Prompt deliquescence and efflorescence of aerosol nanoparticles
|
|
|
- Justina Christina Richards
- 5 years ago
- Views:
Transcription
1 Atmos. Chem. Phys., 6, , 26 Author(s) 26. This work is licensed under a Creative Commons License. Atmospheric Chemistry and Physics Prompt deliquescence and efflorescence of aerosol nanoparticles G. Biskos, D. Paulsen, L. M. Russell 2, P. R. Buseck 3, and S. T. Martin Division of Engineering and Applied Sciences, Harvard University, Cambridge, MA 238, USA 2 Scripps Institution of Oceanography, University of California San Diego, La Jolla, CA 9293, USA 3 Departments of Geological Sciences and Chemistry/Biochemistry, Arizona State University, Tempe, AZ 85287, USA Received: July 26 Published in Atmos. Chem. Phys. Discuss.: 25 July 26 Revised: 5 October 26 Accepted: October 26 Published: 7 October 26 Abstract. Literature reports have differed on the possibilities of discontinuous and continuous (i.e., prompt and nonprompt) deliquescence and efflorescence of aerosol particles in the nanosize regime. Experiments reported herein using a hygroscopic tandem nano-differential mobility analyzer demonstrate prompt deliquescence and efflorescence of ammonium sulfate particles having diameters from 6 to 6 nm. Apparent nonpromptness can be induced both by operation of the experimental apparatus and by interpretation of the measurements, even though the underlying phase transitions of individual particles remain prompt. No nanosize effect on the relative humidity values of deliquescence or efflorescence is observed for the studied size range. Smaller hygroscopic growth factors are, however, observed for the nanoparticles, in agreement with thermodynamic calculations that include the Kelvin effect. A slightly nonspherical shape for dry ammonium sulfate particles is inferred from their hygroscopically induced reconstruction between 5 and 3% relative humidity. Our results provide a further understanding of nanoparticle behavior, especially relevant to the growth rates of atmospheric nanoparticles. Introduction Despite their small mass, the chemical composition of atmospheric aerosol nanoparticles with diameters as small as 2 nm can now be approximately determined, in part due to recent advances in analytical instrumentation (McMurry, 2). Field studies in some locations indicate that ambient particles following nucleation events are composed primarily of ammonium and sulfate ions at variable mixing ratios (Kulmala et al., 24), although organic compounds have been identified as important constituents of atmospheric nanopar- Correspondence to: S. T. Martin (scot martin@harvard.edu) ticle in some cases (O Dowd et al., 22; Allan et al. 26). Aerosol mass spectra recorded during new-particle nucleation events in Pittsburgh (September 22) showed that particles having diameters from 8 to 33 nm were mainly composed of sulfuric acid (Zhang et al., 24). Hygroscopic growth factors of 25-nm particles following a nucleation event in Gosan, Korea (April 2), were similar to those of laboratory-generated ammonium sulfate particles (Buzorius et al., 24). Mass spectra recorded for particles from 6 to 5 nm during nucleation events in Atlanta (August 22) showed that ammonium sulfate accounted for the entire sampled nanoparticle mass (Smith et al., 25). Concurrent measurements of the hygroscopic growth factors were also consistent with ammonium sulfate (Sakurai et al., 25). Ammonium sulfate nanoparticles are believed to form in the atmosphere by nucleation in the presence of gas-phase sulfuric acid, water, and ammonia (Ball et al., 999). Although the exact mechanism of new-particle formation is not yet fully understood, theoretical predictions indicate that ternary nucleation could explain the high particle-formation rates observed in the atmosphere (Napari et al., 22). Once formed, the freshly nucleated particles grow, through processes of condensation and coagulation, to diameters large enough to become optically active. During the growth process, a principal factor affecting the condensation and coagulation rates is particle hygroscopicity because physical state (i.e., solid or liquid) and particle diameter vary with relative humidity (RH). Once large enough to be optically active, the particles influence the radiative properties of the atmosphere and thus directly the climate of the Earth at regional and global scales. The larger particles also indirectly affect the Earth s climate through their action on cloud formation. The hygroscopic behavior of nanoparticles can be different from that of their large-particle counterparts because the relative contribution of the surface term to the free energy of a particle increases markedly for sub- nm particles (Chen, 994; Mirabel et al., 2; Djikaev et al., 2; Russell and Published by Copernicus GmbH on behalf of the European Geosciences Union.
2 4634 G. Biskos et al.: Nanoparticle phase transitions Ming, 22; Biskos et al., 26a). Sodium chloride nanoparticles have been investigated most thoroughly. One effect is that the deliquescence and efflorescence relative humidity values (hereafter, DRH and ERH, respectively) of sodium chloride particles increase for mobility diameters smaller than 4 nm (Hämeri et al., 2; Biskos et al., 26a). A second effect is that the hygroscopic growth factor of the sodium chloride nanoparticles decreases because of the Kelvin effect (Hämeri et al., 2), although quantitative agreement between predictions and observations must also take into account a size-dependent shape factor in the Knudsen regime (Biskos et al., 26b). Measurements of the hygroscopic properties of ammonium sulfate nanoparticles have also been made, and intermediate growth factors were observed for RH values around the DRH (Hämeri et al., 2). The results led Mirabel et al. (2) in a theory paper to introduce the concepts of prompt and nonprompt deliquescence. In an operational definition, prompt and nonprompt deliquescence respectively correspond to discontinuous and continuous increases of particle diameter during the phase transition that accompanies increasing RH. Mirabel et al. (2) hypothesized that nanosized particles have nonprompt behavior in contrast to the prompt behavior of their large-particle counterparts (e.g., as observed in the large-particle experiments of Cohen et al., 987; Tang and Munkelwitz, 994; Weis and Ewing, 996; Cziczo et al., 997; Onasch et al., 999; Wise et al., 25). Djikaev et al. (2) subsequently modeled the deliquescence process for nanosized particles and reported an absence of nonprompt behavior. In further modeling papers, Russell and Ming (22) and Topping et al. (25) also predicted that prompt deliquescence of nanoparticles should occur. In this paper, we provide new experimental measurements of the hygroscopic properties of ammonium sulfate nanoparticles using a tandem nano-differential mobility analyzer (Rader and McMurry, 986; Chen et al., 998). Our results show that both deliquescence and efflorescence of ammonium sulfate nanoparticles are prompt and that the earlier data of Hämeri et al. (2), which has been interpreted to support nonprompt deliquescence, can instead be reproduced by an artifact tied to the operation of the experimental apparatus. 2 Experimental A hygroscopic tandem nano-differential mobility analyzer (TnDMA) was employed to study the deliquescence, the efflorescence, and the hygroscopic growth of monodisperse ammonium sulfate particles having mobility diameters from 6 to 6 nm. Experiments were carried out for particles generated both by electrospray and by atomization to test for any influence of preparation method on the results (e.g., possible chemical impurities enriched in nanoparticles). The electrospray aerosol generator (TSI Model 348) employed - and -mm aqueous solutions of ammonium sulfate (EMD Chemical Inc., 99.5% purity) for nanoparticles of 6 2 nm and 4 6 nm mobility diameter, respectively. High-purity water (Barnstead Model D897; 8 M cm) for the solutions was prepared by consecutive steps including filtration, ultraviolet irradiation, and reverse osmosis. The primary aerosol particles exiting the 4-µm capillary tube of the electrospray generator were diluted with 2 Lpm filtered dry air, resulting in an aerosol stream of below.% RH. The aerosols had lognormal particle number size distributions. From the -mm solution, the mode diameter was nm, the geometric standard deviation was.3, and the integrated particle number concentration was 6 cm 3. The respective quantities for the -mm solution were 5 nm,.6, and 6 6 cm 3. An atomizer (TSI Model 376) was also used to generate aerosol samples. A. wt% solution was atomized in N 2, following a method similar to that of Hämeri et al. (2). The polydisperse aerosol was dried to below % RH by mixing with dry air and passing through a diffusion dryer (TSI Model 362). The mode diameter of the lognormal particle number size distribution was 5 nm, the geometric standard deviation was.4, and the integrated particle number concentration was 4 6 cm 3. Figure shows a schematic layout of the TnDMA used in the experiments. A description of an earlier version of the apparatus was provided in Biskos et al. (26a). In brief, the polydisperse aerosol particles were passed through a 85 Kr neutralizer (TSI Model 377) and a first nano-dma (TSI Model 385). The resulting monodisperse particles were then exposed to an RH history through a series of one or two single-tube Nafion humidity exchangers (Perma Pure Model MD-), after which their number size distribution was measured with a second nano-dma and an ultrafine CPC (TSI Model 325). The RH history allowed either deliquescence- or efflorescence-mode experiments to be carried out. For instance, in deliquescence mode, the RH history of the aerosol prior to a number-size-distribution measurement was 5% X%, where X was increased stepwise as the independent variable of an experiment (i.e., X=RH a of Fig. ). In efflorescence mode, the RH history prior to a number-size-distribution measurement was 5% 95% X%. Modifications to the system described by Biskos et al. (26a) were as follows. DMA-2 employed closedloop recirculation for the sheath flow instead of the openflow circulation. This change somewhat improved the precision of replicate experiments by ensuring that the sheath flow-in was exactly balanced by the sheath flow-out. The recirculation setup depicted in Fig. employed a blower (Minispiral Model SE2RE2SA) that collected the excess flow from the DMA and drove it through () a hydrophobic filter (Whatman Model ) to remove particles, (2) a heat exchanger (Lytron Model C-HX-45GSB) to Atmos. Chem. Phys., 6, , 26
3 Figure (DOUBLE COLUMN) G. Biskos et al.: Nanoparticle phase transitions 4635 Aerosol Deliquescence or Efflorescence NCW NCA.8 Lpm C A B D.8 Lpm 5 to 5 Lpm vent.8 Lpm AG Sheath Air Excess Air 85 Kr vent DMA Air NCW wet dry NCW wet dry PT PID LFE HE HF RB NCA vent RH s RH a.3 or.5 Lpm DMA 2 CPC Monodisperse Aerosol Relative Humidity Control Mobility Measurement Fig.. Schematic layout of the tandem nano-dma. Key: AG, aerosol generator (electrospray or atomizer); 85 Kr, Krypton source aerosol neutralizer; DMA, differential mobility analyzer; CPC, condensation particle counter; LFE, laminar flow element; HE, heat exchanger; HF, high efficiency particulate air (HEPA) filter; PID, proportional-integral-derivative controller; PT, pressure transducer; RB, recirculation blower; NCA, Nafion conditioner with air; NCW, Nafion conditioner with water; RH s, sheath-flow relative-humidity monitor of DMA-2; and RH a, aerosol-flow relative-humidity monitor of DMA-2. Vent is connected to the house hood. Filtered dry house air is used for the sheath flow of DMA- and for the relative humidity control. In a deliquescence-mode experiment, valve A is closed and valve B is open. In an efflorescence-mode experiment, valve A is open and valve B is closed. Valve C is open and valve D is closed to set RH s =RH a. For independent values of RH s and RH a (e.g., RH s =RH a +3%), valve C is closed and valve D is open. Gray and clear paths denote RH-controlling and RH-controlled flows, respectively. See Biskos et al. (26a) for further description of the elements in this figure. equilibrate the temperature (heated by 2 3 K in the compressive blower), (3) a laminar flow element (Furness Controls Model FCO96-2) coupled to a pressure transducer (Aschroft Model CXLdp) and a proportional-integral-derivative (PID) controller (Omega Model CNI6D52-C24-DC) to provide feedback to the blower speed and thereby control the flow rate, and (4) a multiple-tube Nafion conditioner (Permapure Model PD-5T) to rapidly adjust the relative humidity to the desired value. The flow then reentered the DMA as the sheath flow, which was regularly calibrated by use of a Gilibrator bubble flow meter. In the usual mode of operation, we set the relative humidity of the sheath flow (RH s ) equal to the relative humidity of the aerosol (i.e., RH s =RH a ) by opening valve C and closing valve D. For specific experiments designed to compare our results with those of Hämeri et al. (2), we closed valve C and opened valve D and then set RH s =RH a +3% by using an independent RH generator (cf. Fig. ). We employed.5 Lpm for the aerosol flow and 5 Lpm for the sheath flow at 6-nm mobility diameter and steadily decreased the respective flows for increasing mobility diameter, i.e.,.3 Lpm and 5 Lpm at 6-nm mobility diameter. Accuracy and precision of the particle-sizing and relative humidity measurements were as follows. Regarding the accuracy of particle sizing (Kinney et al., 99), the sheath and aerosol flows were calibrated to within % using a Gilibrator bubble flow meter, and the voltage of the central rod was measured to within.% (up to 5 V). These uncertainties, combined with the uncertainties in the geometric dimensions of the DMA, propagated as an uncertainty of 2.5% in the classified particle diameter and, therefore, in the measured growth factor. (An alternative procedure using monodisperse polystyrene latex (PSL) spheres led to an uncertainty of 3%.) The precision of the TnDMA measurements of the particle sizes was within 2% based upon replicate measurements and did not change throughout the course of the experiments. As an experimental check of these accuracies and precisions, the sizing agreement of the two DMAs was within 2.5% from to nm using NaCl particles generated by a vaporizationcondensation method and analyzed below 5% RH (cf. Biskos et al. 26a). The two RH sensors (Omega Model HX93AV) were regularly calibrated against a chilled mirror hygrometer (Kahn Instruments Inc. Model S4) from 5 to 95% RH. Atmos. Chem. Phys., 6, , 26
4 Figure 2 (DOUBLE COLUMN) 4636 G. Biskos et al.: Nanoparticle phase transitions Normalized Concentration (a) Electrospray Deliquescence for RH s = RH a RH = 25 ± 2.5% 76% 78% 79% 8% 82% (b) Atomization Deliquescence for RH s = RH a RH = 27 ± 2.5% 74% 79% 8% 8% 84% (c) Electrospray Deliquescence for RH s = RH a + 3% RH = 4 ± 2.5% 76% 78% 79% 8% 8% Mobility Diameter (nm) Fig. 2. Deliquescence-mode measurements of ammonium sulfate aerosol particles generated by electrospray or atomization. The RH history in each measurement is 5% X%, where X is the value given in each panel. Measured (circles) and fitted (solid lines; one-peak Gaussian function) normalized number size distributions are shown for increasing RH. In (a) and (b), the RH of the sheath flow equals that of the aerosol flow. In (c), the RH of the sheath flow is +3% compared to that of the aerosol flow (Hämeri et al., 2). Temperature is 298 K. The dry mobility diameter is nm. 3 Results and discussion The results of a deliquescence-mode experiment are shown in Fig. 2 for particles having a -nm dry mobility diameter. The normalized number size distributions show that deliquescence occurs at 79±2.5% RH, which is consistent with the large-particle value of 79.5% (cf. Martin, 2). Apparent differences in the deliquescence relative humidity (e.g., 79% in Fig. 2a and 8% in Fig. 2b) lie within the day-to-day variability of the precision of the RH measurements. The hygroscopic responses for particles generated by electrospray and atomization are similar. Provided that RH s =RH a, there is a prompt phase change for a % increase in RH from a mode centered at nm to one centered at 3 nm following deliquescence (Figs. 2a and b). In some measurements, two separate peaks, which correspond to externally mixed populations of aqueous and solid particles, are observed for RH a close to DRH. This effect arises from slight heterogeneities of relative humidity in the apparatus. Once the particles are aqueous above 8% RH, further increase of the RH results in water condensation and, therefore, additional diameter growth. For RH s =RH a +3%, which follows the protocol of Hämeri et al. (2), there is an apparent nonprompt phase change: the apparent mode diameter increases continuously from.3 nm at 76% RH, to.7 nm at 78% RH, to.2 nm at 79% RH, to 2.5 nm at 8% RH (Fig. 2c). Contrary to the experiments in which RH s =RH a, no distinct peaks that correspond to the two phases of the particles (i.e., solid and aqueous) are observed. The size distribution, however, widens for 78 and 79% RH. Similar behavior is also observed by us for -nm NaCl particles (see Fig. S). The apparent nonpromptness, however, does not reflect the true particle behavior but rather is an artifact of the experimental method and analysis, as next explained. The apparent nonprompt deliquescence occurs for RH s =RH a +3% because the particles deliquesce within DMA-2. There is a slow equilibration of the RH between the laminar aerosol and sheath flows, arising from diffusive mixing, as well as limited convective mixing, during transport through the DMA classification column. For instance, consider RH s =8% and RH a =77% when the flows enter the DMA. The particles in the aerosol flow are not immediately exposed to 8% RH when the aerosol and sheath flows merge at the top of the classification column. Rather, after traveling some distance in the classification column as solid particles of smaller mobility diameter, they abruptly deliquesce to form aqueous particles of increased mobility diameter. The classified particles emerging from the DMA therefore have an apparent mobility diameter that is a weighted-average of Atmos. Chem. Phys., 6, , 26
5 G. Biskos et al.: Nanoparticle phase transitions 4637 their transport time as solid and aqueous particles. Mikhailov et al. (24; Fig. 4 therein) reported a similar effect in their study of -nm sodium chloride particles. Numerical modeling by us of air flow and diffusive-convective RH mixing is consistent with deliquescence during particle transport inside the DMA (see Fig. S2). This RH mixing effect inside the DMA leads to the observation of a smoothly increasing growth factor. As the RH values of the sample and sheath flows increase during a scan (e.g., RH s =8% and RH a =78%), deliquescence occurs further upstream in the classification column, and the weightedaveraged apparent mobility diameter increases. According to this explanation, the underlying deliquescence events are prompt, and the apparent nonpromptness results, as an artifact, because these abrupt deliquescence events occur inside the classification column. This explanation makes unnecessary our earlier hypothesis (Biskos et al., 26a, b) of impurities concentrated by atomization as the explanation for literature reports of nonprompt deliquescence of nanoparticles. Furthermore, the apparent widening of the distribution from 77 to 79% RH (Fig. 2c) can be explained by small RH variations in the flow field, causing some particles to deliquesce earlier and some later along their paths within the DMA. Efflorescence-mode experiments for particles having a - nm dry mobility diameter show an apparent nonprompt behavior even for RH s =RH a (Fig. 3). Here, the explanation for apparent nonpromptness differs from that provided above for the deliquescence-mode experiments. In efflorescence mode, the mean mobility diameter of the aqueous particles decreases for decreasing RH, which is explained by the evaporation of water that accompanies reversible hygroscopic growth. For RH>35%, the observations show that the decrease of mobility diameter with RH (i.e., d m =dd m/drh) proceeds rather gently and in agreement with quantitative predictions (see Appendix concerning the hygroscopic growth factor). For 34 to 3% RH, however, d m increases greatly and diverges from predictions. At 3% RH, d m abruptly goes to zero, indicating that crystallization is complete. Unless a tandem-dma data analysis algorithm is used (e.g., Stolzenburg et al., 25; Cubison et al., 25), these measurements could erroneously be interpreted to suggest that particle crystallization for RH s =RH a is nonprompt because no abrupt shift of narrow, distinct peaks is observed during the RH scan from 35 to 3%. The increase both of the width of the size distribution from 34 to 3% RH and of d m over this same range, however, indicates that a different explanation is correct: there are two overlapping, incompletely resolved modes corresponding to solid and aqueous particles. This explanation can be developed further, as follows. For -nm dry mobility-diameter particles of ammonium sulfate, the hygroscopic growth factor at 34% RH is.75 (see Appendix and also Fig. 4). For the typical operating conditions of DMA- (i.e., an aerosol-to-sheath Figure 3 flow ratio of :), the size distribution of the monodisperse aerosol has a onesigma geometric standard deviation (gsd) of.4. The data Normalized Concentration Electrospray Efflorescence for RH s = RH a RH = 44 ± 2.5% 35% 34% 32% 3% 3% 29% 24% Mobility Diameter (nm) Fig. 3. Efflorescence-mode measurements of ammonium sulfate aerosol particles generated by electrospray. The RH history in each measurement is 5% 95% X%, where X is the RH value given in each panel. Measured (circles) and fitted (solid lines) normalized number size distributions are shown for decreasing RH. At 32 and 34% RH, the apparent widening of the normalized number size distributions arises from overlapping peaks of dry and aqueous aerosol particles, which are resolved by the two-peak Gaussian fit shown in the figure (dashed lines). Temperature is 298 K. The dry mobility diameter is nm. Atmos. Chem. Phys., 6, , 26
6 4638 G. Biskos et al.: Nanoparticle phase transitions Mobility Diameter Growth Factor Efflorescence expanded view Deliquescence expanded view Deliquescence Mode d m = nm Electrospray for RH s = RH a Atomization for RH s = RH a Electrospray for RH s = RH a + 3% Hämeri et al. (2) for RH s RH a + 3% Efflorescence Mode Electrospray for RH s = RH a (single peak fit) Hämeri et al. (2) for RH s RH a + 3% Relative Humidity (%) Fig. 4. Hygroscopic growth curves showing that prompt deliquescence and efflorescence occur provided that RH s =RH a (cf. caption to Fig. 2). Temperature is 298 K. The dry mobility diameter is nm. The ordinate values are calculated from the measurements (e.g., Figs. 2 and 3) using Eq. (A). Solid symbols were recorded during efflorescence-mode experiments, and open symbols were recorded during deliquescence-mode measurements. Diamonds show the data of Hämeri et al. (2), for which RH s RH a +3%. The upper panels show expanded views of the efflorescence-mode data from 3 to 4% RH (left panel) and the deliquescence-mode data from 75 to 85% RH (right panel). The solid blue points in the main and upper panels show the efflorescence data analyzed using a one-peak fit whereas the magenta points in the upper panel show the data analyzed using a two-peak fit of overlapping dry and aqueous modes (cf. Fig. 3). for 34% RH in Fig. 3 are successfully fit by two Gaussian peaks having gsd s of.4 and a geometric mode separation of.7. The existence of two modes in the efflorescence data across 3% RH (i.e., 34 to 3% RH), compared to across just % RH for the deliquescence data, arises from either greater RH gradients for lower RH values in the NCA (Fig. ) or stochastic nucleation and crystallization of individual aerosol particles during the residence time in the apparatus (Martin, 2). The hygroscopic behavior of -nm dry mobility-diameter particles is summarized in Fig. 4, including a comparison to the results of Hämeri et al. (2). The points in Fig. 4 correspond to the mode diameters of the fits to the deliquescence Table. Density ρ (kg m 3 ), water activity a w, and surface tension σ aq (N m ) of ammonium sulfate for <w t <78%. Data sources: ρ s (Perry and Green, 997), ρ aq and a w (Tang et al., 997), and σ aq (Pruppacher and Klett, 997). ρ s =77 ρ aq =997.+ A i w i t A =5.92 A 2 = a w =.+ C i w i t σ aq = w t w t A 3 =.24 5 C = C 2 =3.3 5 C 3 = C 4 =.42 8 and efflorescence data. For RH s =RH a, prompt deliquescence occurs for both the electrosprayed and the atomized particles (upper right panel). For RH s =RH a +3%, however, intermediate growth factors are recorded because deliquescence occurs within the classification column. The results of Hämeri et al. (2), also recorded using RH s =RH a +3%, are in qualitative agreement with this nonprompt trend. The efflorescence points in the bottom panel of Fig. 4 are shown for a onepeak fit to the data (i.e., apparent nonprompt efflorescence); the efflorescence points in the upper left panel correspond to solid- and aqueous-modes of a two-peak fit to the data for 3%<RH<34% (cf. discussion of Fig. 3). The slope of the intermediate growth factors depends on several instrumental factors. For instance, an increasing difference between RH a and RH s decreases the slope further. Likewise, the RH mixing rate within DMA-2 is affected both by the design of the DMA and the flow rates through it. Sizing of larger particles typically implies lower flow rates and therefore longer residence times of the particles in the classifier, allowing deliquescence closer to the DMA entrance. Two designs of Hauke-type DMA s (namely, one for particles smaller than 3 nm and another for those larger than 3 nm) are employed by Hämeri et al. (2) (cf. Table therein). In comparison, a TSI nano-dma is used in our apparatus. These factors provide a possible explanation for the difference in the extent of nonpromptness between the results reported in this study (red circles in Fig. 4) and those in Hämeri et al. (2) (black open diamonds). Differences in the design and the operating conditions of the DMA s may explain another observation in Hämeri et al. (2) that 5- and 3-nm particles appear to have a more prompt deliquescence than 8- and -nm particles (cf. Fig. 2 therein). The hygroscopic behavior for 6- to 6-nm dry mobilitydiameter particles generated by electrospray is summarized in Fig. 5, including a comparison to model predictions of hygroscopicity. For fixed relative humidity, the hygroscopic growth of nanoparticles decreases with decreasing particle Atmos. Chem. Phys., 6, , 26
7 G. Biskos et al.: Nanoparticle phase transitions 4639 Figure 5 Mobility Diameter Growth Factor.6 d m = 6 nm d m = 4 nm d m = 2 nm d m = 8 nm.4.2. d m = nm d m = 6 nm Relative Humidity (%) Fig. 5. Hygroscopic growth curves of ammonium sulphate aerosol particles generated by electrospray. Open symbols were recorded during deliquescence-mode measurements. Solid symbols were recorded during efflorescence-mode experiments and were analyzed by using either one- or two-peak fits, as necessary (cf. Figs. 3 and 4). Lines show the growth factors of models that omit (solid line) and include (dot-dashed line) the Kelvin effect (viz. models and 2 described in the Appendix). Models are evaluated for <w t <78%. Figure 6 size. For example, the mobility-diameter growth factor at 8% RH is.22 for 6-nm particles compared to.44 for 6- nm particles. For comparison, for sodium chloride particles the analogous values are.5 and.77, respectively (Biskos et al., 26b). The model predictions in Fig. 5 differ on whether the Kelvin effect is included or not (see Appendix). The measurements agree within 4% with the model predictions that include the Kelvin effect. This agreement suggests that the physical parameters used in the calculations (i.e., the surface tension, the density of the aqueous particles, the density of the dry particles, and the relationship of activity to weight percent composition) are known accurately enough to predict the hygroscopic growth of ammonium sulfate nanoparticles. Even so, there appears to be a systematic bias of to 4% in the comparisons between the model predictions of the growth factor and the measurements, with slightly greater bias for decreasing particle size. The small systematic bias between the model predictions and the measurements can be explained by a shape factor correction of.2, thus implying slightly nonspherical particles. This explanation is corroborated by the decrease of the Mobility Diameter Growth Factor nm 4 nm 2 nm nm 8 nm 6 nm d m because of adsorbed water layers χ because of particle restructuring Relative Humidity (%) Fig. 6. Comparison at intermediate RH values (i.e., below DRH) of the hygroscopic growth factors g of ammonium sulfate aerosol particles of 6- to 6-nm mobility diameter during deliquescence-mode experiments. Particles are generated by electrospray. Equation (A) provides the definition of g(rh). Between 2 and 6% RH, g(rh) decreases because the particle shape factor χ(rh) decreases (i.e., approaching spherical shape). Between 65 and 8% RH, g(rh) increases because adsorbed water layers increase the mobility diameter d m (RH). The RH history of the particles is 5% X%, where X is the abscissa value. The observations do not change for an RH history that includes a pre-treatment step of 5% 9% 5% X% (data not shown), in contrast to the behavior of sodium chloride particles (Biskos et al., 26b). For all the measurements, RH a =RH s. growth factor during deliquescence-mode experiments from. for RH<5% to.99 to.98 for 2%<RH<6% (Fig. 6). This decrease in mobility diameter, most evident for particles smaller than nm, arises from changes in particle shape and is driven by restructuring to more stable forms. The implication is that the mobility diameter for RH<5% is to 4% larger than the volume-equivalent diameter, so that the observed growth factors are systematically to 2% smaller than the predicted growth factors. (The increase in growth factor between 65 and 8% RH arises from adsorbed layers of water; Romakkaniemi et al., 2.) The data in Fig. 5 show that there is no nanosize effect on the deliquescence relative humidity (79.5%) or the efflorescence relative humidity (35%) of ammonium sulfate. This conclusion is subject to the caveats of the size range investigated (6 to 6 nm), the experimental uncertainty (2.5% in RH), the time allowed for a phase transition (between and 2 s), and the particle preparation methods employed. The data of Hämeri et al. (2) also suggest an absence of a nanosize effect provided that the DRH value is taken at the median growth factor of the nonprompt observations. The experimental observations of an absence of a nanosize effect for DRH disagree with theoretical predictions, which have hypothesized based upon assumed physical parameters that Atmos. Chem. Phys., 6, , 26
8 464 G. Biskos et al.: Nanoparticle phase transitions the DRH should increase for decreasing particle size (Chen, 994; Russell and Ming, 22; Topping et al., 25). The observed ERH of ammonium sulfate particles reported in this paper are in agreement, within experimental uncertainty, with those predicted by Gao et al. (26). The absence in the experimental observations of a nanosize effect for the deliquescence and efflorescence phase transitions of ammonium sulfate differs from the presence of an effect for sodium chloride, for which deliquescence shifts from 75% for large particles to 87% RH for a 6-nm particle and efflorescence from 45% to 53% for a similar change in particle size (Biskos et al., 26b). A possible implication is that the surface properties of ammonium sulfate differ from those of sodium chloride. In conclusion, the measurements presented in this paper show that deliquescence and efflorescence of ammonium sulfate nanoparticles are prompt, similar to their large-particle counterparts. Literature reports of nonprompt deliquescence are explained by the operating conditions of the DMA and the interpretation of the data. There is no discernable nanosize effect on the deliquescence and efflorescence relative humidity values of ammonium sulfate particles, in contrast to the phase transitions of sodium chloride nanoparticles. The hygroscopic growth factors observed for ammonium sulfate nanoparticles agree with thermodynamic predictions that take into account the Kelvin effect. An accurate description of the hygroscopic behavior of nanoparticles, including their phase transitions and growth factors, is a foundational component of accurate models of nucleation and growth processes of atmospheric nanoparticles. Supporting information available The data shown in Figs. 2 to 6, as well as the supplementary Figs. S and S2, are available electronically ( acp supplement.zip). Appendix A Description of models and 2 The mobility-diameter growth factor g is calculated from the measurements as follows: g(rh)= d m(rh) d m,dry, (A) where d m,dry is the mobility diameter of the dry particle (viz. RH<5%) and d m (RH) is the mobility diameter at increased relative humidity. Literature accounts suggest that submicron dry ammonium sulfate particles are slightly nonspherical, having a shape factor χ that ranges from.7 to.3 as particle size decreases from 5 to 6 nm (Zelenyuk et al., 26). The restructuring shown in Fig. 6 for increasing RH also demonstrates that the shape factor differs slightly from. (e.g.,.2; see further discussion in main text). The following development of the models nevertheless assumes that the dry ammonium sulfate particles have a unity shape factor, in which case d m,dry =d ve,dry, where d ve denotes the volume-equivalent diameter. After deliquescence, the aqueous particles are also spherical, implying that d m (RH)=d ve (RH). For these conditions, Eq. (A) then simplifies to g(rh)=d ve (RH)/d ve,dry. (For development of the case χ =., see Biskos et al., 26b). We consider two models of the mobility-diameter growth factor: the first omits the Kelvin effect and the second includes it (Biskos et al., 26a). Omission of the Kelvin effect implies that a w =RH/ for model, where a w is the water activity in the aqueous particles. Model is described by the following equation: g(rh)= d ( ) ve(rh) ρ /3 s =, (A2) d ve,dry w t (a w ) ρ(w t (a w )) where ρ s is the density of the dry particle, ρ(w t (a w )) is the density of the aqueous solution droplet, and w t (a w ) is the weight percent of solute in the aqueous particle. Equations for these quantities are given in Table. Model 2 expands on model by including the Kelvin effect, which relates RH to a w as follows: ( ) 4Mw σ aq (w t (a w )) RH= a w exp, (A3) RTρ w d ve (RH) where ρ w is the density of pure water, σ aq (w t (a w )) is the surface tension of the aqueous droplet (Table ), M w is the molar mass of water, R is the universal gas constant, and T is the temperature. Model 2 predicts g(rh) by solving for d ve (RH) and a w (RH) that simultaneously satisfy Eqs. (A2) and (A3). Plots of g(rh) based on models and 2 are shown in Fig. 5 as the solid and dot-dashed lines, respectively. Acknowledgements. This material is based upon work supported by the National Science Foundation under Grant No Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. We thank M. Wise and R. Bahadur for their useful discussion and suggestions. Edited by: J. Abbatt References Allan, J. D., Alfarra, M. R., Bower, K. N., Coe, H., Jayne, J. T., Worsnop, D. R., Aalto, P. P., Kulmala, M., Hyotylainen, T., Cavalli, F., and Laaksonen, A.: Size and composition measurements of background aerosol and new particle growth in a Finnish forest during QUEST 2 using an Aerodyne Aerosol Mass Spectrometer, Atmos. Chem. Phys., 6, , 26, Atmos. Chem. Phys., 6, , 26
9 G. Biskos et al.: Nanoparticle phase transitions 464 Ball, S. M., Hanson, D. R., Eisele, F. L., and McMurry, P. H.: Laboratory studies of particle nucleation: Initial results for H 2 SO 4, H 2 O, and NH 3 vapors, J. Geophys. Res., 4, , 999. Biskos, G., Malinowski, A., Russell, L. M., Buseck, P. R., and Martin, S. T.: Nanosize effect on the deliquescence and the efflorescence of sodium chloride particles, Aerosol Sci. Technol., 4, 97 6, 26a. Biskos, G., Russell, L. M., Buseck, P. R., and Martin, S. T.: Nanosize effect on the hygroscopic growth factor of aerosol particles, Geophys. Res. Lett., 33, L78, doi:.29/25gl2599, 26b. Buzorius, G., McNaughton, C. S., Clarke, A. D., Covert, D. S., Blomquist, B., Nielsen, K., and Brechtel, F. J.: Secondary aerosol formation in continental outflow conditions during ACE-Asia, J. Geophys. Res., 9, D2423, doi:.29/24jd4749, 24. Chen, D. R., Pui, D. Y. H., Hummes, D., Fissan, H., Quant, F. R., and Sem, G. J.: Design and evaluation of a nanometer aerosol differential mobility analyzer (Nano-DMA), J. Aerosol Sci., 29, , 998. Chen, J. P.: Theory of deliquescence and modified Köhler curves, J. Atmos. Sci., 5, , 994. Cohen, M. D., Flagan, R. C., and Seinfeld, J. H.: Studies of concentrated electrolyte solutions using the electrodynamic balance.. Water activities for single-electrolyte solutions, J. Phys. Chem., 9, , 987. Cubison, M. J., Coe, H., and Gysel, M.: A modified hygroscopic tandem DMA and a data retrieval method based on optimal estimation, J. Aerosol Sci., 36, , 25. Cziczo, D. J., Nowak, J. B., Hu, J. H., and Abbatt, J. D. P.: Infrared spectroscopy of model tropospheric aerosols as a function of relative humidity: Observation of deliquescence and crystallization, J. Geophys. Res., 2, , 997. Djikaev, Y. S., Bowles, R., Reiss, H., Hämeri, K., Laaksonen, A., and Väkevä, M.: Theory of size dependent deliquescence of nanoparticles: Relation to heterogeneous nucleation and comparison with experiments, J. Phys. Chem. B, 5, , 2. Gao, Y. G., Chen, S. B., and Yu, L. E.: Efflorescence relative humidity for ammonium sulfate particles, J. Phys. Chem.,, , 26. Hämeri, K., Laaksonen, A., Väkevä, M., and Suni, T.: Hygroscopic growth of ultrafine sodium chloride particles, J. Geophys. Res., 6, , 2. Hämeri, K., Väkevä, M., Hanson, H.-C., and Laaksonen, A.: Hygroscopic growth of ultrafine ammonium sulphate aerosol measured using an ultrafine tandem differential mobility analyzer, J. Geophys. Res., 5, , 2. Kinney, P. D., Pui, D. Y. H., Mulholland, G. W., and Bryner, N. P.: Use of the Electrostatic Classification Method to Size. µm SRM Particles A Feasibility Study, J. Res. Natl. Inst. Stan., 96, 47 76, 99. Kulmala, M., Vehkamäki, H., Petäjdä, T., Dal Maso, M., Lauri, A., Kerminen, V. M., Birmili, W., and McMurry, P. H.: Formation and growth rates of ultrafine atmospheric particles: a review of observations, J. Aerosol Sci., 35, 43 76, 24. Martin, S. T.: Phase transitions of aqueous atmospheric particles, Chem. Rev.,, , 2. McMurry, P. H.: A review of atmospheric aerosol measurements, Atmos. Environ., 34, , 2. Mikhailov, E., Vlasenko, S., Niessner, R., and Pöschl, U.: Interaction of aerosol particles composed of protein and salts with water vapor: hygroscopic growth and microstructural rearrangement, Atmos. Chem. Phys., 4, , 24, Mirabel, P., Reiss, H., and Bowles, R. K.: A theory for the deliquescence of small particles, J. Chem. Phys., 3, , 2. Napari, I., Noppel, M., Vehkamaki, H., and Kulmala, M.: An improved model for ternary nucleation of sulfuric acid-ammoniawater, J. Chem. Phys., 6, , 22. O Dowd, C. D., Aalto, P., Hämeri, K., Kulmala, M., and Hoffmann, T.: Aerosol formation Atmospheric particles from organic vapours, Nature, 46, , 22. Onasch, T. B., Siefert, R. L., Brooks, S. D., Prenni, A. J., Murray, B., Wilson, M. A., and Tolbert, M. A.: Infrared spectroscopic study of the deliquescence and efflorescence of ammonium sulfate aerosol as a function of temperature, J. Geophys. Res., 4, , 999. Perry, R. H. and Green, D. W.: Perry s Chemical Engineers Handbook, McGraw Hill, New York, 997. Pruppacher, H. R. and Klett, J. D.: Microphysics of Clouds and Precipitation, Kluwer Academic Publishers, Boston, 997. Rader, D. J. and McMurry, P. H.: Application of the tandem differential mobility analyzer to studies of droplet growth or evaporation, J. Aerosol Sci., 7, , 986. Romakkaniemi, S., Hämeri, K., Väkevä, M., and Laaksonen, A.: Adsorption of water on 8 5 nm NaCl and (NH 4 ) 2 SO 4 aerosols measured using an ultrafine tandem differential mobility analyzer, J. Phys. Chem. A, 5, , 2. Russell, L. M. and Ming, Y.: Deliquescence of small particles, J. Chem. Phys., 6, 3 32, 22. Sakurai, H., Fink, M. A., McMurry, P. H., Mauldin, L., Moore, K. F., Smith, J. N., and Eisele, F. L.: Hygroscopicity and volatility of 4 nm particles during summertime atmospheric nucleation events in urban Atlanta, J. Geophys. Res.,, D22S4, doi:.29/25jd598, 25. Smith, J. N., Moore, K. F., Eisele, F. L., Voisin, D., Ghimire, A. K., Sakurai, H., and McMurry, P. H.: Chemical composition of atmospheric nanoparticles during nucleation events in Atlanta, J. Geophys. Res.,, D22S3, doi:.29/25jd592, 25. Stolzenburg, M. R. and McMurry, P. H.: TDMAFIT User s Manual, Particle Technology Laboratory, Department of Mechanical Engineering, University of Minnesota, Minneapolis, MN, USA, 988. Tang, I. N. and Munkelwitz, H. R.: Aerosol phase-transformation and growth in the atmosphere, J. Appl. Meteorol., 33, , 994. Tang, I. N., Tridico, A. C., and Fung, K. H.: Thermodynamic and optical properties of sea salt aerosols, J. Geophys. Res., 2, , 997. Topping, D. O., McFiggans, G. B., and Coe, H.: A curved multicomponent aerosol hygroscopicity model framework: Part Inorganic compounds, Atmos. Chem. Phys., 5, , 25, Atmos. Chem. Phys., 6, , 26
10 4642 G. Biskos et al.: Nanoparticle phase transitions Weis, D. D. and Ewing, G. E.: Infrared spectroscopic signatures of (NH 4 ) 2 SO 4 aerosols, J. Geophys. Res.,, , 996. Wise, M. E., Biskos, G., Martin, S. T., Russell, L. M., and Buseck, P. R.: Phase transitions of single salt particles studied using a transmission electron microscope with an environmental cell, Aerosol Sci. Technol., 39, , 25. Zelenyuk, A., Cai, Y., and Imre, D.: From agglomerates of spheres to irregularly shaped particles: Determination of dynamic shape factors from measurements of mobility and vacuum aerodynamic diameters, Aerosol Sci. Technol., 4, 97 27, 26. Zhang, Q., Stanier, C. O., Canagaratna, M. R., Jayner, J. T., Wornsnop, D. R., Pandis, S. N., and Jimenez, J. L.: Insights into the chemistry of new particle formation and growth events in Pittsburgh based on aerosol mass spectrometry, Env. Sci. Technol., 38, , 24. Atmos. Chem. Phys., 6, , 26
Hygroscopicity of Inorganic Aerosols: Size and Relative Humidity Effects on the Growth Factor
 Aerosol and Air Quality Research, 10: 255 264, 2010 Copyright Taiwan Association for Aerosol Research ISSN: 1680-8584 print / 2071-1409 online doi: 10.4209/aaqr.2009.12.0076 Hygroscopicity of Inorganic
Aerosol and Air Quality Research, 10: 255 264, 2010 Copyright Taiwan Association for Aerosol Research ISSN: 1680-8584 print / 2071-1409 online doi: 10.4209/aaqr.2009.12.0076 Hygroscopicity of Inorganic
Title of file for HTML: Supplementary Information Description: Supplementary Figures, Supplementary Tables and Supplementary References
 Title of file for HTML: Supplementary Information Description: Supplementary Figures, Supplementary Tables and Supplementary References Title of file for HTML: Peer Review File Description: g e (RH) (bulk)
Title of file for HTML: Supplementary Information Description: Supplementary Figures, Supplementary Tables and Supplementary References Title of file for HTML: Peer Review File Description: g e (RH) (bulk)
Helsinki, Finland. 1 Aerodyne Research Inc., Billerica, MA, USA. 2 Chemistry Department, Boston College, Chestnut Hill, MA, USA
 Supplementary material to article: Relationship between aerosol oxidation level and hygroscopic properties of laboratory generated secondary organic aerosol (SOA) particles (paper #0GL0 to Geophysical
Supplementary material to article: Relationship between aerosol oxidation level and hygroscopic properties of laboratory generated secondary organic aerosol (SOA) particles (paper #0GL0 to Geophysical
Water adsorption on ammounium sulfate and silica nanoparticles. Keywords: nanoparticles, hygroscopic growth, water monolayers INTRODUCTION
 Water adsorption on ammounium sulfate and silica nanoparticles Keskinen Helmi 1, Jaatinen Antti 1, Miettinen Pasi 1, Romakkaniemi Sami 1, Joutsensaari Jorma 1, Smith James N. 1, and Laaksonen Ari 1,3 1
Water adsorption on ammounium sulfate and silica nanoparticles Keskinen Helmi 1, Jaatinen Antti 1, Miettinen Pasi 1, Romakkaniemi Sami 1, Joutsensaari Jorma 1, Smith James N. 1, and Laaksonen Ari 1,3 1
Jianfei Peng et al. Correspondence to: Jianfei Peng Min Hu and Renyi Zhang
 Supplement of Atmos. Chem. Phys., 17, 10333 10348, 2017 https://doi.org/10.5194/acp-17-10333-2017-supplement Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License.
Supplement of Atmos. Chem. Phys., 17, 10333 10348, 2017 https://doi.org/10.5194/acp-17-10333-2017-supplement Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License.
Particle counting efficiencies of new TSI condensation particle counters
 Aerosol Science 38 (27) 674 682 www.elsevier.com/locate/jaerosci Technical note Particle counting efficiencies of new TSI condensation particle counters M. Hermann a,, B. Wehner a, O. Bischof b, H.-S.
Aerosol Science 38 (27) 674 682 www.elsevier.com/locate/jaerosci Technical note Particle counting efficiencies of new TSI condensation particle counters M. Hermann a,, B. Wehner a, O. Bischof b, H.-S.
COPLEY S C I E N T I F I C. A multi-function aerosol system with aerosol generation, classification and monitoring capabilities for:
 A multi-function aerosol system with aerosol generation, classification and monitoring capabilities for: generating monodisperse aerosol by mobility classification with automatic concentration detection
A multi-function aerosol system with aerosol generation, classification and monitoring capabilities for: generating monodisperse aerosol by mobility classification with automatic concentration detection
Parameterization of the nitric acid effect on CCN activation
 Atmos. Chem. Phys., 5, 879 885, 25 SRef-ID: 168-7324/acp/25-5-879 European Geosciences Union Atmospheric Chemistry and Physics Parameterization of the nitric acid effect on CCN activation S. Romakkaniemi,
Atmos. Chem. Phys., 5, 879 885, 25 SRef-ID: 168-7324/acp/25-5-879 European Geosciences Union Atmospheric Chemistry and Physics Parameterization of the nitric acid effect on CCN activation S. Romakkaniemi,
ELECTROSTATIC CLASSIFIER MODEL 3082
 ELECTROSTATIC CLASSIFIER MODEL 3082 THESE INSTRUMENTS HAVE BEEN USED IN A BROAD VARIETY OF RESEARCH AND HAVE EARNED A WELL-DESERVED REPUTATION FOR BEING HIGHLY RELIABLE AND EXTREMELY VERSATILE. UNDERSTANDING,
ELECTROSTATIC CLASSIFIER MODEL 3082 THESE INSTRUMENTS HAVE BEEN USED IN A BROAD VARIETY OF RESEARCH AND HAVE EARNED A WELL-DESERVED REPUTATION FOR BEING HIGHLY RELIABLE AND EXTREMELY VERSATILE. UNDERSTANDING,
Using TDMAs to make Measurements of Haze
 Welcome! 欢迎! 歓迎! 환영! Добро пожаловать! स व गत! Using TDMAs to make Measurements of Haze This webinar will begin at: Greenwich Mean Time (GMT) Thursday, 1:00am Beijing, China 8:00am Tokyo, Japan 9:00am
Welcome! 欢迎! 歓迎! 환영! Добро пожаловать! स व गत! Using TDMAs to make Measurements of Haze This webinar will begin at: Greenwich Mean Time (GMT) Thursday, 1:00am Beijing, China 8:00am Tokyo, Japan 9:00am
Supplement of Total sulfate vs. sulfuric acid monomer concenterations in nucleation studies
 Supplement of Atmos. Chem. Phys., 15, 3429 3443, 2015 http://www.atmos-chem-phys.net/15/3429/2015/ doi:10.5194/acp-15-3429-2015-supplement Author(s) 2015. CC Attribution 3.0 License. Supplement of Total
Supplement of Atmos. Chem. Phys., 15, 3429 3443, 2015 http://www.atmos-chem-phys.net/15/3429/2015/ doi:10.5194/acp-15-3429-2015-supplement Author(s) 2015. CC Attribution 3.0 License. Supplement of Total
Causes of Concentration Differences Between a Scanning Mobility Particle Sizer and a Condensation Particle Counter
 Aerosol Science and Technology, 37:916 923, 2003 Copyright c American Association for Aerosol Research ISSN: 0278-6826 print / 1521-7388 online DOI: 10.1080/02786820390229057 Causes of Concentration Differences
Aerosol Science and Technology, 37:916 923, 2003 Copyright c American Association for Aerosol Research ISSN: 0278-6826 print / 1521-7388 online DOI: 10.1080/02786820390229057 Causes of Concentration Differences
A New Volatility Tandem Differential Mobility Analyzer to Measure the Volatile Sulfuric Acid Aerosol Fraction
 760 JOURNAL OF ATMOSPHERIC AND OCEANIC TECHNOLOGY VOLUME 16 A New Volatility Tandem Differential Mobility Analyzer to Measure the Volatile Sulfuric Acid Aerosol Fraction D. A. ORSINI, A.WIEDENSOHLER, AND
760 JOURNAL OF ATMOSPHERIC AND OCEANIC TECHNOLOGY VOLUME 16 A New Volatility Tandem Differential Mobility Analyzer to Measure the Volatile Sulfuric Acid Aerosol Fraction D. A. ORSINI, A.WIEDENSOHLER, AND
Effect of ammonium bisulphate formation on atmospheric water-sulphuric acid-ammonia nucleation
 BOREAL ENVIRONMENT RESEARCH 10: 511 523 ISSN 1239-6095 Helsinki 14 December 2005 2005 Effect of ammonium bisulphate formation on atmospheric water-sulphuric acid-ammonia nucleation Tatu Anttila 1)2), Hanna
BOREAL ENVIRONMENT RESEARCH 10: 511 523 ISSN 1239-6095 Helsinki 14 December 2005 2005 Effect of ammonium bisulphate formation on atmospheric water-sulphuric acid-ammonia nucleation Tatu Anttila 1)2), Hanna
Chapter 5 Conclusions and Future Studies
 177 Chapter 5 Conclusions and Future Studies 178 Conclusions and Future Studies The results of the studies presented in this thesis confirm that ambient and laboratory-generated aerosols exhibit complex
177 Chapter 5 Conclusions and Future Studies 178 Conclusions and Future Studies The results of the studies presented in this thesis confirm that ambient and laboratory-generated aerosols exhibit complex
Differential Mobility Particle Sizer (Aerosol measurements)
 Institute for Atmospheric and Climate Science - IACETH Atmospheric Physics Lab Work Differential Mobility Particle Sizer (Aerosol measurements) Abstract A differential mobility particle sizer (DMPS) is
Institute for Atmospheric and Climate Science - IACETH Atmospheric Physics Lab Work Differential Mobility Particle Sizer (Aerosol measurements) Abstract A differential mobility particle sizer (DMPS) is
Results and recommendations from an intercomparison of six Hygroscopicity-TDMA systems
 Atmos. Meas. Tech.,, 85 97, 0 www.atmos-meas-tech.net//85/0/ doi:0.59/amt--85-0 Author(s) 0. CC Attribution.0 License. Atmospheric Measurement Techniques Results and recommendations from an intercomparison
Atmos. Meas. Tech.,, 85 97, 0 www.atmos-meas-tech.net//85/0/ doi:0.59/amt--85-0 Author(s) 0. CC Attribution.0 License. Atmospheric Measurement Techniques Results and recommendations from an intercomparison
CCN activation experiments with adipic acid: effect of particle phase and adipic acid coatings on soluble and insoluble particles
 Atmos. Chem. Phys., 8, 3735 3748, 2008 Author(s) 2008. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics CCN activation experiments with adipic
Atmos. Chem. Phys., 8, 3735 3748, 2008 Author(s) 2008. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics CCN activation experiments with adipic
A novel tandem differential mobility analyzer with organic vapor treatment of aerosol particles
 Atmospheric Chemistry and Physics A novel tandem differential mobility analyzer with organic vapor treatment of aerosol particles J. Joutsensaari 1, P. Vaattovaara 1, M. Vesterinen 1, K. Hämeri 2, and
Atmospheric Chemistry and Physics A novel tandem differential mobility analyzer with organic vapor treatment of aerosol particles J. Joutsensaari 1, P. Vaattovaara 1, M. Vesterinen 1, K. Hämeri 2, and
Rapid Measurements of Aerosol Size Distributions Using a Fast Integrated Mobility Spectrometer (FIMS)
 Rapid Measurements of Aerosol Size Distributions Using a Fast Integrated Mobility Spectrometer (FIMS) Jason Olfert, Brookhaven National Laboratory Jian Wang, Brookhaven National Laboratory Measurement
Rapid Measurements of Aerosol Size Distributions Using a Fast Integrated Mobility Spectrometer (FIMS) Jason Olfert, Brookhaven National Laboratory Jian Wang, Brookhaven National Laboratory Measurement
Mobility-Based Particle Size Classification and Detection down to 2nm Theory to Practice
 Mobility-Based Particle Size Classification and Detection down to 2nm Theory to Practice Robert C. Anderson Presented at SEMATECH November 12, 2012 Current and Future Defectively Issues from Components
Mobility-Based Particle Size Classification and Detection down to 2nm Theory to Practice Robert C. Anderson Presented at SEMATECH November 12, 2012 Current and Future Defectively Issues from Components
Effect of aging on cloud nucleating properties of atmospheric aerosols
 Effect of aging on cloud nucleating properties of atmospheric aerosols Yan Ma Nanjing University of Information Science & Technology The 1st Regional GEOS-Chem Asia Meeting 2015 年 5 月 8 日 Indirect effect
Effect of aging on cloud nucleating properties of atmospheric aerosols Yan Ma Nanjing University of Information Science & Technology The 1st Regional GEOS-Chem Asia Meeting 2015 年 5 月 8 日 Indirect effect
The effect of surface tension (Kelvin effect) on the equilibrium radius of a hygroscopic aqueous aerosol particle
 Aerosol Science 37 (006) 1605 1617 www.elsevier.com/locate/jaerosci The effect of surface tension (Kelvin effect) on the equilibrium radius of a hygroscopic aqueous aerosol particle Ernie R. Lewis Atmospheric
Aerosol Science 37 (006) 1605 1617 www.elsevier.com/locate/jaerosci The effect of surface tension (Kelvin effect) on the equilibrium radius of a hygroscopic aqueous aerosol particle Ernie R. Lewis Atmospheric
Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes
 DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes John H. Seinfeld California Institute of Technology,
DISTRIBUTION STATEMENT A. Approved for public release; distribution is unlimited. Influence of Organic-Containing Aerosols on Marine Boundary Layer Processes John H. Seinfeld California Institute of Technology,
Chasing Aerosol Particles Down to Nano Sizes
 Chasing Aerosol Particles Down to Nano Sizes ERC Seminar 13 June 2013 Armin Sorooshian Chemical & Environmental Engineering Atmospheric Sciences University of Arizona Outline of Talk 1. What are aerosol
Chasing Aerosol Particles Down to Nano Sizes ERC Seminar 13 June 2013 Armin Sorooshian Chemical & Environmental Engineering Atmospheric Sciences University of Arizona Outline of Talk 1. What are aerosol
Comparison of AERONET inverted size distributions to measured distributions from the Aerodyne Aerosol Mass Spectrometer
 Comparison of inverted size distributions to measured distributions from the Aerodyne Aerosol Mass Spectrometer Peter DeCarlo Remote Sensing Project April 28, 23 Introduction The comparison of direct in-situ
Comparison of inverted size distributions to measured distributions from the Aerodyne Aerosol Mass Spectrometer Peter DeCarlo Remote Sensing Project April 28, 23 Introduction The comparison of direct in-situ
Particuology 9 (2011) Contents lists available at SciVerse ScienceDirect. Particuology. j o ur nal homep ag e:
 Particuology 9 (2011) 606 610 Contents lists available at SciVerse ScienceDirect Particuology j o ur nal homep ag e: www.elsevier.com/locate/partic Evaluation of particle growth systems for sampling and
Particuology 9 (2011) 606 610 Contents lists available at SciVerse ScienceDirect Particuology j o ur nal homep ag e: www.elsevier.com/locate/partic Evaluation of particle growth systems for sampling and
Water activity and activation diameters from hygroscopicity data - Part II: Application to organic species
 Water activity and activation diameters from hygroscopicity data - Part II: Application to organic species K. A. Koehler, S. M. Kreidenweis, P. J. Demott, A. J. Prenni, C. M. Carrico, B. Ervens, G. Feingold
Water activity and activation diameters from hygroscopicity data - Part II: Application to organic species K. A. Koehler, S. M. Kreidenweis, P. J. Demott, A. J. Prenni, C. M. Carrico, B. Ervens, G. Feingold
Ammonium Bisulfate/Water Equilibrium and Metastability Phase Diagrams
 J. Phys. Chem. A 1997, 101, 4191-4195 4191 Ammonium Bisulfate/Water Equilibrium and Metastability Phase Diagrams Dan G. Imre,*, Jun Xu, I. N. Tang, and R. McGraw EnVironmental Chemistry DiVision, Department
J. Phys. Chem. A 1997, 101, 4191-4195 4191 Ammonium Bisulfate/Water Equilibrium and Metastability Phase Diagrams Dan G. Imre,*, Jun Xu, I. N. Tang, and R. McGraw EnVironmental Chemistry DiVision, Department
Phase Transformations of the Ternary System (NH 4 ) 2 SO 4 - H 2 SO 4 -H 2 O and the Implications for Cirrus Cloud Formation
 Phase Transformations of the Ternary System (NH 4 ) 2 SO 4 - H 2 SO 4 -H 2 O and the Implications for Cirrus Cloud Formation Scot T. Martin Department of Environmental Sciences and Engineering, The University
Phase Transformations of the Ternary System (NH 4 ) 2 SO 4 - H 2 SO 4 -H 2 O and the Implications for Cirrus Cloud Formation Scot T. Martin Department of Environmental Sciences and Engineering, The University
Phase transitions and hygroscopic growth of aerosol particles containing humic acid and mixtures of humic acid and ammonium sulphate
 Atmos. Chem. Phys., 6, 755 768, 2006 Author(s) 2006. This work is licensed under a Creative Commons License. Atmospheric Chemistry and Physics Phase transitions and hygroscopic growth of aerosol particles
Atmos. Chem. Phys., 6, 755 768, 2006 Author(s) 2006. This work is licensed under a Creative Commons License. Atmospheric Chemistry and Physics Phase transitions and hygroscopic growth of aerosol particles
Aerosol Dynamics. Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008
 Aerosol Dynamics Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of Helsinki Aerosol Dynamics: What? A way to
Aerosol Dynamics Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of Helsinki Aerosol Dynamics: What? A way to
Urban background aerosols: Negative correlations of particle modes and fragmentation mechanism
 Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 34, L11811, doi:10.1029/2006gl029109, 2007 Urban background aerosols: Negative correlations of particle modes and fragmentation mechanism
Click Here for Full Article GEOPHYSICAL RESEARCH LETTERS, VOL. 34, L11811, doi:10.1029/2006gl029109, 2007 Urban background aerosols: Negative correlations of particle modes and fragmentation mechanism
Instrumentational operation and analytical methodology for the reconciliation of aerosol water uptake under sub- and supersaturated conditions
 doi:10.519/amt-3-11-010 Author(s) 010. CC Attribution 3.0 License. Atmospheric Measurement Techniques Instrumentational operation and analytical methodology for the reconciliation of aerosol water uptake
doi:10.519/amt-3-11-010 Author(s) 010. CC Attribution 3.0 License. Atmospheric Measurement Techniques Instrumentational operation and analytical methodology for the reconciliation of aerosol water uptake
Ultrafine Aerosol Size Distribution: A Study of New Particle Formation in the Atmosphere
 . Ultrafine Aerosol Size Distribution: A Study of New Particle Formation in the Atmosphere Report on DOE Grant Number: DE-FG02-9 ler61205 PreDared for: U. S. Department of Energy ATTN: Fred Sienko, Contracting
. Ultrafine Aerosol Size Distribution: A Study of New Particle Formation in the Atmosphere Report on DOE Grant Number: DE-FG02-9 ler61205 PreDared for: U. S. Department of Energy ATTN: Fred Sienko, Contracting
Organic aerosol formation via sulphate cluster activation
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 109,, doi:10.1029/2003jd003961, 2004 Organic aerosol formation via sulphate cluster activation Markku Kulmala, 1 Veli-Matti Kerminen, 2 Tatu Anttila, 1,2 Ari Laaksonen,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 109,, doi:10.1029/2003jd003961, 2004 Organic aerosol formation via sulphate cluster activation Markku Kulmala, 1 Veli-Matti Kerminen, 2 Tatu Anttila, 1,2 Ari Laaksonen,
Supplementary information
 1 Supplementary information Instrument and operation 3 6 7 8 9 10 11 1 13 1 1 16 HTDMA: Briefly, the custom-built HTDMA consists of two long DMAs (3081L, TSI Inc.), a humidifier (PD-0T-1MSS, Perma Pure
1 Supplementary information Instrument and operation 3 6 7 8 9 10 11 1 13 1 1 16 HTDMA: Briefly, the custom-built HTDMA consists of two long DMAs (3081L, TSI Inc.), a humidifier (PD-0T-1MSS, Perma Pure
Evaluation of the NanoAerosol Generator by Kanomax FMT Inc. in Aerosolization of Size Standard Nanoparticle and Protein
 Aerosol and Air Quality Research, 18: 549 554, 2018 Copyright Taiwan Association for Aerosol Research ISSN: 1680-8584 print / 2071-1409 online doi: 10.4209/aaqr.2017.09.0352 Technical Note Evaluation of
Aerosol and Air Quality Research, 18: 549 554, 2018 Copyright Taiwan Association for Aerosol Research ISSN: 1680-8584 print / 2071-1409 online doi: 10.4209/aaqr.2017.09.0352 Technical Note Evaluation of
Hygroscopic growth of ammonium sulfate/dicarboxylic acids
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D20, 4638, doi:10.1029/2003jd003775, 2003 Hygroscopic growth of ammonium sulfate/dicarboxylic acids Matthew E. Wise, Jason D. Surratt, Daniel B. Curtis, John
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D20, 4638, doi:10.1029/2003jd003775, 2003 Hygroscopic growth of ammonium sulfate/dicarboxylic acids Matthew E. Wise, Jason D. Surratt, Daniel B. Curtis, John
Slides partly by Antti Lauri and Hannele Korhonen. Liquid or solid particles suspended in a carrier gas Described by their
 Atmospheric Aerosols Slides partly by Antti Lauri and Hannele Korhonen Aerosol particles Liquid or solid particles suspended in a carrier gas Described by their Size Concentration - Number - Surface -
Atmospheric Aerosols Slides partly by Antti Lauri and Hannele Korhonen Aerosol particles Liquid or solid particles suspended in a carrier gas Described by their Size Concentration - Number - Surface -
An adsorption model of insoluble particle activation: Application to black carbon
 Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2007jd008549, 2007 An adsorption model of insoluble particle activation: Application to black carbon B. F. Henson 1 Received
Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2007jd008549, 2007 An adsorption model of insoluble particle activation: Application to black carbon B. F. Henson 1 Received
Aerosol chemistry and climate: Laboratory studies of the carbonate component of mineral dust and its reaction products
 GEOPHYSICAL RESEARCH LETTERS, VOL. 33, L13811, doi:10.1029/2006gl026386, 2006 Aerosol chemistry and climate: Laboratory studies of the carbonate component of mineral dust and its reaction products Elizabeth
GEOPHYSICAL RESEARCH LETTERS, VOL. 33, L13811, doi:10.1029/2006gl026386, 2006 Aerosol chemistry and climate: Laboratory studies of the carbonate component of mineral dust and its reaction products Elizabeth
LACIS-measurements and parameterization of sea-salt particle hygroscopic growth and activation
 Atmos. Chem. Phys., 8, 579 590, 2008 Author(s) 2008. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics LACIS-measurements and parameterization
Atmos. Chem. Phys., 8, 579 590, 2008 Author(s) 2008. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics LACIS-measurements and parameterization
Generation and Evaluation of Monodisperse Sodium Chloride and Oleic Acid Nanoparticles
 Generation and Evaluation of Monodisperse Sodium Chloride and Oleic Acid Nanoparticles Tzu Ming Chen, Hung Min Chein Energy & Environment Research Laboratories, Industrial Technology Research Institute,
Generation and Evaluation of Monodisperse Sodium Chloride and Oleic Acid Nanoparticles Tzu Ming Chen, Hung Min Chein Energy & Environment Research Laboratories, Industrial Technology Research Institute,
Charged and total particle formation and growth rates during EUCAARI 2007 campaign in Hyytiälä
 Atmos. Chem. Phys., 9, 4077 4089, 2009 Author(s) 2009. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics Charged and total particle formation
Atmos. Chem. Phys., 9, 4077 4089, 2009 Author(s) 2009. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics Charged and total particle formation
Chapter Four: Laboratory Characterisation of the Aerodyne Aerosol Mass Spectrometer
 24 PhD Thesis 79 Chapter Four: Laboratory Characterisation of the Aerodyne Aerosol Mass Spectrometer Laboratory characterisation of the Aerodyne aerosol mass spectrometer has been an important part of
24 PhD Thesis 79 Chapter Four: Laboratory Characterisation of the Aerodyne Aerosol Mass Spectrometer Laboratory characterisation of the Aerodyne aerosol mass spectrometer has been an important part of
APPLICATION OF KOHLER THEORY: MODELING CLOUD CONDENSATION NUCLEI ACTIVITY
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 APPLICATION OF KOHLER THEORY: MODELING CLOUD CONDENSATION NUCLEI ACTIVITY Gavin Cornwell, Katherine Nadler, Alex Nguyen, and Steven Schill Department of
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 APPLICATION OF KOHLER THEORY: MODELING CLOUD CONDENSATION NUCLEI ACTIVITY Gavin Cornwell, Katherine Nadler, Alex Nguyen, and Steven Schill Department of
Modelling the formation of organic particles in the atmosphere
 Atmos. Chem. Phys., 4, 1071 1083, 2004 SRef-ID: 1680-7324/acp/2004-4-1071 Atmospheric Chemistry and Physics Modelling the formation of organic particles in the atmosphere T. Anttila 1,2, V.-M. Kerminen
Atmos. Chem. Phys., 4, 1071 1083, 2004 SRef-ID: 1680-7324/acp/2004-4-1071 Atmospheric Chemistry and Physics Modelling the formation of organic particles in the atmosphere T. Anttila 1,2, V.-M. Kerminen
Measured and modeled equilibrium sizes of NaCl and (NH 4 ) 2 SO 4 particles at relative humidities up to 99.1%
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi:10.1029/2004jd005507, 2005 Measured and modeled equilibrium sizes of NaCl and (NH 4 ) 2 SO 4 particles at relative humidities up to 99.1% H. Wex, A. Kiselev,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi:10.1029/2004jd005507, 2005 Measured and modeled equilibrium sizes of NaCl and (NH 4 ) 2 SO 4 particles at relative humidities up to 99.1% H. Wex, A. Kiselev,
Chapter 4 Hygroscopicity Measurements of Atmospherically Relevant Organic Aerosol Species *
 109 Chapter 4 Hygroscopicity Measurements of Atmospherically Relevant Organic Aerosol Species * * This chapter is prepared for journal submission as Hygroscopicity Measurements of Atmospherically Relevant
109 Chapter 4 Hygroscopicity Measurements of Atmospherically Relevant Organic Aerosol Species * * This chapter is prepared for journal submission as Hygroscopicity Measurements of Atmospherically Relevant
STUDY OF AIR IONS AND NANOMETER PARTICLES IN THE ATMOSPHERE DURING NUCLEATION BURST EVENTS
 STUDY OF AIR IONS AND NANOMETER PARTICLES IN THE ATMOSPHERE DURING NUCLEATION BURST EVENTS U. HÕRRAK 1,2, H. TAMMET 1, P.P. AALTO 2, L. LAAKSO 2 and M. KULMALA 2 1 Institute of Environmental Physics, University
STUDY OF AIR IONS AND NANOMETER PARTICLES IN THE ATMOSPHERE DURING NUCLEATION BURST EVENTS U. HÕRRAK 1,2, H. TAMMET 1, P.P. AALTO 2, L. LAAKSO 2 and M. KULMALA 2 1 Institute of Environmental Physics, University
Particle Size Distribution Measurements with the Novel 1 nm-smps
 Particle Size Distribution Measurements with the Novel 1 nm-smps Fast Scanning at few Nanometers Torsten Tritscher 1 F. Dahlkötter 1, C. Kykal 1, J.H.T. Scheckman 1, J. Spielvogel 1, T. Krinke 1, E. Filimundi
Particle Size Distribution Measurements with the Novel 1 nm-smps Fast Scanning at few Nanometers Torsten Tritscher 1 F. Dahlkötter 1, C. Kykal 1, J.H.T. Scheckman 1, J. Spielvogel 1, T. Krinke 1, E. Filimundi
Analysis of Data from the 2009 SOOT Experiment
 Analysis of Data from the 2009 SOOT Experiment Renyi Zhang Department of Atmospheric Sciences and Department of Chemistry Center for Atmospheric Chemistry and the Environment Texas A&M University College
Analysis of Data from the 2009 SOOT Experiment Renyi Zhang Department of Atmospheric Sciences and Department of Chemistry Center for Atmospheric Chemistry and the Environment Texas A&M University College
Aerosol Basics: Definitions, size distributions, structure
 Aerosol Basics: Definitions, size distributions, structure Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of
Aerosol Basics: Definitions, size distributions, structure Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of
ASSESSMENT OF THE MIXING STATE AND CLOUD NUCLEATING EFFICIENCY OF ASIAN AEROSOLS USING AIRCRAFT-BASED MEASUREMENTS OF HYGROSCOPICITY.
 ASSESSMENT OF THE MIXING STATE AND CLOUD NUCLEATING EFFICIENCY OF ASIAN AEROSOLS USING AIRCRAFT-BASED MEASUREMENTS OF HYGROSCOPICITY A Thesis by TIMOTHY WILLIAM THOMAS Submitted to the Office of Graduate
ASSESSMENT OF THE MIXING STATE AND CLOUD NUCLEATING EFFICIENCY OF ASIAN AEROSOLS USING AIRCRAFT-BASED MEASUREMENTS OF HYGROSCOPICITY A Thesis by TIMOTHY WILLIAM THOMAS Submitted to the Office of Graduate
Atmospheric Chemistry and Physics
 Atmos. Chem. Phys.,, 7489 753, 2 www.atmos-chem-phys.net//7489/2/ doi:.594/acp--7489-2 Author(s) 2. CC Attribution 3. License. Atmospheric Chemistry and Physics Hygroscopicity distribution concept for
Atmos. Chem. Phys.,, 7489 753, 2 www.atmos-chem-phys.net//7489/2/ doi:.594/acp--7489-2 Author(s) 2. CC Attribution 3. License. Atmospheric Chemistry and Physics Hygroscopicity distribution concept for
Water content of ambient aerosol during the Pittsburgh Air Quality Study
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi:10.1029/2004jd004651, 2005 Water content of ambient aerosol during the Pittsburgh Air Quality Study Andrey Khlystov Department of Civil and Environmental
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi:10.1029/2004jd004651, 2005 Water content of ambient aerosol during the Pittsburgh Air Quality Study Andrey Khlystov Department of Civil and Environmental
CHAPTER 8. AEROSOLS 8.1 SOURCES AND SINKS OF AEROSOLS
 1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
Intercomparison of Mobility Particle Size Spectrometers
 Intercomparison of Mobility Particle Size Spectrometers Project No.: MPSS-2015-1-1 Basic information: Location of the quality assurance: TROPOS, lab: 118 Delivery date: October 05, 2015 Setup in the laboratory:
Intercomparison of Mobility Particle Size Spectrometers Project No.: MPSS-2015-1-1 Basic information: Location of the quality assurance: TROPOS, lab: 118 Delivery date: October 05, 2015 Setup in the laboratory:
Heterogeneous ice nucleation ability of crystalline sodium chloride dihydrate particles
 JOURNAL OF GEOPHYSICAL RESEARCH: ATMOSPHERES, VOL. 118, 4610 4622, doi:10.1002/jgrd.50325, 2013 Heterogeneous ice nucleation ability of crystalline sodium chloride dihydrate particles Robert Wagner 1 and
JOURNAL OF GEOPHYSICAL RESEARCH: ATMOSPHERES, VOL. 118, 4610 4622, doi:10.1002/jgrd.50325, 2013 Heterogeneous ice nucleation ability of crystalline sodium chloride dihydrate particles Robert Wagner 1 and
Comprehensive Measurement of Atmospheric Aerosols with a Wide Range Aerosol Spectrometer
 Comprehensive Measurement of Atmospheric Aerosols with a Wide Range Aerosol Spectrometer Juergen Spielvogel, Lothar Keck, Xiaoai Guo, Markus Pesch Email: jsp@grimm-aerosol.com GRIMM Aerosol Technik GmbH
Comprehensive Measurement of Atmospheric Aerosols with a Wide Range Aerosol Spectrometer Juergen Spielvogel, Lothar Keck, Xiaoai Guo, Markus Pesch Email: jsp@grimm-aerosol.com GRIMM Aerosol Technik GmbH
Aerosol hygroscopicity at high (99 to 100%) relative humidities
 Atmos. Chem. Phys., 10, 139 1344, 010 www.atmos-chem-phys.net/10/139/010/ Author(s) 010. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics Aerosol
Atmos. Chem. Phys., 10, 139 1344, 010 www.atmos-chem-phys.net/10/139/010/ Author(s) 010. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics Aerosol
Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables
 Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2008jd010527, 2008 Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables Fangqun Yu 1 Received 2 June
Click Here for Full Article JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 113,, doi:10.1029/2008jd010527, 2008 Updated H 2 SO 4 -H 2 O binary homogeneous nucleation look-up tables Fangqun Yu 1 Received 2 June
Chapter 3 New Particle Formation from Photooxidation of Diiodomethane (CH 2 I 2 )*
 23 Chapter 3 New Particle Formation from Photooxidation of Diiodomethane (CH 2 I 2 )* * This chapter is reproduced by permission from New Particle Formation from Photooxidation of Diiodomethane (CH 2 I
23 Chapter 3 New Particle Formation from Photooxidation of Diiodomethane (CH 2 I 2 )* * This chapter is reproduced by permission from New Particle Formation from Photooxidation of Diiodomethane (CH 2 I
Simulating aerosol nucleation bursts in a coniferous forest
 Boreal Environment Research 12: 421 430 issn 1239-6095 Helsinki 25 June 2007 2007 Simulating aerosol nucleation bursts in a coniferous forest Hannes Tammet 1) and Markku Kulmala 2) 1) Institute of Environmental
Boreal Environment Research 12: 421 430 issn 1239-6095 Helsinki 25 June 2007 2007 Simulating aerosol nucleation bursts in a coniferous forest Hannes Tammet 1) and Markku Kulmala 2) 1) Institute of Environmental
A criterion for new particle formation in the sulfur-rich Atlanta atmosphere
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi:10.1029/2005jd005901, 2005 A criterion for new particle formation in the sulfur-rich Atlanta atmosphere P. H. McMurry, 1 M. Fink, 1 H. Sakurai, 1 M. R. Stolzenburg,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 110,, doi:10.1029/2005jd005901, 2005 A criterion for new particle formation in the sulfur-rich Atlanta atmosphere P. H. McMurry, 1 M. Fink, 1 H. Sakurai, 1 M. R. Stolzenburg,
4 th AMS Users Meeting Brainstorming on (1) Experiments to Nail our Absolute Quantification (2) Best Operating Procedures
 th AMS Users Meeting Brainstorming on (1) Experiments to Nail our Absolute Quantification () Best Operating Procedures Jose-Luis Jimenez Caltech Pasadena, CA Oct. -7, Reminder of the Quantification Issues.
th AMS Users Meeting Brainstorming on (1) Experiments to Nail our Absolute Quantification () Best Operating Procedures Jose-Luis Jimenez Caltech Pasadena, CA Oct. -7, Reminder of the Quantification Issues.
Quasi-unary homogeneous nucleation of H 2 SO 4 -H 2 O
 THE JOURNAL OF CHEMICAL PHYSICS 122, 074501 2005 Quasi-unary homogeneous nucleation of H 2 SO 4 -H 2 O Fangqun Yu a Atmospheric Sciences Research Center, State University of New York at Albany, 251 Fuller
THE JOURNAL OF CHEMICAL PHYSICS 122, 074501 2005 Quasi-unary homogeneous nucleation of H 2 SO 4 -H 2 O Fangqun Yu a Atmospheric Sciences Research Center, State University of New York at Albany, 251 Fuller
Highly Biased Hygroscopicity Derived from Size-Resolved Cloud Condensation Nuclei Activation Ratios without Data Inversion
 ATMOSPHERIC AND OCEANIC SCIENCE LETTERS, 2014, VOL. 7, NO. 3, 254 259 Highly Biased Hygroscopicity Derived from Size-Resolved Cloud Condensation Nuclei Activation Ratios without Data Inversion DENG Zhao-Ze
ATMOSPHERIC AND OCEANIC SCIENCE LETTERS, 2014, VOL. 7, NO. 3, 254 259 Highly Biased Hygroscopicity Derived from Size-Resolved Cloud Condensation Nuclei Activation Ratios without Data Inversion DENG Zhao-Ze
USING THE ELECTRET FILTER TO REMOVE THE SUBMICRON AEROSOLS
 USING THE ELECTRET FILTER TO REMOVE THE SUBMICRON AEROSOLS SH Yang 1,*, GWM Lee 1, CH Luo 2, CC Wu 1, KP Yu 1, CL Lou 1 1 Graduate Institute of Environmental Engineering, National Taiwan University, 71,
USING THE ELECTRET FILTER TO REMOVE THE SUBMICRON AEROSOLS SH Yang 1,*, GWM Lee 1, CH Luo 2, CC Wu 1, KP Yu 1, CL Lou 1 1 Graduate Institute of Environmental Engineering, National Taiwan University, 71,
Intercomparison of Mobility Particle Size Spectrometers
 Intercomparison of Mobility Particle Size Spectrometers Project No.: MPSS- 201- - 1 Basic information: Principal Investigator: Home Institution: Participant: Instrument No.1: Eija Asmi Finnish Meteorological
Intercomparison of Mobility Particle Size Spectrometers Project No.: MPSS- 201- - 1 Basic information: Principal Investigator: Home Institution: Participant: Instrument No.1: Eija Asmi Finnish Meteorological
Hygroscopic Growth of Aerosols and their Optical Properties
 Hygroscopic Growth of Aerosols and their Optical Properties Cynthia Randles Atmospheric & Oceanic Sciences Princeton University V. Ramaswamy and L. M. Russell ! Introduction Presentation Overview! Aerosol
Hygroscopic Growth of Aerosols and their Optical Properties Cynthia Randles Atmospheric & Oceanic Sciences Princeton University V. Ramaswamy and L. M. Russell ! Introduction Presentation Overview! Aerosol
Atmospheric Aerosols: Size Distributions and Composition
 2.1 Atmospheric Aerosols: Size Distributions and Composition The Table below (from Seinfeld and Pandis) lists typical properties of different types of atmospheric model aerosols. Note that the characteristics
2.1 Atmospheric Aerosols: Size Distributions and Composition The Table below (from Seinfeld and Pandis) lists typical properties of different types of atmospheric model aerosols. Note that the characteristics
Supplemental Material for Elemental Composition and Oxidation of Chamber Organic Aerosol
 Supplemental Material for Elemental Composition and Oxidation of Chamber Organic Aerosol P. S. Chhabra 1,N.L.Ng 2, M. R. Canagaratna 2, A. L. Corrigan 3, L. M. Russell 3, D. R. Worsnop 2, R. C. Flagan
Supplemental Material for Elemental Composition and Oxidation of Chamber Organic Aerosol P. S. Chhabra 1,N.L.Ng 2, M. R. Canagaratna 2, A. L. Corrigan 3, L. M. Russell 3, D. R. Worsnop 2, R. C. Flagan
Study of the Mechanism of Nucleation in the Polluted Atmospheric Boundary Layer
 Study of the Mechanism of Nucleation in the Polluted Atmospheric Boundary Layer A DISSERTATION SUBMITTED TO THE FACULTY OF UNIVERSITY OF MINNESOTA BY Modi Chen IN PARTIAL FULFILLMENT OF THE REQUIREMENTS
Study of the Mechanism of Nucleation in the Polluted Atmospheric Boundary Layer A DISSERTATION SUBMITTED TO THE FACULTY OF UNIVERSITY OF MINNESOTA BY Modi Chen IN PARTIAL FULFILLMENT OF THE REQUIREMENTS
A cloud microphysics model including trace gas condensation and sulfate chemistry
 BOREAL ENVIRONMENT RESEARCH 8: 43 424 ISSN 239-695 Helsinki December 23 23 A cloud microphysics model including trace gas condensation and sulfate chemistry Harri Kokkola ), Sami Romakkaniemi ), Markku
BOREAL ENVIRONMENT RESEARCH 8: 43 424 ISSN 239-695 Helsinki December 23 23 A cloud microphysics model including trace gas condensation and sulfate chemistry Harri Kokkola ), Sami Romakkaniemi ), Markku
Generation of monodisperse aerosols through condensation nuclei control
 Air Pollution XV 505 Generation of monodisperse aerosols through condensation nuclei control H. M. Kadlimatti 1, S. Gangamma 2 & S. K. Varghese 3 1 Department of Mechanical Engineering, Basaveshwar Engineering
Air Pollution XV 505 Generation of monodisperse aerosols through condensation nuclei control H. M. Kadlimatti 1, S. Gangamma 2 & S. K. Varghese 3 1 Department of Mechanical Engineering, Basaveshwar Engineering
Formation of highly hygroscopic soot aerosols by atmospheric processing with sulfuric acid vapor
 Formation of highly hygroscopic soot aerosols by atmospheric processing with sulfuric acid vapor Khalizov, Alexei K.; Zhang, Renyi Y.; Zhang, Dan D.; Xue, H.; Pagels, Joakim; McMurry, Peter H.; Chen, J.
Formation of highly hygroscopic soot aerosols by atmospheric processing with sulfuric acid vapor Khalizov, Alexei K.; Zhang, Renyi Y.; Zhang, Dan D.; Xue, H.; Pagels, Joakim; McMurry, Peter H.; Chen, J.
Intercomparison of Mobility Particle Size Spectrometers
 Intercomparison of Mobility Particle Size Spectrometers Project No.: Principal Investigator: Home Institution: Participant: Candidate: Made by: Counter (SN): Software: MPSS-2016-5-1 Christine Braban NERC
Intercomparison of Mobility Particle Size Spectrometers Project No.: Principal Investigator: Home Institution: Participant: Candidate: Made by: Counter (SN): Software: MPSS-2016-5-1 Christine Braban NERC
Mass accommodation coefficient of water: A combined computational fluid dynamics and experimental data analysis
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2007jd008604, 2007 Mass accommodation coefficient of water: A combined computational fluid dynamics and experimental data analysis J. Voigtländer,
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 112,, doi:10.1029/2007jd008604, 2007 Mass accommodation coefficient of water: A combined computational fluid dynamics and experimental data analysis J. Voigtländer,
Laboratory Studies of H2So4/H2O Binary Homogeneous Nucleation from the So2+Oh Reaction: Evaluation of the Experimental Setup and Preliminary Results
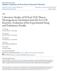 Kent State University Digital Commons @ Kent State University Libraries Biostatistics, Environmental Health, and Epidemiology Publications Biostatistics, Environmental Health and Epidemiology 8 Laboratory
Kent State University Digital Commons @ Kent State University Libraries Biostatistics, Environmental Health, and Epidemiology Publications Biostatistics, Environmental Health and Epidemiology 8 Laboratory
Downloaded By: [Williams, Leah R.] At: 08:55 28 June 2007
![Downloaded By: [Williams, Leah R.] At: 08:55 28 June 2007 Downloaded By: [Williams, Leah R.] At: 08:55 28 June 2007](/thumbs/96/128247824.jpg) Aerosol Science and Technology, 41:721 733, 2007 Copyright c American Association for Aerosol Research ISSN: 0278-6826 print / 1521-7388 online DOI: 10.1080/02786820701422278 Transmission Efficiency of
Aerosol Science and Technology, 41:721 733, 2007 Copyright c American Association for Aerosol Research ISSN: 0278-6826 print / 1521-7388 online DOI: 10.1080/02786820701422278 Transmission Efficiency of
Benjamin A. Nault, Pedro Campuzano-Jost, Doug A. Day, Hongyu Guo, Jason C. Schroder, Jose L. Jimenez, and the Science Teams from KORUS-AQ and ATom
 AMS Quantification Calibrations and Comparisons from Recent Campaigns Benjamin A. Nault, Pedro Campuzano-Jost, Doug A. Day, Hongyu Guo, Jason C. Schroder, Jose L. Jimenez, and the Science Teams from KORUS-AQ
AMS Quantification Calibrations and Comparisons from Recent Campaigns Benjamin A. Nault, Pedro Campuzano-Jost, Doug A. Day, Hongyu Guo, Jason C. Schroder, Jose L. Jimenez, and the Science Teams from KORUS-AQ
The Scanning DMA Transfer Function
 Aerosol Science and Technology, 38:833 850, 2004 Copyright c American Association for Aerosol Research ISSN: 0278-6826 print / 1521-7388 online DOI: 10.1080/027868290503082 The Scanning DMA Transfer Function
Aerosol Science and Technology, 38:833 850, 2004 Copyright c American Association for Aerosol Research ISSN: 0278-6826 print / 1521-7388 online DOI: 10.1080/027868290503082 The Scanning DMA Transfer Function
Insights into the Chemistry of New Particle Formation and Growth Events in Pittsburgh Based on Aerosol Mass Spectrometry
 Environ. Sci. Technol. 2004, 38, 4797-4809 Insights into the Chemistry of New Particle Formation and Growth Events in Pittsburgh Based on Aerosol Mass Spectrometry QI ZHANG, CHARLES O. STANIER, MANJULA
Environ. Sci. Technol. 2004, 38, 4797-4809 Insights into the Chemistry of New Particle Formation and Growth Events in Pittsburgh Based on Aerosol Mass Spectrometry QI ZHANG, CHARLES O. STANIER, MANJULA
Cloud Condensation Nuclei Hygroscopic Parameter Kappa
 Cloud Condensation Nuclei Hygroscopic Parameter Kappa Covers Reading Material in Chapter 17.5 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Cloud Condensation Nuclei (CCN) Form a cloud
Cloud Condensation Nuclei Hygroscopic Parameter Kappa Covers Reading Material in Chapter 17.5 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Cloud Condensation Nuclei (CCN) Form a cloud
Intercomparison of Mobility Particle Size Spectrometers
 Intercomparison of Mobility Particle Size Spectrometers Project No.: MPSS- 01-3- Basic information: Location of the quality assurance:, lab: 11 Delivery date: April 1, 01 Setup in the laboratory: April
Intercomparison of Mobility Particle Size Spectrometers Project No.: MPSS- 01-3- Basic information: Location of the quality assurance:, lab: 11 Delivery date: April 1, 01 Setup in the laboratory: April
High Temperature Condensation Particle Counter (HT- CPC)
 High Temperature Condensation Particle Counter (HT- CPC) Jeng K Rongchai and Nick Collings Outline Motivation The High Temperature CPC (HT-CPC) Desirable characteristics and working fluids Modelling Experiments
High Temperature Condensation Particle Counter (HT- CPC) Jeng K Rongchai and Nick Collings Outline Motivation The High Temperature CPC (HT-CPC) Desirable characteristics and working fluids Modelling Experiments
Insights Into Atmospheric Organic Aerosols Using An Aerosol Mass Spectrometer
 Insights Into Atmospheric Organic Aerosols Using An Aerosol Mass Spectrometer A thesis submitted to the University of Manchester Institute of Science and Technology for the degree of Doctor of Philosophy
Insights Into Atmospheric Organic Aerosols Using An Aerosol Mass Spectrometer A thesis submitted to the University of Manchester Institute of Science and Technology for the degree of Doctor of Philosophy
Ammonium Sulfate: Equilibrium and Metastability Phase Diagrams from 40 to -50 C
 7462 J. Phys. Chem. B 1998, 102, 7462-7469 Ammonium Sulfate: Equilibrium and Metastability Phase Diagrams from 40 to -50 C Jun Xu, Dan Imre,* Robert McGraw, and Ignatius Tang BrookhaVen National Laboratory,
7462 J. Phys. Chem. B 1998, 102, 7462-7469 Ammonium Sulfate: Equilibrium and Metastability Phase Diagrams from 40 to -50 C Jun Xu, Dan Imre,* Robert McGraw, and Ignatius Tang BrookhaVen National Laboratory,
dition of organics to ammonium sulfate particles lowers their DSF.
 Aerosol Science and Technology, 40:197 217, 2006 Copyright c American Association for Aerosol Research ISSN: 0278-6826 print / 1521-7388 online DOI: 10.1080/02786820500529406 From Agglomerates of Spheres
Aerosol Science and Technology, 40:197 217, 2006 Copyright c American Association for Aerosol Research ISSN: 0278-6826 print / 1521-7388 online DOI: 10.1080/02786820500529406 From Agglomerates of Spheres
MEASURING NANOPARTICLE SIZE DISTRIBUTIONS IN REAL-TIME: KEY FACTORS FOR ACCURACY
 MEASURING NANOPARTICLE SIZE DISTRIBUTIONS IN REAL-TIME: KEY FACTORS FOR ACCURACY APPLICATION NOTE SMPS-003 Introduction The benefits of sizing aerosolized submicrometer particles using an electrical mobility
MEASURING NANOPARTICLE SIZE DISTRIBUTIONS IN REAL-TIME: KEY FACTORS FOR ACCURACY APPLICATION NOTE SMPS-003 Introduction The benefits of sizing aerosolized submicrometer particles using an electrical mobility
Intercomparison of Mobility Particle Size Spectrometers
 Intercomparison of Mobility Particle Size Spectrometers Project No.: MPSS-2015-2-1 Basic information: Location of the quality assurance: TROPOS, lab: 118 Delivery date: - Setup in the laboratory: December
Intercomparison of Mobility Particle Size Spectrometers Project No.: MPSS-2015-2-1 Basic information: Location of the quality assurance: TROPOS, lab: 118 Delivery date: - Setup in the laboratory: December
A novel model to predict the physical state of atmospheric H 2 SO 4 /NH 3 /H 2 O aerosol particles
 Atmos. Chem. Phys., 3, 909 924, 2003 Atmospheric Chemistry and Physics A novel model to predict the physical state of atmospheric H 2 SO 4 /NH 3 /H 2 O aerosol particles C. A. Colberg, B. P. Luo, H. Wernli,
Atmos. Chem. Phys., 3, 909 924, 2003 Atmospheric Chemistry and Physics A novel model to predict the physical state of atmospheric H 2 SO 4 /NH 3 /H 2 O aerosol particles C. A. Colberg, B. P. Luo, H. Wernli,
Kinetics of Deliquescence of Ammonium Sulfate Particles
 Kinetics of Deliquescence of Ammonium Sulfate Particles By Rocsana Gabriela Pancescu A thesis presented to the University of Waterloo in fulfillment of the thesis requirement for the degree of Doctor of
Kinetics of Deliquescence of Ammonium Sulfate Particles By Rocsana Gabriela Pancescu A thesis presented to the University of Waterloo in fulfillment of the thesis requirement for the degree of Doctor of
Aerosol-Cloud-Radiation Interactions in Atmospheric Forecast Models
 Aerosol-Cloud-Radiation Interactions in Atmospheric Forecast Models John H. Seinfeld, Principal Investigator California Institute of Technology 1200 E. California Blvd., M/C 210-41 Pasadena, CA 91125 (626)
Aerosol-Cloud-Radiation Interactions in Atmospheric Forecast Models John H. Seinfeld, Principal Investigator California Institute of Technology 1200 E. California Blvd., M/C 210-41 Pasadena, CA 91125 (626)
Supersaturation in the Wyoming CCN Instrument
 OCTOBER 2006 S N I D E R E T A L. 1323 Supersaturation in the Wyoming CCN Instrument JEFFERSON R. SNIDER Department of Atmospheric Science, University of Wyoming, Laramie, Wyoming MARKUS D. PETTERS Department
OCTOBER 2006 S N I D E R E T A L. 1323 Supersaturation in the Wyoming CCN Instrument JEFFERSON R. SNIDER Department of Atmospheric Science, University of Wyoming, Laramie, Wyoming MARKUS D. PETTERS Department
The Atmospheric Chemistry and Physics of Ammonia
 The Atmospheric Chemistry and Physics of Ammonia Russell Dickerson Dept. Meteorology, The University of Maryland Presented at the National Atmospheric Deposition Program Ammonia Workshop October 23, 2003
The Atmospheric Chemistry and Physics of Ammonia Russell Dickerson Dept. Meteorology, The University of Maryland Presented at the National Atmospheric Deposition Program Ammonia Workshop October 23, 2003
Boston College. The Graduate School of Arts and Sciences. Department of Chemistry CHEMICAL KINETICS AND MICROPHYSICS OF ATMOSPHERIC AEROSOLS
 Boston College The Graduate School of Arts and Sciences Department of Chemistry CHEMICAL KINETICS AND MICROPHYSICS OF ATMOSPHERIC AEROSOLS a dissertation by JAMES WALTER MORRIS submitted in partial fulfillment
Boston College The Graduate School of Arts and Sciences Department of Chemistry CHEMICAL KINETICS AND MICROPHYSICS OF ATMOSPHERIC AEROSOLS a dissertation by JAMES WALTER MORRIS submitted in partial fulfillment
Supplement of Multiphase oxidation of SO 2 by NO 2 on CaCO 3 particles
 Supplement of Atmos. Chem. Phys., 18, 2481 2493, 2018 https://doi.org/10.5194/acp-18-2481-2018-supplement Author(s) 2018. This work is distributed under the Creative Commons Attribution 4.0 License. Supplement
Supplement of Atmos. Chem. Phys., 18, 2481 2493, 2018 https://doi.org/10.5194/acp-18-2481-2018-supplement Author(s) 2018. This work is distributed under the Creative Commons Attribution 4.0 License. Supplement
