PREPARATION AND CHARACTERIZATION OF MULTIFUNCTIONAL CHITOSAN MICROPARTICLES FOR LUNG DELIVERY. Swasti Pandey A THESIS
|
|
|
- Jessica Stevenson
- 6 years ago
- Views:
Transcription
1
2 i
3 PREPARATION AND CHARACTERIZATION OF MULTIFUNCTIONAL CHITOSAN MICROPARTICLES FOR LUNG DELIVERY By Swasti Pandey A THESIS Submitted to the faculty of the Graduate School of the Creighton University in Partial Fulfillment of the Requirements for the degree of Master of Science in the Department of Pharmaceutical Sciences Omaha, NE (September 29, 2015) i
4 Swasti Pandey, 2015 ii
5 ABSTRACT The aim of this project was to develop a multifunctional approach with an aim of utilizing both chemotherapeutic agent as well as radio frequency (RF) heating for targeted lung cancer therapy. The system designed for achieving this was a chitosan microparticulate system containing iron oxide nanoparticles and gemcitabine as the chemotherapeutic agent. Iron oxide nanoparticles were prepared by chemical co-precipitation. The iron oxide nanoparticles were made water dispersible by adding an oleic acid-poloxamer coat to the surface of the iron oxide particles. These nanoparticles were incorporated into chitosan microparticles along with gemcitabine by spray drying using a Buchi Mini Spray dryer B-290. The particle size of iron oxide nanoparticles determined by laser light scattering was ± 12.98nm (n=3). However, the TEM results revealed a particle size of nm± 2.15nm. The larger particle size by laser light scattering which measures the hydrodynamic diameter, could be due to oleic acid and poloxamer associated with nanoparticles and its hydration when dispersed in water. The chitosan microparticles prepared had a d0.5 of 1.59 ± 0.35µm (n=3) and a surface charge of ± 0.18mV. The entrapment efficiency of gemcitabine in the chitosan microparticles was found to be ± 5.7%. The loading of iron oxide nanoparticles into chitosan matrix was found to be ± 3.4%, respectively. DSC studies suggest that the drug is present in non- crystalline state in the chitosan matrix. The TGA studies revealed that the percent weight loss from the spray dried particles at 120ᴼC was around 10%. Aerosol testing of the prepared microparticles on the NGI suggested that the microparticles have a polydisperse population with a fine particle fraction of 47.5%. The application of RF exposure for 500 seconds shows a linear increase in temperature with increase in exposure time. The in vitro iii
6 release studies showed a significant release (up to 70%) of Gemcitabine over a period of 72 hours and also indicated that release of Iron oxide from the system was negligible. The magnetometer readings indicated that the magnetic properties of nanoparticles are well retained in the chitosan microparticles. The cellular uptake studies display similar cellular uptake behavior for drug solutions treatments and gemcitabine iron oxide microparticle treatments. However the cellular cytotoxicity observed was higher in the gemcitabine iron oxide microparticle treatments compared to drug solutions treatments. The radiofrequency heating experiments conducted on cell suspensions shows a significant heating due to magnetic hyperthermia causing cellular death. Thus it can be concluded that a combination of radiofrequency and chemotherapy with a single system can be achieved and result in particles that are functional in both aspects iv
7 Dedicated to my family and teachers v
8 Acknowledgements I would like to express my gratitude to my advisor, Dr. Alekha Dash for giving me the opportunity to work on this project and for his guidance and support throughout my Master s degree at Creighton University. I would also like to thank Dr. Justin Tolman for letting me use his laboratory facilities for major work related to this project and for helping understand concepts and guiding me as a member of my committee. I am also grateful to Dr. Jeff North who has always been supportive and encouraging as has also been a very helpful member of my committee. I would like to extend my gratitude to Dr. Manzoor Khan and Dr. Somnath Singh for their supervision as the program directors. I have been lucky to have great help and guidance from Dan Munt who has been an amazing teacher and an awesome support throughout. I am also grateful to my seniors Shantanu Chandratre and Anne Grana for their valuable advice, suggestions and inputs. I am also thankful to Pushkar Saralkar, Akash Patil, Sonal Bhujbal and Dr. Igor Meerovich who have been my labmates and are an imporant part of my journey in getting my master s degree. I earnestly thank Dawn Trojanowski and Kathy Stuhr and entire administrative staff of the Department of Pharmacy Sciences for their assistance and support. I also wish to express gratitude to the entire faculty of the department of Pharmacy Sciences for the knowledge I have gained from them in the last two years. Words are not enough to express the immense love and support I have from my parents, Mrs. Pratima Pandey and Mr. Brajesh Pandey. I also wish to thank my teacher Mr. Vivek Nalawade and my friends and family who have always been my pillars of strength. I would also like to take this opportunity to thank all my friends here in Omaha who made it feel like home, away from home. vi
9 TABLE OF CONTENTS Abstract Acknowledgement Table of contents List of figures List of tables List of equations iii vi vii xii xv xvi Chapter 1: Introduction Lung cancer: Introduction Multifunctional approach to lung cancer treatment Inhalational chemotherapy Chemotherapeutic agent: gemcitabine Mechanism of action of gemcitabine Physicochemical properties of gemcitabine Chitosan Preparation of chitosan microparticles by spray drying Aerodynamic properties of microparticles Magnetic hyperthermia therapy Properties of magnetic particles Hypothesis and specific aims 22 Chapter 2: Synthesis of nanosized iron oxide particles Introduction Materials 25 vii
10 2.3 Methods Synthesis of iron oxide nanoparticles Method Method Synthesis of iron oxide nanoparticles: optimized method Measurement of particle size and zeta potential of nanoparticles Dynamic light scattering Transmission electron microscopy and scanning electron microscopy Results Results: batches from method 1 & Results: optimized batch Measurement of particle size and zeta potential of nanoparticles Transmission electron microscopy Discussion Synthesis by method 1 & Optimized method for nanoparticle synthesis Conclusions 33 Chapter 3: Preparation, characterization and in vitro evaluation of gemcitabine and iron oxide loaded chitosan microparticles Introduction Materials Methods 36 viii
11 3.3.1 Preparation of gemcitabine and iron oxide loaded chitosan microparticles Chromatography Preparation of solutions Standard solutions Calculations Measurement of particle size and zeta potential of spray dried particles Electron microscopy Determination of drug entrapment efficiency Determination of iron loading Thermogravimetric analysis (TGA) Differential scanning calorimetry (DSC) Aerodynamic studies Magnetization studies Radiofrequency heating In vitro release study Determination of cellular uptake MTT toxicity assay Cytotoxicity assay on RF treated cells Statistical data analysis Results Testing of chromatographic method 51 ix
12 Specificity Linearity Precision Accuracy Particle size and zeta potential of spray dried particles Transmission electron microscopy Drug entrapment efficiency Iron loading Thermogravimetric analysis Differential scanning calorimetry (DSC) Aerodynamic studies Magnetization studies Radiofrequency heating In vitro release study Cellular uptake study MTT toxicity assay Cytotoxicity on RF treated cells Discussions Preparation of microparticles Chromatography and spectrophotometry Particle size, zeta potential of microparticles Electron microscopy Drug entrapment efficiency and iron loading 71 x
13 3.5.6 Thermal analysis Aerodynamic studies Magnetization studies Radiofrequency heating In vitro release study Cellular uptake studies In vitro cellular toxicity Cellular RF treatment Conclusion 77 Chapter 4 Summary & future directions Summary Future Studies 82 Bibliography 84 xi
14 LIST OF FIGURES Fig 1 Chemical structure of gemcitabine 6 Fig 2 Structural comparison between gemcitabine and deoxycytidine 7 Fig 3 Metabolism, mechanisms of action and self-potentiation of gemcitabine 8 Fig 4 Chemical structure of chitosan 10 Fig 5 Schematic representation of the spray drying process 12 Fig 6 Components of an NGI 16 Fig 7 Magnetic regimes of magnetite particles as a function of their size (superparamagnetic, single domain, multidomain) 19 Fig 8 M-H curves for (a) ferromagnetic material, (b) superparamagnetic material. M is the magnetization of the material and H is the external magnetic field. 20 Fig 9 Scheme showing the reaction mechanism of magnetite particle formation from an aqueous mixture of ferrous and ferric chloride by addition of a base 24 Fig 10 Schematic representation of synthesis of iron oxide nanoparticles: Method 1 27 Fig 11 Schematic representation of synthesis of iron oxide nanoparticles: Method 2 28 Fig 12 Setup for iron oxide synthesis by optimized method 29 xii
15 Fig 13 Fig 14 (a) Synthesized iron oxide nanoparticles (b) Collapsible stirrer built for synthesis TEM of Iron oxide nanoparticles Fig 15 Setup for spray drying of Gemcitabine-iron oxide loaded chitosan particles 37 Fig 16 A typical RF induction heating setup 45 Fig 17 Radiofrequency induction machine with a. Chiller, b. Compressed Air supply, c. Air jacketing system 46 Fig 18 Set up for in vitro release studies 47 Fig 19 Representative chromatograms for A) Blank B) Peak for Gemcitabine 52 Fig 20 Standard curve for gemcitabine 53 Fig 21 Particle size distribution of chitosan microparticles 56 Fig 22 TEM of spray dried gemcitabine-iron oxide loaded chitosan microparticles 57 Fig 23 SEM of blank spray dried chitosan microparticles 57 Fig 24 TGA of gemcitabine-chitosan particles with and without iron oxide 58 Fig 25 DSC thermograms for drug and various chitosan microparticles 59 xiii
16 Fig 26 Aerosol dispersion performance as % cumulative deposition vs. particle size on the Next Generation Impactor TM for spray dried chitosan iron oxide system (n=3, Ave ± SD) 60 Fig 27 (a): M-H curves for iron oxide nanoparticles (b) M-H curves for iron oxide nanoparticles in chitosan microparticles with gemcitabine 61 Fig 28 (a) ZFC/ FC of iron oxide nanoparticles (b): ZFC/ FC of iron microparticles 62 Fig 29 RF heating curves of iron oxide nanoparticles and chitosan gemcitabine iron oxide particles with water as control 63 Fig 30 Release profiles of gemcitabine from chitosan microparticles 64 Fig 31 Cellular uptake of gemcitabine from drug solutions as well as spray dried chitosan microparticles 65 Fig 32 Cytotoxicity profile of gemcitabine solutions, blank and drug loaded spray dried microparticles after 72 hours of incubation in Wi26 A4 cells 66 Fig 33 Cytotoxicity profile of gemcitabine solutions, blank and drug loaded spray dried microparticles after 72 hours of incubation in A549 cells 67 Fig 34 Cytotoxicity assay reporting percent cell death on RF exposure. [n=3] 68 Fig 35 Cellular localization of rhodamine bound chitosan microparticles in mouse macrophage cell line AMJ2C11 83 xiv
17 LIST OF TABLES Table 1 Physicochemical properties of gemcitabine 9 Table 2 D50 values for NGI stages at air flow rate of 60 L/min 17 Table 3 Particle size, polydispersity index, zeta potential values for nanoparticles. [n=3] 31 Table 4 Spray drying parameters 38 Table 5 Within day and day to day precision of the UPLC analysis of gemcitabine 55 Table 6 Accuracy results for UPLC analysis of gemcitabine 55 Table 7 Particle size and zeta potential of chitosan particles 56 Table 8 Aerodynamic parameters 60 Table 9 LD50 values of different treatments on Wi26VA4 cells 66 Table 10 LD50 values of different treatments on A549 cells 67 xv
18 LIST OF EQUATIONS Equation 1 Drug entrapment efficiency 42 Equation 2 Iron loading 42 Equation 3 % Accuracy 56 xvi
19 CHAPTER 1 Introduction 1
20 1.1 Lung Cancer: Introduction Lung cancer, also known as pulmonary cancer or carcinoma of the lung, is uncontrolled growth of abnormal cells in one or both lungs. These cells grow and form tumors which eventually interfere with the functioning of the lungs 1. The National Cancer Institute lists lung cancer as the second most commonly occurring cancer in the world. In the United States of America, lung cancer has come to be the primary cause of cancer related deaths 2. The occurrence of lung cancer is generally related with prevalence of smoking among the population. Approximately 85% of all lung cancer cases are in cigarette smoking patients. However, the risk of lung cancer differs widely based on a person s age, smoking intensity, and smoking duration. About 15-20% of lung cancer patients have no history of smoking or have smoked minimally 3. The lung cancer risk also involves a variety of other factors like passive smoke cigarette smoke inhalation, air pollutants, exposure to carcinogens such as radon, asbestos, arsenic, radiation and mustard gas 4. Lung Cancer is classified into 2 major categories, namely small cell lung cancer (SCLC) and nonsmall cell lung cancer (NSCLC). According to American Cancer Society (ACS) reports of 2015, SCLC comprised of approximately 15% of all lung cancers 5. The NSCLC type has an occurrence of 85% among all lung cancers. SCLC originates in the bronchial submucosa and is a more aggressive cancer type, when compared to the non-small cell lung cancer Treatment Approaches The conventional approaches for treatment of lung cancer are surgical resection, chemotherapy and radiation therapy. These approaches may be used as monotherapy or in combination with each other. The treatment approach is decided based on the type and stage of the cancer in a particular patient 1. 2
21 The approach of surgical resection of the tumor is difficult in most patients 6. In cases of inoperable tumors, chemotherapy along with thoracic irradiation is the only available option for most patients 7. In case of lung cancer chemotherapy, the chemotherapeutic drugs being delivered systemically are widely distributed and metabolized in the body. As a result, the therapeutic dose in unable to reach the lung to cause the desired anti-cancer effect 6. Hence, the conventional methods of treatment have been found to have a low efficacy. In many cases, the patients are known to have systemic side effects due to chemotherapy. As a result, there is a need to look into more efficacious means for treating lung tumors. The lack of better treatment options has led to a great interest among researchers to explore other options for treatment of pulmonary carcinoma. 1.2 Multifunctional approach to lung cancer treatment The design of a multifunctional system involves incorporation of multiple treatment aspects into a single system which is ultimately able to achieve more than one function. In our goal of achieving a multifunctional system in this project, we have looked at the utility of aerosolized chemotherapy for local administration directly to the lungs, aiming for improvement in lung cancer treatment. Chemotherapy of cancer generally involves administration of a single or multiple agents which are cytotoxic, with an aim of killing cancer cells. However, as discussed in the previous section, lung cancer chemotherapy is known to have low efficacy and shows many side effects due to systemic distribution 8. An ideal approach for this type of cancer would involve direct delivery of the chemotherapeutic agents to the target site for maximum efficacy and low systemic side effects. In this regard, a therapeutic strategy where anticancer agents are delivered directly to the lungs through formulated aerosolization is a novel approach for lung cancer treatment. Inhalational chemotherapy includes designing of particles of appropriate particle size, containing adequate 3
22 amount of drug 9,10,11. All these properties are required for imparting desirable aerodynamic behavior to the particles. Magnetic hyperthermia has also been considered for designing this multifunctional approach. It is currently being looked at as an alternate therapy for cancer treatment. This approach is designed to artificially induce hyperthermia using magnetic nanoparticles causing the heating of malignant cells and resulting in cell death 12. The procedure involves delivery of magnetic nanoparticles to the target site and then applying an alternate current (AC) magnetic field of high frequency to cause nanoparticle heating 12. This heat is above the therapeutic threshold of 42 C. When the cells are heated up to hyperthermia temperatures, it results is initiation of various cell degradation mechanisms such as protein denaturation, aggregation, folding and DNA aggregation. The heating also causes other cellular effects such as induction of apoptosis, tissue level ph changes, perfusion and oxidation of tumor microenvironment The hyperthermia treatment complements currently available treatments for cancers like chemotherapy, radiation therapy and surgical resection 16. Thus an inhalable, hyperthermia inducing approach combined with a chemotherapeutic agent was the basis of this project. These individual concepts have been explained in detail in the following sections. Studies have suggested that hyperthermia is a chemosensitizer for cancer cells by making tumor cells more porous and allowing betting uptake of chemotherapeutic drug 17,18. On the other hand, application of hyperthermia for anti-tumor treatment is limited by tumor size 19. Hyperthermia has been reported to have low performance in large sized tumors. Thus chemotherapy might help in reduction of tumor size and improve hyperthermia performance. Inhalational therapy of cancer provides a great potential in the areas of particle design and aerodynamic behavior. This project involves designing of dry powder microparticle formulations 4
23 via spray drying technique. The system developed here is designed to impart multifunctionality to the inhaled system. These microparticles contain an antineoplastic nucleoside analogue, gemcitabine, as the chemotherapeutic agent and iron oxide as the agents to induce hyperthermia. The polymer being used in the system as a carrier is chitosan. Chitosan has been widely studied for pulmonary drug delivery applications 20. The combination of gemcitabine and iron oxide nanoparticles was used for achieving a combined effect of both therapies. 1.3 Inhalational Chemotherapy The inhalational route of delivery has been explored in recent times for designing treatment approaches for diseases such as tuberculosis, pneumonia, cystic fibrosis, chronic obstructive pulmonary disease (COPD), and lung cancer 9, The lung is an ideal target for drug delivery as it avoids first pass metabolism, shows rapid onset of action, provides localization of delivered drug and minimization of systemic exposure of the delivered agents 11, In the case of lung cancer, the tumors are usually situated in the deep lung. Due to lack of localization of chemotherapeutic drugs in the region of the tumor, possible treatment failure and initiation of chemotherapeutic resistance can be caused 28. Thus inhalational therapy provides greater advantages, increased potential and better approach for treatment of lung cancer. Inhalational therapy has been greatly studied in the recent times. This route requires an understanding of lung physiology, particle design, aerodynamic behavior, drug load and pulmonary delivery method Chemotherapeutic agent: Gemcitabine Gemcitabine is marketed as Gemzar, which is the hydrochloride salt of gemcitabine by Eli Lilly and Company. It is an antineoplastic nucleoside analogue. This drug was synthesized by Hertel et al. in early 1980s (Eli Lilly laboratories, Indianapolis, USA). The molecule was formed by 5
24 replacement of both hydrogen atoms on deoxycytidine by fluorine atoms at the 2 -position of the sugar rings in deoxyribose. Gemcitabine, during its early days of discovery, showed potent antineoplastic activity against human leukemia cells in cell culture studies 29. This led to adaptation of drug into the army of anticancer agents used against several types cancers including metastatic breast cancer 30, non-small cell lung cancer 31,32, pancreatic cancer 33, and ovarian cancer 34. Gemcitabine has been known to be a first line treatment for metastatic breast cancer and also an important drug in gastric cancers or advanced melanoma 29. Gemcitabine has a unique activity against a range of solid tumors 35. Figure 1: Chemical structure of gemcitabine Mechanism of action of gemcitabine Gemcitabine is a prodrug which undergoes conversion to its active form by deoxycytidine kinase. Like most nucleoside analogues, gemcitabine gets transported into the cells via various active nucleoside transporters. 6
25 Figure 2: Structural comparison between gemcitabine and deoxycytidine 36 Upon entering the cell, a nucleoside kinase metabolizes gemcitabine to diphosphate (dfdcdp) and subsequently triphosphate (dfdctp) nucleosides. Gemcitabine diphosphate inhibits ribonucleotide reductase, which is an enzyme responsible for catalyzing the generation of deoxynucleoside triphosphates for DNA synthesis. This results in reductions in deoxynucleotides concentrations, including reduction of cellular deoxycytidine triphosphate (dctp). The other metabolite of the prodrug, which is gemcitabine triphosphate competes with dctp for incorporation into DNA. Since the cellular dctp concentrations are low, the gemcitabine triphosphate is the substrate that DNA polymerases use to incorporate a gemcitabine nucleoside into DNA. After addition of the gemcitabine nucleotide into DNA, one more deoxynucleotide is added and thereafter, the DNA polymerases are unable to continue elongating DNA. This action is called "masked termination." This locks the gemcitabine nucleotide into DNA as the proofreading enzymes are unable to remove gemcitabine from this position 37. Gemcitabine metabolites are also known to have certain unique properties to enhance the overall inhibitory activities on cell growth. This phenomenon is known as self-potentiation of gemcitabine. The mechanism of clearance of gemcitabine is by deamination by cytidine deaminase (CDA) to 2,2-difluorodeoxyuridine (dfdu) (inert metabolite). Gemcitabine triphosphate (dfdc-tp), at low 7
26 concentrations, shows monophasic linear elimination kinetics but at higher concentrations it becomes biphasic with increased terminal half-life. This is because gemcitabine triphosphate (dfdc-tp) inhibits dcmp deaminase and prevents its clearance resulting in retention of gemcitabine by tumor cells 38. Resistance to gemcitabine was found to be related to ATP- binding cassette transporters. ABCC (MRP) transporters in this family mediate efflux of gemcitabine from the cells leading to drug resistance 39. dfdu dfdump - 11 Thymidylate synthase (TS) 7 Gemcitabine ( dfdc ) 3 dcda 4 dcmpda hnts 2 dck/tk2 dfdc dfdcmp dfdcdp dfdctp 5 5 -NT dfdc-dna 8 dfdc-rna Ribonucleotide reductase (RR) CTP-synthetase Figure 3: Metabolism, mechanisms of action and self-potentiation of gemcitabine. 1, transport by nucleoside transporters (hnts); 2, phosphorylation; 3 and 4, deamination; 5, dephosphorylation; 6, accumulation of the triphosphate; 7, incorporation into DNA; 8, incorporation into RNA; 9, inhibition of ribonucleotide reductase (RR); 10, inhibition of CTP-synthetase; 11, inhibition of thymidylate synthase (TS); 12, inhibition of deoxycytidine monophosphate deaminase (dcmpda); inhibitory effect. Other abbreviations: dck, deoxycytidine kinase; TK2, thymidine kinase 2; dcda, deoxycytidine deaminase; 59-NT, 59-nucleotidase 39. The drug is known to be remarkably stable in the solid state, but in its solution at 40 degrees C, deamination of gemcitabine occurs, yielding its uridine analogue 40. 8
27 Gemcitabine is being studied for application in single agent therapy of non-small cell lung cancers. In a study by Zatlaokal et al., they confirmed the findings of previous studies that gemcitabine used as a monotherapy is an effective treatment for NSCLC with respect to both objective response and improved quality of life. The study evaluated responses from 76 patients 35. The response rate observed among the patients in this study was 21.1% which is similar to results reported in other studies using gemcitabine as an active single agent The study also showed that the disease was stabilized in 61.8% of the patients with a median survival of 7.1 months. In another study by U. Gatzemeier et al., gemcitabine has been suggested to be an active, well tolerated and easy to administer drug for use in NSCLC. The most frequent dose limiting toxicity observed was neutropenia. It has also been noted that the incidence and severity of myelosuppression with gemcitabine is lower than that reported for traditional agents used in NSCLC. In this study, almost 22% response rate was seen and independently validated by an extramural Oncology Review Board which confirmed gemcitabine as an effective single agent in NSCLC Physicochemical properties of gemcitabine Gemcitabine hydrochloride White crystalline powder Empirical formula C9H11F2N3O4 HCl Chemical name 2 -Deoxy-2, 2 -difluorocytidine mono hydrochloride Molecular weight g/mol Melting point 250 C 46 9
28 Solubility behavior Soluble in water, slightly soluble in methanol and practically insoluble in ethanol and polar organic solvents Aqueous solubility 22.3 g/l log P value -1.1 to Table 1: Physicochemical properties of gemcitabine Formulation of inhalational particles Chitosan Chitosan linear polysaccharide composed of randomly distributed β-(1-4)-linked D-glucosamine and N-acetyl-D-glucosamine units. As the names suggest, D-glucosamine is the deacetylated unit and N-acetyl-D-glucosamine is the acetylated unit. Chitosan is a polymer derived from natural sources. It made by treating shrimp and other crustacean shells with the alkali sodium hydroxide 48. Figure 4: Chemical structure of chitosan Chitosan is known to be a biocompatible, mucoadhesive polymer with low toxicity and high biodegradability. It has been viewed an important polymer for transmucosal delivery e.g. 10
29 pulmonary administration, nasal, buccal, ocular, intestinal applications This polymer is available in various grades and several types. The grades can be differentiated based on molecular weight, viscosity, area of application, degree of deacetylation, source of chitin. A clear nomenclature based on degree of deacetylation has not been defined for chitosan. On the basis of molecular weight, it has been classified as: low molecular weight chitosan, medium molecular weight chitosan, high molecular weight chitosan. 48. The polymer is odorless and appears white or creamy-white powder or flakes. Chitosan is sparingly soluble in water and practically insoluble in organics. However it is readily soluble in solutions of organic acids. Chitosan is a cationic polymer that influences the mucoadhesive properties to the polymer. Mucous membranes are negatively charged causing ionic interaction to take place between cationic primary amino acid group of chitosan with anionic sialic acid group of mucus membranes 56. For pulmonary delivery applications, chitosan has been reported to have properties of improved drug absorption, protection of the drug against enzymatic degradation 57 and absorption-enhancing effects in the nasal mucosa 57,58. However, the polymer does not have a regulatory approval for pharmaceutical marketing. It is still being widely investigated in different therapeutic areas. Gemcitabine is an antineoplastic nucleoside analogue and has been studied for therapeutic effect on lung cancer cells 59. Ventura et. al. have developed spray dried chitosan microparticles of gemcitabine for lung delivery 59. This method was adapted for spray drying of gemcitabine in chitosan. The method has to be modified to incorporate iron oxide nanoparticles in to the system Preparation of chitosan microparticles by spray drying Spray drying is a high-throughput process for pharmaceutical manufacturing of particles in solid state. This technique has been widely used for efficient production of respirable particles 9,11,60. 11
30 Spray drying is a process of conversion of liquid solutions or suspensions into dry particles through evaporation of the solvent or continuous phase 61. Dry powder inhalation of various drugs like tobramycin, amikacin, capreomycin, ciprofloxacin, salbutamol, budesonide; various proteins and peptides like insulin, albumin, deoxyribonuclease and various excipients like lactose, mannitol, chitosan, insulin have been reported in literature 61. For the purposes of this project, chitosan solutions containing the chemotherapeutic agent, gemcitabine and suspended iron oxide nanoparticles have been used. The principle of spray drying involves evaporation of solvent from liquid droplets to yield a dry solid state powder. The nozzle in the spray dryer is designed to spray tiny droplets of liquid solution, suspension, or emulsion into a heated chamber filled with drying gas, typically air. The drying gas causes the liquid to evaporate and the particles pass through a collection chamber for separating from the drying gas, and finally get deposited in the collector. Figure 5: Schematic representation of Spray Drying Process 62 12
31 The drying time for the spray drying process is approximately 100 milliseconds. This is beneficial as it reduces the chances of heat degradation of gemcitabine in the particles being spray dried for this project. The variables that affect the spray drying process are nature of the sample, inlet temperature, sample feed rate, drying gas flow rate, and spray gas flow rate. These parameters can be altered to optimize the spray drying process. The inlet temperature is the recorded temperature of drying air prior to atomization, during the time it interacts with the feed solution. The outlet temperature is the temperature resulting at the end, after interactions between drying air and atomized droplets. This temperature cannot be regulated and varies depending on the inlet temperature and the product. The drying gas flow rate can also be adjusted to optimize the drying process. It is the volume of the drying gas supplied to the system per unit time and determines the drying level of the product and the degree of product separation in the cyclone. The spray gas flow rate also influences pressure of atomization. The feed rate is the rate at which the feed solution enters the spray dryer by peristaltic pump in unit time. This rate can be controlled based on the nature of drying required and the desired particle properties 63. For the purposes of this project, microparticles belonging to the low micron size range were desired to suit inhalable therapy. The advantage of using spray drying is that it enables us to control the characteristics of the yielded particles and helps us to tailor the system, in terms of particle size, shape and morphology. All these factors are of great importance in pulmonary drug delivery 64, Aerodynamic properties of microparticles The lungs are the most important organ of the human respiratory system. The respiratory tract includes the mouth and throat, trachea, bronchi and alveoli 66. The lungs are organs whose major 13
32 function is to promote and optimize gas exchange. The diameter of the respiratory airway keeps narrowing down from the trachea to the alveoli. The diameter of the tracheal airway is 18 mm whereas the alveolar airway is only 0.4 mm wide 67. The conducting airways are lined by mucus and are composed of ciliated columnar cells which help in upward propulsion of the mucus for expulsion. However, the alveoli are covered with broad and thin squamous cells known as alveolar type I cells. These are cells which primarily cause the function of gas exchange 68. The delivery of drugs to the lungs can be either via solid powder or via liquid aerosols. Irrespective of the form of delivery, the deposition behavior of the particles in the lungs is affected by particle characteristics such as size, shape, density, surface properties and also upon breathing patterns, inhalation rates, state of the lung On application of inhalational delivery on a human body, the aerosolized particles being released into the respiratory system follow a complicated path. The particle momentum along the airway trajectory potentially results in impaction on the airway surfaces every time as the airflow direction changes. The large sized particles in the aerosol tend to deposit on the large airway bifurcation walls by inertial impaction. This phenomenon usually occurs in the oropharynx and large airways with rapid changes in airflow direction 72. This is typically seen in particles having mass median aerodynamic diameter (MMAD) values >5 µm 71. The mass median diameter of an aerosol is a value that refers to a particle diameter that has 50% of the total aerosol mass above it and 50% of its mass below it. For the particles that avoid inertial impaction, the deposition can occur by the mechanism of gravitational sedimentation. This mechanism of deposition is commonly observed in the lower portions of the lung. Particles with an MMAD value that lies between 1-5µm often display deposition in the smaller airways and alveoli 22,70. The particles in aerosol system that are submicron sized usually experience deposition by Brownian motion. This occurs for particles with 14
33 MMAD 0.5 µm leading to their alveolar deposition 73. Also, the particles having submicron aerodynamic sizes can be exhaled out by failing to deposit in the lungs. Thus, in order to achieve a significant particle deposition in the bronchoalveolar region, the aerosol requires the particles be small enough to overcome inertial impaction in the upper airways and penetrate into the lower airways but large enough to avoid exhalation 22. Generally, particles in the 1 5 µm in diameter are deposited in the small airways and alveoli with >50% of the 3 µm diameter particles being deposited in the alveolar region. Thus for deep lung deposition in the case of pulmonary drug delivery, aerosols with a particle size range of 1-5 µm are optimal. Particles with a MMAD of > 3 µm have an approximately 80% chance of reaching the lower airways with 50 60% being deposited in the alveoli Since careful tailoring of particles is required for achieving desires lung deposition behavior, certain guidelines and testing methods have been formed that govern inhalational dosage forms. The aerodynamic diameter of an inhaled particle is of great importance to its deposition behavior and is defined as the diameter of the sphere with a unit density that has the same terminal settling velocity in still air as the particle in consideration 73,77. Most inhaled particles are polydisperse in their aerodynamic size distribution. Thus, in order to estimate the particle size of this polydisperse population, the deposition pattern of aerosolized particles is plotted as a log normal distribution and a cumulative distribution curve. The MMAD is calculated from the cumulative distribution curve at 50%. Another parameter that is calculated is geometric standard deviation (GSD) which is calculated as the square root of the 84 th /16 th percentiles. GSD is a measure of variability of the diameters within the particles of the aerosol. A GSD of 1 is an indication of monodisperse aerosols, whereas a GSD of greater than 1.2 indicates a heterodisperse particle population in aerosols
34 For aerosol testing, the United States Pharmacopoeia (USP) has approved several cascade impactor devices including the Next generation impactor (NGI) 79. For the purpose of aerodynamic testing of the spray dried chitosan microparticles, the NGI was used in this project. The NGI is a cascade impactor having seven stages which are horizontally aligned in a series. It also has a micro-orifice collector (MOC) which is attached after the seven stages. The figure below depicts the various parts of the NGI. Figure 6: Components of an NGI 79 Each stage of the NGI, including the MOC is made up of a removable impaction cup in the bottom frame and these are sprayed on from a nozzle affixed to the seal body. The bottom frame holds the cup, the seal body holds the jets and the lid contains the inter-stage air flow passageways. The NGI has been developed and calibrated for running air flow rates from L/min. The NGI operates at different flow rates with cut sizes spanning a particle size range of μm. The 50% efficiency cut-off diameters of the stages (D50 values) are evenly spaced on a logarithmic scale. For the purpose of this project, the performance of dry powder inhaler (DPI) in the NGI is of importance. Studies have suggested the suitability of the NGI for testing the inhaled dry powders with cut-off diameters of all the stages of the NGI at 60 L/min. 80,81. 16
35 Stages Diameter D50 (μm) at Airflow Rate at 60 L/min Table 2: D50 values for NGI stages at air flow rate of 60 L/min 1.4 Magnetic hyperthermia therapy The aim of this project was to combine inhalational chemotherapy with hyperthermia. The chitosan microparticles that were discussed in the previous section were also loaded with magnetic hyperthermia agents to achieve the objective of the project. Hyperthermia induction using magnetic particles was first reported by Gilchrist et al. in the early 1956 s 82. His work was based on selective inductive heating of lymph nodes after injecting magnetic nanoparticles in the surgical region post resection of tumor 83. The nanoparticles reported by Gilchrist lie in the nm sized range. Further studies were done on combining magnetic particles with radiofrequency heating method. Radiofrequency (RF) refers to electromagnetic waves generated by high frequency 17
36 alternating current (AC). In magnetic hyperthermia, RF induction is used in which alternating current is passed through a coil (RF transmitter) and the magnetic particles are the receivers. Current applications of magnetic hyperthermia involve the use of magnetic nanoparticles in a colloidal suspension. These suspensions are delivered to the tumor site. In order to achieve significant heating in the area, the magnetic particles have to be concentrated at the area of interest. The magnetic particles can be delivered to the target organ via various delivery devices. The next step would be heating of magnetic particles by an application of external AC magnetic field. The phenomenon of tissue heating by applying alternating magnetic fields to magnetic particles is based on conversion of dissipated magnetic energy into thermal energy 84. The magnetic particles used for biomedical application are magnetite or maghemite particles 85. The frequencies used for hyperthermia treatment are chosen such that they pass unaffected through healthy tissues. In order to improve localization of particles, an external magnet field may also be used to concentrate the particles at the desired site 15. It has been noted that hyperthermia modalities, in spite of showing significant cell death, are not enough to replace the current treatment options alone. But it is suitable in combination with chemotherapy and radiotherapy, thus improving the efficacy of the currently available treatments for tumors Properties of magnetic particles: The use of magnetic particles for hyperthermia induction allows the procedure to be localized, target-specific and penetrable to the deep tissues 84. The heating efficiency of particles and their superparamagnetism depends on the size of the particles 87. Mathematical modeling studies have determined that magnetite particles are single domain below 80 nm diameter and are single domain and superparamagnetic below 25 nm diameter
37 Figure 7: Magnetic regimes of magnetite particles as a function of their size (superparamagnetic, single domain, multidomain) The features of magnetic iron oxide particles play a significant role in their properties and performance. The iron oxide particles formed with magnetite display ferrimagnetic at micrometer sizes and above 89. However, at sufficiently small superparamagnetic levels, the magnetite particles (Fe3O4) are present as single domain particles. The magnetic spin of a magnetic is the property of magnetic domains to align themselves in the direction of the magnetic field lines under an applied magnetic field. When a magnetic field is applied across these single domain particles, they are uniformly magnetized with all spins aligned in the same direction 85. In biological applications, these particles are commonly applied as ferrofluids. After being localized at a target site a high frequency alternating magnetic field is applied 90. This leads to heat generation in the magnetic nanoparticles by the mechanisms of Néel relaxation and Brownian relaxation, the most dominant mechanisms by which heat generation is proposed to occur in the superparamagnetic particles 91. The phenomenon of Néel relaxation occurs due to the constant flip of the magnetic moments inside 19
38 the particles. The applied magnetic field also causes rotation of the particles under its influence which leads to heating by Brownian relaxation. On removal of the external field, superparamagnetic particles exhibit no residual magnetism, which is a desired property for their biological application (low risk of embolism and particle aggregation in vivo) 92. The magnetic properties of magnetic particles currently used in research, are generally estimated by plotting M-H curves. The M-H plot yields the hysteresis loop of magnetization. The important features of a hysteresis loop are coercivity (Hc), remnant magnetization (Mr) and saturation magnetization (Ms) 93. Typical superparamagnetic behavior involves existence of particles as individual domains. The magnetic moments of superparamagnetic particles compensate for each other and result in null overall magnetic moment. However on application of an external magnetic field, the particles align themselves to the direction of the field. a b Figure 8: M-H curves for (a) ferromagnetic material, (b) superparamagnetic material. M is the magnetization of the material and H is the external magnetic field. 20
39 As seen in the representative M-H loops above, superparamagnetic particles exhibit negligible coercivity and remnant magnetization. The biomedical application of magnetic nanoparticles, thus combines detailed understanding of the physics of magnetism along with its biological compatibility. 21
40 1.5 Hypothesis and specific aims The objective of the present study was to develop and characterize a multifunctional microparticle system loaded with gemcitabine and magnetic iron oxide nanoparticles intended for inhalational therapy of lung cancer cells. The underlying hypothesis of this project was: A multifunctional inhalational approach combining the application of chemotherapy and hyperthermia will provide an enhanced treatment option for lung cancer The specific aims for this investigation were: 1. To synthesize nanosized iron oxide particles 2. To prepare and characterize gemcitabine and iron oxide loaded chitosan microparticles 3. To study the in vitro application and characterization of these multifunctional microparticles 22
41 CHAPTER 2 Synthesis of nanosized iron oxide particles 23
42 2.1. Introduction Iron oxide nanoparticles can be synthesized by the classic chemical coprecipitation reaction 94. It is the most conventional method for obtaining iron oxide particles. The method involves using ferric and ferrous ions in the ratio of 1:2. These ions are incorporated into solution which are highly basic, with or without elevated temperatures and schematically represented in figure 9. The factors affecting resulting particles are types of salts used, reaction temperature, ionic ratio, ph of the system, and other parameters such as stirring rate, rate of base addition 95. Based on these factors, various methods were tried out in our preliminary studies for synthesizing iron oxide nanoparticles which have particle sizes of superparamagnetic size and are preferentially composed of magnetite. 1. FeCl 3 + H 2 O Fe(OH) 3 2. FeCl 2 + H 2 O Fe(OH) 2 Final step for formation of Fe3O4 non oxidizing environment (ph 9 ) 2Fe(OH) 3 + Fe(OH) 2 Base Fe 3 O H 2 0 Figure 9: Scheme showing the reaction mechanism of magnetite particle formation from an aqueous mixture of ferrous and ferric chloride by addition of a base A different view to the formation of iron oxide particles has also been tried out in this project. The particles formed by coprecipitation method exist under hydrothermal conditions after formation. These conditions provide a favorable environment for crystal growth. It is observed that iron oxide nanoparticles with the size of a few nanometers can coalesce under these hydrothermal conditions 24
43 resulting in larger crystals. These finally formed bulk crystals might not be suitable for biomedical applications due to their large size 95,96. Other methods involving precipitation in highly constrained domains are being looked at for achieving smaller particles that do not have favorable conditions that aid crystal growth. Suggested methods involve use of a microemulsion which can provide a microdroplet sized domain for reactions to occur. These microdroplets are usually aqueous cores, suspended in a non-aqueous dispersant using surfactants 97. Further, studies for designing iron oxide nanoparticles have concentrated on the aspect of forming water dispersible nanoparticles for easy of application in biological systems. Also, water dispersibility is also desirable for pharmaceutical processing of the particles, such as drug incorporation and resuspension for delivery. Use of surfactants for suspending the iron oxide nanoparticles is being examined Oleic acid (OA) which is commonly used non-ionic surfactant can be adsorbed on the surface if iron oxide for steric stabilization and also for protective coating. The hydrophilic-lipophilic balance (HLB) value of oleic acid is 1, resulting in poor water dispersible properties. To improve the water dispersibility, poloxamer can added as a second layer which enhance the dispersion stability of iron oxide particles Materials Ammonium Hydroxide was purchased from Ricca Chemical Company. Iron (III) chloride (FeCl3), Iron (II) chloride (FeCl2), Oleic acid were purchased from Acros Organics, Thermo Fisher Scientific. Poloxamer F127 was also obtained from Sigma Aldrich. Sodium Hydroxide pellets were purchased from Mallinckrodt Inc. Ethanol Anhydrous and n-hexane, 95% (Optima ) were obtained from Fisher Chemicals. 25
44 2.3 Methods Synthesis of Iron Oxide Nanoparticles: Method 1 Iron oxide nanoparticles were prepared by simple chemical coprecipitation method. The procedure was directly designed on the basis of the reactions taking place during the formation of iron oxide nanoparticles. A solution containing 8.6g of FeCl3.6H2O and 3.14g of FeCl2.4H2O was made using 25mL of filtered, (0.45micron) nitrogen purged water (resistance 17.8MΩ). The water oxygen content was reduced by nitrogen gas bubbling for 2 hours. This resulting solution was added dropwise to 200 ml of 2M sodium hydroxide solution under vigorous stirring using a mechanical stirrer. This resulted in formation of a black precipitate. The precipitate was isolated under the influence of a magnet and decanted. The precipitate was washed using nitrogen purged filtered water and isolated using ultracentrifugation at 60,000 g for 35mins. This process was repeated thrice in order to remove any residual base from the precipitated particles. The particles were lyophilized using Labconco Freezone 4.5 freeze dryer for testing particle properties. The samples were pre-frozen to -80ᴼC and lyophilized at mbar vacuum. 26
45 Figure 10: Schematic representation of synthesis of Iron Oxide Nanoparticles: Method Method 2 Nitrogen purged anhydrous ethanol was used to make 0.1M solutions of ferric chloride and ferrous chloride. These solutions were combined in the ratio of 2:1 of ferric and ferrous chlorides respectively. This solution was slowly added to 0.05M solution of dioctyl sulfosuccinate (AOT) in n-hexane and homogenized for 20 minutes. The yielded emulsion was then kept under mechanical stirring with nitrogen purge. The ph was adjusted to ph 9 with addition of nitrogen purged 1M sodium hydroxide solution with vigorous stirring. The particles formed were isolated by ultracentrifugation at 60,000 g for 35mins. 27
46 Figure 11: Schematic representation of synthesis of Iron Oxide Nanoparticles: Method Synthesis of Iron Oxide Nanoparticles: Optimized method The final optimized method used for synthesis was based on the method used by Jain et al 102. A solution containing 1.62g of FeCl3.6H2O and 0.38g of FeCl2.4H2O was made using 90mL of filtered, (0.45micron) nitrogen purged water (resistance 17.8MΩ). In a gas washing bottle attached with nitrogen gas flow, the above solution was added with 500μL of oleic acid. A collapsible stirrer was built and attached to the mechanical overhead stirrer. The solution was stirred under nitrogen gas purge for 10 minutes. 10mL of 5M ammonium hydroxide was injected in to the conical flask (see Figure12). This resulted in saturation of the nitrogen gas which was then slowly purged into the gas washing bottle. This resulted in formation of black precipitate over a period of 2 hours. 28
47 Nitrogen gas Figure 12: Setup for iron oxide synthesis by optimized method This particle suspension was transferred into a beaker and heated to 60ᴼC for 30minutes for removing any residual ammonium hydroxide left in the system. The heating was done on a hot plate (Thermo Scientific series) with an overhead mechanical stirrer for continued agitation. The iron oxide precipitate suspension was then stirrer with 0.38 g of poloxamer 407 for 8hours. The particles formed were isolated by ultracentrifugation at 60,000 g for 35mins. a b Figure 13: (a) Synthesized iron oxide nanoparticles (b) Collapsible stirrer built for synthesis 29
48 2.3.2 Measurement of particle size and zeta potential of nanoparticles Dynamic Light Scattering The particle size (PS) and the zeta potential (ZP) were determined for the all the synthesized trial and optimized batches of nanoparticles using the Brookhaven Zetameter (ZetaPlus, Brookhaven Instruments Corporation, Holtsville, NY). The dilutions were optimized before the actual measurement of PS and ZP. Ten milligrams of nanoparticles were weighed and suspended in 10 ml deionized water followed by sonication using a bath sonicator (Fisher Scientific). This suspension was further diluted 1 to 10. For each sample, five readings were recorded and each reading was taken in triplicate (n=3) Transmission Electron Microscopy & Scanning Electron Microscopy Transmission electron microscopy studies were performed on the aqueous dispersion of the oleic acid-poloxamer stabilized nanoparticles. The microscopy was performed at Electron Microscopy Research Lab, University of Kansas Medical Center, Kansas city, Kansas. Scanning electron microscopy was performed on blank sprayed dried chitosan microparticles at Xavier university of Louisiana. 2.4 Results Results: Batches from method 1 & 2 The above mentioned procedures for synthesis of iron oxide were trial experiments done in order to reach an optimized method of synthesis for iron oxide particles with desired properties. 30
49 Method 1: The synthesis method followed in Trial 1, yielded iron oxide particles which were very large sized. The sizes of these particles measured on the Brookhaven Zetameter (ZetaPlus, Brookhaven Instruments Corporation, Holtsville, NY) were 1150 ± 260 nm (n=3). These particles also had the property of poor aqueous suspendability. Hence the suspensions were made in hexane for PS measurements. As the particle size desired was much smaller, trial 2 was tried for synthesis. Method 2: This trial was employed to achieve small sized nanoparticles, which was the main reason for failure of trial 1. However the particles obtained using this method of synthesis were seen to be in the undesired oxidation state as indicated by the color of the particles. Similar to the particles obtained in trial 1, even these particles had poor aqueous suspendability. Owing to improper oxidation state of the particles, their magnetic properties would have been significantly compromised. These particle size obtained by this method was 655± 520 nm (n=3). The poor particle properties and highly regular particle sizes obtained, led to further designing of synthesis methods to overcome the disadvantages of this method Results: Optimized batch Measurement of particle size and zeta potential of nanoparticles The particle size measurements were performed on nanoparticle suspensions. The data represents an average of three samples. Parameters Particle size Values ± nm Polydispersity index ± Zeta Potential ±1.77 Table 3: Particle size, polydispersity index, zeta potential values for nanoparticles. [n=3] 31
50 Transmission Electron Microscopy Figure 14: Iron oxide nanoparticle TEM 2.5 Discussion Synthesis by Method 1 &2 Various trials were done to primarily achieve desired particle properties. The first method that followed crude coprecipitation method, yielded iron oxide particles which had a large size range of 1150 ± 260 nm and had poor water dispersibility. The use of organic solvents for synthesis was done in method 2 for creating small sized control domains to disallow crystal growth and create small sized nanoparticles. However the particles obtained from this method were highly polydisperse with a particle size of 655± 520 nm. These particles also showed poor water suspendability and quick change of oxidation state which was seen by change in color. 32
51 2.5.2 Optimized method for nanoparticle synthesis Iron oxide nanoparticles were synthesized by chemical coprecipitation method adapted from the method described by Jain et al. The particles synthesized by this method were stabilized using oleic acid and poloxamer 407. The particles obtained were dispersible in water. The particle size obtained by dynamic light scattering was ± nm with a polydispersity index of ± The particles were found to carry a negative surface charge of ±1.77 mv. The TEM images obtained for these particles indicated that the particle size was about ± 2.15 nm. The larger particle size readings obtained using dynamic light scattering may be due to oleic acid and poloxamer layer coating the nanoparticles Conclusions The method of synthesis of iron oxide nanoparticles was optimized. As previously mentioned, the heating efficiency of particles and their superparamagnetism depends on the size of the particles, 87 and it has been determined that magnetite particles are single domain below 80 nm diameter and are single domain and superparamagnetic below 25 nm diameter 88. These synthesized nanoparticles were going to be further subjected to spray drying in an aqueous dispersion system. Thus the parameters such as particle size and aqueous dispersibility were optimized under this specific aim. Iron oxide nanoparticles of low nanosized range were successfully synthesized. These nanoparticles showed desirable particle size distribution. These nanoparticles also have a good aqueous dispersibility. 33
52 CHAPTER 3 Preparation, characterization and in vitro evaluation of gemcitabine and iron oxide loaded chitosan microparticles 34
53 3.1 Introduction The objective of this project was designed with the aim of providing alternative means for targeting lung cancer cells. Dry powder chitosan microparticles were formulated using the spray drying technique as described by Ventura et al 59. The iron oxide particles were suspended in the chitosan solution. All the materials coming in contact with the iron oxide nanoparticles have to be nitrogen purged prior to iron oxide addition. This was done to prevent the oxidation of magnetite to iron (III) oxide which would cause a change in their magnetic properties 103. Chitosan is soluble in solutions of organic acids 56. This property of chitosan is used for preparation of spray drying solutions. The amount of drug and iron oxide being entrapped in the system had to be determined to evaluate the efficiency of the method. A liquid chromatography method was used for gemcitabine detection where as a spectrophotometric method was used for iron oxide loading determination. Both hydrodynamic particle size and aerodynamic particle sizes of the microparticles have to be estimated to understand particle design and aerodynamic behavior. The surface morphology and particle shape of the particles also affects its aerodynamics. To study these characteristics electron microscopy images of the particles can be taken 104. As discussed in the previous section, a superparamagnetic behavior is desired for magnetic hyperthermia treatment. The estimation of magnetic behaviors can be done using magnetometers 105. Radiofrequency induction can be done on a laboratory scale using high frequency RF inductors. These setups can be used to study the effect of RF on prepared particles of iron oxide and chitosan microparticles containing iron oxide. The inductors can also be used for RF heating studies on cells and tissue models 106.The toxicity of a system can be primarily tested on cell lines. The common studies done are MTT cytotoxicity and cellular uptake studies. These are done with treatment of prepared microparticles on the cell lines 35
54 and the results are compared to drug uptake and cytotoxicity yielded with drug solutions of equivalent concentrations. 3.2 Materials Gemcitabine hydrochloride was obtained from LC laboratories, US. Medium Molecular weight Chitosan was obtained from Sigma Aldrich Milwaukee, WI. Glacial Acetic Acid were purchased from Fischer Scientific Fair Lawn, NJ. Hydroxide pellets were purchased from Mallinckrodt Inc. monobasic potassium phosphate was obtained from Spectrum Chemical Mfg. Corp. Optima Grade Methanol and Water were purchased from Fischer Scientific Fair Lawn, NJ. O-phosphoric was also acquired from Fischer Scientific, WI-26 A4, A549 cells were obtained from American Type Culture Collection, Manassas, VA. Modified Eagle Medium (MEM), Ham s F-12 medium, Dulbecco s Phosphate Buffer Saline (DPBS), Trypsin, glutamic acid, non-essential amino acid, penicillin streptomycin and sodium pyruvate were purchased from Cellgro Mediatech Inc, Corning, NY. MTT reagent (3-(4, 5- Dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide), Sodium Dodecyl Sulfate, Sodium bicarbonate, Magnesium Chloride and Dimethylformamide (DMF) were purchased commercially from Sigma Aldrich, Milwaukee. Fetal Bovine Serum (FBS) albumin was acquired from Atlanta Biologicals, GA. 3.3 Methods Preparation of gemcitabine and iron oxide loaded chitosan microparticles A 1 % w/v solution of chitosan was prepared by dissolving 2.5 g of medium molecular weight chitosan in 250mL of 0.6% v/v acetic acid solution. 1.25g of Gemcitabine HCl was solubilized in 10 ml of deionized water. This gemcitabine solution was added to the chitosan solution with magnetic stirring. This solution was adjusted to ph 5.5 using 0.1M 36
55 sodium hydroxide solution. The solution was filtered using 0.45micron filter to remove any undissolved chitosan and transferred to a 500 ml conical flask. This chitosan solution was kept under nitrogen purge for 2 hours to reduce the amount of dissolved oxygen in the solution. The oleic acid coated-poloxamer stabilized-iron oxide nanoparticles suspended in nitrogen purged deionized water are added to chitosan-gemcitabine solution in the 500mL conical flask. This flask was subject to ultra-sonication using a bath sonicator (Fisher Scientific) to create and maintain a homogenous suspension throughout the spray drying process. Figure 15: Setup for spray drying of gemcitabine-iron oxide loaded chitosan particles In order to maintain the homogeneity of the suspension, a bath sonicator was installed near the spray dryer for sonicating the feed suspension. This feed suspension was also kept under nitrogen purge to maintain a low oxygen content in it (prevention of oxidation of the 37
56 suspended iron oxide nanoparticles). This suspension was spray dried using a laboratoryscale mini spray dryer B-290 spray dryer (BÜCHI Labortechnik AG, Flawil, Switzerland). The spray drying parameters are summarized in Table 3. A magnetic trap was attached to prevent any magnetic particles from entering the outlet filter avoiding the risk of aspirator clogging. Spray Drying parameters Inlet temperature Outlet temperature Feed rate Aspiration rate Values C C 2.5 ml/min 37 m 3 /hour Table 4: Spray Drying Parameters The spray dried particles obtained were collected and stored in a sealed container at room temperature for additional analysis Chromatography The Ultra Performance Liquid Chromatography (UPLC) method used for the quantitative estimation of gemcitabine was adapted from a method reported by Malleswararao et al. for UPLC detection of gemcitabine and its components 107. The method was modified with respect to run time and flow rate. The analyses of the samples were performed using Waters Acquity system (Milford, MA). The Waters Acquity HSS T3 (100 mm x 2.1 mm, 1.8 μm) was used for the analysis of gemcitabine. The samples were eluted isocratically. The mobile phase consisted of methanol and M potassium phosphate buffer (ph 2.5) in the ratio 10:90 v/v. The system had a flow rate of 0.4 ml/min. The runtime set for the 38
57 method was 4 minutes. The effluents were monitored at the detector wavelength of 254 nm (λmax of gemcitabine). The injection volume was varied from 10 to 50 µl depending on the requirement of the sample for which the method was applied Preparation of Solutions The aqueous phase consisting of 0.02 M potassium phosphate in water was prepared separately. For the preparation of aqueous phase, g of potassium phosphate monobasic was dissolved in 1000 ml deionized water. The ph of the resulting solution was adjusted to 2.5 using o-phosphoric acid. This solution was filtered through 0.22 µm Nitrocellulose membrane filter (Millipore, Billerica, MA). This solution was mixed with methanol in the ratio of 90:10 respectively and was then degassed for 5 minutes in a bath sonicator. The mobile phase thus prepared was used for the gemcitabine detection method on the UPLC system Standard solutions Standard solutions of gemcitabine were prepared for the purposes of running standard curves prior to every experiment. A 100 μg /ml solution of drug was prepared in 0.1M sodium phosphate buffer ph 7.5. This was achieved by dissolving 10 mg of drug in 100 ml buffer in a volumetric flask. From this stock solution, 5 standards were prepared using 1:2 serial dilutions having concentrations of μg/ml Calculations The standard solutions were injected into the UPLC system. Standard curve was obtained by plotting the peak heights of the drug peak with the corresponding concentrations. The unknown concentrations of drug solutions were determined by interpolating from the equation of the standard curve. 39
58 3.3.3 Measurement of particle size and zeta potential of spray dried particles The particle size of spray dried particles was determined using Malvern Mastersizer Hydro 2000S (Malvern, UK) which works on the principle of laser diffraction. Chitosan is sparingly soluble in water. Sufficient amount of microparticles were dispersed in filtered deionized water (0.45µm filter). The stirring rate was set at 500 rpm with a 50 % sonication for 10 minutes to obtain obscuration between 5-10 %. Three sets of measurements were repeated for three times for each sample. The D (0.5), D (0.1), D (0.9) and D [4, 3] were determined for all samples. The D (0.5) is the median volume diameter at which half of the sample population is smaller and rest of the half of sample is larger than that value. The D (0.9) is the median volume diameter at which 90% of sample is smaller and 10 % of sample is larger. The D (0.1) is the median volume diameter at which 10% of sample is smaller and 90 % of sample is larger. D (0.1) and D (0.9) are mainly used to determine the range of particle size distribution. The D [4, 3] is the volume mean diameter which is the average diameter based on unit volume of particle Electron microscopy Scanning Electron Microscopy was performed on the blank spray dried chitosan microparticles at Xavier University of Louisiana, New Orleans, Louisiana. Transmission Electron Microscopy (TEM)_studies were performed on a dispersion of the chitosan microparticles containing iron oxide and gemcitabine. The dispersion was made in methanol. The microscopy was performed at Electron Microscopy Research Lab, University of Kansas Medical Center, Kansas city, Kansas. 40
59 3.3.5 Determination of drug entrapment efficiency Drug entrapment efficiency of microparticles was determined by dissolving 10 mg of sample in 10ml of 0.1M hydrochloric acid solution. The obtained solution was filtered using 0.2 micron syringe filters. The content of gemcitabine in these solutions was determined using an UPLC method. The description of the UPLC method is provided in section Entrapment efficiency was calculated using Equation 1: Drug entrapment efficiency (% w/w) = Amount entrapped in the formulation Amt. initially added to the formulation Determination of Iron Loading The estimation of the amount of iron loaded into chitosan particles was done by colorimetric assay 108. In this analysis the iron present in the microparticles is extracted to form a solution containing Fe3+ (ferric) ions. These ions are complexed with thiocyanate ions to form blood-red colored complex which be analyzed by UV-visible spectrophotometry at the wavelength 480 nm. Iron loading was determined by dissolving 10 mg of sample in 10ml of 0.1M hydrochloric acid solution. The obtained solution was mixed with 0.1M ammonium thiocyanate in the ratio 1: 1 v/v and allowed to undergo complexation for 2 minutes. The samples were analyzed within 15 minutes to thiocyanate addition. A standard curve of Fe (III) ions was prepared over a range of 50 µm to 800 µm by 1:2 serial dilutions of a 1 mm solution of Iron (III) chloride in 0.1 M hydrochloric acid solution. The standard solutions were also mixed with 0.1 M ammonium thiocyanate in the ratio 1: 1 v/v and allowed to undergo complexation for 2 minutes. The samples were analyzed within 15 minutes to thiocyanate addition and plotted based on molar concentrations of the samples. Iron loading was calculated using Equation 2: 41
60 Iron Loading (%w/w) = Amount of iron oxide entrapped in the formulation Total weight of formulation Thermogravimetric Analysis (TGA) The weight loss on heating was calculated by the method of thermogravimetric analysis using thermogravimetric analyzer (Shimadzu DSC-60, Kyoto, Japan). Approximately 5mg sample was heated from room temperature to 300⁰C at a rate of 10⁰C / minute in a nitrogen environment. The weight loss was analyzed from room temperature to 120⁰C (n=3) Differential Scanning Calorimetry (DSC) To determine the physical state of the drug (gemcitabine) in chitosan particle matrix, the technique of differential scanning calorimetry was used. Pure drug, blank and drug and iron oxide loaded, and only iron oxide loaded chitosan particles were analyzed using the Differential Scanning Calorimeter (Shimadzu, DSC 60, Kyoto, Japan). About 5mg of each sample was weighed into an aluminum pan and crimped. This was the sample pan. A separate pan consisting of air crimped in an aluminum pan was used as the reference pan (n=3). The sample pan was analyzed versus the reference pan from room temperature to 400ºC at a rate of 10ºC using nitrogen purge (flow rate of 20 ml/minute) Aerodynamic studies The aerosolization of the spray dried chitosan particles (dry powder) was carried out using the NGI at an airflow rate of 60 L/min. The NGI was assembled and operated as described by the USP 79 at an airflow rate of 60 L/min. The effective cut off diameters (D50 values) for stages 1-7 of the NGI at flow rate of 60 L/min are reported as 8.06, 4.46, 2.82, 1.66, 0.94, 0.55 and 0.34 μm respectively. A custom made mouthpiece adapter (by Gadgil P.) using Sylgard 184 silicone elastomer kit (Dow Corning, MI, USA) was used to provide an airtight seal between the inhaler and the induction port 109. The mouthpiece adapter is 42
61 attached to the induction port to align the inhaler to the horizontal axis of the induction port. The chitosan is a highly charged cationic polymer and is seen to interact cohesively with gelatin. Thus the dry powder testing could not be done by filling the particles in a capsule as dictated by the protocol for using the Aerolizer. The Aerolizer DPI device was directly filled with 250mg of spray dried chitosan particles containing iron oxide and gemcitabine. The weight of all NGI plates along with the MOC were recorded. After the impactor was assembled, the drug filled capsule was placed in the Aerolizer DPI device attached to the NGI by way of the custom adapter. The NGI was operated for 60 seconds. The plates with particle were weighed to determine the deposition on each stage. The particles deposited in the head, neck, throat and the orifices were scraped off using a plastic spatula and were weighed to determine deposited amount. The aerodynamic particle size distribution was determined by calculating % FPF, MMAD and GSD. The % FPF 5μm was calculated by sum of percent particle deposition from stage 2 to MOC. Thus % FPF is the percentage of particles which have size of 5μm. The MMAD and GSD were determined from the plot of the cumulative percentage of deposited mass in the stages versus the particle size as determined by the stated aerodynamic diameter (D50) (Refer Table 1). The MMAD is the particle size at 50% of the deposited cumulative mass so that 50% of the deposited mass is composed of larger particles and 50 % of the deposited mass has smaller particles. Thus it is the particle size at 50 th percentile in the above mentioned plot. The GSD is the measure of the breadth of the log-normal distribution and gives an indication if the aerosol particle size is monodisperse or polydisperse. GSD is derived by the square root of the particle size at the 84th percentile to the particle size at 16th percentile. 43
62 Magnetization Studies Magnetic measurements were carried out using a Quantum Design MPMS SQUID magnetometer. Zero-field-cooled (ZFC) and field-cooled (FC) magnetization measurements as functions of temperature were performed. The magnetic field applied was measure in Oersted (Oe) and the temperatures were measured in kelvin (K). These studies were done on iron oxide nanoparticles (freeze dried after synthesis) and chitosan particles containing gemcitabine and iron oxide. For the ZFC measurement, each sample was cooled from 300K to 10 K in zero field and the magnetization was measured as a function of temperature while applying a field of 100 Oe as the sample was warmed. For the FC measurement, the sample was cooled in the measuring field and the magnetization was measured as the sample was cooled. Magnetization measurements as a function of field which is the M-H loop, were performed at 300 K Radiofrequency Heating The iron nanoparticles as well as the microparticles were subjected to radiofrequency heating using RDO Model HFI Induction Heating System. A typical setup for an induction heater is shown in Figure 16. The setup consists of a high-frequency supply which has an input of alternating current (AC). The alternating current is converted to high-frequency signal which is sent into the induction heating coil. The high frequency signal in the induction heating coil generates a high frequency magnetic field in the coil. The sample to be testes in placed in the center of this induction coil. The sample can thus interact with the high frequency magnetic field to produce RF heating. 44
63 Figure 16: A typical RF induction heating setup 110 Suspensions of iron oxide nanoparticles (freeze dried after synthesis) were made in water (Optima Grade) at the concentration of 5 mg/ ml. Also, the amount of chitosan particles containing iron oxide particles equivalent to 5 mg/ml was used for making a suspension in water (Optima Grade). The suspensions were made by incorporation of particles into water followed by sonication using bath sonicator (Fisher Scientific). The dispersing phase which is pure optima grade water, was used as a control in these experiments. A volume of 2 ml of the two prepared sample suspensions and water (as control) were subjected to radiofrequency heating. The temperature of all the sample tubes was monitored as a function of RF exposure time using a FOT Fluoroptic Lab Kit (Luxtron Corp, Santa Clara, CA) which is a fiber optic thermocouple and is immune to radiofrequency interference. The experiment was run at 222 khz for 500 seconds. The high frequency current flowing through the induction coil causes the induction coil to heat up. This heat in the induction coil may cause heating of the sample due to radiant heat. To avoid this, the induction heating coil is circulated with cooled water (). However it was 45
64 observed that the heat generated during experiments was high enough to also heat up the water that spirals inside the coil. Additional measures were taken to prevent the coil from heating the sample via radiant heat. A setup was designed such that the sample was suspended in the center of the coil, without any contact with any part of the coil. The materials that were present in the area surrounding the sample in the coil were strictly glass. Also, compressed air was blown through the coil at 1.4 L/min at 28 psi to dissipate any excessive heat from the induction coil without causing cooling of the sample. Figure 17: Radiofrequency Induction Machine with a. Chiller, b. Compressed Air supply, c. Air jacketing system In vitro release study The in vitro release study was done on the spray dried particles in phosphate buffer solution (PBS, ph 7.4) at 37±0.5 C. Dialysis tubing (Biotech CE DialyBiotech CE Dialysis Tubing, kd MWCO, 16 mm Flat-width) was used as a sample holder. The dialysis tubing was cut into 5 cm pieces and wetted in PBS for 20 minutes. An amount of 10 mg particles was 46
65 introduced into the dialysis bag. The dialysis bag was sealed from both ends using custom made dialysis bag clippers. The sealed dialysis bag was introduced in 10 ml PBS (ph 7.4) in a 20mL glass scintillation vial. Figure 18: Set up for release studies Chitosan particles containing only gemcitabine and chitosan particles containing both gemcitabine and iron oxide were prepared for the study. The release assemblies were placed into an incubated orbital shaker for a total of 72 hours at a speed of 100 rpm and kept at a temperature of 37 C. Samples, about 10% (1 ml) of the total volume of dissolution medium, were removed at various time points using a needle and syringe and analyzed using the UPLC analysis method previously stated. Time points for sampling were taken in hours (0, 1, 2, 4, 8, 12, 18, 24, 48, 72, 96, and 120). Sodium phosphate buffer ph 7.4 (1 ml) was used to replace the volume that was extracted. Figure 20 shows the setup for the release study in the scintillation vial. The collected samples were analyzed on 47
66 the UPLC using the method described previously. The amount of iron released was also determined using the colorimetric method described in section Determination of cellular uptake Cellular uptake of gemcitabine from the spray dried particles was studied with respect to drug solution at equivalent concentrations for both the drugs in Wi-26 A4 and A549 cell lines. These cells were cultured in standard Thermo Scientific Nunc 6-well tissue culture plates. The cells were plated and incubated at 37⁰C in a humidified chamber until they were confluent. Solutions of 100 µm gemcitabine were prepared in Minimum essential medium (MEM) for Wi-26 A4 cells and Ham s F-12 media for A549 cells. The confluent cells monolayer was treated with 2 ml of the prepared drug solution of gemcitabine in the medium. Spray dried chitosan particles were used with equivalent drug content for the treatment. The treatment times were set at 0.5, 1, 2, 3 and 6 hours after which the treated cells were washed with ice-cold phosphate buffer saline to wash out the residual drug any non-adherent particles. The plates were then lysed using anhydrous ethanol followed by mechanical scraping and the cell lysates were collected in micro-centrifuge tubes. Homogenized lysate (20μL) was analyzed for the total cellular protein content using the BCA protein assay (Pierce, Rockford, IL). The lysate was then centrifuged 17,000g for 4 minutes at 20 C (accuspin Micro R, Fisher Scientific, Fairlawn, NJ). The supernatant was analyzed by UPLC analysis for gemcitabine content. The method used for analysis has been described in Section The cellular uptake was reported as the mean ± standard deviation of gemcitabine (in µg) content per mg of total cellular protein (n=3). 48
67 MTT toxicity assay The cytotoxicity of gemcitabine drug solutions was compared with that of the spray dried chitosan particles containing gemcitabine and iron oxide using MTT assay. The cell lines used for the assay were A549 and WI-26 A4. Modified Eagle Medium (MEM) was used for growing WI-26 A4 cells. Ham s F-12 medium was used for growing A549 cells. The media were supplemented with 20% FBS, 10% L-glutamine, 10% sodium pyruvate, 10% nonessential amino acids and 10% penicillin streptomycin. Twenty-four hours prior to treatment cells were split in 100μL media/well into 96 well culture plates. After plating, the cells were incubated overnight in a humidified chamber at 37⁰C with 5% regulated CO2. Twenty-four hours after the original plating of cells, treatments consisting of gemcitabine solutions, vehicle control and chitosan particles with equivalent amount of drug were applied on top of existing media in equal volume to the media in the wells (100 μl:100 μl) and allowed to incubate for 4 hours. After 4 hours the media and treatment were removed, 100 μl fresh media was added to each well, and plates were returned to incubation. After incubation period of 24, 48 and 72 hours, cells were treated with 30 μl of a solution of MTT reagent (5mg/mL) in sterile filtered phosphate buffer ph 7.4 and incubated for additional 4 hours. The cells were removed from the incubator after 4 hours; treatment was removed and replaced with 100 μl of a solution of 20% (w/v) SDS solution: Dimethylformamide in 1:1 ratio to solubilize the formazan crystals formed. The plates were incubated for 30 minutes followed by placing in the incubated-shaker for 30 minutes and the absorbance was analyzed on a Thermo Multiskan MCC Unit microplate reader at 540 nm. 49
68 Cytotoxicity assay on RF treated cells The percentage cell death of the cell lines Wi-26 A4 and A549, post radiofrequency induction in presence of iron oxide nanoparticles was estimated by MTT toxicity assay. Since the cells had to be subjected to radiofrequency induction by placing them in induction coil, the MTT toxicity assay had to be modified to suit this experiment. The cell lines used for this study were grown to confluence in a Falcon Tissue Culture Treated Flask (50 ml, canted neck, 0.2 µm vented, 25 cm 2 ) in sets of three for each cell line. The cell lines used for the assay were A549 and WI-26 A4. Modified Eagle Medium (MEM) was used for growing WI-26 A4 cells. Ham s F-12 medium was used for growing A549 cells. The media were supplemented with 20% FBS, 10% L-glutamine, 10% sodium pyruvate, 10% nonessential amino acids and 10% penicillin streptomycin. On the day of the experiment, the confluent cell flasks were trypsinized in the ratio of 1 to 3 and 1 ml of the cell suspension thus formed was transferred into autoclaved glass tubes (Fisherbrand Disposable Borosilicate Glass Tubes 12 mmx75 mm) and were capped using Fisherbrand VersaClosure Tube Closures. Solutions of chitosan particles containing 100 µm gemcitabine were prepared in Minimum essential medium (MEM) for Wi-26 A4 cells and Ham s F-12 media for A549 cells. Cells from each plate were split into three tubes and given 3 different treatments. The three different treatments applied to the cell suspensions were: treatment 1- blank media with no RF treatment; treatment 2- blank media with RF treatment; treatment 3- chitosan particles containing 100 µm gemcitabine in suspended in media and treated on RF. A volume of 1 ml of the above treatments were applied to each tube. The tubes with treatment 2 and 3 were subjected to radiofrequency heating in sealed tubes to maintain sterile conditions for cells. The tubes with treatment 1 50
69 were kept at room temperature in sealed tubes for 500 seconds to simulate blank conditions of the cells exposed to RF heating. The experiment was run at 222 khz for 500 seconds. Post- treatment the cell suspensions were collected in microcentrifuge tubes and centrifuged 17,000g for 4 minutes at 20 C (accuspin Micro R, Fisher Scientific, Fairlawn, NJ). The supernatant was discarded and the cell pellets were resuspended and treated with 200 μl of a solution of MTT reagent (5mg/mL) in sterile filtered phosphate buffer ph 7.4 and incubated for additional 4 hours in an incubator-shaker. The microcentrifuge tubes were again centrifuged to remove the MTT reagent. 1 ml of a solution of 20% (w/v) SDS solution: Dimethylformamide in 1:1 ratio was added to the tubes. The tubes were placed in the incubator-shaker for 60 minutes at 100 rpm. This was cause the formazan crystals to dissolve. The quantification of the dissolved formazan crystals was done by transferring 100μL solution/well from the microcentrifuge tubes into 96 well culture plates and the absorbance was analyzed on a microplate reader at 540 nm Statistical data analysis The experimental data was statistically analyzed for the purpose of comparison using a student s t-test. The differences were termed statistically significant at P< Results Testing of Chromatographic method Specificity According to USP37-NF32, specificity is the ability of analytical method to access the analyte in presence of other interfering components such as excipients, impurities and degradation products 111. The International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) documents define specificity as the ability 51
70 to assess unequivocally the analyte in the presence of components that may be expected to be present, such as impurities, degradation products, and matrix components 112. The reported UPLC method that was used for gemcitabine detection was significantly modified. Thus the method was tested for specificity by comparing the chromatograms obtained by two different injections. Figure 19 A) shows a representative chromatogram of the solvent without any drug (blank). Figure 19 B) shows the representative chromatogram of the solvent with gemcitabine. Figure 19: Representative chromatograms for A) Blank B) Peak for gemcitabine Linearity 52
71 Peak Height As per the USP, linearity is the ability of an analytical procedure to elicit test results that are directly proportional to the concentration of the analyte within the given range. The ICH defines linearity as the ability (within a given range) of an analytical procedure to obtain test results which are directly proportional to the concentration (amount) of analyte in the sample. Thus, for a given UPLC method, the linearity can be referred to as the peak area of peak height obtained for drug concentrations injected into the system, within a range of concentrations. Linearity was tested by injecting standard solutions of drugs and plotting calibration curves of peak height against the concentration of standards. The linearity of this curve was calculated within a specific concentration range, using the slope, y-intercept and coefficient of determination (r 2 ). The standard curves were found to be linear for a concentration range of µg/ ml. The equation of the line was obtained which gave a relationship between the concentration of analyte (x) and the peak height (y). The linear regression equation obtained was y= x , r² = 0.99 for gemcitabine. As the coefficient of determination, r² 1, there is a strong relation between concentrations of drug solutions and the peak height over a given concentration range. Figure 20 shows the standard curve for gemcitabine y = x R² = Figure 20: Standard curve for gemcitabine 53
72 Precision The USP defines precision of an analytical method as the degree of uniformity between individual test results based on multiple samplings of a homogenous sample. The current UPLC method was tested for precision using within day and day to day precision. For within day precision, a set of five standard solutions of the drug were prepared and injected four times in one day. Day to day precision was performed by injecting a set of five standard solutions of the drug, four times over a period of two months. The degree of precision was determined based on calculating the relative percent standard deviation (% RSD) for within day as well as day to day precision. Concentration Within day Day to day (µg/ml) Mean peak height %RSD Mean peak height %RSD ± ± ± ± ± ± Table 5: Within day and day to day precision of the UPLC analysis of gemcitabine Accuracy The USP defines accuracy of analytical as the closeness of test results obtained by that procedure to the true value. The accuracy of an analytical procedure should be established across its range. 54
73 The accuracy of the given UPLC method was determined by comparing the theoretical concentration to the measured concentration. Equation 3: % Accuracy= Measured concentration Theoritical concentration x 100 Standard samples Gemcitabine Theoretical concentration (µg/ml) Measured concentration (µg/ml) % Accuracy ± ± ± Table 6: Accuracy results for UPLC analysis of gemcitabine Particle Size and Zeta Potential of Spray Dried Particles Table 6 summarizes the particle sizes measured by the Malvern Mastersizer including the D (0.5), D (0.1), D (0.9) of the spray dried chitosan particles containing iron oxide and gemcitabine. The D(0.1) and D (0.9) values are a representation of the range of the particle sizes in the sample. The D (0.5) is the median diameter or the medium value of the particle size distribution, it is the value of the particle diameter at 50% in the cumulative distribution. Figure 21 displays the particle size distribution for the chitosan particles measured on the Malvern Mastersizer. The zeta potential of the chitosan particles exhibited highly positively charged reading. The average zeta potential is represented in Table 7 55
74 Parameters Values Particle size d(0.5) 1.59 ± 0.35 µm Particle size d(0.1) 0.838± 0.05 µm Particle size d(0.9) ± 42.5 µm Zeta potential ± 0.18 mv Table 7: Particle size and Zeta potential of chitosan particles Volume (%) Statistics Graph (3 measurements) Mean with max-min error bar Particle Size (µm) Figure 21: Particle size distribution of chitosan microparticles Transmission electron microscopy & Scanning electron microscopy Representative images from the electron microscopy of blank and iron oxide-gemcitabine loaded chitosan microparticles have been represented in Figure 22 and
75 Figure 22: TEM of spray dried gemcitabine-iron oxide loaded chitosan microparticles Figure 23: SEM of blank spray dried chitosan microparticles Drug entrapment efficiency The drug entrapment efficiency of the spray dried chitosan microparticles was tested. All batches of the spray dried particles showed marked ability to encapsulate gemcitabine. The entrapment efficiency of the particles was found to be ± 5.7% w/w. 57
76 3.4.5 Iron Loading The amount of iron loaded into the chitosan particles was calculated to be ± 3.4% w/w. The percentage of iron loading in particles remained consistent from batch to batch Thermogravimetric Analysis The TGA thermograms of spray dried particles are shown in Figure 24. Figure 24: TGA of GEM-Chitosan particles with and w/o Iron oxide Differential Scanning Calorimetry (DSC) An overlay of gemcitabine drug, blank chitosan particles, chitosan microparticles containing gemcitabine and iron oxide, and chitosan microparticles containing only iron oxide in shown in Figure
77 Figure 25: DSC thermograms for drug and various chitosan microparticles Aerodynamic studies The spray dried chitosan microparticles containing iron oxide and gemcitabine were run on the NGI by direct filling into the Aerolizer device to estimate the aerodynamic particle size distribution of the spray dried powder. The deposition of the aerosolized chitosan microparticles were analyzed by mass. Figure 26 represents percentage cumulative particle deposition plotted against the calculated D50 values for each stage. Table 7 summarizes the aerodynamic parameters of MMAD, %FPF and GSD for aerosolized particles. The MMAD of the particles was slightly higher than the desired 1-5 µm range for deep lung deposition. Also, the powders polydisperse aerosols which was evident from the GSD value which was greater than 2. 59
78 Parameters Values Mass median aerodynamic diameter (MMAD) µm Geometric Standard Deviation (GSD) µm Fine Particle Fraction (FPF) (%) 47.5 ± % Table 8: Aerodynamic Parameters 120.0% 100.0% % Cumulative deposition vs. particle size y = x x R² = % 60.0% 40.0% 20.0% 0.0% -20.0% Figure 26: Aerosol dispersion performance as % cumulative deposition vs. particle size on the Next generation Impactor TM for spray dried chitosan iron oxide system (n=3, Ave ± SD) Magnetization Studies The results of the field dependent magnetization studies are depicted in Figure 27 (a) & (b). Figure 27 (a) depicts the M-H curves for iron oxide nanoparticles and figure 24 (b) depicts M-H curves for iron oxide nanoparticles in chitosan microparticles with gemcitabine. Both samples showed negligible coercive field, which indicated low capacity 60
79 M (emu/g) M (emu/g) of particles to have remnant magnetization. The saturation magnetization was found to be higher for chitosan coated iron particles compared to uncoated iron oxide nanoparticles. a Field (Oe) b Field (Oe)) Figure 27 (a): M-H curves for iron oxide nanoparticles (b) M-H curves for iron oxide nanoparticles in chitosan microparticles with gemcitabine 61
80 M (emu/g) M (emu/g) Figure 28 shows the variation of the magnetization M as a function of temperature of both samples in an external magnetic field of 100 Oe recorded in zero-field-cooled (ZFC) and field-cooled (FC). From the curves it is observed the superimposition of the ZFC and FC curves take place with a Tmax K for iron oxide nanoparticles and K for chitosan containing gemcitabine and iron oxide particles. The blocking temperatures recorded for nanoparticles and microparticles were K and K respectively. The superimposition of the curves is a characteristic feature of a superparamagnetic system. a Temperature (K) b Temperature (K) Figure 28: (a) ZFC/ FC of Iron Oxide Nanoparticles (b): ZFC/ FC of Chitosan Iron Microparticles 62
81 ΔT Radiofrequency heating Figure 29 shows the increase in temperature with radiofrequency heating by the influence of alternating magnetic field (222 khz). It was observed that samples containing iron oxide show a thermal response. Significant temperature increase of 13.73K and 19.73K were observed chitosan gemcitabine iron oxide particles and iron oxide nanoparticles respectively in an exposure time of 500seconds. The RF exposure of water did not show a significant increase in temperature with the same exposure time as the samples. The heat generated in the samples was solely from magnetic particle heating. The radiant heat from the surrounding environment was not seen to be affecting the temperature of the sample. 25 WATER IRON OXIDE NANOPARTICLES CHITOSAN GEM IRON OXIDE PARTICLES * 10 5 * Time (seconds) Figure 29: RF heating curves of iron oxide nanoparticles and chitosan gemcitabine iron oxide particles with water as control [n=3], *- P< In vitro release study The release profile of gemcitabine from chitosan microspheres (with and without iron oxide) is shown in figure
82 mcg drug/mg protein Percent Drug Released TIME (hrs) Fe Gem Chitosan Gem Chitosan Figure 30: Release profiles of gemcitabine from chitosan microparticles Also the amount of iron oxide released from the formulation was about 2.5 ± 1.2% w/w at the end of 72 hours. The release of iron oxide was negligible for the lower time points Cellular uptake studies The uptake of gemcitabine from spray dried chitosan particles and from drug solutions is represented in figure Drug Uptake on Wi26 VA4 Gemcitabine 50 Microparticles * * * 10 a Time (hrs) 64
83 mcg drug/mg protein Drug Uptake on A549 * Gemcitabine Microparticles b Time 2(hrs) 3 6 Figure 31: Cellular uptake of gemcitabine from drug solutions as well as spray dried chitosan microparticle formulations (a) Wi26 VA4 cells and (b) A549 cells [n=3], *- P< MTT toxicity assay Figure 30 (a) and (b) represent the plot of percent cell survival with increasing concentration of gemcitabine solution, blank and gemcitabine-iron oxide loaded nanoparticles prepared by spray drying in WI-26A4 and A549 cells. 65
84 Percent cell survival Wi26 VA Gemcitabine concentration (µm) Gemcitabine drug solution Gem Iron Chitosan Particles Blank Chitosan particles Figure 32: Cytotoxicity profile of gemcitabine solutions, blank and drug loaded spray dried microparticles after 72 hours of incubation in Wi26 VA4 cells Treatment LD50 (µm) Drug solution 0.05 Gem-IO- MP Chitosan blank MP 4.75 Table 9: LD50 values of different treatments on Wi26VA4 cells 66
85 Percent cell survival A Gemcitabine concentration (µm) Gemcitabine drug solution Gem Iron Chitosan Particles Blank Chitosan particles Figure 33: Cytotoxicity profile of gemcitabine solutions, blank and drug loaded spray dried microparticles after 72 hours of incubation in A549 cells Treatment LD50 (µm) Drug solution Gem-IO- MP Chitosan blank MP - Table 10: LD50 values of different treatments on A549 cells Cytotoxicity on RF treated cells The cell suspensions of Wi26 VA4 and A549, treated with RF heating with blank and gemcitabine-iron oxide loaded chitosan particles, iron oxide nanoparticles were compared for 67
86 Percent cell survival cell death with a control untreated cell suspension. Figure 31 depicts the cell death observed in these cells with various treatments Wi 26 A * * 20 0 Control Chitosan Microparticles Iron- Gemcitabine loaded Chitosan Microparticles Iron Oxide nanoparticles Treatments Figure 34: Cytotoxicity assay reporting percent cell death on RF exposure. [n=3], *- P< Discussions Preparation of microparticles A solution of medium molecular weight chitosan containing solubilized gemcitabine and suspended iron oxide nanoparticles was prepared. Chitosan was the mucoadhesive carrier matrix, which was dissolved using acetic acid as chitosan is sparingly soluble in water and alcohol, but soluble in acidic solutions 48. Chitosan is a polymer from natural sources. Due to low standards of regulation and less emphasis on purity of the grades, the chitosan solution had to be filtered before use to remove any undissolved extraneous material. The gemcitabine drug has a high aqueous solubility and thus could be directly incorporated in 68
87 to the chitosan solution in solubilized form. Introduction of iron oxide nanoparticles in the chitosan solution was slightly challenging. The nanoparticles were magnetic which restricted the use of any magnetic based stirring for keeping the particles homogenously suspended in the chitosan solution. It was crucial to have the nanoparticles suspended in the chitosan solution throughout the spray drying duration. Hence as bath sonicator was used for this purpose. The bath sonicator was successful in keeping the iron oxide suspended with no settling observed in the suspension while spray drying. The iron oxide nanoparticles being used were in the superparamagnetic size range for iron oxide particles. As a result, there was chance the uncoated nanoparticles escaping the cyclone separator in the spray dryer to the filter and recirculate during a closed loop connection. To avoid the risk that iron nanoparticle recirculation might pose to the aspirator, a magnetic trap was custom made and attached in the spray dryer setup which trapped the escaping nanoparticles preventing their accumulation in the filter Chromatography and spectrophotometry A UPLC method for the determination of gemcitabine was adopted from a previously reported method and modified to suit the requirements of this project. The method was successfully validated and applied for gemcitabine determination. The iron oxide determination was carried out using a complexation reaction followed by spectrophotometric estimation. This method was also successfully tested and applied for iron content determination of the spray dried particles Particle Size, zeta potential of microparticles The particle size distribution of the spray dried particles showed that majority of the particles have a d (0.5) of 8 µm. There is a small population of particles that lie in the 8 69
88 µm to 100 µm range. This distribution can be explained by the high solid content of the spraying solution. This can result in the droplets containing a high solid content which may dry to give large sized particles 113. Also, the particles being highly charged can form agglomerates which can cause an increased particle size reading due to strongly bound aggregates. The zeta potential of the spray dried particles lies in the high positive range. This is an attribute of the cationic polymer properties of chitosan Electron microscopy The principle of electron microscopy uses an electron beam as a source of illumination. In the chitosan microparticle samples containing iron oxide, the iron oxide particles become highly charged in an electron microscopy. As a result, it is difficult to get a good image of the chitosan matrix due to high charge density on the iron oxide particles. An SEM was done on the blank chitosan microparticles to avoid problems due to iron oxide charge density and to study their shape and surface morphology. The SEM images showed that the spray dried particles were roughly spherical in shape. Their particle sizes were similar to the d (0.5) readings obtained on the Malvern Mastersizer. The TEM also encounters the problems associated with high iron oxide charge density. The spray dried chitosan microparticles containing iron oxide and gemcitabine were visualized on the TEM. The chitosan matrix in which the iron oxide particles are embedded is not clearly visible in the TEM image. However, individual particles can be identified as clusters of iron oxide nanoparticles in the image. The nanoparticles seem to be well distributed in the chitosan matrix. These particles also appear to have a roughly spherical 70
89 shape, however this cannot be stated for sure as the chitosan matrix is not well visualized in the image. Both SEM and TEM images showed particle aggregation behavior. Thus it should be noted that the powders do have a tendency to exist as aggregates which might have an influence on their aerodynamic performance Drug entrapment efficiency and iron loading The determination of the iron oxide and gemcitabine that were loaded into chitosan was done to evaluate the efficiency if the spray drying method. A suitable extraction method was designed to extract the drug from chitosan and also dissolve the iron oxide nanoparticles for conducting complexation reaction for colorimetric estimation of iron oxide content. The drug showed a high entrapment efficiency in the microparticles. The iron oxide loading of the particles was consistent from batch to batch Thermal Analysis The DSC thermograms showed an endothermic peak for gemcitabine at C which is close the previously reported value of gemcitabine hydrochloride 115. Melting peaks were not observed for gemcitabine in the chitosan microparticles suggesting that the drugs encapsulated in the microparticles are in amorphous, disordered-crystalline or in solid-state solubilized form in the polymer matrix. The physical state of drug in the microparticles affects the in vitro release patterns. If the drug is present in an amorphous or non-crystalline form, they can diffuse through the polymer matrix faster than their crystalline counterparts. The DSC thermograms show an endothermic peak for all samples containing chitosan at 207 C. This can be the representation of the glass transition temperature of chitosan 116. The thermogravimetric analysis (TGA) was performed to determine the percent weight loss 71
90 in the formulations with increase in temperature. This method could also give us an estimate of the moisture content of the samples. In case chitosan particles containing gemcitabine and iron oxide 7.43% weight loss was seen on heating up to 120 C. The weight loss in blank microparticles was % and weight loss in chitosan particles containing only gemcitabine displays % weight loss. Thus the moisture content of the chitosan particles containing iron oxide was found to lesser than the ones without iron oxide. This may be due presence of iron oxide particles on the surface of chitosan which reduces the surface area available for moisture absorption/ adsorption on the microparticles Aerodynamic Studies The aerodynamic studies were done as a part of developing a proof of concept that the spray dried particles possessed the characteristics needed for pulmonary delivery. The aerodynamic properties of the chitosan particles showed a larger MMAD value of (8.088 ± 3.6 μm) compared to the particle size obtained by laser diffraction (1.59 ± 0.35 μm). This difference can be attributed to the aggregation of the microparticles in the dry powder which caused the deposition of the aggregates in the earlier stages. Another explanation for this difference in particle size can be due to the density of the particles. The chitosan particles have gemcitabine and iron oxide nanoparticles embedded into them which might cause the particles to be highly density and thus show a higher MMAD Magnetization Studies The magnetization studies were performed on the particles to study their magnetic behavior and properties. The desired state of the magnetic particles is the superparamagnetic state. When a superparamagnetic particle is kept under alternating magnetic field, the magnetization directions are quickly reversed since there are no domain walls to move
91 These materials are known to not retain any magnetism like paramagnetic substances and revert to non-magnetic state. These properties were observed in magnetization studies done on both samples: chitosan containing iron oxide particles and the iron oxide nanoparticles. The M-H loops were plotted for both samples. The curves show that once the external magnetic field is removed, the magnetization disappears (the curves showed a negligible remnant magnetization and coercivity). The saturation magnetization for chitosan containing iron oxide particles was significantly higher than iron oxide nanoparticles. This behavior shows that the magnetic particles in the samples display single crystal domain resistance. Also, it can be concluded that the magnetic saturation is related to the amount of iron oxide in the samples. ZFC/ FC curves for the sample were also plotted. The blocking temperature (TB) is defined as the temperature at which the ZFC curve exhibits a cusp. When the samples were subjected to field cooling, the magnetic moment of each nanoparticle was frozen to the field direction as the temperature was decreased below the blocking temperature. The behavior that is displayed below TB is due to the existence of potential energy barriers of magneto-crystalline anisotropy. The superparamagnetic behavior is displayed above blocking temperatures in both samples Radiofrequency heating Magnetic hyperthermia application is based on external radiofrequency heating of particles which have been concentrated in the region of interest, in the body. The RF heating of the particles was done to record the heat generation behavior of the magnetic particles. The use of optima grade water as control showed very low heating after application of radiofrequency. The iron oxide nanoparticles and chitosan microparticles display heating which was significantly higher that the water heating. The heating patterns observed for 73
92 the microparticles and nanoparticles were also significantly different from each other (P<0.05). The magnetic particle suspensions show a magnetically induced thermal response, owing to the energy released through the Néel relaxation process, which is the suggested mechanism contributing for superparamagnetic nanoparticles embedded in a rigid solid. The chitosan microparticle containing the nanoparticles show a reduced heating of the particles compared to uncoated nanoparticles. The reason for that behavior might be the chitosan matrix interfering with the free spin flip movement of the particles embedded into it In vitro release study In vitro release studies were performed on microparticles containing only gemcitabine as well as microparticles containing gemcitabine and iron oxide. These studies were carried out using phosphate buffer (ph 7.4). The release pattern of drugs from a system is largely governed by factors like drug properties, nature of the polymers used, drug polymer interactions. This log p value of gemcitabine is and the solubility in water is 2.23x10 1 g/l 117. These properties suggest the highly hydrophilic nature of gemcitabine. The release of gemcitabine from chitosan was observed over a period of 24 hours after which the release slowed down and completely stopped within hours. The release is seen to be rapid for the first 24 hour of the study causing about 70% (w/w) in that period. The release of the drug from the particles is seen to start within 1 hour of the release. The drug release from iron containing particles continued for the first 48 hours of the study after which the release reached a plateau. This trend is probably due to superficial distribution of drug in the chitosan matrix and also due to the high hydrophilicity of the drug which gets rapidly dissolved and diffused from the chitosan microparticles. Even though chitosan 74
93 does not completely solubilize in water, it behaves as a porous matrix for release of entrapped gemcitabine. The gemcitabine owing to its hydrophilic nature, gets released rapidly from the system. Another possible explanation for the rapid release of the drug is the tendency of the chitosan to swell in aqueous media leading to increased water penetration in the system 118. The release from chitosan gemcitabine particles reaches its maximum in the first 24 hours of the study, but the iron oxide containing particles continue to release for a longer period. This can be an effect of iron oxide presence on the surface of chitosan in the iron containing particles causing a slower diffusion of drug due to reduced porosity. Figure 28 represents the release profile acquired from the study Cellular uptake studies The cellular uptake of gemcitabine from drug solutions in Wi26 VA4 cells was found to be significantly higher from the chitosan particles than from drug solution. In A549 cells the uptake of gemcitabine from drug solutions was found to be higher than the uptake from microparticles. However the overall difference in uptake from the microparticles was not significantly different from drug solutions (P>0.05). The trend observed during uptake studies showed an alternate increase and decrease in the amount of drug uptake by the cells, with respect to time In vitro cellular toxicity MTT assay was performed on lung cancer cells (A549 cells) and normal human lung cells (Wi26 VA4) cells to assess the in vitro cytotoxicity of gemcitabine drug solutions and blank as well as gemcitabine-iron oxide loaded chitosan microparticles. The overall toxicity observed was higher in Wi26 VA4 cells as compared to A549 cells. In individual 75
94 cell lines, the cytotoxicity profiles of the drug solutions were lower than drug-iron oxide loaded chitosan microparticles. The LD50 values observed in gemcitabine-iron oxide loaded microparticles were significantly lower than the LD50 due to drug solutions. The reason for higher cell death observed in cells receiving drug-iron oxide loaded microparticle treatment can be due to added toxicity of chitosan. Blank chitosan microparticles show cellular toxicity in both cell lines. The cell death due to blank chitosan particles was also higher in Wi26 cells than A549 cells. Huang et al. have tested the uptake and cytotoxicity of chitosan molecules on A549 cells 119. Their studies have indicated a dose dependent cytotoxicity of chitosan with a deacetylation and molecular weight similar to the chitosan used by us in this project. Qi et al. have also reported cytotoxic effects of chitosan after 72hrs of treatment Cellular RF treatment The cell suspensions of Wi26 A4 and A549 were treated on the RF induction heater. The percent cell death observed was calculated against a control cell suspension sample which was not subjected to any treatment. The RF treated sample containing blank chitosan microparticles showed a small percent cell death for both cell lines. The percent cell death on blank chitosan treatment was not significant (P>0.05) from the blank in both cell lines. The cells when treated with iron oxide nanoparticles and chitosan microparticles loaded with gemcitabine and iron oxide, show significant cell death post RF heating (P<0.05). There was no significant difference in the cell death observed between the two treatments in both cell lines (P>0.05). The percent cell death was also seen to be similar for both cell lines. (P>0.05). Since the cell death in blank treatments was negligible, it can be said that the cell death observed in the treatments was due to magnetic hyperthermia. 76
95 3.6 Conclusion Chitosan microparticles containing iron oxide nanoparticles and gemcitabine drug were developed and characterized. The quantitative estimation of gemcitabine and iron oxide in the microparticles was successfully done using a UPLC method and a spectrophotometric detection respectively. The particle size of the spray dried chitosan microparticles tested by laser diffraction was around 1.59 µm which was desirable for pulmonary delivery. However, the MMAD of the particles was found to be around 8 µm which may be an attribute of aggregation and high particle density. The magnetic characterization studies carried out on the particles also suggested superparamagnetic behavior on the plot of magnetization as a function of field and temperature. In addition to the magnetic performances, the RF heating studies of the particles show thermal activity caused by magnetic hyperthermia experiments. This reveals that the particles can be applicable for remotely controlled in vivo applications. The release of gemcitabine from the microparticles is found to be rapid, owing to its high solubility. The system displays cellular uptake of drug and cytotoxic behavior by its action. The application of radiofrequency on the cells also shows significant cell death. Thus it can be concluded that a combination of radiofrequency and chemotherapy in a single system can be achieved. 77
96 CHAPTER 4 Summary and Future Directions 78
97 4.1 Summary Iron oxide nanoparticle were chemically synthesized and successfully incorporated into chitosan microparticles along with an anticancer agent (gemcitabine). This approach was designed to combine magnetic hyperthermia and radiotherapy into a single system for a multifunctional anticancer treatment system. The microparticles were prepared by spray drying technique. The particles were formulated with the aim of delivering them to the lung cancer cells via inhalational delivery. Iron oxide nanoparticles were chosen to induce magnetic hyperthermia by excitation using radiofrequency heating 84,85. These nanoparticles were synthesized by chemical coprecipitation. The use of surfactants in the synthesis procedure yielded water dispersible iron oxide nanoparticles in the 17 nm size range, which is estimated to be within the superparamagnetic size range of magnetite 88. The TEM images confirmed the particle size. Superparamagnetic behavior was desired in these nanoparticles for biological application. These nanoparticles were incorporated into chitosan microparticles along with gemcitabine by spray drying. This resulted in a combined system of iron oxide nanoparticles and gemcitabine drug dispersed throughout a chitosan particle. Gemcitabine concentrations in the formulation and various analytical tests was determined using a validated UPLC method that was able to detect drug concentrations as low as 1 µg/ ml. The iron oxide nanoparticles loaded in the chitosan microparticles were quantified using a UV-visible spectrophotometric method. The entrapment of gemcitabine in chitosan was found to be ± 5.7% w/w. The amount of iron oxide nanoparticles loaded into the microparticles was ± 3.4% w/w and was consistent between spray dried batches. The thermogravimetric analysis (TGA) revealed a percent weight loss when heated up to 120ᴼC. The weight loss observed for iron oxide containing microparticles 79
98 was less than both the blank chitosan microparticle and gemcitabine-only microparticles. This weight loss is likely related to the moisture content of the particles. A low moisture content was desired as the stability of gemcitabine is affected by presence of moisture 40. Differential scanning calorimetry (DSC) proved that the drug entrapped with the nanoparticles are found to be in noncrystalline state. The state of the drug has an effect on its solubility and release behavior from the system. This property is desired for reproducible release behaviors in the various batches of microparticles 121. The magnetic properties of iron oxide nanoparticles and nanoparticles dispersed in chitosan microparticles were evaluated using the SQUID magnetometer 93. The particle size of the iron oxide particles were shown to be in the superparamagnetic range and confirmed by the presence of superparamagnetic behavior in the iron oxide nanoparticles and in nanoparticles dispersed in chitosan microparticles. However the magnetic saturation observed in chitosan microparticles was much lesser than the saturation observed in the iron oxide nanoparticle samples, likely due to a smaller concentration of the iron oxide in microparticles comparted to nanoparticles. The magnetic studies confirmed the presence of superparamagnetic behavior in the formulated microparticles which was desired for RF heating applications. The aerodynamic testing of spray dried chitosan microparticles containing iron oxide nanoparticles and gemcitabine demonstrated a proof of concept that this multifunctional microparticle formulation could be prepared for eventual optional lung delivery. Specifically, multifunctional microparticles had an MMAD value of 8.1 μm with a GSD of 3.6 μm. This suggested that the sprayed droplets were polydisperse prior to drying which might be a result of particles aggregation or its density, both affect its aerodynamic performance 78. Although the aerodynamic size distribution of drug and iron oxide containing microparticles was too large and broad for optimal 80
99 particle delivery to the deep lung, formulation and spray drying parameters can likely be modified to reduce the particle size and achieve a narrow size distribution. Suspensions of magnetic nanoparticles and microparticles were subjected to RF heating using RDO Model HFI Induction Heating System. The change in temperature observed was greater for the iron oxide nanoparticles compared to the chitosan microparticles containing iron oxide. However, a significant rise in temperature was observed in both samples. This suggested that the system can be tested for use in RF heating applications in biological systems. Cell suspensions treated with iron oxide nanoparticles and chitosan microparticles containing gemcitabine and iron oxide show significant cell death on RF exposure. The cells did not show significant death on RF treatment with blank chitosan particles, proving the nanoparticles are the source of magnetic hyperthermia and suggesting the microparticle cytotoxicity was due to the iron oxide heating on application of RF. The drug release studies on chitosan microparticles showed rapid release of the drug from the system and eventual drug release of about 70% over 24 hours. The chitosan microparticles demonstrated gemcitabine uptake in both normal lung cells and in lung cancer cells with both cell lines alternating increase and decrease trends in the amount of gemcitabine uptake recorded over six hours. The drug-induced cellular toxicity was higher in the normal lung cells than the lung cancer cell lines while multifunctional microparticles showed higher toxicity than the gemcitabine drug solutions even in the absence of RF-induced heating. This may be due to the added toxicity of chitosan which has been reported to be toxic to some cells 120. These cytotoxicity studies demonstrated multifunctional chitosan microparticles had therapeutic effects due to the magnetic hyperthermia as well as the gemcitabine release and uptake. Thus it can be concluded that this formulation was able to combine both radiofrequency induced heating and chemotherapy with a 81
100 single system to create a multifunctional chitosan microparticle that is capable of being delivered to the lungs. 4.2 Future Studies The present study has designed an inhalable multifunctional chitosan microparticle system. In order to improve the selectivity of the system for lung cancer cells, surface peptide synthesis on chitosan can be evaluated. This surface peptide could potentially cause binding of the particles to the tumor cells resulting in better tumor selectivity and a more targeted delivery. The system designed in this study used gemcitabine as the chemotherapeutic agent. Other chemotherapeutic drugs could be substituted or incorporated into this system. The prepared microparticles tended to aggregate which affected their aerodynamic performance. Typically, dry powder inhaler formulations include excipients to improve their powder flow properties and aerodynamic performance. Excipients could be added to the formulation to improving their aerodynamic performance. The microparticles prepared in this study were tested on only two cell lines. In order to get a better understanding of the effect of particles on cells, other cell line and macrophage studies could be conducted. For example, the interaction of rhodamine coated chitosan microparticles with a mouse macrophage cell line (AMJ2C11) was performed. The images from the study show that the chitosan particles do indeed interact with macrophages (Figure 35). This project has also evaluated cytotoxicity of RF heating studies on suspended cells. Although lung cancer is dissimilar to cell suspensions, magnetic hyperthermia induced cytotoxicity was demonstrated. Future studies could investigate magnetic hyperthermia of multifunctional chitosan microparticles on lung cancer tumor models or solid tumor models such as mouse models for human lung cancer testing and three dimensional tumor models for simulating lung cancer tumors. Berns et al. have described the NSCLC mouse model that can be used for lung cancer studies
101 Another lung cancer model that can be used for this type of study can be the one similar to the model described Mishra et al. which used ex vivo cells 123. Such 3D models can also be designed using primary cells with matrix assisted assembly for 3D structure formation 124. Figure 35: Cellular localization of rhodamine bound chitosan microparticles in Mouse macrophage cell line AMJ2C11 83
MAGNETIC NANOPARTICLES FOR HYPERTHERMIA APPLICATIONS. Mohamed DARWISH and Ivan STIBOR
 MAGNETIC NANOPARTICLES FOR HYPERTHERMIA APPLICATIONS Mohamed DARWISH and Ivan STIBOR Institute for Nanomaterials, Advanced Technology and Innovation, Technical University of Liberec, 461 17Liberec, Czech
MAGNETIC NANOPARTICLES FOR HYPERTHERMIA APPLICATIONS Mohamed DARWISH and Ivan STIBOR Institute for Nanomaterials, Advanced Technology and Innovation, Technical University of Liberec, 461 17Liberec, Czech
Powder. Aulton Chapter 8-10,14,24. Powders
 Powder Aulton Chapter 8-10,14,24 Powders Powders are important in the development of pharmaceutical products since many formulations either is powders or contain powders 1 Pharmaceutical powders Different
Powder Aulton Chapter 8-10,14,24 Powders Powders are important in the development of pharmaceutical products since many formulations either is powders or contain powders 1 Pharmaceutical powders Different
Preparation And Characterization Of Simvastatin Nanosuspension By Homogenization Method
 International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.5, No.1, pp 193-197, Jan-Mar 2013 Preparation And Characterization Of Simvastatin Nanosuspension By Homogenization Method
International Journal of PharmTech Research CODEN (USA): IJPRIF ISSN : 0974-4304 Vol.5, No.1, pp 193-197, Jan-Mar 2013 Preparation And Characterization Of Simvastatin Nanosuspension By Homogenization Method
Process Development & Scale-Up of the AIR Technology
 Process Development & Scale-Up of the AIR Technology Lloyd Johnston, Ph.D. Vice President of Process Development & Manufacturing October 6, 2005 Pharmaceutical Industry o Delivering needed therapeutics
Process Development & Scale-Up of the AIR Technology Lloyd Johnston, Ph.D. Vice President of Process Development & Manufacturing October 6, 2005 Pharmaceutical Industry o Delivering needed therapeutics
Spray Drying of Protein Particles for Pulmonary Delivery Reinhard Vehring Willard R. Foss, John W.Y. Lee, Nazli Egilmez
 Spray Drying of Protein Particles for Pulmonary Delivery Reinhard Vehring Willard R. Foss, John W.Y. Lee, Nazli Egilmez Advanced Technology Development Inhale Therapeutic Systems, Inc. 150 Industrial Road,
Spray Drying of Protein Particles for Pulmonary Delivery Reinhard Vehring Willard R. Foss, John W.Y. Lee, Nazli Egilmez Advanced Technology Development Inhale Therapeutic Systems, Inc. 150 Industrial Road,
Ferrofluid. Student Materials
 Ferrofluid Student Materials Guided Notes: Student Version Investigation 1 -Investigating and Understanding the Unique Properties of Ferrofluids Investigation 2 - Investigating the Structure of Magnetite
Ferrofluid Student Materials Guided Notes: Student Version Investigation 1 -Investigating and Understanding the Unique Properties of Ferrofluids Investigation 2 - Investigating the Structure of Magnetite
429 LIGHT DIFFRACTION MEASUREMENT OF PARTICLE SIZE
 Search USP29 429 LIGHT DIFFRACTION MEASUREMENT OF PARTICLE SIZE Light diffraction is one of the most widely used techniques for measuring the size of a wide range of particles from very fine to very coarse.
Search USP29 429 LIGHT DIFFRACTION MEASUREMENT OF PARTICLE SIZE Light diffraction is one of the most widely used techniques for measuring the size of a wide range of particles from very fine to very coarse.
8. FORMULATION OF LANSOPRAZOLE NANOPARTICLES
 8. FORMULATION OF LANSOPRAZOLE NANOPARTICLES FORMULATION OF LANSOPRAZOLE NANOPARTICLES Preparation of capsule of modified solubility to protect the drug from degradation To protect the drug from degradation
8. FORMULATION OF LANSOPRAZOLE NANOPARTICLES FORMULATION OF LANSOPRAZOLE NANOPARTICLES Preparation of capsule of modified solubility to protect the drug from degradation To protect the drug from degradation
MEASURING NANOPARTICLE EXPOSURE
 MEASURING NANOPARTICLE EXPOSURE APPLICATION NOTE NSAM-001 The Nanoparticle Surface Area Monitor reports the surface area of inhaled particles deposited in the lung Nanoparticle Exposure There is increasing
MEASURING NANOPARTICLE EXPOSURE APPLICATION NOTE NSAM-001 The Nanoparticle Surface Area Monitor reports the surface area of inhaled particles deposited in the lung Nanoparticle Exposure There is increasing
ANTIBIOTIC COATED MAGNETITE NANOPARTICLES FOR BIOLOGICAL APPLICATIONS
 ANTIBIOTIC COATED MAGNETITE NANOPARTICLES FOR BIOLOGICAL APPLICATIONS E.L. Foca-nici Ciurlica a, C. Nadejde a, D.E. Creanga a, A. Carlescu a and V. Badescu b a Faculty of Physics, Alexandru Ioan Cuza University
ANTIBIOTIC COATED MAGNETITE NANOPARTICLES FOR BIOLOGICAL APPLICATIONS E.L. Foca-nici Ciurlica a, C. Nadejde a, D.E. Creanga a, A. Carlescu a and V. Badescu b a Faculty of Physics, Alexandru Ioan Cuza University
Supporting Information
 Supporting Information Amorphous calcium carbonate based-microparticles for peptide pulmonary delivery Frederic Tewes 1, 2 *, Oliviero L. Gobbo 1, Carsten Ehrhardt 1 and Anne Marie Healy 1* 1: School of
Supporting Information Amorphous calcium carbonate based-microparticles for peptide pulmonary delivery Frederic Tewes 1, 2 *, Oliviero L. Gobbo 1, Carsten Ehrhardt 1 and Anne Marie Healy 1* 1: School of
IRON OXIDE NANOPARTICLES FOR BIOMEDICAL APPLICATIONS
 Research Paper ISSN 2278 0149 www.ijmerr.com Vol. 3, No. 2, April, 2014 2014 IJMERR. All Rights Reserved IRON OXIDE NANOPARTICLES FOR BIOMEDICAL APPLICATIONS Aadarsh Mishra* *Corresponding Author: Aadarsh
Research Paper ISSN 2278 0149 www.ijmerr.com Vol. 3, No. 2, April, 2014 2014 IJMERR. All Rights Reserved IRON OXIDE NANOPARTICLES FOR BIOMEDICAL APPLICATIONS Aadarsh Mishra* *Corresponding Author: Aadarsh
Magnetic Nano Particles for In-Vitro Diagnostics
 Magnetic Nano Particles for In-Vitro Diagnostics has utilized its many years of experience in creating a Magnetic Nano Particles Developer Kit for in-vitro biomedical applications. The kit offers a unique
Magnetic Nano Particles for In-Vitro Diagnostics has utilized its many years of experience in creating a Magnetic Nano Particles Developer Kit for in-vitro biomedical applications. The kit offers a unique
Available online at ScienceDirect. Procedia Materials Science 11 (2015 )
 Available online at www.sciencedirect.com ScienceDirect Procedia Materials Science 11 (2015 ) 282 286 5th International Biennial Conference on Ultrafine Grained and Nanostructured Materials, UFGNSM15 Prepartion
Available online at www.sciencedirect.com ScienceDirect Procedia Materials Science 11 (2015 ) 282 286 5th International Biennial Conference on Ultrafine Grained and Nanostructured Materials, UFGNSM15 Prepartion
Chapter 6 Magnetic nanoparticles
 Chapter 6 Magnetic nanoparticles Magnetic nanoparticles (MNPs) are a class of nanoparticle which can be manipulated using magnetic field gradients. Such particles commonly consist of magnetic elements
Chapter 6 Magnetic nanoparticles Magnetic nanoparticles (MNPs) are a class of nanoparticle which can be manipulated using magnetic field gradients. Such particles commonly consist of magnetic elements
In this activity, you will be making your own ferrofluids using three different methods.
 Student Activity Ferrofluids Developed by: Sussan Oladipo, Wells Academy High School Graduate Student Mentor: Faith Boman Faculty Mentor: Franz Geiger Overview Nanotechnology is a cutting-edge field with
Student Activity Ferrofluids Developed by: Sussan Oladipo, Wells Academy High School Graduate Student Mentor: Faith Boman Faculty Mentor: Franz Geiger Overview Nanotechnology is a cutting-edge field with
D'Addio, SM; Chan, JG; Kwok, P; Prud'homme, R; Chan, HK
 Title Spray freeze dried large porous particles for nano drug delivery by inhalation Author(s) D'Addio, SM; Chan, JG; Kwok, P; Prud'homme, R; Chan, HK Citation The 2012 Respiratory Drug Delivery (RDD)
Title Spray freeze dried large porous particles for nano drug delivery by inhalation Author(s) D'Addio, SM; Chan, JG; Kwok, P; Prud'homme, R; Chan, HK Citation The 2012 Respiratory Drug Delivery (RDD)
PHRC 4110 Pharmaceutics I
 CO01: Use interpretive tools for proper data handling CO01.01: Describe basic mathematics and statistic to interpret pharmaceutical data CO01.02: Work with exponents, powers, roots, logarithms, and antilogarithms
CO01: Use interpretive tools for proper data handling CO01.01: Describe basic mathematics and statistic to interpret pharmaceutical data CO01.02: Work with exponents, powers, roots, logarithms, and antilogarithms
Particle size analysis -Chapter 3
 Particle size analysis -Chapter 3 Importance of PSA Size and hence surface area of particles affect: The rate of drug dissolution and release from dosage forms Flow properties of granules and powders.
Particle size analysis -Chapter 3 Importance of PSA Size and hence surface area of particles affect: The rate of drug dissolution and release from dosage forms Flow properties of granules and powders.
(APTES), 3- mercaptopropyltrimethoxysilane (MPTMS), tetraethyl orthosilicate (TEOS), N,Ndimethylformamide
 SUPPRTING INFRMATIN Materials: Iron (II) chloride hydrate ( FeCl 2,4H 2, 99%) was purchased from Alfa Aesar ( Ward Hill, MA). Iron (III) chloride hexahydrate ( FeCl 3,6H 2, 97%), (3-aminopropyl)triethoxysilane
SUPPRTING INFRMATIN Materials: Iron (II) chloride hydrate ( FeCl 2,4H 2, 99%) was purchased from Alfa Aesar ( Ward Hill, MA). Iron (III) chloride hexahydrate ( FeCl 3,6H 2, 97%), (3-aminopropyl)triethoxysilane
Mixtures and Solutions
 Mixtures and Solutions Section 14.1 Heterogeneous and Homogeneous Mixtures In your textbook, read about suspensions and colloids. For each statement below, write true or false. 1. A solution is a mixture
Mixtures and Solutions Section 14.1 Heterogeneous and Homogeneous Mixtures In your textbook, read about suspensions and colloids. For each statement below, write true or false. 1. A solution is a mixture
ProPac WCX-10 Columns
 ProPac WCX-10 Columns Guidance for column use Tips to maximize column lifetime ProPac WCX-10 Column Tips and Tricks This guide provides essential information and invaluable guidelines for mobile phases,
ProPac WCX-10 Columns Guidance for column use Tips to maximize column lifetime ProPac WCX-10 Column Tips and Tricks This guide provides essential information and invaluable guidelines for mobile phases,
Encapsulation. Battelle Technology. Introduction
 Encapsulation Introduction The encapsulation methods reported in the literature 1-7 for the production of microcapsules are generally achieved using one of the following techniques: 1. Phase separation
Encapsulation Introduction The encapsulation methods reported in the literature 1-7 for the production of microcapsules are generally achieved using one of the following techniques: 1. Phase separation
Size and Zeta Potential of Colloidal Gold Particles
 Size and Zeta Potential of Colloidal Gold Particles Mark Bumiller mark.bumiller@horiba.com Colloid Definition Two phases: Dispersed phase (particles) Continuous phase (dispersion medium, solvent) May be
Size and Zeta Potential of Colloidal Gold Particles Mark Bumiller mark.bumiller@horiba.com Colloid Definition Two phases: Dispersed phase (particles) Continuous phase (dispersion medium, solvent) May be
Superparamagnetic Iron Oxide Nanoparticles For Drug Delivery
 Superparamagnetic Iron Oxide Nanoparticles For Drug Delivery Yana Petri C150 Special Topic SPION ferrofluid Background and Motivation Most promising magnetic iron oxide nanoparticles for biomedical applications:
Superparamagnetic Iron Oxide Nanoparticles For Drug Delivery Yana Petri C150 Special Topic SPION ferrofluid Background and Motivation Most promising magnetic iron oxide nanoparticles for biomedical applications:
Spray Drying for Inhaled Dosage Forms CPHI DAVID K. LYON, PH.D. OCTOBER 25, 2017
 Spray Drying for Inhaled Dosage Forms CPHI DAVID K. LYON, PH.D. OCTOBER 25, 2017 Market Drivers Although MDIs are the market leader with 48% of retail sales in Europe versus 39% for DPIs and only 13% for
Spray Drying for Inhaled Dosage Forms CPHI DAVID K. LYON, PH.D. OCTOBER 25, 2017 Market Drivers Although MDIs are the market leader with 48% of retail sales in Europe versus 39% for DPIs and only 13% for
Advanced TRS - ZEOLITE IN A COLLOIDAL SUSPENSION
 Advanced TRS - ZEOLITE IN A COLLOIDAL SUSPENSION The need for this product is summarized in the following graphic Since this data was collected the numbers of deaths have gone up yearly. 25 MARCH 2014
Advanced TRS - ZEOLITE IN A COLLOIDAL SUSPENSION The need for this product is summarized in the following graphic Since this data was collected the numbers of deaths have gone up yearly. 25 MARCH 2014
CEINT/NIST PROTOCOL REPORTING GUIDELINES FOR THE PREPARATION OF AQUEOUS NANOPARTICLE DISPERSIONS FROM DRY MATERIALS. Ver. 2.0
 CEINT/NIST PROTOCOL REPORTING GUIDELINES FOR THE PREPARATION OF AQUEOUS NANOPARTICLE DISPERSIONS FROM DRY MATERIALS Ver. 2.0 July 8, 2010 Protocol Contributors: J. S. Taurozzi 1, V. A. Hackley 1, M. R.
CEINT/NIST PROTOCOL REPORTING GUIDELINES FOR THE PREPARATION OF AQUEOUS NANOPARTICLE DISPERSIONS FROM DRY MATERIALS Ver. 2.0 July 8, 2010 Protocol Contributors: J. S. Taurozzi 1, V. A. Hackley 1, M. R.
Magnetophoresis of colloidal particles in a dispersion of superparamagnetic nanoparticles: Theory and Experiments
 Magnetophoresis of colloidal particles in a dispersion of superparamagnetic nanoparticles: Theory and Experiments M. Benelmekki a*, Ll. M. Martinez b, J. S. Andreu c,d, J. Camacho d, J. Faraudo c. a Centro
Magnetophoresis of colloidal particles in a dispersion of superparamagnetic nanoparticles: Theory and Experiments M. Benelmekki a*, Ll. M. Martinez b, J. S. Andreu c,d, J. Camacho d, J. Faraudo c. a Centro
Supplementary information
 Supplementary information Theoretic approach of magnetic aerosol drug targeting. The following assumptions were made for computer-aided simulation of inhaled SPION aerosol trajectories: (1) Structure of
Supplementary information Theoretic approach of magnetic aerosol drug targeting. The following assumptions were made for computer-aided simulation of inhaled SPION aerosol trajectories: (1) Structure of
PRODUCTION OF PEG SUBMICRON PARTICLES BY THE SOLUTION ENHANCED DISPERSION WITH ENHANCED MASS TRANSFER BY ULTRASOUND IN SUPERCRITICAL CO 2 (SEDS-EM)
 PRODUCTION OF PEG SUBMICRON PARTICLES BY THE SOLUTION ENHANCED DISPERSION WITH ENHANCED MASS TRANSFER BY ULTRASOUND IN SUPERCRITICAL CO 2 (SEDS-EM) Heyang Jin, Sining Li, Daode Hu and Yaping Zhao* Email
PRODUCTION OF PEG SUBMICRON PARTICLES BY THE SOLUTION ENHANCED DISPERSION WITH ENHANCED MASS TRANSFER BY ULTRASOUND IN SUPERCRITICAL CO 2 (SEDS-EM) Heyang Jin, Sining Li, Daode Hu and Yaping Zhao* Email
FLUDEOXYGLUCOSE ( 18 F) INJECTION: Final text for addition to The International Pharmacopoeia (January 2009)
 January 2009 FLUDEOXYGLUCOSE ( 18 F) INJECTION: Final text for addition to The International Pharmacopoeia (January 2009) This monograph was adopted at the Forty-third WHO Expert Committee on Specifications
January 2009 FLUDEOXYGLUCOSE ( 18 F) INJECTION: Final text for addition to The International Pharmacopoeia (January 2009) This monograph was adopted at the Forty-third WHO Expert Committee on Specifications
IMPROVEMENT OF DISSOLUTION PROFILE OF LORNOXICAM BY SOLID DISPERSION USING SPRAY DRYING TECHNIQUE
 66 P a g e International Standard Serial Number (ISSN): 2319-8141 International Journal of Universal Pharmacy and Bio Sciences 3(5): September-October 2014 INTERNATIONAL JOURNAL OF UNIVERSAL PHARMACY AND
66 P a g e International Standard Serial Number (ISSN): 2319-8141 International Journal of Universal Pharmacy and Bio Sciences 3(5): September-October 2014 INTERNATIONAL JOURNAL OF UNIVERSAL PHARMACY AND
Preparation of Iron Oxide Nanoparticles Mixed with Calcinated Laterite for Arsenic Removal
 Preparation of Iron Oxide Nanoparticles Mixed with Calcinated Laterite for Arsenic Removal Wint Myat Shwe 1, Dr. Mya Mya Oo 2, Dr. Su Su Hlaing 3 Abstract-- To overcome arsenic toxicity; which has become
Preparation of Iron Oxide Nanoparticles Mixed with Calcinated Laterite for Arsenic Removal Wint Myat Shwe 1, Dr. Mya Mya Oo 2, Dr. Su Su Hlaing 3 Abstract-- To overcome arsenic toxicity; which has become
The body has three primary lines of defense against changes in hydrogen ion concentration in the body fluids.
 ph and Nucleic acids Hydrogen Ion (H+) concentration is precisely regulated. The H+ concentration in the extracellular fluid is maintained at a very low level, averaging 0.00000004Eq/L. normal variations
ph and Nucleic acids Hydrogen Ion (H+) concentration is precisely regulated. The H+ concentration in the extracellular fluid is maintained at a very low level, averaging 0.00000004Eq/L. normal variations
BIO & PHARMA ANALYTICAL TECHNIQUES. Chapter 5 Particle Size Analysis
 BIO & PHARMA ANALYTICAL TECHNIQUES Chapter 5 by Dr Siti Umairah Mokhtar Faculty of Engineering Technology umairah@ump.edu.my Chapter Description Aims Discuss theory, principles and application of analytical
BIO & PHARMA ANALYTICAL TECHNIQUES Chapter 5 by Dr Siti Umairah Mokhtar Faculty of Engineering Technology umairah@ump.edu.my Chapter Description Aims Discuss theory, principles and application of analytical
Supplementary Figure 1. Fullerence has poor solubility in water while the C60S and LC60S nanoparticles can be stably dispersed in water.
 Supplementary Figure 1. Fullerence has poor solubility in water while the C60S and LC60S nanoparticles can be stably dispersed in water. (a) A typical photograph of fullerence (C60), C60S nanoparticles,
Supplementary Figure 1. Fullerence has poor solubility in water while the C60S and LC60S nanoparticles can be stably dispersed in water. (a) A typical photograph of fullerence (C60), C60S nanoparticles,
Continuous Microfluidic Synthesis of PLGA Nanoparticles by Micromixing
 Continuous Microfluidic Synthesis of PLGA Nanoparticles by Micromixing Dolomite s Nanoparticle Generation System Application Note Page Summary 2 Polymer Nanoparticles 3 Mechanism Micromixing Solvent Diffusion
Continuous Microfluidic Synthesis of PLGA Nanoparticles by Micromixing Dolomite s Nanoparticle Generation System Application Note Page Summary 2 Polymer Nanoparticles 3 Mechanism Micromixing Solvent Diffusion
Continuous Microfluidic Synthesis of PLGA Microparticles by Droplet Method
 Microfluidic Synthesis PLGA Microparticles by Droplet Method - App. Note Continuous Microfluidic Synthesis of PLGA Microparticles by Droplet Method Dolomite s API encapsulation system for PLGA 20 µm to
Microfluidic Synthesis PLGA Microparticles by Droplet Method - App. Note Continuous Microfluidic Synthesis of PLGA Microparticles by Droplet Method Dolomite s API encapsulation system for PLGA 20 µm to
Sanitary Engineering. Coagulation and Flocculation. Week 3
 Sanitary Engineering Coagulation and Flocculation Week 3 1 Coagulation and Flocculation Colloidal particles are too small to be removed by sedimentation or by sand filtration processes. Coagulation: Destabilization
Sanitary Engineering Coagulation and Flocculation Week 3 1 Coagulation and Flocculation Colloidal particles are too small to be removed by sedimentation or by sand filtration processes. Coagulation: Destabilization
HIGH-THROUGHPUT DETERMINATION OF AQUEOUS SOLUBILITY, PRECIPITATION OR COLLOIDAL AGGREGATE FORMATION IN SMALL MOLECULE SCREENING SETS
 HIGH-THROUGHPUT DETERMINATION OF AQUEOUS SOLUBILITY, PRECIPITATION OR COLLOIDAL AGGREGATE FORMATION IN SMALL MOLECULE SCREENING SETS Introduction: Determining the aqueous solubility of a compound is an
HIGH-THROUGHPUT DETERMINATION OF AQUEOUS SOLUBILITY, PRECIPITATION OR COLLOIDAL AGGREGATE FORMATION IN SMALL MOLECULE SCREENING SETS Introduction: Determining the aqueous solubility of a compound is an
EXPERIMENTAL SETUP AND PROCEDURE
 CHAPTER 3 EXPERIMENTAL SETUP AND PROCEDURE 3.1 Determination of vapour-liquid equilibria Isobaric Vapour-Liquid Equilibria date have been obtained, using a Smith and Bonner [39] type still which is a modified
CHAPTER 3 EXPERIMENTAL SETUP AND PROCEDURE 3.1 Determination of vapour-liquid equilibria Isobaric Vapour-Liquid Equilibria date have been obtained, using a Smith and Bonner [39] type still which is a modified
5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 1: EXPERIMENTAL CHEMISTRY 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 1: EXPERIMENTAL CHEMISTRY
 5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 1: EXPERIMENTAL CHEMISTRY 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 1: EXPERIMENTAL CHEMISTRY SUB-TOPIC 1.2 METHODS OF PURIFICATION AND ANALYSIS LEARNING
5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 1: EXPERIMENTAL CHEMISTRY 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 1: EXPERIMENTAL CHEMISTRY SUB-TOPIC 1.2 METHODS OF PURIFICATION AND ANALYSIS LEARNING
Jahresbericht 2003 der Arbeitsgruppe Experimentalphysik Prof. Dr. Michael Farle
 olloidal Synthesis of Magnetic Nanoparticles V. Salgueirino Maceira and M. Farle 1 Institut für Physik, Universität Duisburg-Essen, Lotharstr. 1, 47048 Duisburg 1. Introduction 1 The synthesis of monodisperse
olloidal Synthesis of Magnetic Nanoparticles V. Salgueirino Maceira and M. Farle 1 Institut für Physik, Universität Duisburg-Essen, Lotharstr. 1, 47048 Duisburg 1. Introduction 1 The synthesis of monodisperse
INDBOND 3000 Dry Strength Resin for Paper
 INDBOND 3000 Dry Strength Resin for Paper INDBOND 3000 Dry Strength Resins are specially formulated polymers designed for better paper making and to improve strength characteristics like burst factor,
INDBOND 3000 Dry Strength Resin for Paper INDBOND 3000 Dry Strength Resins are specially formulated polymers designed for better paper making and to improve strength characteristics like burst factor,
NANOMEDICINE. WILEY A John Wiley and Sons, Ltd., Publication DESIGN AND APPLICATIONS OF MAGNETIC NANOMATERIALS, NANOSENSORS AND NANOSYSTEMS
 NANOMEDICINE DESIGN AND APPLICATIONS OF MAGNETIC NANOMATERIALS, NANOSENSORS AND NANOSYSTEMS Vijay K. Varadan Linfeng Chen Jining Xie WILEY A John Wiley and Sons, Ltd., Publication Preface About the Authors
NANOMEDICINE DESIGN AND APPLICATIONS OF MAGNETIC NANOMATERIALS, NANOSENSORS AND NANOSYSTEMS Vijay K. Varadan Linfeng Chen Jining Xie WILEY A John Wiley and Sons, Ltd., Publication Preface About the Authors
Magnetic Iron Oxide Nanoparticles as Long Wavelength Photoinitiators for Free Radical Polymerization
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2015 SUPPORTING INFORMATION Magnetic Iron Oxide Nanoparticles as Long Wavelength Photoinitiators
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2015 SUPPORTING INFORMATION Magnetic Iron Oxide Nanoparticles as Long Wavelength Photoinitiators
CELL STRUCTURE & FUNCTION
 CELL STRUCTURE & FUNCTION CELL TYPES Living cells can be classified into 2 different types on the basis of their internal structure: 4. Prokaryotic Cells 5. Eukaryotic Cells 1. Prokaryotic Cells Are the
CELL STRUCTURE & FUNCTION CELL TYPES Living cells can be classified into 2 different types on the basis of their internal structure: 4. Prokaryotic Cells 5. Eukaryotic Cells 1. Prokaryotic Cells Are the
NANO 243/CENG 207 Course Use Only
 L12: Drug Loading & Quantification May 15, 2018 1. Drug loading techniques 1.1 Physical approaches Nanprecipitation Single emulsion Double emulsion Encapsulation Remote loading 1.2 Chemical approaches
L12: Drug Loading & Quantification May 15, 2018 1. Drug loading techniques 1.1 Physical approaches Nanprecipitation Single emulsion Double emulsion Encapsulation Remote loading 1.2 Chemical approaches
Preview Slides for Introduction to Protein Formulation and Delivery
 Preview Slides for Introduction to Protein Formulation and Delivery Tim Kelly, Ph.D. VP, Biopharmaceutical Development KBI Biopharma, Inc Durham, NC Pooja Arora, Ph.D. Senior Manufacturing Technical Specialist
Preview Slides for Introduction to Protein Formulation and Delivery Tim Kelly, Ph.D. VP, Biopharmaceutical Development KBI Biopharma, Inc Durham, NC Pooja Arora, Ph.D. Senior Manufacturing Technical Specialist
Particle interactions in dry and suspension based formulations
 Finnish Society of Physical Pharmacy, 9 th Feb 2012 Particle interactions in dry and suspension based formulations Pharmaceutical Surface Science Research Group, Department of Pharmacy and Pharmacology,
Finnish Society of Physical Pharmacy, 9 th Feb 2012 Particle interactions in dry and suspension based formulations Pharmaceutical Surface Science Research Group, Department of Pharmacy and Pharmacology,
Chapter 2: Chemical Basis of Life I. Introduction A. The study of chemistry is essential for the study of physiology because
 Shier, Butler, and Lewis: Hole s Human Anatomy and Physiology, 11 th ed. Chapter 2: Chemical Basis of Life Chapter 2: Chemical Basis of Life I. Introduction A. The study of chemistry is essential for the
Shier, Butler, and Lewis: Hole s Human Anatomy and Physiology, 11 th ed. Chapter 2: Chemical Basis of Life Chapter 2: Chemical Basis of Life I. Introduction A. The study of chemistry is essential for the
Overview. Lecture 5 Colloidal Dispersions
 Physical Pharmacy Lecture 5 Colloidal Dispersions Assistant Lecturer in Pharmaceutics Overview Dispersed Systems Classification Colloidal Systems Properties of Colloids Optical Properties Kinetic Properties
Physical Pharmacy Lecture 5 Colloidal Dispersions Assistant Lecturer in Pharmaceutics Overview Dispersed Systems Classification Colloidal Systems Properties of Colloids Optical Properties Kinetic Properties
Magnetic Particles for Phosphorus Adsorption in Simulated Phosphate Solution
 215 4th International Conference on Informatics, Environment, Energy and Applications Volume 82 of IPCBEE (215) DOI: 1.7763/IPCBEE. 215.V82.28 Magnetic Particles for Phosphorus Adsorption in Simulated
215 4th International Conference on Informatics, Environment, Energy and Applications Volume 82 of IPCBEE (215) DOI: 1.7763/IPCBEE. 215.V82.28 Magnetic Particles for Phosphorus Adsorption in Simulated
BRIEFING. Pharmacopeial Discussion Group Sign Off Document Attributes EP JP USP Definition Loss on drying Readily carbonizable substances
 BRIEFING Saccharin, NF 22 page 2825 and page 1711 of PF 29(5) [Sept. Oct. 2003]. The United States Pharmacopeia is the coordinating pharmacopeia for the international harmonization of the compendial standards
BRIEFING Saccharin, NF 22 page 2825 and page 1711 of PF 29(5) [Sept. Oct. 2003]. The United States Pharmacopeia is the coordinating pharmacopeia for the international harmonization of the compendial standards
Introduction to E&Ls 1
 Introduction to E&Ls 1 Overview What industries need to determine E&Ls Define extractables and leachables Basic overview of an E&L study Regulatory landscape 2 A leader in plastics analysis Jordi Labs
Introduction to E&Ls 1 Overview What industries need to determine E&Ls Define extractables and leachables Basic overview of an E&L study Regulatory landscape 2 A leader in plastics analysis Jordi Labs
Formulation and Ocular Bioavailability of Certain Drugs from Ophthalmic Preparations
 Formulation and Ocular Bioavailability of Certain Drugs from Ophthalmic Preparations Thesis presented by Ghada Saad Ahmed El-Moursy B. Pharm. Mansoura University (2007) Submitted in Partial Fulfillment
Formulation and Ocular Bioavailability of Certain Drugs from Ophthalmic Preparations Thesis presented by Ghada Saad Ahmed El-Moursy B. Pharm. Mansoura University (2007) Submitted in Partial Fulfillment
International Journal of Chemistry and Pharmaceutical Sciences
 F. Fathi et al IJCPS, 2014, Vol.2(4): 760-764 Research Article ISSN: 2321-3132 International Journal of Chemistry and Pharmaceutical Sciences www.pharmaresearchlibrary.com/ijcps Synthesis and Surface modification
F. Fathi et al IJCPS, 2014, Vol.2(4): 760-764 Research Article ISSN: 2321-3132 International Journal of Chemistry and Pharmaceutical Sciences www.pharmaresearchlibrary.com/ijcps Synthesis and Surface modification
CDER Risk Assessment to Evaluate Potential Risks from the Use of Nanomaterials in Drug Products
 CDER Risk Assessment to Evaluate Potential Risks from the Use of Nanomaterials in Drug Products Celia N. Cruz, Ph.D. CDER Nanotechnology Working Group Office of Pharmaceutical Science 1 Disclaimer The
CDER Risk Assessment to Evaluate Potential Risks from the Use of Nanomaterials in Drug Products Celia N. Cruz, Ph.D. CDER Nanotechnology Working Group Office of Pharmaceutical Science 1 Disclaimer The
Heat Capacity of Water A) heat capacity amount of heat required to change a substance s temperature by exactly 1 C
 CHEMISTRY Ch. 13 Notes: Water and Its Solutions NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. 13.1 Notes I. Water Molecule Characteristics POLAR molecule (a
CHEMISTRY Ch. 13 Notes: Water and Its Solutions NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. 13.1 Notes I. Water Molecule Characteristics POLAR molecule (a
Multifunctional polyphosphazene-coated multi-walled carbon. nanotubes for the synergistic treatment of redox-responsive
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2017 Supporting information for Multifunctional polyphosphazene-coated multi-walled carbon
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2017 Supporting information for Multifunctional polyphosphazene-coated multi-walled carbon
CHEMISTRY Ch. 14 Notes: Mixtures and Solutions NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 CHEMISTRY Ch. 14 Notes: Mixtures and Solutions NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. 14.1 notes I. Types of mixtures (mixture a physical blend of substances)
CHEMISTRY Ch. 14 Notes: Mixtures and Solutions NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. 14.1 notes I. Types of mixtures (mixture a physical blend of substances)
Magnetic Silica Particles for Catalysis
 4 Magnetic Silica Particles for atalysis Abstract Monodisperse magnetizable colloidal silica particles in a stable dispersion have been functionalized with a homogeneous catalyst: a PP-pincer Pd-complex.
4 Magnetic Silica Particles for atalysis Abstract Monodisperse magnetizable colloidal silica particles in a stable dispersion have been functionalized with a homogeneous catalyst: a PP-pincer Pd-complex.
Val Joly (16-20 June 2014)
 Val Joly (16-20 June 2014) Introduction to drug discovery 1) Bases of solid state pharmaceutics Introduction into Pharmaceutical Technology (M-P Flament 2x45 ) Introduction to Pharmaceutical Technology
Val Joly (16-20 June 2014) Introduction to drug discovery 1) Bases of solid state pharmaceutics Introduction into Pharmaceutical Technology (M-P Flament 2x45 ) Introduction to Pharmaceutical Technology
SPHERO TM Magnetic Particles
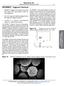 SPHER TM Magnetic Particles SPHER TM Magnetic Microparticles provide high quality and reproducible results for your application Allow for rapid and reliable binding between the target and magnetic particle
SPHER TM Magnetic Particles SPHER TM Magnetic Microparticles provide high quality and reproducible results for your application Allow for rapid and reliable binding between the target and magnetic particle
Formation of valine microcrystals through rapid antisolvent precipitation
 Formation of valine microcrystals through rapid antisolvent precipitation Miroslav Variny a, Sandra Alvarez de Miguel b, Barry D. Moore c, Jan Sefcik b a Department of Chemical and Biochemical Engineering,
Formation of valine microcrystals through rapid antisolvent precipitation Miroslav Variny a, Sandra Alvarez de Miguel b, Barry D. Moore c, Jan Sefcik b a Department of Chemical and Biochemical Engineering,
COCRYSTAL SCREENING USING SUPERCRITICAL FLUID-ASSISTED PROCESSES
 COCRYSTAL SCREENING USING SUPERCRITICAL FLUID-ASSISTED PROCESSES Luis Padrela, Miguel Rodrigues, Sitaram P. Velaga *, Anabela C. Fernandes, Henrique A. Matos, Edmundo Gomes de Azevedo Department of Chemical
COCRYSTAL SCREENING USING SUPERCRITICAL FLUID-ASSISTED PROCESSES Luis Padrela, Miguel Rodrigues, Sitaram P. Velaga *, Anabela C. Fernandes, Henrique A. Matos, Edmundo Gomes de Azevedo Department of Chemical
Biology of Humans: Concepts, Applications, and Issues, 6e (Goodenough) Chapter 2 Chemistry Comes to Life
 Biology of Humans: Concepts, Applications, and Issues, 6e (Goodenough) Chapter 2 Chemistry Comes to Life 2.1 Multiple Choice Questions 1) A neutral atom must contain. A) an equal number of protons and
Biology of Humans: Concepts, Applications, and Issues, 6e (Goodenough) Chapter 2 Chemistry Comes to Life 2.1 Multiple Choice Questions 1) A neutral atom must contain. A) an equal number of protons and
Matter and Substances Section 3-1
 Matter and Substances Section 3-1 Key Idea: All matter is made up of atoms. An atom has a positively charges core surrounded by a negatively charged region. An atom is the smallest unit of matter that
Matter and Substances Section 3-1 Key Idea: All matter is made up of atoms. An atom has a positively charges core surrounded by a negatively charged region. An atom is the smallest unit of matter that
II. The physico-chemical properties of proteins
 II. The physico-chemical properties of proteins Proteins differ by there physical and chemical properties: Molecular mass Total electrical charge Termolability Solubility Molecular weight of the proteins
II. The physico-chemical properties of proteins Proteins differ by there physical and chemical properties: Molecular mass Total electrical charge Termolability Solubility Molecular weight of the proteins
Downloaded from
 Science For Class IX Is Matter Around Us Pure (Q.1) Name the process which can be used to recover sugar from an aqueous sugar solution. (Q.2) What happens when a saturated solution is heated?
Science For Class IX Is Matter Around Us Pure (Q.1) Name the process which can be used to recover sugar from an aqueous sugar solution. (Q.2) What happens when a saturated solution is heated?
The microparticle system has become an indispensable part of the controlled drug delivery fields for the past few decades since it can
 Microencapsulation Microencapsulation is a process or technique by which thin coatings can be applied reproducibly to small particles of solids, droplets of liquids, or dispersions, thus, forming microcapsules.
Microencapsulation Microencapsulation is a process or technique by which thin coatings can be applied reproducibly to small particles of solids, droplets of liquids, or dispersions, thus, forming microcapsules.
Different Biodegradable Silica Structures In Drug Delivery. Mika Jokinen
 Different Biodegradable Silica Structures In Drug Delivery Mika Jokinen & Different Morphologies of Biodegradable Silica - Several levels of morphology ; different forms & structures - monoliths, fibers,
Different Biodegradable Silica Structures In Drug Delivery Mika Jokinen & Different Morphologies of Biodegradable Silica - Several levels of morphology ; different forms & structures - monoliths, fibers,
EVPP 110 Lecture Exam #1 Study Questions Fall 2003 Dr. Largen
 EVPP 110 Lecture Exam #1 Study Questions Fall 2003 Dr. Largen These study questions are meant to focus your study of the material for the first exam. The absence here of a topic or point covered in lecture
EVPP 110 Lecture Exam #1 Study Questions Fall 2003 Dr. Largen These study questions are meant to focus your study of the material for the first exam. The absence here of a topic or point covered in lecture
Encapsulation of enzyme in metal ion-surfactant nanocomposites for
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Supporting information for Encapsulation of enzyme in metal ion-surfactant nanocomposites for catalysis
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Supporting information for Encapsulation of enzyme in metal ion-surfactant nanocomposites for catalysis
Magnetically-driven selective synthesis of Au clusters on Fe 3 O 4 Nanoparticles
 Electronic Supplementary Material (ESI) for Chemical Communications Magnetically-driven selective synthesis of Au clusters on Fe 3 O 4 Nanoparticles Víctor Sebastian, M. Pilar Calatayud, Gerardo F. Goya
Electronic Supplementary Material (ESI) for Chemical Communications Magnetically-driven selective synthesis of Au clusters on Fe 3 O 4 Nanoparticles Víctor Sebastian, M. Pilar Calatayud, Gerardo F. Goya
Applied Surfactants: Principles and Applications
 Applied Surfactants: Principles and Applications Tadros, Tharwat F. ISBN-13: 9783527306299 Table of Contents Preface. 1 Introduction. 1.1 General Classification of Surface Active Agents. 1.2 Anionic Surfactants.
Applied Surfactants: Principles and Applications Tadros, Tharwat F. ISBN-13: 9783527306299 Table of Contents Preface. 1 Introduction. 1.1 General Classification of Surface Active Agents. 1.2 Anionic Surfactants.
Characterization Methods of Manufactured Nanomaterials for EHS Studies
 Characterization Methods of Manufactured Nanomaterials for EHS Studies Steven W Brown, MS, CIH International Standards Organization Technical Committee #229 on Nanotechnologies Convener Work Group #3 Environmental
Characterization Methods of Manufactured Nanomaterials for EHS Studies Steven W Brown, MS, CIH International Standards Organization Technical Committee #229 on Nanotechnologies Convener Work Group #3 Environmental
Synthesis of 12 nm iron oxide nanoparticles
 Electronic Supporting Information for Dendronized iron oxide nanoparticles as contrast agent for MRI Brice Basly, a Delphine Felder-Flesch,* a Pascal Perriat, b Claire Billotey, c Jacqueline Taleb, c Geneviève
Electronic Supporting Information for Dendronized iron oxide nanoparticles as contrast agent for MRI Brice Basly, a Delphine Felder-Flesch,* a Pascal Perriat, b Claire Billotey, c Jacqueline Taleb, c Geneviève
Chapter content. Reference
 Chapter 7 HPLC Instrumental Analysis Rezaul Karim Environmental Science and Technology Jessore University of Science and Technology Chapter content Liquid Chromatography (LC); Scope; Principles Instrumentation;
Chapter 7 HPLC Instrumental Analysis Rezaul Karim Environmental Science and Technology Jessore University of Science and Technology Chapter content Liquid Chromatography (LC); Scope; Principles Instrumentation;
COLLOID CHEMISTRY MD. KHAIRUL ISLAM
 COLLOID CHEMISTRY MD. KHAIRUL ISLAM HISTORICAL BACKGROUND Thomas Graham (1861) observed that crystalline substances such as sugar, urea, and sodium chloride passed through the membrane, while others like
COLLOID CHEMISTRY MD. KHAIRUL ISLAM HISTORICAL BACKGROUND Thomas Graham (1861) observed that crystalline substances such as sugar, urea, and sodium chloride passed through the membrane, while others like
University of Pécs Institute of Pharmaceutical Technology and Biopharmacy
 University of Pécs Institute of Pharmaceutical Technology and Biopharmacy Particle Definition In a continuous phase the particle is an (mostly in gaseous or liquid material) existing, dispersed, interface
University of Pécs Institute of Pharmaceutical Technology and Biopharmacy Particle Definition In a continuous phase the particle is an (mostly in gaseous or liquid material) existing, dispersed, interface
Bis sulfone Reagents. Figure 1.
 Bis sulfone Reagents An intact IgG molecule has four accessible inter chain disulfide bonds that can be reduced to form eight free cysteine thiols, which can serve as sites for conjugation. The reaction
Bis sulfone Reagents An intact IgG molecule has four accessible inter chain disulfide bonds that can be reduced to form eight free cysteine thiols, which can serve as sites for conjugation. The reaction
ELECTROSPRAY: NOVEL FABRICATION METHOD FOR BIODEGRADABLE POLYMERIC NANOPARTICLES FOR FURTHER APPLICATIONS IN DRUG DELIVERY SYSTEMS
 ELECTROSPRAY: NOVEL FABRICATION METHOD FOR BIODEGRADABLE POLYMERIC NANOPARTICLES FOR FURTHER APPLICATIONS IN DRUG DELIVERY SYSTEMS Ali Zarrabi a, Manouchehr Vossoughi b a Institute for Nanscience & Nanotechnology,
ELECTROSPRAY: NOVEL FABRICATION METHOD FOR BIODEGRADABLE POLYMERIC NANOPARTICLES FOR FURTHER APPLICATIONS IN DRUG DELIVERY SYSTEMS Ali Zarrabi a, Manouchehr Vossoughi b a Institute for Nanscience & Nanotechnology,
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1)
 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) 1) Which of the following statements about the atom A) It has 12 neutrons in its nucleus. B) It
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) 1) Which of the following statements about the atom A) It has 12 neutrons in its nucleus. B) It
Hole s Human Anatomy and Physiology Eleventh Edition. Chapter 2
 Hole s Human Anatomy and Physiology Eleventh Edition Shier Butler Lewis Chapter 2 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. CHAPTER 2 CHEMICAL BASIS OF
Hole s Human Anatomy and Physiology Eleventh Edition Shier Butler Lewis Chapter 2 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. CHAPTER 2 CHEMICAL BASIS OF
DOXYCYCLINE HYCLATE Final text to replace published monograph in The International Pharmacopoeia (November 2007)
 November 2007 DXYCYCLINE YCLATE Final text to replace published monograph in The International Pharmacopoeia (November 2007) This monograph was adopted at the Forty-first W Expert Committee on Specifications
November 2007 DXYCYCLINE YCLATE Final text to replace published monograph in The International Pharmacopoeia (November 2007) This monograph was adopted at the Forty-first W Expert Committee on Specifications
Alain Dufresne. Nanocellulose. From Nature to High Performance Tailored Materials OE GRUYTER
 Alain Dufresne Nanocellulose From Nature to High Performance Tailored Materials OE GRUYTER Contents Preface - vii 1 Cellulose and potential reinforcement-1 1.1 Polysaccharides-1 1.2 Chemical structure
Alain Dufresne Nanocellulose From Nature to High Performance Tailored Materials OE GRUYTER Contents Preface - vii 1 Cellulose and potential reinforcement-1 1.1 Polysaccharides-1 1.2 Chemical structure
Chapter 2: Chemical Basis of Life
 Chapter 2: Chemical Basis of Life Chemistry is the scientific study of the composition of matter and how composition changes. In order to understand human physiological processes, it is important to understand
Chapter 2: Chemical Basis of Life Chemistry is the scientific study of the composition of matter and how composition changes. In order to understand human physiological processes, it is important to understand
Karine Chesnel BYU. Idaho State University, Physics department 22 September 2014
 Karine Chesnel BYU Idaho State University, Physics department 22 September 2014 Potential applications of magnetic nanoparticles Magnetic recording Biomedical applications J. Mater. Chem., 19, 6258 6266
Karine Chesnel BYU Idaho State University, Physics department 22 September 2014 Potential applications of magnetic nanoparticles Magnetic recording Biomedical applications J. Mater. Chem., 19, 6258 6266
BIOLOGY 101. CHAPTER 3: Water and Life: The Molecule that supports all Live
 BIOLOGY 101 CHAPTER 3: Water and Life: The Molecule that supports all Live The Molecule that Supports all Life CONCEPTS: 3.1 Polar covalent bonds in water molecules result in hydrogen bonding 3.2 Four
BIOLOGY 101 CHAPTER 3: Water and Life: The Molecule that supports all Live The Molecule that Supports all Life CONCEPTS: 3.1 Polar covalent bonds in water molecules result in hydrogen bonding 3.2 Four
Interface Science. in Pharmaceutical. Development
 Colloid and Interface Science in Pharmaceutical Research and Development Hiroyuki Ohshima Kimiko Makino CT rjjsfc/v IxlK AMSTERDAM BOSTON HEIDELBERG LONDON NEW YORK OXFORD PARIS SAN DIEGO SAN FRANCISCO
Colloid and Interface Science in Pharmaceutical Research and Development Hiroyuki Ohshima Kimiko Makino CT rjjsfc/v IxlK AMSTERDAM BOSTON HEIDELBERG LONDON NEW YORK OXFORD PARIS SAN DIEGO SAN FRANCISCO
Fall Organic Chemistry Experiment #6 Fractional Crystallization (Resolution of Enantiomers)
 Suggested Reading: Fall Organic Chemistry Experiment #6 Fractional Crystallization (Resolution of Enantiomers) Jones Section 4.9 Physical Properties of Diastereomers: Optical Resolution pages 176-178 Introduction
Suggested Reading: Fall Organic Chemistry Experiment #6 Fractional Crystallization (Resolution of Enantiomers) Jones Section 4.9 Physical Properties of Diastereomers: Optical Resolution pages 176-178 Introduction
Chemistry FINAL: CONTENT Review Packet
 Chemistry FINAL: CONTENT Review Packet Name: Period: Date: Classification of Matter & Chemical/ Physical Changes 1. are substances that are made up of two or more elements which are chemically combined
Chemistry FINAL: CONTENT Review Packet Name: Period: Date: Classification of Matter & Chemical/ Physical Changes 1. are substances that are made up of two or more elements which are chemically combined
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) A neutral atom must contain. A) an equal number of protons, neutrons, and electrons B) an equal
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) A neutral atom must contain. A) an equal number of protons, neutrons, and electrons B) an equal
Controlled Assembly of Organic Whispering Gallery Mode Microlasers as Highly Sensitive Chemical Sensors
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Controlled Assembly of Organic Whispering Gallery Mode Microlasers
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Controlled Assembly of Organic Whispering Gallery Mode Microlasers
The Better Way to Characterize Nanoparticles MANTA s ViewSizer 3000
 info@mantainc.com www.mantainc.com The Better Way to Characterize Nanoparticles MANTA s ViewSizer 3000 MANTA s Most Advanced Nanoparticle Tracking Analysis technology performs individual particle analysis
info@mantainc.com www.mantainc.com The Better Way to Characterize Nanoparticles MANTA s ViewSizer 3000 MANTA s Most Advanced Nanoparticle Tracking Analysis technology performs individual particle analysis
ENVR 116 Introduction to Aerosol Science December 17, 2001 FINAL EXAMINATION
 ENVR 116 Introduction to Aerosol Science December 17, 2001 FINAL EXAMINATION Please answer all questions on the attached sheets Answer Question No. 1 and 4 of the remaining 6 questions. No extra credit
ENVR 116 Introduction to Aerosol Science December 17, 2001 FINAL EXAMINATION Please answer all questions on the attached sheets Answer Question No. 1 and 4 of the remaining 6 questions. No extra credit
Generation of monodisperse aerosols through condensation nuclei control
 Air Pollution XV 505 Generation of monodisperse aerosols through condensation nuclei control H. M. Kadlimatti 1, S. Gangamma 2 & S. K. Varghese 3 1 Department of Mechanical Engineering, Basaveshwar Engineering
Air Pollution XV 505 Generation of monodisperse aerosols through condensation nuclei control H. M. Kadlimatti 1, S. Gangamma 2 & S. K. Varghese 3 1 Department of Mechanical Engineering, Basaveshwar Engineering
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. Figure 2.1
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. Figure 2.1 1) Which compound in Figure 2.1 is an ester? 1) A) a b c d e Answer: D 2) A scientist
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. Figure 2.1 1) Which compound in Figure 2.1 is an ester? 1) A) a b c d e Answer: D 2) A scientist
