CHEM1902/ N-8 November Consider the following reaction sequences beginning with the carboxylic acid, E.
|
|
|
- Rhoda Willis
- 5 years ago
- Views:
Transcription
1 CEM1902/ ovember 2014 Consider the following reaction sequences beginning with the carboxylic acid, E. 6 ame compounds E and G. E: propionic acid G: methyl propionate Propose structures for compounds F, and J. F J C 3 Propose a mechanism for step (ii).
2 CEM1902/ ovember 2014 Draw the conjugate bases for the following acids. S T U V 5 Conjugate base of S Conjugate base of T Conjugate base of U Conjugate base of V F S F Which of S and T is the stronger acid? Give a reason for your answer. T will be a stronger acid. In acting as an acid, the conjugate base will be formed. The conjugate base of T is more stable than that of S as the electronegative fluorine atoms will withdraw electron density from the negatively charged atom. Which of U and V is the stronger acid? Give a reason for your answer. U is a stronger acid than V because the conjugate base is more stable. Both are stabilised by resonance which acts to delocalise the negative charge over the electronegative atoms present: S S S For the conjugate base of U, the negative charge is delocalised over 3 atoms. For the conjugate base of V, the negative charge is only delocalised over 2 atoms
3 CEM1902/ ovember 2013 The hydroxide anion can react with chloroethane via a mechanism that is abbreviated S 2, as shown below. Add curly arrows to the reaction scheme to complete a mechanism for this reaction. 7 Explain what each part of the abbreviation S 2 means. S = substitution = nucleophilic 2 = bimolecular The hydroxide anion undergoes an apparently similar reaction with ethanoyl chloride: Draw a mechanism (using curly arrows) for this reaction, thereby demonstrating how it is fundamentally different to the reaction of chloroethane above. In each of these reactions, a full molecular orbital of the hydroxide anion (the M) interacts with an empty molecular orbital of the organic halogen compound (the LUM). Which orbital is the LUM in chloroethane? Which orbital is the LUM in ethanoyl chloride? σ* C- π* C=
4 CEM1902/ ovember 2012 Consider the following reaction sequence beginning with the alkene A. 6 Suggest structures for compounds C F in the reaction sequence above. C D E F Describe the selectivity observed, and briefly explain the reasons for it, in the conversion of alkene A to compound B. Markovnikov s rule: the + of Br adds to C1 leading to a secondary carbocation rather than to C2 which leads to a primary carbocation. The more substituted carbocation is more stable due to hyperconjugation.
5 CEM1902/ ovember 2012 Consider the three nitrogen-containing compounds P, Q and R. Mark s 5 What is the hybridisation at in compound P? sp 3 What is the hybridisation at in compound Q? sp Use this information to decide which of P or Q is more basic. Explain your reasoning. P is more basic. The sp 3 hybridised has more p orbital character (75%) compared to sp (50%). P therefore has a more diffuse lone pair that is more available for protonation. Conversely, the lone pair in Q is more tightly bound and Q is therefore a weaker base. Show curly arrows and another structure to show how compound R is stabilised by resonance. Which is more basic, compound P or compound R? Why? P is more basic. The lone pair in R contributes to the resonance structure and is partially delocalised into the carbonyl group and is therefore unavailable for protonation.
6 CEM1902/ ovember 2012 Benzoic acid, benzyl alcohol I and phenol J are shown below. The pk a values of these three compounds are 15.2, 9.9 and 4.2, but not in that order. Mark s 6 Assign the correct pk a to each of these three compounds. pk a values: = 4.2 I = 15.2 J = 9.9 Draw resonance structures to explain your answer. The conjugate base of is stabilised by delocalisation of the negative charge over two electronegative atoms and over the ring: The conjugate base of J is stabilised by delocalisation of the negative charge over an atom and the ring: As the delocalisation places negative charge over C as well as, the stabilisation is smaller than for. By resonance stabilisation is possible for the conjugate base of I. Would you expect 4-nitrophenol, K, to be more or less acidic than phenol, J? Explain your answer. K will be more acidic than J as the negative charge of the phenoxide ion can be delocalised into the nitro group, increasing the resonance stabilisation.
7 CEM1902/ ovember 2010 Protons next to a carbonyl group can be removed by alkoxide bases as shown below. 6 Apply your understanding of resonance to propose a structure L that explains how the carbonyl group increases the acidity of these hydrogens. Add curly arrows to the reaction scheme above to complete a mechanism for the deprotonation of J to give K, and the stabilisation of K by resonance. The pk a values of compounds J, M and are 9, 13 and 19, but not in that order. Match each compound with the correct pk a, and explain your answer. J M Et Et pk a values: J = 19 M = 9 = 13 Reasoning for above assignments M and are stronger acids than J as the charge on the carbanion can be delocalised onto 2 carbonyl groups rather than 1(i.e. there are more resonance structures). Enolate anions are stabilised by electron withdrawing groups which help reduce the negative charge on the carbon atom. is a weaker acid than M because the Et group of the ester donates electron density towards the enolate anion, thus destabilising it. Also the following resonance form of the ester would mean that the carbonyl group is less available to share the negative charge of the enolate anion.
8 CEM1902/ ovember 2010 Protons next to a carbonyl group can be removed by alkoxide bases as shown below. 6 C 3 C 3 J K L Apply your understanding of resonance to propose a structure L that explains how the carbonyl group increases the acidity of these hydrogens. Add curly arrows to the reaction scheme above to complete a mechanism for the deprotonation of J to give K, and the stabilisation of K by resonance. The pk a values of compounds J, M and are 9, 13 and 19, but not in that order. Match each compound with the correct pk a, and explain your answer. J M Et Et pk a values: J = 19 M = 9 = 13 Reasoning for above assignments M and are stronger acids than J as the charge on the carbanion can be delocalised onto 2 carbonyl groups rather than 1(i.e. there are more resonance structures). Enolate anions are stabilised by electron withdrawing groups which help reduce the negative charge on the carbon atom. is a weaker acid than M because the Et group of the ester donates electron density towards the enolate anion, thus destabilising it. Also the following resonance form of the ester would mean that the carbonyl group is less available to share the negative charge of the enolate anion.
9 CEM1902/ ovember 2009 Suggest reagents to accomplish the following transformations. More than one step is required in all cases. 6 Cr / S 2 excess 2 C 3 dil. 2 S 4 Cr / Br MgBr Mg / dry ether 1. C 2 2. / 2
10 CEM1902/ ovember 2009 Propose a structure for the product of the following reaction. utline a mechanism for its formation. Show all curly arrows and any intermediates. 4 LiAl 4 (excess) then / 2 2 C 2 6 +
11 CEM1902/ ovember 2009 For each of the following pairs of compounds, identify which is the stronger acid and give reasons for your choice. 3 and S (P) (Q) (Q) There is greater resonance stabilisation of the conjugate base (more canonical forms): and (R) (S) (R) There is greater resonance stabilisation of the conjugate base because it is aromatic. ASWER CTIUES TE EXT PAGE
12 CEM1902/ ovember 2009 CF 3 C 2 and C 3 C 2 (T) (U) (T) There is greater resonance stabilisation of the conjugate base due to the inductive electron withdrawal of the very electronegative F atoms. F F C F C
13 CEM1902/ ovember 2008 Complete the following table by drawing the structures of the intermediate and final organic product(s) as required. The intermediate product is formed when the starting material is treated with Reagent 1. The final product is formed when the intermediate product is treated with Reagent 2. 4 Starting material Intermediate product Final product Reagent 1: S 2 Reagent 2: C C Reagent 1: K 2 Cr 2 7 / Reagent 2: C 3 /
14 CEM1902/ ovember 2008 For each of the following pairs of compounds, identify which is the stronger acid and give reasons for your choice. 4 and Phenol is more acidic as the phenoxide ion is resonance stabilised: and Chloroacetic acid is more acidic. The atom is electronegative and pulls electrons from the carboxylic acid, thus weakening the bond. and 2 2 The para isomer is more acidic as the charge on the phenoxide ion can be delocalised into the nitro group. This is not possible with the meta isomer.
15 CEM1902/ ovember 2007 Draw the structure(s) of the major organic product(s) formed in each of the following reactions. Give the names of the products where requested. 3 C 3 C 3 1) C 3 MgBr 2) dil. 3 ame(s): 2-methylhept-5-yn-2-ol TE REMAIDER F TIS PAGE IS FR RUG WRKIG LY.
16 CEM1902/ ovember 2007 Complete the following table. Starting material Reagents / Conditions Major organic product(s) 3 C S 2 C C 1. ab 4 C 2. / 2 C or TE REMAIDER F TIS PAGE IS FR RUG WRKIG LY.
17 CEM1902/ ovember 2006 Draw the structure(s) of the major organic product(s) formed in each of the following reactions. Give the names of the products where requested ab 4 2. / 2 / 2 C 2
18 CEM1902/ ovember 2006 Complete the mechanism for the reaction given below. Draw partial charges and curly arrows as appropriate to illustrate the bonding changes that take place. 3 δ+ δ δ δ+
19 CEM ovember 2005 Draw the structure(s) of the major organic product(s) formed in each of the following reactions. Give the names of the products where requested. 5 B r conc. K heat ame(s): (E)-3-methylhex-3-ene (Z )-3-methylhex-3-ene Cr / / 2 conc. Br Br ame(s): 1-bromo-1-methylcyclohexane / 2 / heat C + 2 C 3 C 3
20 CEM1902/ ovember 2004 Draw the constitutional formula(s) of the major organic product(s) formed in each of the following reactions. 2 C C 3 C 3 C C 3 C 2 C excess C 3 2 C 3 C 2 C C 3 + C
21 CEM ovember 2004 Compound X was isolated as a derivative of a natural product X 3 4 Carbon 4 of X is a stereogenic centre. List the substituents attached to C4 in descending order of priority according to the sequence rules. highest priority -C 2 CC 3 C 2 C=CC 3 -C 3 - lowest priority What is the systematic name for compound X? Make sure you include all relevant stereochemical descriptors. (4S,6Z)-4-methyloct-6-en-2-one. As shown above, the stereochemistry about carbon 4 is (S) (anticlockwise). The C=C bond has the two higher priority groups (-C 3 and C 2 C(C 3 )C 2 CC 3 ) on the same side so it has a (Z) configuration. Reduction of X with sodium borohydride (ab 4 ) followed by quenching the reaction with dilute acid gives Y. Give the constitutional formula for Y. Y Product Y can be separated into two isomers. Explain. The reduction indtroduces a second stereogenic centre into the molecule. The two products are diastereoisomers (not eneantiomers) and hence have different chemical and physical properties and can be separated.
22 CEM1902/ ovember 2004 Complete the three step mechanism for the reaction given below. Draw intermediate structures, curly arrows and partial charges as appropriate to illustrate the bonding changes that take place. 4 C 3 C 3 C 3 C 3
23 CEM1902/ ovember 2004 Draw the repeating unit of the polymer formed in the following reactions. 2 C 3 C 3 + C C 2 2 n n Show clearly the reagents you would use to carry out the following chemical conversion. Draw constitutional formulas for any intermediate compounds. TE: More than one step is necessary. 4 conc. 2 S 4 (dehydration) C 3 C Br (Markovnikov addition across double bond with adding to least substituted end of double bond). Mg / dry ether (i) 2 C Br MgBr (ii) 3 +
CHEM1902/ N-8 November Consider the following reaction sequences beginning with the carboxylic acid, E.
 CHEM1902/4 2014-N-8 November 2014 Consider the following reaction sequences beginning with the carboxylic acid, E. 6 Name compounds E and G. E: G: Propose structures for compounds F, H and J. F H J Propose
CHEM1902/4 2014-N-8 November 2014 Consider the following reaction sequences beginning with the carboxylic acid, E. 6 Name compounds E and G. E: G: Propose structures for compounds F, H and J. F H J Propose
CHEM J-8 June Complete the following table. Make sure you give the name of the starting material where indicated. REAGENTS/ CONDITIONS
 CEM1102 2014-J-8 June 2014 Complete the following table. Make sure you give the name of the starting material where indicated. STARTIG MATERIAL REAGETS/ CDITIS STRUCTURAL FRMULA(S) F MAJR RGAIC PRDUCT(S)
CEM1102 2014-J-8 June 2014 Complete the following table. Make sure you give the name of the starting material where indicated. STARTIG MATERIAL REAGETS/ CDITIS STRUCTURAL FRMULA(S) F MAJR RGAIC PRDUCT(S)
Number of d electrons CO 2 carbon dioxide +IV or +4 0
 CEM1611 2007-J-2 June 2007 Complete the following table. Give, as required, the formula, the systematic name, the oxidation number of the underlined atom and, where indicated, the number of d electrons
CEM1611 2007-J-2 June 2007 Complete the following table. Give, as required, the formula, the systematic name, the oxidation number of the underlined atom and, where indicated, the number of d electrons
CHEM J-10 June The structure of ( )-linalool, a commonly occurring natural product, is shown below.
 CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid
![Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid](/thumbs/95/123929992.jpg) Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
and Stereochemistry) PAPER 1: ORGANIC CHEMISTRY- I (Nature of Bonding and Stereochemistry) MODULE 4: Applications of Electronic Effects
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 1: ORGANIC - I (Nature of Bonding Module 4: Applications of Electronic Effects CHE_P1_M4 PAPER 1: ORGANIC - I (Nature of Bonding
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 1: ORGANIC - I (Nature of Bonding Module 4: Applications of Electronic Effects CHE_P1_M4 PAPER 1: ORGANIC - I (Nature of Bonding
Section Practice Exam II Solutions
 Paul Bracher Chem 30 Section 7 Section Practice Exam II s Whether problems old r problems new, You d better practice, r you ll fail exam II. -- Anonymous TF Problem 1 a) Rank the following series of electrophiles
Paul Bracher Chem 30 Section 7 Section Practice Exam II s Whether problems old r problems new, You d better practice, r you ll fail exam II. -- Anonymous TF Problem 1 a) Rank the following series of electrophiles
CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
Topics in the June 2009 Exam Paper for CHEM1102
 June 009 Topics in the June 009 Exam Paper for CEM110 ick on the links for resources on each topic. 009-J-: 009-J-3: 009-J-4: 009-J-5: 009-J-6: 009-J-7: 009-J-8: 009-J-9: 009-J-10: 009-J-1: 009-J-13: Periodic
June 009 Topics in the June 009 Exam Paper for CEM110 ick on the links for resources on each topic. 009-J-: 009-J-3: 009-J-4: 009-J-5: 009-J-6: 009-J-7: 009-J-8: 009-J-9: 009-J-10: 009-J-1: 009-J-13: Periodic
CHEM1902/ N-9 November 2014
 CEM1902/4 2014-N-9 November 2014 The elimination of 2 O from alcohol A can form the isomeric alkenes B and C. Elimination of Br from the alkyl halide D can generate the same two alkenes. 7 Assign the absolute
CEM1902/4 2014-N-9 November 2014 The elimination of 2 O from alcohol A can form the isomeric alkenes B and C. Elimination of Br from the alkyl halide D can generate the same two alkenes. 7 Assign the absolute
CHEM J-8 June Complete the following table. Make sure you give the name of the starting material where indicated. REAGENTS/ CONDITIONS
 CHEM1102 2014-J-8 June 2014 Complete the following table. Make sure you give the name of the starting material where indicated. STARTING MATERIAL REAGENTS/ CNDITINS STRUCTURAL FRMULA(S) F MAJR RGANIC PRDUCT(S)
CHEM1102 2014-J-8 June 2014 Complete the following table. Make sure you give the name of the starting material where indicated. STARTING MATERIAL REAGENTS/ CNDITINS STRUCTURAL FRMULA(S) F MAJR RGANIC PRDUCT(S)
Lecture Topics: I. Electrophilic Aromatic Substitution (EAS)
 Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
CHE1502. Tutorial letter 203/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry
 E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
11/30/ Substituent Effects in Electrophilic Substitutions. Substituent Effects in Electrophilic Substitutions
 Chapter 9 Problems: 9.1-29, 32-34, 36-37, 39-45, 48-56, 58-59, 61-69, 71-72. 9.8 Substituent effects in the electrophilic substitution of an aromatic ring Substituents affect the reactivity of the aromatic
Chapter 9 Problems: 9.1-29, 32-34, 36-37, 39-45, 48-56, 58-59, 61-69, 71-72. 9.8 Substituent effects in the electrophilic substitution of an aromatic ring Substituents affect the reactivity of the aromatic
Chem 261 Dec 6, 2017
 209 Chem 261 Dec 6, 2017 REVIEW: Example: K!! + 3 C + 3 C K tert-butoxide (an alkoxide) methanol tert-butanol pka = 16 pka = 19 methoxide stronger base stronger acid (lower pka, more acidic) weaker acid
209 Chem 261 Dec 6, 2017 REVIEW: Example: K!! + 3 C + 3 C K tert-butoxide (an alkoxide) methanol tert-butanol pka = 16 pka = 19 methoxide stronger base stronger acid (lower pka, more acidic) weaker acid
Chemistry Final Examinations Summer 2006 answers
 Chemistry 235 - Final Examinations Summer 2006 answers A GEERAL CEMISTRY answers are given from 1-20: no reaction C 3 CC 3 Ph C C C C C 3 Et 2 2 2 B. REACTIS AD REAGETS [32 MARKS] 2. A single substance
Chemistry 235 - Final Examinations Summer 2006 answers A GEERAL CEMISTRY answers are given from 1-20: no reaction C 3 CC 3 Ph C C C C C 3 Et 2 2 2 B. REACTIS AD REAGETS [32 MARKS] 2. A single substance
Chapter 17. Reactions of Aromatic Compounds
 Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
Problem Set #3 Solutions
 Problem Set #3 Solutions 1. a) C 3 (methyl group) Since carbon (E = 2.5) is slightly more electronegative than hydrogen (E = 2.2), there will be a small dipole moment pulling electron density away from
Problem Set #3 Solutions 1. a) C 3 (methyl group) Since carbon (E = 2.5) is slightly more electronegative than hydrogen (E = 2.2), there will be a small dipole moment pulling electron density away from
There are two main electronic effects that substituents can exert:
 Substituent Effects There are two main electronic effects that substituents can exert: RESONANCE effects are those that occur through the π system and can be represented by resonance structures. These
Substituent Effects There are two main electronic effects that substituents can exert: RESONANCE effects are those that occur through the π system and can be represented by resonance structures. These
Week 6 notes CHEM
 Week 6 notes EM1002 2009 Unless otherwise stated, all images in this file have been reproduced from: Blackman, Bottle, Schmid, Mocerino and Wille, hemistry, 2007 (John Wiley) ISBN: 9 78047081 0866 1 Note
Week 6 notes EM1002 2009 Unless otherwise stated, all images in this file have been reproduced from: Blackman, Bottle, Schmid, Mocerino and Wille, hemistry, 2007 (John Wiley) ISBN: 9 78047081 0866 1 Note
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
Lecture Notes Chem 51C S. King. Chapter 20 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation & Reduction
 Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Effect of nucleophile on reaction
 1 Effect of nucleophile on reaction X DS c X c c X DS c + X cleophile not involved in DS of S N 1 so does not effect the reaction (well obviously it controls the formula of the product!) cleophile has
1 Effect of nucleophile on reaction X DS c X c c X DS c + X cleophile not involved in DS of S N 1 so does not effect the reaction (well obviously it controls the formula of the product!) cleophile has
Acid/Base stuff Beauchamp 1
 cid/base stuff Beauchamp 1 Problems You should be able to match a pk a value with its acid in each group below and explain the differences. You should be able to draw an arrow-pushing mechanism with general
cid/base stuff Beauchamp 1 Problems You should be able to match a pk a value with its acid in each group below and explain the differences. You should be able to draw an arrow-pushing mechanism with general
Topics in the November 2007 Exam Paper for CHEM1904
 November 2007 Topics in the November 2007 Exam Paper for CEM1904 Click on the links for resources on each topic. 2007-N-2: 2007-N-3: 2007-N-4: 2007-N-5: 2007-N-6: 2007-N-7: Solubility Equilibrium Intermolecular
November 2007 Topics in the November 2007 Exam Paper for CEM1904 Click on the links for resources on each topic. 2007-N-2: 2007-N-3: 2007-N-4: 2007-N-5: 2007-N-6: 2007-N-7: Solubility Equilibrium Intermolecular
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon
 Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Chapter 7: Alcohols, Phenols and Thiols
 Chapter 7: Alcohols, Phenols and Thiols 45 -Alcohols have the general formula R-OH and are characterized by the presence of a hydroxyl group, -OH. -Phenols have a hydroxyl group attached directly to an
Chapter 7: Alcohols, Phenols and Thiols 45 -Alcohols have the general formula R-OH and are characterized by the presence of a hydroxyl group, -OH. -Phenols have a hydroxyl group attached directly to an
Nucleophilic Addition Reactions of Carboxylic Acid Derivatives
 Lecture 5: bjectives: Nucleophilic Addition eactions of Carboxylic Acid Derivatives By the end of this lecture you will be able to: draw the mechanism of a nucleophilic addition-elimination reaction with
Lecture 5: bjectives: Nucleophilic Addition eactions of Carboxylic Acid Derivatives By the end of this lecture you will be able to: draw the mechanism of a nucleophilic addition-elimination reaction with
Topics in the November 2014 Exam Paper for CHEM1102
 November 2014 Topics in the November 2014 Exam Paper for CEM1102 Click on the links for resources on each topic. 2014-N-2: 2014-N-3: 2014-N-4: 2014-N-5: 2014-N-6: 2014-N-7: 2014-N-8: 2014-N-9: 2014-N-10:
November 2014 Topics in the November 2014 Exam Paper for CEM1102 Click on the links for resources on each topic. 2014-N-2: 2014-N-3: 2014-N-4: 2014-N-5: 2014-N-6: 2014-N-7: 2014-N-8: 2014-N-9: 2014-N-10:
OH OH OH CH 2 CH 2 C(CH 3 ) 2 (a) CH 3 CHCH 2 CHCH(CH 3 ) 2. (b)
 hem 226 Problem Set #8 Fundamentals of rganic hemistry, 4 th edition, John McMurry. hapter 8 1. Give IUPA names for the following alcohols. 2 2 ( 3 ) 2 3 2 ( 3 ) 2 Longest chain = 6 carbons:...hexanediol.
hem 226 Problem Set #8 Fundamentals of rganic hemistry, 4 th edition, John McMurry. hapter 8 1. Give IUPA names for the following alcohols. 2 2 ( 3 ) 2 3 2 ( 3 ) 2 Longest chain = 6 carbons:...hexanediol.
b.p.=100 C b.p.=65 C b.p.=-25 C µ=1.69 D µ=2.0 D µ=1.3 D
 Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Organic Chemistry, 7 L. G. Wade, Jr. Chapter , Prentice Hall
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
Chapter 17 Reactions of Aromatic Compounds. Electrophilic Aromatic Substitution
 Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Electrophile substitutes for a hydrogen on the benzene ring. Chapter 17: Aromatics 2-Reactions Slide 17-2 1 Mechanism Step
Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Electrophile substitutes for a hydrogen on the benzene ring. Chapter 17: Aromatics 2-Reactions Slide 17-2 1 Mechanism Step
Elimination. S N 2 in synthesis. S N 2 and E2. Kinetics. Mechanism bimolecular
 bimolecular B: limination B * 1 Kinetics 2 ate determining step involves both reactants rate = k [base] [-] Second order kinetics 2 B + 2 = limination, 2 nd order 2 3 2 4 Zaitsev s ule In some cases a
bimolecular B: limination B * 1 Kinetics 2 ate determining step involves both reactants rate = k [base] [-] Second order kinetics 2 B + 2 = limination, 2 nd order 2 3 2 4 Zaitsev s ule In some cases a
16. Chemistry of Benzene: Electrophilic Aromatic Substitution. Based on McMurry s Organic Chemistry, 7 th edition
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
16. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry, 7 th edition Substitution Reactions of Benzene and Its Derivatives Benzene is aromatic: a cyclic conjugated
4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY
 4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY During the early 1800's, a group of compounds of natural origin became collectively known as aromatic compounds. As several of these compounds
4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY During the early 1800's, a group of compounds of natural origin became collectively known as aromatic compounds. As several of these compounds
CHEM J-2 June 2012
 CHEM1102 2012-J-2 June 2012 Explain why HClO 4 is a stronger Brønsted acid than HBrO 4, but HCl is a weaker acid than HBr. 2 In Group 17 oxyacids, electron density is drawn away from the O atom as the
CHEM1102 2012-J-2 June 2012 Explain why HClO 4 is a stronger Brønsted acid than HBrO 4, but HCl is a weaker acid than HBr. 2 In Group 17 oxyacids, electron density is drawn away from the O atom as the
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Introduction to Organic Chemistry
 Introduction to rganic hemistry 59 Introduction to rganic hemistry andout 3 - chanism u u http://burton.chem.ox.ac.uk/teaching.html rganic hemistry J. layden,. Greeves, S. Warren Stereochemistry at a Glance
Introduction to rganic hemistry 59 Introduction to rganic hemistry andout 3 - chanism u u http://burton.chem.ox.ac.uk/teaching.html rganic hemistry J. layden,. Greeves, S. Warren Stereochemistry at a Glance
CHEM J-9 June 2014
 CEM1611 2014-J-9 June 2014 Alanine (ala) and lysine (lys) are two amino acids with the structures given below as Fischer projections. The pk a values of the conjugate acid forms of the different functional
CEM1611 2014-J-9 June 2014 Alanine (ala) and lysine (lys) are two amino acids with the structures given below as Fischer projections. The pk a values of the conjugate acid forms of the different functional
ALCOHOLS AND PHENOLS
 ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
Benzenes & Aromatic Compounds
 Benzenes & Aromatic Compounds 1 Structure of Benzene H H C C C H C 6 H 6 H C C C H H A cyclic conjugate molecule Benzene is a colourless odourless liquid, boiling at 80 o C and melting at 5 o C. It is
Benzenes & Aromatic Compounds 1 Structure of Benzene H H C C C H C 6 H 6 H C C C H H A cyclic conjugate molecule Benzene is a colourless odourless liquid, boiling at 80 o C and melting at 5 o C. It is
Reactions. Reactions. Elimination. 2. Elimination Often competes with nucleophilic substitution. 2. Elimination Alkyl halide is treated with a base
 eactions 1 eactions 2 2. limination Alkyl halide is treated with a base B: 2. limination ften competes with nucleophilic substitution LIMINATIN Nu: SUBSTITUTIN Nu Bimolecular B: limination B * * 3 Kinetics
eactions 1 eactions 2 2. limination Alkyl halide is treated with a base B: 2. limination ften competes with nucleophilic substitution LIMINATIN Nu: SUBSTITUTIN Nu Bimolecular B: limination B * * 3 Kinetics
19.4 Physical Properties Key: hydrogen bond strength depends on acidity of the hydrogen and basicity of the N or O
 Chem 360 Jasperse Ch. 19 Notes. Amines 12 19.4 ysical Properties Key: hydrogen bond strength depends on acidity of the hydrogen and basicity of the N or 1. Water Solubility: All amines hydrogen-bond water
Chem 360 Jasperse Ch. 19 Notes. Amines 12 19.4 ysical Properties Key: hydrogen bond strength depends on acidity of the hydrogen and basicity of the N or 1. Water Solubility: All amines hydrogen-bond water
Chem 112A: Final Exam
 Chem 112A: Final Exam December 14th, 2011 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Chem 112A: Final Exam December 14th, 2011 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Chapter 17: Alcohols and Phenols
 hapter 17: Alcohols and Phenols sp 3 alcohol phenol (aromatic alcohol) pka~ 16-18 pka~ 10 Alcohols contain an group connected to a saturated carbon (sp 3 ) Phenols contain an group connected to a carbon
hapter 17: Alcohols and Phenols sp 3 alcohol phenol (aromatic alcohol) pka~ 16-18 pka~ 10 Alcohols contain an group connected to a saturated carbon (sp 3 ) Phenols contain an group connected to a carbon
CHEMISTRY Topic #4: Electrophilic Addition Reactions of Alkenes and Alkynes Fall 2018 Dr. Susan Findlay
 EMISTRY 2600 Topic #4: Electrophilic Addition Reactions of Alkenes and Alkynes Fall 2018 Dr. Susan Findlay rganic Reactions (EM 2500 Review) Most reactions in organic chemistry fall into one (or more)
EMISTRY 2600 Topic #4: Electrophilic Addition Reactions of Alkenes and Alkynes Fall 2018 Dr. Susan Findlay rganic Reactions (EM 2500 Review) Most reactions in organic chemistry fall into one (or more)
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Chapter 23 Phenols CH. 23. Nomenclature. The OH group takes precedence as the parent phenol.
 CH. 23 Chapter 23 Phenols Nomenclature The OH group takes precedence as the parent phenol. Carboxyl and acyl groups take precedence over the OH group. The OH group is a strong electron-donating group through
CH. 23 Chapter 23 Phenols Nomenclature The OH group takes precedence as the parent phenol. Carboxyl and acyl groups take precedence over the OH group. The OH group is a strong electron-donating group through
CHEM J-2 June Complete the following table, giving either the systematic name or the molecular formula as required.
 verall health EM1108 2008-J-2 June 2008 omplete the following table, giving either the systematic name or the molecular formula as required. 2 Formula S 2 ol 2 6 2 Ag 2 r 4 K 3 Systematic name sulfur dioxide
verall health EM1108 2008-J-2 June 2008 omplete the following table, giving either the systematic name or the molecular formula as required. 2 Formula S 2 ol 2 6 2 Ag 2 r 4 K 3 Systematic name sulfur dioxide
Reactions of Aromatic Compounds. Aromatic compounds do not react like other alkenes. With an appropriate catalyst, however, benzene will react
 Reactions of Aromatic Compounds Aromatic compounds do not react like other alkenes 2 Fe 3 2 Does not form A major part of the problem for this reaction is the product has lost all aromatic stabilization,
Reactions of Aromatic Compounds Aromatic compounds do not react like other alkenes 2 Fe 3 2 Does not form A major part of the problem for this reaction is the product has lost all aromatic stabilization,
Alcohols. Alcohol any organic compound containing a hydroxyl (R-OH) group. Alcohols are an extremely important organic source
 Alcohols Alcohol any organic compound containing a hydroxyl (R-OH) group Uses: synthetic intermediate, cleanser, cosmetics, fuel, alcoholic beverages, etc. Alcohols are an extremely important organic source
Alcohols Alcohol any organic compound containing a hydroxyl (R-OH) group Uses: synthetic intermediate, cleanser, cosmetics, fuel, alcoholic beverages, etc. Alcohols are an extremely important organic source
1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound?
 CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
Organic Chemistry Practice Problems: Solutions
 rganic Chemistry Practice Problems: Solutions 1. 2. a. B, A b. D, B c. A, D d. D, A a. Resonance b. Electronegativity of fluorine atoms F F c. Neither is very acidic, but the oxygen will help stabilise
rganic Chemistry Practice Problems: Solutions 1. 2. a. B, A b. D, B c. A, D d. D, A a. Resonance b. Electronegativity of fluorine atoms F F c. Neither is very acidic, but the oxygen will help stabilise
N.b. A catalyst is a species which speeds up a chemical reaction but which remains chemically unchanged. Reverse process of dehydration of an alcohol
 An Introduction to Organic hemistry N.b. A catalyst is a species which speeds up a chemical reaction but which remains chemically unchanged. ydration (Addition) Reverse process of dehydration of an alcohol
An Introduction to Organic hemistry N.b. A catalyst is a species which speeds up a chemical reaction but which remains chemically unchanged. ydration (Addition) Reverse process of dehydration of an alcohol
I5 ELECTROPHILIC SUBSTITUTIONS OF
 Section I Aromatic chemistry I5 ELECTPILIC SUBSTITUTINS F MN-SUBSTITUTED AMATIC INGS Key Notes ortho, meta and para substitution Substituent effect eaction profile Activating groups inductive o/p Deactivating
Section I Aromatic chemistry I5 ELECTPILIC SUBSTITUTINS F MN-SUBSTITUTED AMATIC INGS Key Notes ortho, meta and para substitution Substituent effect eaction profile Activating groups inductive o/p Deactivating
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chemistry 52 Exam #1. Name: 22 January This exam has six (6) questions, two cover pages, six pages, and 2 scratch pages.
 Chemistry 52 Exam #1 Name: 22 January 2003 This exam has six (6) questions, two cover pages, six pages, and 2 scratch pages. Please check before beginning to make sure no questions are missing. 65 minutes
Chemistry 52 Exam #1 Name: 22 January 2003 This exam has six (6) questions, two cover pages, six pages, and 2 scratch pages. Please check before beginning to make sure no questions are missing. 65 minutes
(c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
 KT121 Sem II 09/10 SETIN B: 1.(a) Name of mechanism: E1 elimination. 7 marks (b) (i) + 3 3 3 (ii) + 3 (iii) l 8 marks (c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
KT121 Sem II 09/10 SETIN B: 1.(a) Name of mechanism: E1 elimination. 7 marks (b) (i) + 3 3 3 (ii) + 3 (iii) l 8 marks (c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
CHEMISTRY. Module No and Title Module-, Electrophilic Aromatic Substitution: The ortho/para ipso attack, orientation in other ring systems.
 Subject Chemistry Paper No and Title Paper-5, Organic Chemistry-II Module No and Title Module-, Electrophilic Aromatic Substitution: The ortho/para Module Tag CHE_P5_M29 TABLE OF CONTENTS 1. Learning Outcomes
Subject Chemistry Paper No and Title Paper-5, Organic Chemistry-II Module No and Title Module-, Electrophilic Aromatic Substitution: The ortho/para Module Tag CHE_P5_M29 TABLE OF CONTENTS 1. Learning Outcomes
CHEM120 - ORGANIC CHEMISTRY WORKSHEET 1
 EM120 - RGANI EMISTRY WRKSEET 1 Some of the objectives To understand and know the hybridization concept Be able to distinguish different geometries, including basic bond lengths and angles within organic
EM120 - RGANI EMISTRY WRKSEET 1 Some of the objectives To understand and know the hybridization concept Be able to distinguish different geometries, including basic bond lengths and angles within organic
REASONING QUESTIONS FROM ORGANIC CHEMISTRY (CH. 1 & 2)
 REASONING QUESTIONS FROM ORGANIC CHEMISTRY (CH. 1 & 2) 1.) Why do haloalkenes under go nucleophillic substitution whereas haloarenes under go electophillic substitution. Ans. Due to more electro negative
REASONING QUESTIONS FROM ORGANIC CHEMISTRY (CH. 1 & 2) 1.) Why do haloalkenes under go nucleophillic substitution whereas haloarenes under go electophillic substitution. Ans. Due to more electro negative
Downloaded from
 1 Class XII Chemistry Chapter: Alcohols, Phenols And Ethers Top concepts: 1. Structure of alcohols, phenols and ethers: 2. Preparation of alcohols: 3. Preparation of phenols: 2 4. Physical properties of
1 Class XII Chemistry Chapter: Alcohols, Phenols And Ethers Top concepts: 1. Structure of alcohols, phenols and ethers: 2. Preparation of alcohols: 3. Preparation of phenols: 2 4. Physical properties of
Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water.
 Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water. Alcohols are usually classified as primary, secondary and tertiary. Alcohols with the hydroxyl bound directly
Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water. Alcohols are usually classified as primary, secondary and tertiary. Alcohols with the hydroxyl bound directly
c. Oxidizing agent shown here oxidizes 2º alcohols to ketones and 1º alcohols to carboxylic acids. 3º alcohols DO NOT REACT.
 Exam 1 (Ch 17 and Review of CEM 331) Answer Key: 1. ne-step Questions: You need to know reagents for reagent arrows and to be able to draw products. I know a lot of them seem to look alike its your job
Exam 1 (Ch 17 and Review of CEM 331) Answer Key: 1. ne-step Questions: You need to know reagents for reagent arrows and to be able to draw products. I know a lot of them seem to look alike its your job
Aromatic Compounds II
 2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
Carbon-heteroatom single bonds basic C N C X. X= F, Cl, Br, I Alkyl Halide C O. epoxide Chapter 14 H. alcohols acidic H C S C. thiols.
 hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
Organic Chemistry. M. R. Naimi-Jamal. Faculty of Chemistry Iran University of Science & Technology
 Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 5-2. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry,
Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 5-2. Chemistry of Benzene: Electrophilic Aromatic Substitution Based on McMurry s Organic Chemistry,
Chem 263 March 28, 2006
 Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chapter 17: Alcohols and Phenols. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 17: Alcohols and Phenols Based on McMurry s Organic Chemistry, 7 th edition Alcohols and Phenols Alcohols contain an OH group connected to a a saturated C (sp 3 ) They are important solvents and
Chapter 17: Alcohols and Phenols Based on McMurry s Organic Chemistry, 7 th edition Alcohols and Phenols Alcohols contain an OH group connected to a a saturated C (sp 3 ) They are important solvents and
CHAPTER 23 HW: ENOLS + ENOLATES
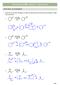 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
R N R N R N. primary secondary tertiary
 Chapter 19 Amines omenclature o assification of amines Amines are classified as 1, 2, or 3 based on how many R groups are attached to the nitrogen R R R R R R primary secondary tertiary When there are
Chapter 19 Amines omenclature o assification of amines Amines are classified as 1, 2, or 3 based on how many R groups are attached to the nitrogen R R R R R R primary secondary tertiary When there are
Chem 263 Nov 24, Properties of Carboxylic Acids
 Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Organic Chemistry. Second Edition. Chapter 19 Aromatic Substitution Reactions. David Klein. Klein, Organic Chemistry 2e
 Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Loudon Chapter 14 Review: Reactions of Alkynes Jacquie Richardson, CU Boulder Last updated 1/16/2018
 An alkyne is any molecule with a triple bond between two carbon atoms. This triple bond consists of one σ bond and two π bonds: the σ bond exists on a straight line between carbon atoms, while one π bond
An alkyne is any molecule with a triple bond between two carbon atoms. This triple bond consists of one σ bond and two π bonds: the σ bond exists on a straight line between carbon atoms, while one π bond
Chapter 8. Acidity, Basicity and pk a
 Chapter 8 Acidity, Basicity and pk a p182 In this reaction water is acting as a base, according to our definition above, by accepting a proton from HCl which in turn is acting as an acid by donating a
Chapter 8 Acidity, Basicity and pk a p182 In this reaction water is acting as a base, according to our definition above, by accepting a proton from HCl which in turn is acting as an acid by donating a
Chapter 7: Alcohols, Phenols and Thiols
 Chapter 7: Alcohols, Phenols and Thiols Nomenclature of Alcohols In the IUPAC system, the hydroxyl group in alcohols is indicated by the ending ol. In common names, the separate word alcohol is placed
Chapter 7: Alcohols, Phenols and Thiols Nomenclature of Alcohols In the IUPAC system, the hydroxyl group in alcohols is indicated by the ending ol. In common names, the separate word alcohol is placed
20 Halogenoalkanes:substitution and elimination reactions
 20 alogenoalkanes:substitution and elimination reactions s to worked examples WE 20.1 Allylic halogenations (on p. 922 in Chemistry 3 ) 1-(Chloromethyl)benzene (benzyl chloride, PhC 2 ) is made on a large
20 alogenoalkanes:substitution and elimination reactions s to worked examples WE 20.1 Allylic halogenations (on p. 922 in Chemistry 3 ) 1-(Chloromethyl)benzene (benzyl chloride, PhC 2 ) is made on a large
Synthesis Using Aromatic Materials
 Chapter 10 Synthesis Using Aromatic Materials ELECTROPHILIC AROMATIC SUBSTITUTION AND DIRECTED ORTHO METALATION Copyright 2018 by Nelson Education Limited 1 10.2 p Bonds Acting as Nucleophiles Copyright
Chapter 10 Synthesis Using Aromatic Materials ELECTROPHILIC AROMATIC SUBSTITUTION AND DIRECTED ORTHO METALATION Copyright 2018 by Nelson Education Limited 1 10.2 p Bonds Acting as Nucleophiles Copyright
Chem 263 Oct. 6, Single bonds, σ. e - donating Activate Activate ortho and para directing ortho and para directing
 Chem 263 ct. 6, 2009 lectrophilic Substitution of Substituted Benzenes Resonance ffect Inductive ffect C=C, π system Single bonds, σ Strong Weak e - donating Activate Activate ortho and para directing
Chem 263 ct. 6, 2009 lectrophilic Substitution of Substituted Benzenes Resonance ffect Inductive ffect C=C, π system Single bonds, σ Strong Weak e - donating Activate Activate ortho and para directing
AND TECHNIQUES. Unit. I. Multiple Choice Questions (Type-I)
 Unit 12 ORGANIC CHEMISTRY SOME BASIC B PRINCIPLES AND TECHNIQUES I. Multiple Choice Questions (Type-I) 1. Which of the following is the correct IUPAC name? 3-Ethyl-4, 4-dimethylheptane 4,4-Dimethyl-3-ethylheptane
Unit 12 ORGANIC CHEMISTRY SOME BASIC B PRINCIPLES AND TECHNIQUES I. Multiple Choice Questions (Type-I) 1. Which of the following is the correct IUPAC name? 3-Ethyl-4, 4-dimethylheptane 4,4-Dimethyl-3-ethylheptane
Examples of Substituted Benzenes
 Organic Chemistry 5 th Edition Paula Yurkanis Bruice Examples of Substituted Benzenes Chapter 15 Reactions of Substituted Benzenes Irene Lee Case Western Reserve University Cleveland, OH 2007, Prentice
Organic Chemistry 5 th Edition Paula Yurkanis Bruice Examples of Substituted Benzenes Chapter 15 Reactions of Substituted Benzenes Irene Lee Case Western Reserve University Cleveland, OH 2007, Prentice
Module 2 Acids and Bases. Lecture 3 Acids and Bases
 Module 2 Acids and Bases Lecture 3 Acids and Bases 2.1 Concepts A compound is classified as an acid or a base based on certain properties. At present there are several theories which define the concepts
Module 2 Acids and Bases Lecture 3 Acids and Bases 2.1 Concepts A compound is classified as an acid or a base based on certain properties. At present there are several theories which define the concepts
CHAPTER 16 - CHEMISTRY OF BENZENE: ELECTROPHILIC AROMATIC SUBSTITUTION
 CAPTR 16 - CMISTRY F BNZN: LCTRPILIC ARMATIC SUBSTITUTIN As stated in the previous chapter, benzene and other aromatic rings do not undergo electrophilic addition reactions of the simple alkenes but rather
CAPTR 16 - CMISTRY F BNZN: LCTRPILIC ARMATIC SUBSTITUTIN As stated in the previous chapter, benzene and other aromatic rings do not undergo electrophilic addition reactions of the simple alkenes but rather
CHEM 240: Survey of Organic Chemistry at North Dakota State University Midterm Exam 02 - Tue, 23 Sep 2014!! Name:! KEY!
 CEM 240: Survey of rganic Chemistry at orth Dakota State University Midterm Exam 02 - Tue, 23 Sep 2014!! ame:! KEY! Please read through each question carefully and answer in the spaces provided. A good
CEM 240: Survey of rganic Chemistry at orth Dakota State University Midterm Exam 02 - Tue, 23 Sep 2014!! ame:! KEY! Please read through each question carefully and answer in the spaces provided. A good
Ch 20 Carboxylic Acids and Nitriles
 Ch 20 Carboxylic Acids and Nitriles Carboxylic Acids (RCO 2 H) are compounds with an OH attached to a carbonyl. Nitriles (RC N) are compounds a carbon-nitrogen triple bond. Naming Carboxylic Acids 1. Replace
Ch 20 Carboxylic Acids and Nitriles Carboxylic Acids (RCO 2 H) are compounds with an OH attached to a carbonyl. Nitriles (RC N) are compounds a carbon-nitrogen triple bond. Naming Carboxylic Acids 1. Replace
Learning Guide for Chapter 17 - Dienes
 Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2
 16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
16. Chemistry of Benzene: Electrophilic Aromatic Substitution جانشینی الکتروندوستی آروماتیک شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based
Chapter 5. Nucleophilic aliphatic substitution mechanism. by G.DEEPA
 Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Topic 6 Alkyl halide and carbonyl compounds Organic compounds containing a halogen
 Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way, eg: 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene
Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way, eg: 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene
Practice Hour Examination # 1-1
 CHEM 346 Organic Chemistry I Fall 2013 Practice Hour Examination # 1-1 Solutions Key Page 1 of 12 CHEM 346 Organic Chemistry I (for Majors) Instructor: Paul J. Bracher Practice Hour Examination # 1-1 Monday,
CHEM 346 Organic Chemistry I Fall 2013 Practice Hour Examination # 1-1 Solutions Key Page 1 of 12 CHEM 346 Organic Chemistry I (for Majors) Instructor: Paul J. Bracher Practice Hour Examination # 1-1 Monday,
Ch 16 Electrophilic Aromatic Substitution
 Ch 16 Electrophilic Aromatic Substitution Mechanism - Aromatic rings typically undergo substitution, where an H is replaced with an electrophile (E+). - The rings do not typically undergo addition across
Ch 16 Electrophilic Aromatic Substitution Mechanism - Aromatic rings typically undergo substitution, where an H is replaced with an electrophile (E+). - The rings do not typically undergo addition across
Chapter 2 Polar Covalent Bonds; Acids and Bases SAMPLE. Chapter Outline
 Chapter 2 Polar Covalent Bonds; Acids and Bases Chapter utline I. Polar covalent bonds (Sections 2.1 2.3). A. Electronegativity (Section 2.1). 1. Although some bonds are totally ionic and some are totally
Chapter 2 Polar Covalent Bonds; Acids and Bases Chapter utline I. Polar covalent bonds (Sections 2.1 2.3). A. Electronegativity (Section 2.1). 1. Although some bonds are totally ionic and some are totally
Chapter 3. Alkenes And Alkynes
 Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
UNIT 11 ALCOHOLS, PHENOLS & ETHERS
 UNIT 11 ALCOHOLS, PHENOLS & ETHERS Alcohols and Phenol are Organic compound with a hydroxyl group (- OH) as functional group. Ethers are identified by a functional group ( 0 ). Cyclic alcohols are named
UNIT 11 ALCOHOLS, PHENOLS & ETHERS Alcohols and Phenol are Organic compound with a hydroxyl group (- OH) as functional group. Ethers are identified by a functional group ( 0 ). Cyclic alcohols are named
CHEM 343 Principles of Organic Chemistry II Summer Instructor: Paul J. Bracher. Quiz # 3. Monday, July 21 st, :30 a.m.
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
Additions to the Carbonyl Groups
 Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
