CALCULATING REACTING QUANTITIES
|
|
|
- Theodora Austin
- 6 years ago
- Views:
Transcription
1 MODULE 2 14 WORKSHEET WORKSHEET For multiple-hoie questions 1 5 irle the letter orresponding to the most orret nswer. 1 The lned eqution for the urning of utnol (C 4 H 9 OH) is given elow: C 4 H 9 OH(l) 6O 2 (g) 4CO 2 (g) 5H 2 The mount of oxygen needed to urn 2 mole of utnol is: d 2 moles 4 moles 6 moles 12 moles 2 For the following retion: Whih of the following is NOT true? d PSO 4 (s) H P 2 HSO 4 One mole of led sulfte produes one mole of led ions. The retion tkes ple in wter. Both produts preipitte out of solution. The eqution is lned. 3 A student hs two identil flsks. One flsk is filled with 1 mole of ron dioxide (CO 2 ) t temperture of 25ºC nd the other flsk is filled with 2 moles of hydrogen (H 2 ) t temperture of 0ºC. The rtio of numer of moleules of CO 2 to the numer of moleules of H 2 is: 4:1 2:1 1:1 d 1:2 4 The lw of omining volumes ws proposed y: d Dlton Gy-Luss Avogrdo Newton CALCULATING REACTING QUANTITIES Syllus referene Copyright 2008 MGrw-Hill Austrli CONQUERINGCHEMISTRY PRELIM MODULE 2 WS 14
2 5 A ompound of mss 2.41 g is otined when 1.77 g olt is reted ompletely with oxygen. The empiril formul of the ompound is: CoO Co 2 O 3 Co 3 O 2 d Co 3 O 4 6 Lrge rystls of white nhydrous opper(ii) nitrte re heted strongly nd prtiulr type of retion ours. The eqution for the retion is: 2Cu(NO 3 (s) 2CuO(s) 4NO 2 (g) O 2 (g) 5.0 moles of opper(ii) nitrte re pled in ruile nd heted. The hnge in the mount of sustne remining s it is heted over period of time is shown elow How would the mss of opper(ii) oxide t the end of the retion ompre with the mss of opper(ii) nitrte t the eginning of the retion? Mss of opper(ii) oxide will e less thn the mss of opper(ii) nitrte. How mny moles of opper(ii) nitrte hve deomposed fter six minutes? Approximtely 4 moles. Clulte the totl numer of moles of oxygen gs formed y the time the retion is ompleted. From the lned eqution, 2 moles Cu(NO 3 produe 1 mole O 2, so 5 moles Cu(NO 3 will produe 2.5 moles O 2. Copyright 2008 MGrw-Hill Austrli CONQUERINGCHEMISTRY PRELIM MODULE 2 WS 14
3 7 The eqution for the omustion of utne is: 2C 4 (g) 13O 2 (g) 8CO 2 (g) 10H 2 Clulte the numer of moles of: CO 2 produed in the omustion of 1 mole of C 4 4 mol H 2 O produed in the omustion of 3 moles of C 4 15 mol O 2 onsumed in the omustion of 0.6 moles of C 4 8 How mny moles of oxygen gs would e otined if: 3.9 mol 0.80 moles of potssium hlorte (KClO 3 ) is deomposed y heting into potssium hloride nd oxygen? 2KClO 3 2KCl 3O mol 0.24 moles of merury(ii) oxide is deomposed y heting into merury nd oxygen gs? 2HgO 2Hg O mol moles of mgnesium oxide is ompletely dissolved in nitri id (HNO 3 ) to form solution of mgnesium nitrte. Clulte the numer of moles nd msses of: nitri id required mgnesium nitrte formed MgO 2HNO 3 Mg(NO 3 H 2 n(hno 3 ) 0.60 mol m (HNO 3 ) 0.60 ( ) 38 g n(mg(no 3 ) 0.30 mol m (Mg(NO 3 ) 0.30 ( ( )) 44 g Copyright 2008 MGrw-Hill Austrli CONQUERINGCHEMISTRY PRELIM MODULE 2 WS 14
4 10 Exess dilute sulfuri id ws dded to 3.00 g of lium ronte (CCO 3 ) to form lium sulfte, ron dioxide nd wter. Clulte the msses of: sulfuri id onsumed 2.94 g ron dioxide produed 1.32 g lium sulfte formed 4.08 g 11 Dilute hydrohlori id ws dded to zin until no further retion ourred. Write lned eqution for the retion. Zn(s) 2HCl(q) ZnCl 2 (q) H 2 (g) If mole of zin hloride ws formed wht mss of zin reted? n(zncl 2 ) mol n(zn) mol m(zn) g Wht mss of zin hloride would e produed if mole of hydrohlori id reted with exess zin? n(hcl) mol n(zncl 2 ) mol m(zncl 2 ) ( ) 2.7 g 12 Wht volume of oxygen is required for the omplete omustion of 125 ml of utene gs (C 4 H 8 ) nd wht volume of ron dioxide is formed? (All volumes re mesured t the sme temperture nd pressure.) C 4 H 8 (g) 6O 2 (g) 4CO 2 (g) 4H 2 V(O 2 ) ml V(CO 2 ) ml Copyright 2008 MGrw-Hill Austrli CONQUERINGCHEMISTRY PRELIM MODULE 2 WS 14
5 13 Nikel rets with ron monoxide to form gseous nikel ronyl. When exess nikel ws reted with 500 ml CO, 125 ml nikel ronyl ws formed t the sme temperture nd pressure. Assuming ll the CO reted, wht is the formul of nikel ronyl? Explin how you dedued this. Ni(CO) ml CO produed 125 ml nikel ronyl; this mens tht 4 mol CO produed 1 mol nikel ronyl so 1 mol nikel ronyl must ontin 4 mol CO hene Ni(CO) Propne is used s fuel in mping stoves. The eqution for the omplete omustion of propne is: C 3 H 8 (g) 50 2 (g) 3CO 2 (g) 4H ml of propne is urnt in n exess of oxygen. Clulte: the volume of oxygen onsumed V(O 2 ) ml the volume of ron dioxide produed V(CO 2 ) ml (All volumes mesured t the sme temperture nd pressure.) 15 A silver oin ws nlysed nd found to ontin only silver nd opper g of this oin ws dissolved in onentrted nitri id nd the resultnt solution diluted. Retion of the solution with exess hydrohlori id yielded g of silver hloride. Clulte the perentge of silver in the smple. m(agcl) g n(agcl) / ( ) mol n(ag) mol m(ag) g %Ag 0.79 / % Copyright 2008 MGrw-Hill Austrli CONQUERINGCHEMISTRY PRELIM MODULE 2 WS 14
6 16 A smple of iron wire rets with oxygen to form iron(iii) oxide. If 3.58 g of the wire yields 5.00 g of the iron(iii) oxide, lulte the perentge purity of the iron. 97.7% 17 The smelting proess of hlopyrite, CuFeS 2, is represented y the following eqution: 2CuFeS 2 (s) 5O 2 (g) 2Cu(s) 2FeO(s) 4SO 2 (g) If 22.0 kg of the hlopyrite rets nd the perentge yield for this proess is 95%, lulte the mss of opper formed. m(cufes 2 ) 22 kg g n(cufes 2 ) / ( ) mol n(cu) (95% yield) mol m(cu) g 7.2 kg Copyright 2008 MGrw-Hill Austrli CONQUERINGCHEMISTRY PRELIM MODULE 2 WS 14
1 This question is about mean bond enthalpies and their use in the calculation of enthalpy changes.
 1 This question is out men ond enthlpies nd their use in the lultion of enthlpy hnges. Define men ond enthlpy s pplied to hlorine. Explin why the enthlpy of tomistion of hlorine is extly hlf the men ond
1 This question is out men ond enthlpies nd their use in the lultion of enthlpy hnges. Define men ond enthlpy s pplied to hlorine. Explin why the enthlpy of tomistion of hlorine is extly hlf the men ond
1 This diagram represents the energy change that occurs when a d electron in a transition metal ion is excited by visible light.
 1 This igrm represents the energy hnge tht ours when eletron in trnsition metl ion is exite y visile light. Give the eqution tht reltes the energy hnge ΔE to the Plnk onstnt, h, n the frequeny, v, of the
1 This igrm represents the energy hnge tht ours when eletron in trnsition metl ion is exite y visile light. Give the eqution tht reltes the energy hnge ΔE to the Plnk onstnt, h, n the frequeny, v, of the
Chemistry Practice Exam
 Chemistry Prtie Exm 1 2 3 Whih of the following is n element? A rop of merury. A splinter of woo. A rystl of sugr. A rop of wter. Whih of the following nswers ontins only elements? Soium, soium hlorie,
Chemistry Prtie Exm 1 2 3 Whih of the following is n element? A rop of merury. A splinter of woo. A rystl of sugr. A rop of wter. Whih of the following nswers ontins only elements? Soium, soium hlorie,
3.15 NMR spectroscopy Different types of NMR There are two main types of NMR 1. C 13 NMR 2. H (proton) NMR
 .5 NMR spetrosopy Different types of NMR There re two min types of NMR. NMR. (proton) NMR There is only round % in orgni moleules ut modern NMR mhines re sensitive enough to give full spetr for The spetr
.5 NMR spetrosopy Different types of NMR There re two min types of NMR. NMR. (proton) NMR There is only round % in orgni moleules ut modern NMR mhines re sensitive enough to give full spetr for The spetr
6.3.2 Spectroscopy. N Goalby chemrevise.org 1 NO 2 CH 3. CH 3 C a. NMR spectroscopy. Different types of NMR
 6.. Spetrosopy NMR spetrosopy Different types of NMR NMR spetrosopy involves intertion of mterils with the lowenergy rdiowve region of the eletromgneti spetrum NMR spetrosopy is the sme tehnology s tht
6.. Spetrosopy NMR spetrosopy Different types of NMR NMR spetrosopy involves intertion of mterils with the lowenergy rdiowve region of the eletromgneti spetrum NMR spetrosopy is the sme tehnology s tht
6.3.2 Spectroscopy. N Goalby chemrevise.org 1 NO 2 H 3 CH3 C. NMR spectroscopy. Different types of NMR
 6.. Spetrosopy NMR spetrosopy Different types of NMR NMR spetrosopy involves intertion of mterils with the lowenergy rdiowve region of the eletromgneti spetrum NMR spetrosopy is the sme tehnology s tht
6.. Spetrosopy NMR spetrosopy Different types of NMR NMR spetrosopy involves intertion of mterils with the lowenergy rdiowve region of the eletromgneti spetrum NMR spetrosopy is the sme tehnology s tht
Thermodynamics. Question 1. Question 2. Question 3 3/10/2010. Practice Questions PV TR PV T R
 /10/010 Question 1 1 mole of idel gs is rought to finl stte F y one of three proesses tht hve different initil sttes s shown in the figure. Wht is true for the temperture hnge etween initil nd finl sttes?
/10/010 Question 1 1 mole of idel gs is rought to finl stte F y one of three proesses tht hve different initil sttes s shown in the figure. Wht is true for the temperture hnge etween initil nd finl sttes?
Review Topic 14: Relationships between two numerical variables
 Review Topi 14: Reltionships etween two numeril vriles Multiple hoie 1. Whih of the following stterplots est demonstrtes line of est fit? A B C D E 2. The regression line eqution for the following grph
Review Topi 14: Reltionships etween two numeril vriles Multiple hoie 1. Whih of the following stterplots est demonstrtes line of est fit? A B C D E 2. The regression line eqution for the following grph
Chemical Equilibrium
 Chpter 16 Questions 5, 7, 31, 33, 35, 43, 71 Chemil Equilibrium Exmples of Equilibrium Wter n exist simultneously in the gs nd liquid phse. The vpor pressure of H O t given temperture is property ssoited
Chpter 16 Questions 5, 7, 31, 33, 35, 43, 71 Chemil Equilibrium Exmples of Equilibrium Wter n exist simultneously in the gs nd liquid phse. The vpor pressure of H O t given temperture is property ssoited
H 4 H 8 N 2. Example 1 A compound is found to have an accurate relative formula mass of It is thought to be either CH 3.
 . Spetrosopy Mss spetrosopy igh resolution mss spetrometry n e used to determine the moleulr formul of ompound from the urte mss of the moleulr ion For exmple, the following moleulr formuls ll hve rough
. Spetrosopy Mss spetrosopy igh resolution mss spetrometry n e used to determine the moleulr formul of ompound from the urte mss of the moleulr ion For exmple, the following moleulr formuls ll hve rough
An excess of concentrated hydrochloric acid is added to separate aqueous solutions containing [Cu(H 2 O) 6 ] 2 and [Co(H 2 O) 6 ] 2.
![An excess of concentrated hydrochloric acid is added to separate aqueous solutions containing [Cu(H 2 O) 6 ] 2 and [Co(H 2 O) 6 ] 2. An excess of concentrated hydrochloric acid is added to separate aqueous solutions containing [Cu(H 2 O) 6 ] 2 and [Co(H 2 O) 6 ] 2.](/thumbs/89/98214175.jpg) 1 An exess of a given reagent is added to eah of the following pairs of aqueous metal ions. For eah metal ion, state the initial olour of the solution and the final oservation that you would make. In eah
1 An exess of a given reagent is added to eah of the following pairs of aqueous metal ions. For eah metal ion, state the initial olour of the solution and the final oservation that you would make. In eah
= mol H O mol H O. 10 mol H O x g C H. 10 mol H O x g O. = 75 mol O. = 34.5 mol O
 1. C 4 H 10 + 13O þ 8CO + 10H O.46 g a).46 g H O x 1 mol H O 18.0 g H O = 0.137 mol H O b).46 g H O x 1 mol H O 18.0 g H O x mol C H 4 10 10 mol H O = 0.073 mol C H 4 10 c).46 g H O x 1 mol H O 18.0 g
1. C 4 H 10 + 13O þ 8CO + 10H O.46 g a).46 g H O x 1 mol H O 18.0 g H O = 0.137 mol H O b).46 g H O x 1 mol H O 18.0 g H O x mol C H 4 10 10 mol H O = 0.073 mol C H 4 10 c).46 g H O x 1 mol H O 18.0 g
Instructions to students: Use your Text Book and attempt these questions.
 Instrutions to students: Use your Text Book nd ttempt these questions. Due Dte: 16-09-2018 Unit 2 Chpter 8 Test Slrs nd vetors Totl mrks 50 Nme: Clss: Dte: Setion A Selet the est nswer for eh question.
Instrutions to students: Use your Text Book nd ttempt these questions. Due Dte: 16-09-2018 Unit 2 Chpter 8 Test Slrs nd vetors Totl mrks 50 Nme: Clss: Dte: Setion A Selet the est nswer for eh question.
Logarithms LOGARITHMS.
 Logrithms LOGARITHMS www.mthletis.om.u Logrithms LOGARITHMS Logrithms re nother method to lulte nd work with eponents. Answer these questions, efore working through this unit. I used to think: In the
Logrithms LOGARITHMS www.mthletis.om.u Logrithms LOGARITHMS Logrithms re nother method to lulte nd work with eponents. Answer these questions, efore working through this unit. I used to think: In the
Name Date Class THE ARITHMETIC OF EQUATIONS
 12.1 THE ARITHMETIC OF EQUATIONS Section Review Objectives Calculate the amount of reactants required or product formed in a nonchemical process Interpret balanced chemical equations in terms of interacting
12.1 THE ARITHMETIC OF EQUATIONS Section Review Objectives Calculate the amount of reactants required or product formed in a nonchemical process Interpret balanced chemical equations in terms of interacting
Chem Homework 11 due Monday, Apr. 28, 2014, 2 PM
 Chem 44 - Homework due ondy, pr. 8, 4, P.. . Put this in eq 8.4 terms: E m = m h /m e L for L=d The degenery in the ring system nd the inresed sping per level (4x bigger) mkes the sping between the HOO
Chem 44 - Homework due ondy, pr. 8, 4, P.. . Put this in eq 8.4 terms: E m = m h /m e L for L=d The degenery in the ring system nd the inresed sping per level (4x bigger) mkes the sping between the HOO
Chemical Equilibrium. Problem Set: Chapter 16 questions 25, 27, 33, 35, 43, 71
 Chemil Equilibrium roblem Set: Chpter 16 questions 5, 7, 33, 35, 43, 71 Exmples of Equilibrium Wter n exists simultneously in the gs nd liquid phse. The vpor pressure of H O t given temperture is property
Chemil Equilibrium roblem Set: Chpter 16 questions 5, 7, 33, 35, 43, 71 Exmples of Equilibrium Wter n exists simultneously in the gs nd liquid phse. The vpor pressure of H O t given temperture is property
ERT 316: REACTION ENGINEERING CHAPTER 3 RATE LAWS & STOICHIOMETRY
 ER 316: REIO EGIEERIG HER 3 RE LWS & SOIHIOMERY 1 OULIE R 1: Rte Lws Reltive Rtes of Retion Retion Orer & Rte Lw Retion Rte onstnt, k R 2: Stoihiometry th System Stoihiometri le low System Stoihiometri
ER 316: REIO EGIEERIG HER 3 RE LWS & SOIHIOMERY 1 OULIE R 1: Rte Lws Reltive Rtes of Retion Retion Orer & Rte Lw Retion Rte onstnt, k R 2: Stoihiometry th System Stoihiometri le low System Stoihiometri
Activities. 4.1 Pythagoras' Theorem 4.2 Spirals 4.3 Clinometers 4.4 Radar 4.5 Posting Parcels 4.6 Interlocking Pipes 4.7 Sine Rule Notes and Solutions
 MEP: Demonstrtion Projet UNIT 4: Trigonometry UNIT 4 Trigonometry tivities tivities 4. Pythgors' Theorem 4.2 Spirls 4.3 linometers 4.4 Rdr 4.5 Posting Prels 4.6 Interloking Pipes 4.7 Sine Rule Notes nd
MEP: Demonstrtion Projet UNIT 4: Trigonometry UNIT 4 Trigonometry tivities tivities 4. Pythgors' Theorem 4.2 Spirls 4.3 linometers 4.4 Rdr 4.5 Posting Prels 4.6 Interloking Pipes 4.7 Sine Rule Notes nd
a) Read over steps (1)- (4) below and sketch the path of the cycle on a P V plot on the graph below. Label all appropriate points.
 Prole 3: Crnot Cyle of n Idel Gs In this prole, the strting pressure P nd volue of n idel gs in stte, re given he rtio R = / > of the volues of the sttes nd is given Finlly onstnt γ = 5/3 is given You
Prole 3: Crnot Cyle of n Idel Gs In this prole, the strting pressure P nd volue of n idel gs in stte, re given he rtio R = / > of the volues of the sttes nd is given Finlly onstnt γ = 5/3 is given You
Perimeter and Area. Mathletics Instant Workbooks. Copyright
 Perimeter nd Are Student Book - Series J- L B Mthletis Instnt Workooks Copyright Student Book - Series J Contents Topis Topi - Plne shpes Topi 2 - Perimeter of regulr shpes Topi 3 - Perimeter of irregulr
Perimeter nd Are Student Book - Series J- L B Mthletis Instnt Workooks Copyright Student Book - Series J Contents Topis Topi - Plne shpes Topi 2 - Perimeter of regulr shpes Topi 3 - Perimeter of irregulr
1 PYTHAGORAS THEOREM 1. Given a right angled triangle, the square of the hypotenuse is equal to the sum of the squares of the other two sides.
 1 PYTHAGORAS THEOREM 1 1 Pythgors Theorem In this setion we will present geometri proof of the fmous theorem of Pythgors. Given right ngled tringle, the squre of the hypotenuse is equl to the sum of the
1 PYTHAGORAS THEOREM 1 1 Pythgors Theorem In this setion we will present geometri proof of the fmous theorem of Pythgors. Given right ngled tringle, the squre of the hypotenuse is equl to the sum of the
AP CALCULUS Test #6: Unit #6 Basic Integration and Applications
 AP CALCULUS Test #6: Unit #6 Bsi Integrtion nd Applitions A GRAPHING CALCULATOR IS REQUIRED FOR SOME PROBLEMS OR PARTS OF PROBLEMS IN THIS PART OF THE EXAMINATION. () The ext numeril vlue of the orret
AP CALCULUS Test #6: Unit #6 Bsi Integrtion nd Applitions A GRAPHING CALCULATOR IS REQUIRED FOR SOME PROBLEMS OR PARTS OF PROBLEMS IN THIS PART OF THE EXAMINATION. () The ext numeril vlue of the orret
AQA Chemistry Paper 2
 AQA hemistry Pper 2 1.1 A student is plnning n investigtion into how the concentrtion of hydrochloric cid ffects the rte of the rection with mrle chips. Wht is the independent vrile? Tick one ox. (1 mrk)
AQA hemistry Pper 2 1.1 A student is plnning n investigtion into how the concentrtion of hydrochloric cid ffects the rte of the rection with mrle chips. Wht is the independent vrile? Tick one ox. (1 mrk)
8 Chemical Equations. Flames and sparks result when aluminum foil is dropped into liquid bromine.
 8 Chemical Equations Flames and sparks result when aluminum foil is dropped into liquid bromine. Chapter Outline 8.1 The Chemical Equation 8.2 Writing and Balancing Chemical Equations 8.3 Types of Chemical
8 Chemical Equations Flames and sparks result when aluminum foil is dropped into liquid bromine. Chapter Outline 8.1 The Chemical Equation 8.2 Writing and Balancing Chemical Equations 8.3 Types of Chemical
PYTHAGORAS THEOREM WHAT S IN CHAPTER 1? IN THIS CHAPTER YOU WILL:
 PYTHAGORAS THEOREM 1 WHAT S IN CHAPTER 1? 1 01 Squres, squre roots nd surds 1 02 Pythgors theorem 1 03 Finding the hypotenuse 1 04 Finding shorter side 1 05 Mixed prolems 1 06 Testing for right-ngled tringles
PYTHAGORAS THEOREM 1 WHAT S IN CHAPTER 1? 1 01 Squres, squre roots nd surds 1 02 Pythgors theorem 1 03 Finding the hypotenuse 1 04 Finding shorter side 1 05 Mixed prolems 1 06 Testing for right-ngled tringles
Which of the following describes the net ionic reaction for the hydrolysis. Which of the following salts will produce a solution with the highest ph?
 95. Which of the following descries the net ionic rection for the hydrolysis of NH4Cl( s)? A. NH4 ( q) Cl & ( q) NH4Cl( s) B. NH Cl & 4 ( s) NH4 ( q) Cl ( q) C. Cl ( q) H O & 2 ( l) HCl( q) OH ( q) D.
95. Which of the following descries the net ionic rection for the hydrolysis of NH4Cl( s)? A. NH4 ( q) Cl & ( q) NH4Cl( s) B. NH Cl & 4 ( s) NH4 ( q) Cl ( q) C. Cl ( q) H O & 2 ( l) HCl( q) OH ( q) D.
Dynamic equilibrium occurs when the forward and reverse reactions occur at the same rate.
 MODULE 2 WORKSHEET8 EQUILIBRIUM Syllus reference 9.3.2 1 Clssify ech of the following sttements s true or flse. For the flse sttements rewrite them so they re true. For chemicl equilirium to e estlished
MODULE 2 WORKSHEET8 EQUILIBRIUM Syllus reference 9.3.2 1 Clssify ech of the following sttements s true or flse. For the flse sttements rewrite them so they re true. For chemicl equilirium to e estlished
Study Guide: Stoichiometry
 Name: Study Guide: Stoichiometry Period: **YOUR ANSWERS MUST INCLUDE THE PROPER NUMBER OF SIG FIGS AND COMPLETE UNITS IN ORDER TO RECEIVE CREDIT FOR THE PROBLEM.** BALANCE THE FOLLOWING EQUATIONS TO USE
Name: Study Guide: Stoichiometry Period: **YOUR ANSWERS MUST INCLUDE THE PROPER NUMBER OF SIG FIGS AND COMPLETE UNITS IN ORDER TO RECEIVE CREDIT FOR THE PROBLEM.** BALANCE THE FOLLOWING EQUATIONS TO USE
CHAPTER 08: MONOPROTIC ACID-BASE EQUILIBRIA
 Hrris: Quntittive Chemicl Anlysis, Eight Edition CHAPTER 08: MONOPROTIC ACIDBASE EQUILIBRIA CHAPTER 08: Opener A CHAPTER 08: Opener B CHAPTER 08: Opener C CHAPTER 08: Opener D CHAPTER 08: Opener E Chpter
Hrris: Quntittive Chemicl Anlysis, Eight Edition CHAPTER 08: MONOPROTIC ACIDBASE EQUILIBRIA CHAPTER 08: Opener A CHAPTER 08: Opener B CHAPTER 08: Opener C CHAPTER 08: Opener D CHAPTER 08: Opener E Chpter
4-cyanopentanoic acid dithiobenzoate (CPADB) was synthesized as reported by Y.
 Eletroni upplementry Mteril (EI) for Journl of Mterils Chemistry B This journl is The Royl oiety of Chemistry 2012 ynthesis of 4-ynopentnoi id dithioenzote (CPADB). 4-ynopentnoi id dithioenzote (CPADB)
Eletroni upplementry Mteril (EI) for Journl of Mterils Chemistry B This journl is The Royl oiety of Chemistry 2012 ynthesis of 4-ynopentnoi id dithioenzote (CPADB). 4-ynopentnoi id dithioenzote (CPADB)
Example Exercise 10.1 Interpreting Chemical Equation Calculations
 Example Exercise 10.1 Interpreting Chemical Equation Calculations Given the chemical equation for the combustion of methane, CH 4, balance the equation and interpret the coefficients in terms of (a) moles
Example Exercise 10.1 Interpreting Chemical Equation Calculations Given the chemical equation for the combustion of methane, CH 4, balance the equation and interpret the coefficients in terms of (a) moles
Compounds and equations SAMPLE
 USING CHEMISTRY 105 ACTIVITY 7.1 Litery Proessing n nlysing t n informtion Compouns n equtions Nming rules for ompouns When two or more elements hemilly join together, ompoun is forme. The nme of the ompoun
USING CHEMISTRY 105 ACTIVITY 7.1 Litery Proessing n nlysing t n informtion Compouns n equtions Nming rules for ompouns When two or more elements hemilly join together, ompoun is forme. The nme of the ompoun
21.1 Using Formulae Construct and Use Simple Formulae Revision of Negative Numbers Substitution into Formulae
 MEP Jmi: STRAND G UNIT 1 Formule: Student Tet Contents STRAND G: Alger Unit 1 Formule Student Tet Contents Setion 1.1 Using Formule 1. Construt nd Use Simple Formule 1.3 Revision of Negtive Numers 1.4
MEP Jmi: STRAND G UNIT 1 Formule: Student Tet Contents STRAND G: Alger Unit 1 Formule Student Tet Contents Setion 1.1 Using Formule 1. Construt nd Use Simple Formule 1.3 Revision of Negtive Numers 1.4
Chemical Reactions Unit
 Name: Hour: Teacher: ROZEMA / Chemistry Chemical Reactions Unit 1 P a g e 2 P a g e 3 P a g e 4 P a g e 5 P a g e 6 P a g e Chemistry Balancing Equations Balance the following equations by inserting the
Name: Hour: Teacher: ROZEMA / Chemistry Chemical Reactions Unit 1 P a g e 2 P a g e 3 P a g e 4 P a g e 5 P a g e 6 P a g e Chemistry Balancing Equations Balance the following equations by inserting the
Homework 04. Acids, Bases, and Salts
 HW04 - Acids, Bses, nd Slts! This is preview of the published version of the quiz Strted: Feb 21 t 8:59m Quiz Instruc!ons Homework 04 Acids, Bses, nd Slts Question 1 In the reversible rection HCN + H O
HW04 - Acids, Bses, nd Slts! This is preview of the published version of the quiz Strted: Feb 21 t 8:59m Quiz Instruc!ons Homework 04 Acids, Bses, nd Slts Question 1 In the reversible rection HCN + H O
Mathematics SKE: STRAND F. F1.1 Using Formulae. F1.2 Construct and Use Simple Formulae. F1.3 Revision of Negative Numbers
 Mthemtis SKE: STRAND F UNIT F1 Formule: Tet STRAND F: Alger F1 Formule Tet Contents Setion F1.1 Using Formule F1. Construt nd Use Simple Formule F1.3 Revision of Negtive Numers F1.4 Sustitution into Formule
Mthemtis SKE: STRAND F UNIT F1 Formule: Tet STRAND F: Alger F1 Formule Tet Contents Setion F1.1 Using Formule F1. Construt nd Use Simple Formule F1.3 Revision of Negtive Numers F1.4 Sustitution into Formule
Funsheet 3.0 [WRITING & BALANCING EQUATIONS] Gu/R. 2017
![Funsheet 3.0 [WRITING & BALANCING EQUATIONS] Gu/R. 2017 Funsheet 3.0 [WRITING & BALANCING EQUATIONS] Gu/R. 2017](/thumbs/80/82478584.jpg) Funsheet 3.0 [WRITING & BALANCING EQUATIONS] Gu/R. 2017 Balance the following chemical equations. Remember, it is not necessary to write "1" if the coefficient is one. 1. N 2 + H 2 NH 3 2. KClO 3 KCl +
Funsheet 3.0 [WRITING & BALANCING EQUATIONS] Gu/R. 2017 Balance the following chemical equations. Remember, it is not necessary to write "1" if the coefficient is one. 1. N 2 + H 2 NH 3 2. KClO 3 KCl +
Outcomes: Interpret a balanced chemical equation in terms of moles, mass and volume of gases. Solve stoichiometric problems involving: moles, mass,
 Stoichiometry Outcomes: Interpret a balanced chemical equation in terms of moles, mass and volume of gases. Solve stoichiometric problems involving: moles, mass, volume, and heat of reaction. Stoichiometry
Stoichiometry Outcomes: Interpret a balanced chemical equation in terms of moles, mass and volume of gases. Solve stoichiometric problems involving: moles, mass, volume, and heat of reaction. Stoichiometry
Reactions in Aqueous Solution
 Reading Assignments: Reactions in Aqueous Solution Chapter 4 Chapter 4 in R. Chang, Chemistry, 9 th Ed., McGraw-Hill, 2006. or previous editions. Or related topics in other textbooks. Consultation outside
Reading Assignments: Reactions in Aqueous Solution Chapter 4 Chapter 4 in R. Chang, Chemistry, 9 th Ed., McGraw-Hill, 2006. or previous editions. Or related topics in other textbooks. Consultation outside
Chemistry Department. The Islamic University of Gaza. General Chemistry B.(CHEMB 1301) Time:2 hours الرقم الجامعي... اسم المدرس...
 The Islmic University of Gz Chemistry Deprtment Generl Chemistry B.(CHEMB 1301) Time:2 hours 60 اسم الطالب... الرقم الجامعي... اسم المدرس... R = 8.314 J/mol.K, or = 0.0821 L.tm/mol.K Q1- True ( ) or flse(
The Islmic University of Gz Chemistry Deprtment Generl Chemistry B.(CHEMB 1301) Time:2 hours 60 اسم الطالب... الرقم الجامعي... اسم المدرس... R = 8.314 J/mol.K, or = 0.0821 L.tm/mol.K Q1- True ( ) or flse(
Stoichiometry is the relationship between the amount of reactants used and/or the amount of products produced in a chemical reaction.
 Unit 7 STOICHIOMETRY 1. Introduction to Stoichiometry 2. Mole Mole Stoichiometry 3. Mass Mole Stoichiometry 4. Mass Mass Stoichiometry 5. Mass Volume & Volume Volume Stoichiometry 6. Excess & Limiting
Unit 7 STOICHIOMETRY 1. Introduction to Stoichiometry 2. Mole Mole Stoichiometry 3. Mass Mole Stoichiometry 4. Mass Mass Stoichiometry 5. Mass Volume & Volume Volume Stoichiometry 6. Excess & Limiting
CHAPTER 8 SALTS. NaCl. A salt is an ionic substance produced when the hydrogen ion of the acid is replaced by metal ion or an ammonium ion.
 CHAPTER 8 SALTS A salt is an ionic substance produced when the hydrogen ion of the acid is replaced by metal ion or an ammonium ion. The salt consists of two parts, cation from base and anion from acid.
CHAPTER 8 SALTS A salt is an ionic substance produced when the hydrogen ion of the acid is replaced by metal ion or an ammonium ion. The salt consists of two parts, cation from base and anion from acid.
Unit 8 Redox 8-1. At the end of this unit, you ll be able to
 8-1 Unit 8 Redox At the end of this unit, you ll be able to Define and identify oxidation reactions Define and identify reduction reactions Assign oxidation numbers to elements in a compound Write and
8-1 Unit 8 Redox At the end of this unit, you ll be able to Define and identify oxidation reactions Define and identify reduction reactions Assign oxidation numbers to elements in a compound Write and
Lecture 27: Diffusion of Ions: Part 2: coupled diffusion of cations and
 Leture 7: iffusion of Ions: Prt : oupled diffusion of tions nd nions s desried y Nernst-Plnk Eqution Tody s topis Continue to understnd the fundmentl kinetis prmeters of diffusion of ions within n eletrilly
Leture 7: iffusion of Ions: Prt : oupled diffusion of tions nd nions s desried y Nernst-Plnk Eqution Tody s topis Continue to understnd the fundmentl kinetis prmeters of diffusion of ions within n eletrilly
GM1 Consolidation Worksheet
 Cmridge Essentils Mthemtis Core 8 GM1 Consolidtion Worksheet GM1 Consolidtion Worksheet 1 Clulte the size of eh ngle mrked y letter. Give resons for your nswers. or exmple, ngles on stright line dd up
Cmridge Essentils Mthemtis Core 8 GM1 Consolidtion Worksheet GM1 Consolidtion Worksheet 1 Clulte the size of eh ngle mrked y letter. Give resons for your nswers. or exmple, ngles on stright line dd up
Stoichiometry is the relationship between the amount of reactants used and the amount of products produced in a chemical reaction.
 Unit 7 STOICHIOMETRY 1. Introduction to Stoichiometry 2. Mole Mole Stoichiometry 3. Mass Mole Stoichiometry 4. Mass Mass Stoichiometry 5. Mass Volume & Volume Volume Stoichiometry 6. Excess & Limiting
Unit 7 STOICHIOMETRY 1. Introduction to Stoichiometry 2. Mole Mole Stoichiometry 3. Mass Mole Stoichiometry 4. Mass Mass Stoichiometry 5. Mass Volume & Volume Volume Stoichiometry 6. Excess & Limiting
ed. Brad Collins Aqueous Chemistry Chapter 5 Some images copyright The McGraw-Hill Companies, Inc. Sunday, August 18, 13
 ed. Brad Collins Aqueous Chemistry Chapter 5 Some images copyright The McGraw-Hill Companies, Inc. A solution is a homogenous mixture of 2 or more substances at the molecular level The solute(s) is(are)
ed. Brad Collins Aqueous Chemistry Chapter 5 Some images copyright The McGraw-Hill Companies, Inc. A solution is a homogenous mixture of 2 or more substances at the molecular level The solute(s) is(are)
Trigonometry Revision Sheet Q5 of Paper 2
 Trigonometry Revision Sheet Q of Pper The Bsis - The Trigonometry setion is ll out tringles. We will normlly e given some of the sides or ngles of tringle nd we use formule nd rules to find the others.
Trigonometry Revision Sheet Q of Pper The Bsis - The Trigonometry setion is ll out tringles. We will normlly e given some of the sides or ngles of tringle nd we use formule nd rules to find the others.
Reaction Writing Sheet #1 Key
 Reaction Writing Sheet #1 Key Write and balance each of the following reactions and indicate the reaction type(s) present: 1. zinc + sulfur zinc sulfide 8 Zn (s) + S 8 (s) 8 ZnS (s) synthesis 2. potassium
Reaction Writing Sheet #1 Key Write and balance each of the following reactions and indicate the reaction type(s) present: 1. zinc + sulfur zinc sulfide 8 Zn (s) + S 8 (s) 8 ZnS (s) synthesis 2. potassium
Chem 1412 SU06 Exam 4
 Chem 1412 SU06 Exam 4 Student: 1. Which of the following is necessary for a process to be spontaneous? A. H sys < 0 B. S sys > 0 C. S surr < 0 D. S univ > 0 E. G sys = 0 2. Which of the following is always
Chem 1412 SU06 Exam 4 Student: 1. Which of the following is necessary for a process to be spontaneous? A. H sys < 0 B. S sys > 0 C. S surr < 0 D. S univ > 0 E. G sys = 0 2. Which of the following is always
Find this material useful? You can help our team to keep this site up and bring you even more content consider donating via the link on our site.
 Find this mteril useful? You cn help our tem to keep this site up nd bring you even more content consider donting vi the link on our site. Still hving trouble understnding the mteril? Check out our Tutoring
Find this mteril useful? You cn help our tem to keep this site up nd bring you even more content consider donting vi the link on our site. Still hving trouble understnding the mteril? Check out our Tutoring
Designing Information Devices and Systems I Discussion 8B
 Lst Updted: 2018-10-17 19:40 1 EECS 16A Fll 2018 Designing Informtion Devices nd Systems I Discussion 8B 1. Why Bother With Thévenin Anywy? () Find Thévenin eqiuvlent for the circuit shown elow. 2kΩ 5V
Lst Updted: 2018-10-17 19:40 1 EECS 16A Fll 2018 Designing Informtion Devices nd Systems I Discussion 8B 1. Why Bother With Thévenin Anywy? () Find Thévenin eqiuvlent for the circuit shown elow. 2kΩ 5V
CHEMISTRY 16 HOUR EXAM III March 26, 1998 Dr. Finklea - Answer key
 CHEMISTRY 16 HOUR EXAM III Mrch 26, 1998 Dr. Finkle - Answer key 1. Phenol, lso known s crbolic cid, is wek monoprotic cid (HA). A 1.0 M solution of phenol hs ph of 4.95. Wht is the K of phenol? 1. 2.0
CHEMISTRY 16 HOUR EXAM III Mrch 26, 1998 Dr. Finkle - Answer key 1. Phenol, lso known s crbolic cid, is wek monoprotic cid (HA). A 1.0 M solution of phenol hs ph of 4.95. Wht is the K of phenol? 1. 2.0
Stoichiometry is the relationship between the amount of reactants used and/or the amount of products produced in a chemical reaction.
 Unit 7 STOICHIOMETRY 1. Introduction to Stoichiometry 2. Mole Mole Stoichiometry 3. Mass Mole Stoichiometry 4. Mass Mass Stoichiometry 5. Mass Volume & Volume Volume Stoichiometry 6. Excess & Limiting
Unit 7 STOICHIOMETRY 1. Introduction to Stoichiometry 2. Mole Mole Stoichiometry 3. Mass Mole Stoichiometry 4. Mass Mass Stoichiometry 5. Mass Volume & Volume Volume Stoichiometry 6. Excess & Limiting
CHAPTER 1 QUANTITATIVE CHEMISTRY
 Page 4 Ex 4 (a) element; (b) mixture; (c) compound; (d) element; (e) compound 5. (a) mixture; (b) compound; (c) mixture; (d) element; (e) compound. Page 5 Ex 1.1 3. C 4. D 5. C 6. D 7. a) 0.20 b) 1.2 10
Page 4 Ex 4 (a) element; (b) mixture; (c) compound; (d) element; (e) compound 5. (a) mixture; (b) compound; (c) mixture; (d) element; (e) compound. Page 5 Ex 1.1 3. C 4. D 5. C 6. D 7. a) 0.20 b) 1.2 10
2 nd Semester Study Guide 2017
 Chemistry 2 nd Semester Study Guide 2017 Name: KEY Unit 6: Chemical Reactions and Balancing 1. Draw the remaining product 2. Write a balanced equation for the following reaction: The reaction between sodium
Chemistry 2 nd Semester Study Guide 2017 Name: KEY Unit 6: Chemical Reactions and Balancing 1. Draw the remaining product 2. Write a balanced equation for the following reaction: The reaction between sodium
1) What is the volume of a tank that can hold Kg of methanol whose density is 0.788g/cm 3?
 1) Convert the following 1) 125 g to Kg 6) 26.9 dm 3 to cm 3 11) 1.8µL to cm 3 16) 4.8 lb to Kg 21) 23 F to K 2) 21.3 Km to cm 7) 18.2 ml to cm 3 12) 2.45 L to µm 3 17) 1.2 m to inches 22) 180 ºC to K
1) Convert the following 1) 125 g to Kg 6) 26.9 dm 3 to cm 3 11) 1.8µL to cm 3 16) 4.8 lb to Kg 21) 23 F to K 2) 21.3 Km to cm 7) 18.2 ml to cm 3 12) 2.45 L to µm 3 17) 1.2 m to inches 22) 180 ºC to K
Unit 5: Chemical Equations and Reactions & Stoichiometry
 pg. 10 Unit 5: Chemical Equations and Reactions & Stoichiometry Chapter 8: Chemical Equations and Reactions 8.1: Describing Chemical Reactions Selected Chemistry Assignment Answers (Section Review on pg.
pg. 10 Unit 5: Chemical Equations and Reactions & Stoichiometry Chapter 8: Chemical Equations and Reactions 8.1: Describing Chemical Reactions Selected Chemistry Assignment Answers (Section Review on pg.
Cu 3 (PO 4 ) 2 (s) 3 Cu 2+ (aq) + 2 PO 4 3- (aq) circle answer: pure water or Na 3 PO 4 solution This is the common-ion effect.
 CHEM 1122011 NAME: ANSWER KEY Vining, Exm # SHORT ANSWER QUESTIONS ============= 4 points ech ============ All work must be shown. 1. Wht re [H O ] nd [OH ] for solution tht hs ph of 9.0? Choose one. ()
CHEM 1122011 NAME: ANSWER KEY Vining, Exm # SHORT ANSWER QUESTIONS ============= 4 points ech ============ All work must be shown. 1. Wht re [H O ] nd [OH ] for solution tht hs ph of 9.0? Choose one. ()
UNIT 9 - STOICHIOMETRY
 General Stoichiometry Notes STOICHIOMETRY: tells relative amts of reactants & products in a chemical reaction Given an amount of a substance involved in a chemical reaction, we can figure out the amount
General Stoichiometry Notes STOICHIOMETRY: tells relative amts of reactants & products in a chemical reaction Given an amount of a substance involved in a chemical reaction, we can figure out the amount
Chemistry 12. Resource Exam B. Exam Booklet
 Chemistry 12 Resource Exam B Exam Booklet Contents: 21 pages Examination: 2 hours 50 multiple-choice questions in the Exam Booklet Additional Time Permitted: 60 minutes Province of British Columbia PART
Chemistry 12 Resource Exam B Exam Booklet Contents: 21 pages Examination: 2 hours 50 multiple-choice questions in the Exam Booklet Additional Time Permitted: 60 minutes Province of British Columbia PART
Intermediate Math Circles Wednesday 17 October 2012 Geometry II: Side Lengths
 Intermedite Mth Cirles Wednesdy 17 Otoer 01 Geometry II: Side Lengths Lst week we disussed vrious ngle properties. As we progressed through the evening, we proved mny results. This week, we will look t
Intermedite Mth Cirles Wednesdy 17 Otoer 01 Geometry II: Side Lengths Lst week we disussed vrious ngle properties. As we progressed through the evening, we proved mny results. This week, we will look t
Electrical Circuits II (ECE233b)
 Eletril Ciruits (ECE2) Polyhse Ciruits Anestis Dounis The Uniersity of Western Ontrio Fulty of Engineering Siene ThreePhse Ciruits Blned three hse iruit: ontins three oltge soures tht re equl in mgnitude
Eletril Ciruits (ECE2) Polyhse Ciruits Anestis Dounis The Uniersity of Western Ontrio Fulty of Engineering Siene ThreePhse Ciruits Blned three hse iruit: ontins three oltge soures tht re equl in mgnitude
CHEMISTRY YEAR 3 REACTIONS SKILL (CHEMIS)TREE
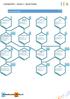 HEMISTRY YER 3 RETIONS SKILL (HEMIS)TREE HEMISTRY YER 3 RETIONS MOLEULR FORMULS E molecular formula tells you which types of atoms are present in a molecule and how many of each are present. For the latter
HEMISTRY YER 3 RETIONS SKILL (HEMIS)TREE HEMISTRY YER 3 RETIONS MOLEULR FORMULS E molecular formula tells you which types of atoms are present in a molecule and how many of each are present. For the latter
SECTION A STUDENT MATERIAL. Part 1. What and Why.?
 SECTION A STUDENT MATERIAL Prt Wht nd Wh.? Student Mteril Prt Prolem n > 0 n > 0 Is the onverse true? Prolem If n is even then n is even. If n is even then n is even. Wht nd Wh? Eploring Pure Mths Are
SECTION A STUDENT MATERIAL Prt Wht nd Wh.? Student Mteril Prt Prolem n > 0 n > 0 Is the onverse true? Prolem If n is even then n is even. If n is even then n is even. Wht nd Wh? Eploring Pure Mths Are
20 b The prime numbers are 2,3,5,7,11,13,17,19.
 Topi : Probbility Short nswer tehnology- free The following my be of use in this test:! 0 0 Two rows of Psl s tringle re: ontiner holds irulr piees eh of the sme size. Written on eh is different number,
Topi : Probbility Short nswer tehnology- free The following my be of use in this test:! 0 0 Two rows of Psl s tringle re: ontiner holds irulr piees eh of the sme size. Written on eh is different number,
2 nd Semester Study Guide 2016
 Chemistry 2 nd Semester Study Guide 2016 Name: Unit 6: Chemical Reactions and Balancing 1. Draw the remaining product 2. Write a balanced equation for the following reaction: The reaction between sodium
Chemistry 2 nd Semester Study Guide 2016 Name: Unit 6: Chemical Reactions and Balancing 1. Draw the remaining product 2. Write a balanced equation for the following reaction: The reaction between sodium
Unit 7: Stoichiometry Homework Packet (85 points)
 Name: Period: By the end of the Unit 7, you should be able to: Chapter 12 1. Use stoichiometry to determine the amount of substance in a reaction 2. Determine the limiting reactant of a reaction 3. Determine
Name: Period: By the end of the Unit 7, you should be able to: Chapter 12 1. Use stoichiometry to determine the amount of substance in a reaction 2. Determine the limiting reactant of a reaction 3. Determine
Formula for Trapezoid estimate using Left and Right estimates: Trap( n) If the graph of f is decreasing on [a, b], then f ( x ) dx
![Formula for Trapezoid estimate using Left and Right estimates: Trap( n) If the graph of f is decreasing on [a, b], then f ( x ) dx Formula for Trapezoid estimate using Left and Right estimates: Trap( n) If the graph of f is decreasing on [a, b], then f ( x ) dx](/thumbs/79/79316452.jpg) Fill in the Blnks for the Big Topis in Chpter 5: The Definite Integrl Estimting n integrl using Riemnn sum:. The Left rule uses the left endpoint of eh suintervl.. The Right rule uses the right endpoint
Fill in the Blnks for the Big Topis in Chpter 5: The Definite Integrl Estimting n integrl using Riemnn sum:. The Left rule uses the left endpoint of eh suintervl.. The Right rule uses the right endpoint
Project 6: Minigoals Towards Simplifying and Rewriting Expressions
 MAT 51 Wldis Projet 6: Minigols Towrds Simplifying nd Rewriting Expressions The distriutive property nd like terms You hve proly lerned in previous lsses out dding like terms ut one prolem with the wy
MAT 51 Wldis Projet 6: Minigols Towrds Simplifying nd Rewriting Expressions The distriutive property nd like terms You hve proly lerned in previous lsses out dding like terms ut one prolem with the wy
Chemical Reactions. Chemical Reactions 5 signs/evidence of chemical reactions:
 Chemical Reactions Chemical Reactions 5 signs/evidence of chemical reactions: Chemical Reaction: a process in which one or more substances are converted into new substances with different chemical and
Chemical Reactions Chemical Reactions 5 signs/evidence of chemical reactions: Chemical Reaction: a process in which one or more substances are converted into new substances with different chemical and
Practice Packet Unit 7: Moles & Stoichiometry
 PRACTICE PACKET: Unit 7 Moles & Stoichiometry Regents Chemistry: Practice Packet Unit 7: Moles & Stoichiometry Vocabulary: Lesson 1: Lesson 6: Lesson 2: Lesson 4A: Lesson 4B: Lesson 3: Lesson 5: www.chempride.weebly.com
PRACTICE PACKET: Unit 7 Moles & Stoichiometry Regents Chemistry: Practice Packet Unit 7: Moles & Stoichiometry Vocabulary: Lesson 1: Lesson 6: Lesson 2: Lesson 4A: Lesson 4B: Lesson 3: Lesson 5: www.chempride.weebly.com
m A 1 1 A ! and AC 6
 REVIEW SET A Using sle of m represents units, sketh vetor to represent: NON-CALCULATOR n eroplne tking off t n ngle of 8 ± to runw with speed of 6 ms displement of m in north-esterl diretion. Simplif:
REVIEW SET A Using sle of m represents units, sketh vetor to represent: NON-CALCULATOR n eroplne tking off t n ngle of 8 ± to runw with speed of 6 ms displement of m in north-esterl diretion. Simplif:
2. Relative molecular mass, M r - The relative molecular mass of a molecule is the average mass of the one molecule when compared with
 Chapter 3: Chemical Formulae and Equations 1. Relative atomic mass, A r - The relative atomic mass of an element is the average mass of one atom of an element when compared with mass of an atom of carbon-12
Chapter 3: Chemical Formulae and Equations 1. Relative atomic mass, A r - The relative atomic mass of an element is the average mass of one atom of an element when compared with mass of an atom of carbon-12
CS311 Computational Structures Regular Languages and Regular Grammars. Lecture 6
 CS311 Computtionl Strutures Regulr Lnguges nd Regulr Grmmrs Leture 6 1 Wht we know so fr: RLs re losed under produt, union nd * Every RL n e written s RE, nd every RE represents RL Every RL n e reognized
CS311 Computtionl Strutures Regulr Lnguges nd Regulr Grmmrs Leture 6 1 Wht we know so fr: RLs re losed under produt, union nd * Every RL n e written s RE, nd every RE represents RL Every RL n e reognized
Unit 1 - Foundations of Chemistry
 Unit 1 - Foundations of Chemistry Chapter 2 - Chemical Reactions Unit 1 - Foundations of Chemistry 1 / 42 2.1 - Chemical Equations Physical and Chemical Changes Physical change: A substance changes its
Unit 1 - Foundations of Chemistry Chapter 2 - Chemical Reactions Unit 1 - Foundations of Chemistry 1 / 42 2.1 - Chemical Equations Physical and Chemical Changes Physical change: A substance changes its
STOICHIOMETRY. Chapter Quiz. Fill in the word(s) that will make each statement true
 STOICHIOMETRY Chapter Quiz Fill in the word(s) that will make each statement true. 1. The 1 in a balanced chemical equation also reveal the mole ratios of the substances involved. 1. 12.1 2. 12.1 2. The
STOICHIOMETRY Chapter Quiz Fill in the word(s) that will make each statement true. 1. The 1 in a balanced chemical equation also reveal the mole ratios of the substances involved. 1. 12.1 2. 12.1 2. The
Name: Unit 9- Stoichiometry Day Page # Description IC/HW
 Name: Unit 9- Stoichiometry Day Page # Description IC/HW Due Date Completed ALL 2 Warm-up IC 1 3 Stoichiometry Notes IC 1 4 Mole Map IC X 1 5 Mole to Mole Practice IC 1 6 Mass to Mole Practice IC 1/2 X
Name: Unit 9- Stoichiometry Day Page # Description IC/HW Due Date Completed ALL 2 Warm-up IC 1 3 Stoichiometry Notes IC 1 4 Mole Map IC X 1 5 Mole to Mole Practice IC 1 6 Mass to Mole Practice IC 1/2 X
Lesson 2.1 Inductive Reasoning
 Lesson 2.1 Inutive Resoning Nme Perio Dte For Eerises 1 7, use inutive resoning to fin the net two terms in eh sequene. 1. 4, 8, 12, 16,, 2. 400, 200, 100, 50, 25,, 3. 1 8, 2 7, 1 2, 4, 5, 4. 5, 3, 2,
Lesson 2.1 Inutive Resoning Nme Perio Dte For Eerises 1 7, use inutive resoning to fin the net two terms in eh sequene. 1. 4, 8, 12, 16,, 2. 400, 200, 100, 50, 25,, 3. 1 8, 2 7, 1 2, 4, 5, 4. 5, 3, 2,
Exam 1 Worksheet Chemistry 102
 Exam 1 Worksheet Chemistry 102 Chapter 1 Introduction 1. Convert: a. 650 nm to inches b. 650 km to miles c. 345 mg to lb d. 145 lb to μg e. 4.50 10 10 km to μm f. 8.09 10 8 mg to Mg g. 9.00 10 5 ml to
Exam 1 Worksheet Chemistry 102 Chapter 1 Introduction 1. Convert: a. 650 nm to inches b. 650 km to miles c. 345 mg to lb d. 145 lb to μg e. 4.50 10 10 km to μm f. 8.09 10 8 mg to Mg g. 9.00 10 5 ml to
The Thermodynamics of Aqueous Electrolyte Solutions
 18 The Thermodynmics of Aqueous Electrolyte Solutions As discussed in Chpter 10, when slt is dissolved in wter or in other pproprite solvent, the molecules dissocite into ions. In queous solutions, strong
18 The Thermodynmics of Aqueous Electrolyte Solutions As discussed in Chpter 10, when slt is dissolved in wter or in other pproprite solvent, the molecules dissocite into ions. In queous solutions, strong
Worksheet 1: REPRESENTATIVE PARTICLES
 Worksheet 1: REPRESENTATIVE PARTICLES Directions: For each substance below, state the representative particle. If the RP is a molecule, state the number of atoms that make up the molecule. If the RP is
Worksheet 1: REPRESENTATIVE PARTICLES Directions: For each substance below, state the representative particle. If the RP is a molecule, state the number of atoms that make up the molecule. If the RP is
Chemical Bonds In elements and compounds, the atoms are held together by chemical bonds.
 Chemical Bonds In elements and compounds, the atoms are held together by chemical bonds. Forming a bond makes an atom more stable, so atoms form as many bonds are they are able to. Bonds are made using
Chemical Bonds In elements and compounds, the atoms are held together by chemical bonds. Forming a bond makes an atom more stable, so atoms form as many bonds are they are able to. Bonds are made using
E20: BALANCING EQUATIONS
 E20: BALANCING EQUATIONS Before we can talk about balancing equations, we need to know how to READ an equation. We've already talked about symbols (like H or Na), and you should know how to read a formula
E20: BALANCING EQUATIONS Before we can talk about balancing equations, we need to know how to READ an equation. We've already talked about symbols (like H or Na), and you should know how to read a formula
PAIR OF LINEAR EQUATIONS IN TWO VARIABLES
 PAIR OF LINEAR EQUATIONS IN TWO VARIABLES. Two liner equtions in the sme two vriles re lled pir of liner equtions in two vriles. The most generl form of pir of liner equtions is x + y + 0 x + y + 0 where,,,,,,
PAIR OF LINEAR EQUATIONS IN TWO VARIABLES. Two liner equtions in the sme two vriles re lled pir of liner equtions in two vriles. The most generl form of pir of liner equtions is x + y + 0 x + y + 0 where,,,,,,
Practice Problems Stoich!
 Practice Problems Stoich! Name: **YOUR ANSWERS MUST INCLUDE THE PROPER NUMBER OF SIG FIGS AND COMPLETE UNITS IN ORDER TO RECEIVE CREDIT FOR THE PROBLEM.** BALANCE THE FOLLOWING EQUATIONS TO USE IN QUESTIONS
Practice Problems Stoich! Name: **YOUR ANSWERS MUST INCLUDE THE PROPER NUMBER OF SIG FIGS AND COMPLETE UNITS IN ORDER TO RECEIVE CREDIT FOR THE PROBLEM.** BALANCE THE FOLLOWING EQUATIONS TO USE IN QUESTIONS
CHAPTER 12: STOICHIOMETRY
 Name: CHAPTER 12: STOICHIOMETRY Period: MOLE TO MOLE RATIO When nitrogen and hydrogen gas are heated under the correct conditions, ammonia gas (NH 3 ) is formed. a. RXN: 1N 2 + 3H 2 2NH 3 b. How many moles
Name: CHAPTER 12: STOICHIOMETRY Period: MOLE TO MOLE RATIO When nitrogen and hydrogen gas are heated under the correct conditions, ammonia gas (NH 3 ) is formed. a. RXN: 1N 2 + 3H 2 2NH 3 b. How many moles
Dr. Steward s CHM152 Exam #2 Review Spring 2014 (Ch ) KEY
 Dr. Stewrd s CHM152 Em #2 Review Spring 2014 (Ch. 16. 17) KEY 1. Eplin the commonion effect. Wek cid or wek bse s % dissocition will decrese if they re plced in solutions with one of the products of dissocition.
Dr. Stewrd s CHM152 Em #2 Review Spring 2014 (Ch. 16. 17) KEY 1. Eplin the commonion effect. Wek cid or wek bse s % dissocition will decrese if they re plced in solutions with one of the products of dissocition.
AP Chemistry Multiple Choice Questions - Chapter 4
 1 Which of the following contains 6.00 x 10 16 atoms? a 6.00 x 10 16 H 2 O molecules b 3.00 x 10 16 Cl 2 molecules c 2.00 x 10 16 P 4 molecules d 1.50 x 10 16 CaSO 4 empirical units 4.1 2 How many atoms
1 Which of the following contains 6.00 x 10 16 atoms? a 6.00 x 10 16 H 2 O molecules b 3.00 x 10 16 Cl 2 molecules c 2.00 x 10 16 P 4 molecules d 1.50 x 10 16 CaSO 4 empirical units 4.1 2 How many atoms
UNIT 31 Angles and Symmetry: Data Sheets
 UNIT 31 Angles nd Symmetry Dt Sheets Dt Sheets 31.1 Line nd Rottionl Symmetry 31.2 Angle Properties 31.3 Angles in Tringles 31.4 Angles nd Prllel Lines: Results 31.5 Angles nd Prllel Lines: Exmple 31.6
UNIT 31 Angles nd Symmetry Dt Sheets Dt Sheets 31.1 Line nd Rottionl Symmetry 31.2 Angle Properties 31.3 Angles in Tringles 31.4 Angles nd Prllel Lines: Results 31.5 Angles nd Prllel Lines: Exmple 31.6
Strategy: Use the Gibbs phase rule (Equation 5.3). How many components are present?
 University Chemistry Quiz 4 2014/12/11 1. (5%) Wht is the dimensionlity of the three-phse coexistence region in mixture of Al, Ni, nd Cu? Wht type of geometricl region dose this define? Strtegy: Use the
University Chemistry Quiz 4 2014/12/11 1. (5%) Wht is the dimensionlity of the three-phse coexistence region in mixture of Al, Ni, nd Cu? Wht type of geometricl region dose this define? Strtegy: Use the
Page 1 Name: 2Al 3+ (aq) + 3Mg(s) 3Mg 2+ (aq) + 2Al(s) Fe 2 O 3 + 2Al Al 2 O 3 + 2Fe
 9666-1 - Page 1 Name: 1) What is the oxidation number of chromium in the chromate ion, CrO 2-4? A) +8 B) +3 C) +2 D) +6 2) What is the oxidation number of sulfur in Na 2 S 2 O 3? A) +6 B) +4 C) +2 D) -1
9666-1 - Page 1 Name: 1) What is the oxidation number of chromium in the chromate ion, CrO 2-4? A) +8 B) +3 C) +2 D) +6 2) What is the oxidation number of sulfur in Na 2 S 2 O 3? A) +6 B) +4 C) +2 D) -1
UNIVERSITY OF MALTA DEPARTMENT OF CHEMISTRY. CH237 - Chemical Thermodynamics and Kinetics. Tutorial Sheet VIII
 UNIVERSITY OF MALTA DEPARTMENT OF CHEMISTRY CH237 - Chemicl Thermodynmics nd Kinetics Tutoril Sheet VIII 1 () (i) The rte of the rection A + 2B 3C + D ws reported s 1.0 mol L -1 s -1. Stte the rtes of
UNIVERSITY OF MALTA DEPARTMENT OF CHEMISTRY CH237 - Chemicl Thermodynmics nd Kinetics Tutoril Sheet VIII 1 () (i) The rte of the rection A + 2B 3C + D ws reported s 1.0 mol L -1 s -1. Stte the rtes of
CEM143 MWF 8:00 8:50 am. October 5, 2018
 CEM43, Fll 208 st Miterm CEM43 MWF 8:00 8:50 m st Miterm toer 5, 208 Nme: Setion: PID: TA: This is lose ook n note exmintion. This exm hs 35 questions. Answer ll questions on the seprte nswer sheet (ule
CEM43, Fll 208 st Miterm CEM43 MWF 8:00 8:50 m st Miterm toer 5, 208 Nme: Setion: PID: TA: This is lose ook n note exmintion. This exm hs 35 questions. Answer ll questions on the seprte nswer sheet (ule
CEM143 MWF 8:00 8:50 am. October 5, 2018
 CEM43, Fll 208 st Miterm CEM43 MWF 8:00 8:50 m st Miterm toer 5, 208 Nme: Setion: PID: TA: This is lose ook n note exmintion. This exm hs 35 questions. Answer ll questions on the seprte nswer sheet (ule
CEM43, Fll 208 st Miterm CEM43 MWF 8:00 8:50 m st Miterm toer 5, 208 Nme: Setion: PID: TA: This is lose ook n note exmintion. This exm hs 35 questions. Answer ll questions on the seprte nswer sheet (ule
QUADRATIC EQUATION. Contents
 QUADRATIC EQUATION Contents Topi Pge No. Theory 0-04 Exerise - 05-09 Exerise - 09-3 Exerise - 3 4-5 Exerise - 4 6 Answer Key 7-8 Syllus Qudrti equtions with rel oeffiients, reltions etween roots nd oeffiients,
QUADRATIC EQUATION Contents Topi Pge No. Theory 0-04 Exerise - 05-09 Exerise - 09-3 Exerise - 3 4-5 Exerise - 4 6 Answer Key 7-8 Syllus Qudrti equtions with rel oeffiients, reltions etween roots nd oeffiients,
Stoichiometry is the relationship between the amount of reactants used and the amount of products produced in a chemical reaction.
 Unit 7 STOICHIOMETRY 1. Introduction to Stoichiometry 2. Mole Mole Stoichiometry 3. Mass Mole Stoichiometry 4. Mass Mass Stoichiometry 5. Mass Volume & Volume Volume Stoichiometry 6. Excess & Limiting
Unit 7 STOICHIOMETRY 1. Introduction to Stoichiometry 2. Mole Mole Stoichiometry 3. Mass Mole Stoichiometry 4. Mass Mass Stoichiometry 5. Mass Volume & Volume Volume Stoichiometry 6. Excess & Limiting
Stoichiometry is the relationship between the amount of reactants used and the amount of products produced in a chemical reaction.
 Unit 7 STOICHIOMETRY 1. Introduction to Stoichiometry 2. Mole Mole Stoichiometry 3. Mass Mole Stoichiometry 4. Mass Mass Stoichiometry 5. Mass Volume & Volume Volume Stoichiometry 6. Excess & Limiting
Unit 7 STOICHIOMETRY 1. Introduction to Stoichiometry 2. Mole Mole Stoichiometry 3. Mass Mole Stoichiometry 4. Mass Mass Stoichiometry 5. Mass Volume & Volume Volume Stoichiometry 6. Excess & Limiting
ISOTHERMAL REACTOR DESIGN (4) Marcel Lacroix Université de Sherbrooke
 ISOTHERML RETOR DESIGN (4) Marcel Lacroix Université de Sherbrooke ISOTHERML RETOR DESIGN: OBJETIVE TO DESIGN VRIOUS TYES OF IDEL ISOTHERML RETORS USING THE FOLLOWING TOOLS: 1. MOLE BLNE OR DESIGN EQUTION:
ISOTHERML RETOR DESIGN (4) Marcel Lacroix Université de Sherbrooke ISOTHERML RETOR DESIGN: OBJETIVE TO DESIGN VRIOUS TYES OF IDEL ISOTHERML RETORS USING THE FOLLOWING TOOLS: 1. MOLE BLNE OR DESIGN EQUTION:
