Fundamentals of Nuclear Power. Original slides provided by Dr. Daniel Holland
|
|
|
- Stuart Johns
- 6 years ago
- Views:
Transcription
1 Fundamentals of Nuclear Power Original slides provided by Dr. Daniel Holland
2 Nuclear Fission We convert mass into energy by breaking large atoms (usually Uranium) into smaller atoms. Note the increases in binding energy per nucleon. Audio Link
3
4 A slow moving neutron induces fission in Uranium 235
5 Fission products The fission products shown are just examples, there are a lot of different possibilities with varying probabilities
6 Expanding Chain Reaction The fission reaction produces more neutrons which can then induce fission in other Uranium atoms.
7
8 Linear Chain Reaction Obviously, an expanding chain reaction cannot be sustained for long (bomb). For controlled nuclear power, once we reach our desired power level we want each fission to produce exactly one additional fission
9 Tricks of the trade Slow moving (thermal) neutrons are more effective at inducing fission, but, fissions produce fast moving electron. We need to slow neutrons down. Fissions typically produce several neutrons but a linear chain reaction only needs one. We need to get rid of a good fraction of our neutrons.
10 Moderator Neutrons are slowed down by having them collide with light atoms (Water in US reactors). Highest level of energy transfer occurs when the masses of the colliding particles are equal (ex: neutron and hydrogen)
11 Control Rods Control rods are made of a material that absorbs excess neutrons (usually Boron or Cadmium). By controlling the number of neutrons, we can control the rate of fissions
12 Basic Ideas The Uranium is both the fuel and the source of neutrons. The neutrons induce the fissions The Water acts as both the moderator and a heat transfer medium. Control rods regulate the energy output by sucking up excess neutrons
13 Practicalities Processing of Uranium Each ton of Uranium ore produces 3-5 lbs of Uranium compounds Uranium ore is processed near the mine to produce yellow cake, a material rich in U3O8. Only 0.7% of U in yellow cake is 235U. Most of the rest is 238U which does not work for fission power. Audio Link
14 US Uranium Deposits
15 World Distribution of Uranium
16 Enrichment To be used in US reactors, fuel must be 3-5% 235U. Yellow cake is converted into UF6 and this compound is enriched using gaseous diffusion and/or centrifuges. There are some reactor designs that run on pure yellow cake.
17 NOTE: A nuclear bomb requires nearly 100% pure 235U or 239Pu. The 3% found in reactor grade Uranium CANNOT create a nuclear explosion!
18 Fuel Pellets The enriched UF6 is converted into UO2 which is then made into fuel pellets. The fuel pellets are collected into long tubes. (~12ft). The fuel rods are collected into bundles (~200 rods per bundle ~175 bundles in the core
19 Cladding The material that the fuel rods are made out of is called cladding. It must be permeable to neutrons and be able to withstand high heats. Typically cladding is made of stainless steel or zircaloy.
20
21 Controlling the chain reaction depends on Arrangement of the fuel/control rods Quality of the moderator Quality of the Uranium fuel Neutron energy required for high probability of fission
22 Two common US reactor types: Boiling Water Reactor and Pressurized Water Reactor. BWR: P=1000 psi T=545 F PWR P=2250 psi T=600 F PWR is most common and is basis of marine nuclear power.
23 Reactor is inside a large containment building
24
25 Other Options Other countries use different reactor designs. Some use heavy water (D2O) as a moderator. Some use Graphite as a moderator. Some are designed to use pure yellow cake without further enrichment Liquid metal such as sodium or gasses such as Helium are possibilities to use for coolants
26 Breeder Reactors A big problem with nuclear power is the creation of Plutonium in the reactor core. This is a long lived radioactive element that is difficult to store. Q: Why not use it as a fuel too?
27 Basic Idea Process that creates the Pu. During fission use one of the extra neutrons to create a Pu atom n+ 92 U 92 U U 23min 93 Np+ 1 β Np 2.4 days 94 Pu+ 1 β
28 Somewhat difficult in that we want fast neutrons to breed the 239Pu out of the 238 U, but we want slow neutrons to induce the fission of 235U. Requires a different design of reactor. Doubling time: Time required to produce twice as many 239Pu atoms as 235U destroyed. A good design will have a 610 doubling time. There are no currently operating breeder reactors in the US.
29 Nuclear Power in the US We currently generate approximately 20% of our electricity using nuclear power. No new nuclear power plants have been ordered since the late 1970 s. Even new plants are nearing 20 years old and will start to need replacing.
30 US Nuclear Power Plants
31
32 World Nuclear Power
33
34
35
36
Term 3 Week 2 Nuclear Fusion & Nuclear Fission
 Term 3 Week 2 Nuclear Fusion & Nuclear Fission Tuesday, November 04, 2014 Nuclear Fusion To understand nuclear fusion & fission Nuclear Fusion Why do stars shine? Stars release energy as a result of fusing
Term 3 Week 2 Nuclear Fusion & Nuclear Fission Tuesday, November 04, 2014 Nuclear Fusion To understand nuclear fusion & fission Nuclear Fusion Why do stars shine? Stars release energy as a result of fusing
AN OVERVIEW OF NUCLEAR ENERGY. Prof. Mushtaq Ahmad, MS, PhD, MIT, USA
 AN OVERVIEW OF NUCLEAR ENERGY Prof. Mushtaq Ahmad, MS, PhD, MIT, USA Outline of the Seminar 2 Motivation and Importance of Nuclear Energy Future Energy Planning in the Kingdom Current Status of Nuclear
AN OVERVIEW OF NUCLEAR ENERGY Prof. Mushtaq Ahmad, MS, PhD, MIT, USA Outline of the Seminar 2 Motivation and Importance of Nuclear Energy Future Energy Planning in the Kingdom Current Status of Nuclear
The discovery of nuclear reactions need not bring about the destruction of mankind any more than the discovery of matches - Albert Einstein
 The world has achieved brilliance without wisdom, power without conscience. Ours is a world of nuclear giants and ethical infants. - Omar Bradley (US general) The discovery of nuclear reactions need not
The world has achieved brilliance without wisdom, power without conscience. Ours is a world of nuclear giants and ethical infants. - Omar Bradley (US general) The discovery of nuclear reactions need not
Step 2: Calculate the total amount of U-238 present at time=0. Step 4: Calculate the rate constant for the decay process.
 LP#9. A meteor contains 0.556 g of Pb-206 to every 1.00g U-238. Determine the age of the meteor. Step 1: Calculate the moles of each nuclide present. 0.566g Pb-206 x 1.00g U-238 x Step 2: Calculate the
LP#9. A meteor contains 0.556 g of Pb-206 to every 1.00g U-238. Determine the age of the meteor. Step 1: Calculate the moles of each nuclide present. 0.566g Pb-206 x 1.00g U-238 x Step 2: Calculate the
Nuclear Chemistry. The nuclei of some unstable isotopes change by releasing energy and particles, collectively known as radiation
 Nuclear Chemistry The nuclei of some unstable isotopes change by releasing energy and particles, collectively known as radiation Spontaneous nuclear reactions - five kinds: ) Emission of α-particles: 4
Nuclear Chemistry The nuclei of some unstable isotopes change by releasing energy and particles, collectively known as radiation Spontaneous nuclear reactions - five kinds: ) Emission of α-particles: 4
Energy. on this world and elsewhere. Visiting today: Prof. Paschke
 Energy on this world and elsewhere Visiting today: Prof. Paschke Instructor: Gordon D. Cates Office: Physics 106a, Phone: (434) 924-4792 email: cates@virginia.edu Course web site available at www.phys.virginia.edu,
Energy on this world and elsewhere Visiting today: Prof. Paschke Instructor: Gordon D. Cates Office: Physics 106a, Phone: (434) 924-4792 email: cates@virginia.edu Course web site available at www.phys.virginia.edu,
Fission and Chain Reactions
 The Harnessed Atom Lesson Five Fission and Chain Reactions What you need to know about Fission and Chain Reactions: Fission Chain reaction Uranium fuel Mining Milling Enrichment Fuel fabrication 2 Nuclear
The Harnessed Atom Lesson Five Fission and Chain Reactions What you need to know about Fission and Chain Reactions: Fission Chain reaction Uranium fuel Mining Milling Enrichment Fuel fabrication 2 Nuclear
Lecture 14, 8/9/2017. Nuclear Reactions and the Transmutation of Elements Nuclear Fission; Nuclear Reactors Nuclear Fusion
 Lecture 14, 8/9/2017 Nuclear Reactions and the Transmutation of Elements Nuclear Fission; Nuclear Reactors Nuclear Fusion Nuclear Reactions and the Transmutation of Elements A nuclear reaction takes place
Lecture 14, 8/9/2017 Nuclear Reactions and the Transmutation of Elements Nuclear Fission; Nuclear Reactors Nuclear Fusion Nuclear Reactions and the Transmutation of Elements A nuclear reaction takes place
Relative abundances of carbon isotopes in our atmosphere are:
 Relative abundances of carbon isotopes in our atmosphere are: - C-12 (stable) - C-13 (stable) - C-14 (radioactive) 0.0000000001% The C-14 is incorporated into compounds such as CO2. This gets photosynthesized
Relative abundances of carbon isotopes in our atmosphere are: - C-12 (stable) - C-13 (stable) - C-14 (radioactive) 0.0000000001% The C-14 is incorporated into compounds such as CO2. This gets photosynthesized
NUCLEI. Atomic mass unit
 13 NUCLEI Atomic mass unit It is a unit used to express the mass of atoms and particles inside it. One atomic mass unit is the mass of atom. 1u = 1.660539 10. Chadwick discovered neutron. The sum of number
13 NUCLEI Atomic mass unit It is a unit used to express the mass of atoms and particles inside it. One atomic mass unit is the mass of atom. 1u = 1.660539 10. Chadwick discovered neutron. The sum of number
Episode 528: Controlling fission
 Episode 528: Controlling fission In this episode, you can look at the different features of the core of a nuclear reactor, and explain its operation using your students knowledge of nuclear physics. Summary
Episode 528: Controlling fission In this episode, you can look at the different features of the core of a nuclear reactor, and explain its operation using your students knowledge of nuclear physics. Summary
Nuclear Energy; Effects and Uses of Radiation
 Nuclear Energy; Effects and Uses of Radiation Nuclear Reactions and the Transmutation of Elements A nuclear reaction takes place when a nucleus is struck by another nucleus or particle. Compare with chemical
Nuclear Energy; Effects and Uses of Radiation Nuclear Reactions and the Transmutation of Elements A nuclear reaction takes place when a nucleus is struck by another nucleus or particle. Compare with chemical
Write down the nuclear equation that represents the decay of neptunium 239 into plutonium 239.
 Q1.A rod made from uranium 238 ( U) is placed in the core of a nuclear reactor where it absorbs free neutrons. When a nucleus of uranium 238 absorbs a neutron it becomes unstable and decays to neptunium
Q1.A rod made from uranium 238 ( U) is placed in the core of a nuclear reactor where it absorbs free neutrons. When a nucleus of uranium 238 absorbs a neutron it becomes unstable and decays to neptunium
Nuclear Reactions. Fission Fusion
 Nuclear Reactions Fission Fusion Nuclear Reactions and the Transmutation of Elements A nuclear reaction takes place when a nucleus is struck by another nucleus or particle. Compare with chemical reactions!
Nuclear Reactions Fission Fusion Nuclear Reactions and the Transmutation of Elements A nuclear reaction takes place when a nucleus is struck by another nucleus or particle. Compare with chemical reactions!
Nuclear Energy Learning Outcomes
 1 Nuclear Energy Learning Outcomes Describe the principles underlying fission and fusion. Interpret nuclear reactions. Discuss nuclear weapons. Describe the structure and operation of a nuclear reactor.
1 Nuclear Energy Learning Outcomes Describe the principles underlying fission and fusion. Interpret nuclear reactions. Discuss nuclear weapons. Describe the structure and operation of a nuclear reactor.
Nuclear Energy Learning Outcomes. Nuclear Fission. Chain Reaction
 by fastfission public domain by fastfission public domain 1 Nuclear Energy Learning Outcomes Describe the principles underlying fission and fusion. Interpret nuclear reactions. Discuss nuclear weapons.
by fastfission public domain by fastfission public domain 1 Nuclear Energy Learning Outcomes Describe the principles underlying fission and fusion. Interpret nuclear reactions. Discuss nuclear weapons.
Nuclear Fission. Conceptual Physics 11 th Edition. Nuclear Fission. Nuclear Fission. Nuclear Fission. This lecture will help you understand:
 Conceptual Physics 11 th Edition A typical uranium fission reaction: Chapter 34: NUCLEAR FISSION AND FUSION Note the mass number as well as atomic numbers balance. This lecture will help you understand:
Conceptual Physics 11 th Edition A typical uranium fission reaction: Chapter 34: NUCLEAR FISSION AND FUSION Note the mass number as well as atomic numbers balance. This lecture will help you understand:
The Physics of Nuclear Reactors. Heather King Physics 420
 The Physics of Nuclear Reactors Heather King Physics 420 Nuclear Reactions A nuclear reaction is a reaction that involves atomic nuclei, or nuclear particles (protons, neutrons), producing products different
The Physics of Nuclear Reactors Heather King Physics 420 Nuclear Reactions A nuclear reaction is a reaction that involves atomic nuclei, or nuclear particles (protons, neutrons), producing products different
WELCOME TO PERIOD 18: CONSEQUENCES OF NUCLEAR ENERGY
 WELCOME TO PERIOD 18: CONSEQUENCES OF NUCLEAR ENERGY Homework #17 is due today. Midterm 2: Weds, Mar 27, 7:45 8:55 pm (Same room as your midterm 1 exam.) Covers periods 10 19 and videos 3 & 4 Review: Tues,
WELCOME TO PERIOD 18: CONSEQUENCES OF NUCLEAR ENERGY Homework #17 is due today. Midterm 2: Weds, Mar 27, 7:45 8:55 pm (Same room as your midterm 1 exam.) Covers periods 10 19 and videos 3 & 4 Review: Tues,
Some nuclear particles:
 1 N U C L E A R R E A C T I O N S Definitions: Atomic Number or Nuclear Charge: The number of protons in the nucleus. This determines which element is present. It s the whole number of the element on the
1 N U C L E A R R E A C T I O N S Definitions: Atomic Number or Nuclear Charge: The number of protons in the nucleus. This determines which element is present. It s the whole number of the element on the
R.A. Chaplin Department of Chemical Engineering, University of New Brunswick, Canada
 NUCLEAR REACTOR CONFIGURATION R.A. Chaplin Department of Chemical Engineering, University of New Brunswick, Canada Keywords: Nuclear Reactors, Reactor Types, Reactor Arrangement, Technical Data Contents
NUCLEAR REACTOR CONFIGURATION R.A. Chaplin Department of Chemical Engineering, University of New Brunswick, Canada Keywords: Nuclear Reactors, Reactor Types, Reactor Arrangement, Technical Data Contents
Introducing nuclear fission The Fizzics Organization
 Nuclear Fission is the splitting of the nucleus of an atom into two or more parts by hitting it with a small particle, almost always a neutron (a proton would be repelled from the positive nucleus and
Nuclear Fission is the splitting of the nucleus of an atom into two or more parts by hitting it with a small particle, almost always a neutron (a proton would be repelled from the positive nucleus and
Chapter 10. Answers to examination-style questions. Answers Marks Examiner s tips. 1 (a) (i) 238. (ii) β particle(s) 1 Electron antineutrinos 1
 (a) (i) 238 92 U + 0 n 239 92 U (ii) β particle(s) Electron antineutrinos (b) For: Natural uranium is 98% uranium-238 which would be otherwise unused. Plutonium-239 would not need to be stored long-term
(a) (i) 238 92 U + 0 n 239 92 U (ii) β particle(s) Electron antineutrinos (b) For: Natural uranium is 98% uranium-238 which would be otherwise unused. Plutonium-239 would not need to be stored long-term
CH0204 Organic Chemical Technology
 CH0204 Organic Chemical Technology Lecture 15 Chapter 5 Nuclear Industries Assistant Professor (OG) Department of Chemical Engineering 1 Overview of topics Chapter 5 Nuclear Industries 1 2 3 4 Nuclear
CH0204 Organic Chemical Technology Lecture 15 Chapter 5 Nuclear Industries Assistant Professor (OG) Department of Chemical Engineering 1 Overview of topics Chapter 5 Nuclear Industries 1 2 3 4 Nuclear
Chemistry 500: Chemistry in Modern Living. Topic 5: The Fires of Nuclear Fission. Atomic Structure, Nuclear Fission and Fusion, and Nuclear.
 Chemistry 500: Chemistry in Modern Living 1 Topic 5: The Fires of Nuclear Fission Atomic Structure, Nuclear Fission and Fusion, and Nuclear Weapons Chemistry in Context, 2 nd Edition: Chapter 8, Pages
Chemistry 500: Chemistry in Modern Living 1 Topic 5: The Fires of Nuclear Fission Atomic Structure, Nuclear Fission and Fusion, and Nuclear Weapons Chemistry in Context, 2 nd Edition: Chapter 8, Pages
nuclear fission nucleus slightly mass
 Nuclear Fuel A nuclear fuel pellet contains about 4 grams of fuel It produces the same amount of energy as a ton of coal or 150 gallons of gasoline It s fairly cheap - $3 per pellet (compare to 150 gallons
Nuclear Fuel A nuclear fuel pellet contains about 4 grams of fuel It produces the same amount of energy as a ton of coal or 150 gallons of gasoline It s fairly cheap - $3 per pellet (compare to 150 gallons
Nuclear Energy ECEG-4405
 Nuclear Energy ECEG-4405 Today s Discussion Technical History and Developments Atom Nuclear Energy concepts and Terms Features Fission Critical Mass Uranium Fission Nuclear Fusion and Fission Fusion Fission
Nuclear Energy ECEG-4405 Today s Discussion Technical History and Developments Atom Nuclear Energy concepts and Terms Features Fission Critical Mass Uranium Fission Nuclear Fusion and Fission Fusion Fission
Nuclear Reactions A Z. Radioactivity, Spontaneous Decay: Nuclear Reaction, Induced Process: x + X Y + y + Q Q > 0. Exothermic Endothermic
 Radioactivity, Spontaneous Decay: Nuclear Reactions A Z 4 P D+ He + Q A 4 Z 2 Q > 0 Nuclear Reaction, Induced Process: x + X Y + y + Q Q = ( m + m m m ) c 2 x X Y y Q > 0 Q < 0 Exothermic Endothermic 2
Radioactivity, Spontaneous Decay: Nuclear Reactions A Z 4 P D+ He + Q A 4 Z 2 Q > 0 Nuclear Reaction, Induced Process: x + X Y + y + Q Q = ( m + m m m ) c 2 x X Y y Q > 0 Q < 0 Exothermic Endothermic 2
Fission Reactors. Alternatives Inappropriate. Fission Reactors
 Page 1 of 5 Fission Reactors The Polywell Reactor Nuclear Reactions Alternatives Inappropriate Hidden Costs of Carbon Web Site Home Page Fission Reactors There are about 438 Neutron Fission Power Reactors
Page 1 of 5 Fission Reactors The Polywell Reactor Nuclear Reactions Alternatives Inappropriate Hidden Costs of Carbon Web Site Home Page Fission Reactors There are about 438 Neutron Fission Power Reactors
1. Which is the most commonly used molten metal for cooling of nuclear reactors? A. Zinc B. Sodium C. Calcium D. Mercury
 1. Which is the most commonly used molten metal for cooling of nuclear reactors? A. Zinc B. Sodium C. Calcium D. Mercury 2. Commercial power generation from fusion reactor is not yet possible, because
1. Which is the most commonly used molten metal for cooling of nuclear reactors? A. Zinc B. Sodium C. Calcium D. Mercury 2. Commercial power generation from fusion reactor is not yet possible, because
Radioactivity is the spontaneous disintegration of nuclei. The first radioactive. elements discovered were the heavy atoms thorium and uranium.
 Chapter 16 What is radioactivity? Radioactivity is the spontaneous disintegration of nuclei. The first radioactive elements discovered were the heavy atoms thorium and uranium. These heavy atoms and others
Chapter 16 What is radioactivity? Radioactivity is the spontaneous disintegration of nuclei. The first radioactive elements discovered were the heavy atoms thorium and uranium. These heavy atoms and others
turbine (a) (i) Which part of the power station provides thermal (heat) energy from a chain reaction?
 Nuclear fission and radiation 1 The diagram shows parts of a nuclear power station. control rods boiler steam generator electricity out turbine condenser nuclear reactor (a) (i) Which part of the power
Nuclear fission and radiation 1 The diagram shows parts of a nuclear power station. control rods boiler steam generator electricity out turbine condenser nuclear reactor (a) (i) Which part of the power
SRI VIDYA COLLEGE OF ENGINEERING & TECHNOLOGY QUESTION BANK UNIT II -TWOMARKS. UNIT-II NUCLEAR POWER PLANTS:
 -TWOMARKS. UNIT-II NUCLEAR POWER PLANTS: 1.What is meant by radioactivity? It refers to the german name of Radio-Activitat. Radioactivity is the spontaneous disintegration of atomic nuclei. The nucleus
-TWOMARKS. UNIT-II NUCLEAR POWER PLANTS: 1.What is meant by radioactivity? It refers to the german name of Radio-Activitat. Radioactivity is the spontaneous disintegration of atomic nuclei. The nucleus
Energy. on this world and elsewhere. Instructor: Gordon D. Cates Office: Physics 106a, Phone: (434)
 Energy on this world and elsewhere Instructor: Gordon D. Cates Office: Physics 106a, Phone: (434) 924-4792 email: cates@virginia.edu Course web site available at www.phys.virginia.edu, click on classes
Energy on this world and elsewhere Instructor: Gordon D. Cates Office: Physics 106a, Phone: (434) 924-4792 email: cates@virginia.edu Course web site available at www.phys.virginia.edu, click on classes
Mechanical Engineering Introduction to Nuclear Engineering /12
 Mechanical Engineering Objectives In this lecture you will learn the following In this lecture the population and energy scenario in India are reviewed. The imminent rapid growth of nuclear power is brought
Mechanical Engineering Objectives In this lecture you will learn the following In this lecture the population and energy scenario in India are reviewed. The imminent rapid growth of nuclear power is brought
NUCLEAR ENGINEERING. 6. Amongst the following, the fissionable materials are (a) U233andPu239 (b) U23iandPu233 (c) U235andPu235 (d) U238andPu239
 NUCLEAR ENGINEERING 1. The efficiency of a nuclear power plant in comparsion to a conventional thermal power plant is (a) same (b) more (c) less (d) may be less or mote depending on size (e) unpredictable.
NUCLEAR ENGINEERING 1. The efficiency of a nuclear power plant in comparsion to a conventional thermal power plant is (a) same (b) more (c) less (d) may be less or mote depending on size (e) unpredictable.
40 Nuclear Fission and Fusion. Nuclear fission and nuclear fusion reactions release huge amounts of energy.
 Nuclear fission and nuclear fusion reactions release huge amounts of energy. In 1939, just at the beginning of World War II, a nuclear reaction was discovered that released much more energy per atom than
Nuclear fission and nuclear fusion reactions release huge amounts of energy. In 1939, just at the beginning of World War II, a nuclear reaction was discovered that released much more energy per atom than
Nuclear 14.notebook. January 26, Radioactivity and Half Life
 Radioactivity and Half Life All elements with more than 82 protons have unstable nuclei (i.e. Bi and beyond!) and are said to be radioactive. This is because there are too many protons in too small a volume
Radioactivity and Half Life All elements with more than 82 protons have unstable nuclei (i.e. Bi and beyond!) and are said to be radioactive. This is because there are too many protons in too small a volume
Nuclear 14.notebook. February 11, Jan 17 1:50 PM. Jan 17 1:51 PM. Jan 17 1:51 PM. Jan 17 1:52 PM. Jan 17 1:53 PM.
 Nuclear Binding Energy Radioactivity and Half Life All elements with more than 82 protons have unstable nuclei (i.e. Bi and beyond!) and are said to be radioactive. This is because there are too many protons
Nuclear Binding Energy Radioactivity and Half Life All elements with more than 82 protons have unstable nuclei (i.e. Bi and beyond!) and are said to be radioactive. This is because there are too many protons
Nuclear Chemistry. Transmutations and the Creation of Elements
 Nuclear Chemistry Transmutations and the Creation of Elements Nuclear Fusion When two smaller elements are fused together to form a larger element. Fusion is Hard! There are two competing forces in an
Nuclear Chemistry Transmutations and the Creation of Elements Nuclear Fusion When two smaller elements are fused together to form a larger element. Fusion is Hard! There are two competing forces in an
UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY
 UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY teacher version www.toppr.com Contents (a) Types of Radiation (b) Properties of Radiation (c) Dangers of Radiation (d) Rates of radioactive decay (e) Nuclear
UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY teacher version www.toppr.com Contents (a) Types of Radiation (b) Properties of Radiation (c) Dangers of Radiation (d) Rates of radioactive decay (e) Nuclear
UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY
 UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY student version www.toppr.com Contents (a) Types of Radiation (b) Properties of Radiation (c) Dangers of Radiation (d) Rates of radioactive decay (e) Nuclear
UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY student version www.toppr.com Contents (a) Types of Radiation (b) Properties of Radiation (c) Dangers of Radiation (d) Rates of radioactive decay (e) Nuclear
Nuclear Fission & Fusion
 Nuclear Fission & Fusion 1 Nuclear Fission 2 There is a delicate balance between nuclear attraction and electrical repulsion between protons in the nucleus. Nuclear Fission If the uranium nucleus is stretched
Nuclear Fission & Fusion 1 Nuclear Fission 2 There is a delicate balance between nuclear attraction and electrical repulsion between protons in the nucleus. Nuclear Fission If the uranium nucleus is stretched
Nuclear Physics and Nuclear Reactions
 Slide 1 / 33 Nuclear Physics and Nuclear Reactions The Nucleus Slide 2 / 33 Proton: The charge on a proton is +1.6x10-19 C. The mass of a proton is 1.6726x10-27 kg. Neutron: The neutron is neutral. The
Slide 1 / 33 Nuclear Physics and Nuclear Reactions The Nucleus Slide 2 / 33 Proton: The charge on a proton is +1.6x10-19 C. The mass of a proton is 1.6726x10-27 kg. Neutron: The neutron is neutral. The
Nuclear Reactions and E = mc 2. L 38 Modern Physics [4] Hazards of radiation. Radiation sickness. Biological effects of nuclear radiation
![Nuclear Reactions and E = mc 2. L 38 Modern Physics [4] Hazards of radiation. Radiation sickness. Biological effects of nuclear radiation Nuclear Reactions and E = mc 2. L 38 Modern Physics [4] Hazards of radiation. Radiation sickness. Biological effects of nuclear radiation](/thumbs/77/76435546.jpg) L 38 Modern Physics [4] Nuclear physics what s s inside the nucleus and what holds it together what is radioactivity, halflife carbon dating Nuclear energy nuclear fission nuclear fusion nuclear reactors
L 38 Modern Physics [4] Nuclear physics what s s inside the nucleus and what holds it together what is radioactivity, halflife carbon dating Nuclear energy nuclear fission nuclear fusion nuclear reactors
Power Installations based on Activated Nuclear Reactions of Fission and Synthesis
 Yu.V. Grigoriev 1,2, A.V. Novikov-Borodin 1 1 Institute for Nuclear Research RAS, Moscow, Russia 2 Joint Institute for Nuclear Research, Dubna, Russia Power Installations based on Activated Nuclear Reactions
Yu.V. Grigoriev 1,2, A.V. Novikov-Borodin 1 1 Institute for Nuclear Research RAS, Moscow, Russia 2 Joint Institute for Nuclear Research, Dubna, Russia Power Installations based on Activated Nuclear Reactions
u d Fig. 6.1 (i) Identify the anti-proton from the table of particles shown in Fig [1]
![u d Fig. 6.1 (i) Identify the anti-proton from the table of particles shown in Fig [1] u d Fig. 6.1 (i) Identify the anti-proton from the table of particles shown in Fig [1]](/thumbs/79/80122691.jpg) 1 (a) Fig. 6.1 shows the quark composition of some particles. proton neutron A B u u d u d d u d u u u u d Fig. 6.1 (i) Identify the anti-proton from the table of particles shown in Fig. 6.1. (ii) State
1 (a) Fig. 6.1 shows the quark composition of some particles. proton neutron A B u u d u d d u d u u u u d Fig. 6.1 (i) Identify the anti-proton from the table of particles shown in Fig. 6.1. (ii) State
SUB-CHAPTER D.1. SUMMARY DESCRIPTION
 PAGE : 1 / 12 CHAPTER D. REACTOR AND CORE SUB-CHAPTER D.1. SUMMARY DESCRIPTION Chapter D describes the nuclear, hydraulic and thermal characteristics of the reactor, the proposals made at the present stage
PAGE : 1 / 12 CHAPTER D. REACTOR AND CORE SUB-CHAPTER D.1. SUMMARY DESCRIPTION Chapter D describes the nuclear, hydraulic and thermal characteristics of the reactor, the proposals made at the present stage
Nuclear Fuel Reprocessing. By Daniel Bolgren Jeff Menees
 Nuclear Fuel Reprocessing By Daniel Bolgren Jeff Menees Goals of the Project 1. Develop a reprocessing technique that can: 1. Reprocess used nuclear fuel. 2. Reduce proliferation concerns. 2. Optimize
Nuclear Fuel Reprocessing By Daniel Bolgren Jeff Menees Goals of the Project 1. Develop a reprocessing technique that can: 1. Reprocess used nuclear fuel. 2. Reduce proliferation concerns. 2. Optimize
Quiz, Physics & Chemistry
 Eight Sessions 1. Pressurized Water Reactor 2. Quiz, Thermodynamics & HTFF 3. Quiz, Physics & Chemistry 4. Exam #1, Electrical Concepts & Systems 5. Quiz, Materials Science 6. Quiz, Strength of Materials
Eight Sessions 1. Pressurized Water Reactor 2. Quiz, Thermodynamics & HTFF 3. Quiz, Physics & Chemistry 4. Exam #1, Electrical Concepts & Systems 5. Quiz, Materials Science 6. Quiz, Strength of Materials
YEAR 11 Physics Unit 1
 Hampton Park Secondary College Student s Name: Senior School Examinations Semester 1 2011 Home Group: Student Number Figures Words YEAR 11 Physics Unit 1 Written Examination QUESTION AND ANSWER BOOK Structure
Hampton Park Secondary College Student s Name: Senior School Examinations Semester 1 2011 Home Group: Student Number Figures Words YEAR 11 Physics Unit 1 Written Examination QUESTION AND ANSWER BOOK Structure
 (C) Number of protons (D) Number of electrons 10. The number of neutrons accompanying the formation of 54 Xe 139 and 38 Sr 94 from the absorption of a slow neutron by 92 U 235, followed by nuclear fission
(C) Number of protons (D) Number of electrons 10. The number of neutrons accompanying the formation of 54 Xe 139 and 38 Sr 94 from the absorption of a slow neutron by 92 U 235, followed by nuclear fission
Nuclear fission and fusion are processes that involve extremely large amounts of energy.
 Nuclear Reactions & Energy Nuclear fission and fusion are processes that involve extremely large amounts of energy. Fission = the splitting of a large nucleus into two smaller nuclei, subatomic particles
Nuclear Reactions & Energy Nuclear fission and fusion are processes that involve extremely large amounts of energy. Fission = the splitting of a large nucleus into two smaller nuclei, subatomic particles
Nuclear Power MORE CHAPTER 11, #6. Nuclear Fission Reactors
 MORE CHAPTER 11, #6 Nuclear Power Nuclear Fission Reactors The discovery that several neutrons are emitted in the fission process led to speculation concerning the possibility of using these neutrons to
MORE CHAPTER 11, #6 Nuclear Power Nuclear Fission Reactors The discovery that several neutrons are emitted in the fission process led to speculation concerning the possibility of using these neutrons to
Lecture PowerPoint. Chapter 31 Physics: Principles with Applications, 6 th edition Giancoli
 Lecture PowerPoint Chapter 31 Physics: Principles with Applications, 6 th edition Giancoli 2005 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the
Lecture PowerPoint Chapter 31 Physics: Principles with Applications, 6 th edition Giancoli 2005 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the
Question to the class: What are the pros, cons, and uncertainties of using nuclear power?
 Energy and Society Week 11 Section Handout Section Outline: 1. Rough sketch of nuclear power (15 minutes) 2. Radioactive decay (10 minutes) 3. Nuclear practice problems or a discussion of the appropriate
Energy and Society Week 11 Section Handout Section Outline: 1. Rough sketch of nuclear power (15 minutes) 2. Radioactive decay (10 minutes) 3. Nuclear practice problems or a discussion of the appropriate
Energy. on this world and elsewhere. Instructor: Gordon D. Cates Office: Physics 106a, Phone: (434)
 Energy on this world and elsewhere Instructor: Gordon D. Cates Office: Physics 106a, Phone: (434) 924-4792 email: cates@virginia.edu Course web site available at www.phys.virginia.edu, click on classes
Energy on this world and elsewhere Instructor: Gordon D. Cates Office: Physics 106a, Phone: (434) 924-4792 email: cates@virginia.edu Course web site available at www.phys.virginia.edu, click on classes
RADIOACTIVITY & HALF-LIFE Part 3
 RADIOACTIVITY & HALF-LIFE Part 3 Half-Life Half-life: is the rate of decay for a radioactive isotope. is the time required for half of an original quantity of an element to decay. is constant and independent
RADIOACTIVITY & HALF-LIFE Part 3 Half-Life Half-life: is the rate of decay for a radioactive isotope. is the time required for half of an original quantity of an element to decay. is constant and independent
PHYS:1200 LECTURE 36 ATOMIC AND NUCLEAR PHYSICS (4)
 1 PHYS:1200 LECTURE 36 ATOMIC AND NUCLEAR PHYSICS (4) This last lecture of the course will focus on nuclear energy. There is an enormous reservoir of energy in the nucleus and it can be released either
1 PHYS:1200 LECTURE 36 ATOMIC AND NUCLEAR PHYSICS (4) This last lecture of the course will focus on nuclear energy. There is an enormous reservoir of energy in the nucleus and it can be released either
Lesson 14: Reactivity Variations and Control
 Lesson 14: Reactivity Variations and Control Reactivity Variations External, Internal Short-term Variations Reactivity Feedbacks Reactivity Coefficients and Safety Medium-term Variations Xe 135 Poisoning
Lesson 14: Reactivity Variations and Control Reactivity Variations External, Internal Short-term Variations Reactivity Feedbacks Reactivity Coefficients and Safety Medium-term Variations Xe 135 Poisoning
17 Neutron Life Cycle
 17 Neutron Life Cycle A typical neutron, from birth as a prompt fission neutron to absorption in the fuel, survives for about 0.001 s (the neutron lifetime) in a CANDU. During this short lifetime, it travels
17 Neutron Life Cycle A typical neutron, from birth as a prompt fission neutron to absorption in the fuel, survives for about 0.001 s (the neutron lifetime) in a CANDU. During this short lifetime, it travels
Nuclear Physics 2. D. atomic energy levels. (1) D. scattered back along the original direction. (1)
 Name: Date: Nuclear Physics 2. Which of the following gives the correct number of protons and number of neutrons in the nucleus of B? 5 Number of protons Number of neutrons A. 5 6 B. 5 C. 6 5 D. 5 2. The
Name: Date: Nuclear Physics 2. Which of the following gives the correct number of protons and number of neutrons in the nucleus of B? 5 Number of protons Number of neutrons A. 5 6 B. 5 C. 6 5 D. 5 2. The
 Nuclear Fission and Fusion A. Nuclear Fission. The process of splitting up of the nucleus of a heavy atom into two nuclei more or less of equal fragments when bombarded with neutron simultaneously releasing
Nuclear Fission and Fusion A. Nuclear Fission. The process of splitting up of the nucleus of a heavy atom into two nuclei more or less of equal fragments when bombarded with neutron simultaneously releasing
One nucleus splits into two smaller nuclei and typically a few neutrons by the bombardment of a neutron. U-235 is the only naturally occurring
 One nucleus splits into two smaller nuclei and typically a few neutrons by the bombardment of a neutron. U-235 is the only naturally occurring nuclide that fissions However, both U-238 and Th-232 can be
One nucleus splits into two smaller nuclei and typically a few neutrons by the bombardment of a neutron. U-235 is the only naturally occurring nuclide that fissions However, both U-238 and Th-232 can be
Neutron reproduction. factor ε. k eff = Neutron Life Cycle. x η
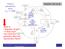 Neutron reproduction factor k eff = 1.000 What is: Migration length? Critical size? How does the geometry affect the reproduction factor? x 0.9 Thermal utilization factor f x 0.9 Resonance escape probability
Neutron reproduction factor k eff = 1.000 What is: Migration length? Critical size? How does the geometry affect the reproduction factor? x 0.9 Thermal utilization factor f x 0.9 Resonance escape probability
Energy production. Stored energy. Another comparison. Fission. Fission and Fusion. Exam 3: Wed. Dec. 1 Covers Chap.
 Exam 3: Wed. Dec. 1 Covers Chap. 13-16, part of 17 No HW assignment over Thanksgiving Energy production Hydroelectric plant Uses 60,000 tons/sec water to produce 1,000 MW Last time: Radioactive decay:
Exam 3: Wed. Dec. 1 Covers Chap. 13-16, part of 17 No HW assignment over Thanksgiving Energy production Hydroelectric plant Uses 60,000 tons/sec water to produce 1,000 MW Last time: Radioactive decay:
INTRODUCTION TO NUCLEAR REACTORS AND NUCLEAR POWER GENERATION. Atsushi TAKEDA & Hisao EDA
 INTRODUCTION TO NUCLEAR REACTORS AND NUCLEAR POWER GENERATION Atsushi TAKEDA & Hisao EDA 1 CONTENTS The first step toward nuclear power Physics of nuclear fission Sustained chain reaction in nuclear reactor
INTRODUCTION TO NUCLEAR REACTORS AND NUCLEAR POWER GENERATION Atsushi TAKEDA & Hisao EDA 1 CONTENTS The first step toward nuclear power Physics of nuclear fission Sustained chain reaction in nuclear reactor
L 36 Atomic and Nuclear Physics-4. Radioactivity. Nuclear reactions: E = mc 2. Hazards of radiation. Biological effects of nuclear radiation
 L 36 Atomic and Nuclear Physics- Nuclear physics what s inside the nucleus and what holds it together what is radioactivity, half-life carbon dating Nuclear energy nuclear fission nuclear fusion nuclear
L 36 Atomic and Nuclear Physics- Nuclear physics what s inside the nucleus and what holds it together what is radioactivity, half-life carbon dating Nuclear energy nuclear fission nuclear fusion nuclear
Energy & Sustainability
 Energy & Sustainability Lecture 20: Nuclear Power April 9, 2009 Radioactive Decay Each radioactive isotope has a characteristic lifetime and decays pathway Each isotope has a given probability of decay
Energy & Sustainability Lecture 20: Nuclear Power April 9, 2009 Radioactive Decay Each radioactive isotope has a characteristic lifetime and decays pathway Each isotope has a given probability of decay
Physics 30 Modern Physics Unit: Fission and Fusion
 Physics 30 Modern Physics Unit: Fission and Fusion Nuclear Energy For years and years scientists struggled to describe where energy came from. They could see the uses of energy and the results of energy
Physics 30 Modern Physics Unit: Fission and Fusion Nuclear Energy For years and years scientists struggled to describe where energy came from. They could see the uses of energy and the results of energy
Nuclear Reactions. Thornton and Rex, Ch. 13. Otto Hahn and Lise Meitner
 Nuclear Reactions Thornton and Rex, Ch. 13 Otto Hahn and Lise Meitner Reaction Kinematics Consider a general reaction, A (x, y) B or A + x Æ y + B with target A at rest. Ex. 9 Be 4 + 4 a 2 Æ 1 n 0 + 12
Nuclear Reactions Thornton and Rex, Ch. 13 Otto Hahn and Lise Meitner Reaction Kinematics Consider a general reaction, A (x, y) B or A + x Æ y + B with target A at rest. Ex. 9 Be 4 + 4 a 2 Æ 1 n 0 + 12
MCRT L8: Neutron Transport
 MCRT L8: Neutron Transport Recap fission, absorption, scattering, cross sections Fission products and secondary neutrons Slow and fast neutrons Energy spectrum of fission neutrons Nuclear reactor safety
MCRT L8: Neutron Transport Recap fission, absorption, scattering, cross sections Fission products and secondary neutrons Slow and fast neutrons Energy spectrum of fission neutrons Nuclear reactor safety
Radioactivity. L 38 Modern Physics [4] Hazards of radiation. Nuclear Reactions and E = mc 2 Einstein: a little mass goes a long way
![Radioactivity. L 38 Modern Physics [4] Hazards of radiation. Nuclear Reactions and E = mc 2 Einstein: a little mass goes a long way Radioactivity. L 38 Modern Physics [4] Hazards of radiation. Nuclear Reactions and E = mc 2 Einstein: a little mass goes a long way](/thumbs/80/81251381.jpg) L 38 Modern Physics [4] Nuclear physics what s inside the nucleus and what holds it together what is radioactivity, halflife carbon dating Nuclear energy nuclear fission nuclear fusion nuclear reactors
L 38 Modern Physics [4] Nuclear physics what s inside the nucleus and what holds it together what is radioactivity, halflife carbon dating Nuclear energy nuclear fission nuclear fusion nuclear reactors
Radiation Damage Effects in Solids. Los Alamos National Laboratory. Materials Science & Technology Division
 Radiation Damage Effects in Solids Kurt Sickafus Los Alamos National Laboratory Materials Science & Technology Division Los Alamos, NM Acknowledgements: Yuri Osetsky, Stuart Maloy, Roger Smith, Scott Lillard,
Radiation Damage Effects in Solids Kurt Sickafus Los Alamos National Laboratory Materials Science & Technology Division Los Alamos, NM Acknowledgements: Yuri Osetsky, Stuart Maloy, Roger Smith, Scott Lillard,
Aim: What are the two types of Nuclear. Reactions? Do Now: 1. Get into your groups and compare your answers to your homework.
 Aim: What are the two types of Nuclear Reactions? Do Now: 1. Get into your groups and compare your answers to your homework. Nuclear Energy In nuclear reaction, mass is converted into energy; there is
Aim: What are the two types of Nuclear Reactions? Do Now: 1. Get into your groups and compare your answers to your homework. Nuclear Energy In nuclear reaction, mass is converted into energy; there is
Lecture PowerPoints. Chapter 31 Physics: Principles with Applications, 7th edition Giancoli
 Lecture PowerPoints Chapter 31 Physics: Principles with Applications, 7th edition Giancoli This work is protected by United States copyright laws and is provided solely for the use of instructors in teaching
Lecture PowerPoints Chapter 31 Physics: Principles with Applications, 7th edition Giancoli This work is protected by United States copyright laws and is provided solely for the use of instructors in teaching
By Tim, John, Shane, Owen
 By Tim, John, Shane, Owen A few refreshers Atoms of the same element, which always have an identical number of protons, that have different numbers of neutrons, is an isotope. Protons and neutrons are
By Tim, John, Shane, Owen A few refreshers Atoms of the same element, which always have an identical number of protons, that have different numbers of neutrons, is an isotope. Protons and neutrons are
NUCLEAR CHEMISTRY. LAST TOPIC OF THE YEAR!! Name: CHANGING THE NUCLEUS OF AN ATOM. 1 P age
 NUCLEAR CHEMISTRY CHANGING THE NUCLEUS OF AN ATOM LAST TOPIC OF THE YEAR!! Name: 1 P age Why do unstable isotopes undergo nuclear reactions? Do Now: Draw Bohr models of three different isotopes of carbon
NUCLEAR CHEMISTRY CHANGING THE NUCLEUS OF AN ATOM LAST TOPIC OF THE YEAR!! Name: 1 P age Why do unstable isotopes undergo nuclear reactions? Do Now: Draw Bohr models of three different isotopes of carbon
Cambridge University Press An Introduction to the Engineering of Fast Nuclear Reactors Anthony M. Judd Excerpt More information
 INTRODUCTION WHAT FAST REACTORS CAN DO Chain Reactions Early in 1939 Meitner and Frisch suggested that the correct interpretation of the results observed when uranium is bombarded with neutrons is that
INTRODUCTION WHAT FAST REACTORS CAN DO Chain Reactions Early in 1939 Meitner and Frisch suggested that the correct interpretation of the results observed when uranium is bombarded with neutrons is that
We completed our discussion of nuclear modeling with a discussion of the liquid drop and shell models We began discussing radioactivity
 Modern Physics (PHY 3305) Lecture Notes Modern Physics (PHY 3305) Lecture Notes Nuclear Physics: Fission and Fusion (11.7) SteveSekula, 19 April 010 (created 1 April 010) Review no tags We completed our
Modern Physics (PHY 3305) Lecture Notes Modern Physics (PHY 3305) Lecture Notes Nuclear Physics: Fission and Fusion (11.7) SteveSekula, 19 April 010 (created 1 April 010) Review no tags We completed our
Unpressurized steam reactor. Controlled Fission Reactors. The Moderator. Global energy production 2000
 From last time Fission of heavy elements produces energy Only works with 235 U, 239 Pu Fission initiated by neutron absorption. Fission products are two lighter nuclei, plus individual neutrons. These
From last time Fission of heavy elements produces energy Only works with 235 U, 239 Pu Fission initiated by neutron absorption. Fission products are two lighter nuclei, plus individual neutrons. These
Nuclear power plants
 Nuclear power plants Introduction: There is a common trend throughout the world to use nuclear energy as a source of power. This is because of the rapid depletion of conventional energy sources. Transportation
Nuclear power plants Introduction: There is a common trend throughout the world to use nuclear energy as a source of power. This is because of the rapid depletion of conventional energy sources. Transportation
Physics 11 Nuclear Process. Nuclear Fusion Reactors Terminology Waste Storage Radiation and living things Nuclear Fission
 Physics 11 Nuclear Process Nuclear Fusion Reactors Terminology Waste Storage Radiation and living things Nuclear Fission Nuclear Reactors Terminology Fission Control Rods, moderator, chain reaction half-life
Physics 11 Nuclear Process Nuclear Fusion Reactors Terminology Waste Storage Radiation and living things Nuclear Fission Nuclear Reactors Terminology Fission Control Rods, moderator, chain reaction half-life
Question Answer Marks Guidance 1 (a) The neutrons interact with other uranium (nuclei) / the neutrons cause further (fission) reactions
 Question Answer Marks Guidance 1 (a) The neutrons interact with other uranium (nuclei) / the neutrons cause further (fission) reactions Not: neutrons interact with uranium atoms / molecules / particles
Question Answer Marks Guidance 1 (a) The neutrons interact with other uranium (nuclei) / the neutrons cause further (fission) reactions Not: neutrons interact with uranium atoms / molecules / particles
Special!Area!of!Study!1! Energy!from!the!nucleus!
 Outcome Year11PhysicsUnit1 SpecialAreaofStudy1 Energyfromthenucleus Chapter12 Oncompletionofthischapter,youshouldbeabletodescribeandexplaintypical fission and fusion reactions, energy transfer and transformation
Outcome Year11PhysicsUnit1 SpecialAreaofStudy1 Energyfromthenucleus Chapter12 Oncompletionofthischapter,youshouldbeabletodescribeandexplaintypical fission and fusion reactions, energy transfer and transformation
Nuclear power plants can generate large amounts of electricity.
 7.3 Nuclear Reactions Nuclear fission and fusion are processes that involve extremely large amounts of energy. Fission = the splitting of nuclei Fusion = the joining of nuclei Nuclear power plants can
7.3 Nuclear Reactions Nuclear fission and fusion are processes that involve extremely large amounts of energy. Fission = the splitting of nuclei Fusion = the joining of nuclei Nuclear power plants can
Cost Analtsis for a Nuclear Power Plant with Standby Redundant Reactor Vessel
 Research Journal of Mathematics and Statistics 2(3): 91-96, 2010 ISSN: 2040-7505 Maxwell Scientific Organization, 2010 Submitted Date: March 09, 2010 Accepted Date: April 30, 2010 Published Date: September
Research Journal of Mathematics and Statistics 2(3): 91-96, 2010 ISSN: 2040-7505 Maxwell Scientific Organization, 2010 Submitted Date: March 09, 2010 Accepted Date: April 30, 2010 Published Date: September
Chemistry Physical, Chemical, and Nuclear Changes
 Chemistry 1010 Physical, Chemical, and Nuclear Changes Review Which state of matter matches the following pictures? gas solid liquid What could the circles in these pictures represent? usually molecules,
Chemistry 1010 Physical, Chemical, and Nuclear Changes Review Which state of matter matches the following pictures? gas solid liquid What could the circles in these pictures represent? usually molecules,
L 36 Modern Physics :006 FINAL EXAM. Nuclear reactions: E = mc 2. Radioactivity. Hazards of radiation. Biological effects of nuclear radiation
 9:006 FINAL EXAM The final exam is on Monday MAY 7:30 AM - 9:30 AM in W90 CB The FE is not cumulative, and will cover lectures 3 through 36. (50 questions) The last regular lecture (Lec. 36) will be given
9:006 FINAL EXAM The final exam is on Monday MAY 7:30 AM - 9:30 AM in W90 CB The FE is not cumulative, and will cover lectures 3 through 36. (50 questions) The last regular lecture (Lec. 36) will be given
General Physics (PHY 2140)
 General Physics (PHY 2140) Lecture 20 Modern Physics Nuclear Energy and Elementary Particles Fission, Fusion and Reactors Elementary Particles Fundamental Forces Classification of Particles Conservation
General Physics (PHY 2140) Lecture 20 Modern Physics Nuclear Energy and Elementary Particles Fission, Fusion and Reactors Elementary Particles Fundamental Forces Classification of Particles Conservation
Nuclear Chemistry. Chapter 24
 Nuclear Chemistry Chapter 24 Radioactivity Radioisotopes are isotopes that have an unstable nucleus. They emit radiation to attain more stable atomic configurations in a process called radioactive decay.
Nuclear Chemistry Chapter 24 Radioactivity Radioisotopes are isotopes that have an unstable nucleus. They emit radiation to attain more stable atomic configurations in a process called radioactive decay.
Nuclear Chemistry Unit
 Nuclear Chemistry Unit January 28th HW Due Thurs. 1/30 Read pages 284 291 Define: Radioactivity Nuclear Radiation Alpha Particle Beta Particle Gamma Ray Half-Life Answer: -Questions 1-3 -Write the symbols
Nuclear Chemistry Unit January 28th HW Due Thurs. 1/30 Read pages 284 291 Define: Radioactivity Nuclear Radiation Alpha Particle Beta Particle Gamma Ray Half-Life Answer: -Questions 1-3 -Write the symbols
Conceptual Physics. Luis A. Anchordoqui. Department of Physics and Astronomy Lehman College, City University of New York. Lesson X November 14, 2017
 Conceptual Physics Luis A. Anchordoqui Department of Physics and Astronomy Lehman College, City University of New York Lesson X November 14, 2017 https://arxiv.org/abs/1711.07445 L. A. Anchordoqui (CUNY)
Conceptual Physics Luis A. Anchordoqui Department of Physics and Astronomy Lehman College, City University of New York Lesson X November 14, 2017 https://arxiv.org/abs/1711.07445 L. A. Anchordoqui (CUNY)
Национальный исследовательский Томский политехнический университет
 ЯДЕРНО ТОПЛИВНЫЙ ЦИКЛ Зяблова Н.Н, Карпова Н.Д. Национальный исследовательский Томский политехнический университет Томск, Россия Данная статья раскрывает понятие ядерно топливного цикла. Объясняет его
ЯДЕРНО ТОПЛИВНЫЙ ЦИКЛ Зяблова Н.Н, Карпова Н.Д. Национальный исследовательский Томский политехнический университет Томск, Россия Данная статья раскрывает понятие ядерно топливного цикла. Объясняет его
Lecture 13. Applications of Nuclear Physics Fission Reactors and Bombs Overview
 Lecture 13 Applications of Nuclear Physics Fission Reactors and Bombs Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 1 12.1 Overview 12.1 Induced fission Fissile nuclei Time scales of
Lecture 13 Applications of Nuclear Physics Fission Reactors and Bombs Dec 2006, Lecture 13 Nuclear Physics Lectures, Dr. Armin Reichold 1 12.1 Overview 12.1 Induced fission Fissile nuclei Time scales of
Production. David Nusbaum Project on Managing the Atom, Belfer Center October 4, 2011
 Production David Nusbaum Project on Managing the Atom, Belfer Center October 4, 2011 Where are we? Nuclear Fuel Cycle Background Pu- Radioactive, chemical element, of the actinoid series of the periodic
Production David Nusbaum Project on Managing the Atom, Belfer Center October 4, 2011 Where are we? Nuclear Fuel Cycle Background Pu- Radioactive, chemical element, of the actinoid series of the periodic
Making the Essential Ingredients of Nuclear Weapons. Matthew Bunn IGA-232, Controlling the World s Most Dangerous Weapons September 12, 2013
 Making the Essential Ingredients of Nuclear Weapons Matthew Bunn IGA-232, Controlling the World s Most Dangerous Weapons September 12, 2013 Two paths to the bomb The plutonium route Reactor: uranium fuel
Making the Essential Ingredients of Nuclear Weapons Matthew Bunn IGA-232, Controlling the World s Most Dangerous Weapons September 12, 2013 Two paths to the bomb The plutonium route Reactor: uranium fuel
The outermost container into which vitrified high level waste or spent fuel rods are to be placed. Made of stainless steel or inert alloy.
 Glossary of Nuclear Waste Terms Atom The basic component of all matter; it is the smallest part of an element having all the chemical properties of that element. Atoms are made up of protons and neutrons
Glossary of Nuclear Waste Terms Atom The basic component of all matter; it is the smallest part of an element having all the chemical properties of that element. Atoms are made up of protons and neutrons
Chapter 18. Nuclear Chemistry
 Chapter 18 Nuclear Chemistry The energy of the sun comes from nuclear reactions. Solar flares are an indication of fusion reactions occurring at a temperature of millions of degrees. Introduction to General,
Chapter 18 Nuclear Chemistry The energy of the sun comes from nuclear reactions. Solar flares are an indication of fusion reactions occurring at a temperature of millions of degrees. Introduction to General,
nuclear chemical change CH4 + 2O2 CO2 + 2H2O carbon dating
 Nuclear Chemistry I. What is nuclear chemistry? a. Nuclear changes vs. chemical changes i. A nuclear change is a change in which the nucleons (things in the nucleus) change. For instance, if the number
Nuclear Chemistry I. What is nuclear chemistry? a. Nuclear changes vs. chemical changes i. A nuclear change is a change in which the nucleons (things in the nucleus) change. For instance, if the number
