PAPER No. 5:Organic Chemistry-2(Reaction Mechanism-1) MODULE No. 6: Generation, Structure, Stability and Reactivity of Carbocations
|
|
|
- Leonard Thompson
- 5 years ago
- Views:
Transcription
1 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper No. 5: Organic Chemistry-II (Reaction Mechanism-1) Generation, Structure, Stability and Reactivity of Carbocations CHE_P5_M6
2 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 2.1 Classification of carbocations 2.2 Non-classical carbocations 3.Generation of carbocation 3.1 Ionization 3.2 Ionization after an initial reaction 3.3 Attack of π-system on electrophiles 3.4 Generation of non-classical carbocations 4. Structure of carbocation 5. Stability of carbocation 5.1 Inductive effect 5.2 Hyperconjugation 5.3 Resonance Effects 6. Reactivity of carbocation 7. Summary
3 1. Learning Outcomes After studying this module, you shall be able to: Understand what is a carbocation. Learn how a carbocation is generated. Identify the various types of carbocations and learn about their stability. Know about the reactivity of carbocations. 2. Introduction The heterolytic cleavage in an organic molecule where carbon donates the shared pair of electrons to the leaving group results in the development of positive charge on the carbon atom. Such species where covalency of carbon is three are called carbenium ions or carbocations (e.g. CH + 3 ). The carbon atom of carbocation is sp 2 hybridised and it uses the three hybridised orbitals for single bonding to three substituents; the remaining p-orbital is empty. However, the pentacoordinated positively charged species such as CH + 5 is called carbonium ion. This nomenclature was proposed by G. A. Olah. Carbocation are intermediates in several kinds of reactions. Some stable carbocation have been synthesized in solution (free or ion pair) and some as solid salts. 5 bonds to C -rare 3 bonds to C -common The carbocation has a flat structure having allthe three covalent bonds are in plane with a bond angle of 120 o between them. 2.1 Classification of carbocations a) Primary carbocation: In a primary carbocation the carbon bearing the positive chargeis attached to onecarbon of an alkyl or aryl group. b) Secondary carbocation: Here carbon with positive charge is attached to two other carbons. c) Tertiary carbocation: As the name suggest, in this case the positively charged carbon is attached to three carbon atoms. While a carbocation with the carbon bearing a positive charge and attached to only three hydrogen atoms and no carbon is known as methyl carbocation.
4 Figure 1: Types of carbocation 2.2 Non-classical Carbocations A cyclic bridged cation involving three-centre two-electron bonding which three atoms share two electronsis unusual and such species are considered as non-classical carbocations or bridged carbocations. In non-classical carbocation, positive charge is delocalised either by sigma electrons of carbon-carbon or carbon-hydrogen bond or by pi electrons of carbon-carbon double bond which is not in allylic position. The examples are 7-norbornenyl cation, norbornyl cation, phenonium ion and cyclopropylmethyl cation. 3. Generation of Carbocation Figure 2: Non classical carbocation 7- norbornenyl cation A number of methods are available to generate stable or unstablecarbocation these are ionization, ionization after an initial reaction, attack of π-system on electrophiles. 3.1 Ionization During direct ionization a leaving group attached to a carbon atom leaves with its pair of electrons resulting in the generation of a carbocation.
5 For example during solvolysis an alkyl halides ionizes to the intermediate carbocation and the corresponding anion. Though, in the case of simple strong acid solution such as H 2 SO 4 the carbocation so formed is not very stable, but they can be stored in solutions of fluorosulfuric acid (FSO 3 H) and antimony pentafluorideto attain stability. Mixtures, of SbF 5 usually dissolved in SO 2 or SO 2 ClF, arestrongest acidic solutions known as super acids. For example the addition of alkyl fluorides to SbF 5 results into the formation of a stable carbocation. Super acid (FSO 3 H SbF 5 ) reacts with alkanes as well. For example, at 140 C (284 F) it converts methane into the tertiary-butyl carbocation, a reaction that begins with the protonation of methane: CH 4 + H + CH 5 + CH 5 + CH H 2 CH CH 4 (CH 3 ) 3 C + + 3H Ionization after an initial reaction Sometimes the functional groups present in an organic compound first undergo an initial reaction which converts the functional group into a good leaving group, which can then ionize into a carbocation after removal of the leaving group. Thus, stable cations can be generated from alcohols in super acid-so 2 at -60 o C. Example: (a) Protonation of an alcohol gives an oxonium ion which ionizes to a carbocation with loss of water molecule. (b) A primary amine gets converted into a diazonium salt in the presence of nitrous acid which then ionizes to a carbocation with loss of nitrogen. +
6 3.3 Attack of π-system on electrophiles When an electrophile add to one of the atoms of a π-system the adjacent atom acquires a positive charge. In case the positive atom is a carbon a carbocation is formed. Stable cations can be generated from alkenes by the addition ofa proton from super acid or HF- SbF 5 in SO 2 or SO 2 ClF at low temperatures. Example: (a) Attack of π-system such as alkene or alkyne on an electrophile like proton, bromonium ion etc results in development of positive charge on the adjacent carbon and leads to the formation of carbocation intermediate. (b) Addition of proton or other positive species to one atom of a C-X bond (X= O, S,N) develops positive charge on adjacent carbon atom. 3.4 Generation of Non-classical Carbocations During acetolysisof anti-tosylate, the tosyl group is removed with strong anchimeric assistance by the double bond, which results into formation of a non-classical carbocation (bridged ion) i.e. 7- norbornenyl cation as shown below. The configuration is retained in such a case.
7 The phenyl group present adjacent to a leaving group (tosyl group, hydroxyl) participate as a neighboring group during acetolysis and form an intermediate nonclassical carbocation called phenonium ion as shown below. 4. Structure of Carbocation There are six valance electrons in a carbocation. Therefore they have deficiency of electron and they act as Lewis acids. Features of a carbocation: The carbon bearing positive charge of a carbocation is sp 2 hybridized and has vacant unhybridized p orbital.
8 It has planar structure having all the three covalent bonds are in plane with the bond angle of 120 o between them. The positively charged carbon is trivalent The positive carbon has sextet of electron thus it is electron deficient. The order of stability of carbocations is 3 o >2 o >1 o > methyl carbocation. 5. Stability of Carbocation The stability of a carbocation is governed by several factors such as inductive effect, hyperconjugation or resonance effect. There effect is discussed below: 5.1 Inductive effect If the groups attached to positively charged carbon are electron releasing they will decrease the intensity of the positive charge on carbon. The electron releasing groups delocalise the positive charge on carbon thereby decreasing its electron deficiency. The alkyl groups are electron releasing in nature (+I effect). More the number of alkyl groups attached more will be the stability of carbocation. Figure 3: Inductive effect in carbocation
9 Thus the order of stability of alkyl carbocations is 3 o >2 o >1 o > methyl carbocation. Thus, +I group increases stability of carbocation and is directly proportional to the +I power of the group. 5.2 Hyperconjugation Hyperconjugation can explain the stability of the carbocation.in case of carbocations the sigma bond in conjugation with vacant p orbital on carbon carrying positive charge participates in delocalisation, thus spreading the charge all over the alkyl groups.this effect is also known as hyperconjugation or no bond resonance. Figure 4: Hyperconjugative overlap of orbital In tert-butyl cation, (CH 3 ) 3 C + there are nine C-H sigma bonds which participate in delocalisation. In iso-propyl cation, (CH 3 ) 2 CH + six C-H sigma bonds and in ethyl cation, CH 3 CH 2 + three C-H bonds are available. Thus (CH 3 ) 3 C + is more stable than (CH 3 ) 2 CH + which in turn is more stable than CH 3 CH 2 + due to greater number of contributing hyperconjugative structures in former than the latter.
10 Figure 5: Hyperconjugation in carbocation 5.3 Resonance Effects Conjugation with multiple bond or lone pair of electron increases the stability of a carbocation. For example, triphenylmethyl cation is more stable than diphenylmethyl cation which in turn is more stable than phenylmethyl cation. This due to greater delocalization of positive charge in case of triphenylmethyl cation as it has more number of resonating structures. The salt of triphenylmethyl cation with boron fluoride is stable and exists as solid.
11 Which is more stable amongst allyl and benzyl carbocations? Benzyl cation is more stable than allyl cation. In both the cases the vacant p orbital of carbon bearing positive charge is in conjugation with pi bonds and delocalisation of positive charge occurs through p-pi overlap.in benzyl cation, the number of contributing structures are more as compared to allyl cation. Figure 6: Resonance in allyl carbocation Thus the order of stability is as follows: Figure 7: Resonance in benzyl carbocation
12 Which is more stable amongst the two tetrahydropyran carbocations? Or If a heteroatom with an unshared pair of electrons is adjacent to the cationic centre then it stabilises the positive charge. Thus the carbocation with positive centre adjacent to the oxygen atom with lone pair of electron is more stable. The lone pair is involved in resonance decrease the electron deficiency of the positive carbon providing it stability. While in the other structure no such stabilisation is present. Similarly, methoxy methyl cation gains stability due to presence of oxygen atom with lone pair of electron.
13 CH 3 -O-CH 2 -CH 2 + CH 3 -O-CH 2 + CH 3 -O + =CH 2 If the vacant p orbital of the carbocation is part of a conjugated cyclic ring, it provides greater stability to the carbocation. Hence,tropylium cation and cyclopropenylium cation being aromatic in nature are more stable than cyclopentadienyl cation. Figure 8: Order of stability of cyclic conjugated carbocation Cyclopropylmethyl cation is more stable than benzyl cations. The additional cyclopropyl ring further stabilise the carbocation, thus the di- and tri- cyclopropylmethylcations are even more stable. This stability is furnished by the bent orbitals of the cyclopropyl ring, which can overlap effectively with the vacant p orbital of the carbocation, thus reducing its electron deficiency and provides more stability. H H H H H H H Cyclopropyl methyl cation Figure 9: Order of Stability of mono-, di- and tri- cyclopropyl methyl cations
14 6. Carbocations are most often short-lived transient species and react further without being isolated. The fate of carbocation depends on its reactivity. Once a carbocation is formed it may: 1) React with a nucleophile to give S N 1 product. 2) Loose a proton (H + ) to give an E1 product. 3) Rearrange to another carbocation. 4) Undergo addition to unsaturated compounds. 5) Undergo reaction with leaving group and results in the formation of starting reactant. Figure 10: Different fates of a carbocation (1) The carbocation may combine with anegative ion orspecies possessing an electron pair (nucleophiles such as OH -, Cl -, Br -, H 2 O).These reactions are very fast. Example: Addition of HBr to propene according to Markovnikov rule proceeds with formation of a more stable 2 o carbocation which further attacked by a bromide which acts as a nucleophile.
15 (2) The carbocation formed may undergo loss of proton from the β-carbonto give an alkene, an E1product. Example: Formation of 2-methylpropene (3) During rearrangement an alkyl or aryl group or hydrogen migrates towards the positively charged carbon with its electron pair, leaving another positive charged more stable carbocation behind.the rearrangement involving migration of hydrogen with its pair of electron is calledthe hydride shiftand alkyl group migration by bonding is called the alkyl shift. 1,2- shifts are the most common type of rearrangements. The Pinacol-pinacolone rearrangement: This is common rearrangement in which 1,2- dihydroxy compounds rearrange to give carbonyl compounds. For example conversion of 2,3- dimethylbutane-2,3-diol (pinacol) to t-butyl methyl ketone (pinacolone)
16 Tiffeneau-Demjanov reaction: This reaction involves conversion of aminomethyl carbinol to ketones on treatment with nitrous acid. It can also be used for synthesis of ring expanded cyclic ketones. (4) A carbocation may add to a double bond, generating a positive charge at a new position:
17 6. Summary In this module you have learnt that: Carbocations are reactive intermediates. Carbocations are electron deficient species with sextet of electron. Stable carbocations can be generated in super acids. The order of stability of carbocations is 3 o >2 o >1 o >methyl. Factors such as inductive effect (+I), hyperconjugation and resonance which can decrease the positive charge on carbon stabilize a carbocation. Carbocation can undergo various types of reactions such as capturing nucleophile, elimination, rearrangement.
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
Lecture Topics: I. Electrophilic Aromatic Substitution (EAS)
 Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
PAPER No. 5: REACTION MECHANISM MODULE No. 2: Types of Organic Reaction Mechanisms
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper No. 5:Organic Chemistry-II Module No. 2: Overview of different types of Organic Reaction Mechanisms CHE_P5_M2 TABLE OF CONTENTS
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper No. 5:Organic Chemistry-II Module No. 2: Overview of different types of Organic Reaction Mechanisms CHE_P5_M2 TABLE OF CONTENTS
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
PAPER No. 05: TITLE: ORGANIC CHEMISTRY-II MODULE No. 12: TITLE: S N 1 Reactions
 Subject hemistry Paper o and Title Module o and Title Module Tag 05, ORGAI EMISTRY-II 12, S 1 Reactions E_P5_M12 EMISTRY PAPER o. 05: TITLE: ORGAI EMISTRY-II TABLE OF OTETS 1. Learning Outcomes 2. Introduction
Subject hemistry Paper o and Title Module o and Title Module Tag 05, ORGAI EMISTRY-II 12, S 1 Reactions E_P5_M12 EMISTRY PAPER o. 05: TITLE: ORGAI EMISTRY-II TABLE OF OTETS 1. Learning Outcomes 2. Introduction
Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) Can act as weak nucleophiles
 Alkenes - Structure, Stability, Nomenclature Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) unsaturated - contain fewer than maximum H's possible per C Can
Alkenes - Structure, Stability, Nomenclature Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) unsaturated - contain fewer than maximum H's possible per C Can
DAV CENTENARY PUBLIC SCHOOL, PASCHIM ENCLAVE, NEW DELHI - 87
 HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
and Stereochemistry) PAPER 1: ORGANIC CHEMISTRY- I (Nature of Bonding and Stereochemistry) MODULE 4: Applications of Electronic Effects
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 1: ORGANIC - I (Nature of Bonding Module 4: Applications of Electronic Effects CHE_P1_M4 PAPER 1: ORGANIC - I (Nature of Bonding
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 1: ORGANIC - I (Nature of Bonding Module 4: Applications of Electronic Effects CHE_P1_M4 PAPER 1: ORGANIC - I (Nature of Bonding
CHEMISTRY. Module No and Title Module-, Electrophilic Aromatic Substitution: The ortho/para ipso attack, orientation in other ring systems.
 Subject Chemistry Paper No and Title Paper-5, Organic Chemistry-II Module No and Title Module-, Electrophilic Aromatic Substitution: The ortho/para Module Tag CHE_P5_M29 TABLE OF CONTENTS 1. Learning Outcomes
Subject Chemistry Paper No and Title Paper-5, Organic Chemistry-II Module No and Title Module-, Electrophilic Aromatic Substitution: The ortho/para Module Tag CHE_P5_M29 TABLE OF CONTENTS 1. Learning Outcomes
But in organic terms: Oxidation: loss of H 2 ; addition of O or O 2 ; addition of X 2 (halogens).
 Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Allylic and Benzylic Reactivity
 17 17 Allylic and Benzylic Reactivity An allylic group is a group on a carbon adjacent to a double bond. A benzylic group is a group on a carbon adjacent to a benzene ring or substituted benzene ring.
17 17 Allylic and Benzylic Reactivity An allylic group is a group on a carbon adjacent to a double bond. A benzylic group is a group on a carbon adjacent to a benzene ring or substituted benzene ring.
REARRANGEMENTS NOTES Mechanistic Aspects of Rearrangements
 - 1 - REARRANGEMENTS NOTES Mechanistic Aspects of Rearrangements Nature of the Rearrangement It can vary from being truly stepwise to migration occurring in concert with initial ionisation. These two situations
- 1 - REARRANGEMENTS NOTES Mechanistic Aspects of Rearrangements Nature of the Rearrangement It can vary from being truly stepwise to migration occurring in concert with initial ionisation. These two situations
Mass Spectrometry. 2000, Paul R. Young University of Illinois at Chicago, All Rights Reserved
 Mass Spectrometry 2000, Paul R. Young University of Illinois at Chicago, All Rights Reserved Mass Spectrometry When a molecule is bombarded with high-energy electrons, one of the process that can occur
Mass Spectrometry 2000, Paul R. Young University of Illinois at Chicago, All Rights Reserved Mass Spectrometry When a molecule is bombarded with high-energy electrons, one of the process that can occur
Physical Properties: Structure:
 Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
Polar Reactions. Indian Institute of Technology Madras CH 3 OH 2 -H 2 O CH 2. HCl. H 3 C CH 3 neopentyl alcohol CH 3 CH 3. 1,2-shift -H + H 3 C
 Polar Reactions Important points to be discussed in this module: 1. Chemistry of Carbocations with respect to: a. 1,2-shifts of carbocations b. Pinacol-pinacolone rearrangement c. Semipinacolone rearrangement
Polar Reactions Important points to be discussed in this module: 1. Chemistry of Carbocations with respect to: a. 1,2-shifts of carbocations b. Pinacol-pinacolone rearrangement c. Semipinacolone rearrangement
CHE1502. Tutorial letter 203/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry
 E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
More Nomenclature: Common Names for Selected Aromatic Groups. Aryl = Ar = aromatic group. It is a broad term, and includes any aromatic rings.
 More Nomenclature: Common Names for Selected Aromatic Groups Phenyl group = or Ph = C 6 H 5 = Aryl = Ar = aromatic group. It is a broad term, and includes any aromatic rings. Benzyl = Bn = It has a -CH
More Nomenclature: Common Names for Selected Aromatic Groups Phenyl group = or Ph = C 6 H 5 = Aryl = Ar = aromatic group. It is a broad term, and includes any aromatic rings. Benzyl = Bn = It has a -CH
Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid
![Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid](/thumbs/95/123929992.jpg) Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
Class XI Chapter 13 Hydrocarbons Chemistry
 Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
S N 1 Displacement Reactions
 S N 1 Displacement Reactions Tertiary alkyl halides cannot undergo S N 2 reactions because of the severe steric hindrance blocking a backside approach of the nucleophile. They can, however, react via an
S N 1 Displacement Reactions Tertiary alkyl halides cannot undergo S N 2 reactions because of the severe steric hindrance blocking a backside approach of the nucleophile. They can, however, react via an
Real life example 1 Let s look at this series of chloroalcohols, and how fast the chloride gets displaced by an external nucleophile.
 Class 2 Carbocations Last time we talked about neighboring group participation in substitution reactions. I want to start today by talking about a few more real life examples. Real life example 1 Let s
Class 2 Carbocations Last time we talked about neighboring group participation in substitution reactions. I want to start today by talking about a few more real life examples. Real life example 1 Let s
Electrophilic Aromatic Substitution. Dr. Mishu Singh Department of chemistry Maharana Pratap Govt.P.G.College Hardoi
 Electrophilic Aromatic Substitution Dr. Mishu Singh Department of chemistry Maharana Pratap Govt.P.G.College Hardoi 1 Recall the electophilic addition of HBr (or Br2) to alkenes H + nu cleophile H Br H
Electrophilic Aromatic Substitution Dr. Mishu Singh Department of chemistry Maharana Pratap Govt.P.G.College Hardoi 1 Recall the electophilic addition of HBr (or Br2) to alkenes H + nu cleophile H Br H
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Module No and Title. PAPER No: 5 ; TITLE : Organic Chemistry-II MODULE No: 25 ; TITLE: S E 1 reactions
 Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 25; S E 1 reactions CHE_P5_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. S E 1 reactions 3.1
Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 25; S E 1 reactions CHE_P5_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. S E 1 reactions 3.1
CHAPTER 7. Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination
 CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
12/27/2010. Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides"
 Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Chapter 17. Reactions of Aromatic Compounds
 Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
Conjugated Systems, Orbital Symmetry and UV Spectroscopy
 Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
16. Chemistry of Benzene: Electrophilic Aromatic Substitution. Based on McMurry s Organic Chemistry, 7 th edition
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
Organic Chemistry, 7 L. G. Wade, Jr. Chapter , Prentice Hall
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
Benzene and Aromatic Compounds. Chapter 15 Organic Chemistry, 8 th Edition John McMurry
 Benzene and Aromatic Compounds Chapter 15 Organic Chemistry, 8 th Edition John McMurry 1 Background Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Four degrees of unsaturation. It
Benzene and Aromatic Compounds Chapter 15 Organic Chemistry, 8 th Edition John McMurry 1 Background Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Four degrees of unsaturation. It
Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution
 John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
John E. McMurry www.cengage.com/chemistry/mcmurry Chapter 16 Chemistry of Benzene: Electrophilic Aromatic Substitution Paul D. Adams University of Arkansas Substitution Reactions of Benzene and Its Derivatives
Chapter 8. Substitution reactions of Alkyl Halides
 Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
1. Radical Substitution on Alkanes. 2. Radical Substitution with Alkenes. 3. Electrophilic Addition
 1. Radical Substitution on Alkanes Only Cl and Br are useful at the laboratory level. Alkane reactivity: tertiary > secondary > primary > methyl Numbers below products give their relative yield. Relative
1. Radical Substitution on Alkanes Only Cl and Br are useful at the laboratory level. Alkane reactivity: tertiary > secondary > primary > methyl Numbers below products give their relative yield. Relative
Chapter 7: Alcohols, Phenols and Thiols
 Chapter 7: Alcohols, Phenols and Thiols 45 -Alcohols have the general formula R-OH and are characterized by the presence of a hydroxyl group, -OH. -Phenols have a hydroxyl group attached directly to an
Chapter 7: Alcohols, Phenols and Thiols 45 -Alcohols have the general formula R-OH and are characterized by the presence of a hydroxyl group, -OH. -Phenols have a hydroxyl group attached directly to an
Benzene and Aromatic Compounds
 1 Background Benzene and Aromatic Compounds Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Benzene has four degrees of unsaturation, making it a highly unsaturated hydrocarbon. Whereas
1 Background Benzene and Aromatic Compounds Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Benzene has four degrees of unsaturation, making it a highly unsaturated hydrocarbon. Whereas
Synthesis Using Aromatic Materials
 Chapter 10 Synthesis Using Aromatic Materials ELECTROPHILIC AROMATIC SUBSTITUTION AND DIRECTED ORTHO METALATION Copyright 2018 by Nelson Education Limited 1 10.2 p Bonds Acting as Nucleophiles Copyright
Chapter 10 Synthesis Using Aromatic Materials ELECTROPHILIC AROMATIC SUBSTITUTION AND DIRECTED ORTHO METALATION Copyright 2018 by Nelson Education Limited 1 10.2 p Bonds Acting as Nucleophiles Copyright
(Neither an oxidation or reduction: Addition or loss of H +, H 2 O, HX).
 eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
Chapter 5. Nucleophilic aliphatic substitution mechanism. by G.DEEPA
 Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy
 Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
PAPER No. : 5; Organic Chemistry-II MODULE No. : 13; Mixed S N 1 and S N 2 Reactions
 Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 13; Mixed S N 1 and S N 2 Reactions CHE_P5_M13 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Nature
Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 13; Mixed S N 1 and S N 2 Reactions CHE_P5_M13 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Nature
The Study of Chemical Reactions. Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order.
 The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
The Study of Chemical Reactions Mechanism: The complete, step by step description of exactly which bonds are broken, formed, and in which order. Thermodynamics: The study of the energy changes that accompany
Chapter 11 - Alcohols and Ethers 1
 Andrew Rosen Chapter 11 - Alcohols and Ethers 1 11.1 - Structure and Nomenclature - The common naming calls alcohols as alkyl alcohols (eg: methyl alcohol) - The common names of ethers have the groups
Andrew Rosen Chapter 11 - Alcohols and Ethers 1 11.1 - Structure and Nomenclature - The common naming calls alcohols as alkyl alcohols (eg: methyl alcohol) - The common names of ethers have the groups
LECTURE #13 Tues., Oct.18, Midterm exam: Tues.Oct.25 during class Ch.1, , 7.10, 2, Sections
 CEM 221 section 01 LECTURE #13 Tues., Oct.18, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSGNED READNGS: TODAY S CLASS: Sections 4.1 4.6 NEXT CLASS: rest of Ch.4 http://artsandscience.concordia.ca/facstaff/p-r/rogers
CEM 221 section 01 LECTURE #13 Tues., Oct.18, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSGNED READNGS: TODAY S CLASS: Sections 4.1 4.6 NEXT CLASS: rest of Ch.4 http://artsandscience.concordia.ca/facstaff/p-r/rogers
Chapter 8 Alkenes and Alkynes II: Addition Reactions. Alkenes are electron rich. Additions to Alkenes
 Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
L substrate (Leaving group,l)
 Aliphatic Nucleophilic Substitution Nu + Nucleophile L substrate (Leaving group,l) conditions products Nucleophiles are chemical species that react with centers of positive ionic character. When the center
Aliphatic Nucleophilic Substitution Nu + Nucleophile L substrate (Leaving group,l) conditions products Nucleophiles are chemical species that react with centers of positive ionic character. When the center
Aryl Halides. Structure
 Aryl Halides Structure Aryl halides are compounds containing halogen attached directly to an aromatic ring. They have the general formula ArX, where Ar is phenyl, substituted phenyl. X= F,Cl,Br,I An aryl
Aryl Halides Structure Aryl halides are compounds containing halogen attached directly to an aromatic ring. They have the general formula ArX, where Ar is phenyl, substituted phenyl. X= F,Cl,Br,I An aryl
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
Chem 251 Fall Learning Objectives
 Learning Objectives Chapter 8 (last semester) 1. Write an electron-pushing mechanism for an SN2 reaction between an alkyl halide and a nucleophile. 2. Describe the rate law and relative rate of reaction
Learning Objectives Chapter 8 (last semester) 1. Write an electron-pushing mechanism for an SN2 reaction between an alkyl halide and a nucleophile. 2. Describe the rate law and relative rate of reaction
Chemistry 2000 Lecture 18: Reactions of organic compounds
 hemistry 2000 Lecture 18: Reactions of organic compounds Marc R. Roussel March 6, 2018 Marc R. Roussel Reactions of organic compounds March 6, 2018 1 / 27 Reactions of organic compounds Organic chemists
hemistry 2000 Lecture 18: Reactions of organic compounds Marc R. Roussel March 6, 2018 Marc R. Roussel Reactions of organic compounds March 6, 2018 1 / 27 Reactions of organic compounds Organic chemists
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Alcohols, Ethers, & Epoxides
 Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
Introduction to Organic Chemistry
 Introduction to rganic hemistry 59 Introduction to rganic hemistry andout 3 - chanism u u http://burton.chem.ox.ac.uk/teaching.html rganic hemistry J. layden,. Greeves, S. Warren Stereochemistry at a Glance
Introduction to rganic hemistry 59 Introduction to rganic hemistry andout 3 - chanism u u http://burton.chem.ox.ac.uk/teaching.html rganic hemistry J. layden,. Greeves, S. Warren Stereochemistry at a Glance
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
Benzenes & Aromatic Compounds
 Benzenes & Aromatic Compounds 1 Structure of Benzene H H C C C H C 6 H 6 H C C C H H A cyclic conjugate molecule Benzene is a colourless odourless liquid, boiling at 80 o C and melting at 5 o C. It is
Benzenes & Aromatic Compounds 1 Structure of Benzene H H C C C H C 6 H 6 H C C C H H A cyclic conjugate molecule Benzene is a colourless odourless liquid, boiling at 80 o C and melting at 5 o C. It is
Organic Mechanisms 1
 Organic Mechanisms 1 Concepts The key ideas required to understand this section are: Concept Book page Chemical properties of alkanes 314 Chemical properties of alkenes 318 Bonding in alkenes 320 Bonding
Organic Mechanisms 1 Concepts The key ideas required to understand this section are: Concept Book page Chemical properties of alkanes 314 Chemical properties of alkenes 318 Bonding in alkenes 320 Bonding
Amines Reading Study Problems Key Concepts and Skills Lecture Topics: Amines: structure and nomenclature
 Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
Double and Triple Bonds. The addition of an electrophile and a
 Chapter 11 Additions to Carbon-Carbon Double and Triple Bonds The addition of an electrophile and a nucleophile to a C-C C double or triple bonds 11.1 The General Mechanism Pi electrons of the double bond
Chapter 11 Additions to Carbon-Carbon Double and Triple Bonds The addition of an electrophile and a nucleophile to a C-C C double or triple bonds 11.1 The General Mechanism Pi electrons of the double bond
CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Text Sections (N0 1.9, 9-11) Homework: Chapter 1:
 CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Atomic Structure - Valence Electrons Chemical Bonds: The Octet Rule - Ionic bond - Covalent bond How to write Lewis
CHEM 261 HOME WORK Lecture Topics: MODULE 1: The Basics: Bonding and Molecular Structure Atomic Structure - Valence Electrons Chemical Bonds: The Octet Rule - Ionic bond - Covalent bond How to write Lewis
11/26/ Polycyclic Aromatic Compounds. Polycyclic Aromatic Compounds. Polycyclic Aromatic Compounds
 9.5 Polycyclic Aromatic Compounds The general concept of aromaticity can be extended to include polycyclic aromatic compounds Benzo[a]pyrene is one of the cancer-causing substances found in tobacco smoke
9.5 Polycyclic Aromatic Compounds The general concept of aromaticity can be extended to include polycyclic aromatic compounds Benzo[a]pyrene is one of the cancer-causing substances found in tobacco smoke
Chapter 13 Conjugated Unsaturated Systems
 Conjugated Unsaturated Systems 13.1 Introduction Allyl radical C 2 C C 2 C C C Allyl cation C 2 C C 2 C C C 1,3-Butadiene C 2 C C C 2 C C C C Molecules with delocalized π bonds are called conjugated unsaturated
Conjugated Unsaturated Systems 13.1 Introduction Allyl radical C 2 C C 2 C C C Allyl cation C 2 C C 2 C C C 1,3-Butadiene C 2 C C C 2 C C C C Molecules with delocalized π bonds are called conjugated unsaturated
Organic Chemistry. Second Edition. Chapter 19 Aromatic Substitution Reactions. David Klein. Klein, Organic Chemistry 2e
 Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
OChem 1 Mechanism Flashcards. Dr. Peter Norris, 2018
 OChem 1 Mechanism Flashcards Dr. Peter Norris, 2018 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2018 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
2016 Pearson Education, Inc. Isolated and Conjugated Dienes
 2016 Pearson Education, Inc. Isolated and Conjugated Dienes 2016 Pearson Education, Inc. Reactions of Isolated Dienes 2016 Pearson Education, Inc. The Mechanism Double Bonds can have Different Reactivities
2016 Pearson Education, Inc. Isolated and Conjugated Dienes 2016 Pearson Education, Inc. Reactions of Isolated Dienes 2016 Pearson Education, Inc. The Mechanism Double Bonds can have Different Reactivities
Chapter 9 Aldehydes and Ketones Excluded Sections:
 Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION
 REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
REACTIONS OF ALCOHOLS Created by: Mohammad Heidarian
 Nucleophilic Substitution, β- Elimination, and Oxidation reactions are the main type of reactions associated with alcohols. Nucleophilic Substitution: A reaction in which a nucleophile replaces a leaving
Nucleophilic Substitution, β- Elimination, and Oxidation reactions are the main type of reactions associated with alcohols. Nucleophilic Substitution: A reaction in which a nucleophile replaces a leaving
Preparation of Alkyl Halides, R-X. Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): R + X X 2.
 Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Aromatic Compounds II
 2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
Chapter 15. Reactions of Aromatic Compounds. Electrophilic Aromatic Substitution on Arenes. The first step is the slow, rate-determining step
 Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
Chemistry of Benzene: Electrophilic Aromatic Substitution
 Chemistry of Benzene: Electrophilic Aromatic Substitution Why this Chapter? Continuation of coverage of aromatic compounds in preceding chapter focus shift to understanding reactions Examine relationship
Chemistry of Benzene: Electrophilic Aromatic Substitution Why this Chapter? Continuation of coverage of aromatic compounds in preceding chapter focus shift to understanding reactions Examine relationship
ALCOHOLS AND PHENOLS
 ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
I. Multiple Choice Questions (Type-I)
 Unit 13 HYDROCARBONS I. Multiple Choice Questions (Type-I) 1. Arrange the following in decreasing order of their boiling points. (A) n butane (B) 2 methylbutane (C) n-pentane (D) 2,2 dimethylpropane A
Unit 13 HYDROCARBONS I. Multiple Choice Questions (Type-I) 1. Arrange the following in decreasing order of their boiling points. (A) n butane (B) 2 methylbutane (C) n-pentane (D) 2,2 dimethylpropane A
Organic Chemistry. M. R. Naimi-Jamal. Faculty of Chemistry Iran University of Science & Technology
 Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 5-2. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry,
Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 5-2. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry,
Chap. 5 Reactive intermediates
 Energy surface hap. 5 Reactive intermediates The plot of energy (potential and kinetic) as a function of 3N- 6 coordinates of the chemical system (reactants, intermediates, products) Reaction oordinate
Energy surface hap. 5 Reactive intermediates The plot of energy (potential and kinetic) as a function of 3N- 6 coordinates of the chemical system (reactants, intermediates, products) Reaction oordinate
KOT 222 Organic Chemistry II
 KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions
 Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Organic Chemistry Lecture 2 - Hydrocarbons, Alcohols, Substitutions
 ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
18.8 Oxidation. Oxidation by silver ion requires an alkaline medium
 18.8 Oxidation Oxidation by silver ion requires an alkaline medium Test for detecting aldehydes Tollens reagent to prevent precipitation of the insoluble silver oxide, a complexing agent is added: ammonia
18.8 Oxidation Oxidation by silver ion requires an alkaline medium Test for detecting aldehydes Tollens reagent to prevent precipitation of the insoluble silver oxide, a complexing agent is added: ammonia
Electrophilic Aromatic Substitution
 Chem 263 ct. 8, 2013 lectrophilic Aromatic Substitution Benzene appears to be a remarkably stable and unreactive compared to alkenes, such as cyclohexene or ethylene, or even alkanes, such as cyclohexane
Chem 263 ct. 8, 2013 lectrophilic Aromatic Substitution Benzene appears to be a remarkably stable and unreactive compared to alkenes, such as cyclohexene or ethylene, or even alkanes, such as cyclohexane
2/26/18. Practice Questions. Practice Questions B F. How many steps are there in this reaction?
 Practice Questions Practice Questions D B F C E A G How many steps are there in this reaction? 1 Practice Questions D B F C E A G What is the highest-energy transitions state? Practice Questions D B F
Practice Questions Practice Questions D B F C E A G How many steps are there in this reaction? 1 Practice Questions D B F C E A G What is the highest-energy transitions state? Practice Questions D B F
Chapter 8 Alkenes and Alkynes II: Addition Reactions
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Chapter 10 Radical Reactions"
 Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
What is the major product of the following reaction?
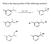 What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
Topic 6 Alkyl halide and carbonyl compounds Organic compounds containing a halogen
 Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene (aryl
Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene (aryl
PAPER No. : Paper-9, Organic Chemistry-III (Reaction Mechanism-2) MODULE No. : Module-10, Hydroboration Reaction CHEMISTRY
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper-9, Organic Chemistry-III (Reaction Mechanism-2) Module-10, Hydroboration Reaction CHE_P9_M10 TABLE OF CONTENTS 1. Learning Outcomes
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper-9, Organic Chemistry-III (Reaction Mechanism-2) Module-10, Hydroboration Reaction CHE_P9_M10 TABLE OF CONTENTS 1. Learning Outcomes
The now-banned diet drug fen-phen is a mixture of two synthetic substituted benzene: fenfluramine and phentermine.
 The now-banned diet drug fen-phen is a mixture of two synthetic substituted benzene: fenfluramine and phentermine. Chemists have synthesized compounds with structures similar to adrenaline, producing amphetamine.
The now-banned diet drug fen-phen is a mixture of two synthetic substituted benzene: fenfluramine and phentermine. Chemists have synthesized compounds with structures similar to adrenaline, producing amphetamine.
Topic 6 Alkyl halide and carbonyl compounds Organic compounds containing a halogen
 Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way, eg: 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene
Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way, eg: 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene
Chapter 1 Reactions of Organic Compounds. Reactions Involving Hydrocarbons
 Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Page 1 of 9. Sessional Examination (November 2017) Max Marks: 20 Date: Time: One Hour. Model Answers
 Page 1 of 9 Sessional Examination (November 2017) Class: B. Pharm-II yr (III sem) Subject: Pharma Org. Chem-II Max Marks: 20 Date: 14.11.2017 Time: One Hour Model Answers Q. 1. Solve the following (ANY
Page 1 of 9 Sessional Examination (November 2017) Class: B. Pharm-II yr (III sem) Subject: Pharma Org. Chem-II Max Marks: 20 Date: 14.11.2017 Time: One Hour Model Answers Q. 1. Solve the following (ANY
11/5/ Conjugated Dienes. Conjugated Dienes. Conjugated Dienes. Heats of Hydrogenation
 8.12 Sites of unsaturation Many compounds have numerous sites of unsaturation If sites are well separated in molecule they react independently If sites are close together they may interact with one another
8.12 Sites of unsaturation Many compounds have numerous sites of unsaturation If sites are well separated in molecule they react independently If sites are close together they may interact with one another
CHEM 2311 HW1. COMPLETELY FILL THE BOXES IN DARK PENCIL, i.e.:, NOT or or STRUCTURE
 EM 2311 W1 Provide answers to the following multiple choice questions on the Scantron form provided in lecture. Make sure that you put your name and bubble in your GTID # on the answer form. MPLETELY FILL
EM 2311 W1 Provide answers to the following multiple choice questions on the Scantron form provided in lecture. Make sure that you put your name and bubble in your GTID # on the answer form. MPLETELY FILL
The C-X bond gets longerand weakergoing down the periodic table.
 Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
Ethers can be symmetrical or not:
 Chapter 14: Ethers, Epoxides, and Sulfides 175 Physical Properties Ethers can be symmetrical or not: linear or cyclic. Ethers are inert and make excellent solvents for organic reactions. Epoxides are very
Chapter 14: Ethers, Epoxides, and Sulfides 175 Physical Properties Ethers can be symmetrical or not: linear or cyclic. Ethers are inert and make excellent solvents for organic reactions. Epoxides are very
