An XPS and ToF-SIMS Investigation of the Outermost Nanometres of a Poly(vinylidene difluoride) Coating.
|
|
|
- Britton Lee
- 6 years ago
- Views:
Transcription
1 An XPS and ToF-SIMS Investigation of the Outermost Nanometres of a Poly(vinylidene difluoride) Coating. Steven J. Hinder 1*, Chris Lowe 2, and John F. Watts 1 1 The Surface Analysis Laboratory, School of Engineering, University of Surrey, Guildford, Surrey GU2 7XH, UK. 2 Becker Industrial Coatings Ltd, Goodlass Road, Speke, Liverpool L24 9HJ, UK. *Correspondence to: Dr Steven J. Hinder, The Surface Analysis Laboratory, School of Engineering (H6), University of Surrey, Guildford, Surrey GU2 7XH, UK. s.hinder@surrey.ac.uk Tel: +44 (0) , Fax: +44 (0)
2 Abstract : The outermost nanometres of a poly(vinylidene difluoride) (PVdF) based coil coating have been investigated using X-ray photoelectron spectroscopy (XPS) and time-offlight secondary ion mass spectrometry (ToF-SIMS). A reference PVdF based coating formulation and three variations of this formulation were characterised by XPS. The addition of flow agent and/or acrylic co-polymers induced significant changes in the elemental and chemical composition of the coating s air/coating surface. The XPS results indicate that both the flow agent and acrylic co-polymers segregate towards the coating s air/coating surface. The XPS results also suggest that in the fully formulated coating, segregation of the flow agent and co-polymers results in the formation of a surface/sub-surface acrylic layer of sufficient thickness to mask the fluorine signal originating from PVdF. Using a Buckminsterfullerene (C 60 ) ion source as an etch source for polymers, depth profiles were obtained of the outermost nanometres of the fully formulated PVdF coating. Fluorine and oxygen depth profiles revealed compositional changes in the coating with respect to depth. The oxygen depth profile revealed the presence of an oxygen rich sub-surface layer within the coating. Molecular depth profiles, acquired for fragment ions diagnostic of the PVdF and acrylic co-polymer components of the coating formulation, revealed the presence of an acrylic co-polymers rich layer in the coating s sub-surface region. All depth profiles suggest that the PVdF coating bulk possesses a homogeneous material composition. The XPS and SIMS results suggest the fully formulated coating is composed of three distinct layers; a thin flow agent 2
3 layer at the air/coating surface, an acrylic co-polymer rich sub-surface layer and the coating bulk. Keywords : Time-of-Flight Secondary Ion Mass Spectrometry; X-ray Photoelectron Spectroscopy; PVdF coating; Depth Profiling; C 60 ; Polymer. 3
4 Introduction : Coil coatings are considered to be one of the most technologically advanced of all coatings/paint systems. As such they must possess a wide range of diverse properties. In the sheet forming processes that the coated stock undergoes, coil coatings are able to withstand, without cracking or loss of coating-metal adhesion, the severe mechanical deformations applied. Coil coatings are also able to resist corrosion and photodegradation for 20 years or more. Steel and aluminium substrates protected by coil coatings have a wide range of commercial and industrial uses. Applications include architectural cladding, caravans, household/domestic appliances and agricultural machinery. PVdF based coil coatings have seen increasing use in the protection of structures exposed to the environment. The strength of the C-F bond ensures that PVdF molecules are unreactive towards many aggressive environments. The bulk polymer is capable of yielding under applied stress rather than breaking. Finally the electrons in the C-F bond can only be excited by the very short wavelength UV light not found terrestrially as a result of the ozone layer. PVdF based coil coatings follow the contours of formed metal during the forming process without cracking except on the most severe bends. They are resistant to chemical attack and have good gloss retention when they are exposed to UV light. However, PVdF coil coatings typically require that the PVdF component be blended with a secondary polymer, often poly(methyl methacrylate) (PMMA), so as to optimise the performance of the coating [1]. Experimental research has established that the surface composition and structure 4
5 of a polymer blend is generally different to that of the bulk [2,3], presumably as a result of Gibbsian segregation because of differences in the free energy of the different polymeric components within a formulation. Thus a greater understanding of the nature of the surface and sub-surface regions of coatings based on mixed polymer systems, such as those found in PVdF/acrylic co-polymer coatings, will greatly assist the coatings formulator in areas such as intercoat adhesion and weathering. The use of XPS to investigate PVdF based materials is well documented. Areas of particularly strong research interest in PVdF materials includes PVdF based membranes and PVdF as a biocompatible material. The XPS analyses of PVdF based membranes has generated much research interest in areas such as drug permeation [4], modifying membrane chemical reactivity [5] and microfiltration [6]. PVdF has also received increasing attention as a biocompatible polymer due to its durability, chemical inertness and lack of toxicity. However, due to the lack of functional groups on a PVdF surface, routes to functionalise PVdF and immobilise molecules on its surface have had to be developed. Biomolecules have been immobilised on PVdF surfaces (and characterised by XPS) to improve cell adhesion [7] and to improve biocompatibility [8]. Conversely, procedures to minimise protein interactions with PVdF surfaces have also been developed [9]. The use of XPS to investigate phenomena associated with PVdF based coating formulations has also been reported. Hinder et al have investigated a variety of topics including intercoat adhesion between a PVdF topcoat and a poly(urethane) primer [10], the migration and 5
6 segregation of a silicone additive through a PVdF topcoat [11] and the investigation of a PVdF topcoat/poly(urethane) primer interface buried 20 µm beneath a PVdF topcoats air/coating surface [12]. It has long been a desire of polymer and organic coatings scientists to perform depth profiling experiments on polymeric/organic materials using analytical techniques such as ToF-SIMS. However, ion induced damage to polymeric (and other organic) surfaces, typically leads to a loss of chemical and molecular specificity with respect to depth within depth profile layers. Metallic and inorganic materials however are routinely investigated using ToF-SIMS depth profiling techniques. Recently, with the availability of cluster ion based etch sources, the capability to perform ToF-SIMS depth profiling of a number of polymeric (or organic) materials has been possible. The use of a SF + 5 cluster ion as a depth profiling etch tool for use on polymeric samples has been demonstrated by Fuoco et al employing PMMA thin film on silicon [13], Norrman et al also used PMMA and poly(vinyl chloride) films spin coated onto Si wafer [14] and by Mahoney et al who investigated poly(l-lactic acid) (PLLA) doped with 4-acetaminodophenol [15] and PLLA blended with a triblock co-polymer [16]. Most recently, with the introduction of the Buckminsterfullerene (C 60 ) cluster ion source as an etch tool, the depth profiling of polymeric (or organic) materials has taken great strides forward. Much of the development work of the C 60 ion source as a depth profiling etch tool has been undertaken by Winograd and his colleagues. Winograd has demonstrated C 60 depth profiling of peptides in a trehalose matrix [17], 6
7 Langmuir-Blodgett films [18,19], histamine in an ice matrix [20,21] and PMMA on silicon wafer as a model polymeric system [22]. In this paper, changes in the material composition and chemistry of the surface and sub-surface regions of a PVdF based coating formulation have been investigated. Four variations of a PVdF based coating formulations air/coating surfaces have been analysed using XPS. ToF-SIMS, using a C 60 etch source, has been employed to obtain elemental and molecular depth profiles through the outermost nanometres of the coating enabling compositional changes from the air/coating surface, through the subsurface region and down into the bulk of the coating have been investigated. Experimental : Materials and Methods The PVdF coating formulations used in this work were applied to aluminium substrate. The PVdF coatings employed were built up stepwise from one containing mainly PVdF to one containing many of the components used in a real world formulation. The real world coating formulation is principally composed of a PVdF resin blended with acrylic co-polymers. The fluoropolymer provides high durability performance and chemical resistance, whilst the acrylics enhance the film forming properties and hardness. The PVdF coating formulations employed in these studies were clear coat formulations containing no pigmentation. The formulation variations employed are described in Table 1. The PVdF coating was applied so as to obtain a 7
8 dry film thickness of µm and cured by stoving with an oven dwell time of ~30 s at a peak metal temperature of ~249 C. The samples analysed were cured PVdF based coatings applied to aluminium panels (~16 cm 10 cm 0.5 cm). For XPS characterisation of the coating surfaces a disc ~1 cm in diameter was punched from the sample panel and analysed immediately. To prepare specimens for ToF-SIMS analysis samples ~1 cm 2 were cut from the panel using an industrial guillotine. Surface Analysis by XPS. XPS analyses were performed on a Thermo VG Scientific (East Grinstead, UK) Sigma Probe spectrometer. The instrument employs a monochromated AlKα X-ray source (hν = ev) which was used at a power of 140 W. The area of analysis was approximately 500 µm diameter for the coating surfaces analysed. The pass energy was set at 20 ev for core level high-resolution spectra of all elements of interest and at 100 ev for all survey spectra. Charge compensation was achieved using an electron flood gun. The coating samples were held in place on the instruments sample stage by a sprung Cu/Be clip. Quantitative surface chemical analyses were calculated from the high resolution core level spectra, following the removal of a non-linear Shirley background. The manufacturer s Avantage software was used which incorporates the appropriate sensitivity factors and corrects for the electron energy analyser transmission function. 8
9 Surface Analysis by ToF-SIMS. ToF-SIMS analyses were carried out on an ION-TOF GmbH (Münster, Germany) TOF.SIMS 5 system. The instrument is equipped with a reflectron type analyser and microchannel plate detector with 20kV post-acceleration capability. A Bi liquid metal ion source (LMIS) was employed for mass data acquisition. Mass data was acquired using the Bi + 3 cluster ion. Such clusters ions provide superior secondary ion yields to those available from the more conventional Ga + source. Mass data acquisition was performed by raster scanning over a µm 2 area. The area analysed was at the centre of the etch crater formed using the rastered C 60 beam. A 25 kev Bi + 3 primary ion beam delivering 0.8 pa of current was used. A cycle time of 100µs was employed for mass data acquisition. A Buckminsterfullerene ion source was employed to etch the polymeric coating. The C + 60 ion was used for etching in the depth profiling experiments described here. The C + 60 etch area was µm 2 +. A 10 kv C 60 primary ion beam with a fluence of 4 x ions cm -2 was employed. An etch interval of 10 ms was used for all of the depth profile studies described here. Charge compensation was achieved using a low energy electron flood gun for a duration of 20 ms. The depth profiling analyses were performed in the IONTOF non-interlaced mode of operation. That is, each depth profiling cycle starts with mass data acquisition by the Bi + 3 LMIS; this is then is followed by etching of the coating sample using the C 60 source, finally charge compensation using low energy electron flooding completes the depth profiling cycle. 9
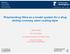
10 Results and Discussion : Today s coil coating formulation is a complex mix, typically comprising some different components. The majority of the formulation comprises resins, co-polymers and solvents. However, many additives including pigments, dispersants, flow-aids and matting agents will be added to the formulation to provide unique properties. In Figure 1 the ev binding energy region taken from XPS survey spectra of PVdF based sample coatings, S1-S4 (see Table 1) respectively, are presented. This region of the survey spectrum contains the C1s, O1s and F1s XPS peaks, these being the only elements present in the S1-S4 coating formulations investigated. Figure 1a contains the C1s (51.5 atomic %), O1s (2.6 atomic %) and F1s (45.9 atomic %) XPS peaks for the S1 coating (PVdF and plasticiser). It is observed in Figure 1a that the F1s peak (~686 ev) is the most intense peak in the spectrum and that the O1s peak (~531 ev) is the least intense. The C1s peak in Figure 1a is observed to possess two distinct component peaks. The C1s component peak at lower binding energy (~285 ev) is attributed to the hydrocarbon components within the coating materials. The C1s component peak at higher binding energy (~291 ev) in Figure 1a is attributed to the CF 2 function of the PVdF [23]. As indicated above there were only two nonsolvent components added to the S1 coating; PVdF and plasticiser. By comparing the actual measured atomic concentrations to theoretical values calculated from a knowledge of the components (Table 2), and further comparing to the calculated values of the pure components in Table 3, it is clear that the surface is not composed of a stoichiometric mixture of plasticiser and PVdF. However the fact that the oxygen 10
11 concentration is almost correct indicates that either the PVdF or the plasticiser or both are made up of components with more carbon in them. In Figure 1b the C1s, O1s and F1s peaks for the S2 coating formulation (S1 base formulation plus acrylic flow agent) are presented in the same way they were for the S1 coating sample in Figure 1a. However, for the S2 coating the intensity of the F1s peak (36.3 atomic %) is much diminished while the intensity of the O1s peak (5.1 atomic %) has increased, when compared to the S1 reference formulation (see Table 2). There are also significant differences between these values and the theoretical ones. It is also observed in Figure 1b that there is an increase in the C1s concentration (58.6 atomic %) when compared to the S1 reference coating in Figure 1a, but this is associated with a substantial change in the C1s peak shape. In Figure 1b the C1s peak now possesses a more intense lower binding energy component peak than in Figure 1a, indicating an increase in the hydrocarbon component of the S2 coating. This increase in the intensity of the hydrocarbon component peak is accompanied by a reduction in the intensity of the component peak at higher binding energy (CF 2 ) in the C1s peak. Although the component peak at higher binding energy in the C1s peak is still clearly observed in Figure 1b, it is not as distinct as in Figure 1a. The decrease in F1s concentration, reduction in intensity of the CF 2 component of the C1s peak, the increase in O1s concentration over and above the theoretical value and the greater intensity of the hydrocarbon component of the C1s peak in Figure 1b 11
12 are all attributed to the surface segregation of the acrylic flow agent included in the S2 coating formulation. See Table 3 for the theoretical values for the flow agent. It is well documented that the addition of a flow agent to a coating formulation alters the surface composition and chemistry of a coating [24], due to the formation of a flow agent segregation layer. Such flow agent segregation layers are typically ~1 nm thick [25]. This XPS data suggests that the F1s signal is being attenuated by the formation of a flow agent segregation layer at the coating surface. That is, for the S2 coating in Figure 1b, the addition of the acrylic flow agent not only results in changes in the chemical and material composition of the surface but also leads to an attenuation of the XPS signal associated with the fluorine in the PVdF, indicating that the PVdF component of the coating is buried below a flow agent segregation layer in the S2 coating. In Figure 1c C1s, O1s and F1s XPS peaks are again observed, this time for the S3 coating formulation (S1 reference formulation plus acrylic copolymers). In Figure 1c a substantial decrease in the intensity of the F1s peak (from 45.9 to 15.4 atomic %) is now observed, when compared to the S1 coating. This is accompanied by a large increase in the intensity of the O1s peak (from 2.6 to 16.4 atomic %). There is also an increase in the intensity of the C1s peak (from 51.5 to 68.3 atomic %) when compared to the S1 coating in Figures 1a (see Table 2). Perhaps more noticeable in Figure 1c is the change in the C1s peak shape. Strong attenuation of the F1s signal in the S3 coating results in a loss of the distinct CF 2 component peak at higher binding energy (as observed in Figures 1a and 1b for the S1 and S2 coatings) in the C1s XPS 12
13 peak. The CF 2 component peak is now only observed as part of the asymmetry to the higher binding energy side of the carbon peak. When compared to the theoretical values the carbon concentration is 40% higher (68.3 vs. 48.9) where as that of oxygen is only 30% higher (16.4 vs. 12.1). This could be explained by a higher PMMA concentration at the surface caused by some surface segregation. The excess carbon could be due to adsorption of adventitious carbon containing contaminants. Thus like the flow agent added to the S2 coating formulation, the acrylic copolymers (or a substantial fraction of them) segregate to the S3 coatings air/coating surface. This segregation of the acrylic copolymers both dramatically changes the material and chemical composition of the S3 coatings air/coating surface and results in the strong attenuation of the F1s signal. The attenuation of the F1s XPS signal is due to the formation of an acrylic copolymer segregation layer at the S3 coatings air/coating surface. Given that the decrease in F1s intensity observed for the S3 coating in Figure 1c is greater than that observed for the S2 coating in Figure 1b it is reasonable to assume that the acrylic copolymer segregation layer formed for the S3 coating is thicker than the segregation layer formed by the acrylic flow agent in the S2 coating. For the S4 coating the flow agent used to produce the S2 coating and the acrylic copolymers included in the S3 coating were added to the S1 reference coating formulation. The ev region of an XPS survey spectrum of the S4 coating is presented in Figure 1d. The F1s peak (0.l atomic %) is now so heavily attenuated 13
14 when compared to the F1s peaks in Figures 1a, 1b and 1c that the F1s XPS peak is now barely observed in the S4 coating survey spectrum in Figure 1d. The intensity of the O1s peak (18.8 atomic %) in Figure 1d is greater than that observed for the S3 coating in Figure 1c. There is a further substantial increase in the C1s concentration (81.1 atomic %) and hence peak intensity in the S4 coating in Figure 1d. The C1s peak shape now shows no discernible contribution from the CF 2 component peak associated with the PVdF component of the formulation and the peak shape in Figure 1d is now that of a dominant hydrocarbon peak with some minor asymmetry to the higher binding energy side of the peak. The lack of an F1s peak in Figure 1d indicates that the PVdF component of the S4 coating is now buried beneath a significant segregation layer. The concentration ratios are now very similar to those that would be observed from the pure acrylic flow aid, indicating that the surface consists largely of a fairly thick layer of the flow aid. The analysis depth of XPS is generally taken to approximate to 3λ (where λ is the electron attenuation length of the material being investigated), although one must remember that the electron intensity decays exponentially within this sampling volume, in accord with the well known Beer- Lambert formalism. Frey et al have demonstrated that λ for a fluorinated material is ~3-4 nm [26]. The total thickness of this segregation layer (flow aid plus acrylic copolymer) in the S4 coating is great enough (<<10 nm) to almost completely mask the PVdF component of the coating. This suggests the segregation layer must be at least 8 nm thick i.e. occupying most if not all of the XPS analysis depth. The very small amount of F emanates not from the underlying PVdF coating but from a very small amount of fluorinated material that is incorporated into the segregated layer. 14
15 The XPS spectra in Figures 1a-d suggest that both the acrylic flow agent included in the S2 coating and the acrylic copolymers included in the S3 coating have a tendency to segregate towards the coating s air/coating surface. However, the flow agent is designed to segregate to the coating surface, by virtue of its incompatibility, and thus aids the coating aesthetics by equilibrating the surface free energy areas that vary in this quantity. It has previously been demonstrated that the air/coating surface of a fully formulated PVdF coating is dominated by the acrylic flow agent even when acrylic copolymers are included in the formulation [27]. It is therefore reasonable to propose that the S4 coating surface is multilayer. That is, the air/coating surface of the S4 coating is composed of a flow agent segregation layer, beneath this is a subsurface layer either formed by, or rich in, the segregating acrylic copolymers. Finally, there is the coating bulk, which presumably possesses a homogeneous PVdF/acrylic copolymer composition. However, this hypothesis of a multilayer coating surface cannot be easily validated using the XPS results. To validate this hypothesis depth profiling of the PVdF coating is desired. In Figure 2 negative ion elemental depth profiles from the outermost nanometres of the S4 coating s air/coating surface are presented. Variations in the intensity (and thus concentration) of O and F have been followed with respect to etch time and thus depth into the S4 coating. The O and F depth profiles in Figure 2 were obtained using + Bi 3 cluster ions from a Bi liquid metal ion source as the analysis source and C + 60 ions from a Buckminsterfullerene (C 60 ) source as the etch source. A C + 60 etch interval of 15
16 10 ms was employed. Szakal et al have previously demonstrated that employing a C 60 source for etching enables chemical and molecular specificity to be maintained when etching through polymeric materials such as PMMA [22]. An apparent disparity exists between the XPS results and the ToF-SIMS depth profile results which is immediately obvious to any one but the casual observer! That is the XPS results predict zero or almost zero fluorine in the top few nanometers of the surface of S4 and only once the flow aid layer/acrylic copolymer layer has been eroded by the C60 source should the PVdF be revealed. Thus a zero level plateau should be observed in Figure 2a for the first 0.5 to 1s of erosion time (assuming 4-8 nm of erosion per second). However, given the differences in the elemental cross sections (the detection limit for fluorine in ToF-SIMS is far superior to that in XPS) and the analysis depths for XPS and SIMS (~10 nm and 1 nm respectively) it is possible that a F concentration of 0.1 atomic % as measured by XPS at the S4 coating surface is equal to an F - intensity of ~7x 10-2 counts/s as observed in SIMS. Additionally, it is likely that the Bi + 3 ions used for the initial analysis removes a substantial portion of the loosely bound low molecular weight flow aid, which is sitting on the surface, resulting in the phenomena observed. The concentration of F is seen to increases steadily with depth through the subsurface region of the coating (0-1 s etch) in Figure 2a. The concentration of F in the depth profile in Figure 2a then continues to increase but at a slower rate (between 1 and 2.5 s etch) before reaching a stable concentration (after 2.5 s etch) in the S4 16
17 coating bulk. That the intensity of the F depth profile is at a minimum at the air/coating surface of the S4 coating is consistent with the interpretation of the XPS data in Figures 1-4 which indicated that the S4 coating s air/coating surface is composed of the acrylic flow agent. That the F intensity increases in the sub-surface region of the S4 coating (0.1-2 s etch) is also consistent with the XPS data in Figures 1a-d in that the sub-surface region is rich in the acrylic copolymers included in the formulation but this acrylic rich layer has a finite thickness. The shape of the O depth profile in Figure 2b is substantially different to that of the F depth profile in Figure 2a. It is observed in Figure 2b that the O depth profile exhibits an intensity maximum close to the coating surface (within s etch). The O intensity then gradually decreases with etch time and thus depth until the O intensity stabilises in the bulk of the S4 coating (after 2.5 s etch). The shape of the O depth profile in Figure 2b suggests that the surface and sub-surface regions of the S4 coating are rich in O containing materials, when compared to the bulk of the S4 coating (the region beyond 2.5 s etch in Figure 2b). Given that the acrylic copolymers have a greater oxygen content than the flow aid (see Table 3), the O profile is in keeping with the interpretation of the XPS results, which suggest the formation of a flow agent surface layer over an acrylic copolymers rich subsurface segregation layer in the S4 coating. Such segregation layers would be expected to be rich in O when compared to the S4 coating bulk, as is observed in Figure 2b. 17
18 In the sub-surface region of the S4 coating the F concentration in the F depth profile in Figure 2a is increasing with depth while that in the O depth profile in Figure 2b reaches a concentration maximum in the sub-surface region of the S4 coating. The stability of the intensity of the O and F depth profiles after 2.5 s etch in Figure 2 suggests that the S4 coating bulk possesses a homogeneous material composition. This stability of the material composition of the S4 coatings bulk contrasts sharply with the material composition of the sub-surface region of the S4 coating, which exhibits considerable variation. This variation in material composition in the subsurface is due to materials included in the coating formulation segregating towards the S4 coating s air/coating surface. This tendency of components included in the coating formulation to segregate towards the S4 coating s air/coating surface is driven by the differences in the compatibility of the different components that make up the formulation. To further investigate the possible formation of an acrylic co-polymers rich subsurface layer in the S4 coating, positive ion molecular depth profiles were obtained. The molecular depth profiles in Figures 3 and 4 were obtained in the manner previously described. In Figure 3 positive ion depth profiles of the molecular fragments C 3 HF + 4, C 5 H 2 F + 5, and CF + 3, diagnostic of the PVdF component of the S4 coating [28,29], are presented. It is observed in Figure 3 that the depth profiles for the C 3 HF + 4, C 5 H 2 F + 5, and CF + 3 molecular ions originating from the PVdF are identical in shape to the F depth profile in Figure 2a (although of varying intensity). All of the depth profiles presented in Figure 3 exhibit an increasing F intensity with depth in the 18
19 surface and sub-surface regions of the S4 coating. This is followed by a region of stable F intensity in the S4 coating bulk. In Figure 4 positive ion molecular depth profiles of the fragments, C 4 H 5 O +, C 3 H 5 O +, and C 8 H 11 O + 2, diagnostic of the acrylic copolymers included in the S4 coating formulation are presented [30]. It is noted that the shapes of the depth profiles in Figure 4 are similar to those observed for the O depth profile in Figure 2b. All of the depth profiles in Figure 4 exhibit an intensity maximum in the sub-surface region of the S4 coating ( s etch). The intensity of the depth profiles in Figure 4 then falls gradually with etch time and thus depth into the coating ( s etch). The depth profiles in Figure 4 then assume more stable intensity values in the bulk of the S4 coating (beyond 1.0 s etch). The shape of the acrylic copolymer derived depth profiles in Figure 4 reveals that the O rich sub-surface layer suggested by the XPS data and the elemental depth profiles does indeed result from the segregation of the acrylic copolymers towards the S4 coating s air/coating surface. This new information concerning the surface and subsurface morphologies will have an impact on the interpretation of durability as measured by gloss retention and certainly opens the way for investigations into how the surface changes after weathering in specific environments and locations. Furthermore the thick layer of flow agent and acrylic co-polymer may explain why the well documented resistance of PVdF coatings to dirt pick up is not as good as might be anticipated from a clean PVdF surface. Precise quantification of the layer thickness may help in determining 19
20 how an inter-phase could be set up if it is desired to overcoat the PVdF. That is fluorine polymers are notoriously difficult to wet out, hence the chances of a good inter-phase of over 50nm being set up are small resulting in poor inter-coat adhesion. Knowledge of how to alter the composition of the near surface region could help in the design of a third-coat. The elemental and molecular ion depth profiles in Figures 2, 3 and 4 provide confirmatory evidence for the formation of an acrylic copolymer rich layer within the sub-surface region of the S4 coating, resulting from segregation of the acrylic copolymers towards the S4 coating s air/coating surface. The XPS analyses of the S1- S4 coating formulations and the elemental and molecular ion SIMS depth profiles of the S4 coating all indicate that the S4 coating s outermost nanometres are composed of three distinct layers. The S4 coating s air/coating surface is composed of the acrylic flow agent included in the S2 and S4 coating formulations. It has previously been demonstrated by Perruchot et al that flow agent layers are typically nm thick [25], depending on the nature and concentration of the flow agent employed. Below this flow agent layer is a sub-surface layer rich in the acrylic co-polymer included in the S3 and S4 coating formulations. The acrylic copolymers rich subsurface layer is the result of the segregation of the acrylic co-polymers towards the S4 coating s air/coating surface. Although it might be viewed that the flow agent and acrylic copolymers are both segregating to the S4 coating s air/coating surface, this is a competitive process. The levelling capability of the flow agent is better than that of the acrylic copolymers and so it is the flow agent that reaches the air/coating surface, 20
21 probably due to its molecular weight differences causing incompatibility. Some of the more compatible acrylic co-polymers may segregate towards the coating s air/coating surface alongside the flow agent, leading to the proposed structure of the near-surface region, which is somewhat different to that suggested by Gu et al who apparently observed a fluorine rich surface [31]. The segregating acrylic copolymers are therefore unable to reach the air/coating surface and thus form an acrylic co-polymers rich sub-surface layer within the S4 coating. The final layer in the coating is the S4 coating bulk, which all analyses suggest possesses a homogeneous material composition. Conclusions: The XPS and TOF-SIMS depth profiling characterisation of the outermost nanometres of a PVdF/acrylic copolymers coating based on a real world commercial formulation has been demonstrated. The XPS analyses reveal that both the flow agent and acrylic copolymers included in the formulation segregate towards the coating s air/coating surface. Addition of flow agent or acrylic copolymers to the coating formulation leads to attenuation of the F1s XPS signal. When both the flow agent and acrylic co-polymers are added to the same coating formulation the segregation layer may be sufficiently thick to mask the F1s signal from the underlying PVdF component of the formulation. The XPS results suggest the more fully formulated coating (S4) possesses an acrylic rich layer at the surface and in the sub-surface region of the coating. 21
22 We have demonstrated that a Bi analysis source and a C 60 etch source can be used in combination to obtain elemental and molecular SIMS depth profiles from complex PVdF based coating formulations. The O depth profile revealed the existence of an oxygen rich layer in the sub-surface region of the S4 coating. The F depth profile showed that the surface and sub-surface regions of the coating were depleted in F when compared to the bulk of the coating. SIMS positive ion molecular depth profiles revealed that the sub-surface region of the S4 coating is rich in the acrylic copolymers included in the coating formulation. These results confirm the segregation of the acrylic co-polymers towards the air/coating surface of the S4 coating. Overall the XPS and SIMS depth profiling results suggest the outermost nanometres of the S4 coating is composed of three distinct layers; a thin flow agent layer at the air/coating surface, an acrylic co-polymer rich sub-surface layer and the coating bulk which possesses a homogeneous material composition. 22
23 References: 1) J. Scheirs, S. Burks, A. Locaspi, Trends Polym. Sci. 3 (1995) 74. 2) H.R. Thomas, J.J. OMalley, Macromolecules 12 (1979) ) W. Lee, W.Cho, C. Ha, A. Takahara, T. Kajiyama, Polymer 36 (1995) ) L. Ying, E.T. Kang, K.G. Neoh, K.Kato, H. Iwata, J. Membrane Sci. 243 (2004) ) M. Momtaz, J-L. Dewez, J. Marchand-Brynaert, J. Membrane Sci. 250 (2005) 29. 6) J. Mueller, R.H. Davis, J. Membrane Sci. 116 (1996) 47. 7) D. Klee, Z. Ademovic, A. Bosserhoff, H. Hoecker, G. Maziolis, H-J Erli, Biomaterials 24 (2003) ) Z. Ademovic, A. Gonera, P. Mischnick D. Klee, Biomacromolecules 7 (2006) ) Z. Ademovic, D. Klee, P. Kingshott, R. Kaufmann, H. Höcker, Bimolecular Eng. 19 (2002) ) S.J. Hinder, C. Lowe, J.T. Maxted, J.F. Watts, Prog. Org. Coat. 54 (2005) ) S.J. Hinder, C. Lowe, J.T. Maxted, J.F. Watts, Prog. Org. Coat. 54 (2005) ) S.J. Hinder, C. Lowe, J.T. Maxted, J.F. Watts, Surf. Interface Anal. 36 (2004) ) E.R. Fuoco, G. Gillen, M.B.J. Wijesundara, W.E. Wallace, L. Hanley. J. Phys. Chem. B 105 (2001) ) K. Norrman, K.B. Haugshøj, N.B. Larsen. J. Phys. Chem. B 106 (2002) ) C.M. Mahoney, S.V. Robertson, G. Gillen. Anal. Chem. 76 (2004) ) C.M. Mahoney, J. Yu, J.A. Gardella Jr. Anal. Chem. 77 (2005) ) J. Cheng, N. Winograd. Anal. Chem. 77 (2005) ) A.G. Sostarecz, S. Sun, C. Szakal, A. Wucher, N. Winograd. Appl. Surf. Sci (2004)
24 19) A.G. Sostarecz, C.M. McQuaw, A. Wucher, N. Winograd. Anal. Chem. 76 (2004) ) A. Wucher, S. Sun, C. Szakal, N. Winograd. Appl. Surf. Sci (2004) ) A. Wucher, S. Sun, C. Szakal, N. Winograd. Anal. Chem. 76 (2004) ) C. Szakal, S. Sun, A.Wucher, N. Winograd. Appl. Surf. Sci (2004) ) G. Beamson, D. Briggs, High Resolution XPS of Organic Polymers; The Scienta ESCA300 Database, John Wiley & Sons Ltd: Chichester, (1992) ) S.R. Leadley, J.F. Watts, C.J. Blomfield, C. Lowe, Surf. Interface Anal. 26 (1998) ) C. Perruchot, J.F. Watts, C. Lowe, R.G. White, P.J. Cumpson, Surf. Interface Anal. 34 (2002) ) S. Frey, K. Heister, M. Zharnikov, M. Grunze, K. Tamada, R. Colarado Jr. Isr. J. Chem (2000) ) S.J. Hinder, C. Lowe, J.F. Watts, Accepted for publication in Surf. Interface Anal ) J. Feng, C-M. Chan, L-T. Weng, Polymer 41 (2000) ) P. Spevack, Y. Deslandes, Appl. Surf. Sci. 99 (1996) ) A.M. Leeson, M.R.Alexander, R.D. Short, D. Briggs, M.J. Hearn, Surf. Interface Anal. 25 (1997) ) X. Gu, C.A. Michaels, D. Nguyen, Y.C. Jean, J.W. Martin, T. Nguyen, App. Surf. Sci. 252 (2006)
25 a) F1s C o u n ts / s C1s O1s b) F1s B in d in g E n e r g y / e V C o u n t s / s O1s C1s c) F1s B i n d i n g E n e r g y / e V C1s O1s C o u n t s / s d) B in d in g E n e r g y / e V C1s C o u n t s / s O1s F1s B i n d i n g E n e r g y / e V Figure 1. The ev region of a series of XPS survey spectra of a) S1 coating (reference formulation), b) S2 coating (S1 reference formulation plus flow agent), c) S3 coating (S1 reference formulation plus acrylic co-polymers) and d) S4 coating (S1 Reference formulation plus flow agent and acrylic co-polymers). 25
26 a) 1.00E+05 F E+04 Counts / s 1.00E+03 b) 1.00E Etch Time / s 1.00E+04 Counts / s O E Etch Time / s Figure 2. ToF-SIMS negative ion mode, elemental depth profiles for a) fluorine and b) oxygen. The depth profiles were obtained from the air/coating surface of the S4 coating sample. 26
27 1.00E E+04 + C 3 HF 4 Counts / s CF + 3 C 5 H 2 F E E Etch Time / s Figure 3. ToF-SIMS positive ion mode, molecular depth profiles for the C 3 HF 4 +, CF 3 + and C 5 H 2 F 5 + fragments characteristic of PVdF. The depth profiles were obtained from the air/coating surface of the S4 coating sample. 27
28 1.00E+04 C 4 H 5 O + Counts / s 1.00E+03 C 3 H 5 O + + C 8 H 11 O E Etch Time / s Figure 4. ToF-SIMS positive ion mode, molecular depth profiles for the C 4 H 5 O +, C 5 H 9 O + and C 8 H 11 O 2 + fragments characteristic of the acrylic co-polymers included in the S4 coating formulation. The depth profiles were obtained from the air/coating surface of the S4 coating sample. 28
29 Coating S1 S2 S3 S4 Formulation PVdF Topcoat Reference Formulation As in S1 + flow agent. As in S1 + acrylic co-polymers As in S1 + flow agent and acrylic copolymers. Table 1. PVdF coating sample formulations. 29
30 Coating Surface Composition/Atomic % C1s F1s O1s Actual Theory Actual Theory Actual Theory S S S S Table 2. XPS and theoretical (from formulation) quantification data of the PVdF coating samples analysed. 30
31 Material C1s F1s O1s PVdF Plasticiser Flow Agent Acrylic Co-polymer Table 3. Theoretical values for compositions of components used in coatings formulations. 31
Interfacial Chemistry and Adhesion Phenomena: How to Analyse and How to Optimise
 Interfacial Chemistry and Adhesion Phenomena: How to Analyse and How to Optimise John F Watts Department of Mechanical Engineering Sciences The Role of Surface Analysis in Adhesion Studies Assessing surface
Interfacial Chemistry and Adhesion Phenomena: How to Analyse and How to Optimise John F Watts Department of Mechanical Engineering Sciences The Role of Surface Analysis in Adhesion Studies Assessing surface
A ToF-SIMS Investigation of a Buried Polymer-Polymer Interface Exposed by Ultra-Low-Angle Microtomy.
 *Correspondence to: Steven J. Hinder, The Surface Analysis Laboratory, School of Engineering, Mail Stop H6, University of Surrey, Guildford, Surrey GU2 7XH, UK. Email: s.hinder@surrey.ac.uk Fax: +44 (0)
*Correspondence to: Steven J. Hinder, The Surface Analysis Laboratory, School of Engineering, Mail Stop H6, University of Surrey, Guildford, Surrey GU2 7XH, UK. Email: s.hinder@surrey.ac.uk Fax: +44 (0)
The Rôle of the Adhesion Promoter in a Model Water-Borne Primer
 The Rôle of the Adhesion Promoter in a Model Water-Borne Primer Siavash Adhami, Marie-Laure Abel, Chris Lowe, John F. Watts Department of Mechanical Engineering Sciences October 13-18, 213 Cagliari, Sardinia
The Rôle of the Adhesion Promoter in a Model Water-Borne Primer Siavash Adhami, Marie-Laure Abel, Chris Lowe, John F. Watts Department of Mechanical Engineering Sciences October 13-18, 213 Cagliari, Sardinia
Reduced preferential sputtering of TiO 2 (and Ta 2 O 5 ) thin films through argon cluster ion bombardment.
 NATIOMEM Reduced preferential sputtering of TiO 2 (and Ta 2 O 5 ) thin films through argon cluster ion bombardment. R. Grilli *, P. Mack, M.A. Baker * * University of Surrey, UK ThermoFisher Scientific
NATIOMEM Reduced preferential sputtering of TiO 2 (and Ta 2 O 5 ) thin films through argon cluster ion bombardment. R. Grilli *, P. Mack, M.A. Baker * * University of Surrey, UK ThermoFisher Scientific
Surface and Interface Analysis. Investigations of Molecular Depth Profiling with Dual Beam Sputtering. Journal: Surface and Interface Analysis
 Surface and Interface Analysis Investigations of Molecular Depth Profiling with Dual Beam Sputtering Journal: Surface and Interface Analysis Manuscript ID: Draft Wiley - Manuscript type: SIMS proceedings
Surface and Interface Analysis Investigations of Molecular Depth Profiling with Dual Beam Sputtering Journal: Surface and Interface Analysis Manuscript ID: Draft Wiley - Manuscript type: SIMS proceedings
PHI Model 06-C60 Sputter Ion Gun
 PHI Model 6-C6 Sputter Ion Gun Introduction: Physical Electronics introduced the model 6-C6 C 6 sputter ion gun and its unique capabilities for surface cleaning and depth profiling of soft materials (figure
PHI Model 6-C6 Sputter Ion Gun Introduction: Physical Electronics introduced the model 6-C6 C 6 sputter ion gun and its unique capabilities for surface cleaning and depth profiling of soft materials (figure
David Jesson, School of Engineering (H6), University of Surrey, Guildford, Surrey, UK, GU2 7XH ORGANIC-INORGANIC HYBRID NANOPARTICLES: ADSORPTION
 David Jesson, School of Engineering (H6), University of Surrey, Guildford, Surrey, UK, GU2 7XH Tel: 00 44 1483 689627 Fax: 00 44 1483 686291 e-mail: d.jesson@surrey.ac.uk RGANIC-INRGANIC HYBRID NANPARTICLES:
David Jesson, School of Engineering (H6), University of Surrey, Guildford, Surrey, UK, GU2 7XH Tel: 00 44 1483 689627 Fax: 00 44 1483 686291 e-mail: d.jesson@surrey.ac.uk RGANIC-INRGANIC HYBRID NANPARTICLES:
A Sustainable Synthesis of Nitrogen-Doped Carbon Aerogels
 A Sustainable Synthesis of Nitrogen-Doped Carbon Aerogels Supporting Information By Robin J. White, a, * Noriko Yoshizawa, b Markus Antonietti, a and Maria-Magdalena Titirici. a * e-mail: robin.white@mpikg.mpg.de
A Sustainable Synthesis of Nitrogen-Doped Carbon Aerogels Supporting Information By Robin J. White, a, * Noriko Yoshizawa, b Markus Antonietti, a and Maria-Magdalena Titirici. a * e-mail: robin.white@mpikg.mpg.de
PHI 5000 Versaprobe-II Focus X-ray Photo-electron Spectroscopy
 PHI 5000 Versaprobe-II Focus X-ray Photo-electron Spectroscopy The very basic theory of XPS XPS theroy Surface Analysis Ultra High Vacuum (UHV) XPS Theory XPS = X-ray Photo-electron Spectroscopy X-ray
PHI 5000 Versaprobe-II Focus X-ray Photo-electron Spectroscopy The very basic theory of XPS XPS theroy Surface Analysis Ultra High Vacuum (UHV) XPS Theory XPS = X-ray Photo-electron Spectroscopy X-ray
Plasma polymers can be used to modify the surface chemistries of materials in a controlled fashion (without effecting bulk chemistry).
 Plasma polymers can be used to modify the surface chemistries of materials in a controlled fashion (without effecting bulk chemistry). An example used here is the modification of the alumina surface of
Plasma polymers can be used to modify the surface chemistries of materials in a controlled fashion (without effecting bulk chemistry). An example used here is the modification of the alumina surface of
X-Ray Photoelectron Spectroscopy (XPS) Prof. Paul K. Chu
 X-Ray Photoelectron Spectroscopy (XPS) Prof. Paul K. Chu X-ray Photoelectron Spectroscopy Introduction Qualitative analysis Quantitative analysis Charging compensation Small area analysis and XPS imaging
X-Ray Photoelectron Spectroscopy (XPS) Prof. Paul K. Chu X-ray Photoelectron Spectroscopy Introduction Qualitative analysis Quantitative analysis Charging compensation Small area analysis and XPS imaging
Surface and Interface Characterization of Polymer Films
 Surface and Interface Characterization of Polymer Films Jeff Shallenberger, Evans Analytical Group 104 Windsor Center Dr., East Windsor NJ Copyright 2013 Evans Analytical Group Outline Introduction to
Surface and Interface Characterization of Polymer Films Jeff Shallenberger, Evans Analytical Group 104 Windsor Center Dr., East Windsor NJ Copyright 2013 Evans Analytical Group Outline Introduction to
ToF-SIMS or XPS? Xinqi Chen Keck-II
 ToF-SIMS or XPS? Xinqi Chen Keck-II 1 Time of Flight Secondary Ion Mass Spectrometry (ToF-SIMS) Not ToF MS (laser, solution) X-ray Photoelectron Spectroscopy (XPS) 2 3 Modes of SIMS 4 Secondary Ion Sputtering
ToF-SIMS or XPS? Xinqi Chen Keck-II 1 Time of Flight Secondary Ion Mass Spectrometry (ToF-SIMS) Not ToF MS (laser, solution) X-ray Photoelectron Spectroscopy (XPS) 2 3 Modes of SIMS 4 Secondary Ion Sputtering
Secondary Ion Mass Spectroscopy (SIMS)
 Secondary Ion Mass Spectroscopy (SIMS) Analyzing Inorganic Solids * = under special conditions ** = semiconductors only + = limited number of elements or groups Analyzing Organic Solids * = under special
Secondary Ion Mass Spectroscopy (SIMS) Analyzing Inorganic Solids * = under special conditions ** = semiconductors only + = limited number of elements or groups Analyzing Organic Solids * = under special
Peak-Fitting of High Resolution ToF-SIMS Spectra: A Preliminary Study
 Peak-Fitting of High Resolution ToF-SIMS Spectra: A Preliminary Study M-L. Abel*, K. Shimizu, M. Holliman and J.F. Watts The Surface Analysis Laboratory, Faculty of Engineering and Physical Sciences, University
Peak-Fitting of High Resolution ToF-SIMS Spectra: A Preliminary Study M-L. Abel*, K. Shimizu, M. Holliman and J.F. Watts The Surface Analysis Laboratory, Faculty of Engineering and Physical Sciences, University
An Introduction to Auger Electron Spectroscopy
 An Introduction to Auger Electron Spectroscopy Spyros Diplas MENA3100 SINTEF Materials & Chemistry, Department of Materials Physics & Centre of Materials Science and Nanotechnology, Department of Chemistry,
An Introduction to Auger Electron Spectroscopy Spyros Diplas MENA3100 SINTEF Materials & Chemistry, Department of Materials Physics & Centre of Materials Science and Nanotechnology, Department of Chemistry,
XPS Surface Characterization of Disposable Laboratory Gloves and the Transfer of Glove Components to Other Surfaces
 Application Note: 52287 XPS Surface Characterization of Disposable Laboratory Gloves and the Transfer of Glove Components to Other Surfaces Brian R. Strohmeier, Thermo Fisher Scientific, Madison, WI, USA
Application Note: 52287 XPS Surface Characterization of Disposable Laboratory Gloves and the Transfer of Glove Components to Other Surfaces Brian R. Strohmeier, Thermo Fisher Scientific, Madison, WI, USA
The design of an integrated XPS/Raman spectroscopy instrument for co-incident analysis
 The design of an integrated XPS/Raman spectroscopy instrument for co-incident analysis Tim Nunney The world leader in serving science 2 XPS Surface Analysis XPS +... UV Photoelectron Spectroscopy UPS He(I)
The design of an integrated XPS/Raman spectroscopy instrument for co-incident analysis Tim Nunney The world leader in serving science 2 XPS Surface Analysis XPS +... UV Photoelectron Spectroscopy UPS He(I)
Methods of surface analysis
 Methods of surface analysis Nanomaterials characterisation I RNDr. Věra Vodičková, PhD. Surface of solid matter: last monoatomic layer + absorbed monolayer physical properties are effected (crystal lattice
Methods of surface analysis Nanomaterials characterisation I RNDr. Věra Vodičková, PhD. Surface of solid matter: last monoatomic layer + absorbed monolayer physical properties are effected (crystal lattice
Case Study of Electronic Materials Packaging with Poor Metal Adhesion and the Process for Performing Root Cause Failure Analysis
 Case Study of Electronic Materials Packaging with Poor Metal Adhesion and the Process for Performing Root Cause Failure Analysis Dr. E. A. Leone BACKGRUND ne trend in the electronic packaging industry
Case Study of Electronic Materials Packaging with Poor Metal Adhesion and the Process for Performing Root Cause Failure Analysis Dr. E. A. Leone BACKGRUND ne trend in the electronic packaging industry
Analysis of Poly(dimethylsiloxane) on Solid Surfaces Using Silver Deposition/TOF-SIMS
 Special Issue Surface and Micro-Analysis of Organic Materials 21 Research Report Analysis of Poly(dimethylsiloxane) on Solid Surfaces Using Silver Deposition/TOF-SIMS Masae Inoue, Atsushi Murase Abstract
Special Issue Surface and Micro-Analysis of Organic Materials 21 Research Report Analysis of Poly(dimethylsiloxane) on Solid Surfaces Using Silver Deposition/TOF-SIMS Masae Inoue, Atsushi Murase Abstract
Secondaryionmassspectrometry
 Secondaryionmassspectrometry (SIMS) 1 Incident Ion Techniques for Surface Composition Analysis Mass spectrometric technique 1. Ionization -Electron ionization (EI) -Chemical ionization (CI) -Field ionization
Secondaryionmassspectrometry (SIMS) 1 Incident Ion Techniques for Surface Composition Analysis Mass spectrometric technique 1. Ionization -Electron ionization (EI) -Chemical ionization (CI) -Field ionization
Application of Surface Analysis for Root Cause Failure Analysis
 Application of Surface Analysis for Root Cause Failure Analysis David A. Cole Evans Analytical Group East Windsor, NJ Specialists in Materials Characterization Outline Introduction X-Ray Photoelectron
Application of Surface Analysis for Root Cause Failure Analysis David A. Cole Evans Analytical Group East Windsor, NJ Specialists in Materials Characterization Outline Introduction X-Ray Photoelectron
The Benefit of Wide Energy Range Spectrum Acquisition During Sputter Depth Profile Measurements
 The Benefit of Wide Energy Range Spectrum Acquisition During Sputter Depth Profile Measurements Uwe Scheithauer, 82008 Unterhaching, Germany E-Mail: scht.uhg@googlemail.com Internet: orcid.org/0000-0002-4776-0678;
The Benefit of Wide Energy Range Spectrum Acquisition During Sputter Depth Profile Measurements Uwe Scheithauer, 82008 Unterhaching, Germany E-Mail: scht.uhg@googlemail.com Internet: orcid.org/0000-0002-4776-0678;
Practical Surface Analysis
 Practical Surface Analysis SECOND EDITION Volume 1 Auger and X-ray Photoelectron Spectroscopy Edited by D. BRIGGS ICI PLC, Wilton Materials Research Centre, Wilton, Middlesbrough, Cleveland, UK and M.
Practical Surface Analysis SECOND EDITION Volume 1 Auger and X-ray Photoelectron Spectroscopy Edited by D. BRIGGS ICI PLC, Wilton Materials Research Centre, Wilton, Middlesbrough, Cleveland, UK and M.
IV. Surface analysis for chemical state, chemical composition
 IV. Surface analysis for chemical state, chemical composition Probe beam Detect XPS Photon (X-ray) Photoelectron(core level electron) UPS Photon (UV) Photoelectron(valence level electron) AES electron
IV. Surface analysis for chemical state, chemical composition Probe beam Detect XPS Photon (X-ray) Photoelectron(core level electron) UPS Photon (UV) Photoelectron(valence level electron) AES electron
Secondary ion mass spectrometry (SIMS)
 Secondary ion mass spectrometry (SIMS) ELEC-L3211 Postgraduate Course in Micro and Nanosciences Department of Micro and Nanosciences Personal motivation and experience on SIMS Offers the possibility to
Secondary ion mass spectrometry (SIMS) ELEC-L3211 Postgraduate Course in Micro and Nanosciences Department of Micro and Nanosciences Personal motivation and experience on SIMS Offers the possibility to
Hiden SIMS Secondary Ion Mass Spectrometers. Analysers for surface, elemental and molecular analysis
 Hiden SIMS Secondary Ion Mass Spectrometers Analysers for surface, elemental and molecular analysis vacuum analysis surface science plasma diagnostics gas analysis SIMS Versatility SIMS is a high sensitivity
Hiden SIMS Secondary Ion Mass Spectrometers Analysers for surface, elemental and molecular analysis vacuum analysis surface science plasma diagnostics gas analysis SIMS Versatility SIMS is a high sensitivity
Introduction. Photoresist : Type: Structure:
 Photoresist SEM images of the morphologies of meso structures and nanopatterns on (a) a positively nanopatterned silicon mold, and (b) a negatively nanopatterned silicon mold. Introduction Photoresist
Photoresist SEM images of the morphologies of meso structures and nanopatterns on (a) a positively nanopatterned silicon mold, and (b) a negatively nanopatterned silicon mold. Introduction Photoresist
Polymer/drug films as a model system for a drug eluting coronary stent coating layer
 Polymer/drug films as a model system for a drug eluting coronary stent coating layer Valeria Ciarnelli Prof. Clive Roberts Prof. Morgan Alexander, Prof. Martyn Davies School of Pharmacy The University
Polymer/drug films as a model system for a drug eluting coronary stent coating layer Valeria Ciarnelli Prof. Clive Roberts Prof. Morgan Alexander, Prof. Martyn Davies School of Pharmacy The University
Creating New Barriers with Graphene
 Creating New Barriers with Graphene Authors: Richard Akam, Lynn Chikosha & Tim von Werne Introduction Graphene was first isolated in 2004 by Andre Geim and Konstantin Novoselov at Manchester University.
Creating New Barriers with Graphene Authors: Richard Akam, Lynn Chikosha & Tim von Werne Introduction Graphene was first isolated in 2004 by Andre Geim and Konstantin Novoselov at Manchester University.
Surface Analytical Techniques for Analysis of Coatings Mary Jane Walzak, Mark Biesinger and Brad Kobe The University of Western Ontario, Surface
 Surface Analytical Techniques for Analysis of Coatings Mary Jane Walzak, Mark Biesinger and Brad Kobe The University of Western Ontario, Surface Science Western 999 Collip Circle, Room LL31, London, ON
Surface Analytical Techniques for Analysis of Coatings Mary Jane Walzak, Mark Biesinger and Brad Kobe The University of Western Ontario, Surface Science Western 999 Collip Circle, Room LL31, London, ON
Improving Adhesion: Examining the Electrochemistry of Organic Inhibitors
 Improving Adhesion: Examining the Electrochemistry of rganic Inhibitors Benefits of rganics Chemisorb onto metallic substrates Complex with metal ions at substrate Neutralize & absorb the corrodents Decrease
Improving Adhesion: Examining the Electrochemistry of rganic Inhibitors Benefits of rganics Chemisorb onto metallic substrates Complex with metal ions at substrate Neutralize & absorb the corrodents Decrease
Theta Probe: A tool for characterizing ultra thin films and self assembled monolayers using parallel angle resolved XPS (ARXPS)
 Theta Probe: A tool for characterizing ultra thin films and self assembled monolayers using parallel angle resolved XPS (ARXPS) C. E. Riley, P. Mack, T. S. Nunney and R. G. White Thermo Fisher Scientific
Theta Probe: A tool for characterizing ultra thin films and self assembled monolayers using parallel angle resolved XPS (ARXPS) C. E. Riley, P. Mack, T. S. Nunney and R. G. White Thermo Fisher Scientific
Lecture 11 Surface Characterization of Biomaterials in Vacuum
 1 Lecture 11 Surface Characterization of Biomaterials in Vacuum The structure and chemistry of a biomaterial surface greatly dictates the degree of biocompatibility of an implant. Surface characterization
1 Lecture 11 Surface Characterization of Biomaterials in Vacuum The structure and chemistry of a biomaterial surface greatly dictates the degree of biocompatibility of an implant. Surface characterization
Large Area TOF-SIMS Imaging of the Antibacterial Distribution in Frozen-Hydrated Contact Lenses
 Large Area TOF-SIMS Imaging of the Antibacterial Distribution in Frozen-Hydrated Contact Lenses Overview: Imaging by time-of-flight secondary ion mass spectrometry (TOF-SIMS) is accomplished in a vacuum
Large Area TOF-SIMS Imaging of the Antibacterial Distribution in Frozen-Hydrated Contact Lenses Overview: Imaging by time-of-flight secondary ion mass spectrometry (TOF-SIMS) is accomplished in a vacuum
X- ray Photoelectron Spectroscopy and its application in phase- switching device study
 X- ray Photoelectron Spectroscopy and its application in phase- switching device study Xinyuan Wang A53073806 I. Background X- ray photoelectron spectroscopy is of great importance in modern chemical and
X- ray Photoelectron Spectroscopy and its application in phase- switching device study Xinyuan Wang A53073806 I. Background X- ray photoelectron spectroscopy is of great importance in modern chemical and
IONTOF. Latest Developments in 2D and 3D TOF-SIMS Analysis. Surface Analysis Innovations and Solutions for Industry 2017 Coventry
 Latest Developments in 2D and 3D TOF-SIMS Analysis Surface Analysis Innovations and Solutions for Industry 2017 Coventry 12.10.2017 Matthias Kleine-Boymann Regional Sales Manager matthias.kleine-boymann@iontof.com
Latest Developments in 2D and 3D TOF-SIMS Analysis Surface Analysis Innovations and Solutions for Industry 2017 Coventry 12.10.2017 Matthias Kleine-Boymann Regional Sales Manager matthias.kleine-boymann@iontof.com
Figure 1: Graphene release, transfer and stacking processes. The graphene stacking began with CVD
 Supplementary figure 1 Graphene Growth and Transfer Graphene PMMA FeCl 3 DI water Copper foil CVD growth Back side etch PMMA coating Copper etch in 0.25M FeCl 3 DI water rinse 1 st transfer DI water 1:10
Supplementary figure 1 Graphene Growth and Transfer Graphene PMMA FeCl 3 DI water Copper foil CVD growth Back side etch PMMA coating Copper etch in 0.25M FeCl 3 DI water rinse 1 st transfer DI water 1:10
A DIVISION OF ULVAC-PHI. Quantera II. Scanning XPS Microprobe
 A DIVISION OF ULVAC-PHI Quantera II Scanning XPS Microprobe X-ray Photoelectron Spectroscopy (XPS/ESCA) is the most widely used surface analysis technique and has many well established industrial and
A DIVISION OF ULVAC-PHI Quantera II Scanning XPS Microprobe X-ray Photoelectron Spectroscopy (XPS/ESCA) is the most widely used surface analysis technique and has many well established industrial and
A DIVISION OF ULVAC-PHI. Time-of-Flight Secondary Ion Mass Spectrometer with Parallel Imaging MS/MS for Confident Molecular Identification
 A DIVISION OF ULVAC-PHI Time-of-Flight Secondary Ion Mass Spectrometer with Parallel Imaging MS/MS for Confident Molecular Identification Designed for Confident Molecular Identification and Superior Imaging
A DIVISION OF ULVAC-PHI Time-of-Flight Secondary Ion Mass Spectrometer with Parallel Imaging MS/MS for Confident Molecular Identification Designed for Confident Molecular Identification and Superior Imaging
Three-dimensional depth profiling of molecular structures
 Anal Bioanal Chem (2009) 393:1835 1842 DOI 10.1007/s00216-008-2596-5 REVIEW Three-dimensional depth profiling of molecular structures A. Wucher & J. Cheng & L. Zheng & N. Winograd Received: 5 September
Anal Bioanal Chem (2009) 393:1835 1842 DOI 10.1007/s00216-008-2596-5 REVIEW Three-dimensional depth profiling of molecular structures A. Wucher & J. Cheng & L. Zheng & N. Winograd Received: 5 September
1 Introduction COPYRIGHTED MATERIAL. 1.1 HowdoweDefinetheSurface?
 1 Introduction JOHN C. VICKERMAN Manchester Interdisciplinary Biocentre, School of Chemical Engineering and Analytical Science, The University of Manchester, Manchester, UK The surface behaviour of materials
1 Introduction JOHN C. VICKERMAN Manchester Interdisciplinary Biocentre, School of Chemical Engineering and Analytical Science, The University of Manchester, Manchester, UK The surface behaviour of materials
X-ray photoelectron spectroscopic characterization of molybdenum nitride thin films
 Korean J. Chem. Eng., 28(4), 1133-1138 (2011) DOI: 10.1007/s11814-011-0036-2 INVITED REVIEW PAPER X-ray photoelectron spectroscopic characterization of molybdenum nitride thin films Jeong-Gil Choi Department
Korean J. Chem. Eng., 28(4), 1133-1138 (2011) DOI: 10.1007/s11814-011-0036-2 INVITED REVIEW PAPER X-ray photoelectron spectroscopic characterization of molybdenum nitride thin films Jeong-Gil Choi Department
Colloidal Particles with Complex Microstructures via Phase Separation in Swelled Polymer Microspheres
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Supporting Information Colloidal Particles with Complex Microstructures via Phase Separation in
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Supporting Information Colloidal Particles with Complex Microstructures via Phase Separation in
Control of Optical Properties by the Stepwise Chemical and Plasma Spray Treatment of Polycarbonate
 Appl. Sci. Converg. Technol. 27(6): 135-139 (2018) https://doi.org/10.5757/asct.2018.27.6.135 Research Paper Control of Optical Properties by the Stepwise Chemical and Plasma Spray Treatment of Polycarbonate
Appl. Sci. Converg. Technol. 27(6): 135-139 (2018) https://doi.org/10.5757/asct.2018.27.6.135 Research Paper Control of Optical Properties by the Stepwise Chemical and Plasma Spray Treatment of Polycarbonate
Molecular depth profiling with reactive ions, or why chemistry matters in sputtering.
 Molecular depth profiling with reactive ions, or why chemistry matters in sputtering. L. Houssiau, N. Mine, N. Wehbe Research Centre in Physics of Matter and Radiation (PMR), University of Namur (FUNDP),
Molecular depth profiling with reactive ions, or why chemistry matters in sputtering. L. Houssiau, N. Mine, N. Wehbe Research Centre in Physics of Matter and Radiation (PMR), University of Namur (FUNDP),
Supplementary Figure 1: AFM topography of the graphene/sio 2 [(a) and (c)] and graphene/h BN [(b) and (d)] surfaces acquired before [(a) and (b)],
![Supplementary Figure 1: AFM topography of the graphene/sio 2 [(a) and (c)] and graphene/h BN [(b) and (d)] surfaces acquired before [(a) and (b)], Supplementary Figure 1: AFM topography of the graphene/sio 2 [(a) and (c)] and graphene/h BN [(b) and (d)] surfaces acquired before [(a) and (b)],](/thumbs/94/121705311.jpg) Supplementary Figure 1: AFM topography of the graphene/sio 2 [(a) and (c)] and graphene/h BN [(b) and (d)] surfaces acquired before [(a) and (b)], and after [(c) and (d)], respectively, 35 seconds of Cs
Supplementary Figure 1: AFM topography of the graphene/sio 2 [(a) and (c)] and graphene/h BN [(b) and (d)] surfaces acquired before [(a) and (b)], and after [(c) and (d)], respectively, 35 seconds of Cs
ARGON RF PLASMA TREATMENT OF PET FILMS FOR SILICON FILMS ADHESION IMPROVEMENT
 Journal of Optoelectronics and Advanced Materials Vol. 7, No. 5, October 2005, p. 2529-2534 ARGON RF PLASMA TREATMENT OF FILMS FOR SILICON FILMS ADHESION IMPROVEMENT I. A. Rusu *, G. Popa, S. O. Saied
Journal of Optoelectronics and Advanced Materials Vol. 7, No. 5, October 2005, p. 2529-2534 ARGON RF PLASMA TREATMENT OF FILMS FOR SILICON FILMS ADHESION IMPROVEMENT I. A. Rusu *, G. Popa, S. O. Saied
Supporting Information s for
 Supporting Information s for # Self-assembling of DNA-templated Au Nanoparticles into Nanowires and their enhanced SERS and Catalytic Applications Subrata Kundu* and M. Jayachandran Electrochemical Materials
Supporting Information s for # Self-assembling of DNA-templated Au Nanoparticles into Nanowires and their enhanced SERS and Catalytic Applications Subrata Kundu* and M. Jayachandran Electrochemical Materials
QUESTIONS AND ANSWERS
 QUESTIONS AND ANSWERS (1) For a ground - state neutral atom with 13 protons, describe (a) Which element this is (b) The quantum numbers, n, and l of the inner two core electrons (c) The stationary state
QUESTIONS AND ANSWERS (1) For a ground - state neutral atom with 13 protons, describe (a) Which element this is (b) The quantum numbers, n, and l of the inner two core electrons (c) The stationary state
S. Ichikawa*, R. Kuze, T. Shimizu and H. Shimaoka INTRODUCTION
 Journal of Surface Analysis,Vol.12 No.2 (2005); S.Ichikawa, et al., Coverage Estimation of Silane. Coverage Estimation of Silane Functionalized Perfluoropolyether Layer by using Time of Flight Secondary
Journal of Surface Analysis,Vol.12 No.2 (2005); S.Ichikawa, et al., Coverage Estimation of Silane. Coverage Estimation of Silane Functionalized Perfluoropolyether Layer by using Time of Flight Secondary
SPECIALTY MONOMERS FOR ENHANCED FUNCTIONALITY IN EMULSION POLYMERIZATION
 SPECIALTY MONOMERS FOR ENHANCED FUNCTIONALITY IN EMULSION POLYMERIZATION Pierre Hennaux, Nemesio Martinez-Castro, Jose P. Ruiz, Zhihua Zhang and Michael D. Rhodes Solvay Inc. Centre for Research & Technology-
SPECIALTY MONOMERS FOR ENHANCED FUNCTIONALITY IN EMULSION POLYMERIZATION Pierre Hennaux, Nemesio Martinez-Castro, Jose P. Ruiz, Zhihua Zhang and Michael D. Rhodes Solvay Inc. Centre for Research & Technology-
for XPS surface analysis
 Thermo Scientific Avantage XPS Software Powerful instrument operation and data processing for XPS surface analysis Avantage Software Atomic Concentration (%) 100 The premier software for surface analysis
Thermo Scientific Avantage XPS Software Powerful instrument operation and data processing for XPS surface analysis Avantage Software Atomic Concentration (%) 100 The premier software for surface analysis
PHI. Scanning XPS Microprobe
 PHI Scanning XPS Microprobe Unique Scanning XPS Microprobe X-ray photoelectron spectroscopy (XPS/ESA) is the most widely used surface analysis technique and has many well established industrial and research
PHI Scanning XPS Microprobe Unique Scanning XPS Microprobe X-ray photoelectron spectroscopy (XPS/ESA) is the most widely used surface analysis technique and has many well established industrial and research
NOVEL APPROACHES TO THE TACKIFICATION OF PRESSURE SENSITIVE ADHESIVES
 NOVEL APPROACHES TO THE TACKIFICATION OF PRESSURE SENSITIVE ADHESIVES By: Laura J. Donkus, Dr. William B. Griffith, Melissa A. Lane, and David Keely The Dow Chemical Company, Spring House, PA Introduction
NOVEL APPROACHES TO THE TACKIFICATION OF PRESSURE SENSITIVE ADHESIVES By: Laura J. Donkus, Dr. William B. Griffith, Melissa A. Lane, and David Keely The Dow Chemical Company, Spring House, PA Introduction
EE 527 MICROFABRICATION. Lecture 25 Tai-Chang Chen University of Washington
 EE 527 MICROFABRICATION Lecture 25 Tai-Chang Chen University of Washington ION MILLING SYSTEM Kaufmann source Use e-beam to strike plasma A magnetic field applied to increase ion density Drawback Low etch
EE 527 MICROFABRICATION Lecture 25 Tai-Chang Chen University of Washington ION MILLING SYSTEM Kaufmann source Use e-beam to strike plasma A magnetic field applied to increase ion density Drawback Low etch
Special Properties of Au Nanoparticles
 Special Properties of Au Nanoparticles Maryam Ebrahimi Chem 7500/750 March 28 th, 2007 1 Outline Introduction The importance of unexpected electronic, geometric, and chemical properties of nanoparticles
Special Properties of Au Nanoparticles Maryam Ebrahimi Chem 7500/750 March 28 th, 2007 1 Outline Introduction The importance of unexpected electronic, geometric, and chemical properties of nanoparticles
XPS/UPS and EFM. Brent Gila. XPS/UPS Ryan Davies EFM Andy Gerger
 XPS/UPS and EFM Brent Gila XPS/UPS Ryan Davies EFM Andy Gerger XPS/ESCA X-ray photoelectron spectroscopy (XPS) also called Electron Spectroscopy for Chemical Analysis (ESCA) is a chemical surface analysis
XPS/UPS and EFM Brent Gila XPS/UPS Ryan Davies EFM Andy Gerger XPS/ESCA X-ray photoelectron spectroscopy (XPS) also called Electron Spectroscopy for Chemical Analysis (ESCA) is a chemical surface analysis
The Controlled Evolution of a Polymer Single Crystal
 Supporting Online Material The Controlled Evolution of a Polymer Single Crystal Xiaogang Liu, 1 Yi Zhang, 1 Dipak K. Goswami, 2 John S. Okasinski, 2 Khalid Salaita, 1 Peng Sun, 1 Michael J. Bedzyk, 2 Chad
Supporting Online Material The Controlled Evolution of a Polymer Single Crystal Xiaogang Liu, 1 Yi Zhang, 1 Dipak K. Goswami, 2 John S. Okasinski, 2 Khalid Salaita, 1 Peng Sun, 1 Michael J. Bedzyk, 2 Chad
Characterization of Secondary Emission Materials for Micro-Channel Plates. S. Jokela, I. Veryovkin, A. Zinovev
 Characterization of Secondary Emission Materials for Micro-Channel Plates S. Jokela, I. Veryovkin, A. Zinovev Secondary Electron Yield Testing Technique We have incorporated XPS, UPS, Ar-ion sputtering,
Characterization of Secondary Emission Materials for Micro-Channel Plates S. Jokela, I. Veryovkin, A. Zinovev Secondary Electron Yield Testing Technique We have incorporated XPS, UPS, Ar-ion sputtering,
SIMS: Secondary Ion Mass Spectrometry
 SIMS: Secondary Ion Mass Spectrometry SIMS is based on the emission of ions (secondary ions) from the first monolayers of a solid surface after bombardment by high energy primary ions. The cascade collision
SIMS: Secondary Ion Mass Spectrometry SIMS is based on the emission of ions (secondary ions) from the first monolayers of a solid surface after bombardment by high energy primary ions. The cascade collision
Surface Science Spectra
 Surface Science Spectra WCF Submission 226 Proof - SSS Submission # 14-015 (20141102)V22 Analysis of Silicon Germanium Standards for the Quantification of SiGe Microelectronic Devices using AES SECTION
Surface Science Spectra WCF Submission 226 Proof - SSS Submission # 14-015 (20141102)V22 Analysis of Silicon Germanium Standards for the Quantification of SiGe Microelectronic Devices using AES SECTION
Mass spectrometric determination of the surface compositions of ethanol water mixtures
 International Journal of Mass Spectrometry 212 (2001) 267 271 www.elsevier.com/locate/ijms Cluster/kinetic method Mass spectrometric determination of the surface compositions of ethanol water mixtures
International Journal of Mass Spectrometry 212 (2001) 267 271 www.elsevier.com/locate/ijms Cluster/kinetic method Mass spectrometric determination of the surface compositions of ethanol water mixtures
Formation of Halogen Bond-Based 2D Supramolecular Assemblies by Electric Manipulation
 Formation of Halogen Bond-Based 2D Supramolecular Assemblies by Electric Manipulation Qing-Na Zheng, a,b Xuan-He Liu, a,b Ting Chen, a Hui-Juan Yan, a Timothy Cook, c Dong Wang* a, Peter J. Stang, c Li-Jun
Formation of Halogen Bond-Based 2D Supramolecular Assemblies by Electric Manipulation Qing-Na Zheng, a,b Xuan-He Liu, a,b Ting Chen, a Hui-Juan Yan, a Timothy Cook, c Dong Wang* a, Peter J. Stang, c Li-Jun
A DIVISION OF ULVAC-PHI
 A DIVISION OF ULVAC-PHI X-ray photoelectron spectroscopy (XPS/ESCA) is the most widely used surface analysis technique and has many well established industrial and research applications. XPS provides
A DIVISION OF ULVAC-PHI X-ray photoelectron spectroscopy (XPS/ESCA) is the most widely used surface analysis technique and has many well established industrial and research applications. XPS provides
ETCHING Chapter 10. Mask. Photoresist
 ETCHING Chapter 10 Mask Light Deposited Substrate Photoresist Etch mask deposition Photoresist application Exposure Development Etching Resist removal Etching of thin films and sometimes the silicon substrate
ETCHING Chapter 10 Mask Light Deposited Substrate Photoresist Etch mask deposition Photoresist application Exposure Development Etching Resist removal Etching of thin films and sometimes the silicon substrate
PHYSICAL AND CHEMICAL PROPERTIES OF ATMOSPHERIC PRESSURE PLASMA POLYMER FILMS
 PHYSICAL AND CHEMICAL PROPERTIES OF ATMOSPHERIC PRESSURE PLASMA POLYMER FILMS O. Goossens, D. Vangeneugden, S. Paulussen and E. Dekempeneer VITO Flemish Institute for Technological Research, Boeretang
PHYSICAL AND CHEMICAL PROPERTIES OF ATMOSPHERIC PRESSURE PLASMA POLYMER FILMS O. Goossens, D. Vangeneugden, S. Paulussen and E. Dekempeneer VITO Flemish Institute for Technological Research, Boeretang
Surface Analysis by XPS & ToF-SIMS Basics, Strengths, and Limitations
 Surface Analysis by XPS & ToF-SIMS Basics, Strengths, and Limitations Michael Bruns michael.bruns@kit.edu KIT University of the State of Baden-Württemberg and National Laboratory of the Helmholtz Association
Surface Analysis by XPS & ToF-SIMS Basics, Strengths, and Limitations Michael Bruns michael.bruns@kit.edu KIT University of the State of Baden-Württemberg and National Laboratory of the Helmholtz Association
Thin and Ultrathin Plasma Polymer Films and Their Characterization
 WDS'13 Proceedings of Contributed Papers, Part III, 134 138, 2013. ISBN 978-80-7378-252-8 MATFYZPRESS Thin and Ultrathin Plasma Polymer Films and Their Characterization M. Petr, O. Kylián, J. Hanuš, A.
WDS'13 Proceedings of Contributed Papers, Part III, 134 138, 2013. ISBN 978-80-7378-252-8 MATFYZPRESS Thin and Ultrathin Plasma Polymer Films and Their Characterization M. Petr, O. Kylián, J. Hanuš, A.
Auger Electron Spectroscopy (AES)
 1. Introduction Auger Electron Spectroscopy (AES) Silvia Natividad, Gabriel Gonzalez and Arena Holguin Auger Electron Spectroscopy (Auger spectroscopy or AES) was developed in the late 1960's, deriving
1. Introduction Auger Electron Spectroscopy (AES) Silvia Natividad, Gabriel Gonzalez and Arena Holguin Auger Electron Spectroscopy (Auger spectroscopy or AES) was developed in the late 1960's, deriving
Protocols for Three-Dimensional Molecular Imaging Using Mass Spectrometry
 Anal. Chem. 2007, 79, 5529-5539 Accelerated Articles Protocols for Three-Dimensional Molecular Imaging Using Mass Spectrometry Andreas Wucher, Juan Cheng,, and Nicholas Winograd*, Department of Chemistry,
Anal. Chem. 2007, 79, 5529-5539 Accelerated Articles Protocols for Three-Dimensional Molecular Imaging Using Mass Spectrometry Andreas Wucher, Juan Cheng,, and Nicholas Winograd*, Department of Chemistry,
Photoelectron Spectroscopy using High Order Harmonic Generation
 Photoelectron Spectroscopy using High Order Harmonic Generation Alana Ogata Yamanouchi Lab, University of Tokyo ABSTRACT The analysis of photochemical processes has been previously limited by the short
Photoelectron Spectroscopy using High Order Harmonic Generation Alana Ogata Yamanouchi Lab, University of Tokyo ABSTRACT The analysis of photochemical processes has been previously limited by the short
Surface and Micro-Analysis of Organic Materials
 Special Issue Surface and Micro-Analysis of Organic Materials 1 Surface and Micro-Analysis of Organic Materials Review Atsushi Murase Abstract This paper is a review of the technical approaches taken at
Special Issue Surface and Micro-Analysis of Organic Materials 1 Surface and Micro-Analysis of Organic Materials Review Atsushi Murase Abstract This paper is a review of the technical approaches taken at
Spin-resolved photoelectron spectroscopy
 Spin-resolved photoelectron spectroscopy Application Notes Spin-resolved photoelectron spectroscopy experiments were performed in an experimental station consisting of an analysis and a preparation chamber.
Spin-resolved photoelectron spectroscopy Application Notes Spin-resolved photoelectron spectroscopy experiments were performed in an experimental station consisting of an analysis and a preparation chamber.
Surface Chemistry and Reaction Dynamics of Electron Beam Induced Deposition Processes
 Surface Chemistry and Reaction Dynamics of Electron Beam Induced Deposition Processes e -? 2 nd FEBIP Workshop Thun, Switzerland 2008 Howard Fairbrother Johns Hopkins University Baltimore, MD, USA Outline
Surface Chemistry and Reaction Dynamics of Electron Beam Induced Deposition Processes e -? 2 nd FEBIP Workshop Thun, Switzerland 2008 Howard Fairbrother Johns Hopkins University Baltimore, MD, USA Outline
Development of a Standards Base for Static SIMS
 Development of a Standards Base for Static SIMS I S Gilmore * Quality of Life Division, National Physical Laboratory, Teddington, Middlesex TW LW, UK * ian.gilmore@npl.co.uk (Received 7 November, 7) Reliability
Development of a Standards Base for Static SIMS I S Gilmore * Quality of Life Division, National Physical Laboratory, Teddington, Middlesex TW LW, UK * ian.gilmore@npl.co.uk (Received 7 November, 7) Reliability
Materials Characterization and Preparation Facility, Hong Kong University of Science and Technology, Clear Water Bay, Hong Kong
 7248 Macromolecules 1998, 31, 7248-7255 Specific Interaction between Poly(styrene-co-4-vinylphenol) and Poly(styrene-co-4-vinylpyridine) Studied by X-ray Photoelectron Spectroscopy and Time-of-Flight Secondary
7248 Macromolecules 1998, 31, 7248-7255 Specific Interaction between Poly(styrene-co-4-vinylphenol) and Poly(styrene-co-4-vinylpyridine) Studied by X-ray Photoelectron Spectroscopy and Time-of-Flight Secondary
Dr. Tim Nunney Thermo Fisher Scientific, East Grinstead, UK Dr. Nick Bulloss Thermo Fisher Scientific, Madison, WI, USA Dr. Harry Meyer III Oak Ridge
 Dr. Tim Nunney Thermo Fisher Scientific, East Grinstead, UK Dr. Nick Bulloss Thermo Fisher Scientific, Madison, WI, USA Dr. Harry Meyer III Oak Ridge National Laboratory, TN, USA Introduction New materials
Dr. Tim Nunney Thermo Fisher Scientific, East Grinstead, UK Dr. Nick Bulloss Thermo Fisher Scientific, Madison, WI, USA Dr. Harry Meyer III Oak Ridge National Laboratory, TN, USA Introduction New materials
Supporting information
 Supporting information A Facile and Large-area Fabrication Method of Superhydrophobic Self-cleaning Flourinated Polysiloxane/TiO 2 Nanocomposite Coatings with Long-term Durability Xiaofeng Ding, Shuxue
Supporting information A Facile and Large-area Fabrication Method of Superhydrophobic Self-cleaning Flourinated Polysiloxane/TiO 2 Nanocomposite Coatings with Long-term Durability Xiaofeng Ding, Shuxue
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and
 This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
Portable type TXRF analyzer: Ourstex 200TX
 Excerpted from Adv. X-Ray. Chem. Anal., Japan: 42, pp. 115-123 (2011) H. Nagai, Y. Nakajima, S. Kunimura, J. Kawai Improvement in Sensitivity and Quantification by Using a Portable Total Reflection X-Ray
Excerpted from Adv. X-Ray. Chem. Anal., Japan: 42, pp. 115-123 (2011) H. Nagai, Y. Nakajima, S. Kunimura, J. Kawai Improvement in Sensitivity and Quantification by Using a Portable Total Reflection X-Ray
Low temperature anodically grown silicon dioxide films for solar cell. Nicholas E. Grant
 Low temperature anodically grown silicon dioxide films for solar cell applications Nicholas E. Grant Outline 1. Electrochemical cell design and properties. 2. Direct-current current anodic oxidations-part
Low temperature anodically grown silicon dioxide films for solar cell applications Nicholas E. Grant Outline 1. Electrochemical cell design and properties. 2. Direct-current current anodic oxidations-part
NOVEL PHENYLETHYNYL IMIDE SILANES AS COUPLING AGENTS FOR TITANIUM ALLOY
 NOVEL PHENYLETHYNYL IMIDE SILANES AS OUPLING AGENTS FOR TITANIUM ALLOY. Park*, S. E. Lowther, J. G. Smith Jr., J. W. onnell, P. M. Hergenrother, and T. L. St. lair *National Research ouncil, omposites
NOVEL PHENYLETHYNYL IMIDE SILANES AS OUPLING AGENTS FOR TITANIUM ALLOY. Park*, S. E. Lowther, J. G. Smith Jr., J. W. onnell, P. M. Hergenrother, and T. L. St. lair *National Research ouncil, omposites
Introduction to X-ray Photoelectron Spectroscopy (XPS) XPS which makes use of the photoelectric effect, was developed in the mid-1960
 Introduction to X-ray Photoelectron Spectroscopy (XPS) X-ray Photoelectron Spectroscopy (XPS), also known as Electron Spectroscopy for Chemical Analysis (ESCA) is a widely used technique to investigate
Introduction to X-ray Photoelectron Spectroscopy (XPS) X-ray Photoelectron Spectroscopy (XPS), also known as Electron Spectroscopy for Chemical Analysis (ESCA) is a widely used technique to investigate
Volume # 7 Issue # 8 August 2015 Rs.5/- Volume # 7 Issue # 8 August-September 2015 INTERNATIONAL.
 Rs.5/- Volume # 7 Issue # 8 August 2015 Rs.5/- Volume # 7 Issue # 8 August-September 2015 INTERNATIONAL www.eqmaglive.com www.eqmaglive.com Materials Matter For Reliable Performance And Power Output Of
Rs.5/- Volume # 7 Issue # 8 August 2015 Rs.5/- Volume # 7 Issue # 8 August-September 2015 INTERNATIONAL www.eqmaglive.com www.eqmaglive.com Materials Matter For Reliable Performance And Power Output Of
Highly efficient SERS test strips
 Electronic Supplementary Information (ESI) for Highly efficient SERS test strips 5 Ran Zhang, a Bin-Bin Xu, a Xue-Qing Liu, a Yong-Lai Zhang, a Ying Xu, a Qi-Dai Chen, * a and Hong-Bo Sun* a,b 5 10 Experimental
Electronic Supplementary Information (ESI) for Highly efficient SERS test strips 5 Ran Zhang, a Bin-Bin Xu, a Xue-Qing Liu, a Yong-Lai Zhang, a Ying Xu, a Qi-Dai Chen, * a and Hong-Bo Sun* a,b 5 10 Experimental
Review. Surfaces of Biomaterials. Characterization. Surface sensitivity
 Surfaces of Biomaterials Three lectures: 1.23.05 Surface Properties of Biomaterials 1.25.05 Surface Characterization 1.27.05 Surface and Protein Interactions Review Bulk Materials are described by: Chemical
Surfaces of Biomaterials Three lectures: 1.23.05 Surface Properties of Biomaterials 1.25.05 Surface Characterization 1.27.05 Surface and Protein Interactions Review Bulk Materials are described by: Chemical
Chemical Reactions Induced by Ionizing and Electron-beam Irradiation in Freon/Water (Ice) Films
 Chemical Reactions Induced by Ionizing and Electron-beam Irradiation in Freon/Water (Ice) Films Johns Hopkins University (founded in 1876) Dr. C.C. Perry Prof. D.H. Fairborther School of Arts & Sciences
Chemical Reactions Induced by Ionizing and Electron-beam Irradiation in Freon/Water (Ice) Films Johns Hopkins University (founded in 1876) Dr. C.C. Perry Prof. D.H. Fairborther School of Arts & Sciences
Supporting Information: Analysis of protein coatings on gold nanoparticles by XPS and liquid-based particle sizing techniques
 Supporting Information: Analysis of protein coatings on gold nanoparticles by XPS and liquid-based particle sizing techniques Natalie A. Belsey, a) Alex G. Shard a) and Caterina Minelli a),b) National
Supporting Information: Analysis of protein coatings on gold nanoparticles by XPS and liquid-based particle sizing techniques Natalie A. Belsey, a) Alex G. Shard a) and Caterina Minelli a),b) National
Damage to Molecular Solids Irradiated by X-ray Laser Beam
 WDS'11 Proceedings of Contributed Papers, Part II, 247 251, 2011. ISBN 978-80-7378-185-9 MATFYZPRESS Damage to Molecular Solids Irradiated by X-ray Laser Beam T. Burian, V. Hájková, J. Chalupský, L. Juha,
WDS'11 Proceedings of Contributed Papers, Part II, 247 251, 2011. ISBN 978-80-7378-185-9 MATFYZPRESS Damage to Molecular Solids Irradiated by X-ray Laser Beam T. Burian, V. Hájková, J. Chalupský, L. Juha,
High-Precision Evaluation of Ultra-Shallow Impurity Profiles by Secondary Ion Mass Spectrometry
 High-Precision Evaluation of Ultra-Shallow Impurity Profiles by Secondary Ion Mass Spectrometry Yoko Tada Kunihiro Suzuki Yuji Kataoka (Manuscript received December 28, 2009) As complementary metal oxide
High-Precision Evaluation of Ultra-Shallow Impurity Profiles by Secondary Ion Mass Spectrometry Yoko Tada Kunihiro Suzuki Yuji Kataoka (Manuscript received December 28, 2009) As complementary metal oxide
Encapsulation. Battelle Technology. Introduction
 Encapsulation Introduction The encapsulation methods reported in the literature 1-7 for the production of microcapsules are generally achieved using one of the following techniques: 1. Phase separation
Encapsulation Introduction The encapsulation methods reported in the literature 1-7 for the production of microcapsules are generally achieved using one of the following techniques: 1. Phase separation
Defense Technical Information Center Compilation Part Notice
 UNCLASSIFIED Defense Technical Information Center Compilation Part Notice ADP012830 TITLE: XPS Study of Cu-Clusters and Atoms in Cu/SiO2 Composite Films DISTRIBUTION: Approved for public release, distribution
UNCLASSIFIED Defense Technical Information Center Compilation Part Notice ADP012830 TITLE: XPS Study of Cu-Clusters and Atoms in Cu/SiO2 Composite Films DISTRIBUTION: Approved for public release, distribution
Applications of XPS, AES, and TOF-SIMS
 Applications of XPS, AES, and TOF-SIMS Scott R. Bryan Physical Electronics 1 Materials Characterization Techniques Microscopy Optical Microscope SEM TEM STM SPM AFM Spectroscopy Energy Dispersive X-ray
Applications of XPS, AES, and TOF-SIMS Scott R. Bryan Physical Electronics 1 Materials Characterization Techniques Microscopy Optical Microscope SEM TEM STM SPM AFM Spectroscopy Energy Dispersive X-ray
Secondary Ion Mass Spectrometry of Proteins
 World Academy of Science, Engineering and Technology 2 211 Secondary Ion Mass Spectrometry of Proteins Santanu Ray and Alexander G. Shard Abstract The adsorption of bovine serum albumin (BSA), immunoglobulin
World Academy of Science, Engineering and Technology 2 211 Secondary Ion Mass Spectrometry of Proteins Santanu Ray and Alexander G. Shard Abstract The adsorption of bovine serum albumin (BSA), immunoglobulin
Introduction to SIMS Basic principles Components Techniques Drawbacks Figures of Merit Variations Resources
 Introduction to SIMS Basic principles Components Techniques Drawbacks Figures of Merit Variations Resources New technique for surface chemical analysis. SIMS examines the mass of ions, instead of energy
Introduction to SIMS Basic principles Components Techniques Drawbacks Figures of Merit Variations Resources New technique for surface chemical analysis. SIMS examines the mass of ions, instead of energy
XPS studies of octadecylphosphonic acid (OPA) monolayer interactions with some metal and mineral surfaces
 SURFACE AND INTERFACE ANALYSIS Surf. Interface Anal. 2005; 37: 749 754 Published online in Wiley InterScience (www.interscience.wiley.com). DOI: 10.1002/sia.2075 XPS studies of octadecylphosphonic acid
SURFACE AND INTERFACE ANALYSIS Surf. Interface Anal. 2005; 37: 749 754 Published online in Wiley InterScience (www.interscience.wiley.com). DOI: 10.1002/sia.2075 XPS studies of octadecylphosphonic acid
raw materials C V Mn Mg S Al Ca Ti Cr Si G H Nb Na Zn Ni K Co A B C D E F
 Today s advanced batteries require a range of specialized analytical tools to better understand the electrochemical processes that occur during battery cycling. Evans Analytical Group (EAG) offers a wide-range
Today s advanced batteries require a range of specialized analytical tools to better understand the electrochemical processes that occur during battery cycling. Evans Analytical Group (EAG) offers a wide-range
White Paper Adhesives Sealants Tapes
 Fundamental principles of UV reactive manufacturing processes Introduction While the UV systems technology and the appropriate chemistry have been developing continuously, the principle of irradiation
Fundamental principles of UV reactive manufacturing processes Introduction While the UV systems technology and the appropriate chemistry have been developing continuously, the principle of irradiation
