferent, more complicate quintic, rather than quartic) equation for L in the case I = 0, α=, a similar simplification occurs at the same value L = R + /2 as in case ii). The system is governe by couple equations [ ] Z R = 4LZ ) 2 + 2L) 2 + 8L2 Z K intra 4LZ ), 4) Z = L L + R + 4LR L ), 42)
13 Analysis of Biochemical Equilibria Relevant to the Immune Response 83 set α = in28) an 3)). Substitution of Z from 42) into4) leas to a quintic for L, which factorizes in the special case L = R + /2, with a triple factor 2L ) emerging: 0 = 2L ) 3 4L 2 2LK intra + 2R ) + K intra + 2R ) ). 43) This equation has five real roots for L: L = /2 with multiplicity 3), an two others, neither of which are physically relevant they violate the convergence conition 0 < 4LZ <, leaing to Z = /2in42)). Again, with L = R +/2 an L = /2, Z is unefine in 42) an we nee a local analysis. Setting L = R + /2 + ɛ, L = /2 + ɛl + ɛ 2 L 2 +, ɛ in43), we can etermine L, L 2, successively; substitution in 42) then gives Z correct to orer ɛ just as in case ii), Z = L )/2 + L )) + Oɛ)). The correction L is foun to satisfy a cubic equation, 2K intra + 2R ) ) L K intra + 2R ) ) L K intra) L + 2K intra = 0. 44) With L = /2 + ɛl + Oɛ 2 ), Z = L )/2 + L )) + Oɛ), only one of the three roots L satisfies the convergence criterion 0 < 4LZ < ; this is the real root of 44) that lies in the interval 0, ). We substitute the physically-relevant expressions in = Z + 2L) 2 45) R which follows from 26) with I = 0 an α = ). Again explicit calculation reveals a local maximum in at ɛ = 0L = /2). The value of at this maximum is obtaine by setting L = R + /2, L = /2, Z = L )/2 + L )) in 45), giving,max = 2 L ) R + L ), 46) with L the unique root of 44) lying in 0, ). Ientifying the coefficients in 44) by c 0 = 2K intra + 2R ), c = 2 + K intra + 2R ) ), c 2 = + 2K intra, c 3 = 2K intra, the roots are foun in terms of parameters Q = 3c 2c 0 c 2 9c0 2, R= 9c 0c c 2 27c0 2c 3 2c 3 54c0 3, D = Q 3 + R 2, S = R + D ) /3 ) /3,, T = R D with ST = Q). The three solutions are L, = S + T c, 3c 0 L,2 = 2 S + T) c + i 3 S T), 3c 0 2 L,3 = 2 S + T) c i 3 S T), 3c 0 2
14 84 L.J. Cummings et al. an here D 0 always, so all roots are real. Which root lies in 0, ) varies accoring to the values of K intra an R. The results may be summarize as follows: Consiere as a function of al ligan concentration L, the maximum value of occurs for L = R +/2. The maximal value at this value of L is given by the following expressions from 46)): 0 <K intra < 2 + 2R ) :,max = 2 L,3) R + L,3 ), 47) K intra > 2 + 2R ) : f,max = 2 L,) R + L, ). 48) 4.2. Determining the Dissociation Constants As explaine for case ii) above, if cooperativity is close to unity, these results can be use to estimate the issociation constants K inter an K intra.thevalueofk inter is obtaine exactly as explaine at the en of Sect. 4., using the relation L = R + K inter 2 that hols when the concentration of complexes of size n 2, C, is maximize. The measure value of Cmax must equal R f,max, where,max = Cmax /R is givenby47) or48). Either of these relations gives the value of L at the maximum remember that R = R /K inter ). It is known that L satisfies 44); with L an R known, 44) is a linear equation for the remaining unknown, K intra,giving Hence, finally, K intra K intra = 4.3 A Posteriori Checking L L ) L )[L 2 + 2R ) ]. = K inter K intra. Without prior information, it is ifficult to know whether or not the cooperativity α. Some form of check may be provie by carrying out more than one experiment of the kin escribe in Sects. 4.., 4.2., with ifferent values of R. Each such experiment shoul yiel the same or approximately the same) values for the issociation constants K inter, K intra the first is easily checke by looking at the values of L R ) at the maximum of for all experiments; the values shoul be the same). Large eviations in the values obtaine for K inter an K intra woul suggest that α is not close to unity. However, if the values are close for all experiments, then the approximation is likely goo. 5 General Case Numerical Simulations Before consiering asympic limits that permit analytical progress enabling explicit preictions to be mae) we first present sample numerical computations that
15 Analysis of Biochemical Equilibria Relevant to the Immune Response 85 Fig. 2 Fraction of complexes with more than one bivalent receptor ) as a function of the al concentration of bivalent ligan L. Each curve correspons to a ifferent value of the al concentration of bivalent receptor R ; from the bottom curve upwar the values are: R = 0.0, 0.,, 0, 00 allow us to investigate the effect of iniviual system parameters for the general case in which 2) 24) hol. Such simulations corroborate the analytical results of the preceing section, an provie guiance as to which asympic limits might usefully be investigate. 5. Depenence on Normalize Total Receptor Concentration R Figure 2 shows the fraction of complexes with more than one bivalent receptor ) as a function of the al concentration of bivalent ligan L, at fixe values of the intramolecular issociation constant Kn intra an the cooperativity parameter α. This simulation correspons to the analysis of Sect. 4.2, since we simulate the simple case in which al monovalent ligan inhibitor) concentration I = 0; cooperativity α =, an K intra =, with all other Kn intra = K intra constant for n 2 short bivalent ligan). For this figure, we set K intra = 0., an use five ifferent values of normalize bivalent receptor concentration R as etaile in the caption. The figure bears out the analytical fining of a maximum in at L = R + / Depenence on Cooperativity Parameter α Figure 3 shows as a function of al bivalent ligan concentration L,atfixe values of intramolecular issociation constant K intra an al bivalent receptor concentration R, an al inhibitor concentration I = 0. We again consier case i) short bivalent ligan), with K intra =, an all other Kn intra = K intra = 0. n 2), an R = 0.. The plot shows curves for six ifferent values of the cooperativity α; from the bottom curve upwars the values are: α = 0.,, 0, 00, 000, We observe that the curves become left right asymmetric as α becomes large. This effect is not observe with the moel of Dembo an Golstein 978), an is attributable
16 86 L.J. Cummings et al. Fig. 3 Fraction of complexes with more than one bivalent receptor f ) as a function of the al concentration of bivalent ligan L. Each curve correspons to a ifferent value of cooperativity α; from the bottom curve upwar the values are: α = 0.,, 0, 00, 000, 0000 to our ealing explicitly with the concentration of free ligan L, which those authors assume fixe. 5.3 Depenence on Intramolecular Dissociation Constant K intra Figures 4, 5 show as a function of al bivalent ligan concentration L,atfixe values of α an R. Total receptor concentration R = 0.; al inhibitor concentration I = 0, an cooperativity α = in both plots. For Fig. 4, we consier case i), with intramolecular issociation constants K intra =, an all other Kn intra = K intra, constant for n 2; thus we are in the situation of Sect. 4.2 short bivalent ligan). The plot shows curves for seven ifferent values of K intra as etaile in the caption. For Fig. 5, we consier case ii), with all Kn intra equal for n ; thus we are in the situation of Sect. 4. long bivalent ligan). The plot shows curves for six ifferent values of K intra as etaile in the caption. Again, both Figs. 4 an 5 corroborate the analytical result of a maximum in at L = R + /2 with no inhibitor an α =. The epenence on K intra varies ramatically in the two cases i) an ii), with opposite trens being observe. In case i), as we ecrease the issociation constant K intra, the cyclic complexes are much more likely to form, an here, since the singleton cyclic complex C ) cannot form, cyclic complexes with size n 2 preominate in fact, the n = 2 cyclic complex will ominate all others). Hence, increases. In case ii), as we ecrease K intra it is easier for all cyclic complexes, incluing the singleton, to form. Since the singleton forms first, an is unlikely to issociate at small K intra, this will be the ominant complex, meaning that which measures complexes containing two or more receptors) goes to zero. This is iscusse in etail in Sects. 6. an 6.2 below.
17 Analysis of Biochemical Equilibria Relevant to the Immune Response 87 Fig. 4 Fraction of complexes with more than one bivalent receptor f ) as a function of the al concentration of bivalent ligan L. Here, the intramolecular issociation parameter for mono-receptor complexes K intra =, while the intramolecular issociation constant for complexes with more than one bivalent receptor are all equal, K intra n> = Kintra case i)). Each curve correspons to a ifferent value of K intra ; from the bottom curve upwar the values are: K intra = 00, 0,, 0., 0.0, 0.00, Fig. 5 Fraction of complexes with more than one bivalent receptor f ) as a function of the al concentration of bivalent ligan L. Here, all the intramolecular issociation constants K intra are equal case ii)), an each curve correspons to a ifferent value; from the bottom curve upwar the values are: K intra = 0.00, 0.0, 0.,, 0, Depenence on Normalize Total Inhibitor Concentration I Figure 6 shows f as a function of al bivalent ligan concentration L,atfixe values of K intra, K mono the imensionless form of the issociation constant relating to complexes between monovalent inhibitor an bivalent receptor, as escribe in Sect. 2), an R. Total inhibitor concentration I iffers for each curve in the plot.
18 88 L.J. Cummings et al. Fig. 6 Fraction of complexes with more than one bivalent receptor ) as a function of the al concentration of bivalent ligan L. Each curve correspons to a ifferent value of the al concentration of monovalent ligan inhibitor) I ; from bottom curve upwar the values are: I = 00, 0,, 0., 0.0, 0.00 We assume case i), short bivalent ligans, with K intra =, an all other Kn intra = 0. for n 2; the normalize inhibitor issociation constant K mono =, an R = 0.. The plot shows curves for five ifferent values of I as etaile in the caption. Strong inhibition suppresses the number of large complexes forme, as we woul expect, an also shifts the value of the maximum complex concentration to larger values of ae ligan. 6 Asympic Analysis The general case governe by 2) 24) is highly nonlinear, but consierable analytical progress may be mae usefully in certain asympic limits. We explore these below, noting the physical interpretation of the limits, an comparing results to the numerical computations of the previous section. 6. K intra = ɛ Asympically Small, Case i) Short Bivalent Ligan) If the parameter K intra is small cyclic complexes are favorable an form preferentially compare with linear complexes. In case i), ligans are short, an the singleton cyclic complex cannot form, so the ominant complex is the cyclic 2-form C 2 ). Figure 4 suggests that this asympic limit is nontrivial an might yiel a useful preictive result. 2 We consier K intra = ɛ in2), 23), an 24). Solutions epen on the 2 In case ii), consiere next, the ligan is long enough to form cyclic singletons, an this is the ominant complex in the equilibrium mixture; Fig. 5 suggests that in this limit 0 uniformly in K intra an that therefore this limit may be ifficult to use preictively.
19 Analysis of Biochemical Equilibria Relevant to the Immune Response 89 relative proportions of receptor an ligan present in the original mixture, since one of these will be entirely use up at leaing orer), so that either L orz. 6.. L <R In this case, all ligan is boun up in cyclic complexes, leaving only asympically small amounts of free ligan: L, Z = O), I = O), so that the asympic expansions procee as Z = Z 0 + o), L = o), I = I 0 + o). Equations 23) an 24) are simple at leaing orer, R L = Z 0 + I ) 2 0, I = I 0 + 2I 0Z 0 K mono K mono Eliminating Z 0, I 0 satisfies a quaratic equation, + I 0 K mono I0 2 + I 0 2R L ) I + K mono) I K mono = 0, 49) with unique positive solution I 0. Hence, Z 0 = R L + I 0 K mono ). 50) 2 Stuy of 2) suggests that ligan concentration L scales with ɛ /2 : L = ɛ /2 L 0 + Oɛ), an 0 is given by the leaing-orer terms in 26). Since L, it is clear that in this case 0 is given by 0 = L 0, ), R an we o not nee to calculate L 0 unless we wish to etermine higher-orer terms. This expression for 0 makes intuitive sense: with short ligans, but cyclic complexes favore, the ominant reaction will be the formation of the cyclic 2-complex, limite only by availability of ligan when L <R. So, if all available ligan ens up in cyclic 2-complexes then these will constitute all complexes of size n 2, an hence account for the entirety of 0. To fin the next orer correction, we expan variables in powers of ɛ /2 : Z = Z 0 + ɛ /2 Z +, L= ɛ /2 L 0 +, I = I 0 + ɛ /2 I +, in 2), 23), 24), an in the expression 26) for. After solving for Z, L 0, an I in terms of known) leaing orer quantities, substitution in 26) gives ɛ /2 L = L + R 2 2R K mono ) 2 L R ) α [ I 0 2R L ) 2K mono) I K mono) + K mono) 2 4αR L ) 2 4R L ) ) I K mono] + Oɛ), 5) ).
20 90 L.J. Cummings et al. Fig. 7 Comparison of numerical soli lines) an asympic solutions for f as a function of al concentration of bivalent ligan L. The four ifferent asympic regions are istinguishe as from left to right) L <R : ashe; L R : otte; L >R : ash-otte; L R : ashe. Parameter values are: al concentration of bivalent receptor R = 0.25; al concentration of monovalent inhibitor I = 0; intramolecular issociation constant K intra = 0 5 inepenent of the complex size, case ii)); monovalent issociation constant K mono = 0.25; an cooperativity parameter α = with I 0 as given by 49). For ɛ = K intra, this expression gives very goo agreement with the full numerical solution in the region L <R see the whole range of asympic approximations constructe in Fig. 7) L >R Here, ligan is in excess, so to leaing orer) all receptor is boun up in cyclic complexes, leaving only asympically small amounts: Z, L = O), I = O). Leaing-orer behavior is trivial, 23) an 24)giving L 0 = L R, I 0 = I 52) the only free ligan is the excess ligan; an since cyclic complexes are favore, inhibitor is left with nothing to bin to). The leaing-orer balance in 2) isr 8αL 2 Z 2/ɛ, suggesting that Z scales as ɛ /2 : Z = ɛ /2 Z 0 + Oɛ), an L = L 0 + ɛ /2 L + Oɛ), I = I 0 + ɛ /2 I + Oɛ). This leaing-orer balance, together with 52) gives R Z 0 = 8α L R ). Equations 23) an 24) at orer ɛ /2 give expressions for the corrections L, I, an substitution of the expansions into 26) gives the fraction of receptor boun up in complexes of size n 2 correct to orer ɛ /2 )as = ɛ/2 2L R ) + + 8αR L R ) I K mono ) 2 + Oɛ). 53)
21 Analysis of Biochemical Equilibria Relevant to the Immune Response L R The asympic expansions constructe in the regions L <R an L >R break own when L R. In this case, at leaing orer, all free receptor an ligan are use up in making the cyclic complex, so both Z an L are asympically small; an no inhibitor can bin to receptors. While many possible balances coul be explore, the crossover region between L <R an L >R is aequately escribe by stuying the asympic regime Equations 2) an 23) lea to L 0 = Z 0 which together yiel Z 0 = L = R + ɛ /4 l, Z = ɛ /4 Z 0 +, L = ɛ /4 L 0 +, I = I +. R 8α ) /2, L 0 = l + Z 0 + I ) 2 K mono, { [ ) /2 2 + I l + l 2 K ) R + I ) 2 ] /2 } 8α K mono, 54) where recall l = L R )/K intra ) /4 = O). The expression 26) for then gives = ɛ /4 Z 0 + I R K mono 2 + I )) K mono + O ɛ /2), 55) with Z 0 as given by 54) above L R When L is asympically large, the scaling for Z changes. With L = O/ɛ /2 ), leaing orer in 2) gives ) Z R 4αLZ ) 2 4L 2 + 8αL2 Z 4αLZ ). ɛ The exact balance of terms here epens on the size of LZ which, recall, must always be less than /4α)). Note first that 4αLZ ) cannot be asympically small, since this can give no balance of terms. Neither can we have both LZ an 4αLZ ) orer-one, since then 4L 2 Z / 4αLZ ) 2 with nothing to balance it. So, we must have LZ, an R 4L 2 Z + 8αL2 Z 2, ɛ this new balance is where we will first epart from the previous case, where R 8αL 2 Z 2/ɛ 4L2 Z ). The asympic expansions now procee as
22 92 L.J. Cummings et al. L = L 0 ɛ /2 + L + ɛ /2 L 2 + Oɛ), Z = ɛz 0 + O ɛ 3/2), I = I 0 + ɛ /2 I + Oɛ), an also L = L /ɛ /2, with L O). Leaing-orer analysis of 2) 24) yiels L 0 = L, I 0 = I, Z 0 = 4α + + 2αR ) /2 4α L 2, an substitution in 26) gives the leaing-orer expression for as = + L 2 + 2αR ) /2 ) αr L 2 + o). 56) Figure 7 shows all four asympic expressions for, plotte as functions of L ) against the full numerical solution, on the appropriate omains of L.Asthe figure shows, to the orers obtaine, the asympic expressions are quite accurate on their regions of applicability Determining the Dissociation Constants Since a primary goal of our analysis is to erive results that experimentalists might use to extract information about a given system, we now consier how the expressions erive above can be use if it is suspecte that K intra = K intra /K inter. The most easily-ientifie experimental regimes in Fig. 7 appear to be L <R, an L R ; accoringly, we focus on these regimes. We again consier a titration experiment in which ligan is ae to a receptor/inhibitor mixture, an the concentration, C = R, of complexes of size n 2, is measure at selecte stages. In the regime L R, the late stages of a titration, the expression 56) is vali, an thus in the imensional variables C = R + K intra L 2 αk inter ) 2 or, rearranging to make α/k intra α K intra = 2L 3 [ the subject, K inter ) 3 C R ) 2 + 2αK inter K intra R L R + C R ) L K inter ) /2 ], 57) ). 58) In a given experiment, the quantities C, R, an L can be measure at any stage, an we woul like to extract the unknowns K inter, K intra, an α that appear in 57), 58). Suppose we carry out an experiment with a fixe amount of receptor R, an make measurements at two ifferent concentrations of ae ligan, L an L 2.We measure the two corresponing values of complex concentration, C an C 2.The expression 58) applies to each measurement: if we take the ratio of the two expressions for the two measurements, we obtain a relation that contains only one unknown, K inter. Solving this relation for K inter an simplifying, we fin
23 Analysis of Biochemical Equilibria Relevant to the Immune Response 93 K inter = R [L 3 C 2 R )2 L 3 2 C R )2 ] C R )C2 R )[L 2 2 C R ) L 2 C 2 R )]. 59) With K inter etermine, we can now return to 58) an use either of the experimental measurements to etermine the ratio α/k intra. We cannot, however, etermine the two constants separately using these results. To make further progress we consier another experiment with less ligan than receptor, L <R, so that the expression 5) is vali this coul simply be a measurement taken from the earlier stages of the same experiment iscusse above). Consier first an experiment in which no inhibitor is present. Equation 5) then simplifies, an in terms of imensional quantities takes the form ) /2 L )/2 K intra 2α C = L 2 K inter R L ) [ 4α R ) 2 L 4K inter R L ) ) K inter 2 ]. 60) For a given measurement of C, at a ligan concentration L <R, since we alreay know K inter an K intra /α by the proceure outline above, this relation 60) is reaily solve for α. Finally, if we wish also to etermine the issociation constant K mono,wemust carry out a further experiment with nonzero concentration of monovalent ligan inhibitor), I 0. Rewriting 5) in imensional form, the only unknown is now K mono. The problem reuces to solving a cubic equation for K mono, the physicallyrelevant root of which must be selecte. 6.2 K intra = ɛ Asympically Small, Case ii) Long Bivalent Ligan) In this case, regarless of the relative sizes of L an R, the fraction of receptor boun up in complexes of size n 2,, is always asympically small. This makes intuitive sense because if there is less ligan than receptor in the initial mixture then all available ligan is immeiately taken up to form the cyclic -complex, leaving none free to form larger complexes. Available inhibitor can bin to remaining receptor that is not taken up in singleton cyclic complexes C ). On the other han, if there is more ligan than receptor in the initial mix then it will bin all available receptor into singleton cyclic complexes; there will be excess free ligan, an no inhibitor will be able to bin to receptor. No receptor is left over that can form any of the larger complexes. Since is always asympically small an reliable estimates of issociation constants woul therefore be ifficult to obtain), we o not pursue this case in etail; but we briefly emonstrate the above statements in the cases L <R, L >R, below L <R Here, stuy of 22) reveals ligan concentration L to scale with ɛ: L = ɛl 0 + Oɛ 2 ), an 0 is given by the leaing-orer terms in 27). In this case, we cannot simply neglect all terms in L at leaing orer, since 27) contains a term in L/ɛ; so we nee to fin L 0. This we o from 22), which gives
24 94 L.J. Cummings et al. [ R = Z 0 + 2L 0 + I 0 K mono 2 + I )] 0 K mono. 6) The fraction of receptor boun up in complexes of size n 2, 0, is then given to leaing orer) by 0 = Z [ 0 + 2L 0 + I 0 R K mono 2 + I )] 0 K mono = 0, using 6) in the final step. The ominant complex forme is the -cyclic complex; no complexes of size n 2 are forme to leaing orer) L >R In this case, ligan is present in excess an free-receptor concentration is asympically small. The leaing-orer balance in 22) isr 2LZ /ɛ, suggesting that Z scales as ɛ. It is then easily euce from 23) an 24) that Z = ɛz 0 + Oɛ 2 ), L = L R + Oɛ) an I = I + Oɛ);22) then gives Z 0 = R /2R L )). Hence, in 27), retaining only leaing orer terms, 0 = High Cooperativity α, Case i) Short Bivalent Ligan) Here, the formation of large complexes is favore. Figure 3 suggests that this is a nontrivial asympic limit worth exploring, which may yiel useful preictions. In this case, the conition 4αLZ < shows that one or both of L or Z must be asympically small. Again, the asympic scalings epen on the relative quantities of receptor an ligan in the system L <R With L <R receptor is in excess, an all free ligan is taken up to form larger complexes. In this regime, L is asympically small, while Z = O). We know that 0 < 4αLZ <, but we o not know whether this quantity is O) or asympically small. However, the ominant balance in 2) an 23) noting that αl 2 Z must be asympically small because αlz is at most O)) is Z R + I ) 2, 62) 4αLZ ) 2 K mono Z R L + I ) 2 4αLZ ) K mono, 63) showing that take the ratio of 63) an 62)) 4αLZ L. R So, with Z = O), L is of orer /α, an asympic expansions procee as Z = Z 0 + α Z +, L= α L 0 +, I = I 0 + α I +.
25 Analysis of Biochemical Equilibria Relevant to the Immune Response 95 Substitution into 2), 23), 24), an retaining only leaing-orer terms gives Z 0 = x2, L 0 = L ) R 4x 2, I 0 = K mono R L, 64) x where x is the unique positive root of 2 I K mono x2 + x K mono 2 ) K mono R L ) + R L ) = 0. The concentration of nontrivial complexes,, efine in 26), then has a simple leaing-orer expression in terms of L : = L ) 2 + o). 65) R L >R In this case, ligan is in excess, an all available free receptor is boun, leaving only asympically small amounts. Free ligan an inhibitor are present in O) concentrations, however. With R = O), 2) shows that 4αLZ must also be asympically small. Careful consieration of 2) an 23) reveals the correct scalings to be Z = α Z 0 + α 3/2 Z +, L= L 0 + α /2 L +, I = I ) α /2 I +, an substitution of these expansions into 2) an 23) leas to ) Z = 4αL R ) + O α 3/2, L= L R + O I = I + O α /2 ). Equation 26) then gives correct to O/α) as f = 4αR L R ) + 2L R ) + I K mono ) α /2, 67) ) ) This expression is clearly not vali as L R,orasL becomes asympically large. In these cases, a separate analysis is require below) L R When al ligan an receptor concentrations are approximately equal, the concentrations of free ligan an receptor are both asympically small, an the scalings iffer from the cases L <R an L >R consiere in Sects an above. Stuy of 23) shows that, as we transition between these cases, we require
26 96 L.J. Cummings et al. Z 4αLZ. The transition region may be aequately escribe by the istinguishe limit in which R L =Oα /4 ), L Oα /2 ), Z Oα /2 ), 4αLZ Oα /4 ), an I O). With expansions proceeing as Z = Z 0 α /2 + Z α 3/4 +, L= L 0 α /2 + L α 3/4 +, I = I 0 + I, 69) α/4 an 4αLZ = O α /4), 70) the conition 70) givesl 0 Z 0 = /4; 24) trivially gives I 0 = I at leaing orer, while 2) an 23) give, respectively, Z 0 R = 6L 0 Z + L Z 0 ) 2 + I ) 2 K mono, Z 0 α /4 R L = + I ) 2 4L 0 Z + L Z 0 ) K mono. Together these results give Z 0 = α/2 R L ) 2 R + I K mono ), L 2 0 =, I 0 = I. 7) 4Z 0 While these asympic solutions for Z, L, an I are clearly ifferent to those foun in regions L <R an L >R, we nonetheless fin on substitution in 26)) that = L ) 2 + o) vali when L R O α /4), 72) R an expression ientical to 65) from the region L <R L R The expressions erive in Sect for L >R cease to be vali when L Oα). Since ligan is greatly in excess assuming R = O)), L = Oα) also, an writing L = α L where L O)) we assume asympic expansions Z = Z 0 α 2 + Z α 3 +, L= αl 0 + L + L 2 α +, I = I 0 + I α + the scaling for Z comes from the requirement that 4αLZ O)). Leaing orer in 2) then gives R = while Oα) an O) in 23) give 4Z 0 L 2 0 4L 0 Z 0 ) 2, 73) L 0 = L, R = 4L2 0 Z 0 4L 0 Z 0 L
Linear First-Order Equations
 5 Linear First-Orer Equations Linear first-orer ifferential equations make up another important class of ifferential equations that commonly arise in applications an are relatively easy to solve (in theory)
5 Linear First-Orer Equations Linear first-orer ifferential equations make up another important class of ifferential equations that commonly arise in applications an are relatively easy to solve (in theory)
APPROXIMATE SOLUTION FOR TRANSIENT HEAT TRANSFER IN STATIC TURBULENT HE II. B. Baudouy. CEA/Saclay, DSM/DAPNIA/STCM Gif-sur-Yvette Cedex, France
 APPROXIMAE SOLUION FOR RANSIEN HEA RANSFER IN SAIC URBULEN HE II B. Bauouy CEA/Saclay, DSM/DAPNIA/SCM 91191 Gif-sur-Yvette Ceex, France ABSRAC Analytical solution in one imension of the heat iffusion equation
APPROXIMAE SOLUION FOR RANSIEN HEA RANSFER IN SAIC URBULEN HE II B. Bauouy CEA/Saclay, DSM/DAPNIA/SCM 91191 Gif-sur-Yvette Ceex, France ABSRAC Analytical solution in one imension of the heat iffusion equation
Implicit Differentiation
 Implicit Differentiation Thus far, the functions we have been concerne with have been efine explicitly. A function is efine explicitly if the output is given irectly in terms of the input. For instance,
Implicit Differentiation Thus far, the functions we have been concerne with have been efine explicitly. A function is efine explicitly if the output is given irectly in terms of the input. For instance,
19 Eigenvalues, Eigenvectors, Ordinary Differential Equations, and Control
 19 Eigenvalues, Eigenvectors, Orinary Differential Equations, an Control This section introuces eigenvalues an eigenvectors of a matrix, an iscusses the role of the eigenvalues in etermining the behavior
19 Eigenvalues, Eigenvectors, Orinary Differential Equations, an Control This section introuces eigenvalues an eigenvectors of a matrix, an iscusses the role of the eigenvalues in etermining the behavior
The Exact Form and General Integrating Factors
 7 The Exact Form an General Integrating Factors In the previous chapters, we ve seen how separable an linear ifferential equations can be solve using methos for converting them to forms that can be easily
7 The Exact Form an General Integrating Factors In the previous chapters, we ve seen how separable an linear ifferential equations can be solve using methos for converting them to forms that can be easily
Agmon Kolmogorov Inequalities on l 2 (Z d )
 Journal of Mathematics Research; Vol. 6, No. ; 04 ISSN 96-9795 E-ISSN 96-9809 Publishe by Canaian Center of Science an Eucation Agmon Kolmogorov Inequalities on l (Z ) Arman Sahovic Mathematics Department,
Journal of Mathematics Research; Vol. 6, No. ; 04 ISSN 96-9795 E-ISSN 96-9809 Publishe by Canaian Center of Science an Eucation Agmon Kolmogorov Inequalities on l (Z ) Arman Sahovic Mathematics Department,
'HVLJQ &RQVLGHUDWLRQ LQ 0DWHULDO 6HOHFWLRQ 'HVLJQ 6HQVLWLYLW\,1752'8&7,21
 Large amping in a structural material may be either esirable or unesirable, epening on the engineering application at han. For example, amping is a esirable property to the esigner concerne with limiting
Large amping in a structural material may be either esirable or unesirable, epening on the engineering application at han. For example, amping is a esirable property to the esigner concerne with limiting
θ x = f ( x,t) could be written as
 9. Higher orer PDEs as systems of first-orer PDEs. Hyperbolic systems. For PDEs, as for ODEs, we may reuce the orer by efining new epenent variables. For example, in the case of the wave equation, (1)
9. Higher orer PDEs as systems of first-orer PDEs. Hyperbolic systems. For PDEs, as for ODEs, we may reuce the orer by efining new epenent variables. For example, in the case of the wave equation, (1)
The Principle of Least Action
 Chapter 7. The Principle of Least Action 7.1 Force Methos vs. Energy Methos We have so far stuie two istinct ways of analyzing physics problems: force methos, basically consisting of the application of
Chapter 7. The Principle of Least Action 7.1 Force Methos vs. Energy Methos We have so far stuie two istinct ways of analyzing physics problems: force methos, basically consisting of the application of
Thermal conductivity of graded composites: Numerical simulations and an effective medium approximation
 JOURNAL OF MATERIALS SCIENCE 34 (999)5497 5503 Thermal conuctivity of grae composites: Numerical simulations an an effective meium approximation P. M. HUI Department of Physics, The Chinese University
JOURNAL OF MATERIALS SCIENCE 34 (999)5497 5503 Thermal conuctivity of grae composites: Numerical simulations an an effective meium approximation P. M. HUI Department of Physics, The Chinese University
Time-of-Arrival Estimation in Non-Line-Of-Sight Environments
 2 Conference on Information Sciences an Systems, The Johns Hopkins University, March 2, 2 Time-of-Arrival Estimation in Non-Line-Of-Sight Environments Sinan Gezici, Hisashi Kobayashi an H. Vincent Poor
2 Conference on Information Sciences an Systems, The Johns Hopkins University, March 2, 2 Time-of-Arrival Estimation in Non-Line-Of-Sight Environments Sinan Gezici, Hisashi Kobayashi an H. Vincent Poor
Lecture 2 Lagrangian formulation of classical mechanics Mechanics
 Lecture Lagrangian formulation of classical mechanics 70.00 Mechanics Principle of stationary action MATH-GA To specify a motion uniquely in classical mechanics, it suffices to give, at some time t 0,
Lecture Lagrangian formulation of classical mechanics 70.00 Mechanics Principle of stationary action MATH-GA To specify a motion uniquely in classical mechanics, it suffices to give, at some time t 0,
Qubit channels that achieve capacity with two states
 Qubit channels that achieve capacity with two states Dominic W. Berry Department of Physics, The University of Queenslan, Brisbane, Queenslan 4072, Australia Receive 22 December 2004; publishe 22 March
Qubit channels that achieve capacity with two states Dominic W. Berry Department of Physics, The University of Queenslan, Brisbane, Queenslan 4072, Australia Receive 22 December 2004; publishe 22 March
Polynomial Inclusion Functions
 Polynomial Inclusion Functions E. e Weert, E. van Kampen, Q. P. Chu, an J. A. Muler Delft University of Technology, Faculty of Aerospace Engineering, Control an Simulation Division E.eWeert@TUDelft.nl
Polynomial Inclusion Functions E. e Weert, E. van Kampen, Q. P. Chu, an J. A. Muler Delft University of Technology, Faculty of Aerospace Engineering, Control an Simulation Division E.eWeert@TUDelft.nl
6 General properties of an autonomous system of two first order ODE
 6 General properties of an autonomous system of two first orer ODE Here we embark on stuying the autonomous system of two first orer ifferential equations of the form ẋ 1 = f 1 (, x 2 ), ẋ 2 = f 2 (, x
6 General properties of an autonomous system of two first orer ODE Here we embark on stuying the autonomous system of two first orer ifferential equations of the form ẋ 1 = f 1 (, x 2 ), ẋ 2 = f 2 (, x
Unit #6 - Families of Functions, Taylor Polynomials, l Hopital s Rule
 Unit # - Families of Functions, Taylor Polynomials, l Hopital s Rule Some problems an solutions selecte or aapte from Hughes-Hallett Calculus. Critical Points. Consier the function f) = 54 +. b) a) Fin
Unit # - Families of Functions, Taylor Polynomials, l Hopital s Rule Some problems an solutions selecte or aapte from Hughes-Hallett Calculus. Critical Points. Consier the function f) = 54 +. b) a) Fin
Optimization of Geometries by Energy Minimization
 Optimization of Geometries by Energy Minimization by Tracy P. Hamilton Department of Chemistry University of Alabama at Birmingham Birmingham, AL 3594-140 hamilton@uab.eu Copyright Tracy P. Hamilton, 1997.
Optimization of Geometries by Energy Minimization by Tracy P. Hamilton Department of Chemistry University of Alabama at Birmingham Birmingham, AL 3594-140 hamilton@uab.eu Copyright Tracy P. Hamilton, 1997.
Equilibrium in Queues Under Unknown Service Times and Service Value
 University of Pennsylvania ScholarlyCommons Finance Papers Wharton Faculty Research 1-2014 Equilibrium in Queues Uner Unknown Service Times an Service Value Laurens Debo Senthil K. Veeraraghavan University
University of Pennsylvania ScholarlyCommons Finance Papers Wharton Faculty Research 1-2014 Equilibrium in Queues Uner Unknown Service Times an Service Value Laurens Debo Senthil K. Veeraraghavan University
Assignment 1. g i (x 1,..., x n ) dx i = 0. i=1
 Assignment 1 Golstein 1.4 The equations of motion for the rolling isk are special cases of general linear ifferential equations of constraint of the form g i (x 1,..., x n x i = 0. i=1 A constraint conition
Assignment 1 Golstein 1.4 The equations of motion for the rolling isk are special cases of general linear ifferential equations of constraint of the form g i (x 1,..., x n x i = 0. i=1 A constraint conition
Math Notes on differentials, the Chain Rule, gradients, directional derivative, and normal vectors
 Math 18.02 Notes on ifferentials, the Chain Rule, graients, irectional erivative, an normal vectors Tangent plane an linear approximation We efine the partial erivatives of f( xy, ) as follows: f f( x+
Math 18.02 Notes on ifferentials, the Chain Rule, graients, irectional erivative, an normal vectors Tangent plane an linear approximation We efine the partial erivatives of f( xy, ) as follows: f f( x+
Short Intro to Coordinate Transformation
 Short Intro to Coorinate Transformation 1 A Vector A vector can basically be seen as an arrow in space pointing in a specific irection with a specific length. The following problem arises: How o we represent
Short Intro to Coorinate Transformation 1 A Vector A vector can basically be seen as an arrow in space pointing in a specific irection with a specific length. The following problem arises: How o we represent
1 dx. where is a large constant, i.e., 1, (7.6) and Px is of the order of unity. Indeed, if px is given by (7.5), the inequality (7.
 Lectures Nine an Ten The WKB Approximation The WKB metho is a powerful tool to obtain solutions for many physical problems It is generally applicable to problems of wave propagation in which the frequency
Lectures Nine an Ten The WKB Approximation The WKB metho is a powerful tool to obtain solutions for many physical problems It is generally applicable to problems of wave propagation in which the frequency
Table of Common Derivatives By David Abraham
 Prouct an Quotient Rules: Table of Common Derivatives By Davi Abraham [ f ( g( ] = [ f ( ] g( + f ( [ g( ] f ( = g( [ f ( ] g( g( f ( [ g( ] Trigonometric Functions: sin( = cos( cos( = sin( tan( = sec
Prouct an Quotient Rules: Table of Common Derivatives By Davi Abraham [ f ( g( ] = [ f ( ] g( + f ( [ g( ] f ( = g( [ f ( ] g( g( f ( [ g( ] Trigonometric Functions: sin( = cos( cos( = sin( tan( = sec
u t v t v t c a u t b a v t u t v t b a
 Nonlinear Dynamical Systems In orer to iscuss nonlinear ynamical systems, we must first consier linear ynamical systems. Linear ynamical systems are just systems of linear equations like we have been stuying
Nonlinear Dynamical Systems In orer to iscuss nonlinear ynamical systems, we must first consier linear ynamical systems. Linear ynamical systems are just systems of linear equations like we have been stuying
Sparse Reconstruction of Systems of Ordinary Differential Equations
 Sparse Reconstruction of Systems of Orinary Differential Equations Manuel Mai a, Mark D. Shattuck b,c, Corey S. O Hern c,a,,e, a Department of Physics, Yale University, New Haven, Connecticut 06520, USA
Sparse Reconstruction of Systems of Orinary Differential Equations Manuel Mai a, Mark D. Shattuck b,c, Corey S. O Hern c,a,,e, a Department of Physics, Yale University, New Haven, Connecticut 06520, USA
Euler equations for multiple integrals
 Euler equations for multiple integrals January 22, 2013 Contents 1 Reminer of multivariable calculus 2 1.1 Vector ifferentiation......................... 2 1.2 Matrix ifferentiation........................
Euler equations for multiple integrals January 22, 2013 Contents 1 Reminer of multivariable calculus 2 1.1 Vector ifferentiation......................... 2 1.2 Matrix ifferentiation........................
The derivative of a function f(x) is another function, defined in terms of a limiting expression: f(x + δx) f(x)
 Y. D. Chong (2016) MH2801: Complex Methos for the Sciences 1. Derivatives The erivative of a function f(x) is another function, efine in terms of a limiting expression: f (x) f (x) lim x δx 0 f(x + δx)
Y. D. Chong (2016) MH2801: Complex Methos for the Sciences 1. Derivatives The erivative of a function f(x) is another function, efine in terms of a limiting expression: f (x) f (x) lim x δx 0 f(x + δx)
ensembles When working with density operators, we can use this connection to define a generalized Bloch vector: v x Tr x, v y Tr y
 Ph195a lecture notes, 1/3/01 Density operators for spin- 1 ensembles So far in our iscussion of spin- 1 systems, we have restricte our attention to the case of pure states an Hamiltonian evolution. Toay
Ph195a lecture notes, 1/3/01 Density operators for spin- 1 ensembles So far in our iscussion of spin- 1 systems, we have restricte our attention to the case of pure states an Hamiltonian evolution. Toay
Calculus of Variations
 16.323 Lecture 5 Calculus of Variations Calculus of Variations Most books cover this material well, but Kirk Chapter 4 oes a particularly nice job. x(t) x* x*+ αδx (1) x*- αδx (1) αδx (1) αδx (1) t f t
16.323 Lecture 5 Calculus of Variations Calculus of Variations Most books cover this material well, but Kirk Chapter 4 oes a particularly nice job. x(t) x* x*+ αδx (1) x*- αδx (1) αδx (1) αδx (1) t f t
Math 342 Partial Differential Equations «Viktor Grigoryan
 Math 342 Partial Differential Equations «Viktor Grigoryan 6 Wave equation: solution In this lecture we will solve the wave equation on the entire real line x R. This correspons to a string of infinite
Math 342 Partial Differential Equations «Viktor Grigoryan 6 Wave equation: solution In this lecture we will solve the wave equation on the entire real line x R. This correspons to a string of infinite
Lectures - Week 10 Introduction to Ordinary Differential Equations (ODES) First Order Linear ODEs
 Lectures - Week 10 Introuction to Orinary Differential Equations (ODES) First Orer Linear ODEs When stuying ODEs we are consiering functions of one inepenent variable, e.g., f(x), where x is the inepenent
Lectures - Week 10 Introuction to Orinary Differential Equations (ODES) First Orer Linear ODEs When stuying ODEs we are consiering functions of one inepenent variable, e.g., f(x), where x is the inepenent
05 The Continuum Limit and the Wave Equation
 Utah State University DigitalCommons@USU Founations of Wave Phenomena Physics, Department of 1-1-2004 05 The Continuum Limit an the Wave Equation Charles G. Torre Department of Physics, Utah State University,
Utah State University DigitalCommons@USU Founations of Wave Phenomena Physics, Department of 1-1-2004 05 The Continuum Limit an the Wave Equation Charles G. Torre Department of Physics, Utah State University,
Lagrangian and Hamiltonian Mechanics
 Lagrangian an Hamiltonian Mechanics.G. Simpson, Ph.. epartment of Physical Sciences an Engineering Prince George s Community College ecember 5, 007 Introuction In this course we have been stuying classical
Lagrangian an Hamiltonian Mechanics.G. Simpson, Ph.. epartment of Physical Sciences an Engineering Prince George s Community College ecember 5, 007 Introuction In this course we have been stuying classical
Calculus of Variations
 Calculus of Variations Lagrangian formalism is the main tool of theoretical classical mechanics. Calculus of Variations is a part of Mathematics which Lagrangian formalism is base on. In this section,
Calculus of Variations Lagrangian formalism is the main tool of theoretical classical mechanics. Calculus of Variations is a part of Mathematics which Lagrangian formalism is base on. In this section,
Math 1B, lecture 8: Integration by parts
 Math B, lecture 8: Integration by parts Nathan Pflueger 23 September 2 Introuction Integration by parts, similarly to integration by substitution, reverses a well-known technique of ifferentiation an explores
Math B, lecture 8: Integration by parts Nathan Pflueger 23 September 2 Introuction Integration by parts, similarly to integration by substitution, reverses a well-known technique of ifferentiation an explores
Separation of Variables
 Physics 342 Lecture 1 Separation of Variables Lecture 1 Physics 342 Quantum Mechanics I Monay, January 25th, 2010 There are three basic mathematical tools we nee, an then we can begin working on the physical
Physics 342 Lecture 1 Separation of Variables Lecture 1 Physics 342 Quantum Mechanics I Monay, January 25th, 2010 There are three basic mathematical tools we nee, an then we can begin working on the physical
A simple model for the small-strain behaviour of soils
 A simple moel for the small-strain behaviour of soils José Jorge Naer Department of Structural an Geotechnical ngineering, Polytechnic School, University of São Paulo 05508-900, São Paulo, Brazil, e-mail:
A simple moel for the small-strain behaviour of soils José Jorge Naer Department of Structural an Geotechnical ngineering, Polytechnic School, University of São Paulo 05508-900, São Paulo, Brazil, e-mail:
18 EVEN MORE CALCULUS
 8 EVEN MORE CALCULUS Chapter 8 Even More Calculus Objectives After stuing this chapter you shoul be able to ifferentiate an integrate basic trigonometric functions; unerstan how to calculate rates of change;
8 EVEN MORE CALCULUS Chapter 8 Even More Calculus Objectives After stuing this chapter you shoul be able to ifferentiate an integrate basic trigonometric functions; unerstan how to calculate rates of change;
Discrete Mathematics
 Discrete Mathematics 309 (009) 86 869 Contents lists available at ScienceDirect Discrete Mathematics journal homepage: wwwelseviercom/locate/isc Profile vectors in the lattice of subspaces Dániel Gerbner
Discrete Mathematics 309 (009) 86 869 Contents lists available at ScienceDirect Discrete Mathematics journal homepage: wwwelseviercom/locate/isc Profile vectors in the lattice of subspaces Dániel Gerbner
Schrödinger s equation.
 Physics 342 Lecture 5 Schröinger s Equation Lecture 5 Physics 342 Quantum Mechanics I Wenesay, February 3r, 2010 Toay we iscuss Schröinger s equation an show that it supports the basic interpretation of
Physics 342 Lecture 5 Schröinger s Equation Lecture 5 Physics 342 Quantum Mechanics I Wenesay, February 3r, 2010 Toay we iscuss Schröinger s equation an show that it supports the basic interpretation of
SYNCHRONOUS SEQUENTIAL CIRCUITS
 CHAPTER SYNCHRONOUS SEUENTIAL CIRCUITS Registers an counters, two very common synchronous sequential circuits, are introuce in this chapter. Register is a igital circuit for storing information. Contents
CHAPTER SYNCHRONOUS SEUENTIAL CIRCUITS Registers an counters, two very common synchronous sequential circuits, are introuce in this chapter. Register is a igital circuit for storing information. Contents
Semiclassical analysis of long-wavelength multiphoton processes: The Rydberg atom
 PHYSICAL REVIEW A 69, 063409 (2004) Semiclassical analysis of long-wavelength multiphoton processes: The Ryberg atom Luz V. Vela-Arevalo* an Ronal F. Fox Center for Nonlinear Sciences an School of Physics,
PHYSICAL REVIEW A 69, 063409 (2004) Semiclassical analysis of long-wavelength multiphoton processes: The Ryberg atom Luz V. Vela-Arevalo* an Ronal F. Fox Center for Nonlinear Sciences an School of Physics,
Computing Exact Confidence Coefficients of Simultaneous Confidence Intervals for Multinomial Proportions and their Functions
 Working Paper 2013:5 Department of Statistics Computing Exact Confience Coefficients of Simultaneous Confience Intervals for Multinomial Proportions an their Functions Shaobo Jin Working Paper 2013:5
Working Paper 2013:5 Department of Statistics Computing Exact Confience Coefficients of Simultaneous Confience Intervals for Multinomial Proportions an their Functions Shaobo Jin Working Paper 2013:5
Lecture 6: Calculus. In Song Kim. September 7, 2011
 Lecture 6: Calculus In Song Kim September 7, 20 Introuction to Differential Calculus In our previous lecture we came up with several ways to analyze functions. We saw previously that the slope of a linear
Lecture 6: Calculus In Song Kim September 7, 20 Introuction to Differential Calculus In our previous lecture we came up with several ways to analyze functions. We saw previously that the slope of a linear
JUST THE MATHS UNIT NUMBER DIFFERENTIATION 2 (Rates of change) A.J.Hobson
 JUST THE MATHS UNIT NUMBER 10.2 DIFFERENTIATION 2 (Rates of change) by A.J.Hobson 10.2.1 Introuction 10.2.2 Average rates of change 10.2.3 Instantaneous rates of change 10.2.4 Derivatives 10.2.5 Exercises
JUST THE MATHS UNIT NUMBER 10.2 DIFFERENTIATION 2 (Rates of change) by A.J.Hobson 10.2.1 Introuction 10.2.2 Average rates of change 10.2.3 Instantaneous rates of change 10.2.4 Derivatives 10.2.5 Exercises
Convective heat transfer
 CHAPTER VIII Convective heat transfer The previous two chapters on issipative fluis were evote to flows ominate either by viscous effects (Chap. VI) or by convective motion (Chap. VII). In either case,
CHAPTER VIII Convective heat transfer The previous two chapters on issipative fluis were evote to flows ominate either by viscous effects (Chap. VI) or by convective motion (Chap. VII). In either case,
Some vector algebra and the generalized chain rule Ross Bannister Data Assimilation Research Centre, University of Reading, UK Last updated 10/06/10
 Some vector algebra an the generalize chain rule Ross Bannister Data Assimilation Research Centre University of Reaing UK Last upate 10/06/10 1. Introuction an notation As we shall see in these notes the
Some vector algebra an the generalize chain rule Ross Bannister Data Assimilation Research Centre University of Reaing UK Last upate 10/06/10 1. Introuction an notation As we shall see in these notes the
Chapter 9 Method of Weighted Residuals
 Chapter 9 Metho of Weighte Resiuals 9- Introuction Metho of Weighte Resiuals (MWR) is an approimate technique for solving bounary value problems. It utilizes a trial functions satisfying the prescribe
Chapter 9 Metho of Weighte Resiuals 9- Introuction Metho of Weighte Resiuals (MWR) is an approimate technique for solving bounary value problems. It utilizes a trial functions satisfying the prescribe
Conservation Laws. Chapter Conservation of Energy
 20 Chapter 3 Conservation Laws In orer to check the physical consistency of the above set of equations governing Maxwell-Lorentz electroynamics [(2.10) an (2.12) or (1.65) an (1.68)], we examine the action
20 Chapter 3 Conservation Laws In orer to check the physical consistency of the above set of equations governing Maxwell-Lorentz electroynamics [(2.10) an (2.12) or (1.65) an (1.68)], we examine the action
Final Exam Study Guide and Practice Problems Solutions
 Final Exam Stuy Guie an Practice Problems Solutions Note: These problems are just some of the types of problems that might appear on the exam. However, to fully prepare for the exam, in aition to making
Final Exam Stuy Guie an Practice Problems Solutions Note: These problems are just some of the types of problems that might appear on the exam. However, to fully prepare for the exam, in aition to making
Diophantine Approximations: Examining the Farey Process and its Method on Producing Best Approximations
 Diophantine Approximations: Examining the Farey Process an its Metho on Proucing Best Approximations Kelly Bowen Introuction When a person hears the phrase irrational number, one oes not think of anything
Diophantine Approximations: Examining the Farey Process an its Metho on Proucing Best Approximations Kelly Bowen Introuction When a person hears the phrase irrational number, one oes not think of anything
Calculus and optimization
 Calculus an optimization These notes essentially correspon to mathematical appenix 2 in the text. 1 Functions of a single variable Now that we have e ne functions we turn our attention to calculus. A function
Calculus an optimization These notes essentially correspon to mathematical appenix 2 in the text. 1 Functions of a single variable Now that we have e ne functions we turn our attention to calculus. A function
Applications of First Order Equations
 Applications of First Orer Equations Viscous Friction Consier a small mass that has been roppe into a thin vertical tube of viscous flui lie oil. The mass falls, ue to the force of gravity, but falls more
Applications of First Orer Equations Viscous Friction Consier a small mass that has been roppe into a thin vertical tube of viscous flui lie oil. The mass falls, ue to the force of gravity, but falls more
Chapter 6: Energy-Momentum Tensors
 49 Chapter 6: Energy-Momentum Tensors This chapter outlines the general theory of energy an momentum conservation in terms of energy-momentum tensors, then applies these ieas to the case of Bohm's moel.
49 Chapter 6: Energy-Momentum Tensors This chapter outlines the general theory of energy an momentum conservation in terms of energy-momentum tensors, then applies these ieas to the case of Bohm's moel.
ALGEBRAIC AND ANALYTIC PROPERTIES OF ARITHMETIC FUNCTIONS
 ALGEBRAIC AND ANALYTIC PROPERTIES OF ARITHMETIC FUNCTIONS MARK SCHACHNER Abstract. When consiere as an algebraic space, the set of arithmetic functions equippe with the operations of pointwise aition an
ALGEBRAIC AND ANALYTIC PROPERTIES OF ARITHMETIC FUNCTIONS MARK SCHACHNER Abstract. When consiere as an algebraic space, the set of arithmetic functions equippe with the operations of pointwise aition an
Hyperbolic Systems of Equations Posed on Erroneous Curved Domains
 Hyperbolic Systems of Equations Pose on Erroneous Curve Domains Jan Norström a, Samira Nikkar b a Department of Mathematics, Computational Mathematics, Linköping University, SE-58 83 Linköping, Sween (
Hyperbolic Systems of Equations Pose on Erroneous Curve Domains Jan Norström a, Samira Nikkar b a Department of Mathematics, Computational Mathematics, Linköping University, SE-58 83 Linköping, Sween (
Least-Squares Regression on Sparse Spaces
 Least-Squares Regression on Sparse Spaces Yuri Grinberg, Mahi Milani Far, Joelle Pineau School of Computer Science McGill University Montreal, Canaa {ygrinb,mmilan1,jpineau}@cs.mcgill.ca 1 Introuction
Least-Squares Regression on Sparse Spaces Yuri Grinberg, Mahi Milani Far, Joelle Pineau School of Computer Science McGill University Montreal, Canaa {ygrinb,mmilan1,jpineau}@cs.mcgill.ca 1 Introuction
4. Important theorems in quantum mechanics
 TFY4215 Kjemisk fysikk og kvantemekanikk - Tillegg 4 1 TILLEGG 4 4. Important theorems in quantum mechanics Before attacking three-imensional potentials in the next chapter, we shall in chapter 4 of this
TFY4215 Kjemisk fysikk og kvantemekanikk - Tillegg 4 1 TILLEGG 4 4. Important theorems in quantum mechanics Before attacking three-imensional potentials in the next chapter, we shall in chapter 4 of this
Calculus in the AP Physics C Course The Derivative
 Limits an Derivatives Calculus in the AP Physics C Course The Derivative In physics, the ieas of the rate change of a quantity (along with the slope of a tangent line) an the area uner a curve are essential.
Limits an Derivatives Calculus in the AP Physics C Course The Derivative In physics, the ieas of the rate change of a quantity (along with the slope of a tangent line) an the area uner a curve are essential.
Lower Bounds for the Smoothed Number of Pareto optimal Solutions
 Lower Bouns for the Smoothe Number of Pareto optimal Solutions Tobias Brunsch an Heiko Röglin Department of Computer Science, University of Bonn, Germany brunsch@cs.uni-bonn.e, heiko@roeglin.org Abstract.
Lower Bouns for the Smoothe Number of Pareto optimal Solutions Tobias Brunsch an Heiko Röglin Department of Computer Science, University of Bonn, Germany brunsch@cs.uni-bonn.e, heiko@roeglin.org Abstract.
A note on the Mooney-Rivlin material model
 A note on the Mooney-Rivlin material moel I-Shih Liu Instituto e Matemática Universiae Feeral o Rio e Janeiro 2945-97, Rio e Janeiro, Brasil Abstract In finite elasticity, the Mooney-Rivlin material moel
A note on the Mooney-Rivlin material moel I-Shih Liu Instituto e Matemática Universiae Feeral o Rio e Janeiro 2945-97, Rio e Janeiro, Brasil Abstract In finite elasticity, the Mooney-Rivlin material moel
How to Minimize Maximum Regret in Repeated Decision-Making
 How to Minimize Maximum Regret in Repeate Decision-Making Karl H. Schlag July 3 2003 Economics Department, European University Institute, Via ella Piazzuola 43, 033 Florence, Italy, Tel: 0039-0-4689, email:
How to Minimize Maximum Regret in Repeate Decision-Making Karl H. Schlag July 3 2003 Economics Department, European University Institute, Via ella Piazzuola 43, 033 Florence, Italy, Tel: 0039-0-4689, email:
Math 1271 Solutions for Fall 2005 Final Exam
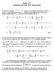 Math 7 Solutions for Fall 5 Final Eam ) Since the equation + y = e y cannot be rearrange algebraically in orer to write y as an eplicit function of, we must instea ifferentiate this relation implicitly
Math 7 Solutions for Fall 5 Final Eam ) Since the equation + y = e y cannot be rearrange algebraically in orer to write y as an eplicit function of, we must instea ifferentiate this relation implicitly
Survey Sampling. 1 Design-based Inference. Kosuke Imai Department of Politics, Princeton University. February 19, 2013
 Survey Sampling Kosuke Imai Department of Politics, Princeton University February 19, 2013 Survey sampling is one of the most commonly use ata collection methos for social scientists. We begin by escribing
Survey Sampling Kosuke Imai Department of Politics, Princeton University February 19, 2013 Survey sampling is one of the most commonly use ata collection methos for social scientists. We begin by escribing
Analytic Scaling Formulas for Crossed Laser Acceleration in Vacuum
 October 6, 4 ARDB Note Analytic Scaling Formulas for Crosse Laser Acceleration in Vacuum Robert J. Noble Stanfor Linear Accelerator Center, Stanfor University 575 San Hill Roa, Menlo Park, California 945
October 6, 4 ARDB Note Analytic Scaling Formulas for Crosse Laser Acceleration in Vacuum Robert J. Noble Stanfor Linear Accelerator Center, Stanfor University 575 San Hill Roa, Menlo Park, California 945
1 Introuction In the past few years there has been renewe interest in the nerson impurity moel. This moel was originally propose by nerson [2], for a
![1 Introuction In the past few years there has been renewe interest in the nerson impurity moel. This moel was originally propose by nerson [2], for a 1 Introuction In the past few years there has been renewe interest in the nerson impurity moel. This moel was originally propose by nerson [2], for a](/thumbs/77/74534741.jpg) Theory of the nerson impurity moel: The Schrieer{Wol transformation re{examine Stefan K. Kehrein 1 an nreas Mielke 2 Institut fur Theoretische Physik, uprecht{karls{universitat, D{69120 Heielberg, F..
Theory of the nerson impurity moel: The Schrieer{Wol transformation re{examine Stefan K. Kehrein 1 an nreas Mielke 2 Institut fur Theoretische Physik, uprecht{karls{universitat, D{69120 Heielberg, F..
Introduction to variational calculus: Lecture notes 1
 October 10, 2006 Introuction to variational calculus: Lecture notes 1 Ewin Langmann Mathematical Physics, KTH Physics, AlbaNova, SE-106 91 Stockholm, Sween Abstract I give an informal summary of variational
October 10, 2006 Introuction to variational calculus: Lecture notes 1 Ewin Langmann Mathematical Physics, KTH Physics, AlbaNova, SE-106 91 Stockholm, Sween Abstract I give an informal summary of variational
A Path Planning Method Using Cubic Spiral with Curvature Constraint
 A Path Planning Metho Using Cubic Spiral with Curvature Constraint Tzu-Chen Liang an Jing-Sin Liu Institute of Information Science 0, Acaemia Sinica, Nankang, Taipei 5, Taiwan, R.O.C., Email: hartree@iis.sinica.eu.tw
A Path Planning Metho Using Cubic Spiral with Curvature Constraint Tzu-Chen Liang an Jing-Sin Liu Institute of Information Science 0, Acaemia Sinica, Nankang, Taipei 5, Taiwan, R.O.C., Email: hartree@iis.sinica.eu.tw
On the number of isolated eigenvalues of a pair of particles in a quantum wire
 On the number of isolate eigenvalues of a pair of particles in a quantum wire arxiv:1812.11804v1 [math-ph] 31 Dec 2018 Joachim Kerner 1 Department of Mathematics an Computer Science FernUniversität in
On the number of isolate eigenvalues of a pair of particles in a quantum wire arxiv:1812.11804v1 [math-ph] 31 Dec 2018 Joachim Kerner 1 Department of Mathematics an Computer Science FernUniversität in
arxiv:physics/ v2 [physics.ed-ph] 23 Sep 2003
![arxiv:physics/ v2 [physics.ed-ph] 23 Sep 2003 arxiv:physics/ v2 [physics.ed-ph] 23 Sep 2003](/thumbs/79/79115961.jpg) Mass reistribution in variable mass systems Célia A. e Sousa an Vítor H. Rorigues Departamento e Física a Universiae e Coimbra, P-3004-516 Coimbra, Portugal arxiv:physics/0211075v2 [physics.e-ph] 23 Sep
Mass reistribution in variable mass systems Célia A. e Sousa an Vítor H. Rorigues Departamento e Física a Universiae e Coimbra, P-3004-516 Coimbra, Portugal arxiv:physics/0211075v2 [physics.e-ph] 23 Sep
Quantum mechanical approaches to the virial
 Quantum mechanical approaches to the virial S.LeBohec Department of Physics an Astronomy, University of Utah, Salt Lae City, UT 84112, USA Date: June 30 th 2015 In this note, we approach the virial from
Quantum mechanical approaches to the virial S.LeBohec Department of Physics an Astronomy, University of Utah, Salt Lae City, UT 84112, USA Date: June 30 th 2015 In this note, we approach the virial from
MATH 205 Practice Final Exam Name:
 MATH 205 Practice Final Eam Name:. (2 points) Consier the function g() = e. (a) (5 points) Ientify the zeroes, vertical asymptotes, an long-term behavior on both sies of this function. Clearly label which
MATH 205 Practice Final Eam Name:. (2 points) Consier the function g() = e. (a) (5 points) Ientify the zeroes, vertical asymptotes, an long-term behavior on both sies of this function. Clearly label which
FLUCTUATIONS IN THE NUMBER OF POINTS ON SMOOTH PLANE CURVES OVER FINITE FIELDS. 1. Introduction
 FLUCTUATIONS IN THE NUMBER OF POINTS ON SMOOTH PLANE CURVES OVER FINITE FIELDS ALINA BUCUR, CHANTAL DAVID, BROOKE FEIGON, MATILDE LALÍN 1 Introuction In this note, we stuy the fluctuations in the number
FLUCTUATIONS IN THE NUMBER OF POINTS ON SMOOTH PLANE CURVES OVER FINITE FIELDS ALINA BUCUR, CHANTAL DAVID, BROOKE FEIGON, MATILDE LALÍN 1 Introuction In this note, we stuy the fluctuations in the number
THE VAN KAMPEN EXPANSION FOR LINKED DUFFING LINEAR OSCILLATORS EXCITED BY COLORED NOISE
 Journal of Soun an Vibration (1996) 191(3), 397 414 THE VAN KAMPEN EXPANSION FOR LINKED DUFFING LINEAR OSCILLATORS EXCITED BY COLORED NOISE E. M. WEINSTEIN Galaxy Scientific Corporation, 2500 English Creek
Journal of Soun an Vibration (1996) 191(3), 397 414 THE VAN KAMPEN EXPANSION FOR LINKED DUFFING LINEAR OSCILLATORS EXCITED BY COLORED NOISE E. M. WEINSTEIN Galaxy Scientific Corporation, 2500 English Creek
arxiv: v4 [math.pr] 27 Jul 2016
![arxiv: v4 [math.pr] 27 Jul 2016 arxiv: v4 [math.pr] 27 Jul 2016](/thumbs/93/113740839.jpg) The Asymptotic Distribution of the Determinant of a Ranom Correlation Matrix arxiv:309768v4 mathpr] 7 Jul 06 AM Hanea a, & GF Nane b a Centre of xcellence for Biosecurity Risk Analysis, University of Melbourne,
The Asymptotic Distribution of the Determinant of a Ranom Correlation Matrix arxiv:309768v4 mathpr] 7 Jul 06 AM Hanea a, & GF Nane b a Centre of xcellence for Biosecurity Risk Analysis, University of Melbourne,
Chapter 2 Lagrangian Modeling
 Chapter 2 Lagrangian Moeling The basic laws of physics are use to moel every system whether it is electrical, mechanical, hyraulic, or any other energy omain. In mechanics, Newton s laws of motion provie
Chapter 2 Lagrangian Moeling The basic laws of physics are use to moel every system whether it is electrical, mechanical, hyraulic, or any other energy omain. In mechanics, Newton s laws of motion provie
Quantum Mechanics in Three Dimensions
 Physics 342 Lecture 20 Quantum Mechanics in Three Dimensions Lecture 20 Physics 342 Quantum Mechanics I Monay, March 24th, 2008 We begin our spherical solutions with the simplest possible case zero potential.
Physics 342 Lecture 20 Quantum Mechanics in Three Dimensions Lecture 20 Physics 342 Quantum Mechanics I Monay, March 24th, 2008 We begin our spherical solutions with the simplest possible case zero potential.
3.7 Implicit Differentiation -- A Brief Introduction -- Student Notes
 Fin these erivatives of these functions: y.7 Implicit Differentiation -- A Brief Introuction -- Stuent Notes tan y sin tan = sin y e = e = Write the inverses of these functions: y tan y sin How woul we
Fin these erivatives of these functions: y.7 Implicit Differentiation -- A Brief Introuction -- Stuent Notes tan y sin tan = sin y e = e = Write the inverses of these functions: y tan y sin How woul we
TMA 4195 Matematisk modellering Exam Tuesday December 16, :00 13:00 Problems and solution with additional comments
 Problem F U L W D g m 3 2 s 2 0 0 0 0 2 kg 0 0 0 0 0 0 Table : Dimension matrix TMA 495 Matematisk moellering Exam Tuesay December 6, 2008 09:00 3:00 Problems an solution with aitional comments The necessary
Problem F U L W D g m 3 2 s 2 0 0 0 0 2 kg 0 0 0 0 0 0 Table : Dimension matrix TMA 495 Matematisk moellering Exam Tuesay December 6, 2008 09:00 3:00 Problems an solution with aitional comments The necessary
Diagonalization of Matrices Dr. E. Jacobs
 Diagonalization of Matrices Dr. E. Jacobs One of the very interesting lessons in this course is how certain algebraic techniques can be use to solve ifferential equations. The purpose of these notes is
Diagonalization of Matrices Dr. E. Jacobs One of the very interesting lessons in this course is how certain algebraic techniques can be use to solve ifferential equations. The purpose of these notes is
Analytical accuracy of the one dimensional heat transfer in geometry with logarithmic various surfaces
 Cent. Eur. J. Eng. 4(4) 014 341-351 DOI: 10.478/s13531-013-0176-8 Central European Journal of Engineering Analytical accuracy of the one imensional heat transfer in geometry with logarithmic various surfaces
Cent. Eur. J. Eng. 4(4) 014 341-351 DOI: 10.478/s13531-013-0176-8 Central European Journal of Engineering Analytical accuracy of the one imensional heat transfer in geometry with logarithmic various surfaces
Linear and quadratic approximation
 Linear an quaratic approximation November 11, 2013 Definition: Suppose f is a function that is ifferentiable on an interval I containing the point a. The linear approximation to f at a is the linear function
Linear an quaratic approximation November 11, 2013 Definition: Suppose f is a function that is ifferentiable on an interval I containing the point a. The linear approximation to f at a is the linear function
Kolmogorov spectra of weak turbulence in media with two types of interacting waves
 3 Decemer 2001 Physics Letters A 291 (2001) 139 145 www.elsevier.com/locate/pla Kolmogorov spectra of wea turulence in meia with two types of interacting waves F. Dias a P. Guyenne V.E.Zaharov c a Centre
3 Decemer 2001 Physics Letters A 291 (2001) 139 145 www.elsevier.com/locate/pla Kolmogorov spectra of wea turulence in meia with two types of interacting waves F. Dias a P. Guyenne V.E.Zaharov c a Centre
PDE Notes, Lecture #11
 PDE Notes, Lecture # from Professor Jalal Shatah s Lectures Febuary 9th, 2009 Sobolev Spaces Recall that for u L loc we can efine the weak erivative Du by Du, φ := udφ φ C0 If v L loc such that Du, φ =
PDE Notes, Lecture # from Professor Jalal Shatah s Lectures Febuary 9th, 2009 Sobolev Spaces Recall that for u L loc we can efine the weak erivative Du by Du, φ := udφ φ C0 If v L loc such that Du, φ =
LATTICE-BASED D-OPTIMUM DESIGN FOR FOURIER REGRESSION
 The Annals of Statistics 1997, Vol. 25, No. 6, 2313 2327 LATTICE-BASED D-OPTIMUM DESIGN FOR FOURIER REGRESSION By Eva Riccomagno, 1 Rainer Schwabe 2 an Henry P. Wynn 1 University of Warwick, Technische
The Annals of Statistics 1997, Vol. 25, No. 6, 2313 2327 LATTICE-BASED D-OPTIMUM DESIGN FOR FOURIER REGRESSION By Eva Riccomagno, 1 Rainer Schwabe 2 an Henry P. Wynn 1 University of Warwick, Technische
Sturm-Liouville Theory
 LECTURE 5 Sturm-Liouville Theory In the three preceing lectures I emonstrate the utility of Fourier series in solving PDE/BVPs. As we ll now see, Fourier series are just the tip of the iceberg of the theory
LECTURE 5 Sturm-Liouville Theory In the three preceing lectures I emonstrate the utility of Fourier series in solving PDE/BVPs. As we ll now see, Fourier series are just the tip of the iceberg of the theory
Vectors in two dimensions
 Vectors in two imensions Until now, we have been working in one imension only The main reason for this is to become familiar with the main physical ieas like Newton s secon law, without the aitional complication
Vectors in two imensions Until now, we have been working in one imension only The main reason for this is to become familiar with the main physical ieas like Newton s secon law, without the aitional complication
On Characterizing the Delay-Performance of Wireless Scheduling Algorithms
 On Characterizing the Delay-Performance of Wireless Scheuling Algorithms Xiaojun Lin Center for Wireless Systems an Applications School of Electrical an Computer Engineering, Purue University West Lafayette,
On Characterizing the Delay-Performance of Wireless Scheuling Algorithms Xiaojun Lin Center for Wireless Systems an Applications School of Electrical an Computer Engineering, Purue University West Lafayette,
Physics 5153 Classical Mechanics. The Virial Theorem and The Poisson Bracket-1
 Physics 5153 Classical Mechanics The Virial Theorem an The Poisson Bracket 1 Introuction In this lecture we will consier two applications of the Hamiltonian. The first, the Virial Theorem, applies to systems
Physics 5153 Classical Mechanics The Virial Theorem an The Poisson Bracket 1 Introuction In this lecture we will consier two applications of the Hamiltonian. The first, the Virial Theorem, applies to systems
arxiv: v2 [cond-mat.stat-mech] 11 Nov 2016
![arxiv: v2 [cond-mat.stat-mech] 11 Nov 2016 arxiv: v2 [cond-mat.stat-mech] 11 Nov 2016](/thumbs/75/72611610.jpg) Noname manuscript No. (will be inserte by the eitor) Scaling properties of the number of ranom sequential asorption iterations neee to generate saturate ranom packing arxiv:607.06668v2 [con-mat.stat-mech]
Noname manuscript No. (will be inserte by the eitor) Scaling properties of the number of ranom sequential asorption iterations neee to generate saturate ranom packing arxiv:607.06668v2 [con-mat.stat-mech]
water adding dye partial mixing homogenization time
 iffusion iffusion is a process of mass transport that involves the movement of one atomic species into another. It occurs by ranom atomic jumps from one position to another an takes place in the gaseous,
iffusion iffusion is a process of mass transport that involves the movement of one atomic species into another. It occurs by ranom atomic jumps from one position to another an takes place in the gaseous,
Switching Time Optimization in Discretized Hybrid Dynamical Systems
 Switching Time Optimization in Discretize Hybri Dynamical Systems Kathrin Flaßkamp, To Murphey, an Sina Ober-Blöbaum Abstract Switching time optimization (STO) arises in systems that have a finite set
Switching Time Optimization in Discretize Hybri Dynamical Systems Kathrin Flaßkamp, To Murphey, an Sina Ober-Blöbaum Abstract Switching time optimization (STO) arises in systems that have a finite set
NON-ADIABATIC COMBUSTION WAVES FOR GENERAL LEWIS NUMBERS: WAVE SPEED AND EXTINCTION CONDITIONS
 ANZIAM J. 46(2004), 1 16 NON-ADIABATIC COMBUSTION WAVES FOR GENERAL LEWIS NUMBERS: WAVE SPEED AND EXTINCTION CONDITIONS A. C. MCINTOSH 1,R.O.WEBER 2 ang.n.mercer 2 (Receive 14 August, 2002) Abstract This
ANZIAM J. 46(2004), 1 16 NON-ADIABATIC COMBUSTION WAVES FOR GENERAL LEWIS NUMBERS: WAVE SPEED AND EXTINCTION CONDITIONS A. C. MCINTOSH 1,R.O.WEBER 2 ang.n.mercer 2 (Receive 14 August, 2002) Abstract This
Modeling the effects of polydispersity on the viscosity of noncolloidal hard sphere suspensions. Paul M. Mwasame, Norman J. Wagner, Antony N.
 Submitte to the Journal of Rheology Moeling the effects of polyispersity on the viscosity of noncolloial har sphere suspensions Paul M. Mwasame, Norman J. Wagner, Antony N. Beris a) epartment of Chemical
Submitte to the Journal of Rheology Moeling the effects of polyispersity on the viscosity of noncolloial har sphere suspensions Paul M. Mwasame, Norman J. Wagner, Antony N. Beris a) epartment of Chemical
One-dimensional I test and direction vector I test with array references by induction variable
 Int. J. High Performance Computing an Networking, Vol. 3, No. 4, 2005 219 One-imensional I test an irection vector I test with array references by inuction variable Minyi Guo School of Computer Science
Int. J. High Performance Computing an Networking, Vol. 3, No. 4, 2005 219 One-imensional I test an irection vector I test with array references by inuction variable Minyi Guo School of Computer Science
II. First variation of functionals
 II. First variation of functionals The erivative of a function being zero is a necessary conition for the etremum of that function in orinary calculus. Let us now tackle the question of the equivalent
II. First variation of functionals The erivative of a function being zero is a necessary conition for the etremum of that function in orinary calculus. Let us now tackle the question of the equivalent
Strength Analysis of CFRP Composite Material Considering Multiple Fracture Modes
 5--XXXX Strength Analysis of CFRP Composite Material Consiering Multiple Fracture Moes Author, co-author (Do NOT enter this information. It will be pulle from participant tab in MyTechZone) Affiliation
5--XXXX Strength Analysis of CFRP Composite Material Consiering Multiple Fracture Moes Author, co-author (Do NOT enter this information. It will be pulle from participant tab in MyTechZone) Affiliation
q = F If we integrate this equation over all the mass in a star, we have q dm = F (M) F (0)
 Astronomy 112: The Physics of Stars Class 4 Notes: Energy an Chemical Balance in Stars In the last class we introuce the iea of hyrostatic balance in stars, an showe that we coul use this concept to erive
Astronomy 112: The Physics of Stars Class 4 Notes: Energy an Chemical Balance in Stars In the last class we introuce the iea of hyrostatic balance in stars, an showe that we coul use this concept to erive
Mathematical Biosciences
 Mathematical Biosciences 26 (2008) 40 49 Contents lists available at ScienceDirect Mathematical Biosciences journal homepage: www.elsevier.com/late/mbs Homotopy methos for counting reaction network equilibria
Mathematical Biosciences 26 (2008) 40 49 Contents lists available at ScienceDirect Mathematical Biosciences journal homepage: www.elsevier.com/late/mbs Homotopy methos for counting reaction network equilibria
Characterizing Real-Valued Multivariate Complex Polynomials and Their Symmetric Tensor Representations
 Characterizing Real-Value Multivariate Complex Polynomials an Their Symmetric Tensor Representations Bo JIANG Zhening LI Shuzhong ZHANG December 31, 2014 Abstract In this paper we stuy multivariate polynomial
Characterizing Real-Value Multivariate Complex Polynomials an Their Symmetric Tensor Representations Bo JIANG Zhening LI Shuzhong ZHANG December 31, 2014 Abstract In this paper we stuy multivariate polynomial
