Diffusional Growth of Liquid Phase Hydrometeros.
|
|
|
- Allan Waters
- 5 years ago
- Views:
Transcription
1 Diffusional Growth of Liquid Phase Hydrometeros. I. Diffusional Growth of Liquid Phase Hydrometeors A. Basic concepts of diffusional growth. 1. To understand the diffusional growth of a droplet, we must consider two important processes a. Water vapor is transferred to the droplet by vapor diffusion i. A gradient of vapor develops around a drop that is growing or evaporating - the droplet is not in equilibrium b. Condensation (evaporation) results in latent heat release (absorption) i. The net effect is that the droplet is warmed (cooled) ii. The warming (cooling) slows the growth (evaporation ) of the droplet as the saturation vapor density is directly related to the temperature iii. If the drop is to grow, the excess heat must be removed. This can occur by conduction with the environment. B. In summary, we must be concerned with 1. The mass flux of water molecules toward/away from the droplet 2. The conduction of heat away/toward the droplet 3. We will solve these individually as well as simultaneously with the help of the Clausius - Clapeyron equation. a. Alternative methods are also possible; however, we will not look at these. C. Assumptions: 1. Initial work will be based on the following assumptions a. Critical radius droplet b. Stationary droplet c. Isolated droplet d. Spherical (symmetric) Area = 4πa 2, where a is droplet radius D. Mass flux of vapor Now the flux of vapor molecules to / from the surface of a droplet is 1. The mass rate of change a. The mass flux toward the drop through radius r from the drop is proportional to i. the gradient of vapor density through any radial ii. diffusivity of water vapor iii. area of a shell with radius r b. with ρ = mn dm / dt = 4π D a[ρ v, - ρ v,a ] i. The droplet mass change is a function of i. radius a ii. diffusivity iii. [ρ v, - ρ v,a ] if [ρ v, - ρ v,a ] > 1; dm/dt > 0 if [ρ v, - ρ v,a ] < 1; dm/dt < 0 2. Growth due to mass flux in terms of radius 1
2 M = ρ w V = ρ w π 4/3 a 3 (ρ w is density of water) dm / dt = 4π D a [ρ v, - ρ v,a ] ρ w 4/3 π da 3 / dt = 4π D a [ρ v, - ρ v,a ] ρ w (4/3) π 3a 2 da / dt = 4π D a [ρ v, - ρ v,a ] ρ w 4π a 2 da / dt = 4π D a [ρ v, - ρ v,a ] ρ w a da / dt = D [ρ v, - ρ v,a ] a da / dt = D [ρ v, - ρ v,a ] / ρ w a. We can integrate this to get a as a function of time t for mass flux of water vapor... [a 2 (t1) - a 2 (t0)]/ 2 = D [ρ v, - ρ v,a ] (t1-t0) / ρ w t1 = t0 + ρ w [a 2 (t1) - a 2 (t0)] {2 D [ρ v, - ρ v,a ] } b. The time for a droplet to grow by vapor diffusion given SS, T, and initial radius i. Initial conditions D = 2.11x10-5 m 2 /s ρ w = 1000 kg / m 3 SS = 1.01 ρ v, = 4.895x10-3 kg/m 3 T = K p = Pa r(t0) = 5x10-6 m radius dt t+dt (m) (sec) (accumulated) 5x10-6 m x10-6 m 20x10-6 m 30x10-6 m 40x10-6 m 50x10-6 m 100x10-6 m 400x10-6 m 1000x10-6 m 5000x10-6 m E. The conduction of heat away / toward the droplet As a droplet grows, the droplet heats. This increases its saturation vapor pressure for equilibrium over the drop surface. For a steady state drop growth, we find the steady state droplet temperature. 1. This derivation is analogous to that for mass flux of water vapor 2. The heat flux equation necessary to keep droplet T constant a. The conduction (diffusion) of heat toward the droplet at radius r from the drop is proportional to i. the gradient of temperature through any radial 2
3 ii. thermal conductivity iii. area of a shell with radius r (see picture from before) -dq / dt = 4π a 2 K {a[t a - T ]/r + T }/ r (r=a) d. T / r = 0, so -dq / dt = 4π a 2 K {a[t a - T ]/r}/ r (r=a) e. or, noting (1/r)/ r = -1/r 2 r/ r -dq / dt = 4π a 2 K {-a[t a - T ]/r 2 r/ r} (r=a) f. thus, with r = a (droplet surface) -dq / dt = 4π a 2 K {-a[t a - T ]/a 2 } -dq / dt = 4π K a[t - T a ] g. The droplet heat change is a function of i. radius a ii. conductivity iii. Thermal gradient if [T - T a ]< 1; -dq/dt > 0 if [T - T a ]> 1; -dq/dt < 0 3 This equation can be re-written in terms of mass change noting that -dq/dt = -L dm/dt where L is the latent heat of vaporization -L dm / dt = 4π K a[t - T a ] F. Now we need to discuss the balance conditions for the heats associated with condensation and conduction. 1. We have an equation for mass flux of vapor (which multiplied by L yields heat released during condensation) a. dm / dt = 4π D a [ρ v, - ρ v,a ] 2. We also have an equation for the heat conduction a. -dq / dt = 4π K a [T - T a ] 3. The rate of change of surface temperature of the droplet is a. 4/3 π r 3 ρ w C dt r / dt = L dm/dt - dq/dt i. C is the specific heat capacity ii. L dm/dt is the mass flux of vapor times L iii. dq/dt is the heat conduction rate iv. Steady state growth; so dt r / dt = 0 4. The latent heat owing to mass flux and the heat conduction terms oppose each other. While latent heating warms it reduces the vapor density gradient, which reduces the vapor flux. To solve for the true mass growth, these need to be balanced (iterate) 4. Begin by assuming steady state growth, L dm/dt - dq/dt = 0 5. From this we can state (the particle wet bulb relationship) 4π L D a [ρ v, - ρ v,a ] = -4π K a [T - T a ] 6. Or, L D [ρ v, - ρ v,a ] = -K [T - T a ] 7. Thus, the following must be balanced by the thermal and vapor fields [ρ v, - ρ v,a ] / [T - T a ] = -K / (L D) 8. This equation can be solved by iterating as the vapor density at the droplet surface is a function of T at the surface. a. Others, such as Young (1974: JAS pg 1735) suggest that we can linearize the saturation vapor density relation to get ρ v,a = s T a + b (where s and b are constant)to derive T a 3
4 T a = [D L (ρ v, -b) + KT ] / [D L s + K] 9. Upon arriving at the equilibrium T and vapor density, we can solve the above mass flux equation for the mass growth. 10. An estimate of the influence of the of heating can be made as follows. a. Re-compute the drop temperature from 7 above. b. From this compute a new vapor density. c. The time for a droplet to grow based on the heat conduction equation can be computed given the following information: Compare this rate to that fromthe mass flux equation. i. Initial conditions D = 2.11x10-5 m 2 /s ρ w = 1000 kg / m 3 SS = 1.01 ρ v, = 4.895x10-3 kg/m 3 T = K p = Pa r(t0) = 5x10-6 m radius dt t+dt (m) (sec) (accumulated) 5x10-6 m x10-6 m 20x10-6 m 30x10-6 m 40x10-6 m 50x10-6 m 100x10-6 m 400x10-6 m 1000x10-6 m 5000x10-6 m G. The previous equations are tedious to solve. It is possible to solve simultaneously for the growth rate in terms of mass flux and heat conduction using the Clausius-Clapeyron equation. This is what is shown next (There are a number of ways of proceeding. I will choose that presented by Byers.) 1. We begin by writing the mass flux and thermal conduction equations a. L dm / dt = 4π L D a [ρ v, - ρ v,a ] b. -dq / dt = 4π K a [T - T a ] 2. Now from the particle wet-bulb relation, and the equation of state a. [ρ v, - ρ v,a ] / [T - T a ] = -K / (L D) b. ρ v = e v / (R w T) (R w is the gas constant for water vapor) 3. We get, approximately (assuming T T a in the equation of state; also note I moved a minus sign) [e v, - e v,a ] / {T [T a - T ]} = K R w / (L D) a. When the ambient vapor is at supersaturation with respect to the drop, e v, > e v,a, then T a > T 4. Or, we get the following equation a da / dt = (S-1) / {ρ w L 2 / (K R w T 2 )] + ρw R w T / (e sv, D) } 5. Using dm/dt = ρ w 4 π a 2 da/dt 4
5 a da/dt = [1/(ρ w 4 π a)] dm/dt 6. We get dm/dt = 4 π a (S-1) / {L 2 / (K R w T 2 )] + Rw T / (e sv, D) } 7. Notes a. This equation is entirely in terms of the ambient conditions b. It is very accurate for S near 1 (RH = 101%) c. It can be in error for S-1 > 0.1 H. The influence of radius and solutions on diffusional growth. 1. In the previous section we computed the growth rate of a spherical liquid hydrometeor by steady state diffusion of water vapor and temperature assuming a balance between latent heat release and heat conduction. 2. We neglected the influence of radius and solutions. These can be easily included by using the principles discussed in deriving the Kohler equation. a. The Kohler equation is given as e r=a,w,aw = e s {1 + c a -1 - b (a 3 - a x 3 ) -1 } or e r=a,w,aw / e s = {1 + c a -1 - b (a 3 - a x 3 ) -1 } i. e r=a,w,aw is the equilibrium vapor pressure over a solution droplet with activity a w and radius a. ii. e s is the saturation vapor pressure for a plane, pure water surface. iii. The c a -1 term takes into account the influence of radius a via the Kelvin equation iv. The b (a 3 - a x 3 ) -1 term takes into account the influence of solutions and solid particulate matter v. constants b and c are described in Section 4 3. Our diffusional growth equation is a. In terms of radius growth rate a da / dt = (S-1) / {ρ w L 2 / (K R w T 2 )] + ρw R w T / (e sv, D) } i. which is often written (S-1) G(T,p) where G(T,P) is given as 1 / (A+B) or 1 / {ρ w L 2 / (K R w T 2 )] + ρw R w T / (e sv, D) } b. In terms of mass growth rate dm/dt = 4 π a (S-1) / {L 2 / (K R w T 2 )] + Rw T / (e sv, D) } 4. Now, (S-1) in this equation is just (e v, / e sv, - 1) I. Ventilation effects. 1. In deriving our vapor diffusion equation for droplet growth we assumed a spherically symmetric vapor and thermal gradient around the drop. a. This is accurate for a drop at rest: no relative flow. 2. For falling droplets (or droplets with a relative flow around them), the vapor and temperature fields are distorted 2. The vapor and thermal gradients will be enhanced ahead of the drop (upstream) and spread out behind the drop (downstream). 3. If the distortion is symmetric as shown in the attached figure, the effect will be to enhance growth or evaporation. Can you explain this 5
6 physically? a. During condensation, heat is convected away from the droplet, and the vapor supply (or rather gradient) is enhanced b. During evaporation, heat is convected toward the droplet, and vapor from evaporation is removed 4. These influences in our latent heat and conduction equations are included by multiplying by a correction factor called the ventilation coefficient. a. The latent heat equation becomes L dm / dt = 4π L D a fv [ρ v, - ρ v,a ] where fv is the vapor ventilation coefficient b. The conduction equation becomes -dq / dt = 4π K a fh [T - T a ] where fh is the heat ventilation coefficient 5. For a droplet at rest, we would expect these coefficients to be unity (1) 6. Relaxing the stationary constraint, we expect small droplets to fall slowly, and large droplets to fall relatively fast. Therefore, we expect that the ventilation coefficients would be near unity for small droplets and large for large droplets. 7. It is very difficult to compute these numbers analytically. Therefore, scientists resort to making estimates based on observations in terms of physical quantities important to the problem: They parameterize. a. One way to develop parameterizions of the ventilation coefficients is to measure the vapor diffusion mass growth rate and the heat conduction rate as a function of radius for stationary and falling droplets b. We define the vapor ventilation coefficient as fv = dm/dt / dmo/dt i. dm/dt is the rate for a moving drop ii. dmo/dt is the rate for a stationary drop 6
Generating cloud drops from CCN. Wallace & Hobbs (1977)
 Generating cloud drops from CCN Wallace & Hobbs (1977) Cloud Drops and Equilibrium Considera3ons: review We discussed how to compute the equilibrium vapor pressure over a pure water drop, or a solu3on
Generating cloud drops from CCN Wallace & Hobbs (1977) Cloud Drops and Equilibrium Considera3ons: review We discussed how to compute the equilibrium vapor pressure over a pure water drop, or a solu3on
Graupel and Hail Growth
 Graupel and Hail Growth I. Growth of large ice particles In this section we look at some basics of graupeln and hail growth. Important components of graupeln and hail growth models include production of
Graupel and Hail Growth I. Growth of large ice particles In this section we look at some basics of graupeln and hail growth. Important components of graupeln and hail growth models include production of
1. Droplet Growth by Condensation
 1. Droplet Growth by Condensation It was shown before that a critical size r and saturation ratio S must be exceeded for a small solution droplet to become a cloud droplet. Before the droplet reaches the
1. Droplet Growth by Condensation It was shown before that a critical size r and saturation ratio S must be exceeded for a small solution droplet to become a cloud droplet. Before the droplet reaches the
Summary of riming onset conditions for different crystal habits. Semi-dimension: width / lateral dimension (perpendicular to c-axis)
 Summary of riming onset conditions for different crystal habits Semi-dimension: width / lateral dimension (perpendicular to c-axis) HEAT BALANCE FOR GRAUPEL PARTICLES Consider a graupel particle growing
Summary of riming onset conditions for different crystal habits Semi-dimension: width / lateral dimension (perpendicular to c-axis) HEAT BALANCE FOR GRAUPEL PARTICLES Consider a graupel particle growing
2σ e s (r,t) = e s (T)exp( rr v ρ l T ) = exp( ) 2σ R v ρ l Tln(e/e s (T)) e s (f H2 O,r,T) = f H2 O
 Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Formulas/Constants, Physics/Oceanography 4510/5510 B Atmospheric Physics II N A = 6.02 10 23 molecules/mole (Avogadro s number) 1 mb = 100 Pa 1 Pa = 1 N/m 2 Γ d = 9.8 o C/km (dry adiabatic lapse rate)
Lecture Ch. 6. Condensed (Liquid) Water. Cloud in a Jar Demonstration. How does saturation occur? Saturation of Moist Air. Saturation of Moist Air
 Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
Lecture Ch. 6 Saturation of moist air Relationship between humidity and dewpoint Clausius-Clapeyron equation Dewpoint Temperature Depression Isobaric cooling Moist adiabatic ascent of air Equivalent temperature
Kelvin Effect. Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics
 Kelvin Effect Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Vapor Pressure (e) e < e # e = e # Vapor Pressure e > e # Relative humidity RH =
Kelvin Effect Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Vapor Pressure (e) e < e # e = e # Vapor Pressure e > e # Relative humidity RH =
1. Water Vapor in Air
 1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
1. Water Vapor in Air Water appears in all three phases in the earth s atmosphere - solid, liquid and vapor - and it is one of the most important components, not only because it is essential to life, but
Clouds associated with cold and warm fronts. Whiteman (2000)
 Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
Clouds associated with cold and warm fronts Whiteman (2000) Dalton s law of partial pressures! The total pressure exerted by a mixture of gases equals the sum of the partial pressure of the gases! Partial
Greenhouse Steady State Energy Balance Model
 Greenhouse Steady State Energy Balance Model The energy balance for the greenhouse was obtained by applying energy conservation to the greenhouse system as a control volume and identifying the energy terms.
Greenhouse Steady State Energy Balance Model The energy balance for the greenhouse was obtained by applying energy conservation to the greenhouse system as a control volume and identifying the energy terms.
Part I.
 Part I bblee@unimp . Introduction to Mass Transfer and Diffusion 2. Molecular Diffusion in Gasses 3. Molecular Diffusion in Liquids Part I 4. Molecular Diffusion in Biological Solutions and Gels 5. Molecular
Part I bblee@unimp . Introduction to Mass Transfer and Diffusion 2. Molecular Diffusion in Gasses 3. Molecular Diffusion in Liquids Part I 4. Molecular Diffusion in Biological Solutions and Gels 5. Molecular
Chapter 17. Fundamentals of Atmospheric Modeling
 Overhead Slides for Chapter 17 of Fundamentals of Atmospheric Modeling by Mark Z. Jacobson Department of Civil & Environmental Engineering Stanford University Stanford, CA 94305-4020 August 21, 1998 Mass
Overhead Slides for Chapter 17 of Fundamentals of Atmospheric Modeling by Mark Z. Jacobson Department of Civil & Environmental Engineering Stanford University Stanford, CA 94305-4020 August 21, 1998 Mass
Chapter 4 Water Vapor
 Chapter 4 Water Vapor Chapter overview: Phases of water Vapor pressure at saturation Moisture variables o Mixing ratio, specific humidity, relative humidity, dew point temperature o Absolute vs. relative
Chapter 4 Water Vapor Chapter overview: Phases of water Vapor pressure at saturation Moisture variables o Mixing ratio, specific humidity, relative humidity, dew point temperature o Absolute vs. relative
12.009/ Problem Set 2
 12.009/18.352 Problem Set 2 Due Thursday, 26 February 2015 100 points total Problem 1: 15 pts (a,b)=(10,5) Problem 2: 45 pts (a,b,c,d,e,f)=(5,5,5,10,10,10) Problem 3: 40 pts (a,b,c,d,e,f)=(5,5,5,5,10,10)
12.009/18.352 Problem Set 2 Due Thursday, 26 February 2015 100 points total Problem 1: 15 pts (a,b)=(10,5) Problem 2: 45 pts (a,b,c,d,e,f)=(5,5,5,10,10,10) Problem 3: 40 pts (a,b,c,d,e,f)=(5,5,5,5,10,10)
Solutions to questions from chapter 8 in GEF Cloud Physics
 Solutions to questions from chapter 8 in GEF4310 - Cloud Physics i.h.h.karset@geo.uio.no Problem 1 a) What is expressed by the equation below? Answer: The left side is the time rate of change of the mass
Solutions to questions from chapter 8 in GEF4310 - Cloud Physics i.h.h.karset@geo.uio.no Problem 1 a) What is expressed by the equation below? Answer: The left side is the time rate of change of the mass
Parcel Model. Atmospheric Sciences September 30, 2012
 Parcel Model Atmospheric Sciences 6150 September 30, 2012 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature,
Parcel Model Atmospheric Sciences 6150 September 30, 2012 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature,
Chemical Potential. Combining the First and Second Laws for a closed system, Considering (extensive properties)
 Chemical Potential Combining the First and Second Laws for a closed system, Considering (extensive properties) du = TdS pdv Hence For an open system, that is, one that can gain or lose mass, U will also
Chemical Potential Combining the First and Second Laws for a closed system, Considering (extensive properties) du = TdS pdv Hence For an open system, that is, one that can gain or lose mass, U will also
Combustion MATHEMATICAL MODEL FOR TRANSIENT. S. M. Frolov Λ,F.S.Frolov Λ, and B. Basara y
 Combustion MATHEMATICAL MODEL FOR TRANSIENT DROPLET VAPORIZATION S. M. Frolov Λ,F.S.Frolov Λ, and B. Basara y Λ N. N. Semenov Institute of Chemical Physics Russian Academy of Sciences Moscow, Russia y
Combustion MATHEMATICAL MODEL FOR TRANSIENT DROPLET VAPORIZATION S. M. Frolov Λ,F.S.Frolov Λ, and B. Basara y Λ N. N. Semenov Institute of Chemical Physics Russian Academy of Sciences Moscow, Russia y
The Clausius-Clapeyron and the Kelvin Equations
 PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics The Clausius-Clapeyron and the Kelvin Equations by Dario B. Giaiotti and Fulvio
PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics The Clausius-Clapeyron and the Kelvin Equations by Dario B. Giaiotti and Fulvio
Convective Heat and Mass Transfer Prof. A.W. Date Department of Mechanical Engineering Indian Institute of Technology, Bombay
 Convective Heat and Mass Transfer Prof. A.W. Date Department of Mechanical Engineering Indian Institute of Technology, Bombay Module No. # 01 Lecture No. # 32 Stefan Flow Model We are now familiar with
Convective Heat and Mass Transfer Prof. A.W. Date Department of Mechanical Engineering Indian Institute of Technology, Bombay Module No. # 01 Lecture No. # 32 Stefan Flow Model We are now familiar with
A droplet of colloidal solution is left to evaporate on a superhydrophobic surface. Avijit Baidya
 A droplet of colloidal solution is left to evaporate on a superhydrophobic surface. Avijit Baidya 14.03.15 In this paper Evaporation-driven particle self-assembly can be used to generate three-dimensional
A droplet of colloidal solution is left to evaporate on a superhydrophobic surface. Avijit Baidya 14.03.15 In this paper Evaporation-driven particle self-assembly can be used to generate three-dimensional
Köhler Curve. Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics
 Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
ATMO 551a Moist Adiabat Fall Change in internal energy: ΔU
 Enthalpy and the Moist Adiabat We have described the dry adiabat where an air parcel is lifted rapidly causing the air parcel to expand as the environmental pressure decreases and the air parcel does work
Enthalpy and the Moist Adiabat We have described the dry adiabat where an air parcel is lifted rapidly causing the air parcel to expand as the environmental pressure decreases and the air parcel does work
CAE 331/513 Building Science Fall 2017
 CAE 331/513 Building Science Fall 2017 October 5, 2017 Psychrometrics (equations) Advancing energy, environmental, and sustainability research within the built environment www.built-envi.com Twitter: @built_envi
CAE 331/513 Building Science Fall 2017 October 5, 2017 Psychrometrics (equations) Advancing energy, environmental, and sustainability research within the built environment www.built-envi.com Twitter: @built_envi
Level 7 Post Graduate Diploma in Engineering Heat and mass transfer
 9210-221 Level 7 Post Graduate Diploma in Engineering Heat and mass transfer 0 You should have the following for this examination one answer book non programmable calculator pen, pencil, drawing instruments
9210-221 Level 7 Post Graduate Diploma in Engineering Heat and mass transfer 0 You should have the following for this examination one answer book non programmable calculator pen, pencil, drawing instruments
The perturbation pressure, p, can be represented as the sum of a hydrostatic pressure perturbation p h and a nonhydrostatic pressure perturbation p nh
 z = The perturbation pressure, p, can be represented as the sum of a hydrostatic pressure perturbation p h and a nonhydrostatic pressure perturbation p nh, that is, p = p h + p nh. (.1) The former arises
z = The perturbation pressure, p, can be represented as the sum of a hydrostatic pressure perturbation p h and a nonhydrostatic pressure perturbation p nh, that is, p = p h + p nh. (.1) The former arises
df dz = dp dt Essentially, this is just a statement of the first law in one of the forms we derived earlier (expressed here in W m 3 ) dq p dt dp
 A problem with using entropy as a variable is that it is not a particularly intuitive concept. The mechanics of using entropy for evaluating system evolution is well developed, but it sometimes feels a
A problem with using entropy as a variable is that it is not a particularly intuitive concept. The mechanics of using entropy for evaluating system evolution is well developed, but it sometimes feels a
Properties of Vapors
 Properties of Vapors Topics for Discussion The Pressure/Temperature Relationship Vaporization Condensation Enthalpy Properties of Vapors Topics for Discussion Entropy Properties of Substances Saturated
Properties of Vapors Topics for Discussion The Pressure/Temperature Relationship Vaporization Condensation Enthalpy Properties of Vapors Topics for Discussion Entropy Properties of Substances Saturated
Parcel Model. Meteorology September 3, 2008
 Parcel Model Meteorology 5210 September 3, 2008 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature, θ, mixing
Parcel Model Meteorology 5210 September 3, 2008 1 Governing Equations for Precipitating Convection For precipitating convection, we have the following set of equations for potential temperature, θ, mixing
Heat processes. Heat exchange
 Heat processes Heat exchange Heat energy transported across a surface from higher temperature side to lower temperature side; it is a macroscopic measure of transported energies of molecular motions Temperature
Heat processes Heat exchange Heat energy transported across a surface from higher temperature side to lower temperature side; it is a macroscopic measure of transported energies of molecular motions Temperature
Weather & Atmospheric Variables Review
 Weather & Atmospheric Variables Review Words that are bold, italicized and/or underlined are vocabulary you must KNOW! A) Atmospheric variables: a) Temperature as it relates to: 1) duration of insolation...longer
Weather & Atmospheric Variables Review Words that are bold, italicized and/or underlined are vocabulary you must KNOW! A) Atmospheric variables: a) Temperature as it relates to: 1) duration of insolation...longer
Differential equations of mass transfer
 Differential equations of mass transfer Definition: The differential equations of mass transfer are general equations describing mass transfer in all directions and at all conditions. How is the differential
Differential equations of mass transfer Definition: The differential equations of mass transfer are general equations describing mass transfer in all directions and at all conditions. How is the differential
G109 Midterm Exam (Version A) October 10, 2006 Instructor: Dr C.M. Brown 1. Time allowed 50 mins. Total possible points: 40 number of pages: 5
 G109 Midterm Exam (Version A) October 10, 2006 Instructor: Dr C.M. Brown 1 Time allowed 50 mins. Total possible points: 40 number of pages: 5 Part A: Short Answer & Problems (12), Fill in the Blanks (6).
G109 Midterm Exam (Version A) October 10, 2006 Instructor: Dr C.M. Brown 1 Time allowed 50 mins. Total possible points: 40 number of pages: 5 Part A: Short Answer & Problems (12), Fill in the Blanks (6).
P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C.
![P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C. P sat = A exp [B( 1/ /T)] B= 5308K. A=6.11 mbar=vapor press. 0C.](/thumbs/79/79184286.jpg) Lecture 5. Water and water vapor in the atmosphere 14 Feb 2008 Review of buoyancy, with an unusual demonstration of Archimedes principle. Water is a polar molecule that forms hydrogen bonds. Consequently
Lecture 5. Water and water vapor in the atmosphere 14 Feb 2008 Review of buoyancy, with an unusual demonstration of Archimedes principle. Water is a polar molecule that forms hydrogen bonds. Consequently
Outline. Aim. Gas law. Pressure. Scale height Mixing Column density. Temperature Lapse rate Stability. Condensation Humidity.
 Institute of Applied Physics University of Bern Outline A planetary atmosphere consists of different gases hold to the planet by gravity The laws of thermodynamics hold structure as vertical coordinate
Institute of Applied Physics University of Bern Outline A planetary atmosphere consists of different gases hold to the planet by gravity The laws of thermodynamics hold structure as vertical coordinate
Diabatic Processes. Diabatic processes are non-adiabatic processes such as. entrainment and mixing. radiative heating or cooling
 Diabatic Processes Diabatic processes are non-adiabatic processes such as precipitation fall-out entrainment and mixing radiative heating or cooling Parcel Model dθ dt dw dt dl dt dr dt = L c p π (C E
Diabatic Processes Diabatic processes are non-adiabatic processes such as precipitation fall-out entrainment and mixing radiative heating or cooling Parcel Model dθ dt dw dt dl dt dr dt = L c p π (C E
Measuring State Parameters of the Atmosphere
 Measuring State Parameters of the Atmosphere Some Applications of Atmospheric Thermodynamics Earth Observing Laboratory, NCAR IDEAS-4 Tutorial Introduction Goals of This Presentation Present two complementary
Measuring State Parameters of the Atmosphere Some Applications of Atmospheric Thermodynamics Earth Observing Laboratory, NCAR IDEAS-4 Tutorial Introduction Goals of This Presentation Present two complementary
MT3230 Supplemental Data 4.2 Spring, 2018 Dr. Sam Miller COMPUTING THE LATENT HEAT OF VAPORIZATION OF WATER AS A FUNCTION OF TEMPERATURE.
 MT3230 Supplemental Data 4.2 Spring, 2018 Dr. Sam Miller COMPUTING THE LATENT HEAT OF VAPORIZATION OF WATER AS A FUNCTION OF TEMPERATURE Abstract The latent heat of vaporization parameterizes the amount
MT3230 Supplemental Data 4.2 Spring, 2018 Dr. Sam Miller COMPUTING THE LATENT HEAT OF VAPORIZATION OF WATER AS A FUNCTION OF TEMPERATURE Abstract The latent heat of vaporization parameterizes the amount
EXPERIMENT 1 DETERMINATION OF GAS DIFFUSION COEFFICIENT
 EXPERIMENT 1 DETERMINATION OF GAS DIFFUSION COEFFICIENT Objective: The objective of this experiment is to calculate diffusion coefficient of a volatile organic compound in air by means of Chapman Enskog
EXPERIMENT 1 DETERMINATION OF GAS DIFFUSION COEFFICIENT Objective: The objective of this experiment is to calculate diffusion coefficient of a volatile organic compound in air by means of Chapman Enskog
Lecture 9: Climate Sensitivity and Feedback Mechanisms
 Lecture 9: Climate Sensitivity and Feedback Mechanisms Basic radiative feedbacks (Plank, Water Vapor, Lapse-Rate Feedbacks) Ice albedo & Vegetation-Climate feedback Cloud feedback Biogeochemical feedbacks
Lecture 9: Climate Sensitivity and Feedback Mechanisms Basic radiative feedbacks (Plank, Water Vapor, Lapse-Rate Feedbacks) Ice albedo & Vegetation-Climate feedback Cloud feedback Biogeochemical feedbacks
z g + F w (2.56) p(x, y, z, t) = p(z) + p (x, y, z, t) (2.120) ρ(x, y, z, t) = ρ(z) + ρ (x, y, z, t), (2.121)
 = + dw dt = 1 ρ p z g + F w (.56) Let us describe the total pressure p and density ρ as the sum of a horizontally homogeneous base state pressure and density, and a deviation from this base state, that
= + dw dt = 1 ρ p z g + F w (.56) Let us describe the total pressure p and density ρ as the sum of a horizontally homogeneous base state pressure and density, and a deviation from this base state, that
Thermodynamics Introduction and Basic Concepts
 Thermodynamics Introduction and Basic Concepts by Asst. Prof. Channarong Asavatesanupap Mechanical Engineering Department Faculty of Engineering Thammasat University 2 What is Thermodynamics? Thermodynamics
Thermodynamics Introduction and Basic Concepts by Asst. Prof. Channarong Asavatesanupap Mechanical Engineering Department Faculty of Engineering Thammasat University 2 What is Thermodynamics? Thermodynamics
Lecture 4: Global Energy Balance. Global Energy Balance. Solar Flux and Flux Density. Blackbody Radiation Layer Model.
 Lecture : Global Energy Balance Global Energy Balance S/ * (1-A) terrestrial radiation cooling Solar radiation warming T S Global Temperature Blackbody Radiation ocean land Layer Model energy, water, and
Lecture : Global Energy Balance Global Energy Balance S/ * (1-A) terrestrial radiation cooling Solar radiation warming T S Global Temperature Blackbody Radiation ocean land Layer Model energy, water, and
Lecture 4: Global Energy Balance
 Lecture : Global Energy Balance S/ * (1-A) T A T S T A Blackbody Radiation Layer Model Greenhouse Effect Global Energy Balance terrestrial radiation cooling Solar radiation warming Global Temperature atmosphere
Lecture : Global Energy Balance S/ * (1-A) T A T S T A Blackbody Radiation Layer Model Greenhouse Effect Global Energy Balance terrestrial radiation cooling Solar radiation warming Global Temperature atmosphere
Entropy and the Second Law of Thermodynamics
 Entropy and the Second Law of Thermodynamics Reading Problems 7-1 7-3 7-88, 7-131, 7-135 7-6 7-10 8-24, 8-44, 8-46, 8-60, 8-73, 8-99, 8-128, 8-132, 8-1 8-10, 8-13 8-135, 8-148, 8-152, 8-166, 8-168, 8-189
Entropy and the Second Law of Thermodynamics Reading Problems 7-1 7-3 7-88, 7-131, 7-135 7-6 7-10 8-24, 8-44, 8-46, 8-60, 8-73, 8-99, 8-128, 8-132, 8-1 8-10, 8-13 8-135, 8-148, 8-152, 8-166, 8-168, 8-189
Exam 2: Cloud Physics April 16, 2008 Physical Meteorology Questions 1-10 are worth 5 points each. Questions are worth 10 points each.
 Exam : Cloud Physics April, 8 Physical Meteorology 344 Name Questions - are worth 5 points each. Questions -5 are worth points each.. Rank the concentrations of the following from lowest () to highest
Exam : Cloud Physics April, 8 Physical Meteorology 344 Name Questions - are worth 5 points each. Questions -5 are worth points each.. Rank the concentrations of the following from lowest () to highest
Step 1. Step 2. g l = g v. dg = 0 We have shown that over a plane surface of water. g v g l = ρ v R v T ln e/e sat. this can be rewritten
 The basic question is what makes the existence of a droplet thermodynamically preferable to the existence only of water vapor. We have already derived an expression for the saturation vapor pressure over
The basic question is what makes the existence of a droplet thermodynamically preferable to the existence only of water vapor. We have already derived an expression for the saturation vapor pressure over
PHASE CHANGE. Freezing Sublimation
 Melting Graphic Organizer Deposition PHASE CHANGE Freezing Sublimation Boiling Evaporation Condensation PHASE CHANGE Phase change happens as the temperature changes. All matter can move from one state
Melting Graphic Organizer Deposition PHASE CHANGE Freezing Sublimation Boiling Evaporation Condensation PHASE CHANGE Phase change happens as the temperature changes. All matter can move from one state
2 D. Terminal velocity can be solved for by equating Fd and Fg Fg = 1/6πd 3 g ρ LIQ = 1/8 Cd π d 2 ρ air u
 ecture 8 Collection Growth of iquid Hydrometeors. I. Terminal velocity The terminal velocity of an object is the maximum speed an object will accelerate to by gravity. At terminal velocity, a balance of
ecture 8 Collection Growth of iquid Hydrometeors. I. Terminal velocity The terminal velocity of an object is the maximum speed an object will accelerate to by gravity. At terminal velocity, a balance of
Consider a volume Ω enclosing a mass M and bounded by a surface δω. d dt. q n ds. The Work done by the body on the surroundings is
 The Energy Balance Consider a volume enclosing a mass M and bounded by a surface δ. δ At a point x, the density is ρ, the local velocity is v, and the local Energy density is U. U v The rate of change
The Energy Balance Consider a volume enclosing a mass M and bounded by a surface δ. δ At a point x, the density is ρ, the local velocity is v, and the local Energy density is U. U v The rate of change
The linear additivity of the forcings' responses in the energy and water cycles. Nathalie Schaller, Jan Cermak, Reto Knutti and Martin Wild
 The linear additivity of the forcings' responses in the energy and water cycles Nathalie Schaller, Jan Cermak, Reto Knutti and Martin Wild WCRP OSP, Denver, 27th October 2011 1 Motivation How will precipitation
The linear additivity of the forcings' responses in the energy and water cycles Nathalie Schaller, Jan Cermak, Reto Knutti and Martin Wild WCRP OSP, Denver, 27th October 2011 1 Motivation How will precipitation
Course , General Circulation of the Earth's Atmosphere Prof. Peter Stone Section 4: Water Vapor Budget
 Course 12.812, General Circulation of the Earth's Atmosphere Prof. Peter Stone Section 4: Water Vapor Budget Water Vapor Distribution First let us look at the distribution of specific humidity, q. The
Course 12.812, General Circulation of the Earth's Atmosphere Prof. Peter Stone Section 4: Water Vapor Budget Water Vapor Distribution First let us look at the distribution of specific humidity, q. The
Theory. Humidity h of an air-vapor mixture is defined as the mass ratio of water vapor and dry air,
 Theory Background In a cooling tower with open water circulation, heat is removed from water because of the material and heat exchange between the water and the ambient air. The cooling tower is a special
Theory Background In a cooling tower with open water circulation, heat is removed from water because of the material and heat exchange between the water and the ambient air. The cooling tower is a special
Lecture notes: Interception and evapotranspiration
 Lecture notes: Interception and evapotranspiration I. Vegetation canopy interception (I c ): Portion of incident precipitation (P) physically intercepted, stored and ultimately evaporated from vegetation
Lecture notes: Interception and evapotranspiration I. Vegetation canopy interception (I c ): Portion of incident precipitation (P) physically intercepted, stored and ultimately evaporated from vegetation
ATMO 551a Homework 2 Solutions Fall r planet orbit
 1. Pluto s orbit is far more eccentric than those of the major planets orbits: Aphelion: 7,375,927,931 km Perihelion: 4,436,824,613 km a. Determine the solar flux (watts/m 2 ) at each of these distances.
1. Pluto s orbit is far more eccentric than those of the major planets orbits: Aphelion: 7,375,927,931 km Perihelion: 4,436,824,613 km a. Determine the solar flux (watts/m 2 ) at each of these distances.
Radiation, Sensible Heat Flux and Evapotranspiration
 Radiation, Sensible Heat Flux and Evapotranspiration Climatological and hydrological field work Figure 1: Estimate of the Earth s annual and global mean energy balance. Over the long term, the incoming
Radiation, Sensible Heat Flux and Evapotranspiration Climatological and hydrological field work Figure 1: Estimate of the Earth s annual and global mean energy balance. Over the long term, the incoming
Steady One-Dimensional Diffusion of One Species A through a Second Non-Transferring Species B. z y x. Liquid A
 Steady One-Dimensional Diffusion of One Species through a Second on-transferring Species B R. Shankar Subramanian Department of Chemical and Biomolecular Engineering Clarkson University Consider a simple
Steady One-Dimensional Diffusion of One Species through a Second on-transferring Species B R. Shankar Subramanian Department of Chemical and Biomolecular Engineering Clarkson University Consider a simple
Løsningsforslag: oppgavesett kap. 9 (2 av 3) GEF2200
 Løsningsforslag: oppgavesett kap. 9 (2 av 3) GEF2200 s.m.blichner@geo.uio.no Oppgave 1 a) The turbulent vertical flux of sensible heat (Q H ) in the atmospheric boundary layer often takes place through
Løsningsforslag: oppgavesett kap. 9 (2 av 3) GEF2200 s.m.blichner@geo.uio.no Oppgave 1 a) The turbulent vertical flux of sensible heat (Q H ) in the atmospheric boundary layer often takes place through
Lecture 10: Climate Sensitivity and Feedback
 Lecture 10: Climate Sensitivity and Feedback Human Activities Climate Sensitivity Climate Feedback 1 Climate Sensitivity and Feedback (from Earth s Climate: Past and Future) 2 Definition and Mathematic
Lecture 10: Climate Sensitivity and Feedback Human Activities Climate Sensitivity Climate Feedback 1 Climate Sensitivity and Feedback (from Earth s Climate: Past and Future) 2 Definition and Mathematic
Mid High Latitude Cirrus Precipitation Processes. Jon Sauer, Dan Crocker, Yanice Benitez
 Mid High Latitude Cirrus Precipitation Processes Jon Sauer, Dan Crocker, Yanice Benitez Department of Chemistry and Biochemistry, University of California, San Diego, CA 92093, USA *To whom correspondence
Mid High Latitude Cirrus Precipitation Processes Jon Sauer, Dan Crocker, Yanice Benitez Department of Chemistry and Biochemistry, University of California, San Diego, CA 92093, USA *To whom correspondence
Fundamental Stellar Parameters. Radiative Transfer. Stellar Atmospheres
 Fundamental Stellar Parameters Radiative Transfer Stellar Atmospheres Equations of Stellar Structure Basic Principles Equations of Hydrostatic Equilibrium and Mass Conservation Central Pressure, Virial
Fundamental Stellar Parameters Radiative Transfer Stellar Atmospheres Equations of Stellar Structure Basic Principles Equations of Hydrostatic Equilibrium and Mass Conservation Central Pressure, Virial
1/2/2016 WEATHER DEFINITION
 WEATHER DEFINITION Weather state or condition of the variables of the atmosphere at a given time Weather variables temperature, air pressure, wind, moisture, cloud cover, precipitation, storms Weather
WEATHER DEFINITION Weather state or condition of the variables of the atmosphere at a given time Weather variables temperature, air pressure, wind, moisture, cloud cover, precipitation, storms Weather
First Law of Thermodynamics
 First Law of Thermodynamics E int = Q + W other state variables E int is a state variable, so only depends on condition (P, V, T, ) of system. Therefore, E int only depends on initial and final states
First Law of Thermodynamics E int = Q + W other state variables E int is a state variable, so only depends on condition (P, V, T, ) of system. Therefore, E int only depends on initial and final states
12. Heat of melting and evaporation of water
 VS 12. Heat of melting and evaporation of water 12.1 Introduction The change of the physical state of a substance in general requires the absorption or release of heat. In this case, one speaks of a first
VS 12. Heat of melting and evaporation of water 12.1 Introduction The change of the physical state of a substance in general requires the absorption or release of heat. In this case, one speaks of a first
Heat and Mass Transfer Unit-1 Conduction
 1. State Fourier s Law of conduction. Heat and Mass Transfer Unit-1 Conduction Part-A The rate of heat conduction is proportional to the area measured normal to the direction of heat flow and to the temperature
1. State Fourier s Law of conduction. Heat and Mass Transfer Unit-1 Conduction Part-A The rate of heat conduction is proportional to the area measured normal to the direction of heat flow and to the temperature
Modeling the Process of Drying Stationary Objects inside a Tumble Dryer Using COMSOL Multiphysics
 Presented at the COMSOL Conference 2008 Hannover Modeling the Process of Drying Stationary Objects inside a Tumble Dryer Using COMSOL Multiphysics Tarek H.M. Zeineldin Outline Introduction - Drying processes
Presented at the COMSOL Conference 2008 Hannover Modeling the Process of Drying Stationary Objects inside a Tumble Dryer Using COMSOL Multiphysics Tarek H.M. Zeineldin Outline Introduction - Drying processes
2.1 Effects of a cumulus ensemble upon the large scale temperature and moisture fields by induced subsidence and detrainment
 Atmospheric Sciences 6150 Cloud System Modeling 2.1 Effects of a cumulus ensemble upon the large scale temperature and moisture fields by induced subsidence and detrainment Arakawa (1969, 1972), W. Gray
Atmospheric Sciences 6150 Cloud System Modeling 2.1 Effects of a cumulus ensemble upon the large scale temperature and moisture fields by induced subsidence and detrainment Arakawa (1969, 1972), W. Gray
CHAPTER 8 ENTROPY. Blank
 CHAPER 8 ENROPY Blank SONNAG/BORGNAKKE SUDY PROBLEM 8-8. A heat engine efficiency from the inequality of Clausius Consider an actual heat engine with efficiency of η working between reservoirs at and L.
CHAPER 8 ENROPY Blank SONNAG/BORGNAKKE SUDY PROBLEM 8-8. A heat engine efficiency from the inequality of Clausius Consider an actual heat engine with efficiency of η working between reservoirs at and L.
Introduction to Mass Transfer
 Introduction to Mass Transfer Introduction Three fundamental transfer processes: i) Momentum transfer ii) iii) Heat transfer Mass transfer Mass transfer may occur in a gas mixture, a liquid solution or
Introduction to Mass Transfer Introduction Three fundamental transfer processes: i) Momentum transfer ii) iii) Heat transfer Mass transfer Mass transfer may occur in a gas mixture, a liquid solution or
Meteorology 6150 Cloud System Modeling
 Meteorology 6150 Cloud System Modeling Steve Krueger Spring 2009 1 Fundamental Equations 1.1 The Basic Equations 1.1.1 Equation of motion The movement of air in the atmosphere is governed by Newton s Second
Meteorology 6150 Cloud System Modeling Steve Krueger Spring 2009 1 Fundamental Equations 1.1 The Basic Equations 1.1.1 Equation of motion The movement of air in the atmosphere is governed by Newton s Second
R13 SET - 1 '' ''' '' ' '''' Code No RT21033
 SET - 1 II B. Tech I Semester Supplementary Examinations, June - 2015 THERMODYNAMICS (Com. to ME, AE, AME) Time: 3 hours Max. Marks: 70 Note: 1. Question Paper consists of two parts (Part-A and Part-B)
SET - 1 II B. Tech I Semester Supplementary Examinations, June - 2015 THERMODYNAMICS (Com. to ME, AE, AME) Time: 3 hours Max. Marks: 70 Note: 1. Question Paper consists of two parts (Part-A and Part-B)
1. Heterogeneous Systems and Chemical Equilibrium
 1. Heterogeneous Systems and Chemical Equilibrium The preceding section involved only single phase systems. For it to be in thermodynamic equilibrium, a homogeneous system must be in thermal equilibrium
1. Heterogeneous Systems and Chemical Equilibrium The preceding section involved only single phase systems. For it to be in thermodynamic equilibrium, a homogeneous system must be in thermal equilibrium
Examination Heat Transfer
 Examination Heat Transfer code: 4B680 date: 17 january 2006 time: 14.00-17.00 hours NOTE: There are 4 questions in total. The first one consists of independent sub-questions. If necessary, guide numbers
Examination Heat Transfer code: 4B680 date: 17 january 2006 time: 14.00-17.00 hours NOTE: There are 4 questions in total. The first one consists of independent sub-questions. If necessary, guide numbers
Melting of ice particles:
 Melting of ice particles: When ice particles fall below 0 C they begin to melt, but the process takes some time since heat transfer needs to occur (heat from ambient environment has to supply the latent
Melting of ice particles: When ice particles fall below 0 C they begin to melt, but the process takes some time since heat transfer needs to occur (heat from ambient environment has to supply the latent
Temp 54 Dew Point 41 Relative Humidity 63%
 Temp 54 Dew Point 41 Relative Humidity 63% Water in the Atmosphere Evaporation Water molecules change from the liquid to gas phase Molecules in liquids move slowly Heat energy makes them move faster When
Temp 54 Dew Point 41 Relative Humidity 63% Water in the Atmosphere Evaporation Water molecules change from the liquid to gas phase Molecules in liquids move slowly Heat energy makes them move faster When
Atmospheric Composition הרכב האטמוספירה
 Atmospheric Composition הרכב האטמוספירה N 2 O 2 Trace Gases Water Vapor (H 2 O) Argon (Ar) Carbon Dioxide (CO 2 ) Neon (Ne) Helium (He) Methane (CH 4 ) Nitrous Oxide (N 2 O) Ozone (O 3 ) Nitrogen and oxygen
Atmospheric Composition הרכב האטמוספירה N 2 O 2 Trace Gases Water Vapor (H 2 O) Argon (Ar) Carbon Dioxide (CO 2 ) Neon (Ne) Helium (He) Methane (CH 4 ) Nitrous Oxide (N 2 O) Ozone (O 3 ) Nitrogen and oxygen
One-dimensional Spray Combustion Optimization with a Sequential Linear Quadratic Algorithm
 One-dimensional Spray Combustion Optimization with a Sequential Linear Quadratic Algorithm Justin A. Sirignano, Luis Rodriguez, Athanasios Sideris, and William A. Sirignano Department of Mechanical and
One-dimensional Spray Combustion Optimization with a Sequential Linear Quadratic Algorithm Justin A. Sirignano, Luis Rodriguez, Athanasios Sideris, and William A. Sirignano Department of Mechanical and
A CONVENIENT NUCLEUS PARAMETER FOR CONSIDERATIONS OF DROPLET GROWTH
 A CONVENIENT NUCLEUS PARAMETER FOR CONSIDERATIONS OF DROPLET GROWTH by E. X BERRY Desert Research Institute, Reno, Nevada, U.S.A. RESUME La quantite r 0 est definie comme le rayon d'une gouttelette quand
A CONVENIENT NUCLEUS PARAMETER FOR CONSIDERATIONS OF DROPLET GROWTH by E. X BERRY Desert Research Institute, Reno, Nevada, U.S.A. RESUME La quantite r 0 est definie comme le rayon d'une gouttelette quand
Radiative-Convective Models. The Hydrological Cycle Hadley Circulation. Manabe and Strickler (1964) Course Notes chapter 5.1
 Climate Modeling Lecture 8 Radiative-Convective Models Manabe and Strickler (1964) Course Notes chapter 5.1 The Hydrological Cycle Hadley Circulation Prepare for Mid-Term (Friday 9 am) Review Course Notes
Climate Modeling Lecture 8 Radiative-Convective Models Manabe and Strickler (1964) Course Notes chapter 5.1 The Hydrological Cycle Hadley Circulation Prepare for Mid-Term (Friday 9 am) Review Course Notes
3.1and 3.2 Thermal. Rise in temperature in deg C Final temperature in C A B C D
 Name: Date: 3.1and 3.2 Thermal 1. During an experiment, a solid is heated from 285 K to 298 K. Which one of the following gives the rise in temperature, in deg C, and the final temperature, in C, of the
Name: Date: 3.1and 3.2 Thermal 1. During an experiment, a solid is heated from 285 K to 298 K. Which one of the following gives the rise in temperature, in deg C, and the final temperature, in C, of the
WUFI Workshop at NTNU /SINTEF Fundamentals
 WUFI Workshop at NTNU /SINTEF 2008 Fundamentals Contents: From steady-state to transient Heat storage and -transport Moisture storage and -transport Calculation of coupled transport Model limitations 2
WUFI Workshop at NTNU /SINTEF 2008 Fundamentals Contents: From steady-state to transient Heat storage and -transport Moisture storage and -transport Calculation of coupled transport Model limitations 2
Applied Thermodynamics for Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, Kharagpur
 Applied Thermodynamics for Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture - 21 Psychometric Processes Good afternoon, yesterday we
Applied Thermodynamics for Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture - 21 Psychometric Processes Good afternoon, yesterday we
An alternative, less empirical approach (though still full of brazen assumptions) is the following:
 ERTH 500: More Notes on Final Project: Dr. Dave Dempsey Earth Systems II Modeling the Dept. of (Spring 2016) Cenozoic Icehouse Earth Earth & Climate Sciences More notes on Upslope/Monsoon Precipitation
ERTH 500: More Notes on Final Project: Dr. Dave Dempsey Earth Systems II Modeling the Dept. of (Spring 2016) Cenozoic Icehouse Earth Earth & Climate Sciences More notes on Upslope/Monsoon Precipitation
PROBLEM 14.6 ( )( ) (b) Applying a species balance to a control volume about the hydrogen, dt 6 dt 6RAT dt 6RT dt
 PROBLEM 14.6 KNOWN: Pressure and temperature of hydrogen stored in a spherical steel tank of prescribed diameter and thickness. FIND: (a) Initial rate of hydrogen mass loss from the tank, (b) Initial rate
PROBLEM 14.6 KNOWN: Pressure and temperature of hydrogen stored in a spherical steel tank of prescribed diameter and thickness. FIND: (a) Initial rate of hydrogen mass loss from the tank, (b) Initial rate
Quasi-equilibrium transitions
 Quasi-equilibrium transitions We have defined a two important equilibrium conditions. he first is one in which there is no heating, or the system is adiabatic, and dh/ =0, where h is the total enthalpy
Quasi-equilibrium transitions We have defined a two important equilibrium conditions. he first is one in which there is no heating, or the system is adiabatic, and dh/ =0, where h is the total enthalpy
Module 8: BoiIing Lecture 34: Analysis of Bubble Formation on a Heated Wall. The Lecture Contains: Bubble Growth with Heat and Mass Transfer
 The Lecture Contains: Bubble Growth with Heat and Mass Transfer Simple Analysis of Bubble Growth in a Uniform Temperature Field file:///d /Web%20Course%20(Ganesh%20Rana)/Dr.%20gautam%20biswas/Final/convective_heat_and_mass_transfer/lecture34/34_1.html[12/24/2014
The Lecture Contains: Bubble Growth with Heat and Mass Transfer Simple Analysis of Bubble Growth in a Uniform Temperature Field file:///d /Web%20Course%20(Ganesh%20Rana)/Dr.%20gautam%20biswas/Final/convective_heat_and_mass_transfer/lecture34/34_1.html[12/24/2014
( ) = 1005 J kg 1 K 1 ;
 Problem Set 3 1. A parcel of water is added to the ocean surface that is denser (heavier) than any of the waters in the ocean. Suppose the parcel sinks to the ocean bottom; estimate the change in temperature
Problem Set 3 1. A parcel of water is added to the ocean surface that is denser (heavier) than any of the waters in the ocean. Suppose the parcel sinks to the ocean bottom; estimate the change in temperature
Warm Cloud Processes. Some definitions. Two ways to make big drops: Effects of cloud condensation nuclei
 Warm Cloud Processes Dr. Christopher M. Godfrey University of North Carolina at Asheville Warm clouds lie completely below the 0 isotherm 0 o C Some definitions Liquid water content (LWC) Amount of liquid
Warm Cloud Processes Dr. Christopher M. Godfrey University of North Carolina at Asheville Warm clouds lie completely below the 0 isotherm 0 o C Some definitions Liquid water content (LWC) Amount of liquid
Precipitation Processes METR σ is the surface tension, ρ l is the water density, R v is the Gas constant for water vapor, T is the air
 Precipitation Processes METR 2011 Introduction In order to grow things on earth, they need water. The way that the earth naturally irrigates is through snowfall and rainfall. Therefore, it is important
Precipitation Processes METR 2011 Introduction In order to grow things on earth, they need water. The way that the earth naturally irrigates is through snowfall and rainfall. Therefore, it is important
Aircraft Icing Icing Physics
 Aircraft Icing Icing Physics Prof. Dr. Dept. Aerospace Engineering, METU Fall 2015 Outline Formation of ice in the atmosphere Supercooled water droplets Mechanism of aircraft icing Icing variations Ice
Aircraft Icing Icing Physics Prof. Dr. Dept. Aerospace Engineering, METU Fall 2015 Outline Formation of ice in the atmosphere Supercooled water droplets Mechanism of aircraft icing Icing variations Ice
Physics 5D PRACTICE FINAL EXAM Fall 2013
 Print your name: Physics 5D PRACTICE FINAL EXAM Fall 2013 Real Exam is Wednesday December 11 Thimann Lecture 3 4:00-7:00 pm Closed book exam two 8.5x11 sheets of notes ok Note: Avogadro s number N A =
Print your name: Physics 5D PRACTICE FINAL EXAM Fall 2013 Real Exam is Wednesday December 11 Thimann Lecture 3 4:00-7:00 pm Closed book exam two 8.5x11 sheets of notes ok Note: Avogadro s number N A =
Introduction to Heat and Mass Transfer. Week 5
 Introduction to Heat and Mass Transfer Week 5 Critical Resistance Thermal resistances due to conduction and convection in radial systems behave differently Depending on application, we want to either maximize
Introduction to Heat and Mass Transfer Week 5 Critical Resistance Thermal resistances due to conduction and convection in radial systems behave differently Depending on application, we want to either maximize
New Methods for Measuring Water Desorption and Vapour Permeation Rates in Membranes
 New Methods for Measuring Water Desorption and Vapour Permeation Rates in Membranes L. I. iortea, D. O Driscoll, E. P. Berg, P. Xiao, F.. Pascut and R. E. Imhof School of Engineering, South Bank University,
New Methods for Measuring Water Desorption and Vapour Permeation Rates in Membranes L. I. iortea, D. O Driscoll, E. P. Berg, P. Xiao, F.. Pascut and R. E. Imhof School of Engineering, South Bank University,
Chapiter VII: Ionization chamber
 Chapiter VII: Ionization chamber 1 Types of ionization chambers Sensitive volume: gas (most often air direct measurement of exposure) ionization chamber Sensitive volume: semiconductor (silicon, germanium,
Chapiter VII: Ionization chamber 1 Types of ionization chambers Sensitive volume: gas (most often air direct measurement of exposure) ionization chamber Sensitive volume: semiconductor (silicon, germanium,
SAIOH Tutorial Ventilation 1 pressures and basic air flow calculations
 SAIOH Tutorial Ventilation 1 pressures and basic air flow calculations Acknowledgement This tutorial was provided by SAIOH as an assessment support aid for prospective candidates. The tutorial is free
SAIOH Tutorial Ventilation 1 pressures and basic air flow calculations Acknowledgement This tutorial was provided by SAIOH as an assessment support aid for prospective candidates. The tutorial is free
Measuring State Parameters of the Atmosphere
 Measuring State Parameters of the Atmosphere Some Applications of Atmospheric Thermodynamics Earth Observing Laboratory, NCAR IDEAS-4 Tutorial Introduction Goals of This Presentation Present two complementary
Measuring State Parameters of the Atmosphere Some Applications of Atmospheric Thermodynamics Earth Observing Laboratory, NCAR IDEAS-4 Tutorial Introduction Goals of This Presentation Present two complementary
5) The amount of heat needed to raise the temperature of 1 gram of a substance by 1 C is called: Page Ref: 69
 Homework #2 Due 9/19/14 1) If the maximum temperature for a particular day is 26 C and the minimum temperature is 14 C, what would the daily mean temperature be? (Page Ref: 66) 2) How is the annual mean
Homework #2 Due 9/19/14 1) If the maximum temperature for a particular day is 26 C and the minimum temperature is 14 C, what would the daily mean temperature be? (Page Ref: 66) 2) How is the annual mean
By ablation we mean the recession of a surface due to heating, usually by a hot gas. It is the key process for
 16.50 Lecture 15 Subject: Ablative cooling By ablation we mean the recession of a surface due to heating, usually by a hot gas. It is the key process for a) Re-entry heat shields b) Solid propellant nozzles
16.50 Lecture 15 Subject: Ablative cooling By ablation we mean the recession of a surface due to heating, usually by a hot gas. It is the key process for a) Re-entry heat shields b) Solid propellant nozzles
Physics and Thermodynamics of Water and Ice. Ottmar Möhler
 Forschungszentrum Karlsruhe in der Helmholtz-Gemeinschaft Physics and Thermodynamics of Water and Ice Ottmar Möhler Institute for Meteorology and Climate Research (IMK-AAF) ESF workshop on Microbiological
Forschungszentrum Karlsruhe in der Helmholtz-Gemeinschaft Physics and Thermodynamics of Water and Ice Ottmar Möhler Institute for Meteorology and Climate Research (IMK-AAF) ESF workshop on Microbiological
Simplified Microphysics. condensation evaporation. evaporation
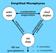 Simplified Microphysics water vapor condensation evaporation cloud droplets evaporation condensation collection rain drops fall out (precipitation) = 0 (reversible) = (irreversible) Simplified Microphysics
Simplified Microphysics water vapor condensation evaporation cloud droplets evaporation condensation collection rain drops fall out (precipitation) = 0 (reversible) = (irreversible) Simplified Microphysics
