D T IC. HENII~IHl K? AD-A OFFICE OF NAVAL RESEARCH Contract N/N J-1893 R&T Code TECHNICAL REPORT NO.
|
|
|
- Kelly Bruce
- 6 years ago
- Views:
Transcription
1 AD-A OFFICE OF NAVAL RESEARCH Contract N/N J-1893 R&T Code TECHNICAL REPORT NO. 5 Intramolecular vs. Intermolecular Condensation Rates in the Acidic Polymerization of Octaethoxytrisiloxane by S J. Sanchez and A.V. McCormick Dept. of Chemical Engineering & Materials Science University of Minnesota 421 Washington Ave. SE D T IC Minneapolis, MN ELECTE JUL July 12,1993 A" Reproduction in whole or in part is permitted for any purpose of the U.S. government. This document has been approved for public release and sale; its distribution is unlimited HENII~IHl K?
2 REPORT DOCUMENTATION PAGE,2 : -- : ' ' " - " ' - :.... ' - " p " = AGENCY JSE ONLY,ea,4, 0, dnk) 2. REPORT DATE 13 REPORT TYPE AND OATES COVERED I 7/12/93 ITechnical 4 TITLE AND SUBTITLE S. FUNDING NUMBERS Intramolecular vs. Intermolecular Condensation Rates in the Acidic Polymerization of Octaethoxytrisiloxane N/NO J AUTHORýS) J. Sanchez and A.V. McCormick 7 PERFORMING ORGANIZATION NAME(S) AND ADDRESS(ES) S. PERFORMING ORGANIZAT:ON Dept. of Chemical Engineering & Materials Science REPORT NUMBER University of Minnesota 421 Washington Ave., SE Minneapolis, MN SPONSORING, MONITORING AGENCY NAME(S) AND AODORESS(ES) 10. SPONSORING 'MONITORING Office of Naval Research AGENCY REPORT NUMBER 800 N. Quincy Street Arlington, VA SUPPLEMENTARY NOTES Submitted JNCS 12a. DISTRIBUTION/ AVAILABILITY STATEMENT 12b. DISTRIBUTION CODE Reproduction in whole or in part is permitted for any purpose of the U.S. Government. This document has been approved for public release and sale; its distribution is unlimited. 13. ABSTRACT (Maximum 200 words) Octaethoxytrisiloxane, (EtO) 3 Si-O-Si(OEt) -O-Si(OEt) 3, was synthesized and then polymerized in water/alcohol 2 4olution in ihe presence of an acid catalyst. The reaction was monitored with Si-NMR. The rate constants of linear and cyclic condensation were estimated by curve-fitting and were compared. No evidence of depolymerization was found, indicating that condensation is irreversible in our conditions. In addition, it was found that the formation of trimeric cyclic species by ring closure is fast and competes effectively with chain extension condensation. These observations are consistent with previous suggestions, but in addition they provide a quantitative measure of selectivity that is much needed. Quantitative kinetic models for silicon alkoxide sol-gel processes need to account for cyclization at very early stages of the polymerization. 14. SUBJECT TERMS 1%,-UMBER OF PAGES Si0 2, solgel, NMR, kinetics, trisiloxane, gelization 15 I& POEI CODE 17. SECURITY CLASSIFICATION I1. SECURITY CLASSIFICATION 19. SECURITY CLASSIF AITION 20. LIANTATION OF ABSTRACT OF REPORT OF THIS PAGE OF ABSTRACT unclassified unclassified unclassified UL %S% ' Standard Form 298 2ev, ig) "I"I' 'a
3 4 Intramolecular vs. intermolecular condensation rates in the acidic polymerization of octaethoxytrisiloxane Jorge Sanchez and Alon V. McCormick* Accesion For NTIS University of Minnesota DTIC CRA&I IA8" - Unrriou,;ced [] Department of Chemical Engineering and Materials Science 0,,h c : e do0 jusfhý.ýtjo' Washington Ave. SE By Minneapolis, MN Dist'ibution S...Availab~ilty "Dist "Aval Codies a:ar I or Special Abstract I A-i Octaethoxytrisiloxane, (EtO) 3 Si-O-Si(OEt)2-O-Si(OEt)3, was synthesized and then polymerized in water/alcohol solution in the presence of an acid catalyst. The reaction was monitored with 2 9 Si-NMR. The rate constants of linear and cyclic condensation were estimated by curvefitting and were compared. No evidence of depolymerization was found, indicating that condensation is irreversible in our conditions. In addition, it was found that the formation of trimeric cyclic species by ring closure is fast and competes effectively with chain extension condensation. These observations are consistent with previous suggestions, but in addition they provide a quantitative measure of selectivity that is much needed. Quantitative kinetic models for silicon alkoxide sol-gel processes need to account for cyclization at very early stages of the polymerization. to whom correspondence should be addressed
4 Introduction The polymerization of alkoxides in alcohol/water solutions is a promising route to the synthesis of advanced ceramics [11. In principle, tailoring the structure and properties of the product can be achieved by controlling the reaction kinetics. However, the large number of possible reaction pathways has been a major obstacle to the development of quantitative models for sol-gel processes. Moreover, most studies of solgel reactions have provided only qualitative understanding. Silicon alkoxides constitute the most studied type of sol-gel systems. The reactions involved in the polymerization of silicon alkoxides are (i) the hydrolysis of alkoxide groups to form silanol groups and (ii) the condensation of the silanol groups to yield siloxane bonds which constitute the backbone of the polymer. Attempts have been made to use 29 Si-NMR to measure the kinetics of the polymerization of Si(OEt) 4 (TEOS) [2,3], Si(OMe)4 (TMOS) [3] and even (MeO)3Si-O-Si(OMe)3 [4], which is one of the dimeric intermediates formed during the polymerization of TMOS. It has been shown that in acidic conditions hydrolysis is reversible[5] and fast. Much less is known about condensation. Based on the very low solubility of amorphous silica in acidic alcoholic solutions [6], it is generally assumed that condensation is irreversible in acidic conditions - thereby greatly simplifying kinetics models. This seems to be confirmed by some studies [71. An area of controversy concerns the importance of intramolecular reactions, also known as ring closure or cyclization. Most polymerization models do not consider cyclization because it is extremely difficult to account for. Besides, intramolecular reactions are often negligible in organic polymerization [8]. However, it has been shown that cyclic intermediates are formed during the polymerization of TEOS and TMOS in acidic conditions [9]. Cyclization seems to be more prevalent in TEOS than in TMOS systems [31. Indeed, the failure of quantitative kinetic
5 models for sol-gel polymerization has been explained by cyclization effects in these systems [10. To estimate the relative rate of formation of small rings versus chain extension, we used 2 9 Si-NMR to study the kinetics of polymerization of octaethoxytrisiloxane, (EtO])3Si-O-Si(OEt) 2 -O-Si(OEt) 3, a trimeric oligomer that appears as intermediate in the sol-gel polymerization of TEOS. This molecule is the smallest oligomer that can undergo chain extension and ring closure; it thus provides us the opportunity to unambiguously measure the selectivity of intra- versus intermolecular polymerization rates. 2. Experimental Procedures 2.1 Synthesis of octaethoxytrisiloxane Octaethoxytrisiloxane was synthesized by adapting a procedure described by Dougthy et al. [4]. Octachlorotrisiloxane (97%, Hiuls America) was slowly added to a solution of ethylorthoformate (Aldrich) in heptane. The reaction, carried out in an inert atmosphere and monitored by gas chromatography, was carried out at 600C for two hours. Octaethoxytrisiloxane was isolated and purified by vacuum distillation. Remaining traces of HCI formed during the reaction were removed by degzssing with dry nitrogen. A final purification was done with an alumina packed chromatography column. The product was identified by 2 9 Si-NMR as octaethoxytrisiloxane (Figure 1) with a purity greater than 96% from gas chromatography. 2.2 Kinetic Measurements In a 10 mm NMR tube, 2 ml of octaethoxytrisiloxane were mixed with 2 ml 1 wt.%. Cr(acac)3 (paramagnetic relaxant agent) in absolute ethanol, 300gl deionized H20 and 23 gtl of a calibrated N HC! solution. The
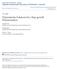
6 composition of the resultant solution was thus: 0.97 M octaethoxytrisiloxane/7.94 M EtOH/4.15 M H20/ M HCI. The reaction was monitored at 250C with 29 Si-NMR. Each spectrum was acquired with 32 scans at MHz and with a pulse delay of 12 s. 3. Results and Discussion Figure 2 shows the sequence of 29 Si-NMR spectra recorded during the reaction. The NMR spectra show peaks assigned to silanol groups formed by hydrolysis, and peaks attributed to silicon sites in cyclic trisiloxanes [9]. The concentrations of silicon end (Qi), middle (Q2) and cyclic units (Qc) are plotted in Figure 3. The lack of silicon units with three siloxane bonds (branching units Q3) in the 8-hour period studied shows that branching condensation is very slow for the cycles and chains produced. In particular, the middle unit of the trimeric precursor is much less reactive than its end units. No depolymerization of the trimer occurs as evidenced by the lack of smaller fragments such as monomeric units (Qo). This is confirmed by size exclusion chromatography (Figure 4). It appears then that in these conditions, condensation is virtually irreversible and the polymeric intermediates cannot undergo rearrangement; these data are thus akin to a polyfunctional organic polymerization [6]. Since only the end units react during the period studied, the overall extent of polymerization is given by (1) [Q1]o[QI] [Q1]0 In addition, three cyclic units appear each time two end units disappear when a trimeric cycle is formed. Neglecting the formation of larger cycles, the extent of ring closure is
7 (2) =3 [Q ll0 Finally, the extent of chain extension polymerization is (3) a= -o a and a are plotted in Figure 5. It can be seen that during the first two hours of the reaction, chain extension is predominant. During this period, the polymers grow rapidly. However, ring closure then becomes the most important reaction, and polymer growth slows. A possible explanation for this behavior is that since the number of chains in the system decreases during polymerization, the probability for a reactive group to react intermolecularly diminishes as it becomes increasingly difficult for an end unit to find an outside reaction partner as the reaction proceeds. This increased competition of cyclization and the very slow rate of branching can explain the remarkably long gel time (on the order of two months at room temperature) of this system, and perhaps the observation of exceedingly high conversion at the gel point [9]. To quantitatively assess the ring-chain competition, the following simple kinetic equation is written (4) d[ = -2kct[QI] 2 "- ko[q] where ka and ka are rate constants for inter- and intramolecular condensation of the end units. The Si end sites may actually carry from 0 to 3 -OH groups and a great number of reactions involving molecules with various numbers of silanol groups are possible. Since the rate of hydrolysis is much faster than the rate of condensation, to simplify the analytical treatment the rate constants ka and ka are averaged over the extent of hydrolysis on the Si groups. Though we expect these apparent rate constants to depend on the amount of water and the catalyst concentration, the relative values of ka and ka should not be affected and they will be a good measure of the ring-chain competition. The rate constants should also be affected by
8 the initial precursor concentration, as intramolecular reactions are more probable in dilute systems. The solution of (4) is 1_ 1 +2keo (5) (Q(e-Qi0 k at - 1) or (6) + 2 (ekat where X = k is a dimensionless number describing the tendency to [Q1]oka form rings [ 11]. A curve-fitting of.-- versus time (shown in Figure 6) gives: ka = mol l-hh-1 ± 4% ka= h- 1 ± 9% where mol refers to moles of silicon end units (Qi), and X = 2.14 This remarkably high value of X indicates that octaethoxytrisiloxane has a strong tendency to form small cycles. 4. Conclusion The study of the sol-gel polymerization of octaethoxytrisiloxane in acidic ethanol/water solution has shown that in our conditions, polymerization is irreversible. In addition, ring closure condensation (cyclization) is relatively fast and actively competes with chain extension condensation. The rapid formation of small rings during silicon alkoxide sol-gel polymerization should considerably affect the gel characteristics, since
9 intramolecular reactions "waste" bonds from the point of view of polymer growth. Moreover, these small cycles branch very slowly and their formation slows down the gelation. It is thus important to consider cyclization effects in any attempt to develop a realistic quantitative model of silica sol-gel polymerization. This work was supported by a grant from the Office of Naval Research and with a fellowship to J. S. from the Center for Interfacial Engineering at the University of Minnesota. The authors gratefully acknowledge helpful discussions with Prof. C. W. Macosko, Prof. L. E. Scriven, Anshu Gupta and Rich Cairncross at the University of Minnesota. References [1] C.J. Brinker and G.W. Scherer, Sol-Gel Science (Academic Press, New York, 1990). [2] J.C. Pouxviel and J.P. Boilot, J. Non-Cryst. Solids 94 (1987) 374. [31 B.D. Kay awid R.A. Assink, J. Non-Cryst. Solids 99 (1988) 359; 104 (1988)112; 107 (1988) 35. [41 D.H. Doughty, R.A. Assink and B.D. Kay, Adv. Chem. Ser. 224 (1990) 241. [5] J. Sanchez and A. V. McCormick, J. Phys. Chem. 96 (1992) [6] R. H1er, The Chemistry of Silica (John Wiley & Sons, New York, 1979). [7] W. G. Klemperer, V. V. Mainz and D. M. Millar, Mat. Res. Soc.Symp. Proc. 73 (1986) 3.
10 [81 P.J. Flory, Principles of Polymer Chemistry (Cornell University Press, Ithaca, New York, 1953). [9] L.W. Kelts and N.J. Armstrong, J. Mater. Res. 4 (1989) 423. [10] J.K. Bailey, M.L. MeCartney and C.W. Macosko, J. Non-Cryst. Solids 125 (1990) 208. [111 M. Gordon and G. R. Scantlebury, J. Chem. Soc. (B) (1967) 1.
11 Figure Captions Figure Si-NMR spectrum of synthesized octaethoxysilane. Figure S; NMR for the polymerization of octaethoxytrisiloxane. rhe first,)ectrum was recorded after 25 minutes of reaction and the following spectra were acquired every 15 minutes, up to eight hours of reaction. Figure 3. Concentrations of silicon end units (Qi), middle units (Q2) and trimeric cyclic units (Qc). Figure 4. Size exclusion chromatograph after 6 hours of reaction with proposed peak assignements. The elution solvent was tetrahydrofuran. Note the peak inversion from trimer to hexamer. Figure 5. Plot of the extent of cylization (a) and chain extension condensation (a ). Figure 6. Plot of 1- rate constants. and curve-fitting for the determination of the
12 LLi4: LU 0 (0 0) 0 C 0 4J ugu -_ uj 0 0 0
13 ... \40 CI 9I
14 C.f c O0.8 S Time (h)
15 Nonamer Hexamer Dodecamer Trimer Elution Volume (ml)
16 35-40I % T Time (h)
17 Ii S%~ Time (h)
Defense Technical Information Center Compilation Part Notice
 UNCLASSIFIED Defense Technical Information Center Compilation Part Notice ADP014281 TITLE: Structure and Characterization of Sol-gel and Aerogel Materials and Oxidation Products from the Reaction of [CH30]4Si
UNCLASSIFIED Defense Technical Information Center Compilation Part Notice ADP014281 TITLE: Structure and Characterization of Sol-gel and Aerogel Materials and Oxidation Products from the Reaction of [CH30]4Si
How Silica Aerogels Are Made
 Page 1 of 7 How Silica Aerogels Are Made Back to the Table of Contents The discussion below relies upon the following terms: Hydrolysis: The reaction of a metal alkoxide (M-OR) with water, forming a metal
Page 1 of 7 How Silica Aerogels Are Made Back to the Table of Contents The discussion below relies upon the following terms: Hydrolysis: The reaction of a metal alkoxide (M-OR) with water, forming a metal
Sol-Gel Methods. Hydrolysis Condensation Gelation Ageing Drying Densification
 Sol-Gel Methods Sol-gel process: Hydrolysis Condensation Gelation Ageing Drying Densification Powders: microcrystalline, nanocrystalline, amorphous Monoliths, Coatings, Films, Fibers Aerogels Glasses,
Sol-Gel Methods Sol-gel process: Hydrolysis Condensation Gelation Ageing Drying Densification Powders: microcrystalline, nanocrystalline, amorphous Monoliths, Coatings, Films, Fibers Aerogels Glasses,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION Supporting Information Kinetic Resolution of Constitutional Isomers Controlled by Selective Protection inside a Supramolecular Nanocapsule Simin Liu, Haiying Gan, Andrew T. Hermann,
SUPPLEMENTARY INFORMATION Supporting Information Kinetic Resolution of Constitutional Isomers Controlled by Selective Protection inside a Supramolecular Nanocapsule Simin Liu, Haiying Gan, Andrew T. Hermann,
ISPUB.COM. In-Situ Thiol-Modified Silica Nanoparticles. G Hernández-Padrón, R Rodríguez, V Castañro INTRODUCTION EXPERIMENTS
 ISPUB.COM The Internet Journal of Nanotechnology Volume 1 Number 2 G Hernández-Padrón, R Rodríguez, V Castañro Citation G Hernández-Padrón, R Rodríguez, V Castañro.. The Internet Journal of Nanotechnology.
ISPUB.COM The Internet Journal of Nanotechnology Volume 1 Number 2 G Hernández-Padrón, R Rodríguez, V Castañro Citation G Hernández-Padrón, R Rodríguez, V Castañro.. The Internet Journal of Nanotechnology.
SYNTHESIS, CHARACTERISATION AND APPLICATIONS OF SILICA AEROGELS
 SYNTHESIS, CHARACTERISATION AND APPLICATIONS OF SILICA AEROGELS Luc Van Ginneken 1,*, René E. Van de Leest 2, Louis Willems 1, Kathy Elst 1, Herman Weyten 1 1 Vito, Process Technology, Boeretang 200, B-2400
SYNTHESIS, CHARACTERISATION AND APPLICATIONS OF SILICA AEROGELS Luc Van Ginneken 1,*, René E. Van de Leest 2, Louis Willems 1, Kathy Elst 1, Herman Weyten 1 1 Vito, Process Technology, Boeretang 200, B-2400
IMPROVED SYNTHESIS AND REACTION CHEMISTRY OF FN 3
 IMPROVED SYNTHESIS AND REACTION CHEMISTRY OF FN 3 William W. Wilson,, Karl O. Christe, Ashwani Vij, Ralf Haiges ERC, Inc and Propellants Branch, Propulsion Directorate, Air Force Research Laboratory, Edwards
IMPROVED SYNTHESIS AND REACTION CHEMISTRY OF FN 3 William W. Wilson,, Karl O. Christe, Ashwani Vij, Ralf Haiges ERC, Inc and Propellants Branch, Propulsion Directorate, Air Force Research Laboratory, Edwards
Coating of Tetraethylorthosilicate (TEOS)/Vinyltriethoxysilane (VTES) Hybrid Solution on Polymer Films
 Journal of Sol-Gel Science and Technology 13, 409 413 (1998) c 1998 Kluwer Academic Publishers. Manufactured in The Netherlands. Coating of Tetraethylorthosilicate (TEOS)/Vinyltriethoxysilane (VTES) Hybrid
Journal of Sol-Gel Science and Technology 13, 409 413 (1998) c 1998 Kluwer Academic Publishers. Manufactured in The Netherlands. Coating of Tetraethylorthosilicate (TEOS)/Vinyltriethoxysilane (VTES) Hybrid
Surface Modification of PTMS Particles with Organosilanes: TEOS-, VTMS-, and MTMS-Modified Particles
 Journal of Sol-Gel Science and Technology 31, 117 121, 2004 c 2004 Kluwer Academic Publishers. Manufactured in The United States. Surface Modification of PTMS Particles with Organosilanes: TEOS-, VTMS-,
Journal of Sol-Gel Science and Technology 31, 117 121, 2004 c 2004 Kluwer Academic Publishers. Manufactured in The United States. Surface Modification of PTMS Particles with Organosilanes: TEOS-, VTMS-,
The Effects of Organotin Catalysts on Hydrolytic Condensation of
 Original Research Paper J. Jpn. Sco. Colour Mater. (SHIKIZAI), 76(10),373-379 (2003) Polymethylsiloxane Oligomer and Moisture Cure of the Coatings Akira IWASAWA*, Ryuichi Ape, Hiroharu SASAKI*, Toshiya
Original Research Paper J. Jpn. Sco. Colour Mater. (SHIKIZAI), 76(10),373-379 (2003) Polymethylsiloxane Oligomer and Moisture Cure of the Coatings Akira IWASAWA*, Ryuichi Ape, Hiroharu SASAKI*, Toshiya
Report on Preparation of Nanotemplates for mab Crystallization
 Deliverable number D2.1 Due date 30/09/2017 Deliverable title Report on Preparation of Nanotemplates for mab Crystallization Issue date 21/09/2017 WP number WP2 Author(s) J. Heng, W. Chen, H. Yang Lead
Deliverable number D2.1 Due date 30/09/2017 Deliverable title Report on Preparation of Nanotemplates for mab Crystallization Issue date 21/09/2017 WP number WP2 Author(s) J. Heng, W. Chen, H. Yang Lead
48 Journal of Non-Crystalline Solids 111 (1989) North-Holland, Amsterdam
 48 Journal of Non-Crystalline Solids 111 (1989) 48-54 North-Holland, Amsterdam SPINNABILITY OF SILICA SOLS Structural and rheological criteria C.J. BRINKER and R.A. ASSINK Sandia National Laboratories,
48 Journal of Non-Crystalline Solids 111 (1989) 48-54 North-Holland, Amsterdam SPINNABILITY OF SILICA SOLS Structural and rheological criteria C.J. BRINKER and R.A. ASSINK Sandia National Laboratories,
geeeo. I UNCLASSIFIED NO 814-B5-K-88 F/G 716 NL SCENCESECTION L F HANCOCK ET AL 81 AUG 87 TR-i
 -AiS4 819 PROTON ABSTRACTION AS A ROUTE TO CONDUCTIVE POLYMERS 1/1 (U) PENNSYLVANIA STATE UNIV UNIVERSITY PARK PA POLYMER SCENCESECTION L F HANCOCK ET AL 81 AUG 87 TR-i I UNCLASSIFIED NO 814-B5-K-88 F/G
-AiS4 819 PROTON ABSTRACTION AS A ROUTE TO CONDUCTIVE POLYMERS 1/1 (U) PENNSYLVANIA STATE UNIV UNIVERSITY PARK PA POLYMER SCENCESECTION L F HANCOCK ET AL 81 AUG 87 TR-i I UNCLASSIFIED NO 814-B5-K-88 F/G
Nanostructures Materials Seminar
 CHE499 Nanostructures Materials Seminar Supported by 5FW Spring 2007 National Science Foundation Anita LaSalle, Program Manager (CISE- EIA 0203481) Aerogels AEROGELS: Strcture (TEM) 3IV TEM: Link-&-Blob
CHE499 Nanostructures Materials Seminar Supported by 5FW Spring 2007 National Science Foundation Anita LaSalle, Program Manager (CISE- EIA 0203481) Aerogels AEROGELS: Strcture (TEM) 3IV TEM: Link-&-Blob
MetE 215 Sol-Gel Processing
 MetE 215 Sol-Gel Processing 1. Introduction 1.1 Glass from liquid precursors: What is sol-gel? Glasses are usually prepared by mixing the different oxides precursors (carbonates, nitrates, sulfates, oxides..)
MetE 215 Sol-Gel Processing 1. Introduction 1.1 Glass from liquid precursors: What is sol-gel? Glasses are usually prepared by mixing the different oxides precursors (carbonates, nitrates, sulfates, oxides..)
Combined metallocene catalysts: an efficient technique to manipulate long-chain branching frequency of polyethylene
 Macromol. Rapid Commun. 20, 541 545 (1999) 541 Combined metallocene catalysts: an efficient technique to manipulate long-chain branching frequency of polyethylene Daryoosh Beigzadeh, João B. P. Soares*,
Macromol. Rapid Commun. 20, 541 545 (1999) 541 Combined metallocene catalysts: an efficient technique to manipulate long-chain branching frequency of polyethylene Daryoosh Beigzadeh, João B. P. Soares*,
Chemically recyclable alternating copolymers with low polydispersity from
 Electronic Supplementary Information Chemically recyclable alternating copolymers with low polydispersity from conjugated/aromatic aldehydes with vinyl ethers: selective degradation to another monomer
Electronic Supplementary Information Chemically recyclable alternating copolymers with low polydispersity from conjugated/aromatic aldehydes with vinyl ethers: selective degradation to another monomer
Synthesis of Tethered Chromium Carbene Complexes
 SYNTHESIS OF TETHERED CHROMIUM CARBENE COMPLEXES 375 Synthesis of Tethered Chromium Carbene Complexes Nicole S. Lueck Faculty Sponsor: Curtis Czerwinski, Department of Chemistry ABSTRACT Hydroxycarbene
SYNTHESIS OF TETHERED CHROMIUM CARBENE COMPLEXES 375 Synthesis of Tethered Chromium Carbene Complexes Nicole S. Lueck Faculty Sponsor: Curtis Czerwinski, Department of Chemistry ABSTRACT Hydroxycarbene
(07) 2 (c) 2 (c) (i) Calculate the ph of this buffer solution at 25 oc (3 marks) (Extra space)
 7 The value of Ka for methanoic acid is 1.78 10 4 mol dm 3 at 25 oc. A buffer solution is prepared containing 2.35 10 2 mol of methanoic acid and Question 1: N/A 1.84 10 2 mol of sodium methanoate in 1.00
7 The value of Ka for methanoic acid is 1.78 10 4 mol dm 3 at 25 oc. A buffer solution is prepared containing 2.35 10 2 mol of methanoic acid and Question 1: N/A 1.84 10 2 mol of sodium methanoate in 1.00
GO-CATALYSIS IN FRIEDEL-CRAFTS REACTIONS
 GO-CATALYSIS IN FRIEDEL-CRAFTS REACTIONS VI. POLYMERIZATION OF 2-BUTENE BY BORON FLUORIDE - METHANOL'. H. R. ALL COCK^ AND A. M. EASTHAM Division of Applied Chemistry, National Research Council, Ottawa,
GO-CATALYSIS IN FRIEDEL-CRAFTS REACTIONS VI. POLYMERIZATION OF 2-BUTENE BY BORON FLUORIDE - METHANOL'. H. R. ALL COCK^ AND A. M. EASTHAM Division of Applied Chemistry, National Research Council, Ottawa,
Factors Affecting Reaction Rate
 Factors Affecting Reaction Rate Outcomes: Formulate an operational definition of reaction rate. State the collision theory. Perform a lab to identify factors that affect reaction rate. Describe, qualitatively,
Factors Affecting Reaction Rate Outcomes: Formulate an operational definition of reaction rate. State the collision theory. Perform a lab to identify factors that affect reaction rate. Describe, qualitatively,
A novel smart polymer responsive to CO 2
 A novel smart polymer responsive to CO 2 Zanru Guo, a,b Yujun Feng,* a Yu Wang, a Jiyu Wang, a,b Yufeng Wu, a,b and Yongmin Zhang a,b a Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences,
A novel smart polymer responsive to CO 2 Zanru Guo, a,b Yujun Feng,* a Yu Wang, a Jiyu Wang, a,b Yufeng Wu, a,b and Yongmin Zhang a,b a Chengdu Institute of Organic Chemistry, Chinese Academy of Sciences,
SYNTHESIS AND CHARACTERIZATION OF COMMON OPAL. Pimthong Thongnopkun, 1,* Sukanya Pramol 2
 SYNTHESIS AND CHARACTERIZATION OF COMMON OPAL Pimthong Thongnopkun, 1,* Sukanya Pramol 2 1,2 Gems and Jewelry Research Unit, Faculty of Gems, Burapha University, Chanthaburi 22170, Thailand. *e-mail: pimthong@buu.ac.th,
SYNTHESIS AND CHARACTERIZATION OF COMMON OPAL Pimthong Thongnopkun, 1,* Sukanya Pramol 2 1,2 Gems and Jewelry Research Unit, Faculty of Gems, Burapha University, Chanthaburi 22170, Thailand. *e-mail: pimthong@buu.ac.th,
Optimizing Ion Transport in Polyether-based Electrolytes for Lithium Batteries
 Supporting Information Optimizing Ion Transport in Polyether-based Electrolytes for Lithium Batteries Qi Zheng, 1 Danielle M. Pesko, 1 Brett M. Savoie, Ksenia Timachova, Alexandra L. Hasan, Mackensie C.
Supporting Information Optimizing Ion Transport in Polyether-based Electrolytes for Lithium Batteries Qi Zheng, 1 Danielle M. Pesko, 1 Brett M. Savoie, Ksenia Timachova, Alexandra L. Hasan, Mackensie C.
Preparation of Hydrophobic Monolithic Silica Aerogels through Surface Modification Using Hexamethyldisilazane in Supercritical CO 2
 Preparation of Hydrophobic Monolithic Silica Aerogels through Surface Modification Using Hexamethyldisilazane in Supercritical CO 2 Can Erkey* and Ayse Meric Kartal Department of Chemical and Biological
Preparation of Hydrophobic Monolithic Silica Aerogels through Surface Modification Using Hexamethyldisilazane in Supercritical CO 2 Can Erkey* and Ayse Meric Kartal Department of Chemical and Biological
Size exclusion chromatography of branched polymers: Star and comb polymers
 Macromol. Theory Simul. 8, 513 519 (1999) 513 Size exclusion chromatography of branched polymers: Star and comb polymers Hidetaka Tobita*, Sadayuki Saito Department of Materials Science and Engineering,
Macromol. Theory Simul. 8, 513 519 (1999) 513 Size exclusion chromatography of branched polymers: Star and comb polymers Hidetaka Tobita*, Sadayuki Saito Department of Materials Science and Engineering,
AD--A251 '109 IENTATION PAGE IIIIII
 AD--A251 '109 IENTATION PAGE IIIIII111 1111 1mii 111i ~men FomApoe Int ra "1e.0 ettr a e 1O1 cctif 10su o snlorrate i IS en A Co WfA t f u buiden est:mate of any other auoect of trr hn oufco to ahington.4eadquarters
AD--A251 '109 IENTATION PAGE IIIIII111 1111 1mii 111i ~men FomApoe Int ra "1e.0 ettr a e 1O1 cctif 10su o snlorrate i IS en A Co WfA t f u buiden est:mate of any other auoect of trr hn oufco to ahington.4eadquarters
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/NCHEM.2633 Mechanically controlled radical polymerization initiated by ultrasound Hemakesh Mohapatra, Maya Kleiman, Aaron P. Esser-Kahn Contents 1. Materials and methods 2 2. Procedure for
DOI: 10.1038/NCHEM.2633 Mechanically controlled radical polymerization initiated by ultrasound Hemakesh Mohapatra, Maya Kleiman, Aaron P. Esser-Kahn Contents 1. Materials and methods 2 2. Procedure for
AD-A i ELECTE. (*Sandia National Laboratories, Livermore, CA) OFFICE OF NAVAL RESEARCH. R&T Project Code 413 a 001
 AD-A262 1 1 5 OFFICE OF NAVAL RESEARCH R&T Project Code 413 a 001 Contract No. NOOO14-89-J-1235 Technical Report No. 16 Kinetics and Thermochemistry of Tetramethyl and Tetraethyl Orthosilicates In the
AD-A262 1 1 5 OFFICE OF NAVAL RESEARCH R&T Project Code 413 a 001 Contract No. NOOO14-89-J-1235 Technical Report No. 16 Kinetics and Thermochemistry of Tetramethyl and Tetraethyl Orthosilicates In the
AD-A ~ Cop. telecte SNOVO 10O. OFFICE OF NAVAL RESEARCH Contract N K-0517 R&T Code TECHNICAL REPORT NO.
 Cop AD-A228 202 OFFICE OF NAVAL RESEARCH Contract N00014-87-K-0517 R&T Code 4132001---02 TECHNICAL REPORT NO. 11 Gas Solubility in Glassy Polymers - A Correlation with Excess Enthalpy by D.H. Rein, R.F.
Cop AD-A228 202 OFFICE OF NAVAL RESEARCH Contract N00014-87-K-0517 R&T Code 4132001---02 TECHNICAL REPORT NO. 11 Gas Solubility in Glassy Polymers - A Correlation with Excess Enthalpy by D.H. Rein, R.F.
Adsorption Processes. Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad
 Adsorption Processes Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad Contents Introduction Principles of adsorption Types of adsorption Definitions Brief history Adsorption isotherms Mechanism
Adsorption Processes Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad Contents Introduction Principles of adsorption Types of adsorption Definitions Brief history Adsorption isotherms Mechanism
Siloxane Network Formation from the Si 8 O 20 8 Silicate Species and Dimethyldichlorosilane
 APPLIED ORGANOMETALLIC CHEMISTRY Appl. Organometal. Chem. 13, 273 277 (1999) Siloxane Network Formation from the Si 8 O 20 8 Silicate Species and Dimethyldichlorosilane Isao Hasegawa, 1 * Yasutaka Nakane
APPLIED ORGANOMETALLIC CHEMISTRY Appl. Organometal. Chem. 13, 273 277 (1999) Siloxane Network Formation from the Si 8 O 20 8 Silicate Species and Dimethyldichlorosilane Isao Hasegawa, 1 * Yasutaka Nakane
Synthesis; sol-gel. Helmer Fjellvåg and Anja Olafsen Sjåstad. Lectures at CUTN spring 2016
 Synthesis; sol-gel Helmer Fjellvåg and Anja Olafsen Sjåstad Lectures at CUTN spring 2016 Inorganic Materials Synthesis In January: Solid State Reactions Our text book has extended the definition to any
Synthesis; sol-gel Helmer Fjellvåg and Anja Olafsen Sjåstad Lectures at CUTN spring 2016 Inorganic Materials Synthesis In January: Solid State Reactions Our text book has extended the definition to any
E6 PROPERTIES OF GASES Flow-times, density, phase changes, solubility
 E6 PROPERTIES OF GASES Flow-times, density, phase changes, solubility Introduction Kinetic-Molecular Theory The kinetic energy of an object is dependent on its mass and its speed. The relationship, given
E6 PROPERTIES OF GASES Flow-times, density, phase changes, solubility Introduction Kinetic-Molecular Theory The kinetic energy of an object is dependent on its mass and its speed. The relationship, given
Surface modification of silica particles with organoalkoxysilanes through two-step (acid-base) process in aqueous solution
 Journal of Ceramic Processing Research. Vol. 3, No. 3, pp. 216~221 (2002) J O U R N A L O F Surface modification of silica particles with organoalkoxysilanes through two-step (acid-base) process in aqueous
Journal of Ceramic Processing Research. Vol. 3, No. 3, pp. 216~221 (2002) J O U R N A L O F Surface modification of silica particles with organoalkoxysilanes through two-step (acid-base) process in aqueous
Fast Nucleation for Silica Nanoparticle Synthesis in. Sol-Gel Method
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2016 Fast Nucleation for lica Nanoparticle Synthesis in Sol-Gel Method Chandra K. Dixit*, Snehasis
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2016 Fast Nucleation for lica Nanoparticle Synthesis in Sol-Gel Method Chandra K. Dixit*, Snehasis
DTIC ELECTE S JUL u AD-A '
 AD-A266 957 OFFICE OF NAVAL RESEARCH Contract N/N00014-91-J-1893 R&T Code 4132062 TECHNICAL REPORT NO. 2 Self-Diffusion Coefficients of Sol-Gel Intermediates L. Ng and A.V. McCormick Dept. of Chemical
AD-A266 957 OFFICE OF NAVAL RESEARCH Contract N/N00014-91-J-1893 R&T Code 4132062 TECHNICAL REPORT NO. 2 Self-Diffusion Coefficients of Sol-Gel Intermediates L. Ng and A.V. McCormick Dept. of Chemical
The Surface Chemistry of Hydrophobic Silica Aerogels
 Supplementary Information The Surface Chemistry of Hydrophobic Silica Aerogels Wim J. Malfait, Shanyu Zhao, Rene Verel, Subramaniam Iswar, Daniel Rentsch, Resul Fener, Yucheng Zhang, Barbara Milow and
Supplementary Information The Surface Chemistry of Hydrophobic Silica Aerogels Wim J. Malfait, Shanyu Zhao, Rene Verel, Subramaniam Iswar, Daniel Rentsch, Resul Fener, Yucheng Zhang, Barbara Milow and
Deterministic Solutions for a Step-growth Polymerization
 University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Molecular Chemistry Chemical and Biomolecular Engineering Research and Publications 10-17-2003 Deterministic Solutions
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Molecular Chemistry Chemical and Biomolecular Engineering Research and Publications 10-17-2003 Deterministic Solutions
An Investigation of Molecular Templating in Amorphous Silicas by Cross-Polarization NMR Spectroscopy
 An Investigation of Molecular Templating in Amorphous Silicas by Cross-Polarization NMR Spectroscopy C. A. Click,l R. A. Assink,2* C. J. Brinker,2 3 and S. J. Naik3 1. University of Missouri, Rolls, MO
An Investigation of Molecular Templating in Amorphous Silicas by Cross-Polarization NMR Spectroscopy C. A. Click,l R. A. Assink,2* C. J. Brinker,2 3 and S. J. Naik3 1. University of Missouri, Rolls, MO
CHEM4. (JAN13CHEM401) WMP/Jan13/CHEM4. General Certificate of Education Advanced Level Examination January 2013
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination January 2013 Question 1 2 Mark
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination January 2013 Question 1 2 Mark
FACILE AND ECONOMIC SYNTHESIS OF SILICA NANOPARTICLES
 Journal of Ovonic Research Vol. 5, No. 5, October 2009, p. 129-133 FACILE AND ECONOMIC SYNTHESIS OF SILICA NANOPARTICLES M. F. ZAWRAH* 1, A. A. EL-KHESHEN 2 AND HAITHAM M. ABD-EL- AAL1 1 1 Ceramics Department,
Journal of Ovonic Research Vol. 5, No. 5, October 2009, p. 129-133 FACILE AND ECONOMIC SYNTHESIS OF SILICA NANOPARTICLES M. F. ZAWRAH* 1, A. A. EL-KHESHEN 2 AND HAITHAM M. ABD-EL- AAL1 1 1 Ceramics Department,
June 1, [994 Technical 9/93-6/94. N J Methylcvclopropene
 "AD -A280 185 ATION PAGE... No 0704-0188 **..- fll-:f...,... :, -...,
"AD -A280 185 ATION PAGE... No 0704-0188 **..- fll-:f...,... :, -...,
Supplementary Material (ESI) for Chemical Communications This journal is (c) The Royal Society of Chemistry 2008
 Supplementary Information for: Scrambling Reaction between Polymers Prepared by Step-growth and Chain-growth Polymerizations: Macromolecular Cross-metathesis between 1,4-Polybutadiene and Olefin-containing
Supplementary Information for: Scrambling Reaction between Polymers Prepared by Step-growth and Chain-growth Polymerizations: Macromolecular Cross-metathesis between 1,4-Polybutadiene and Olefin-containing
Multistep Synthesis of 5-isopropyl-1,3-cyclohexanedione
 Multistep Synthesis of 5-isopropyl-1,3-cyclohexanedione The purpose of this experiment was to synthesize 5-isopropyl-1,3-cyclohexanedione from commercially available compounds. To do this, acetone and
Multistep Synthesis of 5-isopropyl-1,3-cyclohexanedione The purpose of this experiment was to synthesize 5-isopropyl-1,3-cyclohexanedione from commercially available compounds. To do this, acetone and
Pore structure evolution in silica gel during aging/drying II. Effect of pore fluids
 Journal of Non-Crystalline Solids 142 (1992) 197-207 North-Holland ]OURNA L OF NON-CRYSTALLINE SOLIDS Pore structure evolution in silica gel during aging/drying II. Effect of pore fluids Pamela J. Davis
Journal of Non-Crystalline Solids 142 (1992) 197-207 North-Holland ]OURNA L OF NON-CRYSTALLINE SOLIDS Pore structure evolution in silica gel during aging/drying II. Effect of pore fluids Pamela J. Davis
New Sol-Gel Solution with 45 Days Stability for Preparation Silica Thin Films
 Iran. J. Chem. Chem. Eng. Vol. 26, No.3, 2007 New Sol-Gel Solution with 45 Days Stability for Preparation Silica Thin Films Adelkhani, Hadi* + ; Mellatnavaz, Bahram; Roohi, Hossein; Noorbakhsh, Mansoor
Iran. J. Chem. Chem. Eng. Vol. 26, No.3, 2007 New Sol-Gel Solution with 45 Days Stability for Preparation Silica Thin Films Adelkhani, Hadi* + ; Mellatnavaz, Bahram; Roohi, Hossein; Noorbakhsh, Mansoor
Kinetic Isotope Effects
 1 Experiment 31 Kinetic Isotope Effects Isotopic substitution is a useful technique for the probing of reaction mechanisms. The change of an isotope may affect the reaction rate in a number of ways, providing
1 Experiment 31 Kinetic Isotope Effects Isotopic substitution is a useful technique for the probing of reaction mechanisms. The change of an isotope may affect the reaction rate in a number of ways, providing
F G C G H H C B B A A DD E E
 a D E F G H D E I F G H F F I DD E E G H H b c [1] 0 (M) [2a] 0 (M) [1] (M) [2a] (M) [3a] (M) K (M -1 ) a) 0.160 0.139 0.049 0.028 0.111 81 b) 0.147 0.180 0.025 0.058 0.122 84 c) 0.157 0.270 0.013 0.126
a D E F G H D E I F G H F F I DD E E G H H b c [1] 0 (M) [2a] 0 (M) [1] (M) [2a] (M) [3a] (M) K (M -1 ) a) 0.160 0.139 0.049 0.028 0.111 81 b) 0.147 0.180 0.025 0.058 0.122 84 c) 0.157 0.270 0.013 0.126
Ceramic Processing Research
 Journal of Ceramic Processing Research. Vol. 7, No. 1, pp. 83~89 (2006) J O U R N A L O F Ceramic Processing Research Effect of Alcohol Chain Length on Particle Growth in a Mixed Solvent System Joon Sang
Journal of Ceramic Processing Research. Vol. 7, No. 1, pp. 83~89 (2006) J O U R N A L O F Ceramic Processing Research Effect of Alcohol Chain Length on Particle Growth in a Mixed Solvent System Joon Sang
Supporting Information
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Nanoparticle-to-vesicle and nanoparticle-to-toroid transitions of ph-sensitive
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Nanoparticle-to-vesicle and nanoparticle-to-toroid transitions of ph-sensitive
Metallurgical and Materials Engineering Department MME 2509 Materials Processing Laboratory SOL-GEL DIP COATING
 Metallurgical and Materials Engineering Department MME 2509 Materials Processing Laboratory SOL-GEL DIP COATING Assist. Prof. Dr. Tolga TAVŞANOĞLU 1. Sol-gel Process Sol-gel process is used for production
Metallurgical and Materials Engineering Department MME 2509 Materials Processing Laboratory SOL-GEL DIP COATING Assist. Prof. Dr. Tolga TAVŞANOĞLU 1. Sol-gel Process Sol-gel process is used for production
Ceramic Processing Research
 Journal of Ceramic Processing Research. Vol. 19, No. 4, pp. 321~326 (2018) J O U R N A L O F Ceramic Processing Research Effects of alcohol solvents on formation of the hydrated silica gel Sang-Eun Lee
Journal of Ceramic Processing Research. Vol. 19, No. 4, pp. 321~326 (2018) J O U R N A L O F Ceramic Processing Research Effects of alcohol solvents on formation of the hydrated silica gel Sang-Eun Lee
Palladium-Catalyzed Alkylarylation of Acrylamides with
 Supporting Information Palladium-Catalyzed Alkylarylation of Acrylamides with Unactivated Alkyl Halides Hua Wang, Li-a Guo, and Xin-Hua Duan* Department of Chemistry, School of Science and ME Key Laboratory
Supporting Information Palladium-Catalyzed Alkylarylation of Acrylamides with Unactivated Alkyl Halides Hua Wang, Li-a Guo, and Xin-Hua Duan* Department of Chemistry, School of Science and ME Key Laboratory
Supporting Information.
 Supporting Information. Materials. Polyethyleneglycol monomethylether methacrylate (PEGMA) (~475 Da), trifluoroethyl acrylate (tfea) and ethyleneglycol dimethacrylate (EGDMA) were purchased from Sigma
Supporting Information. Materials. Polyethyleneglycol monomethylether methacrylate (PEGMA) (~475 Da), trifluoroethyl acrylate (tfea) and ethyleneglycol dimethacrylate (EGDMA) were purchased from Sigma
Light irradiation experiments with coumarin [1]
![Light irradiation experiments with coumarin [1] Light irradiation experiments with coumarin [1]](/thumbs/88/114731970.jpg) Materials and instruments All the chemicals were purchased from commercial suppliers and used as received. Thin-layer chromatography (TLC) analysis was carried out on pre-coated silica plates. Column chromatography
Materials and instruments All the chemicals were purchased from commercial suppliers and used as received. Thin-layer chromatography (TLC) analysis was carried out on pre-coated silica plates. Column chromatography
This paper is part of the following report: UNCLASSIFIED
 UNCLASSIFIED Defense Technical Information Center Compilation Part Notice ADP023624 TITLE: Ignition Kinetics in Fuels Oxidation DISTRIBUTION: Approved for public release, distribution unlimited This paper
UNCLASSIFIED Defense Technical Information Center Compilation Part Notice ADP023624 TITLE: Ignition Kinetics in Fuels Oxidation DISTRIBUTION: Approved for public release, distribution unlimited This paper
Polymers 2017; doi: 1. Structural Characterisation of the Prepared Iniferters, BDC and SBDC
 S1/S15 Supplementary Materials: Optimisation of surfaceinitiated photoiniferter-mediated polymerisation under confinement, and the formation of block copolymers in mesoporous films Jessica C. Tom 1, Robert
S1/S15 Supplementary Materials: Optimisation of surfaceinitiated photoiniferter-mediated polymerisation under confinement, and the formation of block copolymers in mesoporous films Jessica C. Tom 1, Robert
Sol-Gel Methods. Hydrolysis Condensation Gelation Ageing Drying Densification
 Sol-Gel Methods Sol-gel process: Hydrolysis Condensation Gelation Ageing Drying Densification Powders: microcrystalline, nanocrystalline, amorphous Monoliths, Coatings, Films, Fibers Aerogels Glasses,
Sol-Gel Methods Sol-gel process: Hydrolysis Condensation Gelation Ageing Drying Densification Powders: microcrystalline, nanocrystalline, amorphous Monoliths, Coatings, Films, Fibers Aerogels Glasses,
Sumio Sakka On the Occasion of His.
 A SAXS Characterization of Particle TitleSol-Gel System (Commemoration Issue Sumio Sakka On the Occasion of His Author(s) Kamiyama, Tomoaki; Yoshida, Naozumi Citation Bulletin of the Institute for Chemi
A SAXS Characterization of Particle TitleSol-Gel System (Commemoration Issue Sumio Sakka On the Occasion of His Author(s) Kamiyama, Tomoaki; Yoshida, Naozumi Citation Bulletin of the Institute for Chemi
IUNCLSSIME N E F/G 11?2 NL
 AD-RI58 658 BORON-NITROGEN POLYNERS(U) ULTRASYSTENS INC IRVINE CA III 1 K L PACIOREK ET AL. 01 JUL 85 SN-2822-F NS9914-82-C-9482 IUNCLSSIME N E F/G 11?2 NL 1.0 16 2.: w1111342 1.8 11111125 1111.4 L MICROCOPY
AD-RI58 658 BORON-NITROGEN POLYNERS(U) ULTRASYSTENS INC IRVINE CA III 1 K L PACIOREK ET AL. 01 JUL 85 SN-2822-F NS9914-82-C-9482 IUNCLSSIME N E F/G 11?2 NL 1.0 16 2.: w1111342 1.8 11111125 1111.4 L MICROCOPY
MODIFICATION WITH A SULFONATE MONOMER
 Thesis - MOLECULAR STRUCTURES AND FUNCTIONAL MODIFICATIONS OF POLY(VINYL ALCOHOL) CHAPTER 8 BY TOHEI MORITANI MODIFICATION WITH A SULFONATE MONOMER A functional monomer containing sodium sulfonate group,
Thesis - MOLECULAR STRUCTURES AND FUNCTIONAL MODIFICATIONS OF POLY(VINYL ALCOHOL) CHAPTER 8 BY TOHEI MORITANI MODIFICATION WITH A SULFONATE MONOMER A functional monomer containing sodium sulfonate group,
One-pot polymer brush synthesis via simultaneous isocyanate coupling chemistry and grafting from RAFT polymerization
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2014 One-pot polymer brush synthesis via simultaneous isocyanate coupling chemistry and grafting
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2014 One-pot polymer brush synthesis via simultaneous isocyanate coupling chemistry and grafting
Scheme 1: Reaction scheme for the synthesis of p(an-co-mma) copolymer
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Design and Development of Poly (acrylonitrile-co-methyl methacrylate) Copolymer to Improve
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Design and Development of Poly (acrylonitrile-co-methyl methacrylate) Copolymer to Improve
Rule 2. Rule 1. Rule 4. Rule 3. Rule 5. Rule 6. Rule 7. Rule 8
 Rule 1 Follow the directions in your course reader, of your teaching assistant and of your instructor. They are usually much more experienced doing chemistry. Rule 3 When in doubt, ask. This will make
Rule 1 Follow the directions in your course reader, of your teaching assistant and of your instructor. They are usually much more experienced doing chemistry. Rule 3 When in doubt, ask. This will make
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds.
 This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
Olefin Metathesis ROMP. L n Ru= ROMP n RCM. dilute
 lefin Metathesis MP: ing-opening metathesis polymerization Thermodynamically favored for 3,4, 8, larger ring systems Bridging groups (bicyclic olefins) make ΔG polymerization more favorable as a result
lefin Metathesis MP: ing-opening metathesis polymerization Thermodynamically favored for 3,4, 8, larger ring systems Bridging groups (bicyclic olefins) make ΔG polymerization more favorable as a result
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Supporting Information
 Supporting Information Controlled Radical Polymerization and Quantification of Solid State Electrical Conductivities of Macromolecules Bearing Pendant Stable Radical Groups Lizbeth Rostro, Aditya G. Baradwaj,
Supporting Information Controlled Radical Polymerization and Quantification of Solid State Electrical Conductivities of Macromolecules Bearing Pendant Stable Radical Groups Lizbeth Rostro, Aditya G. Baradwaj,
Advanced Physical Chemistry CHAPTER 18 ELEMENTARY CHEMICAL KINETICS
 Experimental Kinetics and Gas Phase Reactions Advanced Physical Chemistry CHAPTER 18 ELEMENTARY CHEMICAL KINETICS Professor Angelo R. Rossi http://homepages.uconn.edu/rossi Department of Chemistry, Room
Experimental Kinetics and Gas Phase Reactions Advanced Physical Chemistry CHAPTER 18 ELEMENTARY CHEMICAL KINETICS Professor Angelo R. Rossi http://homepages.uconn.edu/rossi Department of Chemistry, Room
Fabrication of SiO 2, Al 2 O 3, and TiO 2 Microcapsules with Hollow Core and Mesoporous Shell Structure
 Fabrication of SiO 2, Al 2 O 3, and TiO 2 Microcapsules with Hollow Core and Mesoporous Shell Structure Xiao-Feng Guo, Yong-Suk Kim, and Geon-Joong Kim Department of Chemical Engineering, Inha UniVersity,
Fabrication of SiO 2, Al 2 O 3, and TiO 2 Microcapsules with Hollow Core and Mesoporous Shell Structure Xiao-Feng Guo, Yong-Suk Kim, and Geon-Joong Kim Department of Chemical Engineering, Inha UniVersity,
Surface Science and Stability of Networks Prepared from Hydroxy-Terminated Polydimethylsiloxane and Methyltriethoxysilane
 APPLIED ORGANOMETALLIC CHEMISTRY Appl. Organometal. Chem. 12, 763 770 (1998) Surface Science and Stability of Networks Prepared from Hydroxy-Terminated Polydimethylsiloxane and Methyltriethoxysilane Kenneth
APPLIED ORGANOMETALLIC CHEMISTRY Appl. Organometal. Chem. 12, 763 770 (1998) Surface Science and Stability of Networks Prepared from Hydroxy-Terminated Polydimethylsiloxane and Methyltriethoxysilane Kenneth
The Identification of Chemical Species of Silica in Sodium Hydroxide, Potassium Hydroxide and Sodium Chloride Solutions by FAB-MS
 ANALYTICAL SCIENCES DECEMBER 1999, VOL. 15 1999 The Japan Society for Analytical Chemistry 1241 The Identification of Chemical Species of Silica in Sodium Hydroxide, Potassium Hydroxide and Sodium Chloride
ANALYTICAL SCIENCES DECEMBER 1999, VOL. 15 1999 The Japan Society for Analytical Chemistry 1241 The Identification of Chemical Species of Silica in Sodium Hydroxide, Potassium Hydroxide and Sodium Chloride
Preparation of monodisperse silica particles with controllable size and shape
 Preparation of monodisperse silica particles with controllable size and shape J.H. Zhang, a) P. Zhan, Z.L. Wang, W.Y. Zhang, and N.B. Ming National Laboratory of Solid State Microstructures, Department
Preparation of monodisperse silica particles with controllable size and shape J.H. Zhang, a) P. Zhan, Z.L. Wang, W.Y. Zhang, and N.B. Ming National Laboratory of Solid State Microstructures, Department
Supporting Information
 Supporting Information Branched polyethylene mimicry by metathesis copolymerization of fatty acid-based α,ω-dienes. Thomas Lebarbé, a,b,d Mehdi Neqal, a,b Etienne Grau, a,b Carine Alfos, c and Henri Cramail
Supporting Information Branched polyethylene mimicry by metathesis copolymerization of fatty acid-based α,ω-dienes. Thomas Lebarbé, a,b,d Mehdi Neqal, a,b Etienne Grau, a,b Carine Alfos, c and Henri Cramail
Amphiphilic diselenide-containing supramolecular polymers
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2014 Amphiphilic diselenide-containing supramolecular polymers Xinxin Tan, Liulin Yang, Zehuan
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2014 Amphiphilic diselenide-containing supramolecular polymers Xinxin Tan, Liulin Yang, Zehuan
Accelerated Cure of Phenol-Formaldehyde Resins: Studies With Model Compounds
 Accelerated Cure of Phenol-Formaldehyde Resins: Studies With Model Compounds Anthony H. Conner, Linda F. Lorenz, Kolby C. Hirth USDA Forest Service, Forest Products Laboratory, One Gifford Pinchot Drive,
Accelerated Cure of Phenol-Formaldehyde Resins: Studies With Model Compounds Anthony H. Conner, Linda F. Lorenz, Kolby C. Hirth USDA Forest Service, Forest Products Laboratory, One Gifford Pinchot Drive,
Ulm 1.00 &L6 MICROCOPY RESOLUTION4 TEST CHART. 4-_IU44k 1URE-AU OF STANOARM-19WA - -W ~ -. EW W W.~ %
 ND-R193 591 A ROTATIONAL ISOMERIC STATE MODEL FOR THE POLYCARBONATE 1/'1 OF 22' - BIS (4-H.. (U) MASSACHUSETTS INST OF TECH CAMIRIDGE DEPT OF CHEMICAL ENOINEE.. A HtITNII( ET AL. UNCLASSIFIED MEu"M. 31
ND-R193 591 A ROTATIONAL ISOMERIC STATE MODEL FOR THE POLYCARBONATE 1/'1 OF 22' - BIS (4-H.. (U) MASSACHUSETTS INST OF TECH CAMIRIDGE DEPT OF CHEMICAL ENOINEE.. A HtITNII( ET AL. UNCLASSIFIED MEu"M. 31
SUPPORTING INFORMATION
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2014 SUPPORTING INFORMATION Fast and Accurate Partial Hydrolysis of Poly(2-ethyl-2- oxazoline)
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2014 SUPPORTING INFORMATION Fast and Accurate Partial Hydrolysis of Poly(2-ethyl-2- oxazoline)
BAE 820 Physical Principles of Environmental Systems
 BAE 820 Physical Principles of Environmental Systems Catalysis of environmental reactions Dr. Zifei Liu Catalysis and catalysts Catalysis is the increase in the rate of a chemical reaction due to the participation
BAE 820 Physical Principles of Environmental Systems Catalysis of environmental reactions Dr. Zifei Liu Catalysis and catalysts Catalysis is the increase in the rate of a chemical reaction due to the participation
Supporting Information
 Supporting Information Syntheses and characterizations: Compound 1 was synthesized according to Scheme S-1. Scheme S-1 2 N N 5 i N 4 P Et Et iii N 6 ii P Et Et iv v, vi N N i) Fmoc-Su, DIPEA, Acetone;
Supporting Information Syntheses and characterizations: Compound 1 was synthesized according to Scheme S-1. Scheme S-1 2 N N 5 i N 4 P Et Et iii N 6 ii P Et Et iv v, vi N N i) Fmoc-Su, DIPEA, Acetone;
SUPPORTING INFORMATION
 SUPPORTING INFORMATION Polymerization-induced Self-Assembly of Homopolymer and Diblock copolymer: A Facile Approach for preparing Polymer Nano-objects with Higher Order Morphologies Jianbo Tan *a,b, Chundong
SUPPORTING INFORMATION Polymerization-induced Self-Assembly of Homopolymer and Diblock copolymer: A Facile Approach for preparing Polymer Nano-objects with Higher Order Morphologies Jianbo Tan *a,b, Chundong
IMMOBILIZATION AND PHOTOCHEMICAL STUDIES OF BROMOCRESOL PURPLE IN SOL-GEL MEMBRANE Buronov A.O., Tashpulatov Kh.Sh., Nasimov A.M.
 GSJ: VOLUME 6, ISSUE 8, August 2018 599 GSJ: Volume 6, Issue 8, August 2018, Online: IMMOBILIZATION AND PHOTOCHEMICAL STUDIES OF BROMOCRESOL PURPLE IN SOL-GEL MEMBRANE Buronov A.O., Tashpulatov Kh.Sh.,
GSJ: VOLUME 6, ISSUE 8, August 2018 599 GSJ: Volume 6, Issue 8, August 2018, Online: IMMOBILIZATION AND PHOTOCHEMICAL STUDIES OF BROMOCRESOL PURPLE IN SOL-GEL MEMBRANE Buronov A.O., Tashpulatov Kh.Sh.,
Effects of Solvent Acidity on the Free-Radical-Initiated Synthesis of Methanesulfonic Acid from CH 4 and SO 3
 Ind. Eng. Chem. Res. 2002, 41, 5901-5905 5901 APPLIED CHEMISTRY Effects of Solvent Acidity on the Free-Radical-Initiated Synthesis of Methanesulfonic Acid from CH 4 and SO 3 Sudip Mukhopadhyay and Alexis
Ind. Eng. Chem. Res. 2002, 41, 5901-5905 5901 APPLIED CHEMISTRY Effects of Solvent Acidity on the Free-Radical-Initiated Synthesis of Methanesulfonic Acid from CH 4 and SO 3 Sudip Mukhopadhyay and Alexis
Electronic supporting information for
 Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Electronic supporting information for The effects of an ionic liquid on
Electronic Supplementary Material (ESI) for Organic & Biomolecular Chemistry. This journal is The Royal Society of Chemistry 2016 Electronic supporting information for The effects of an ionic liquid on
Chemical Kinetics of HC Combustion
 Spark Ignition Engine Combustion MAK65E Chemical Kinetics of HC Combustion Prof.Dr. Cem Soruşbay Istanbul Technical University Chemical Kinetics of HC Combustion Introduction Elementary reactions Multi-step
Spark Ignition Engine Combustion MAK65E Chemical Kinetics of HC Combustion Prof.Dr. Cem Soruşbay Istanbul Technical University Chemical Kinetics of HC Combustion Introduction Elementary reactions Multi-step
A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility
 (P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
(P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
Silver-catalyzed decarboxylative acylfluorination of styrenes in aqueous media
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Silver-catalyzed decarboxylative acylfluorination of styrenes in aqueous
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Silver-catalyzed decarboxylative acylfluorination of styrenes in aqueous
Babak Karimi* and Majid Vafaeezadeh
 Electronic upplementary Material (EI) for RC Advances This journal is The Royal ociety of Chemistry 2013 BA-15 functionalized sulfonic acid confined hydrophobic and acidic ionic liquid: a highly efficient
Electronic upplementary Material (EI) for RC Advances This journal is The Royal ociety of Chemistry 2013 BA-15 functionalized sulfonic acid confined hydrophobic and acidic ionic liquid: a highly efficient
AD-A PORT DOCUMENTATION PAGE *mii m~. 2. REPORT DATE 3 EPORT TYPE AND SATES COVERED
 UNCLASSIFIED AD-A278 940 PORT DOCUMENTATION PAGE *mii m~. 2. REPORT DATE 3 EPORT TYPE AND SATES COVERED April 8, 1994 IFinal Report, Feb. 1, 1992 through Jan. 30, 1094 4. TITLE AND SUBTITLE 5. FUNDING
UNCLASSIFIED AD-A278 940 PORT DOCUMENTATION PAGE *mii m~. 2. REPORT DATE 3 EPORT TYPE AND SATES COVERED April 8, 1994 IFinal Report, Feb. 1, 1992 through Jan. 30, 1094 4. TITLE AND SUBTITLE 5. FUNDING
GCSE CHEMISTRY REVISION LIST
 GCSE CHEMISTRY REVISION LIST OCR Gateway Chemistry (J248) from 2016 Topic C1: Particles C1.1 Describe the main features of the particle model in terms of states of matter and change of state Explain, in
GCSE CHEMISTRY REVISION LIST OCR Gateway Chemistry (J248) from 2016 Topic C1: Particles C1.1 Describe the main features of the particle model in terms of states of matter and change of state Explain, in
Soluble Precursor of Hexacene and its Application on Thin Film Transistor
 Soluble Precursor of Hexacene and its Application on Thin Film Transistor Supplementary Information Motonori Watanabe, a Wei-Ting Su, b Kew-Yu Chen,* c Ching-Ting Chien, a Ting-Han Chao, a Yuan Jay Chang,
Soluble Precursor of Hexacene and its Application on Thin Film Transistor Supplementary Information Motonori Watanabe, a Wei-Ting Su, b Kew-Yu Chen,* c Ching-Ting Chien, a Ting-Han Chao, a Yuan Jay Chang,
Methodology and Discussion
 The objective of this work is the syntheses of low-density spherical silica particles, which will be used to further enhance our knowledge of the fundamental physics of the particle movements in fluids.
The objective of this work is the syntheses of low-density spherical silica particles, which will be used to further enhance our knowledge of the fundamental physics of the particle movements in fluids.
STUDY OF DETECTION LIMITS AND QUANTITATION ACCURACY USING 300 MHZ NMR
 STUDY OF DETECTION LIMITS AND QUANTITATION ACCURACY USING 300 MHZ NMR William R. Creasy EAI Corporation, 1308 Continental Drive, Suite J, Abingdon MD 21009 David J. McGarvey, Jeffrey S. Rice, Richard O'Connor,
STUDY OF DETECTION LIMITS AND QUANTITATION ACCURACY USING 300 MHZ NMR William R. Creasy EAI Corporation, 1308 Continental Drive, Suite J, Abingdon MD 21009 David J. McGarvey, Jeffrey S. Rice, Richard O'Connor,
EXPERIMENT 1 REACTION RATE, RATE LAW, AND ACTIVATION ENERGY THE IODINE CLOCK REACTION
 PURPOSE: To determine the Rate Law and the Activation Energy for a reaction from experimental data. PRINCIPLES: The Rate Law is a mathematical expression that predicts the rate of a reaction from the concentration
PURPOSE: To determine the Rate Law and the Activation Energy for a reaction from experimental data. PRINCIPLES: The Rate Law is a mathematical expression that predicts the rate of a reaction from the concentration
AD-A FATION PAGE cmb No SUPPLEMENTARY NOTESlIII lllll II ili JUN PRICE CODE
 Form Approved AD-A252 270 FATION PAGE cmb No. 0704-0188 ge I~ uour oer resorse. including tile time for reviewing instructions. searchng existing cata sourcf-.. 'r, P 'llecicon o0 of information Send Commintsr
Form Approved AD-A252 270 FATION PAGE cmb No. 0704-0188 ge I~ uour oer resorse. including tile time for reviewing instructions. searchng existing cata sourcf-.. 'r, P 'llecicon o0 of information Send Commintsr
arxiv:chem-ph/ v1 22 Nov 1995
 Influence of surfactants on the structure of titanium oxide gels : experiments and simulations F. Molino arxiv:chem-ph/9511002v1 22 Nov 1995 J. M. Barthez A. Ayral, C. Guizard, R. Jullien*, J. Marignan
Influence of surfactants on the structure of titanium oxide gels : experiments and simulations F. Molino arxiv:chem-ph/9511002v1 22 Nov 1995 J. M. Barthez A. Ayral, C. Guizard, R. Jullien*, J. Marignan
Chapter 8. Substitution reactions of Alkyl Halides
 Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
, i, ~. '~~~* a F- WI W U V U S S S S S S It
 AD-A194 365 REACTION DYNAMICS ON SEMICONDUCTOR SURFACES(U) h RENSSELAER POLYTECHNIC INST TROY NY DEPT OF MATERIALS ENGINEERING J B HUDSON 29 FEB 86 NOB8e4-86-K-0259 UNCLASSIFIED F/G 7/4 NI @MonossonE ,
AD-A194 365 REACTION DYNAMICS ON SEMICONDUCTOR SURFACES(U) h RENSSELAER POLYTECHNIC INST TROY NY DEPT OF MATERIALS ENGINEERING J B HUDSON 29 FEB 86 NOB8e4-86-K-0259 UNCLASSIFIED F/G 7/4 NI @MonossonE ,
Supporting Information
 Supporting Information Molecular Weight Dependence of Zero-Shear Viscosity in Atactic Polypropylene Bottlebrush Polymers Samuel J. Dalsin, Marc A. Hillmyer,*, and Frank S. Bates*, Department of Chemical
Supporting Information Molecular Weight Dependence of Zero-Shear Viscosity in Atactic Polypropylene Bottlebrush Polymers Samuel J. Dalsin, Marc A. Hillmyer,*, and Frank S. Bates*, Department of Chemical
