Boardworks A2 Chemistry
|
|
|
- Kerry Webb
- 5 years ago
- Views:
Transcription
1 Boardworks A2 Chemistry Organic Synthesis 34 slides 13 Flash activities Optical isomerism Introduction to light waves Animation illustrating the nature of polarized light Optically active compounds, enantiomers and chiral carbon atoms Identifying chiral carbons Animation illustrating how the optical activity of compounds can be measured Properties of optical isomers Optically active compounds Organic Animation illustrating how nucleophilic attack of carbonyl compounds can lead to the formation of optical isomers Optical isomers and drug manufacture, including the thalidomide tragedy Separating optical isomers and methods of obtaining optically pure compounds, including chiral catalysts Identifying true-or-false statements relating to optically active compounds Synthetic routes The importance of organic synthesis Overview of different functional groups Activity to identify the different functional groups present in a compound Overview of how compound properties can be predicted from the functional groups present in its structure Summary of organic reactions and synthetic routes Devising a synthetic route 1/22
2 Carbonyl Compounds 38 slides 16 Flash activities Aldehydes & ketones Introduction to the carbonyl group Representing and naming aldehydes and ketones Identifying the names of aldehydes and ketones Animations illustrating the synthesis of aldehydes and ketones from alcohols Properties of aldehydes and ketones Animated graph comparing the boiling points of aldehydes and ketones with alcohols and alkanes of similar M r Reactions of aldehydes & ketones Orgnaic Overview of the polarity of the carbonyl group as the basis for the reactivity of carbonyl compounds Animation illustrating the nucleophilic addition reaction mechanism of cyanide and the carbonyl group Animations illustrating the reaction mechanisms of the reduction of aldehydes and ketones with a reducing agent such as sodium tetrahydridoborate Virtual experiments illustrating the use of Tollens reagent and Fehling s solution to distinguish between aldehydes and ketones Animation illustrating the use of 2,4-DNPH (Brady s reagent) to identify an aldehyde or ketone Identifying the conditions needed for various reactions involving aldehydes and ketones Identifying true-or-false statements about reactions involving aldehydes and ketones Carboxylic acids Overview of the carboxyl group Identifying the names of carboxylic acids Animated graph comparing the boiling points of carboxylic acids with alcohols, aldehydes and alkanes of similar M r. Preparation of carboxylic acids by the oxidation of primary alcohols or aldehydes, and by the hydrolysis of nitriles Virtual experiment illustrating the preparation of carboxylic acids by the oxidation of primary alcohols 2/22
3 Reactions of carboxylic acids Overview of the type of reactions involving carboxylic acids Formation of the carboxylate ion, and the reaction of carboxylic acids with carbonates and alkalis Identifying missing words relating to carboxylic acids 3/22
4 Acyl Compounds 38 slides 17 Flash activities Esters Organic Introduction to esters Animation illustrating the formation of esters from carboxylic acids and alcohols Nomenclature of esters Activity to identify the names of esters from their structures Animations illustrating the hydrolysis of esters by hydrochloric acid and by sodium hydroxide Uses of esters Animation illustrating the structure of triglycerides in terms of glycerol and fatty acids Identifying true-or-false statements about esters Guide to saturated and unsaturated fatty acids, and the cis/trans configuration Trans fats, saturated fats, cholesterol and health Animations illustrating the formation of soap and biodiesel from triglycerides Acid chlorides & acid anhydrides Overview of acylation and acid derivatives Overview and nomenclature of acid chlorides and acid anhydrides Identifying structures as being acid chlorides, acid anhydrides or esters Activity to identify the names of acid chlorides and acid anhydrides from their structures Addition elimination reactions Overview of the reactivity of acid chlorides and acid anhydrides Animation illustrating the reaction mechanism of acid chlorides and nucleophiles Animations illustrating the reaction mechanisms of acid chlorides with water, alcohols, ammonia and amines Identifying the relative reactivity of nucleophiles in their reactions with acid chlorides Applications of acylation reactions Animations illustrating the reaction mechanisms of the formation of paracetamol and aspirin Identifying missing words relating to acid derivatives 4/22
5 Aromatic Chemistry 36 slides 15 Flash activities Bonding in benzene The history of the structure of benzene The Kekulé structure of benzene and problems with the Kekulé structure: evidence from hydrogenation, bromination and isomers of dibromobenzene Animation illustrating the role of thermochemical data in determining the structure of benzene: comparing hydrogenation enthalpies of benzene and cyclohexene Bond lengths in benzene: evidence from X-ray crystallography Identifying missing words relating to problems with the Kekulé structure of benzene Animation illustrating the delocalized structure of benzene Identifying true-or-false statements relating to the structure of benzene The evolution of scientific knowledge as illustrated by the history of benzene Organic Properties of arenes Overview of the arenes: physical properties and nomenclature Animation illustrating the conventions for naming aromatic compounds Activity to identify the names of arenes from their structures Reactions of arenes Overview of how delocalization affects the reactivity of arenes Animation illustrating the general mechanism of electrophilic substitution reactions Animation illustrating the mechanism of the nitration of benzene Uses of nitrated arenes Overview of Friedel Crafts reactions Animations illustrating the reaction mechanisms of Friedel Crafts alkylation and Friedel Crafts acylation Animation illustrating the reaction mechanism of benzene with bromine Animation illustrating the reaction of phenol and sodium Reaction of phenol with bromine Identifying components of electrophilic substitution reactions involving arenes 5/22
6 Amines 34 slides 10 Flash activities Properties of amines Overview of amines: structure and shape Identifying primary, secondary and tertiary amines from their structures Nomenclature of amines Activity to identify the names of amines from their structures Physical properties of amines Synthesis of amines Organic Synthesis of amines from ammonia and halogenoalkanes Synthesis of amines via the reduction of nitriles The preparation of phenylamine Identifying which conditions are needed to synthesize amines in different types of reactions Reactions of amines Aliphatic and aromatic amines Animation illustrating the action of amines as Brønsted Lowry bases Relative base strength of the amines, and the reactions of amines as bases to form salts Animation illustrating the action of amines as nucleophiles: reaction with halogenoalkanes to form secondary, tertiary and quaternary amines Uses of quaternary ammonium salts Animations illustrating the reaction of amines with acyl compounds (acid chlorides and acid anhydrides) The synthesis of diazonium salts and azo compounds, and uses of azo compounds Identifying true-or-false statements relating to the reactions of amines 6/22
7 Polymers and Amino Acids Boardworks A2 Chemistry Contents Guide 36 slides 16 Flash activities Addition polymers Overview of different types of polymer Naming addition polymers Guide to common addition polymers Identifying addition polymers Disposal of addition polymers Condensation polymers Organic Overview of condensation polymers Animation illustrating the formation of esters from carboxylic acids and alcohols, and the formation of polyesters from dicarboxylic acids and diols, including the formation of PET Animation illustrating the formation of amides from carboxylic acids and amines, and the formation of polyesters from dicarboxylic acids and diamines, including the formation of nylon-6,6 and Kevlar Disposal of condensation polymers Acidic and basic hydrolysis of polyesters and polyamides Identifying statements as referring to either addition polymers or condensation polymers Identifying types of polymer: addition, polyester or polyamide Identifying polymers from monomers Amino acids and proteins Overview of amino acids Guide to the 22 proteinogenic amino acids Animation illustrating the formation of zwitterions Overview of the acid base nature of amino acids Virtual experiment exploring the effect of changing ph on amino acids Animation illustrating the formation of a peptide bond between two amino acids Animation illustrating the primary, secondary, tertiary and quaternary structure of proteins The types of bonds in proteins The two main types of protein: fibrous and globular, including the structure of enzymes Identifying true-or-false statements relating to amino acids and proteins 7/22
8 Structure Determination 45 slides 21 Flash activities Mass spectrometry Overview of mass spectrometry Animation illustrating how fragmentation can help to identify compounds Animation illustrating the fragmentation of carbonyl compounds Activity to interpret mass spectra Uses of mass spectrometry Infrared spectroscopy Overview of infrared spectroscopy Infrared spectra of different functional groups Activity to interpret infrared spectra Uses of infrared spectroscopy Organic Nuclear magnetic resonance spectroscopy Animation illustrating how NMR spectroscopy works Uses of NMR spectroscopy Animation illustrating carbon-13 NMR spectroscopy Activity to interpret 13 C NMR spectra Chemical shift and the use of TMS as a reference compound Activity to assign peaks on 13 C NMR spectra Animation illustrating proton NMR spectroscopy Activity to interpret 1 H NMR spectra Integration of 1 H NMR spectra Animation illustrating the process of spin coupling, including the n+1 rule Activity to interpret splitting patterns on 1 H NMR spectra Activity to assign peaks on 1 H NMR spectra Uses of NMR spectroscopy Chromatography Overview of chromatography Animation illustrating thin layer chromatography Animation illustrating column chromatography Overview of high performance liquid chromatography Animation illustrating gas liquid chromatography Identifying true-or-false statements relating to chromatography 8/22
9 Boardworks A2 Chemistry Contents Guide 9/22
10 Kinetics 26 slides 15 Flash activities Introduction to rates of reaction Introduction to rate of reaction and activation energy Guide to factors affecting the rate of reaction Identifying true-or-false statements about rates of reaction Introduction to measuring rates of reaction Animation illustrating three common methods for measuring rate of reaction Identifying missing words relating to rate of reaction Rate equations Physical Introduction to the rate equation Animations illustrating zero, first and second order reactions Completing a table showing the effect of changing concentration on rate for zero, first and second order reactions The effect of temperature on the rate constant, k Identifying true-or-false statements about rate equations Calculations and experiments Example worked calculations relating to experimental data and the rate equation Calculations relating to experimental data and the rate equation, including full working out Introduction to the rate-determining step Animation explaining the rate-determining step in more detail Guide to using the rate equation to suggest a possible mechanism for a reaction Identifying the correct answer to questions about the rate-determining step 10/22
11 Equilibria Boardworks A2 Chemistry Contents Guide 33 slides 16 Flash activities The equilibrium constant, K c Recap of dynamic equilibria Animated graph explaining the position of equilibrium Introduction to the equilibrium constant, K c Guide to calculating the units of K c for a reaction, including full working out Multiple choice quiz about K c expressions and units Animation illustrating how to calculate the value of K c for a reaction Interpreting K c values Calculations involving K c, including full working out Physical The equilibrium constant, K p Overview of gas equilibria Guide to calculating partial pressures of gases Calculations involving partial pressures, including full working out Introduction to the equilibrium constant, K p Units of K p Multiple choice quiz about K p units Animation illustrating how to calculate the value of K p for a reaction Calculations involving K p, including full working out Factors affecting equilibria Recap of Le Chatelier s principle Overview of the effect of temperature and concentration on the value of K c Completing a table showing the effect of pressure changes on the equilibrium position The effect of a catalyst on K c Identifying true-or-false statements about Le Chatelier s principle Application of equilibria Guide to the effect of different factors on the value of K p in an industrial equilibrium Atom economy in industrial equilibria 11/22
12 Acids and Bases Boardworks A2 Chemistry Contents Guide 51 slides 31 Flash activities Acid base theory Guide to definitions of acids and bases Monoprotic and diprotic acids Conjugate acids and bases Identifying acids and bases, and conjugate acids and bases ph, K a and K w Physical Introduction to ph and strong and weak acids Guide to calculating the ph of a strong acid Calculations of the ph of strong acids, including full working out Calculating [H + ] from ph Calculations of [H + ] from ph, including full working out Weak acids, K a and pk a Animation illustrating how to derive the equation for calculating the ph of a weak acid Guide to calculating the ph of a weak acid Calculations of the ph of weak acids, including full working out Introduction to K w Animation illustrating the use of K w to calculate the ph of water and the variation of K w and ph with temperature. Guide to calculating the ph of a strong base using K w Calculations involving the ph of strong bases, including full working out Summary guide to the methods of calculating ph for strong and weak acids and strong bases Summary ph calculations, including full working out ph curves and indicators Introduction to acid base titration Animations illustrating ph curves for the following titrations: strong acid vs. strong base, weak acid vs. strong base, strong acid vs. weak base and weak acid vs. weak base Example worked calculations from the results of titration experiments Selecting an appropriate indicator Guide to selecting an appropriate indicator based on ph curves Animation illustrating approximations that can be made at the half-neutralization point, and how this can be used to find ph and K a Identifying missing words relating to titrations Example worked calculations of ph for strong acid strong base titrations 12/22
13 Calculations of ph for strong acid strong base titrations, including full working out Buffer solutions Introduction to buffer solutions Animations illustrating how acidic buffers work Buffers in the body Example worked calculations of ph for buffer solutions Calculations involving the ph of buffer solutions, including full working out Making a buffer from strong base and weak acid Example worked calculations of ph for buffers made from strong base and weak acid Step-by-step calculation of the ph for a buffer solution made from a strong base and a weak acid, including full working out Changes in buffer ph when small amounts of acid or base are added Example worked calculations of change in buffer ph when small amounts of acid or base are added Calculations involving the change in buffer ph when small amounts of acid or base are added, including full working out Guide to selecting the correct method to use for ph calculations 13/22
14 Thermodynamics 43 slides 24 Flash activities Enthalpy changes Introduction to enthalpy changes Guide to different types of enthalpy change Identifying the correct equation for various enthalpy changes Introduction to bond enthalpies Calculations involving enthalpy change using bond enthalpies, including full working out Born Haber cycles Animation explaining Born Haber cycles Guide to constructing a Born Haber cycle for NaCl Physical Born Haber cycle for MgCl 2 Completing Born Haber cycles and calculations based on them Identifying the correct answer to calculations based on Born Haber cycles Lattice formation enthalpies Animation illustrating polarizability, covalent character and their affect on lattice enthalpies Identifying missing words relating to polarization and lattice enthalpies Enthalpies of solution Standard enthalpy of solution and standard enthalpy of hydration Animation illustrating the use of Hess s Law to calculate enthalpy of solution Calculations involving enthalpies of solution Factors affecting enthalpy of hydration Entropy Guide to the need for another factor as well as enthalpy to explain spontaneous reactions Introduction to entropy Animation illustrating entropy changes for melting and evaporation, and for reactions involving changes in the complexity of molecules Identifying whether entropy changes for particular reactions are positive or negative Calculating entropy change for a reaction Example worked calculations of entropy changes for various reactions Calculations involving entropy changes for various reactions, including full working out Animation explaining what the entropy change for the surroundings is and how to calculate it Calculations involving the entropy change for the surroundings for various reactions, including full working out 14/22
15 Introduction to Gibbs free energy Example worked calculations of Gibbs free energy change for various reactions Calculations involving Gibbs free energy change for various reactions, including full working out Discussion of when a reaction is feasible and how to find the lowest temperature at which a reaction will be feasible Calculations involving temperatures at which various reactions become feasible, including full working out Using G sol to work out whether a substance will be soluble in water at a particular temperature Using both thermodynamic and kinetic considerations to decide whether a reaction will occur 15/22
16 Periodicity in Period 3 35 slides 16 Flash activities Physical properties Guide to the physical properties of period 3 elements Identifying the physical properties of period 3 elements Reactions with water and oxygen Inorganic Animations illustrating the reaction of sodium, magnesium and chlorine with water Summary of the reactions of period 3 elements with water Animations illustrating the reaction of period 3 elements (Na S) with oxygen Summary of the reactions of period 3 elements with oxygen Calculating the oxidation states in reactions of period 3 elements and oxygen Identifying missing words relating to the reactions of period 3 elements with water and oxygen Reactions of oxides Guide to the physical properties of period 3 oxides Virtual experiment investigating the reactivity of period 3 (Na P) oxides with water Virtual experiment investigating the reactivity of sulfur oxides with water Na 2 O and MgO as alkaline oxides Al 2 O 3 and SiO 2 as insoluble oxides P 4 O 10, SO 2 and SO 3 as acidic oxides Matching period 3 oxides to the ph of the solution they form after reacting with water Identifying true-or-false statements about period 3 oxides and water The reactions of Na 2 O and MgO with bases The amphoteric nature of Al 2 O 3 The reactions of SiO 2, P 4 O 10 and SO 2 with bases Identifying missing substances in the reactions of period 3 oxides with acids and bases Identifying key trends in period 3 elements and their oxides 16/22
17 Redox Chemistry and Electrode Potentials 35 slides 15 Flash activities Redox reactions Identifying reactions as being redox or not Identifying statements as referring to either oxidation or reduction Guide to the general rules for assigning oxidation states Identifying the oxidation states of different elements Identifying missing words relating to oxidation states Inorganic Balancing redox reactions Guide on how to combine and balance half equations Balancing equations when given two half equations Half cells Introduction to half cells Animation introducing electrode potentials and the creation electrochemical cells using two half cells, a salt bridge and a voltmeter Virtual experiment to combine different half cells to create electrochemical cells Animation introducing the standard hydrogen electrode Virtual experiment to combine different half cells with a standard hydrogen electrode Cell diagrams and he electrochemical series Guide on how to calculate E cell by combining half cell equations Activity to calculate E cell for different combinations of half cells Feasibility of reactions Guide on how to determine the feasibility of reactions using standard electrode potentials Identifying whether different redox reactions are feasible or not feasible 17/22
18 Applications of Redox Chemistry 30 slides 16 Flash activities Cells and batteries Inorganic Overview of electrochemical cells How an electrochemical cell works Introduction to primary cells Guide to different types of secondary cells Identifying the reactions in a zinc carbon cell Identifying primary and secondary cells Definition of a battery Guide to a lead acid battery Calculating the e.m.f. of cells and batteries Fuel cells Overview of fuel cells, and the reactions taking place in a hydrogen fuel cell Animation illustrating how a hydrogen fuel cell works Labelling species in a hydrogen fuel cell Methods of producing hydrogen gas Guide to different methods of storing hydrogen Introduction to hydrogen-rich fuels Animation illustrating how a direct methanol fuel cell works Identifying the reactions taking place in a direct methanol fuel cell Development of an ethanol fuel cell Identifying properties of fuel cell vehicles and conventional vehicles Guide to different opinions about a hydrogen economy How fuel cell technology has been applied to modern breath alcohol testers 18/22
19 Introducing Transition Metals 33 slides 16 Flash activities Properties of transition metals Definitions of a transition metal Completing the electron configurations of transition metal atoms and ions Successive ionization energies as evidence of transition metal electronic structure Graphs of successive ionization energies of vanadium, chromium and manganese Distinguishing between transition metals and d block elements Identifying transition metals and d block elements Inorganic Variable oxidation states Variable oxidation states in transition metals Ordering the oxidation states of transition metal compounds Ordering the oxidation states of vanadium compounds How oxidation state affects bonding Guide to common oxidation states of transition metals Transition metal complexes Introduction to ligands Features of bidentate and multidentate ligands Identifying the bonding atoms in ligands Identifying unidentate, bidentate and multidentate ligands Co-ordination numbers Guide to complex ions Identifying properties of a complex ion from a structural diagram Guide to the different shapes of complex ions Cis trans and optical isomerism Identifying true-or-false statements relating to transition metal complex ions Identifying the shape and co-ordination number of complex ions 19/22
20 Transition Metals: Colours and Reactions 43 slides 14 Flash activities Transition metal colours How we see colour Why transition metal ions absorb light Animation of energy changes in d orbitals Factors affecting the colours of transition metal complexes Identifying true-or-false statements relating to colours of complex ions Using UV Vis spectroscopy to determine concentrations of complex ion solutions Animation illustrating how to determine the concentration of a solution using spectroscopy Improving UV Vis spectroscopy by exchanging ligands Inorganic Hydrolysis reactions Overview of hydrolysis reactions Differing acidity of aqua ions Ordering aqua ions by acidity, using charge and ionic radius Hydrolysis reactions of M 2+ and M 3+ ions Reactions with bases, including ammonia, hydroxide ions and carbonate ions Virtual experiment exploring the reaction of bases with aqua ion solutions Selecting the correct numbers to balance hydrolysis equations Amphoteric character Matching reaction terms with their definitions Ligand substitution reactions Introduction to ligand substitution reactions Virtual experiment exploring ligand substitution reactions Identifying reactants and conditions in acid base and ligand substitution reactions An overview of stability constants The chelate effect Predicting the direction of reversible ligand substitution reactions based on entropy Ligand substitution in haemoglobin 20/22
21 Redox reactions Redox reactions in transition metals Oxidation of cobalt(ii) Reduction of chromium(vi) Identifying the type of reaction Boardworks A2 Chemistry Contents Guide 21/22
22 Uses of Transition Metals 40 slides 14 Flash activities Catalysis Inorganic Introduction to transition metals as catalysts; heterogeneous and homogeneous catalysis Animation illustrating the process of heterogeneous catalysis Improving catalytic efficiency Catalysts in the Haber and Contact processes, and the production of methanol Identifying missing words relating heterogeneous catalysts Introduction to homogeneous catalysis Reaction of iodine and peroxodisulfate ions Introduction to autocatalysis Oxidation of ethanedioic acid Matching industrial products to the catalysts used in their production Development of new catalysts Identifying true-or-false statements relating to industrial catalysts Redox titrations Introduction to redox titrations Identifying missing words relating to the oxidation states of manganese Virtual experiment exploring a potassium manganate(vii) titration Guide to calculating potassium manganate(vii) titration results, including full working out Introduction to potassium dichromate(vi) titrations Identifying missing numbers in ionic equations for the reaction of potassium dichromate(vi) with Fe 2+ ions Guide to calculating potassium dichromate(vi) titration results, including full working out The reaction of iodine and thiosulfate ions Calculations involving the reaction of iodine and thiosulfate ions, including full working out Applications of transition metals Uses of transition metal in catalytic converters, polychromic sunglasses, chemotherapy drugs specifically cisplatin paints and dyes and alloys Matching transition metal properties to their uses 22/22
UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry
 UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry Topic 4.1 Kinetics a) Define the terms: rate of a reaction, rate constant, order of reaction and overall order of reaction b) Deduce the orders of reaction
UNIT 4 REVISION CHECKLIST CHEM 4 AS Chemistry Topic 4.1 Kinetics a) Define the terms: rate of a reaction, rate constant, order of reaction and overall order of reaction b) Deduce the orders of reaction
GCSE to A-level progression
 . GCSE to A-level progression AQA A-level Chemistry is a natural next stage from the GCSE course and so there are many recognisable topics that are taken a stage further. Some topics, such as atomic structure,
. GCSE to A-level progression AQA A-level Chemistry is a natural next stage from the GCSE course and so there are many recognisable topics that are taken a stage further. Some topics, such as atomic structure,
TUTORIAL LIST 2017/18
 TUTORIAL LIST 2017/18 ATOMIC STRUCTURE & MOLE CALCULATIONS BONDING & INORGANIC CHEMISTRY PHYSICAL CHEMISTRY ORGANIC CHEMISTRY PRACTICAL CHEMISTRY PAST PAPER WALKTHROUGHS ATOMIC STRUCTURE & MOLE CALCULATIONS
TUTORIAL LIST 2017/18 ATOMIC STRUCTURE & MOLE CALCULATIONS BONDING & INORGANIC CHEMISTRY PHYSICAL CHEMISTRY ORGANIC CHEMISTRY PRACTICAL CHEMISTRY PAST PAPER WALKTHROUGHS ATOMIC STRUCTURE & MOLE CALCULATIONS
A-LEVEL A-LEVEL CHEMISTRY CHEMISTRY NOTES
 A-LEVEL A-LEVEL CHEMISTRY CHEMISTRY NOTES snaprevise.co.uk I have designed and compiled these beautiful notes to provide a detailed but concise summary of this module. I have spent a lot of time perfecting
A-LEVEL A-LEVEL CHEMISTRY CHEMISTRY NOTES snaprevise.co.uk I have designed and compiled these beautiful notes to provide a detailed but concise summary of this module. I have spent a lot of time perfecting
Topic 1: Quantitative chemistry
 covered by A-Level Chemistry products Topic 1: Quantitative chemistry 1.1 The mole concept and Avogadro s constant 1.1.1 Apply the mole concept to substances. Moles and Formulae 1.1.2 Determine the number
covered by A-Level Chemistry products Topic 1: Quantitative chemistry 1.1 The mole concept and Avogadro s constant 1.1.1 Apply the mole concept to substances. Moles and Formulae 1.1.2 Determine the number
AQA A-level Chemistry Year 2 Scheme of Work
 AQA A-level Chemistry Year 2 Scheme of Work Scheme of Work AQA A-level Chemistry Year 2 of A-level This course covers the requirements of the second year of the AQA AS and A-level Chemistry specification.
AQA A-level Chemistry Year 2 Scheme of Work Scheme of Work AQA A-level Chemistry Year 2 of A-level This course covers the requirements of the second year of the AQA AS and A-level Chemistry specification.
Introduction. A1.1 (a) Shell number and number of subshells 1. A1.1 (b) Orbitals 2. A1.1 (c ) Orbital shapes (s, p & d) 2
 Preface Table of Contents Introduction i A1.1 (a) Shell number and number of subshells 1 A1.1 (b) Orbitals 2 A1.1 (c ) Orbital shapes (s, p & d) 2 A1.1 (d) Relative energies of s,p,d,f sub-shells 4 A 1.1
Preface Table of Contents Introduction i A1.1 (a) Shell number and number of subshells 1 A1.1 (b) Orbitals 2 A1.1 (c ) Orbital shapes (s, p & d) 2 A1.1 (d) Relative energies of s,p,d,f sub-shells 4 A 1.1
Pearson Edexcel AS and A level Chemistry
 Pearson Edexcel AS and A level Chemistry What s Changed? Level Topic Spec Points New Content Included Content Not Included in the New Specification Implications of the New Spec 1-5 Greater clarity in terms
Pearson Edexcel AS and A level Chemistry What s Changed? Level Topic Spec Points New Content Included Content Not Included in the New Specification Implications of the New Spec 1-5 Greater clarity in terms
Teaching scheme. Student book links. Practical activity links. Weekly learning outcomes
 Teaching scheme Week 1 1. Revision of AS 2.1.3 2. Benzene s structure historical to modern 3. The evidence for and against the delocalised model 4. The extra stability of the benzene molecule and its reluctance
Teaching scheme Week 1 1. Revision of AS 2.1.3 2. Benzene s structure historical to modern 3. The evidence for and against the delocalised model 4. The extra stability of the benzene molecule and its reluctance
Department Curriculum and Assessment Outline
 Timing Department: Science Year Group: 0 Teaching, learning and assessment during the course: Chemistry (Combined) C/C States of matter/methods of Separating and purifying substances C3 Atomic structure
Timing Department: Science Year Group: 0 Teaching, learning and assessment during the course: Chemistry (Combined) C/C States of matter/methods of Separating and purifying substances C3 Atomic structure
3.4 A2 Unit F324: Rings, Polymers and Analysis
 3.4 A2 Unit F324: Rings, Polymers and Analysis This unit builds upon the chemical concepts that have been developed during AS Chemistry. This unit consists of three teaching modules: Module 1: Rings, Acids
3.4 A2 Unit F324: Rings, Polymers and Analysis This unit builds upon the chemical concepts that have been developed during AS Chemistry. This unit consists of three teaching modules: Module 1: Rings, Acids
Subject Overview Curriculum pathway
 Subject Overview Curriculum pathway Course Summary Course: A Level Chemistry Overall Summary Unit / Module Exam / Controlled % of course UMS allocation Marks available UMS / RAW mark grade boundaries from
Subject Overview Curriculum pathway Course Summary Course: A Level Chemistry Overall Summary Unit / Module Exam / Controlled % of course UMS allocation Marks available UMS / RAW mark grade boundaries from
NANYANG TECHNOLOGICAL UNIVERSITY ENTRANCE EXAMINATION SYLLABUS FOR INTERNATIONAL STUDENTS CHEMISTRY
 NANYANG TECHNOLOGICAL UNIVERSITY ENTRANCE EXAMINATION SYLLABUS FOR INTERNATIONAL STUDENTS OAFA/01/07 STRUCTURE OF EXAMINATION PAPER CHEMISTRY 1. There will be one 2-hour paper consisting of two sections.
NANYANG TECHNOLOGICAL UNIVERSITY ENTRANCE EXAMINATION SYLLABUS FOR INTERNATIONAL STUDENTS OAFA/01/07 STRUCTURE OF EXAMINATION PAPER CHEMISTRY 1. There will be one 2-hour paper consisting of two sections.
ADVANCED CHEMISTRY 2
 ADVANCED CHEMISTRY 2 Philip Matthews ±m±l CAMBRIDGE UNIVERSITY PRESS Acknowledgements How to use this book INORGANIC CHEMISTRY 88 Periodicity of physical properties 88.1 Periodicity of ionisation energies
ADVANCED CHEMISTRY 2 Philip Matthews ±m±l CAMBRIDGE UNIVERSITY PRESS Acknowledgements How to use this book INORGANIC CHEMISTRY 88 Periodicity of physical properties 88.1 Periodicity of ionisation energies
A selection of resources to start advanced Chemistry lessons for year olds
 A selection of resources to start advanced Chemistry lessons for 14-18 year olds Kristy Turner and Catherine Smith RSC School Teacher Fellows 2011-12 Foreword Kristy graduated from the University of Bradford
A selection of resources to start advanced Chemistry lessons for 14-18 year olds Kristy Turner and Catherine Smith RSC School Teacher Fellows 2011-12 Foreword Kristy graduated from the University of Bradford
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Boardworks GCSE Separate Sciences: Chemistry
 Boardworks GCSE Separate Sciences: Contents Guide Boardworks GCSE Separate Sciences: CFCs and Alcohols 51 slides 21 Flash activities What are CFCs? Introducing CFCs Naming CFCs Properties and uses of CFCs
Boardworks GCSE Separate Sciences: Contents Guide Boardworks GCSE Separate Sciences: CFCs and Alcohols 51 slides 21 Flash activities What are CFCs? Introducing CFCs Naming CFCs Properties and uses of CFCs
Switching to OCR A from AQA
 Switching to OCR A from AQA The content within the OCR Chemistry A specification covers the key concepts of chemistry and will be very familiar. We ve laid it out in a logical progression to support co-teaching
Switching to OCR A from AQA The content within the OCR Chemistry A specification covers the key concepts of chemistry and will be very familiar. We ve laid it out in a logical progression to support co-teaching
TOK: The relationship between a reaction mechanism and the experimental evidence to support it could be discussed. See
 Option G: Further organic chemistry (15/22 hours) SL students study the core of these options and HL students study the whole option (the core and the extension material). TOK: The relationship between
Option G: Further organic chemistry (15/22 hours) SL students study the core of these options and HL students study the whole option (the core and the extension material). TOK: The relationship between
OCR (A) Chemistry A-level. Module 6: Organic Chemistry and Analysis
 OCR (A) Chemistry A-level Module 6: Organic Chemistry and Analysis Organic Synthesis Notes by Adam Robertson DEFINITIONS Heterolytic fission: The breaking of a covalent bond when one of the bonded atoms
OCR (A) Chemistry A-level Module 6: Organic Chemistry and Analysis Organic Synthesis Notes by Adam Robertson DEFINITIONS Heterolytic fission: The breaking of a covalent bond when one of the bonded atoms
Organic Chemistry Review: Topic 10 & Topic 20
 Organic Structure Alkanes C C σ bond Mechanism Substitution (Incoming atom or group will displace an existing atom or group in a molecule) Examples Occurs with exposure to ultraviolet light or sunlight,
Organic Structure Alkanes C C σ bond Mechanism Substitution (Incoming atom or group will displace an existing atom or group in a molecule) Examples Occurs with exposure to ultraviolet light or sunlight,
Name/CG: 2012 Term 2 Organic Chemistry Revision (Session II) Deductive Question
 Name/G: 2012 Term 2 rganic hemistry Revision (Session II) Deductive Question 1(a) A yellow liquid A, 7 7 N 2, reacts with alkaline potassium manganate (VII) and on acidification gives a yellow solid B,
Name/G: 2012 Term 2 rganic hemistry Revision (Session II) Deductive Question 1(a) A yellow liquid A, 7 7 N 2, reacts with alkaline potassium manganate (VII) and on acidification gives a yellow solid B,
F324:$$ Rings,$ Polymers$and$ Analysis$
 Dr.$Faisal$Rana$ $ www.biochemtuition.com$ OCR Advanced GCE Chemistry A (H434) F324:$$ Rings,$ Polymers$and$ Analysis$ OCRA2Chemistry F324 F325 F326 Dr.FaisalRana Telephone:07783919244 www.biochemtuition.com
Dr.$Faisal$Rana$ $ www.biochemtuition.com$ OCR Advanced GCE Chemistry A (H434) F324:$$ Rings,$ Polymers$and$ Analysis$ OCRA2Chemistry F324 F325 F326 Dr.FaisalRana Telephone:07783919244 www.biochemtuition.com
Mechanisms. . CCl2 F + Cl.
 Mechanisms 1) Free radical substitution Alkane à halogenoalkane Initiation: Propagation: Termination: Overall: 2) Ozone depletion UV light breaks the C Cl bond releasing chlorine radical CFCl 3 F à. CCl2
Mechanisms 1) Free radical substitution Alkane à halogenoalkane Initiation: Propagation: Termination: Overall: 2) Ozone depletion UV light breaks the C Cl bond releasing chlorine radical CFCl 3 F à. CCl2
Contents. 1 Matter: Its Properties and Measurement 1. 2 Atoms and the Atomic Theory Chemical Compounds Chemical Reactions 111
 Ed: Pls provide art About the Authors Preface xvii xvi 1 Matter: Its Properties and Measurement 1 1-1 The Scientific Method 2 1-2 Properties of Matter 4 1-3 Classification of Matter 5 1-4 Measurement of
Ed: Pls provide art About the Authors Preface xvii xvi 1 Matter: Its Properties and Measurement 1 1-1 The Scientific Method 2 1-2 Properties of Matter 4 1-3 Classification of Matter 5 1-4 Measurement of
Page 7 of 30 CHEMISTRY
 Page 7 of 30 2 CHEMISTRY Page 8 of 30 SYLLABUS PHYSICAL CHEMISTRY 1A. Fundamental Concepts A. Define relative atomic, isotopic, molecular and formula masses, based on the 12-C scale B. Explain mole in
Page 7 of 30 2 CHEMISTRY Page 8 of 30 SYLLABUS PHYSICAL CHEMISTRY 1A. Fundamental Concepts A. Define relative atomic, isotopic, molecular and formula masses, based on the 12-C scale B. Explain mole in
I can calculate the rate of reaction from graphs of a changing property versus time, e.g. graphs of volume against time
 UNIT 1 CONTROLLING THE RATE OF REACTION I can calculate the rate of reaction from graphs of a changing property versus time, e.g. graphs of volume against time I can use the reciprocal of to calculate
UNIT 1 CONTROLLING THE RATE OF REACTION I can calculate the rate of reaction from graphs of a changing property versus time, e.g. graphs of volume against time I can use the reciprocal of to calculate
Cambridge International Examinations Cambridge International Advanced Subsidiary and Advanced Level
 Cambridge International Examinations Cambridge International Advanced Subsidiary and Advanced Level *0516596213* CEMISTRY 9701/42 Paper 4 A Level Structured Questions February/March 2016 2 hours Candidates
Cambridge International Examinations Cambridge International Advanced Subsidiary and Advanced Level *0516596213* CEMISTRY 9701/42 Paper 4 A Level Structured Questions February/March 2016 2 hours Candidates
Switching to OCR A from Pearson (Edexcel)
 Switching to OCR A from Pearson (Edexcel) The content within the OCR Chemistry A specification covers the key concepts of chemistry and will be very familiar. We ve laid it out in a logical progression
Switching to OCR A from Pearson (Edexcel) The content within the OCR Chemistry A specification covers the key concepts of chemistry and will be very familiar. We ve laid it out in a logical progression
CHERRY HILL TUITION AQA CHEMISTRY A2 PAPER Section A. Answer all questions in the spaces provided.
 2 Section A Answer all questions in the spaces provided. 1 This question is about bond dissociation enthalpies and their use in the calculation of enthalpy changes. 1 (a) Define bond dissociation enthalpy
2 Section A Answer all questions in the spaces provided. 1 This question is about bond dissociation enthalpies and their use in the calculation of enthalpy changes. 1 (a) Define bond dissociation enthalpy
Option G: Further organic chemistry (15/22 hours)
 Option G: Further organic chemistry (15/) TOK: The relationship between a reaction mechanism and the experimental evidence to support it could be discussed. See 16... Core material: G1 G8 are core material
Option G: Further organic chemistry (15/) TOK: The relationship between a reaction mechanism and the experimental evidence to support it could be discussed. See 16... Core material: G1 G8 are core material
Organic Chemistry SL IB CHEMISTRY SL
 Organic Chemistry SL IB CHEMISTRY SL 10.1 Fundamentals of organic chemistry Understandings: A homologous series is a series of compounds of the same family, with the same general formula, which differ
Organic Chemistry SL IB CHEMISTRY SL 10.1 Fundamentals of organic chemistry Understandings: A homologous series is a series of compounds of the same family, with the same general formula, which differ
The Basics of General, Organic, and Biological Chemistry
 The Basics of General, Organic, and Biological Chemistry By Ball, Hill and Scott Download PDF at https://open.umn.edu/opentextbooks/bookdetail.aspx?bookid=40 Page 5 Chapter 1 Chemistry, Matter, and Measurement
The Basics of General, Organic, and Biological Chemistry By Ball, Hill and Scott Download PDF at https://open.umn.edu/opentextbooks/bookdetail.aspx?bookid=40 Page 5 Chapter 1 Chemistry, Matter, and Measurement
2 Answer all the questions. 1 Born Haber cycles can be used to calculate enthalpy changes indirectly.
 2 Answer all the questions. 1 Born Haber cycles can be used to calculate enthalpy changes indirectly. (a) The table below shows enthalpy changes for a Born Haber cycle involving potassium sulfide, K 2
2 Answer all the questions. 1 Born Haber cycles can be used to calculate enthalpy changes indirectly. (a) The table below shows enthalpy changes for a Born Haber cycle involving potassium sulfide, K 2
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
SKILLS FOR PRACTICAL INVESTIGATIONS
 INDEX Unit Page SKILLS 17 SKILLS FOR PRACTICAL INVESTIGATIONS 17 1.1 Development of sciences 17 1.2 Scientific research 18 1.2.1 Types of research 20 1.2.2 Variables 20 1.2.3 Relationship between variables
INDEX Unit Page SKILLS 17 SKILLS FOR PRACTICAL INVESTIGATIONS 17 1.1 Development of sciences 17 1.2 Scientific research 18 1.2.1 Types of research 20 1.2.2 Variables 20 1.2.3 Relationship between variables
Year 13 Chemistry (NCEA) Student Information
 Year 13 Chemistry (NCEA) Student Information Science Department Onslow College 2015 Introduction Year 13 Chemistry is a full year course, of five topics, that works towards gaining Level Three credits
Year 13 Chemistry (NCEA) Student Information Science Department Onslow College 2015 Introduction Year 13 Chemistry is a full year course, of five topics, that works towards gaining Level Three credits
21.1 Introduction Carboxylic Acids Nomenclature of Carboxylic Acids. Acids Structure and Properties of Carboxylic Acids.
 21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
Chemistry 2
 WTCS Repository 10-806-129 Chemistry 2 Course Outcome Summary Course Information Description Total Credits 4.00 Further study of basic chemical principles (e.g. atomic and molecular structure, reactions,
WTCS Repository 10-806-129 Chemistry 2 Course Outcome Summary Course Information Description Total Credits 4.00 Further study of basic chemical principles (e.g. atomic and molecular structure, reactions,
Module9. Nuclear Magnetic Resonance Spectroscopy Nuclear Magnetic Resonance (NMR) spectroscopy - Chemical shift - Integration of signal area
 1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
(07) 3 (e) Calculate the ph of this buffer solution at 298 K. Give your answer to 2 decimal places
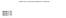 7 3 (e) An acidic buffer solution is formed when 10.0 cm3 of 0.125 mol dm 3 aqueous sodium hydroxide are added to 15.0 cm3 of 0.174 mol dm 3 aqueous HX. The value of Ka for the weak acid HX is 3.01 10
7 3 (e) An acidic buffer solution is formed when 10.0 cm3 of 0.125 mol dm 3 aqueous sodium hydroxide are added to 15.0 cm3 of 0.174 mol dm 3 aqueous HX. The value of Ka for the weak acid HX is 3.01 10
CHEMISTRY 263 HOME WORK
 Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
AP Chemistry Common Ion Effect; 16.6 ionization constants, will. Equilibria with Weak Acids and and the preparation of buffer
 Instructional Unit Acid-Base Equibria 16.1 Acid-Ionizaation Equilibria; Students will perform Students will distinguish Oral response, written 3.1.12C, 16.2 Polyprotic Acids; 16.3 Base- calculations involving
Instructional Unit Acid-Base Equibria 16.1 Acid-Ionizaation Equilibria; Students will perform Students will distinguish Oral response, written 3.1.12C, 16.2 Polyprotic Acids; 16.3 Base- calculations involving
Core. Topic 10: Organic chemistry. Essential idea: Organic chemistry focuses on the chemistry of compounds containing carbon.
 Core Chemistry guide 67 Essential idea: Organic chemistry focuses on the chemistry of compounds containing carbon. 10.1 Fundamentals of organic chemistry Nature of science: Serendipity and scientific discoveries
Core Chemistry guide 67 Essential idea: Organic chemistry focuses on the chemistry of compounds containing carbon. 10.1 Fundamentals of organic chemistry Nature of science: Serendipity and scientific discoveries
Student Achievement. Chemistry 12
 Student Achievement Chemistry 12 Key Elements: Reaction Kinetics Estimated Time: 14 16 hours By the end of this course, students will be able to explain the significance of reaction rates, demonstrate
Student Achievement Chemistry 12 Key Elements: Reaction Kinetics Estimated Time: 14 16 hours By the end of this course, students will be able to explain the significance of reaction rates, demonstrate
Name Date Class. aryl halides substitution reaction
 23.1 INTRODUCTION TO FUNCTIONAL GROUPS Section Review Objectives Explain how organic compounds are classified Identify the IUPAC rules for naming halocarbons Describe how halocarbons can be prepared Vocabulary
23.1 INTRODUCTION TO FUNCTIONAL GROUPS Section Review Objectives Explain how organic compounds are classified Identify the IUPAC rules for naming halocarbons Describe how halocarbons can be prepared Vocabulary
Amines Reading Study Problems Key Concepts and Skills Lecture Topics: Amines: structure and nomenclature
 Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
(a) (i) Give the equation representing the overall reaction. (1) Give the equation representing the formation of the electrophile.
 1. Benzene reacts with concentrated nitric acid in the presence of concentrated sulphuric acid at about 50 º in an electrophilic substitution reaction to give nitrobenzene. (a) Give the equation representing
1. Benzene reacts with concentrated nitric acid in the presence of concentrated sulphuric acid at about 50 º in an electrophilic substitution reaction to give nitrobenzene. (a) Give the equation representing
About the Authors Preface Student's Guide to Using this Text Matter-Its Properties and Measurement The Scientific Method Properties of Matter
 About the Authors Preface Student's Guide to Using this Text Matter-Its Properties and Measurement The Scientific Method Properties of Matter Classification of Matter Measurement of Matter: SI (Metric)
About the Authors Preface Student's Guide to Using this Text Matter-Its Properties and Measurement The Scientific Method Properties of Matter Classification of Matter Measurement of Matter: SI (Metric)
A-level CHEMISTRY 7405/1. Paper 1: Inorganic and Physical Chemistry. SPECIMEN MATERIAL v1.2
 SPECIMEN MATERIAL v1.2 Please write clearly in block capitals. Centre number Candidate number Surname Forename(s) Candidate signature A-level CHEMISTRY Paper 1: Inorganic and Physical Chemistry Specimen
SPECIMEN MATERIAL v1.2 Please write clearly in block capitals. Centre number Candidate number Surname Forename(s) Candidate signature A-level CHEMISTRY Paper 1: Inorganic and Physical Chemistry Specimen
Unit title: Chemistry for Applied Biologists
 Unit title: Chemistry for Applied Biologists Unit code: K/601/0292 QCF level: 5 Credit value: 15 Aim This unit covers bonding, thermodynamics, reaction rates, equilibrium, oxidation and reduction and organic
Unit title: Chemistry for Applied Biologists Unit code: K/601/0292 QCF level: 5 Credit value: 15 Aim This unit covers bonding, thermodynamics, reaction rates, equilibrium, oxidation and reduction and organic
Chapter 24. Amines. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
6.10 Amines. Naming. N Goalby chemrevise.org 1 CH2 CH2
 6.10 Amines aming Amines These end in amine. There is, however, rather confusingly two ways of using this suffix. The exam board tend to use the common version where the name stem ends in -yl propylamine.
6.10 Amines aming Amines These end in amine. There is, however, rather confusingly two ways of using this suffix. The exam board tend to use the common version where the name stem ends in -yl propylamine.
WJEC Eduqas AS Chemistry - Component 2 THERMOCHEMISTRY
 WJEC Eduqas AS Chemistry - Component 2 THERMOCHEMISTRY enthalpy change of reaction, enthalpy change of combustion and standard molar enthalpy change of formation, Δ fh ϴ Hess s law and energy cycles concept
WJEC Eduqas AS Chemistry - Component 2 THERMOCHEMISTRY enthalpy change of reaction, enthalpy change of combustion and standard molar enthalpy change of formation, Δ fh ϴ Hess s law and energy cycles concept
KOT 222 Organic Chemistry II
 KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
UNIT 3 CHEMISTRY. Fundamental Principles in Chemistry
 UNIT 3 CHEMISTRY NOTE: This list has been compiled based on the topics covered in the 2016 Master Class program. Once all of the 2017 Chemistry program materials have been finalised, this summary will
UNIT 3 CHEMISTRY NOTE: This list has been compiled based on the topics covered in the 2016 Master Class program. Once all of the 2017 Chemistry program materials have been finalised, this summary will
3.5 A2 Unit F325: Equilibria, Energetics and Elements
 3.5 A2 Unit F325: Equilibria, Energetics and Elements This unit builds upon the chemical concepts that have been developed during AS Chemistry. This unit consists of three teaching modules: Module 1: Rates,
3.5 A2 Unit F325: Equilibria, Energetics and Elements This unit builds upon the chemical concepts that have been developed during AS Chemistry. This unit consists of three teaching modules: Module 1: Rates,
CHEM4. General Certificate of Education Advanced Level Examination June Unit 4 Kinetics, Equilibria and Organic Chemistry
 Centre Number Surname Candidate Number For Examiner s Use ther Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination June 2012 Question 1 2 Mark Chemistry
Centre Number Surname Candidate Number For Examiner s Use ther Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination June 2012 Question 1 2 Mark Chemistry
CHERRY HILL TUITION OCR (SALTERS) CHEMISTRY A2 PAPER Answer all the questions. O, is formed in the soil by denitrifying bacteria. ...
 2 Answer all the questions. 1 itrous oxide gas, 2, is formed in the soil by denitrifying bacteria. (a) Give the systematic name for nitrous oxide. ne model of the bonding in nitrous oxide includes a dative
2 Answer all the questions. 1 itrous oxide gas, 2, is formed in the soil by denitrifying bacteria. (a) Give the systematic name for nitrous oxide. ne model of the bonding in nitrous oxide includes a dative
Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers
 Chapter Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers Ch 1-Structure and bonding Ch 2-Polar covalent bonds: Acids and bases McMurry, J. (2004) Organic Chemistry 6 th Edition
Chapter Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers Ch 1-Structure and bonding Ch 2-Polar covalent bonds: Acids and bases McMurry, J. (2004) Organic Chemistry 6 th Edition
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry
 Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
1 Principles of chemistry
 1 Principles of chemistry The following sub-topics are covered in this section. (a) States of matter (b) Elements, compounds and mixtures (c) Atomic structure (d) The Periodic Table (e) Chemical formulae,
1 Principles of chemistry The following sub-topics are covered in this section. (a) States of matter (b) Elements, compounds and mixtures (c) Atomic structure (d) The Periodic Table (e) Chemical formulae,
AP Chemistry Standards and Benchmarks
 Standard: Understands and applies the principles of Scientific Inquiry Benchmark 1: Scientific Reasoning Course Level Benchmarks A. Formulates and revises scientific explanations and models B. Understands
Standard: Understands and applies the principles of Scientific Inquiry Benchmark 1: Scientific Reasoning Course Level Benchmarks A. Formulates and revises scientific explanations and models B. Understands
Module 4 revision guide: Compounds with C=O group
 opyright N Goalby Bancroft's School Module 4 revision guide: ompounds with = group arbonyls: Aldehydes and Ketones arbonyls are compounds with a = bond, they can be either aldehydes or ketones. 3 ethanal
opyright N Goalby Bancroft's School Module 4 revision guide: ompounds with = group arbonyls: Aldehydes and Ketones arbonyls are compounds with a = bond, they can be either aldehydes or ketones. 3 ethanal
CONTENTS. Introduction...x. 1. Atomic Structure Radioactivity...10
 CONTENTS Introduction...x Exam paper analysis...x Structure of exam and advice on core questions...x Timing for the exam...xii Exam strategy...xii Sample exam question: 2005 Q8 Higher level...xiii List
CONTENTS Introduction...x Exam paper analysis...x Structure of exam and advice on core questions...x Timing for the exam...xii Exam strategy...xii Sample exam question: 2005 Q8 Higher level...xiii List
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS
 AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
A-Level Chemistry (AQA) Exam Dates 2018: Note: the practical endorsement is reported as a pass or fail separately. Exam Structure:
 A-Level Chemistry (AQA) Exam Dates 2018: Note: the practical endorsement is reported as a pass or fail separately Exam Structure: 1 Inorganic Chemistry Year 1 Topic 2.1: Periodicity 2.1.1 Classification
A-Level Chemistry (AQA) Exam Dates 2018: Note: the practical endorsement is reported as a pass or fail separately Exam Structure: 1 Inorganic Chemistry Year 1 Topic 2.1: Periodicity 2.1.1 Classification
Section A. 1 at a given temperature. The rate was found to be first order with respect to the ester and first order with respect to hydroxide ions.
 2 Section A Answer all questions in the spaces provided. Question 1:The N/Arate of hydrolysis of an ester X (HCOOCH2CH2CH3) was studied in alkaline 1 at a given temperature. The rate was found to be first
2 Section A Answer all questions in the spaces provided. Question 1:The N/Arate of hydrolysis of an ester X (HCOOCH2CH2CH3) was studied in alkaline 1 at a given temperature. The rate was found to be first
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding.
 Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Alkyl phenyl ketones are usually named by adding the acyl group as prefix to phenone.
 Aldehydes, Ketones and Carboxylic Acids Nomenclature of aldehydes and ketones Aldehydes: Often called by their common names instead of IUPAC names. Ketones: Derived by naming two alkyl or aryl groups bonded
Aldehydes, Ketones and Carboxylic Acids Nomenclature of aldehydes and ketones Aldehydes: Often called by their common names instead of IUPAC names. Ketones: Derived by naming two alkyl or aryl groups bonded
Candidate Name. School Name CHEMISTRY. Saturday 27 February hour 30 minutes INSTRUCTIONS TO CANDIDATES
 Candidate Name School Name CHEMISTRY Saturday 27 February 2016 1 hour 30 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. You are not permitted access to
Candidate Name School Name CHEMISTRY Saturday 27 February 2016 1 hour 30 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. You are not permitted access to
About the GRE Chemistry Subject Test p. 1 About the GRE Chemistry Subject Test GRE Chemistry Topics Test Dates Testing Fee Test Format Testing Time
 About the GRE Chemistry Subject Test p. 1 About the GRE Chemistry Subject Test GRE Chemistry Topics Test Dates Testing Fee Test Format Testing Time Scoring To Guess or Not to Guess On the Day of the Test
About the GRE Chemistry Subject Test p. 1 About the GRE Chemistry Subject Test GRE Chemistry Topics Test Dates Testing Fee Test Format Testing Time Scoring To Guess or Not to Guess On the Day of the Test
Chapter 19: Amines. Introduction
 Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
Chapter 19: Amines Chap 19 HW: (be able to name amines); 37, 39, 41, 42, 44, 46, 47, 48, 53-55, 57, 58 Introduction Organic derivatives of ammonia. Many are biologically active. Chap 19: Amines Slide 19-2
NANYANG TECHNOLOGICAL UNIVERSITY ENTRANCE EXAMINATION SYLLABUS FOR INTERNATIONAL STUDENTS CHEMISTRY
 NANYANG TECHNOLOGICAL UNIVERSITY ENTRANCE EXAMINATION SYLLABUS FOR INTERNATIONAL STUDENTS CHEMISTRY STRUCTURE OF EXAMINATION PAPER 1. There will be one 2-hour paper consisting of two sections: Section
NANYANG TECHNOLOGICAL UNIVERSITY ENTRANCE EXAMINATION SYLLABUS FOR INTERNATIONAL STUDENTS CHEMISTRY STRUCTURE OF EXAMINATION PAPER 1. There will be one 2-hour paper consisting of two sections: Section
Appendix 1. Periodic Table and Atomic Structure. History of the idea of elements.
 Appendix 1 Detailed list of additions and deletions This appendix provides a detailed list of additions and deletions compared with the former (1983) Leaving Certificate Chemistry syllabus. Completely
Appendix 1 Detailed list of additions and deletions This appendix provides a detailed list of additions and deletions compared with the former (1983) Leaving Certificate Chemistry syllabus. Completely
Chemistry Assessment Unit A2 1
 Centre Number 71 Candidate Number ADVANCED General Certificate of Education January 2013 Chemistry Assessment Unit A2 1 assessing Periodic Trends and Further Organic, Physical and Inorganic Chemistry AC212
Centre Number 71 Candidate Number ADVANCED General Certificate of Education January 2013 Chemistry Assessment Unit A2 1 assessing Periodic Trends and Further Organic, Physical and Inorganic Chemistry AC212
2 Answer all the questions. 1 Nitrogen monoxide is formed when nitrogen and oxygen from the air combine. (g) + O 2
 2 Answer all the questions. 1 Nitrogen monoxide is formed when nitrogen and oxygen from the air combine. N 2 (g) + 2 (g) 2N(g) equation 1.1 Under normal atmospheric conditions, a further reaction occurs
2 Answer all the questions. 1 Nitrogen monoxide is formed when nitrogen and oxygen from the air combine. N 2 (g) + 2 (g) 2N(g) equation 1.1 Under normal atmospheric conditions, a further reaction occurs
Grade 12 Chemistry, University I
 Grade 12 Chemistry, University I Monarch Park Collegiate SCH 4U Credit Value: 1 Course Outline prepared by: Monarch Park Chemistry Teachers Text Book: Nelson "Chemistry 12" Department Head: G. Nakashima
Grade 12 Chemistry, University I Monarch Park Collegiate SCH 4U Credit Value: 1 Course Outline prepared by: Monarch Park Chemistry Teachers Text Book: Nelson "Chemistry 12" Department Head: G. Nakashima
National 5 Whole Course Revision Questions
 National 5 Whole Course Revision Questions Unit 1 Chemical changes 1. Describe how temperature, concentration and particle size affect the rate of a chemical reaction- mention collision theory in your
National 5 Whole Course Revision Questions Unit 1 Chemical changes 1. Describe how temperature, concentration and particle size affect the rate of a chemical reaction- mention collision theory in your
Chemistry: The Central Science Twelfth Edition, AP* Edition 2012
 A Correlation of The Central Science Twelfth Edition, AP* Edition 2012 to the AP* Chemistry Topics I. Structure of Matter A. Atomic theory and atomic structure 1. Evidence for the atomic theory SECTIONS:
A Correlation of The Central Science Twelfth Edition, AP* Edition 2012 to the AP* Chemistry Topics I. Structure of Matter A. Atomic theory and atomic structure 1. Evidence for the atomic theory SECTIONS:
Chirality, Carbonyls and Carboxylic Acids
 hirality, arbonyls and arboxylic Acids Questions on this unit may include material from UNIT 2 see syllabus Isomerism Structural isomerism. Structural isomerism was dealt with in UNIT 2. All isomers are
hirality, arbonyls and arboxylic Acids Questions on this unit may include material from UNIT 2 see syllabus Isomerism Structural isomerism. Structural isomerism was dealt with in UNIT 2. All isomers are
2 Answer all the questions. CH(NH 2. )COOH, R is CH [1] (ii) Draw the structures of the ions formed by alanine at ph 6.0 and at ph 1.5.
![2 Answer all the questions. CH(NH 2. )COOH, R is CH [1] (ii) Draw the structures of the ions formed by alanine at ph 6.0 and at ph 1.5. 2 Answer all the questions. CH(NH 2. )COOH, R is CH [1] (ii) Draw the structures of the ions formed by alanine at ph 6.0 and at ph 1.5.](/thumbs/94/121313851.jpg) 2 Answer all the questions. 1 This question looks at the properties and chemistry of some α-amino acids. The general formula of an α-amino acid is R(N 2 ). (a) In the α-amino acid alanine, 3 (N 2 ), R
2 Answer all the questions. 1 This question looks at the properties and chemistry of some α-amino acids. The general formula of an α-amino acid is R(N 2 ). (a) In the α-amino acid alanine, 3 (N 2 ), R
2A - Amines. 2 H atoms replaced: 2 attached C's to N. 3 H atom replaced: 3 attached C's to N Ammonia, NH3 Primary amine Secondary amine Tertiary amine
 2A - Amines Something fishy about amines: Have an NH 2, amine group. Amines are derivatives of ammonia: 3 H atoms 1 H atom replaced: 1 attached C to N 2 H atoms replaced: 2 attached C's to N 3 H atom replaced:
2A - Amines Something fishy about amines: Have an NH 2, amine group. Amines are derivatives of ammonia: 3 H atoms 1 H atom replaced: 1 attached C to N 2 H atoms replaced: 2 attached C's to N 3 H atom replaced:
ORGANIC CHEMISTRY. Fifth Edition. Stanley H. Pine
 ORGANIC CHEMISTRY Fifth Edition Stanley H. Pine Professor of Chemistry California State University, Los Angeles McGraw-Hill, Inc. New York St. Louis San Francisco Auckland Bogota Caracas Lisbon London
ORGANIC CHEMISTRY Fifth Edition Stanley H. Pine Professor of Chemistry California State University, Los Angeles McGraw-Hill, Inc. New York St. Louis San Francisco Auckland Bogota Caracas Lisbon London
Isaac Newton Academy. A-Level Chemistry Summer Learning (Triple Science Version) Name: Page 1
 A-Level Chemistry Summer Learning (Triple Science Version) Name: Page 1 If you want to study Chemistry at A-level you really have to own your learning. It is important to care about your study as you will
A-Level Chemistry Summer Learning (Triple Science Version) Name: Page 1 If you want to study Chemistry at A-level you really have to own your learning. It is important to care about your study as you will
Chapter 22 Amines. Nomenclature Amines are classified according to the degree of substitution at nitrogen.
 CH. 22 Chapter 22 Amines Amines are very important in biological chemistry. Most of the bases in biological acid-base reactions are amines. They are also very important nucleophiles in biochemical reactions.
CH. 22 Chapter 22 Amines Amines are very important in biological chemistry. Most of the bases in biological acid-base reactions are amines. They are also very important nucleophiles in biochemical reactions.
Unit 4: General Principles of Chemistry I Rates, Equilibria and Further Organic Chemistry (including synoptic assessment)
 Write your name here Surname Other names Edexcel GCE Centre Number Candidate Number Chemistry Advanced Unit 4: General Principles of Chemistry I Rates, Equilibria and Further Organic Chemistry (including
Write your name here Surname Other names Edexcel GCE Centre Number Candidate Number Chemistry Advanced Unit 4: General Principles of Chemistry I Rates, Equilibria and Further Organic Chemistry (including
CHEMISTRY. HIGHER 2 (Syllabus 9647) CONTENTS
 CHEMISTRY HIGHER 2 (Syllabus 9647) CONTENTS Page INTRODUCTION 1 AIMS 1 ASSESSMENT OBJECTIVES 2 SCHEME OF ASSESSMENT 4 ADDITIONAL INFORMATION 6 SUBJECT CONTENT 7 PRACTICAL ASSESSMENT 27 QUALITATIVE ANALYSIS
CHEMISTRY HIGHER 2 (Syllabus 9647) CONTENTS Page INTRODUCTION 1 AIMS 1 ASSESSMENT OBJECTIVES 2 SCHEME OF ASSESSMENT 4 ADDITIONAL INFORMATION 6 SUBJECT CONTENT 7 PRACTICAL ASSESSMENT 27 QUALITATIVE ANALYSIS
2 Answer all the questions. 1 Alkenes and benzene both react with bromine but alkenes are much more reactive.
 2 Answer all the questions. 1 Alkenes and benzene both react with bromine but alkenes are much more reactive. (a) Explain the relative resistance to bromination of benzene compared with alkenes. In your
2 Answer all the questions. 1 Alkenes and benzene both react with bromine but alkenes are much more reactive. (a) Explain the relative resistance to bromination of benzene compared with alkenes. In your
Pearson Edexcel Level 3 GCE Chemistry Advanced Paper 2: Advanced Organic and Physical Chemistry
 Write your name here Surname Other names Pearson Edexcel Level 3 GCE Centre Number Candidate Number Chemistry Advanced Paper 2: Advanced Organic and Physical Chemistry Specimen Paper for first teaching
Write your name here Surname Other names Pearson Edexcel Level 3 GCE Centre Number Candidate Number Chemistry Advanced Paper 2: Advanced Organic and Physical Chemistry Specimen Paper for first teaching
SYLLABUS OF THE CHEMISTRY PRACTICALS ( ) CLASS-XII
 Experiment 1. SYLLABUS OF THE CHEMISTRY PRACTICALS (28-19) CLASS-XII Qualitative analysis: To analyze the given salt for acidic and basic radicals. (08 marks) Experiment 2. Volumetric analysis: (08 marks)
Experiment 1. SYLLABUS OF THE CHEMISTRY PRACTICALS (28-19) CLASS-XII Qualitative analysis: To analyze the given salt for acidic and basic radicals. (08 marks) Experiment 2. Volumetric analysis: (08 marks)
*AC212* *28AC21201* Chemistry. Assessment Unit A2 1 [AC212] FRIDAY 27 MAY, MORNING
![*AC212* *28AC21201* Chemistry. Assessment Unit A2 1 [AC212] FRIDAY 27 MAY, MORNING *AC212* *28AC21201* Chemistry. Assessment Unit A2 1 [AC212] FRIDAY 27 MAY, MORNING](/thumbs/78/76733119.jpg) Centre Number ADVANCED General Certificate of Education 2016 Candidate Number Chemistry Assessment Unit A2 1 assessing Periodic Trends and Further Organic, Physical and Inorganic Chemistry [AC212] *AC212*
Centre Number ADVANCED General Certificate of Education 2016 Candidate Number Chemistry Assessment Unit A2 1 assessing Periodic Trends and Further Organic, Physical and Inorganic Chemistry [AC212] *AC212*
Course Syllabus. Department: Science & Technology. Date: April I. Course Prefix and Number: CHM 212. Course Name: Organic Chemistry II
 Department: Science & Technology Date: April 2012 I. Course Prefix and Number: CHM 212 Course Name: Organic Chemistry II Course Syllabus Credit Hours and Contact Hours: 5 credit hours and 7 (3:3:1) contact
Department: Science & Technology Date: April 2012 I. Course Prefix and Number: CHM 212 Course Name: Organic Chemistry II Course Syllabus Credit Hours and Contact Hours: 5 credit hours and 7 (3:3:1) contact
Miami Dade College CHM Second Semester General Chemistry
 Miami Dade College CHM 1046 - Second Semester General Chemistry Course Description: CHM 1046 is the second semester of a two-semester general chemistry course for science, premedical science and engineering
Miami Dade College CHM 1046 - Second Semester General Chemistry Course Description: CHM 1046 is the second semester of a two-semester general chemistry course for science, premedical science and engineering
Calculate a rate given a species concentration change.
 Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
Chemistry *P46941A0128* Pearson Edexcel P46941A
 Write your name here Surname ther names Pearson Edexcel International Advanced Level Centre Number Candidate Number Chemistry Advanced Unit 5: General Principles of Chemistry II Transition Metals and rganic
Write your name here Surname ther names Pearson Edexcel International Advanced Level Centre Number Candidate Number Chemistry Advanced Unit 5: General Principles of Chemistry II Transition Metals and rganic
Worksheet Chapter 10: Organic chemistry glossary
 Worksheet 10.1 Chapter 10: Organic chemistry glossary Addition elimination reaction A reaction in which two molecules combine with the release of a small molecule, often water. This type of reaction is
Worksheet 10.1 Chapter 10: Organic chemistry glossary Addition elimination reaction A reaction in which two molecules combine with the release of a small molecule, often water. This type of reaction is
5.3.1 Transition Elements
 5.3.1 Transition Elements General properties of transition metals transition metal characteristics of elements Ti u arise from an incomplete d sub-level in ions these characteristics include formation
5.3.1 Transition Elements General properties of transition metals transition metal characteristics of elements Ti u arise from an incomplete d sub-level in ions these characteristics include formation
Unit 4: General Principles of Chemistry I Rates, Equilibria and Further Organic Chemistry (including synoptic assessment)
 Write your name here Surname Other names Edexcel GCE Centre Number Chemistry Advanced Unit 4: General Principles of Chemistry I Rates, Equilibria and Further Organic Chemistry (including synoptic assessment)
Write your name here Surname Other names Edexcel GCE Centre Number Chemistry Advanced Unit 4: General Principles of Chemistry I Rates, Equilibria and Further Organic Chemistry (including synoptic assessment)
