The Clausius-Clapeyron Equation
|
|
|
- Tyler Cory Spencer
- 5 years ago
- Views:
Transcription
1 The Clausius-Clapeyron Equation stepwise Frank W Seeberger The Clausius-Clapeyron Equation 1 / 8
2 Outline 1 Application Abbreviations 2 3 Frank W Seeberger The Clausius-Clapeyron Equation 2 / 8
3 Application Abbreviations Application of the For calculating... Bsp.: phase diagram of water the phase boundary line H (melt pressure curve given) the melting point at p 2 (T = constant) with given p 1 and H f 221 bar pressure 1,013 bar 0,006 bar ice triple point water vapour critical point 0,01 C 100 C temperature 374 C Frank W Seeberger The Clausius-Clapeyron Equation 3 / 8
4 Abbreviations Application Abbreviations G V p S T Free Enthalpy Volume Pressure Entropy Temperature Frank W Seeberger The Clausius-Clapeyron Equation 4 / 8
5 dg = V SdT (1) dg 2 = V 2 S 2 dt and V 1 S 1 dt = dg 1 (2) V 2 S 2 dt = V 1 S 1 dt (3) V 2 V 1 = S 2 dt S 1 dt (4) (V 2 V 1 ) = (S 2 S 1 ) dt (5) dt = S 2 S 1 Frank W Seeberger The Clausius-Clapeyron Equation 5 / 8
6 dg = V SdT (1) dg 2 = V 2 S 2 dt and V 1 S 1 dt = dg 1 (2) V 2 S 2 dt = V 1 S 1 dt (3) V 2 V 1 = S 2 dt S 1 dt (4) (V 2 V 1 ) = (S 2 S 1 ) dt (5) dt = S 2 S 1 Frank W Seeberger The Clausius-Clapeyron Equation 5 / 8
7 dg = V SdT (1) dg 2 = V 2 S 2 dt and V 1 S 1 dt = dg 1 (2) V 2 S 2 dt = V 1 S 1 dt (3) V 2 V 1 = S 2 dt S 1 dt (4) (V 2 V 1 ) = (S 2 S 1 ) dt (5) dt = S 2 S 1 Frank W Seeberger The Clausius-Clapeyron Equation 5 / 8
8 dg = V SdT (1) dg 2 = V 2 S 2 dt and V 1 S 1 dt = dg 1 (2) V 2 S 2 dt = V 1 S 1 dt (3) V 2 V 1 = S 2 dt S 1 dt (4) (V 2 V 1 ) = (S 2 S 1 ) dt (5) dt = S 2 S 1 Frank W Seeberger The Clausius-Clapeyron Equation 5 / 8
9 dg = V SdT (1) dg 2 = V 2 S 2 dt and V 1 S 1 dt = dg 1 (2) V 2 S 2 dt = V 1 S 1 dt (3) V 2 V 1 = S 2 dt S 1 dt (4) (V 2 V 1 ) = (S 2 S 1 ) dt (5) dt = S 2 S 1 Frank W Seeberger The Clausius-Clapeyron Equation 5 / 8
10 dg = V SdT (1) dg 2 = V 2 S 2 dt and V 1 S 1 dt = dg 1 (2) V 2 S 2 dt = V 1 S 1 dt (3) V 2 V 1 = S 2 dt S 1 dt (4) (V 2 V 1 ) = (S 2 S 1 ) dt (5) dt = S 2 S 1 Frank W Seeberger The Clausius-Clapeyron Equation 5 / 8
11 dt = S 2 S 1 G = H T S and G = 0 leads to (7) S = H T dt = H T V and (8) (9) Frank W Seeberger The Clausius-Clapeyron Equation 6 / 8
12 dt = S 2 S 1 G = H T S and G = 0 leads to (7) S = H T dt = H T V and (8) (9) Frank W Seeberger The Clausius-Clapeyron Equation 6 / 8
13 dt = S 2 S 1 G = H T S and G = 0 leads to (7) S = H T dt = H T V and (8) (9) Frank W Seeberger The Clausius-Clapeyron Equation 6 / 8
14 dt = S 2 S 1 G = H T S and G = 0 leads to (7) S = H T dt = H T V and (8) (9) Frank W Seeberger The Clausius-Clapeyron Equation 6 / 8
15 of the p2 dt = H T V p 1 = H V T2 T 1 dt T (9) and (10) p 2 p 1 = H V ln T 2 T 1 (11) Frank W Seeberger The Clausius-Clapeyron Equation 7 / 8
16 of the p2 dt = H T V p 1 = H V T2 T 1 dt T (9) and (10) p 2 p 1 = H V ln T 2 T 1 (11) Frank W Seeberger The Clausius-Clapeyron Equation 7 / 8
17 of the p2 dt = H T V p 1 = H V T2 T 1 dt T (9) and (10) p 2 p 1 = H V ln T 2 T 1 (11) Frank W Seeberger The Clausius-Clapeyron Equation 7 / 8
18 Walter J. Moore, Dieter O. Hummel Physikalische Chemie, 4. Aufl., S. 250f Walter de Gruyter, Clausius-Clapeyron-Gleichung Frank W Seeberger The Clausius-Clapeyron Equation 8 / 8
The Second Law of Thermodynamics (Chapter 4)
 The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
Thermodynamic condition for equilibrium between two phases a and b is G a = G b, so that during an equilibrium phase change, G ab = G a G b = 0.
 CHAPTER 5 LECTURE NOTES Phases and Solutions Phase diagrams for two one component systems, CO 2 and H 2 O, are shown below. The main items to note are the following: The lines represent equilibria between
CHAPTER 5 LECTURE NOTES Phases and Solutions Phase diagrams for two one component systems, CO 2 and H 2 O, are shown below. The main items to note are the following: The lines represent equilibria between
 3.012 PS 7 3.012 Issued: 11.05.04 Fall 2004 Due: 11.12.04 THERMODYNAMICS 1. single-component phase diagrams. Shown below is a hypothetical phase diagram for a single-component closed system. Answer the
3.012 PS 7 3.012 Issued: 11.05.04 Fall 2004 Due: 11.12.04 THERMODYNAMICS 1. single-component phase diagrams. Shown below is a hypothetical phase diagram for a single-component closed system. Answer the
Application of Thermodynamics in Phase Diagrams. Today s Topics
 Lecture 23 Application of Thermodynamics in Phase Diagrams The Clausius Clapeyron Equation A. K. M. B. Rashid Professor, Department of MME BUET, Dhaka Today s Topics The Clapeyron equation Integration
Lecture 23 Application of Thermodynamics in Phase Diagrams The Clausius Clapeyron Equation A. K. M. B. Rashid Professor, Department of MME BUET, Dhaka Today s Topics The Clapeyron equation Integration
 Physical Chemistry Physical chemistry is the branch of chemistry that establishes and develops the principles of Chemistry in terms of the underlying concepts of Physics Physical Chemistry Main book: Atkins
Physical Chemistry Physical chemistry is the branch of chemistry that establishes and develops the principles of Chemistry in terms of the underlying concepts of Physics Physical Chemistry Main book: Atkins
Phase Diagrams. NC State University
 Chemistry 433 Lecture 18 Phase Diagrams NC State University Definition of a phase diagram A phase diagram is a representation of the states of matter, solid, liquid, or gas as a function of temperature
Chemistry 433 Lecture 18 Phase Diagrams NC State University Definition of a phase diagram A phase diagram is a representation of the states of matter, solid, liquid, or gas as a function of temperature
MS212 Thermodynamics of Materials ( 소재열역학의이해 ) Lecture Note: Chapter 7
 2017 Spring Semester MS212 Thermodynamics of Materials ( 소재열역학의이해 ) Lecture Note: Chapter 7 Byungha Shin ( 신병하 ) Dept. of MSE, KAIST Largely based on lecture notes of Prof. Hyuck-Mo Lee and Prof. WooChul
2017 Spring Semester MS212 Thermodynamics of Materials ( 소재열역학의이해 ) Lecture Note: Chapter 7 Byungha Shin ( 신병하 ) Dept. of MSE, KAIST Largely based on lecture notes of Prof. Hyuck-Mo Lee and Prof. WooChul
Thermodynamics Free E and Phase D. J.D. Price
 Thermodynamics Free E and Phase D J.D. Price Force - the acceleration of matter (N, kg m/s 2 ) Pressure (P)( ) - a force applied over an area (N/m 2 ) Work (W) - force multiplied by distance (kg( m 2 /s
Thermodynamics Free E and Phase D J.D. Price Force - the acceleration of matter (N, kg m/s 2 ) Pressure (P)( ) - a force applied over an area (N/m 2 ) Work (W) - force multiplied by distance (kg( m 2 /s
Exam 3, Chemistry 481, 8 December 2017
 1 Exam 3, Chemistry 481, 8 December 2017 Show all work for full credit Useful constants: k B = 1.3807 10 23 J K 1 ; R (molar gas constant) = 8.314 J K 1 mol 1 Helmholz free energy: A = U S, so that da
1 Exam 3, Chemistry 481, 8 December 2017 Show all work for full credit Useful constants: k B = 1.3807 10 23 J K 1 ; R (molar gas constant) = 8.314 J K 1 mol 1 Helmholz free energy: A = U S, so that da
Lecture Phase transformations. Fys2160,
 Lecture 12 01.10.2018 Phase transformations Fys2160, 2018 1 A phase transformation Discontinuous change in the properties of substance when the environent is changed infinitesimaly. Change between phases
Lecture 12 01.10.2018 Phase transformations Fys2160, 2018 1 A phase transformation Discontinuous change in the properties of substance when the environent is changed infinitesimaly. Change between phases
CHAPTER 4 Physical Transformations of Pure Substances.
 I. Generalities. CHAPTER 4 Physical Transformations of Pure Substances. A. Definitions: 1. A phase of a substance is a form of matter that is uniform throughout in chemical composition and physical state.
I. Generalities. CHAPTER 4 Physical Transformations of Pure Substances. A. Definitions: 1. A phase of a substance is a form of matter that is uniform throughout in chemical composition and physical state.
Problem Set 10 Solutions
 Chemistry 360 Dr Jean M Standard Problem Set 10 Solutions 1 Sketch (roughly to scale) a phase diagram for molecular oxygen given the following information: the triple point occurs at 543 K and 114 torr;
Chemistry 360 Dr Jean M Standard Problem Set 10 Solutions 1 Sketch (roughly to scale) a phase diagram for molecular oxygen given the following information: the triple point occurs at 543 K and 114 torr;
Chapter 8 Phase Diagram, Relative Stability of Solid, Liquid, and Gas
 Chapter 8 Phase Diagram, Relative Stability of Solid, Liquid, and Gas Three states of matter: solid, liquid, gas (plasma) At low T: Solid is most stable. At high T: liquid or gas is most stable. Ex: Most
Chapter 8 Phase Diagram, Relative Stability of Solid, Liquid, and Gas Three states of matter: solid, liquid, gas (plasma) At low T: Solid is most stable. At high T: liquid or gas is most stable. Ex: Most
ln( P vap(s) / torr) = T / K ln( P vap(l) / torr) = T / K
 Chem 4501 Introduction to Thermodynamics, 3 Credits Kinetics, and Statistical Mechanics Fall Semester 2017 Homework Problem Set Number 9 Solutions 1. McQuarrie and Simon, 9-4. Paraphrase: Given expressions
Chem 4501 Introduction to Thermodynamics, 3 Credits Kinetics, and Statistical Mechanics Fall Semester 2017 Homework Problem Set Number 9 Solutions 1. McQuarrie and Simon, 9-4. Paraphrase: Given expressions
CHEM-UA 652: Thermodynamics and Kinetics
 1 CHEM-UA 652: hermodynamics and Kinetics Notes for Lecture 14 I. HE CLAEYRON EQUAION he Clapeyron attempts to answer the question of what the shape of a two-phase coexistence line is. In the - plane,
1 CHEM-UA 652: hermodynamics and Kinetics Notes for Lecture 14 I. HE CLAEYRON EQUAION he Clapeyron attempts to answer the question of what the shape of a two-phase coexistence line is. In the - plane,
Introduction to Chemical Thermodynamics. (10 Lectures) Michaelmas Term
 Introduction to Chemical Thermodynamics Dr. D. E. Manolopoulos First Year (0 Lectures) Michaelmas Term Lecture Synopsis. Introduction & Background. Le Chatelier s Principle. Equations of state. Systems
Introduction to Chemical Thermodynamics Dr. D. E. Manolopoulos First Year (0 Lectures) Michaelmas Term Lecture Synopsis. Introduction & Background. Le Chatelier s Principle. Equations of state. Systems
Free energy dependence along the coexistence curve
 Free energy dependence along the coexistence curve In a system where two phases (e.g. liquid and gas) are in equilibrium the Gibbs energy is G = GG l + GG gg, where GG l and GG gg are the Gibbs energies
Free energy dependence along the coexistence curve In a system where two phases (e.g. liquid and gas) are in equilibrium the Gibbs energy is G = GG l + GG gg, where GG l and GG gg are the Gibbs energies
Introduction to Chemical Thermodynamics. D. E. Manolopoulos First Year (13 Lectures) Michaelmas Term
 Introduction to Chemical Thermodynamics D. E. Manolopoulos First Year (13 Lectures) Michaelmas Term Lecture Synopsis 1. Introduction & Background. Le Chatelier s Principle. Equations of state. Systems
Introduction to Chemical Thermodynamics D. E. Manolopoulos First Year (13 Lectures) Michaelmas Term Lecture Synopsis 1. Introduction & Background. Le Chatelier s Principle. Equations of state. Systems
Chemistry 163B. One-Component. Phase Diagram Basics
 Chemistry 163B One-Component Phase Diagram Basics 1 qualitative factors in phase changes solid melting liquid freezing/fusion vaporization ENDOTHERMIC liquid gas condensation EXOTHERMIC sublimation solid
Chemistry 163B One-Component Phase Diagram Basics 1 qualitative factors in phase changes solid melting liquid freezing/fusion vaporization ENDOTHERMIC liquid gas condensation EXOTHERMIC sublimation solid
Phase Equilibrium: Preliminaries
 Phase Equilibrium: Preliminaries Phase diagrams for two one component systems, CO 2 and H 2 O, are shown below. The main items to note are the following: The lines represent equilibria between two phases.
Phase Equilibrium: Preliminaries Phase diagrams for two one component systems, CO 2 and H 2 O, are shown below. The main items to note are the following: The lines represent equilibria between two phases.
A 3 Vapor pressure of volatile liquids
 Versuchsanleitungen zum Praktikum Physikalische Chemie für Anfänger 1 A 3 Vapor pressure of volatile liquids Purpose The purpose of this experiment is to determine the vaporization enthalpy, entropy and
Versuchsanleitungen zum Praktikum Physikalische Chemie für Anfänger 1 A 3 Vapor pressure of volatile liquids Purpose The purpose of this experiment is to determine the vaporization enthalpy, entropy and
Chapter 5. On-line resource
 Chapter 5 The water-air heterogeneous system On-line resource on-line analytical system that portrays the thermodynamic properties of water vapor and many other gases http://webbook.nist.gov/chemistry/fluid/
Chapter 5 The water-air heterogeneous system On-line resource on-line analytical system that portrays the thermodynamic properties of water vapor and many other gases http://webbook.nist.gov/chemistry/fluid/
UNIT 15: THERMODYNAMICS
 UNIT 15: THERMODYNAMICS ENTHALPY, DH ENTROPY, DS GIBBS FREE ENERGY, DG ENTHALPY, DH Energy Changes in Reactions Heat is the transfer of thermal energy between two bodies that are at different temperatures.
UNIT 15: THERMODYNAMICS ENTHALPY, DH ENTROPY, DS GIBBS FREE ENERGY, DG ENTHALPY, DH Energy Changes in Reactions Heat is the transfer of thermal energy between two bodies that are at different temperatures.
The Gibbs Phase Rule F = 2 + C - P
 The Gibbs Phase Rule The phase rule allows one to determine the number of degrees of freedom (F) or variance of a chemical system. This is useful for interpreting phase diagrams. F = 2 + C - P Where F
The Gibbs Phase Rule The phase rule allows one to determine the number of degrees of freedom (F) or variance of a chemical system. This is useful for interpreting phase diagrams. F = 2 + C - P Where F
Vapor Pressure is determined primarily from!vaph!vaph depends on the intermolecular forces
 What do you remember from last time? What do you remember from last time? You have two containers. one has a total volume of 2 L and one has a total volume of 1 L Into each you place 500 ml of liquid ether
What do you remember from last time? What do you remember from last time? You have two containers. one has a total volume of 2 L and one has a total volume of 1 L Into each you place 500 ml of liquid ether
Introduction into thermodynamics
 Introduction into thermodynamics Solid-state thermodynamics, J. Majzlan Chemical thermodynamics deals with reactions between substances and species. Mechanical thermodynamics, on the other hand, works
Introduction into thermodynamics Solid-state thermodynamics, J. Majzlan Chemical thermodynamics deals with reactions between substances and species. Mechanical thermodynamics, on the other hand, works
More on phase diagram, chemical potential, and mixing
 More on phase diagram, chemical potential, and mixing Narayanan Kurur Department of Chemistry IIT Delhi 13 July 2013 Melting point changes with P ( ) Gα P T = V α V > 0 = G α when P Intersection point
More on phase diagram, chemical potential, and mixing Narayanan Kurur Department of Chemistry IIT Delhi 13 July 2013 Melting point changes with P ( ) Gα P T = V α V > 0 = G α when P Intersection point
Phase Equilibria in a One-Component System I
 5.60 spring 2005 Lecture #17 page 1 Phase Equilibria in a One-Component System I Goal: Understand the general phenomenology of phase transitions and phase coexistence conditions for a single component
5.60 spring 2005 Lecture #17 page 1 Phase Equilibria in a One-Component System I Goal: Understand the general phenomenology of phase transitions and phase coexistence conditions for a single component
For an incompressible β and k = 0, Equations (6.28) and (6.29) become:
 Internal Energy and Entropy as Functions of T and V These are general equations relating the internal energy and entropy of homogeneous fluids of constant composition to temperature and volume. Equation
Internal Energy and Entropy as Functions of T and V These are general equations relating the internal energy and entropy of homogeneous fluids of constant composition to temperature and volume. Equation
Practice Examinations Chem 393 Fall 2005 Time 1 hr 15 min for each set.
 Practice Examinations Chem 393 Fall 2005 Time 1 hr 15 min for each set. The symbols used here are as discussed in the class. Use scratch paper as needed. Do not give more than one answer for any question.
Practice Examinations Chem 393 Fall 2005 Time 1 hr 15 min for each set. The symbols used here are as discussed in the class. Use scratch paper as needed. Do not give more than one answer for any question.
Thermodynamics of solids 5. Unary systems. Kwangheon Park Kyung Hee University Department of Nuclear Engineering
 Thermodynamics of solids 5. Unary systems Kwangheon ark Kyung Hee University Department of Nuclear Engineering 5.1. Unary heterogeneous system definition Unary system: one component system. Unary heterogeneous
Thermodynamics of solids 5. Unary systems Kwangheon ark Kyung Hee University Department of Nuclear Engineering 5.1. Unary heterogeneous system definition Unary system: one component system. Unary heterogeneous
12. Heat of melting and evaporation of water
 VS 12. Heat of melting and evaporation of water 12.1 Introduction The change of the physical state of a substance in general requires the absorption or release of heat. In this case, one speaks of a first
VS 12. Heat of melting and evaporation of water 12.1 Introduction The change of the physical state of a substance in general requires the absorption or release of heat. In this case, one speaks of a first
B. Correct! Good work. F = C P + 2 = = 2 degrees of freedom. Good try. Hint: Think about the meaning of components and phases.
 Physical Chemistry - Problem Drill 06: Phase Equilibrium No. 1 of 10 1. The Gibbs Phase Rule is F = C P + 2, how many degrees of freedom does a system have that has two independent components and two phases?
Physical Chemistry - Problem Drill 06: Phase Equilibrium No. 1 of 10 1. The Gibbs Phase Rule is F = C P + 2, how many degrees of freedom does a system have that has two independent components and two phases?
Mixtures. Partial Molar Quantities
 CHEM 331 Physical Chemistry Fall 2017 Mixtures Our current discussion takes up some general results for systems that are mixtures and/or open. The former involve systems that contain multiple components;
CHEM 331 Physical Chemistry Fall 2017 Mixtures Our current discussion takes up some general results for systems that are mixtures and/or open. The former involve systems that contain multiple components;
The Clausius-Clapeyron and the Kelvin Equations
 PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics The Clausius-Clapeyron and the Kelvin Equations by Dario B. Giaiotti and Fulvio
PhD Environmental Fluid Mechanics Physics of the Atmosphere University of Trieste International Center for Theoretical Physics The Clausius-Clapeyron and the Kelvin Equations by Dario B. Giaiotti and Fulvio
Lecture 4-6 Equilibrium
 Lecture 4-6 Equilibrium Discontinuity in the free energy, G verses T graph is an indication of phase transition. For one-component system, existing in two phases, the chemical potentials of each of these
Lecture 4-6 Equilibrium Discontinuity in the free energy, G verses T graph is an indication of phase transition. For one-component system, existing in two phases, the chemical potentials of each of these
Physical transformations of pure substances Boiling, freezing, and the conversion of graphite to diamond examples of phase transitions changes of
 Physical transformations of pure substances Boiling, freezing, and the conversion of graphite to diamond examples of phase transitions changes of phase without change of chemical composition. In this chapter
Physical transformations of pure substances Boiling, freezing, and the conversion of graphite to diamond examples of phase transitions changes of phase without change of chemical composition. In this chapter
1. Heterogeneous Systems and Chemical Equilibrium
 1. Heterogeneous Systems and Chemical Equilibrium The preceding section involved only single phase systems. For it to be in thermodynamic equilibrium, a homogeneous system must be in thermal equilibrium
1. Heterogeneous Systems and Chemical Equilibrium The preceding section involved only single phase systems. For it to be in thermodynamic equilibrium, a homogeneous system must be in thermal equilibrium
4) It is a state function because enthalpy(h), entropy(s) and temperature (T) are state functions.
 Chemical Thermodynamics S.Y.BSc. Concept of Gibb s free energy and Helmholtz free energy a) Gibb s free energy: 1) It was introduced by J.Willard Gibb s to account for the work of expansion due to volume
Chemical Thermodynamics S.Y.BSc. Concept of Gibb s free energy and Helmholtz free energy a) Gibb s free energy: 1) It was introduced by J.Willard Gibb s to account for the work of expansion due to volume
Liquids and Solids. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Liquids and Solids Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Gases, Liquids and Solids Gases are compressible fluids. They have no proper volume and proper
Liquids and Solids Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Gases, Liquids and Solids Gases are compressible fluids. They have no proper volume and proper
Gibb s free energy change with temperature in a single component system
 Gibb s free energy change with temperature in a single component system An isolated system always tries to maximize the entropy. That means the system is stable when it has maximum possible entropy. Instead
Gibb s free energy change with temperature in a single component system An isolated system always tries to maximize the entropy. That means the system is stable when it has maximum possible entropy. Instead
CHEMICAL THERMODYNAMICS
 DEPARTMENT OF APPLIED CHEMISTRY LECTURE NOTES 6151- ENGINEERING CHEMISTRY-II UNIT II CHEMICAL THERMODYNAMICS Unit syllabus: Terminology of thermodynamics - Second law: Entropy - entropy change for an ideal
DEPARTMENT OF APPLIED CHEMISTRY LECTURE NOTES 6151- ENGINEERING CHEMISTRY-II UNIT II CHEMICAL THERMODYNAMICS Unit syllabus: Terminology of thermodynamics - Second law: Entropy - entropy change for an ideal
Courtesy of Marc De Graef. Used with permission.
 Courtesy of Marc De Graef. Used with permission. 3.01 PS 5 3.01 Issued: 10.31.04 Fall 005 Due: 10..04 1. Electrochemistry. a. What voltage is measured across the electrodes of a Zn/Cu Daniell galvanic
Courtesy of Marc De Graef. Used with permission. 3.01 PS 5 3.01 Issued: 10.31.04 Fall 005 Due: 10..04 1. Electrochemistry. a. What voltage is measured across the electrodes of a Zn/Cu Daniell galvanic
HONOUR SCHOOL OF NATURAL SCIENCE. Final Examination GENERAL PHYSICAL CHEMISTRY I. Answer FIVE out of nine questions
 HONOUR SCHOOL OF NATURAL SCIENCE Final Examination GENERAL PHYSICAL CHEMISTRY I Monday, 12 th June 2000, 9.30 a.m. - 12.30 p.m. Answer FIVE out of nine questions The numbers in square brackets indicate
HONOUR SCHOOL OF NATURAL SCIENCE Final Examination GENERAL PHYSICAL CHEMISTRY I Monday, 12 th June 2000, 9.30 a.m. - 12.30 p.m. Answer FIVE out of nine questions The numbers in square brackets indicate
* The actual temperature dependence for the enthalpy and entropy of reaction is given by the following two equations:
 CHM 3400 Problem Set 5 Due date: Tuesday, October 7 th Do all of the following problems. Show your work. "The first essential in chemistry is that you should perform practical work and conduct experiments,
CHM 3400 Problem Set 5 Due date: Tuesday, October 7 th Do all of the following problems. Show your work. "The first essential in chemistry is that you should perform practical work and conduct experiments,
At this point, we've developed the tools and basic concepts necessary to apply
 18 Lecture 0 At this point, we've developed the tools and basic concepts necessary to apply thermodynamics to a number of different systems, with the ultimate goal of describing chemically reacting systems.
18 Lecture 0 At this point, we've developed the tools and basic concepts necessary to apply thermodynamics to a number of different systems, with the ultimate goal of describing chemically reacting systems.
Phase Change (State Change): A change in physical form but not the chemical identity of a substance.
 CHM 123 Chapter 11 11.1-11.2 Phase change, evaporation, vapor pressure, and boiling point Phase Change (State Change): A change in physical form but not the chemical identity of a substance. Heat (Enthalpy)
CHM 123 Chapter 11 11.1-11.2 Phase change, evaporation, vapor pressure, and boiling point Phase Change (State Change): A change in physical form but not the chemical identity of a substance. Heat (Enthalpy)
Practice Midterm Exam 1 March, 2011
 NAME: MSE 508: Solid State Thermodynamics Department of Materials Science & Engineering Boise State University Spring 2011 Practice Midterm Exam 1 March, 2011 Problem Total Points Points Obtained 1. 2.
NAME: MSE 508: Solid State Thermodynamics Department of Materials Science & Engineering Boise State University Spring 2011 Practice Midterm Exam 1 March, 2011 Problem Total Points Points Obtained 1. 2.
Liquids. properties & structure
 Liquids properties & structure Energetics of Vaporization when the high energy molecules are lost from the liquid, it lowers the average kinetic energy if energy is not drawn back into the liquid, its
Liquids properties & structure Energetics of Vaporization when the high energy molecules are lost from the liquid, it lowers the average kinetic energy if energy is not drawn back into the liquid, its
We can see from the gas phase form of the equilibrium constant that pressure of species depend on pressure. For the general gas phase reaction,
 Pressure dependence Equilibrium constant We can see from the gas phase form of the equilibrium constant that the equilibrium concentrations of species depend on pressure. This dependence is inside the
Pressure dependence Equilibrium constant We can see from the gas phase form of the equilibrium constant that the equilibrium concentrations of species depend on pressure. This dependence is inside the
Phase Equilibria I. Introduction. Heat and Phase Changes
 Phase Equilibria I 2 Introduction In the previous chapter, it was discussed the thermodynamics principles that are the basis of thermochemistry. It was shown how to calculate the energy involved in any
Phase Equilibria I 2 Introduction In the previous chapter, it was discussed the thermodynamics principles that are the basis of thermochemistry. It was shown how to calculate the energy involved in any
General Physical Chemistry I
 General Physical Chemistry I Lecture 11 Aleksey Kocherzhenko March 12, 2015" Last time " W Entropy" Let be the number of microscopic configurations that correspond to the same macroscopic state" Ø Entropy
General Physical Chemistry I Lecture 11 Aleksey Kocherzhenko March 12, 2015" Last time " W Entropy" Let be the number of microscopic configurations that correspond to the same macroscopic state" Ø Entropy
Lecture Notes 2: Physical Equilibria Phase Diagrams
 Lecture Notes 2: Physical Equilibria Phase Diagrams There are number of graphical means to help to understand the relationships between the different phases of a particular substance. The first thing we
Lecture Notes 2: Physical Equilibria Phase Diagrams There are number of graphical means to help to understand the relationships between the different phases of a particular substance. The first thing we
NAME and Section No. b). A refrigerator is a Carnot cycle run backwards. That is, heat is now withdrawn from the cold reservoir at T cold
 Chemistry 391 Fall 007 Exam II KEY 1. (30 Points) ***Do 5 out of 7***(If 6 or 7 are done only the first 5 will be graded)*** a). How does the efficiency of a reversible engine compare with that of an irreversible
Chemistry 391 Fall 007 Exam II KEY 1. (30 Points) ***Do 5 out of 7***(If 6 or 7 are done only the first 5 will be graded)*** a). How does the efficiency of a reversible engine compare with that of an irreversible
Thermodynamics SCQF Level 9, PHYS Wednesday 9th May, :30-16:30
 College of Science and Engineering School of Physics & Astronomy H T O F E E U D N I I N V E B R U S I R T Y H G Thermodynamics SCQF Level 9, PHYS09021 Wednesday 9th May, 2012 14:30-16:30 Chairman of Examiners
College of Science and Engineering School of Physics & Astronomy H T O F E E U D N I I N V E B R U S I R T Y H G Thermodynamics SCQF Level 9, PHYS09021 Wednesday 9th May, 2012 14:30-16:30 Chairman of Examiners
THERMODYNAMICS CONTENTS
 1. Introduction HERMODYNAMICS CONENS. Maxwell s thermodynamic equations.1 Derivation of Maxwell s equations 3. Function and derivative 3.1 Differentiation 4. Cyclic Rule artial Differentiation 5. State
1. Introduction HERMODYNAMICS CONENS. Maxwell s thermodynamic equations.1 Derivation of Maxwell s equations 3. Function and derivative 3.1 Differentiation 4. Cyclic Rule artial Differentiation 5. State
HR-XRPD and Polymorph Stability
 HR-XRPD and Polymorph Stability HR-XRPD, A CRUCIAL FACTOR IN THE DETERMINATION OF THE STABILITY HIERARCHY OF POLYMORPHS BY TOPOLOGICAL AND EXPERIMENTAL PRESSURE- TEMPERATURE DIAGRAMS Ivo B. Rietveld, M.
HR-XRPD and Polymorph Stability HR-XRPD, A CRUCIAL FACTOR IN THE DETERMINATION OF THE STABILITY HIERARCHY OF POLYMORPHS BY TOPOLOGICAL AND EXPERIMENTAL PRESSURE- TEMPERATURE DIAGRAMS Ivo B. Rietveld, M.
WEEK 6. Multiphase systems
 WEEK 6 Multiphase systems Multiphase systems P 237. Processes usually deal with material being transferred from one phase (gas, liquid, or solid) to another. 6.1a Phase diagrams F = force on piston Water
WEEK 6 Multiphase systems Multiphase systems P 237. Processes usually deal with material being transferred from one phase (gas, liquid, or solid) to another. 6.1a Phase diagrams F = force on piston Water
Chapter 11 Spontaneous Change and Equilibrium
 Chapter 11 Spontaneous Change and Equilibrium 11-1 Enthalpy and Spontaneous Change 11-2 Entropy 11-3 Absolute Entropies and Chemical Reactions 11-4 The Second Law of Thermodynamics 11-5 The Gibbs Function
Chapter 11 Spontaneous Change and Equilibrium 11-1 Enthalpy and Spontaneous Change 11-2 Entropy 11-3 Absolute Entropies and Chemical Reactions 11-4 The Second Law of Thermodynamics 11-5 The Gibbs Function
Physics and Thermodynamics of Water and Ice. Ottmar Möhler
 Forschungszentrum Karlsruhe in der Helmholtz-Gemeinschaft Physics and Thermodynamics of Water and Ice Ottmar Möhler Institute for Meteorology and Climate Research (IMK-AAF) ESF workshop on Microbiological
Forschungszentrum Karlsruhe in der Helmholtz-Gemeinschaft Physics and Thermodynamics of Water and Ice Ottmar Möhler Institute for Meteorology and Climate Research (IMK-AAF) ESF workshop on Microbiological
Lecture 6 Free energy and its uses
 Lecture 6 Free energy and its uses dg = VdP G - G o = PoP VdP G = G o (T) + RT ln P/P o for gases and G = G o (T) + V (P-P o ) for solids and liquids µ = µ o + RT ln P (for one mole) G = G o + RT ln Q
Lecture 6 Free energy and its uses dg = VdP G - G o = PoP VdP G = G o (T) + RT ln P/P o for gases and G = G o (T) + V (P-P o ) for solids and liquids µ = µ o + RT ln P (for one mole) G = G o + RT ln Q
OCN 623: Thermodynamic Laws & Gibbs Free Energy. or how to predict chemical reactions without doing experiments
 OCN 623: Thermodynamic Laws & Gibbs Free Energy or how to predict chemical reactions without doing experiments Definitions Extensive properties Depend on the amount of material e.g. # of moles, mass or
OCN 623: Thermodynamic Laws & Gibbs Free Energy or how to predict chemical reactions without doing experiments Definitions Extensive properties Depend on the amount of material e.g. # of moles, mass or
Melting of ice particles:
 Melting of ice particles: When ice particles fall below 0 C they begin to melt, but the process takes some time since heat transfer needs to occur (heat from ambient environment has to supply the latent
Melting of ice particles: When ice particles fall below 0 C they begin to melt, but the process takes some time since heat transfer needs to occur (heat from ambient environment has to supply the latent
CHEM-UA 652: Thermodynamics and Kinetics
 1 CHEM-UA 652: Thermodynamics and Kinetics Notes for Lecture 13 I. PHASE DIAGRAMS The different phases of substances are characterized by different ranges of thermodynamic variables in which these phasesarethestablephases.
1 CHEM-UA 652: Thermodynamics and Kinetics Notes for Lecture 13 I. PHASE DIAGRAMS The different phases of substances are characterized by different ranges of thermodynamic variables in which these phasesarethestablephases.
Lecture 4 Clausius Inequality
 Lecture 4 Clausius Inequality Entropy distinguishes between irreversible and reversible processes. irrev S > 0 rev In a spontaneous process, there should be a net increase in the entropy of the system
Lecture 4 Clausius Inequality Entropy distinguishes between irreversible and reversible processes. irrev S > 0 rev In a spontaneous process, there should be a net increase in the entropy of the system
The Thermodynamic Properties of Platinum
 DOI: 10.1595/147106705X54262 The Thermodynamic Properties of Platinum REVISED DATA FOR THE LIQUID STATE AND VAPOUR PRESSURE By J. W. Arblaster Coleshill Laboratories, Gorsey Lane, Coleshill, West Midlands
DOI: 10.1595/147106705X54262 The Thermodynamic Properties of Platinum REVISED DATA FOR THE LIQUID STATE AND VAPOUR PRESSURE By J. W. Arblaster Coleshill Laboratories, Gorsey Lane, Coleshill, West Midlands
Physics 408 Final Exam
 Physics 408 Final Exam Name You are graded on your work, with partial credit where it is deserved. Please give clear, well-organized solutions. 1. Consider the coexistence curve separating two different
Physics 408 Final Exam Name You are graded on your work, with partial credit where it is deserved. Please give clear, well-organized solutions. 1. Consider the coexistence curve separating two different
Outline Review Example Problem 1. Thermodynamics. Review and Example Problems: Part-2. X Bai. SDSMT, Physics. Fall 2014
 Review and Example Problems: Part- SDSMT, Physics Fall 014 1 Review Example Problem 1 Exponents of phase transformation : contents 1 Basic Concepts: Temperature, Work, Energy, Thermal systems, Ideal Gas,
Review and Example Problems: Part- SDSMT, Physics Fall 014 1 Review Example Problem 1 Exponents of phase transformation : contents 1 Basic Concepts: Temperature, Work, Energy, Thermal systems, Ideal Gas,
Problem Set #10 Assigned November 8, 2013 Due Friday, November 15, 2013 Please show all work for credit To Hand in
 Problem Set #10 Assigned November 8, 2013 Due Friday, November 15, 2013 Please show all work for credit To Hand in 1. 2. 1 3. 4. The vapor pressure of an unknown solid is approximately given by ln(p/torr)
Problem Set #10 Assigned November 8, 2013 Due Friday, November 15, 2013 Please show all work for credit To Hand in 1. 2. 1 3. 4. The vapor pressure of an unknown solid is approximately given by ln(p/torr)
m m 3 mol Pa = Pa or bar At this pressure the system must also be at approximately 1000 K.
 5. PHASES AND SOLUTIONS n Thermodynamics of Vapor Pressure 5.. At equilibrium, G(graphite) G(diamond); i.e., G 2 0. We are given G 2900 J mol. ( G/ P) T V V 2.0 g mol.95 0 6 m 3 mol Holding T constant
5. PHASES AND SOLUTIONS n Thermodynamics of Vapor Pressure 5.. At equilibrium, G(graphite) G(diamond); i.e., G 2 0. We are given G 2900 J mol. ( G/ P) T V V 2.0 g mol.95 0 6 m 3 mol Holding T constant
UNIVERSITY OF SOUTHAMPTON
 UNIVERSIY OF SOUHAMPON PHYS1013W1 SEMESER 2 EXAMINAION 2013-2014 Energy and Matter Duration: 120 MINS (2 hours) his paper contains 9 questions. Answers to Section A and Section B must be in separate answer
UNIVERSIY OF SOUHAMPON PHYS1013W1 SEMESER 2 EXAMINAION 2013-2014 Energy and Matter Duration: 120 MINS (2 hours) his paper contains 9 questions. Answers to Section A and Section B must be in separate answer
Name: Discussion Section:
 CBE 141: Chemical Engineering Thermodynamics, Spring 2017, UC Berkeley Midterm 2 FORM B March 23, 2017 Time: 80 minutes, closed-book and closed-notes, one-sided 8 ½ x 11 equation sheet allowed lease show
CBE 141: Chemical Engineering Thermodynamics, Spring 2017, UC Berkeley Midterm 2 FORM B March 23, 2017 Time: 80 minutes, closed-book and closed-notes, one-sided 8 ½ x 11 equation sheet allowed lease show
Lecture Notes 2014March 13 on Thermodynamics A. First Law: based upon conservation of energy
 Dr. W. Pezzaglia Physics 8C, Spring 2014 Page 1 Lecture Notes 2014March 13 on Thermodynamics A. First Law: based upon conservation of energy 1. Work 1 Dr. W. Pezzaglia Physics 8C, Spring 2014 Page 2 (c)
Dr. W. Pezzaglia Physics 8C, Spring 2014 Page 1 Lecture Notes 2014March 13 on Thermodynamics A. First Law: based upon conservation of energy 1. Work 1 Dr. W. Pezzaglia Physics 8C, Spring 2014 Page 2 (c)
CHEMISTRY. CHM202 Class #2 CHEMISTRY. Chapter 10. Chapter Outline for Class #2
 CHEMISTRY Fifth Edition Gilbert Kirss Foster Bretz Davies CHM202 Class #2 1 Chemistry, 5 th Edition Copyright 2017, W. W. Norton & Company CHEMISTRY Fifth Edition Gilbert Kirss Foster Bretz Davies Chapter
CHEMISTRY Fifth Edition Gilbert Kirss Foster Bretz Davies CHM202 Class #2 1 Chemistry, 5 th Edition Copyright 2017, W. W. Norton & Company CHEMISTRY Fifth Edition Gilbert Kirss Foster Bretz Davies Chapter
3.012 PS 7 Thermo solutions Issued: Fall 2003 Graded problems due:
 3.012 PS 7 Thermo solutions 3.012 Issued: 11.17.03 Fall 2003 Graded problems due: 11.26.03 Graded problems: 1. Analysis of equilibrium phases with a binary phase diagram. Shown below is the phase diagram
3.012 PS 7 Thermo solutions 3.012 Issued: 11.17.03 Fall 2003 Graded problems due: 11.26.03 Graded problems: 1. Analysis of equilibrium phases with a binary phase diagram. Shown below is the phase diagram
Chemistry 360 Spring 2017 Dr. Jean M. Standard April 19, Exam points
 Chemistry 360 pring 2017 Dr. Jean M. tandard April 19, 2017 Name Exam 3 100 points Note: You must show your work on problems in order to receive full credit for any answers. You must turn in your equation
Chemistry 360 pring 2017 Dr. Jean M. tandard April 19, 2017 Name Exam 3 100 points Note: You must show your work on problems in order to receive full credit for any answers. You must turn in your equation
Spontaneous Change.! Although exothermic processes tend to be spontaneous, spontaneous reactions can be exothermic or endothermic:
 Spontaneous Change! Any process, once initiated, that continues without further intervention is spontaneous.! Although exothermic processes tend to be spontaneous, spontaneous reactions can be exothermic
Spontaneous Change! Any process, once initiated, that continues without further intervention is spontaneous.! Although exothermic processes tend to be spontaneous, spontaneous reactions can be exothermic
This follows from the Clausius inequality as a consequence of the second law of thermodynamics. Therefore. (for reversible process only) (22.
 Entropy Clausius inequality can be used to analyze the cyclic process in a quantitative manner. The second law became a law of wider applicability when Clausius introduced the property called entropy.
Entropy Clausius inequality can be used to analyze the cyclic process in a quantitative manner. The second law became a law of wider applicability when Clausius introduced the property called entropy.
Lecture 4 Clausius Inequality
 Lecture 4 Clausius Inequality We know: Heat flows from higher temperature to lower temperature. T A V A U A + U B = constant V A, V B constant S = S A + S B T B V B Diathermic The wall insulating, impermeable
Lecture 4 Clausius Inequality We know: Heat flows from higher temperature to lower temperature. T A V A U A + U B = constant V A, V B constant S = S A + S B T B V B Diathermic The wall insulating, impermeable
Basic Thermodynamics Module 1
 Basic Thermodynamics Module 1 Lecture 9: Thermodynamic Properties of Fluids Thermodynamic Properties of fluids Most useful properties: Properties like pressure, volume and temperature which can be measured
Basic Thermodynamics Module 1 Lecture 9: Thermodynamic Properties of Fluids Thermodynamic Properties of fluids Most useful properties: Properties like pressure, volume and temperature which can be measured
Let's look at how different properties affect vapor pressure. P =0 P =vapor pressure P =vapor pressure. first all liquid
 Let's look at how different properties affect vapor pressure P =0 P =vapor pressure P =vapor pressure Quick Quiz You have two containers. one has a total volume of 2 L and one has a total volume of 1 L
Let's look at how different properties affect vapor pressure P =0 P =vapor pressure P =vapor pressure Quick Quiz You have two containers. one has a total volume of 2 L and one has a total volume of 1 L
Identify the intensive quantities from the following: (a) enthalpy (b) volume (c) refractive index (d) none of these
 Q 1. Q 2. Q 3. Q 4. Q 5. Q 6. Q 7. The incorrect option in the following table is: H S Nature of reaction (a) negative positive spontaneous at all temperatures (b) positive negative non-spontaneous regardless
Q 1. Q 2. Q 3. Q 4. Q 5. Q 6. Q 7. The incorrect option in the following table is: H S Nature of reaction (a) negative positive spontaneous at all temperatures (b) positive negative non-spontaneous regardless
Lecture 3 Clausius Inequality
 Lecture 3 Clausius Inequality Rudolf Julius Emanuel Clausius 2 January 1822 24 August 1888 Defined Entropy Greek, en+tropein content transformative or transformation content The energy of the universe
Lecture 3 Clausius Inequality Rudolf Julius Emanuel Clausius 2 January 1822 24 August 1888 Defined Entropy Greek, en+tropein content transformative or transformation content The energy of the universe
NENG 301 Week 8 Unary Heterogeneous Systems (DeHoff, Chap. 7, Chap )
 NENG 301 Week 8 Unary Heterogeneous Systems (DeHoff, Chap. 7, Chap. 5.3-5.4) Learning objectives for Chapter 7 At the end of this chapter you will be able to: Understand the general features of a unary
NENG 301 Week 8 Unary Heterogeneous Systems (DeHoff, Chap. 7, Chap. 5.3-5.4) Learning objectives for Chapter 7 At the end of this chapter you will be able to: Understand the general features of a unary
Problem Set #10 Assigned November 8, 2013 Due Friday, November 15, 2013 Please show all work for credit. To Hand in
 Problem Set #10 Assigned November 8, 013 Due Friday, November 15, 013 Please show all work or credit To Hand in 1. 1 . A least squares it o ln P versus 1/T gives the result 3. Hvaporization = 5.8 kj mol
Problem Set #10 Assigned November 8, 013 Due Friday, November 15, 013 Please show all work or credit To Hand in 1. 1 . A least squares it o ln P versus 1/T gives the result 3. Hvaporization = 5.8 kj mol
Clausius Clapeyron Equation
 Course - BSc. Applied Physical Science (Computer Science) Year & Semester - Ist, IInd Subject - Physics Paper No. - 6 Paper Title - Thermal Physics Lecture No. 18 Clausius Clapeyron Equation Hello friends,
Course - BSc. Applied Physical Science (Computer Science) Year & Semester - Ist, IInd Subject - Physics Paper No. - 6 Paper Title - Thermal Physics Lecture No. 18 Clausius Clapeyron Equation Hello friends,
Vapour pressure of water at high temperature
 Vapour pressure of water at high temperature (Item No.: P2340100) Curricular Relevance Area of Expertise: Physics Education Level: University Topic: Thermodynamics Subtopic: Thermal Properties and Processes
Vapour pressure of water at high temperature (Item No.: P2340100) Curricular Relevance Area of Expertise: Physics Education Level: University Topic: Thermodynamics Subtopic: Thermal Properties and Processes
States of Matter; Liquids and Solids. Condensation - change of a gas to either the solid or liquid state
 States of Matter; Liquids and Solids Phase transitions - a change in substance from one state to another Melting - change from a solid to a liquid state Freezing - change of a liquid to the solid state
States of Matter; Liquids and Solids Phase transitions - a change in substance from one state to another Melting - change from a solid to a liquid state Freezing - change of a liquid to the solid state
Solutions to Problem Set 9
 Solutions to Problem Set 9 1. When possible, we want to write an equation with the quantity on the ordinate in terms of the quantity on the abscissa for each pf the labeled curves. A B C p CHCl3 = K H
Solutions to Problem Set 9 1. When possible, we want to write an equation with the quantity on the ordinate in terms of the quantity on the abscissa for each pf the labeled curves. A B C p CHCl3 = K H
Lecture 3 Evaluation of Entropy
 Lecture 3 Evaluation of Entropy If we wish to designate S by a proper name we can say of it that it is the transformation content of the body, in the same way that we say of the quantity U that it is the
Lecture 3 Evaluation of Entropy If we wish to designate S by a proper name we can say of it that it is the transformation content of the body, in the same way that we say of the quantity U that it is the
Outline Review Example Problem 1 Example Problem 2. Thermodynamics. Review and Example Problems. X Bai. SDSMT, Physics. Fall 2013
 Review and Example Problems SDSMT, Physics Fall 013 1 Review Example Problem 1 Exponents of phase transformation 3 Example Problem Application of Thermodynamic Identity : contents 1 Basic Concepts: Temperature,
Review and Example Problems SDSMT, Physics Fall 013 1 Review Example Problem 1 Exponents of phase transformation 3 Example Problem Application of Thermodynamic Identity : contents 1 Basic Concepts: Temperature,
Are Ionic Liquids Pairwise In Gas Phase?
 TG/g DTG/(g/min) TG/g DTG/( g/min) Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics Supporting Information Are Ionic Liuids Pairwise In Gas Phase? A cluster Approach and
TG/g DTG/(g/min) TG/g DTG/( g/min) Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics Supporting Information Are Ionic Liuids Pairwise In Gas Phase? A cluster Approach and
Module 5 : Electrochemistry Lecture 21 : Review Of Thermodynamics
 Module 5 : Electrochemistry Lecture 21 : Review Of Thermodynamics Objectives In this Lecture you will learn the following The need for studying thermodynamics to understand chemical and biological processes.
Module 5 : Electrochemistry Lecture 21 : Review Of Thermodynamics Objectives In this Lecture you will learn the following The need for studying thermodynamics to understand chemical and biological processes.
Data Provided: A formula sheet and table of physical constants are attached to this paper.
 Data Provided: A formula sheet and table of physical constants are attached to this paper. DEPARTMENT OF PHYSICS AND ASTRONOMY Spring Semester (2016-2017) From Thermodynamics to Atomic and Nuclear Physics
Data Provided: A formula sheet and table of physical constants are attached to this paper. DEPARTMENT OF PHYSICS AND ASTRONOMY Spring Semester (2016-2017) From Thermodynamics to Atomic and Nuclear Physics
Chapter 12 Intermolecular Forces of Attraction
 Chapter 12 Intermolecular Forces of Attraction Intermolecular Forces Attractive or Repulsive Forces between molecules. Molecule - - - - - - Molecule Intramolecular Forces bonding forces within the molecule.
Chapter 12 Intermolecular Forces of Attraction Intermolecular Forces Attractive or Repulsive Forces between molecules. Molecule - - - - - - Molecule Intramolecular Forces bonding forces within the molecule.
Four states of matter (Aggregatzustand) a. solid b. liquid c. gaseous d. plasma
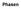 Phasen Four states of matter (Aggregatzustand) a. solid b. liquid c. gaseous d. plasma T Phase diagram H 2 0 Lines = equilibrium = phase boundary = co-existence Sublimate/Condense Evaporate/Condense Melt/Freeze
Phasen Four states of matter (Aggregatzustand) a. solid b. liquid c. gaseous d. plasma T Phase diagram H 2 0 Lines = equilibrium = phase boundary = co-existence Sublimate/Condense Evaporate/Condense Melt/Freeze
Thermodynamics of phase transitions
 Thermodynamics of phase transitions Katarzyna Sznajd-Weron Department of Theoretical of Physics Wroc law University of Science and Technology, Poland March 12, 2017 Katarzyna Sznajd-Weron (WUST) Thermodynamics
Thermodynamics of phase transitions Katarzyna Sznajd-Weron Department of Theoretical of Physics Wroc law University of Science and Technology, Poland March 12, 2017 Katarzyna Sznajd-Weron (WUST) Thermodynamics
Solutions to Problem Set 5
 Cornell University, Physics Department Fall 204 PHYS-334 Statistical Physics Prof. Itai Cohen Solutions to Problem Set 5 David C. sang, Woosong Choi 5. Refrigeration Reif 5.22: Refrigeration cycles have
Cornell University, Physics Department Fall 204 PHYS-334 Statistical Physics Prof. Itai Cohen Solutions to Problem Set 5 David C. sang, Woosong Choi 5. Refrigeration Reif 5.22: Refrigeration cycles have
PHASE CHANGES EVAPORATION EVAPORATION PHYSICAL PROPERTIES OF LIQUID PHYSICAL PROPERTIES OF LIQUID SOME PROPERTIES OF LIQUIDS 2014/08/08
 PHYSICAL PROPERTIES OF LIQUID PHYSICAL PROPERTIES OF LIQUID A physical property is a property that can be changed without changing the fundamental components of a substance. SOME PROPERTIES OF LIQUIDS
PHYSICAL PROPERTIES OF LIQUID PHYSICAL PROPERTIES OF LIQUID A physical property is a property that can be changed without changing the fundamental components of a substance. SOME PROPERTIES OF LIQUIDS
MME 2010 METALLURGICAL THERMODYNAMICS II. Fundamentals of Thermodynamics for Systems of Constant Composition
 MME 2010 METALLURGICAL THERMODYNAMICS II Fundamentals of Thermodynamics for Systems of Constant Composition Thermodynamics addresses two types of problems: 1- Computation of energy difference between two
MME 2010 METALLURGICAL THERMODYNAMICS II Fundamentals of Thermodynamics for Systems of Constant Composition Thermodynamics addresses two types of problems: 1- Computation of energy difference between two
