Physics Nov The Direction of a Reaction A + B C,
|
|
|
- Lindsey Norris
- 5 years ago
- Views:
Transcription
1 Physcs Nov Suppose we have a reacton such as The Drecton of a Reacton A + B C whch has come to equlbrum at some temperature τ. Now we rase the temperature. Does the equlbrum shft to the left (more A and B) or to the rght (more C)? The heat of reacton at constant pressure Q p s the heat that must be suppled to the system f the reacton goes from left to rght. If Q p > 0 heat s absorbed and the reacton s called endothermc. If Q p < 0 heat s released and the reacton s called exothermc. For a reacton at constant pressure the heat s the change n the enthalpy of the system Q p = H. We have H = G + τσ and so H = G τ σ = ( ) G pn ( ) ( G = τ 2 pn ( )) G. τ pn What we actually want to do s to change the temperature slghtly. Then the system s no longer n equlbrum and the reacton (n the forward or reverse drecton) wll have to occur n order to restore equlbrum. When the reacton occurs from left to rght the change n partcle number s N = ν and the change n G s G = µ ν. If ths s 0 we have the equlbrum condton (but we ve taken t out of equlbrum by changng the temperature). The change n H s Q p = H = τ 2 ( ( )) ( ( G = +τ 2 τ pn µ ν τ )) pn. The chemcal potental s ( ) x p/τ µ = τ log n Q Z nt.
2 Physcs Nov We substtute nto our expresson for Q p and obtan Q p = τ 2 = τ 2 = τ 2 = τ 2 log (ν log (x p) ν log (τ n Q Z.nt )) (ν log (τ n Q Z.nt )) (log(τ n Q Z.nt ) ν ) = τ 2 log K p(τ). ( ) (τ n Q Z.nt ) ν We ve related the heat of reacton to the equlbrum constant! Ths s called van t Hoff s equaton. A note on sgns: I ve assumed that the ν are postve on the left hand sde of the reacton and negatve for the rght hand sde of the reacton. Mandl (who provdes the bass for ths secton) assumes the opposte so we wnd up wth our equlbrum constants beng nverses of each other and opposte sgns n the van t Hoff equaton. In any case our law of mass acton has the concentratons of the left hand sde reactants n the numerator and the rght hand sde reactants n the denomnator. So an ncrease n the equlbrum constant means the reacton goes to the left and a decrease means the reacton goes to the rght. We see that f Q p s postve (we have to add heat to go from left to rght an endothermc reacton) then our equlbrum constant decreases wth temperature. Ths means ncreasng the temperature moves the reacton to the rght. Rule of thumb: ncreasng the temperature causes the reacton to go towards whatever drecton t can absorb energy. We ve just shown that ncreasng the temperature drves an endothermc reacton to the rght. It wll drve an exothermc reacton to the left.
3 Physcs Nov Applcaton: the Saha Equaton Ths secton s related to K&K chapter 9 problem 2. Consder the onzaton of atomc hydrogen p + + e H. Ionzng hydrogen from ts ground state requres an energy of 13.6 ev and as the above reacton s wrtten t s exothermc from left to rght. If we are consderng low densty gases we can treat them as classcal deal gases and apply our law of mass acton: [p + ][e ] [H] = (n pq Z pnt )(n eq Z ent ) n HQ Z Hnt exp(i/τ) where the partton functon for the hydrogen atom s to be computed wth the ground state at the zero of energy as we ve taken explct account of the bndng energy I = 13.6 ev. Ths (or more properly some of the forms we wll derve below) s called the Saha equaton. Some of the factors n the equlbrum constant are easy to calculate and others are hard to calculate! Let s do the easy ones. Frst of all the mass of a proton and the mass of a hydrogen atom are almost the same so the quantum concentratons of the proton and the hydrogen are almost the same and we can cancel them out. The quantum concentraton of the electron s ( ) 3/2 me τ n eq = 2π h 2. The nternal partton functons for the electron and proton are both just 2 snce each has spn 1/2. Ths leaves us wth the nternal partton functon of the hydrogen atom. Ths s complcated. Frst of all the electron and proton each have two spn states so whatever else s gong on there s a factor of four due to the spns. Asde: n fact the spns can combne wth the orbtal angular momentum to gve a total angular momentum. In the ground state the orbtal angular momentum s zero and the spns can be parallel to gve a total angular momentum of 1 h wth 3 states or ant-parallel to gve a total angular momentum of 0 wth 1 state. The parallel states are slghtly hgher n energy than the ant-parallel state. Transtons between these states are called hyperfne transtons and result n the 21 cm lne whch s radated and absorbed by neutral hydrogen throughout our galaxy and others. In any case the energy dfference between these states s small enough to be gnored n computng the nternal partton functon for the purposes of the Saha equaton. When all s sad and done we have [p + ][e ] [H] ( ) 3/2 me τ = 4 2π h 2 e I/τ 1 Z Hnt
4 Physcs Nov where the factor of four accounts for the two spn states of the proton and the two spn states of the electron (there s a factor of four n the hydrogen partton functon as well). If the temperature s small compared to the bndng energy of hydrogen (whch means t s small compared to the dfference between the frst excted state and the ground state) then we mght as well approxmate the partton functon as 4. Ths gves [p + ][e ] [H] ( ) 3/2 me τ 2π h 2 e I/τ. If we have only hydrogen and onzed hydrogen [p + ] = [e ] and [e ] ( ) 3/4 me τ [H] 2π h 2 e I/2τ. Some ponts to note: the fact that the exponental has I/2τ ndcates that ths s a mass acton effect not a Boltzmann factor effect. If there s another source of electrons (for example heaver elements whose outer electrons are loosely bound) the reacton would shft to favor more hydrogen and fewer protons. The Saha equaton apples to gases n space or stars as well as donor atoms n sem-conductors (modfed for the approprate physcal characterstcs of the atom and the medum). In fact we can do a lttle more wth the Saha equaton. Let s consder an atom whch has several electrons and ask about the onzaton equlbrum between the ons that have been onzed tmes and those that have been onzed + 1 tmes n +1 [e ] = (n +1Q Z +1nt )(n eq Z ent ) n n Q Z nt exp(i +1 /τ) where n +1 and n are the concentratons of the two ons n +1Q and n Q are the quantum concentratons of the two ons whch are essentally the same so we cancel them out Z +1nt and Z nt are the nternal partton functons of the two ons and I +1 s the dfference n bndng energy between the two ons. That s I +1 s the energy requred to remove an electron from on and produce on +1. Now each on wll have some nternal structure and energy levels. We let ɛ +1j be the energy (relatve to 0 for the on ground state) of the j th state of on + 1. Ths state has multplcty g +1j. (If there s more than one state at a gven energy we say that energy s degenerate and the multplcty s the number of such states. Sometmes the multplcty s called the degeneracy or the statstcal weght.) Smlarly ɛ k and g k are the energy and multplcty of the k th state of on. The fracton of ons + 1 whch are n state j s gven by a Boltzmann factor n +1j n +1 = g +1j e ɛ +1j/τ Z +1nt.
5 Physcs Nov If we substtute ths expresson nto the Saha equaton and also substtute the quantum concentraton of the electrons and the nternal partton functon of the electrons (2) we get n +1j [e ] n k = 2g +1j g k ( ) 3/2 me τ 2π h 2 e (I +1 + ɛ +1j ɛ k )/τ. Ths form of the Saha equaton connects the concentraton of ons n varous energy levels to the electron concentraton and the temperature. Note that we managed to get rd of the nternal partton functons. Of course now we have a relaton connectng concentratons of states of a gven energy level rather than concentratons of ons of a gven onzaton. We can apply the above expresson to hydrogen (agan!). There are only two onzaton states. We let = 0 and k = 0 so n k s the concentraton of hydrogen atoms n the ground state (whch has multplcty g 00 = 4 and energy ɛ 00 = 0. The onzed state s just a proton whch has a multplcty of 2 and no excted states. So [p + ][e ( ) 3/2 ] me τ = n 00 2π h 2 e I/τ whch s essentally the same equaton we had before except that now t ncludes only hydrogen atoms n the ground state and t s exact. Phase Transtons Phase transtons occur throughout physcs. We are all famlar wth meltng ce and bolng water. But other knds of phase transtons occur as well. Some solds when heated through certan temperatures change ther crystal structure. For example sulfur can exst n monoclnc or rhombc forms. When ron s cooled below the Cure pont t spontaneously magnetzes. The Cure pont of ron s T c = 1043 K. A typcal chunk of ron has no net magnetzaton because t magnetzes n small domans wth the drecton of the magnetc feld orented at random. The magnetzaton even n the small domans dsappears above the Cure temperature. The transton between the normal and superflud states of 4 He s a phase transton as are the transtons between normal and superconductng states n superconductors. You ve probably heard about the symmetry breakng phase transtons that mght have occurred n the very early unverse as the unverse cooled from ts extremely hot ntal state. Such transtons broke the symmetry of the fundamental forces causng there to be dfferent couplngs for the strong weak electromagnetc and gravtatonal force. The latent heat released n such a transton mght have drven the unverse nto a state of very rapd expanson (nflaton).
6 Physcs Nov The spontaneous magnetzaton of ron as t s cooled below the Cure temperature s an example of a symmetry breakng transton. Above the Cure pont the atomc magnets (spns) are orented at random (by thermal fluctuatons). So any drecton s the same as any other drecton and there s rotatonal symmetry. Below the Cure pont (and wthn a sngle doman) all the atomc magnets are lned up so a sngle drecton s pcked out and the rotatonal symmetry s broken. Ths s not an exhaustve lst of phase transtons! Even so we wll not have tme to dscuss all these knds of phase transtons. We wll start wth somethng smple lke the lqud to gas transton. Phase Dagrams Suppose we do some very smple experments. We place pure water nsde a contaner whch keeps the amount of water constant and doesn t allow any other knds of molecules to enter. The contaner s n contact wth adjustable temperature and pressure reservors. We dal n a temperature and a pressure wat for equlbrum to be establshed and then see what we have. For most pressures and temperatures we wll fnd that the water s all sold (ce) all lqud or all vapor (steam). For some temperatures and pressures we wll fnd mxtures of sold and vapor or sold and lqud or lqud and vapor. The fgure shows a schematc plot of a phase dagram for water. I ddn t put any numbers on the axes whch s why t s schematc. (Also there are several knds of ce whch we re gnorng!) K&K gve a dagram but t doesn t have any resoluton at the trple pont. Note that the frst fgure (whch we ll talk about some more n a mnute) s somethng lke a map: t says here we have vapor there we have sold etc. The second fgure s a schematc of a pv dagram showng an sotherm. For an deal gas we would have a hyperbola. For the sotherm as shown we have pure lqud on the branch to the left of pont a pure vapor to the rght of pont b and along the segment from a to b we have a mxture of lqud and vapor. If we move along ths sotherm from left to rght we are essentally movng down a vertcal lne n the pτ dagram. To the left of pont a we are movng to lower pressures wth lqud water. from a to b we are stuck at the lne n the pτ dagram that dvdes the lqud from the vapor regon and to the rght of b we are movng down n the vapor regon. So the
7 Physcs Nov entre transton from all lqud to all vapor whch s a to b n the pv dagram happens n a sngle pont n the pτ dagram. At ths pont the water has a fxed temperature and pressure and what adjusts to match the volume s the relatve amounts of lqud and vapor. Now at each locaton n the pτ dagram we fx the temperature and pressure and let the system come to equlbrum. The equlbrum condton s that the Gbbs free energy s mnmzed. Ignorng for the moment the fact that the water can be a sold the Gbbs free energy s G(pτN l N v ) = N l µ l (pτ) + N v µ v (pτ) where the subscrpts l and v refer to the lqud and vapor and we ve made use of the fact that for a sngle component substance the chemcal potental can be wrtten as a functon of p and τ only. There are several ways we mght mnmze G. Frst of all f µ l (pτ) < µ v (pτ) then we mnmze G by settng N l = N and N v = 0 where N s the total number of water molecules. In other words the system s entrely lqud. If µ v (pτ) < µ l (pτ) we mnmze the free energy by makng the system entrely vapor. Fnally f µ l (pτ) = µ v (pτ) we can t change the free energy by changng the amount of vapor and lqud so we can have a mxture wth the exact amounts of lqud and vapor determned by other constrants (such as the volume to be occuped). So what we ve just shown s that where lqud and vapor coexst n equlbrum we must have µ l (pτ) = µ v (pτ) whch s exactly the same condton we would have come up wth had we consdered the reacton H 2 O lqud H 2 O vapor. Ths s a relaton between p and τ and t descrbes a curve on the pτ dagram. It s called the vapor pressure curve. by Wth smlar arguments we deduce that sold and vapor coexst along the curve defned µ s (pτ) = µ v (pτ) whch s called the sublmaton curve and sold and lqud coexst along the curve µ s (pτ) = µ l (pτ) whch s the meltng curve. If we have all three chemcal potentals equal smultaneously µ s (pτ) = µ l (pτ) = µ v (pτ)
8 Physcs Nov we have two condtons on p and τ and ths defnes a pont. Ths unque (for each substance) pont where sold lqud and vapor all coexst s called the trple pont. For water T t = K p t = 4.58 mm Hg. Actually ths s now used to defne the Kelvn scale. If a substance has more than three phases t can have more than one trple pont. For example the two crystallne phases of sulfur gve t four phases and t has three trple ponts. The vapor pressure curve eventually ends at a pont called the crtcal pont. At ths pont one can t tell the dfference between the lqud phase and the vapor phase. We ll say more about ths later but for now consder that as you go up n temperature you get suffcently volent motons that bndng to neghborng molecules (a lqud) becomes a neglgble contrbuton to the energy. As one goes up n temperature the heat of vaporzaton decreases. At the crtcal pont t s zero. The crtcal pont for water occurs at T c = K p c = atm. Another way to thnk of the phase dagram and the coexstence curves s to magne a 3D plot. Pressure and temperature are measured n a horzontal plane whle µ(pτ) s plotted as heght above the plane. Ths defnes a surface. In fact we have several surfaces one for µ s µ l and µ v. We take the overall surface to be the lowest of all the surfaces remember we re tryng to mnmze G. Where µ v s the lowest we have pure vapor etc. Where two surfaces ntersect we have a coexstence curve. Of course the phase dagram corresponds to equlbrum. It s possble to have lqud n the vapor regon (superheated) or sold regon (supercooled) etc. but these stuatons are unstable and the system wll try to get to equlbrum. Whether ths happens rapdly or slowly depends on the detals of the partcular stuaton.
Introduction to Vapor/Liquid Equilibrium, part 2. Raoult s Law:
 CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
CE304, Sprng 2004 Lecture 4 Introducton to Vapor/Lqud Equlbrum, part 2 Raoult s Law: The smplest model that allows us do VLE calculatons s obtaned when we assume that the vapor phase s an deal gas, and
( ) 1/ 2. ( P SO2 )( P O2 ) 1/ 2.
 Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
Chemstry 360 Dr. Jean M. Standard Problem Set 9 Solutons. The followng chemcal reacton converts sulfur doxde to sulfur troxde. SO ( g) + O ( g) SO 3 ( l). (a.) Wrte the expresson for K eq for ths reacton.
Thermodynamics General
 Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
Thermodynamcs General Lecture 1 Lecture 1 s devoted to establshng buldng blocks for dscussng thermodynamcs. In addton, the equaton of state wll be establshed. I. Buldng blocks for thermodynamcs A. Dmensons,
G4023 Mid-Term Exam #1 Solutions
 Exam1Solutons.nb 1 G03 Md-Term Exam #1 Solutons 1-Oct-0, 1:10 p.m to :5 p.m n 1 Pupn Ths exam s open-book, open-notes. You may also use prnt-outs of the homework solutons and a calculator. 1 (30 ponts,
Exam1Solutons.nb 1 G03 Md-Term Exam #1 Solutons 1-Oct-0, 1:10 p.m to :5 p.m n 1 Pupn Ths exam s open-book, open-notes. You may also use prnt-outs of the homework solutons and a calculator. 1 (30 ponts,
Temperature. Chapter Heat Engine
 Chapter 3 Temperature In prevous chapters of these notes we ntroduced the Prncple of Maxmum ntropy as a technque for estmatng probablty dstrbutons consstent wth constrants. In Chapter 9 we dscussed the
Chapter 3 Temperature In prevous chapters of these notes we ntroduced the Prncple of Maxmum ntropy as a technque for estmatng probablty dstrbutons consstent wth constrants. In Chapter 9 we dscussed the
University of Washington Department of Chemistry Chemistry 452/456 Summer Quarter 2014
 Lecture 12 7/25/14 ERD: 7.1-7.5 Devoe: 8.1.1-8.1.2, 8.2.1-8.2.3, 8.4.1-8.4.3 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 2014 A. Free Energy and Changes n Composton: The
Lecture 12 7/25/14 ERD: 7.1-7.5 Devoe: 8.1.1-8.1.2, 8.2.1-8.2.3, 8.4.1-8.4.3 Unversty o Washngton Department o Chemstry Chemstry 452/456 Summer Quarter 2014 A. Free Energy and Changes n Composton: The
Open Systems: Chemical Potential and Partial Molar Quantities Chemical Potential
 Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
Open Systems: Chemcal Potental and Partal Molar Quanttes Chemcal Potental For closed systems, we have derved the followng relatonshps: du = TdS pdv dh = TdS + Vdp da = SdT pdv dg = VdP SdT For open systems,
NAME and Section No. it is found that 0.6 mol of O
 NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
NAME and Secton No. Chemstry 391 Fall 7 Exam III KEY 1. (3 Ponts) ***Do 5 out of 6***(If 6 are done only the frst 5 wll be graded)*** a). In the reacton 3O O3 t s found that.6 mol of O are consumed. Fnd
Robert Eisberg Second edition CH 09 Multielectron atoms ground states and x-ray excitations
 Quantum Physcs 量 理 Robert Esberg Second edton CH 09 Multelectron atoms ground states and x-ray exctatons 9-01 By gong through the procedure ndcated n the text, develop the tme-ndependent Schroednger equaton
Quantum Physcs 量 理 Robert Esberg Second edton CH 09 Multelectron atoms ground states and x-ray exctatons 9-01 By gong through the procedure ndcated n the text, develop the tme-ndependent Schroednger equaton
Why? Chemistry Crunch #4.1 : Name: KEY Phase Changes. Success Criteria: Prerequisites: Vocabulary:
 Chemstry Crunch #4.1 : Name: KEY Phase Changes Why? Most substances wll eventually go through a phase change when heated or cooled (sometmes they chemcally react nstead). Molecules of a substance are held
Chemstry Crunch #4.1 : Name: KEY Phase Changes Why? Most substances wll eventually go through a phase change when heated or cooled (sometmes they chemcally react nstead). Molecules of a substance are held
CHAPTER 14 GENERAL PERTURBATION THEORY
 CHAPTER 4 GENERAL PERTURBATION THEORY 4 Introducton A partcle n orbt around a pont mass or a sphercally symmetrc mass dstrbuton s movng n a gravtatonal potental of the form GM / r In ths potental t moves
CHAPTER 4 GENERAL PERTURBATION THEORY 4 Introducton A partcle n orbt around a pont mass or a sphercally symmetrc mass dstrbuton s movng n a gravtatonal potental of the form GM / r In ths potental t moves
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM
 ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
ELASTIC WAVE PROPAGATION IN A CONTINUOUS MEDIUM An elastc wave s a deformaton of the body that travels throughout the body n all drectons. We can examne the deformaton over a perod of tme by fxng our look
Physics 207: Lecture 20. Today s Agenda Homework for Monday
 Physcs 207: Lecture 20 Today s Agenda Homework for Monday Recap: Systems of Partcles Center of mass Velocty and acceleraton of the center of mass Dynamcs of the center of mass Lnear Momentum Example problems
Physcs 207: Lecture 20 Today s Agenda Homework for Monday Recap: Systems of Partcles Center of mass Velocty and acceleraton of the center of mass Dynamcs of the center of mass Lnear Momentum Example problems
8.1 Arc Length. What is the length of a curve? How can we approximate it? We could do it following the pattern we ve used before
 .1 Arc Length hat s the length of a curve? How can we approxmate t? e could do t followng the pattern we ve used before Use a sequence of ncreasngly short segments to approxmate the curve: As the segments
.1 Arc Length hat s the length of a curve? How can we approxmate t? e could do t followng the pattern we ve used before Use a sequence of ncreasngly short segments to approxmate the curve: As the segments
Thermodynamics Second Law Entropy
 Thermodynamcs Second Law Entropy Lana Sherdan De Anza College May 8, 2018 Last tme the Boltzmann dstrbuton (dstrbuton of energes) the Maxwell-Boltzmann dstrbuton (dstrbuton of speeds) the Second Law of
Thermodynamcs Second Law Entropy Lana Sherdan De Anza College May 8, 2018 Last tme the Boltzmann dstrbuton (dstrbuton of energes) the Maxwell-Boltzmann dstrbuton (dstrbuton of speeds) the Second Law of
Week3, Chapter 4. Position and Displacement. Motion in Two Dimensions. Instantaneous Velocity. Average Velocity
 Week3, Chapter 4 Moton n Two Dmensons Lecture Quz A partcle confned to moton along the x axs moves wth constant acceleraton from x =.0 m to x = 8.0 m durng a 1-s tme nterval. The velocty of the partcle
Week3, Chapter 4 Moton n Two Dmensons Lecture Quz A partcle confned to moton along the x axs moves wth constant acceleraton from x =.0 m to x = 8.0 m durng a 1-s tme nterval. The velocty of the partcle
Physics 181. Particle Systems
 Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
Physcs 181 Partcle Systems Overvew In these notes we dscuss the varables approprate to the descrpton of systems of partcles, ther defntons, ther relatons, and ther conservatons laws. We consder a system
A particle in a state of uniform motion remain in that state of motion unless acted upon by external force.
 The fundamental prncples of classcal mechancs were lad down by Galleo and Newton n the 16th and 17th centures. In 1686, Newton wrote the Prncpa where he gave us three laws of moton, one law of gravty,
The fundamental prncples of classcal mechancs were lad down by Galleo and Newton n the 16th and 17th centures. In 1686, Newton wrote the Prncpa where he gave us three laws of moton, one law of gravty,
Physics 607 Exam 1. ( ) = 1, Γ( z +1) = zγ( z) x n e x2 dx = 1. e x2
 Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
Physcs 607 Exam 1 Please be well-organzed, and show all sgnfcant steps clearly n all problems. You are graded on your wor, so please do not just wrte down answers wth no explanaton! Do all your wor on
Conservation of Angular Momentum = "Spin"
 Page 1 of 6 Conservaton of Angular Momentum = "Spn" We can assgn a drecton to the angular velocty: drecton of = drecton of axs + rght hand rule (wth rght hand, curl fngers n drecton of rotaton, thumb ponts
Page 1 of 6 Conservaton of Angular Momentum = "Spn" We can assgn a drecton to the angular velocty: drecton of = drecton of axs + rght hand rule (wth rght hand, curl fngers n drecton of rotaton, thumb ponts
Frequency dependence of the permittivity
 Frequency dependence of the permttvty February 7, 016 In materals, the delectrc constant and permeablty are actually frequency dependent. Ths does not affect our results for sngle frequency modes, but
Frequency dependence of the permttvty February 7, 016 In materals, the delectrc constant and permeablty are actually frequency dependent. Ths does not affect our results for sngle frequency modes, but
PHYS 705: Classical Mechanics. Newtonian Mechanics
 1 PHYS 705: Classcal Mechancs Newtonan Mechancs Quck Revew of Newtonan Mechancs Basc Descrpton: -An dealzed pont partcle or a system of pont partcles n an nertal reference frame [Rgd bodes (ch. 5 later)]
1 PHYS 705: Classcal Mechancs Newtonan Mechancs Quck Revew of Newtonan Mechancs Basc Descrpton: -An dealzed pont partcle or a system of pont partcles n an nertal reference frame [Rgd bodes (ch. 5 later)]
Homework Chapter 21 Solutions!!
 Homework Chapter 1 Solutons 1.7 1.13 1.17 1.19 1.6 1.33 1.45 1.51 1.71 page 1 Problem 1.7 A mole sample of oxygen gas s confned to a 5 lter vessel at a pressure of 8 atm. Fnd the average translatonal knetc
Homework Chapter 1 Solutons 1.7 1.13 1.17 1.19 1.6 1.33 1.45 1.51 1.71 page 1 Problem 1.7 A mole sample of oxygen gas s confned to a 5 lter vessel at a pressure of 8 atm. Fnd the average translatonal knetc
Physics 4B. A positive value is obtained, so the current is counterclockwise around the circuit.
 Physcs 4B Solutons to Chapter 7 HW Chapter 7: Questons:, 8, 0 Problems:,,, 45, 48,,, 7, 9 Queston 7- (a) no (b) yes (c) all te Queston 7-8 0 μc Queston 7-0, c;, a;, d; 4, b Problem 7- (a) Let be the current
Physcs 4B Solutons to Chapter 7 HW Chapter 7: Questons:, 8, 0 Problems:,,, 45, 48,,, 7, 9 Queston 7- (a) no (b) yes (c) all te Queston 7-8 0 μc Queston 7-0, c;, a;, d; 4, b Problem 7- (a) Let be the current
Physics 5153 Classical Mechanics. Principle of Virtual Work-1
 P. Guterrez 1 Introducton Physcs 5153 Classcal Mechancs Prncple of Vrtual Work The frst varatonal prncple we encounter n mechancs s the prncple of vrtual work. It establshes the equlbrum condton of a mechancal
P. Guterrez 1 Introducton Physcs 5153 Classcal Mechancs Prncple of Vrtual Work The frst varatonal prncple we encounter n mechancs s the prncple of vrtual work. It establshes the equlbrum condton of a mechancal
Ionization fronts in HII regions
 Ionzaton fronts n HII regons Intal expanson of HII onzaton front s supersonc, creatng a shock front. Statonary frame: front advances nto neutral materal In frame where shock front s statonary, neutral
Ionzaton fronts n HII regons Intal expanson of HII onzaton front s supersonc, creatng a shock front. Statonary frame: front advances nto neutral materal In frame where shock front s statonary, neutral
THERMAL DISTRIBUTION IN THE HCL SPECTRUM OBJECTIVE
 ame: THERMAL DISTRIBUTIO I THE HCL SPECTRUM OBJECTIVE To nvestgate a system s thermal dstrbuton n dscrete states; specfcally, determne HCl gas temperature from the relatve occupatons of ts rotatonal states.
ame: THERMAL DISTRIBUTIO I THE HCL SPECTRUM OBJECTIVE To nvestgate a system s thermal dstrbuton n dscrete states; specfcally, determne HCl gas temperature from the relatve occupatons of ts rotatonal states.
PHY688, Statistical Mechanics
 Department of Physcs & Astronomy 449 ESS Bldg. Stony Brook Unversty January 31, 2017 Nuclear Astrophyscs James.Lattmer@Stonybrook.edu Thermodynamcs Internal Energy Densty and Frst Law: ε = E V = Ts P +
Department of Physcs & Astronomy 449 ESS Bldg. Stony Brook Unversty January 31, 2017 Nuclear Astrophyscs James.Lattmer@Stonybrook.edu Thermodynamcs Internal Energy Densty and Frst Law: ε = E V = Ts P +
3. Be able to derive the chemical equilibrium constants from statistical mechanics.
 Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
Lecture #17 1 Lecture 17 Objectves: 1. Notaton of chemcal reactons 2. General equlbrum 3. Be able to derve the chemcal equlbrum constants from statstcal mechancs. 4. Identfy how nondeal behavor can be
Be true to your work, your word, and your friend.
 Chemstry 13 NT Be true to your work, your word, and your frend. Henry Davd Thoreau 1 Chem 13 NT Chemcal Equlbrum Module Usng the Equlbrum Constant Interpretng the Equlbrum Constant Predctng the Drecton
Chemstry 13 NT Be true to your work, your word, and your frend. Henry Davd Thoreau 1 Chem 13 NT Chemcal Equlbrum Module Usng the Equlbrum Constant Interpretng the Equlbrum Constant Predctng the Drecton
Electrical double layer: revisit based on boundary conditions
 Electrcal double layer: revst based on boundary condtons Jong U. Km Department of Electrcal and Computer Engneerng, Texas A&M Unversty College Staton, TX 77843-318, USA Abstract The electrcal double layer
Electrcal double layer: revst based on boundary condtons Jong U. Km Department of Electrcal and Computer Engneerng, Texas A&M Unversty College Staton, TX 77843-318, USA Abstract The electrcal double layer
Structure and Drive Paul A. Jensen Copyright July 20, 2003
 Structure and Drve Paul A. Jensen Copyrght July 20, 2003 A system s made up of several operatons wth flow passng between them. The structure of the system descrbes the flow paths from nputs to outputs.
Structure and Drve Paul A. Jensen Copyrght July 20, 2003 A system s made up of several operatons wth flow passng between them. The structure of the system descrbes the flow paths from nputs to outputs.
Math1110 (Spring 2009) Prelim 3 - Solutions
 Math 1110 (Sprng 2009) Solutons to Prelm 3 (04/21/2009) 1 Queston 1. (16 ponts) Short answer. Math1110 (Sprng 2009) Prelm 3 - Solutons x a 1 (a) (4 ponts) Please evaluate lm, where a and b are postve numbers.
Math 1110 (Sprng 2009) Solutons to Prelm 3 (04/21/2009) 1 Queston 1. (16 ponts) Short answer. Math1110 (Sprng 2009) Prelm 3 - Solutons x a 1 (a) (4 ponts) Please evaluate lm, where a and b are postve numbers.
...Thermodynamics. If Clausius Clapeyron fails. l T (v 2 v 1 ) = 0/0 Second order phase transition ( S, v = 0)
 If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
If Clausus Clapeyron fals ( ) dp dt pb =...Thermodynamcs l T (v 2 v 1 ) = 0/0 Second order phase transton ( S, v = 0) ( ) dp = c P,1 c P,2 dt Tv(β 1 β 2 ) Two phases ntermngled Ferromagnet (Excess spn-up
The equation of motion of a dynamical system is given by a set of differential equations. That is (1)
 Dynamcal Systems Many engneerng and natural systems are dynamcal systems. For example a pendulum s a dynamcal system. State l The state of the dynamcal system specfes t condtons. For a pendulum n the absence
Dynamcal Systems Many engneerng and natural systems are dynamcal systems. For example a pendulum s a dynamcal system. State l The state of the dynamcal system specfes t condtons. For a pendulum n the absence
Moments of Inertia. and reminds us of the analogous equation for linear momentum p= mv, which is of the form. The kinetic energy of the body is.
 Moments of Inerta Suppose a body s movng on a crcular path wth constant speed Let s consder two quanttes: the body s angular momentum L about the center of the crcle, and ts knetc energy T How are these
Moments of Inerta Suppose a body s movng on a crcular path wth constant speed Let s consder two quanttes: the body s angular momentum L about the center of the crcle, and ts knetc energy T How are these
Open string operator quantization
 Open strng operator quantzaton Requred readng: Zwebach -4 Suggested readng: Polchnsk 3 Green, Schwarz, & Wtten 3 upto eq 33 The lght-cone strng as a feld theory: Today we wll dscuss the quantzaton of an
Open strng operator quantzaton Requred readng: Zwebach -4 Suggested readng: Polchnsk 3 Green, Schwarz, & Wtten 3 upto eq 33 The lght-cone strng as a feld theory: Today we wll dscuss the quantzaton of an
NUMERICAL DIFFERENTIATION
 NUMERICAL DIFFERENTIATION 1 Introducton Dfferentaton s a method to compute the rate at whch a dependent output y changes wth respect to the change n the ndependent nput x. Ths rate of change s called the
NUMERICAL DIFFERENTIATION 1 Introducton Dfferentaton s a method to compute the rate at whch a dependent output y changes wth respect to the change n the ndependent nput x. Ths rate of change s called the
Chapter 8. Potential Energy and Conservation of Energy
 Chapter 8 Potental Energy and Conservaton of Energy In ths chapter we wll ntroduce the followng concepts: Potental Energy Conservatve and non-conservatve forces Mechancal Energy Conservaton of Mechancal
Chapter 8 Potental Energy and Conservaton of Energy In ths chapter we wll ntroduce the followng concepts: Potental Energy Conservatve and non-conservatve forces Mechancal Energy Conservaton of Mechancal
Section 8.3 Polar Form of Complex Numbers
 80 Chapter 8 Secton 8 Polar Form of Complex Numbers From prevous classes, you may have encountered magnary numbers the square roots of negatve numbers and, more generally, complex numbers whch are the
80 Chapter 8 Secton 8 Polar Form of Complex Numbers From prevous classes, you may have encountered magnary numbers the square roots of negatve numbers and, more generally, complex numbers whch are the
A quote of the week (or camel of the week): There is no expedience to which a man will not go to avoid the labor of thinking. Thomas A.
 A quote of the week (or camel of the week): here s no expedence to whch a man wll not go to avod the labor of thnkng. homas A. Edson Hess law. Algorthm S Select a reacton, possbly contanng specfc compounds
A quote of the week (or camel of the week): here s no expedence to whch a man wll not go to avod the labor of thnkng. homas A. Edson Hess law. Algorthm S Select a reacton, possbly contanng specfc compounds
A how to guide to second quantization method.
 Phys. 67 (Graduate Quantum Mechancs Sprng 2009 Prof. Pu K. Lam. Verson 3 (4/3/2009 A how to gude to second quantzaton method. -> Second quantzaton s a mathematcal notaton desgned to handle dentcal partcle
Phys. 67 (Graduate Quantum Mechancs Sprng 2009 Prof. Pu K. Lam. Verson 3 (4/3/2009 A how to gude to second quantzaton method. -> Second quantzaton s a mathematcal notaton desgned to handle dentcal partcle
Non-Ideality Through Fugacity and Activity
 Non-Idealty Through Fugacty and Actvty S. Patel Deartment of Chemstry and Bochemstry, Unversty of Delaware, Newark, Delaware 19716, USA Corresondng author. E-mal: saatel@udel.edu 1 I. FUGACITY In ths dscusson,
Non-Idealty Through Fugacty and Actvty S. Patel Deartment of Chemstry and Bochemstry, Unversty of Delaware, Newark, Delaware 19716, USA Corresondng author. E-mal: saatel@udel.edu 1 I. FUGACITY In ths dscusson,
Rate of Absorption and Stimulated Emission
 MIT Department of Chemstry 5.74, Sprng 005: Introductory Quantum Mechancs II Instructor: Professor Andre Tokmakoff p. 81 Rate of Absorpton and Stmulated Emsson The rate of absorpton nduced by the feld
MIT Department of Chemstry 5.74, Sprng 005: Introductory Quantum Mechancs II Instructor: Professor Andre Tokmakoff p. 81 Rate of Absorpton and Stmulated Emsson The rate of absorpton nduced by the feld
Spring 2002 Lecture #13
 44-50 Sprng 00 ecture # Dr. Jaehoon Yu. Rotatonal Energy. Computaton of oments of nerta. Parallel-as Theorem 4. Torque & Angular Acceleraton 5. Work, Power, & Energy of Rotatonal otons Remember the md-term
44-50 Sprng 00 ecture # Dr. Jaehoon Yu. Rotatonal Energy. Computaton of oments of nerta. Parallel-as Theorem 4. Torque & Angular Acceleraton 5. Work, Power, & Energy of Rotatonal otons Remember the md-term
Comparative Studies of Law of Conservation of Energy. and Law Clusters of Conservation of Generalized Energy
 Comparatve Studes of Law of Conservaton of Energy and Law Clusters of Conservaton of Generalzed Energy No.3 of Comparatve Physcs Seres Papers Fu Yuhua (CNOOC Research Insttute, E-mal:fuyh1945@sna.com)
Comparatve Studes of Law of Conservaton of Energy and Law Clusters of Conservaton of Generalzed Energy No.3 of Comparatve Physcs Seres Papers Fu Yuhua (CNOOC Research Insttute, E-mal:fuyh1945@sna.com)
Physics 53. Rotational Motion 3. Sir, I have found you an argument, but I am not obliged to find you an understanding.
 Physcs 53 Rotatonal Moton 3 Sr, I have found you an argument, but I am not oblged to fnd you an understandng. Samuel Johnson Angular momentum Wth respect to rotatonal moton of a body, moment of nerta plays
Physcs 53 Rotatonal Moton 3 Sr, I have found you an argument, but I am not oblged to fnd you an understandng. Samuel Johnson Angular momentum Wth respect to rotatonal moton of a body, moment of nerta plays
The Feynman path integral
 The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
The Feynman path ntegral Aprl 3, 205 Hesenberg and Schrödnger pctures The Schrödnger wave functon places the tme dependence of a physcal system n the state, ψ, t, where the state s a vector n Hlbert space
Linear Momentum. Center of Mass.
 Lecture 6 Chapter 9 Physcs I 03.3.04 Lnear omentum. Center of ass. Course webste: http://faculty.uml.edu/ndry_danylov/teachng/physcsi Lecture Capture: http://echo360.uml.edu/danylov03/physcssprng.html
Lecture 6 Chapter 9 Physcs I 03.3.04 Lnear omentum. Center of ass. Course webste: http://faculty.uml.edu/ndry_danylov/teachng/physcsi Lecture Capture: http://echo360.uml.edu/danylov03/physcssprng.html
Chapter 11: Angular Momentum
 Chapter 11: ngular Momentum Statc Equlbrum In Chap. 4 we studed the equlbrum of pontobjects (mass m) wth the applcaton of Newton s aws F 0 F x y, 0 Therefore, no lnear (translatonal) acceleraton, a0 For
Chapter 11: ngular Momentum Statc Equlbrum In Chap. 4 we studed the equlbrum of pontobjects (mass m) wth the applcaton of Newton s aws F 0 F x y, 0 Therefore, no lnear (translatonal) acceleraton, a0 For
Applied Nuclear Physics (Fall 2004) Lecture 23 (12/3/04) Nuclear Reactions: Energetics and Compound Nucleus
 .101 Appled Nuclear Physcs (Fall 004) Lecture 3 (1/3/04) Nuclear Reactons: Energetcs and Compound Nucleus References: W. E. Meyerhof, Elements of Nuclear Physcs (McGraw-Hll, New York, 1967), Chap 5. Among
.101 Appled Nuclear Physcs (Fall 004) Lecture 3 (1/3/04) Nuclear Reactons: Energetcs and Compound Nucleus References: W. E. Meyerhof, Elements of Nuclear Physcs (McGraw-Hll, New York, 1967), Chap 5. Among
Week 9 Chapter 10 Section 1-5
 Week 9 Chapter 10 Secton 1-5 Rotaton Rgd Object A rgd object s one that s nondeformable The relatve locatons of all partcles makng up the object reman constant All real objects are deformable to some extent,
Week 9 Chapter 10 Secton 1-5 Rotaton Rgd Object A rgd object s one that s nondeformable The relatve locatons of all partcles makng up the object reman constant All real objects are deformable to some extent,
between standard Gibbs free energies of formation for products and reactants, ΔG! R = ν i ΔG f,i, we
 hermodynamcs, Statstcal hermodynamcs, and Knetcs 4 th Edton,. Engel & P. ed Ch. 6 Part Answers to Selected Problems Q6.. Q6.4. If ξ =0. mole at equlbrum, the reacton s not ery far along. hus, there would
hermodynamcs, Statstcal hermodynamcs, and Knetcs 4 th Edton,. Engel & P. ed Ch. 6 Part Answers to Selected Problems Q6.. Q6.4. If ξ =0. mole at equlbrum, the reacton s not ery far along. hus, there would
ˆ (0.10 m) E ( N m /C ) 36 ˆj ( j C m)
 7.. = = 3 = 4 = 5. The electrc feld s constant everywhere between the plates. Ths s ndcated by the electrc feld vectors, whch are all the same length and n the same drecton. 7.5. Model: The dstances to
7.. = = 3 = 4 = 5. The electrc feld s constant everywhere between the plates. Ths s ndcated by the electrc feld vectors, whch are all the same length and n the same drecton. 7.5. Model: The dstances to
Review of Classical Thermodynamics
 Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
Revew of Classcal hermodynamcs Physcs 4362, Lecture #1, 2 Syllabus What s hermodynamcs? 1 [A law] s more mpressve the greater the smplcty of ts premses, the more dfferent are the knds of thngs t relates,
Kernel Methods and SVMs Extension
 Kernel Methods and SVMs Extenson The purpose of ths document s to revew materal covered n Machne Learnng 1 Supervsed Learnng regardng support vector machnes (SVMs). Ths document also provdes a general
Kernel Methods and SVMs Extenson The purpose of ths document s to revew materal covered n Machne Learnng 1 Supervsed Learnng regardng support vector machnes (SVMs). Ths document also provdes a general
Physics 111: Mechanics Lecture 11
 Physcs 111: Mechancs Lecture 11 Bn Chen NJIT Physcs Department Textbook Chapter 10: Dynamcs of Rotatonal Moton q 10.1 Torque q 10. Torque and Angular Acceleraton for a Rgd Body q 10.3 Rgd-Body Rotaton
Physcs 111: Mechancs Lecture 11 Bn Chen NJIT Physcs Department Textbook Chapter 10: Dynamcs of Rotatonal Moton q 10.1 Torque q 10. Torque and Angular Acceleraton for a Rgd Body q 10.3 Rgd-Body Rotaton
Physics 3 (PHYF144) Chap 2: Heat and the First Law of Thermodynamics System. Quantity Positive Negative
 Physcs (PHYF hap : Heat and the Frst aw of hermodynamcs -. Work and Heat n hermodynamc Processes A thermodynamc system s a system that may exchange energy wth ts surroundngs by means of heat and work.
Physcs (PHYF hap : Heat and the Frst aw of hermodynamcs -. Work and Heat n hermodynamc Processes A thermodynamc system s a system that may exchange energy wth ts surroundngs by means of heat and work.
STATISTICAL MECHANICS
 STATISTICAL MECHANICS Thermal Energy Recall that KE can always be separated nto 2 terms: KE system = 1 2 M 2 total v CM KE nternal Rgd-body rotaton and elastc / sound waves Use smplfyng assumptons KE of
STATISTICAL MECHANICS Thermal Energy Recall that KE can always be separated nto 2 terms: KE system = 1 2 M 2 total v CM KE nternal Rgd-body rotaton and elastc / sound waves Use smplfyng assumptons KE of
C/CS/Phy191 Problem Set 3 Solutions Out: Oct 1, 2008., where ( 00. ), so the overall state of the system is ) ( ( ( ( 00 ± 11 ), Φ ± = 1
 C/CS/Phy9 Problem Set 3 Solutons Out: Oct, 8 Suppose you have two qubts n some arbtrary entangled state ψ You apply the teleportaton protocol to each of the qubts separately What s the resultng state obtaned
C/CS/Phy9 Problem Set 3 Solutons Out: Oct, 8 Suppose you have two qubts n some arbtrary entangled state ψ You apply the teleportaton protocol to each of the qubts separately What s the resultng state obtaned
Lecture 14: Forces and Stresses
 The Nuts and Bolts of Frst-Prncples Smulaton Lecture 14: Forces and Stresses Durham, 6th-13th December 2001 CASTEP Developers Group wth support from the ESF ψ k Network Overvew of Lecture Why bother? Theoretcal
The Nuts and Bolts of Frst-Prncples Smulaton Lecture 14: Forces and Stresses Durham, 6th-13th December 2001 CASTEP Developers Group wth support from the ESF ψ k Network Overvew of Lecture Why bother? Theoretcal
Module 1 : The equation of continuity. Lecture 1: Equation of Continuity
 1 Module 1 : The equaton of contnuty Lecture 1: Equaton of Contnuty 2 Advanced Heat and Mass Transfer: Modules 1. THE EQUATION OF CONTINUITY : Lectures 1-6 () () () (v) (v) Overall Mass Balance Momentum
1 Module 1 : The equaton of contnuty Lecture 1: Equaton of Contnuty 2 Advanced Heat and Mass Transfer: Modules 1. THE EQUATION OF CONTINUITY : Lectures 1-6 () () () (v) (v) Overall Mass Balance Momentum
First Law: A body at rest remains at rest, a body in motion continues to move at constant velocity, unless acted upon by an external force.
 Secton 1. Dynamcs (Newton s Laws of Moton) Two approaches: 1) Gven all the forces actng on a body, predct the subsequent (changes n) moton. 2) Gven the (changes n) moton of a body, nfer what forces act
Secton 1. Dynamcs (Newton s Laws of Moton) Two approaches: 1) Gven all the forces actng on a body, predct the subsequent (changes n) moton. 2) Gven the (changes n) moton of a body, nfer what forces act
ψ ij has the eigenvalue
 Moller Plesset Perturbaton Theory In Moller-Plesset (MP) perturbaton theory one taes the unperturbed Hamltonan for an atom or molecule as the sum of the one partcle Foc operators H F() where the egenfunctons
Moller Plesset Perturbaton Theory In Moller-Plesset (MP) perturbaton theory one taes the unperturbed Hamltonan for an atom or molecule as the sum of the one partcle Foc operators H F() where the egenfunctons
Physics 5153 Classical Mechanics. D Alembert s Principle and The Lagrangian-1
 P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
P. Guterrez Physcs 5153 Classcal Mechancs D Alembert s Prncple and The Lagrangan 1 Introducton The prncple of vrtual work provdes a method of solvng problems of statc equlbrum wthout havng to consder the
r i r j 3. (2) Gm j m i r i (r i r j ) r i r j 3. (3)
 N-body problem There s a lot of nterestng physcs related to the nteractons of a few bodes, but there are also many systems that can be well-approxmated by a large number of bodes that nteract exclusvely
N-body problem There s a lot of nterestng physcs related to the nteractons of a few bodes, but there are also many systems that can be well-approxmated by a large number of bodes that nteract exclusvely
5.60 Thermodynamics & Kinetics Spring 2008
 MIT OpenCourseWare http://ocw.mt.edu 5.60 Thermodynamcs & Knetcs Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.60 Sprng 2008 Lecture #29 page 1
MIT OpenCourseWare http://ocw.mt.edu 5.60 Thermodynamcs & Knetcs Sprng 2008 For nformaton about ctng these materals or our Terms of Use, vst: http://ocw.mt.edu/terms. 5.60 Sprng 2008 Lecture #29 page 1
π e ax2 dx = x 2 e ax2 dx or x 3 e ax2 dx = 1 x 4 e ax2 dx = 3 π 8a 5/2 (a) We are considering the Maxwell velocity distribution function: 2πτ/m
 Homework Solutons Problem In solvng ths problem, we wll need to calculate some moments of the Gaussan dstrbuton. The brute-force method s to ntegrate by parts but there s a nce trck. The followng ntegrals
Homework Solutons Problem In solvng ths problem, we wll need to calculate some moments of the Gaussan dstrbuton. The brute-force method s to ntegrate by parts but there s a nce trck. The followng ntegrals
Workshop: Approximating energies and wave functions Quantum aspects of physical chemistry
 Workshop: Approxmatng energes and wave functons Quantum aspects of physcal chemstry http://quantum.bu.edu/pltl/6/6.pdf Last updated Thursday, November 7, 25 7:9:5-5: Copyrght 25 Dan Dll (dan@bu.edu) Department
Workshop: Approxmatng energes and wave functons Quantum aspects of physcal chemstry http://quantum.bu.edu/pltl/6/6.pdf Last updated Thursday, November 7, 25 7:9:5-5: Copyrght 25 Dan Dll (dan@bu.edu) Department
Prof. Dr. I. Nasser Phys 630, T Aug-15 One_dimensional_Ising_Model
 EXACT OE-DIMESIOAL ISIG MODEL The one-dmensonal Isng model conssts of a chan of spns, each spn nteractng only wth ts two nearest neghbors. The smple Isng problem n one dmenson can be solved drectly n several
EXACT OE-DIMESIOAL ISIG MODEL The one-dmensonal Isng model conssts of a chan of spns, each spn nteractng only wth ts two nearest neghbors. The smple Isng problem n one dmenson can be solved drectly n several
CHEMICAL REACTIONS AND DIFFUSION
 CHEMICAL REACTIONS AND DIFFUSION A.K.A. NETWORK THERMODYNAMICS BACKGROUND Classcal thermodynamcs descrbes equlbrum states. Non-equlbrum thermodynamcs descrbes steady states. Network thermodynamcs descrbes
CHEMICAL REACTIONS AND DIFFUSION A.K.A. NETWORK THERMODYNAMICS BACKGROUND Classcal thermodynamcs descrbes equlbrum states. Non-equlbrum thermodynamcs descrbes steady states. Network thermodynamcs descrbes
ENGN 40 Dynamics and Vibrations Homework # 7 Due: Friday, April 15
 NGN 40 ynamcs and Vbratons Homework # 7 ue: Frday, Aprl 15 1. Consder a concal hostng drum used n the mnng ndustry to host a mass up/down. A cable of dameter d has the mass connected at one end and s wound/unwound
NGN 40 ynamcs and Vbratons Homework # 7 ue: Frday, Aprl 15 1. Consder a concal hostng drum used n the mnng ndustry to host a mass up/down. A cable of dameter d has the mass connected at one end and s wound/unwound
PART I: MULTIPLE CHOICE (32 questions, each multiple choice question has a 2-point value, 64 points total).
 CHEMISTRY 123-07 Mdterm #2 answer key November 04, 2010 Statstcs: Average: 68 p (68%); Hghest: 91 p (91%); Lowest: 37 p (37%) Number of students performng at or above average: 58 (53%) Number of students
CHEMISTRY 123-07 Mdterm #2 answer key November 04, 2010 Statstcs: Average: 68 p (68%); Hghest: 91 p (91%); Lowest: 37 p (37%) Number of students performng at or above average: 58 (53%) Number of students
Gasometric Determination of NaHCO 3 in a Mixture
 60 50 40 0 0 5 15 25 35 40 Temperature ( o C) 9/28/16 Gasometrc Determnaton of NaHCO 3 n a Mxture apor Pressure (mm Hg) apor Pressure of Water 1 NaHCO 3 (s) + H + (aq) Na + (aq) + H 2 O (l) + CO 2 (g)
60 50 40 0 0 5 15 25 35 40 Temperature ( o C) 9/28/16 Gasometrc Determnaton of NaHCO 3 n a Mxture apor Pressure (mm Hg) apor Pressure of Water 1 NaHCO 3 (s) + H + (aq) Na + (aq) + H 2 O (l) + CO 2 (g)
Problem Set #6 solution, Chem 340, Fall 2013 Due Friday, Oct 11, 2013 Please show all work for credit
 Problem Set #6 soluton, Chem 340, Fall 2013 Due Frday, Oct 11, 2013 Please show all work for credt To hand n: Atkns Chap 3 Exercses: 3.3(b), 3.8(b), 3.13(b), 3.15(b) Problems: 3.1, 3.12, 3.36, 3.43 Engel
Problem Set #6 soluton, Chem 340, Fall 2013 Due Frday, Oct 11, 2013 Please show all work for credt To hand n: Atkns Chap 3 Exercses: 3.3(b), 3.8(b), 3.13(b), 3.15(b) Problems: 3.1, 3.12, 3.36, 3.43 Engel
Army Ants Tunneling for Classical Simulations
 Electronc Supplementary Materal (ESI) for Chemcal Scence. Ths journal s The Royal Socety of Chemstry 2014 electronc supplementary nformaton (ESI) for Chemcal Scence Army Ants Tunnelng for Classcal Smulatons
Electronc Supplementary Materal (ESI) for Chemcal Scence. Ths journal s The Royal Socety of Chemstry 2014 electronc supplementary nformaton (ESI) for Chemcal Scence Army Ants Tunnelng for Classcal Smulatons
Lectures - Week 4 Matrix norms, Conditioning, Vector Spaces, Linear Independence, Spanning sets and Basis, Null space and Range of a Matrix
 Lectures - Week 4 Matrx norms, Condtonng, Vector Spaces, Lnear Independence, Spannng sets and Bass, Null space and Range of a Matrx Matrx Norms Now we turn to assocatng a number to each matrx. We could
Lectures - Week 4 Matrx norms, Condtonng, Vector Spaces, Lnear Independence, Spannng sets and Bass, Null space and Range of a Matrx Matrx Norms Now we turn to assocatng a number to each matrx. We could
Chemical Equilibrium. Chapter 6 Spontaneity of Reactive Mixtures (gases) Taking into account there are many types of work that a sysem can perform
 Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
Ths chapter deals wth chemcal reactons (system) wth lttle or no consderaton on the surroundngs. Chemcal Equlbrum Chapter 6 Spontanety of eactve Mxtures (gases) eactants generatng products would proceed
University of Washington Department of Chemistry Chemistry 453 Winter Quarter 2015
 Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
Lecture 2. 1/07/15-1/09/15 Unversty of Washngton Department of Chemstry Chemstry 453 Wnter Quarter 2015 We are not talkng about truth. We are talkng about somethng that seems lke truth. The truth we want
PHYSICS - CLUTCH CH 28: INDUCTION AND INDUCTANCE.
 !! www.clutchprep.com CONCEPT: ELECTROMAGNETIC INDUCTION A col of wre wth a VOLTAGE across each end wll have a current n t - Wre doesn t HAVE to have voltage source, voltage can be INDUCED V Common ways
!! www.clutchprep.com CONCEPT: ELECTROMAGNETIC INDUCTION A col of wre wth a VOLTAGE across each end wll have a current n t - Wre doesn t HAVE to have voltage source, voltage can be INDUCED V Common ways
CinChE Problem-Solving Strategy Chapter 4 Development of a Mathematical Model. formulation. procedure
 nhe roblem-solvng Strategy hapter 4 Transformaton rocess onceptual Model formulaton procedure Mathematcal Model The mathematcal model s an abstracton that represents the engneerng phenomena occurrng n
nhe roblem-solvng Strategy hapter 4 Transformaton rocess onceptual Model formulaton procedure Mathematcal Model The mathematcal model s an abstracton that represents the engneerng phenomena occurrng n
DUE: WEDS FEB 21ST 2018
 HOMEWORK # 1: FINITE DIFFERENCES IN ONE DIMENSION DUE: WEDS FEB 21ST 2018 1. Theory Beam bendng s a classcal engneerng analyss. The tradtonal soluton technque makes smplfyng assumptons such as a constant
HOMEWORK # 1: FINITE DIFFERENCES IN ONE DIMENSION DUE: WEDS FEB 21ST 2018 1. Theory Beam bendng s a classcal engneerng analyss. The tradtonal soluton technque makes smplfyng assumptons such as a constant
Poisson brackets and canonical transformations
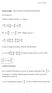 rof O B Wrght Mechancs Notes osson brackets and canoncal transformatons osson Brackets Consder an arbtrary functon f f ( qp t) df f f f q p q p t But q p p where ( qp ) pq q df f f f p q q p t In order
rof O B Wrght Mechancs Notes osson brackets and canoncal transformatons osson Brackets Consder an arbtrary functon f f ( qp t) df f f f q p q p t But q p p where ( qp ) pq q df f f f p q q p t In order
MAGNETISM MAGNETIC DIPOLES
 MAGNETISM We now turn to magnetsm. Ths has actually been used for longer than electrcty. People were usng compasses to sal around the Medterranean Sea several hundred years BC. However t was not understood
MAGNETISM We now turn to magnetsm. Ths has actually been used for longer than electrcty. People were usng compasses to sal around the Medterranean Sea several hundred years BC. However t was not understood
Gravitational Acceleration: A case of constant acceleration (approx. 2 hr.) (6/7/11)
 Gravtatonal Acceleraton: A case of constant acceleraton (approx. hr.) (6/7/11) Introducton The gravtatonal force s one of the fundamental forces of nature. Under the nfluence of ths force all objects havng
Gravtatonal Acceleraton: A case of constant acceleraton (approx. hr.) (6/7/11) Introducton The gravtatonal force s one of the fundamental forces of nature. Under the nfluence of ths force all objects havng
10. Canonical Transformations Michael Fowler
 10. Canoncal Transformatons Mchael Fowler Pont Transformatons It s clear that Lagrange s equatons are correct for any reasonable choce of parameters labelng the system confguraton. Let s call our frst
10. Canoncal Transformatons Mchael Fowler Pont Transformatons It s clear that Lagrange s equatons are correct for any reasonable choce of parameters labelng the system confguraton. Let s call our frst
PHYS 1101 Practice problem set 12, Chapter 32: 21, 22, 24, 57, 61, 83 Chapter 33: 7, 12, 32, 38, 44, 49, 76
 PHYS 1101 Practce problem set 1, Chapter 3: 1,, 4, 57, 61, 83 Chapter 33: 7, 1, 3, 38, 44, 49, 76 3.1. Vsualze: Please reer to Fgure Ex3.1. Solve: Because B s n the same drecton as the ntegraton path s
PHYS 1101 Practce problem set 1, Chapter 3: 1,, 4, 57, 61, 83 Chapter 33: 7, 1, 3, 38, 44, 49, 76 3.1. Vsualze: Please reer to Fgure Ex3.1. Solve: Because B s n the same drecton as the ntegraton path s
Supplementary Notes for Chapter 9 Mixture Thermodynamics
 Supplementary Notes for Chapter 9 Mxture Thermodynamcs Key ponts Nne major topcs of Chapter 9 are revewed below: 1. Notaton and operatonal equatons for mxtures 2. PVTN EOSs for mxtures 3. General effects
Supplementary Notes for Chapter 9 Mxture Thermodynamcs Key ponts Nne major topcs of Chapter 9 are revewed below: 1. Notaton and operatonal equatons for mxtures 2. PVTN EOSs for mxtures 3. General effects
Problem Points Score Total 100
 Physcs 450 Solutons of Sample Exam I Problem Ponts Score 1 8 15 3 17 4 0 5 0 Total 100 All wor must be shown n order to receve full credt. Wor must be legble and comprehensble wth answers clearly ndcated.
Physcs 450 Solutons of Sample Exam I Problem Ponts Score 1 8 15 3 17 4 0 5 0 Total 100 All wor must be shown n order to receve full credt. Wor must be legble and comprehensble wth answers clearly ndcated.
Electrochemical Equilibrium Electromotive Force
 CHM465/865, 24-3, Lecture 5-7, 2 th Sep., 24 lectrochemcal qulbrum lectromotve Force Relaton between chemcal and electrc drvng forces lectrochemcal system at constant T and p: consder Gbbs free energy
CHM465/865, 24-3, Lecture 5-7, 2 th Sep., 24 lectrochemcal qulbrum lectromotve Force Relaton between chemcal and electrc drvng forces lectrochemcal system at constant T and p: consder Gbbs free energy
and Statistical Mechanics Material Properties
 Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Statstcal Mechancs and Materal Propertes By Kuno TAKAHASHI Tokyo Insttute of Technology, Tokyo 15-855, JAPA Phone/Fax +81-3-5734-3915 takahak@de.ttech.ac.jp http://www.de.ttech.ac.jp/~kt-lab/ Only for
Solution Thermodynamics
 Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
Soluton hermodynamcs usng Wagner Notaton by Stanley. Howard Department of aterals and etallurgcal Engneerng South Dakota School of nes and echnology Rapd Cty, SD 57701 January 7, 001 Soluton hermodynamcs
Thermodynamics and statistical mechanics in materials modelling II
 Course MP3 Lecture 8/11/006 (JAE) Course MP3 Lecture 8/11/006 Thermodynamcs and statstcal mechancs n materals modellng II A bref résumé of the physcal concepts used n materals modellng Dr James Ellott.1
Course MP3 Lecture 8/11/006 (JAE) Course MP3 Lecture 8/11/006 Thermodynamcs and statstcal mechancs n materals modellng II A bref résumé of the physcal concepts used n materals modellng Dr James Ellott.1
Week 11: Chapter 11. The Vector Product. The Vector Product Defined. The Vector Product and Torque. More About the Vector Product
 The Vector Product Week 11: Chapter 11 Angular Momentum There are nstances where the product of two vectors s another vector Earler we saw where the product of two vectors was a scalar Ths was called the
The Vector Product Week 11: Chapter 11 Angular Momentum There are nstances where the product of two vectors s another vector Earler we saw where the product of two vectors was a scalar Ths was called the
THE CURRENT BALANCE Physics 258/259
 DSH 1988, 005 THE CURRENT BALANCE Physcs 58/59 The tme average force between two parallel conductors carryng an alternatng current s measured by balancng ths force aganst the gravtatonal force on a set
DSH 1988, 005 THE CURRENT BALANCE Physcs 58/59 The tme average force between two parallel conductors carryng an alternatng current s measured by balancng ths force aganst the gravtatonal force on a set
Adiabatic Sorption of Ammonia-Water System and Depicting in p-t-x Diagram
 Adabatc Sorpton of Ammona-Water System and Depctng n p-t-x Dagram J. POSPISIL, Z. SKALA Faculty of Mechancal Engneerng Brno Unversty of Technology Techncka 2, Brno 61669 CZECH REPUBLIC Abstract: - Absorpton
Adabatc Sorpton of Ammona-Water System and Depctng n p-t-x Dagram J. POSPISIL, Z. SKALA Faculty of Mechancal Engneerng Brno Unversty of Technology Techncka 2, Brno 61669 CZECH REPUBLIC Abstract: - Absorpton
Lecture Note 3. Eshelby s Inclusion II
 ME340B Elastcty of Mcroscopc Structures Stanford Unversty Wnter 004 Lecture Note 3. Eshelby s Incluson II Chrs Wenberger and We Ca c All rghts reserved January 6, 004 Contents 1 Incluson energy n an nfnte
ME340B Elastcty of Mcroscopc Structures Stanford Unversty Wnter 004 Lecture Note 3. Eshelby s Incluson II Chrs Wenberger and We Ca c All rghts reserved January 6, 004 Contents 1 Incluson energy n an nfnte
1 Derivation of Rate Equations from Single-Cell Conductance (Hodgkin-Huxley-like) Equations
 Physcs 171/271 -Davd Klenfeld - Fall 2005 (revsed Wnter 2011) 1 Dervaton of Rate Equatons from Sngle-Cell Conductance (Hodgkn-Huxley-lke) Equatons We consder a network of many neurons, each of whch obeys
Physcs 171/271 -Davd Klenfeld - Fall 2005 (revsed Wnter 2011) 1 Dervaton of Rate Equatons from Sngle-Cell Conductance (Hodgkn-Huxley-lke) Equatons We consder a network of many neurons, each of whch obeys
EPR Paradox and the Physical Meaning of an Experiment in Quantum Mechanics. Vesselin C. Noninski
 EPR Paradox and the Physcal Meanng of an Experment n Quantum Mechancs Vesseln C Nonnsk vesselnnonnsk@verzonnet Abstract It s shown that there s one purely determnstc outcome when measurement s made on
EPR Paradox and the Physcal Meanng of an Experment n Quantum Mechancs Vesseln C Nonnsk vesselnnonnsk@verzonnet Abstract It s shown that there s one purely determnstc outcome when measurement s made on
4.2 Chemical Driving Force
 4.2. CHEMICL DRIVING FORCE 103 4.2 Chemcal Drvng Force second effect of a chemcal concentraton gradent on dffuson s to change the nature of the drvng force. Ths s because dffuson changes the bondng n a
4.2. CHEMICL DRIVING FORCE 103 4.2 Chemcal Drvng Force second effect of a chemcal concentraton gradent on dffuson s to change the nature of the drvng force. Ths s because dffuson changes the bondng n a
