Atomic Spectra for Atoms and Ions. Light is made up of different wavelengths
|
|
|
- Ambrose Reeves
- 6 years ago
- Views:
Transcription
1 Atomic Spectra for Atoms and Ions What will you be doing in lab next week? Recording the line spectra of several different substances in discharge tubes. Recording the line spectra of several ions from salts present in a flame. Understanding the correlation between emission spectra & atomic structure. Identifying unknowns by their atomic fingerprints. Light is made up of different wavelengths White light is the combination of all wavelengths of visible radiation resulting in the colors of the rainbow 1
2 Line Spectra Not all radiation is a continuous spectrum of energies (colors). Neon sign it gives off the equivalent of orange light to our eye So, why do line spectra occur? Max Planck (1900) proposed that atoms in a heated solid could absorb or emit electromagnetic energy He observed that a quantum of energy is related to the frequency of that radiation: 2
3 Einstein's Description of Events Einstein (1905) considered energy as packets he called photons. He observed that when photons of light hit a substance, only photons of a certain energy were absorbed by the atom. Electrons may only become excited by input of a specific amount of energy. The energized electrons overcome their attraction to the nucleus and escape from their normal ground state. Bohr Model of the Atom Niels Bohr (1913) described the structure of the atom based on Planck s and Einstein s theories. Bohr proposed: The H atom has only certain allowable energy levels, called stationary states. The atom does not radiate energy while in one of its stationary states. The atom changes to another stationary state (one electron moves to another orbit) only when absorbing or emitting a photon whose energy equals the difference of energy between the two states. 3
4 Bohr s Theory Bohr proposed that the H atom had only specific energy states E = B 2 n What happens when the electron is far away? Bohr explains line spectra 4
5 Bohr s Equation predicts H atom lines B Ef = 2 nf Final State minus B Ei = 2 ni Initial State Transitions are Quantized on the H atom 5
6 Atoms emit various kinds of radiation Visible light emission n = 6 n = 5 n = 4 n = 3 n = 2 n = 1 Ultraviolet light emission Infrared light emission Ultraviolet light = electron relaxing from n > 1 to an n = 1 orbital. Visible light = electron relaxing from n > 2 to an n = 2 orbital. Infrared light = electron relaxing from n > 3 to an n = 3 orbital. Terminology When an atom has its electrons in their lowest possible energy levels, it is in its ground state. When an electron has been promoted to a higher level, it is in an excited state. Electrons are promoted through an electric discharge, heat, or some other source of energy An atom in an excited state can emit photons as the electron relaxes to the ground state. 6
7 The hydrogen atom All other atoms Multi-electron atoms In the hydrogen atom, energy levels with the same integer number are of the same energy In multi-electron atoms, ALL of the orbitals have different energy levels. 7
8 Discharge tubes & emission spectra Line Spectra 8
9 All elements show emission spectra Every element has a unique emission spectrum. The spectral lines identify the element like an atomic fingerprint. (e.g., atomic absorption) Flame ionization also provides enough energy, but heat is used to excite the elements instead of electricity. MA/ma_s_kennedy3.html What you will do in experiment 2? You will use discharge tubes in the lab to evaluate line spectra of various gases. You will also perform flame ionization to evaluate the emission spectra of ions once they are excited in a flame. 9
10 Q: Calculate the energy of a photon with wavelength 540 nm (green light). You are performing Spectroscopy! Spectroscopy Quantitative measurement of absorption & emission of energy Atomic spectroscopy information about the structure of the atom based on energy information Emission Spectroscopy analysis of light emitted from a energized atom or ion, giving an emission spectrum 10
11 Let s wrap up what we know about electrons 1. An electron in an atom occupies an orbital. 2. Each orbital has a specific energy. 3. The energies available to electrons are quantized. 4. Absorption of energy by an atom causes an electron to move from a lower-energy orbital to a higher energy orbital (the atom is now in an excited state). 5. An excited atom will relax to the ground state. 6. Electrons may return to a lower-energy level by emission of an amount of energy equal to the energy difference between the higher and lower energy levels. 7. Since the energies of the orbitals are quantized, the amount of energy emitted is quantized. 8. One can calculate the energy differences between orbitals by measuring the frequency, wavelength, or energy of light emitted. 11
12 Safety & Waste Disposal Absolutely NO substances found in a chemistry lab should be ingested. Wear gloves while using the metal salts. Prevent contamination of burners by using a small amount of water and metal salt. They will drip!! Turn off the Bunsen burners when finished and ensure the gas is turned off. Do not touch any part of the power supply unless it is unplugged. Do not look directly at the discharge lamps while they are illuminated; look only off to the sides of the lamp to observe the line spectra. Before next week s lab Print the prelab for Atomic Spectra for Atoms and Ions from the website and complete the problems. Prepare final draft of the Introduction section of the formal lab report. 12
Atomic Spectroscopy. Objectives
 Atomic Spectroscopy Name Objectives explain the difference between emission and absorption spectra calculate the energy of orbits in the Bohr model of hydrogen calculate E for energy transitions in the
Atomic Spectroscopy Name Objectives explain the difference between emission and absorption spectra calculate the energy of orbits in the Bohr model of hydrogen calculate E for energy transitions in the
Chemistry 212 ATOMIC SPECTROSCOPY
 Chemistry 212 ATOMIC SPECTROSCOPY The emission and absorption of light energy of particular wavelengths by atoms and molecules is a common phenomenon. The emissions/absorptions are characteristic for each
Chemistry 212 ATOMIC SPECTROSCOPY The emission and absorption of light energy of particular wavelengths by atoms and molecules is a common phenomenon. The emissions/absorptions are characteristic for each
Emission Spectroscopy
 Objectives Emission Spectroscopy Observe spectral lines from a hydrogen gas discharge tube Determine the initial and final energy levels for the electronic transitions associated with the visible portion
Objectives Emission Spectroscopy Observe spectral lines from a hydrogen gas discharge tube Determine the initial and final energy levels for the electronic transitions associated with the visible portion
The relationship between these aspects is described by the following equation: E = hν =
 1 Learning Outcomes EXPERIMENT A10: LINE SPECTRUM Upon completion of this lab, the student will be able to: 1) Examine the line spectrum of the hydrogen atom. 2) Calculate the frequency and energy of the
1 Learning Outcomes EXPERIMENT A10: LINE SPECTRUM Upon completion of this lab, the student will be able to: 1) Examine the line spectrum of the hydrogen atom. 2) Calculate the frequency and energy of the
Lab: Excited Electrons
 Part A: EMISSION SPECTROSCOPY Lab: Excited Electrons According to the Bohr atomic model, electrons orbit the nucleus within specific energy levels. These levels are defined by unique amounts of energy.
Part A: EMISSION SPECTROSCOPY Lab: Excited Electrons According to the Bohr atomic model, electrons orbit the nucleus within specific energy levels. These levels are defined by unique amounts of energy.
Higher -o-o-o- Past Paper questions o-o-o- 3.4 Spectra
 Higher -o-o-o- Past Paper questions 1991-2010 -o-o-o- 3.4 Spectra 1992 Q37 The diagram below shows the energy levels for the hydrogen atom. (a) Between which two energy levels would an electron transition
Higher -o-o-o- Past Paper questions 1991-2010 -o-o-o- 3.4 Spectra 1992 Q37 The diagram below shows the energy levels for the hydrogen atom. (a) Between which two energy levels would an electron transition
Laboratory Atomic Emission Spectrum
 Laboratory Atomic Emission Spectrum Pre-Lab Questions: Answer the following questions in complete sentences by reading through the Overview and Background sections below. 1. What is the purpose of the
Laboratory Atomic Emission Spectrum Pre-Lab Questions: Answer the following questions in complete sentences by reading through the Overview and Background sections below. 1. What is the purpose of the
Atomic Spectra Introduction
 Atomic Spectra Introduction: Light and all other electromagnetic radiation is energy that is emitted in the form of waves. Thus light behaves like a wave, and the energy of light varies with the wavelength
Atomic Spectra Introduction: Light and all other electromagnetic radiation is energy that is emitted in the form of waves. Thus light behaves like a wave, and the energy of light varies with the wavelength
Quick Review. 1. Kinetic Molecular Theory. 2. Average kinetic energy and average velocity. 3. Graham s Law of Effusion. 4. Real Gas Behavior.
 Quick Review 1. Kinetic Molecular Theory. 2. Average kinetic energy and average velocity. 3. Graham s Law of Effusion. 4. Real Gas Behavior. Emission spectra Every element has a unique emission spectrum
Quick Review 1. Kinetic Molecular Theory. 2. Average kinetic energy and average velocity. 3. Graham s Law of Effusion. 4. Real Gas Behavior. Emission spectra Every element has a unique emission spectrum
Chapter 7. Quantum Theory and Atomic Structure
 Chapter 7 Quantum Theory and Atomic Structure Outline 1. The Nature of Light 2. Atomic Spectra 3. The Wave-Particle Duality of Matter and Energy 4. The Quantum-Mechanical Model of the Atom 3 September
Chapter 7 Quantum Theory and Atomic Structure Outline 1. The Nature of Light 2. Atomic Spectra 3. The Wave-Particle Duality of Matter and Energy 4. The Quantum-Mechanical Model of the Atom 3 September
where n = (an integer) =
 5.111 Lecture Summary #5 Readings for today: Section 1.3 (1.6 in 3 rd ed) Atomic Spectra, Section 1.7 up to equation 9b (1.5 up to eq. 8b in 3 rd ed) Wavefunctions and Energy Levels, Section 1.8 (1.7 in
5.111 Lecture Summary #5 Readings for today: Section 1.3 (1.6 in 3 rd ed) Atomic Spectra, Section 1.7 up to equation 9b (1.5 up to eq. 8b in 3 rd ed) Wavefunctions and Energy Levels, Section 1.8 (1.7 in
Experiment #9. Atomic Emission Spectroscopy
 Introduction Experiment #9. Atomic Emission Spectroscopy Spectroscopy is the study of the interaction of light with matter. This interaction can be in the form of the absorption or the emission of electromagnetic
Introduction Experiment #9. Atomic Emission Spectroscopy Spectroscopy is the study of the interaction of light with matter. This interaction can be in the form of the absorption or the emission of electromagnetic
Spectroscopy Minneapolis Community and Technical College v.10.17
 Spectroscopy Minneapolis Community and Technical College v.10.17 Objective: To observe, measure and compare line spectra from various elements and to determine the energies of those electronic transitions
Spectroscopy Minneapolis Community and Technical College v.10.17 Objective: To observe, measure and compare line spectra from various elements and to determine the energies of those electronic transitions
Chapter 6. Quantum Theory and the Electronic Structure of Atoms Part 1
 Chapter 6 Quantum Theory and the Electronic Structure of Atoms Part 1 The nature of light Quantum theory Topics Bohr s theory of the hydrogen atom Wave properties of matter Quantum mechanics Quantum numbers
Chapter 6 Quantum Theory and the Electronic Structure of Atoms Part 1 The nature of light Quantum theory Topics Bohr s theory of the hydrogen atom Wave properties of matter Quantum mechanics Quantum numbers
Electron Energy and Light
 Why? Electron Energy and Light How does light reveal the behavior of electrons in an atom? From fireworks to stars, the color of light is useful in finding out what s in matter. The emission of light by
Why? Electron Energy and Light How does light reveal the behavior of electrons in an atom? From fireworks to stars, the color of light is useful in finding out what s in matter. The emission of light by
I understand the relationship between energy and a quanta I understand the difference between an electron s ground state and an electron s excited
 NCCS 1.1.2 & 1.1.3 I understand the relationship between energy and a quanta I understand the difference between an electron s ground state and an electron s excited state I will describe how an electron
NCCS 1.1.2 & 1.1.3 I understand the relationship between energy and a quanta I understand the difference between an electron s ground state and an electron s excited state I will describe how an electron
UNIT : QUANTUM THEORY AND THE ATOM
 Name St.No. Date(YY/MM/DD) / / Section UNIT 102-10: QUANTUM THEORY AND THE ATOM OBJECTIVES Atomic Spectra for Hydrogen, Mercury and Neon. 1. To observe various atomic spectra with a diffraction grating
Name St.No. Date(YY/MM/DD) / / Section UNIT 102-10: QUANTUM THEORY AND THE ATOM OBJECTIVES Atomic Spectra for Hydrogen, Mercury and Neon. 1. To observe various atomic spectra with a diffraction grating
CSUS Department of Chemistry Experiment 9 Chem. 1A
 CSUS Department of Chemistry xperiment 9 Chem. 1A xp. 9 PR-Lab ASSIGNMNT Name: Lab Section (1) Use equation (2) [see the discussion on the next page] to calculate the energies of the ten lowest states
CSUS Department of Chemistry xperiment 9 Chem. 1A xp. 9 PR-Lab ASSIGNMNT Name: Lab Section (1) Use equation (2) [see the discussion on the next page] to calculate the energies of the ten lowest states
P O G I L E L E C T R O N E N E R G Y A N D L I G H T
 South Pasadena Honors Chemistry Name 9 Atomic Structure Period Date Why? P O G I L E L E C T R O N E N E R G Y A N D L I G H T How does light reveal the behavior of electrons in an atom? From fireworks
South Pasadena Honors Chemistry Name 9 Atomic Structure Period Date Why? P O G I L E L E C T R O N E N E R G Y A N D L I G H T How does light reveal the behavior of electrons in an atom? From fireworks
The Bohr Model of the Atom
 Unit 4: The Bohr Model of the Atom Properties of light Before the 1900 s, light was thought to behave only as a wave. Light is a type of electromagnetic radiation - a form of energy that exhibits wave
Unit 4: The Bohr Model of the Atom Properties of light Before the 1900 s, light was thought to behave only as a wave. Light is a type of electromagnetic radiation - a form of energy that exhibits wave
Emission of Light: Discharge Lamps & Flame Tests 1
 Emission of Light: Discharge Lamps & Flame Tests 1 Objectives At the end of this activity you should be able to: o Describe how discharge lamps emit photons following electrical excitation of gaseous atoms.
Emission of Light: Discharge Lamps & Flame Tests 1 Objectives At the end of this activity you should be able to: o Describe how discharge lamps emit photons following electrical excitation of gaseous atoms.
Experiment 3 Electromagnetic Radiation and Atom Interaction
 Experiment 3 Electromagnetic Radiation and Atom Interaction B OBJECTIVES To be familiar with the relationship between emission line spectra and the energy levels of electrons in various atoms. B INTRODUCTION
Experiment 3 Electromagnetic Radiation and Atom Interaction B OBJECTIVES To be familiar with the relationship between emission line spectra and the energy levels of electrons in various atoms. B INTRODUCTION
Electromagnetic spectrum Electromagnetic radiation
 Chapter 4 Section 1 Electromagnetic spectrum includes all the different wave lengths of radiation. Electromagnetic radiation is a form of energy that exhibits wave like behavior as it travels through space.
Chapter 4 Section 1 Electromagnetic spectrum includes all the different wave lengths of radiation. Electromagnetic radiation is a form of energy that exhibits wave like behavior as it travels through space.
Do Now: Bohr Diagram, Lewis Structures, Valence Electrons 1. What is the maximum number of electrons you can fit in each shell?
 Chemistry Ms. Ye Name Date Block Do Now: Bohr Diagram, Lewis Structures, Valence Electrons 1. What is the maximum number of electrons you can fit in each shell? 1 st shell 2 nd shell 3 rd shell 4 th shell
Chemistry Ms. Ye Name Date Block Do Now: Bohr Diagram, Lewis Structures, Valence Electrons 1. What is the maximum number of electrons you can fit in each shell? 1 st shell 2 nd shell 3 rd shell 4 th shell
Modern Physics- Introduction. L 35 Modern Physics [1] ATOMS and classical physics. Newton s Laws have flaws! accelerated charges radiate energy
![Modern Physics- Introduction. L 35 Modern Physics [1] ATOMS and classical physics. Newton s Laws have flaws! accelerated charges radiate energy Modern Physics- Introduction. L 35 Modern Physics [1] ATOMS and classical physics. Newton s Laws have flaws! accelerated charges radiate energy](/thumbs/87/96907347.jpg) L 35 Modern Physics [1] Introduction- quantum physics Particles of light PHOTONS The photoelectric effect Photocells & intrusion detection devices The Bohr atom emission & absorption of radiation LASERS
L 35 Modern Physics [1] Introduction- quantum physics Particles of light PHOTONS The photoelectric effect Photocells & intrusion detection devices The Bohr atom emission & absorption of radiation LASERS
Atoms and Spectroscopy
 Atoms and Spectroscopy Lecture 3 1 ONE SMALL STEP FOR MAN ONE GIANT LEAP FOR MANKIND 2 FROM ATOMS TO STARS AND GALAXIES HOW DO WE KNOW? Observations The Scientific Method Hypothesis Verifications LAW 3
Atoms and Spectroscopy Lecture 3 1 ONE SMALL STEP FOR MAN ONE GIANT LEAP FOR MANKIND 2 FROM ATOMS TO STARS AND GALAXIES HOW DO WE KNOW? Observations The Scientific Method Hypothesis Verifications LAW 3
high energy state for the electron in the atom low energy state for the electron in the atom
 Atomic Spectra Objectives The objectives of this experiment are to: 1) Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. 2) Measure several wavelengths of light
Atomic Spectra Objectives The objectives of this experiment are to: 1) Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. 2) Measure several wavelengths of light
Duncan. Electrons, Energy, & the Electromagnetic Spectrum Notes Simplified, 2-D Bohr Model: Figure 1. Figure 2. Figure 3
 Electrons, Energy, & the Electromagnetic Spectrum Notes Simplified, 2-D Bohr Model: Figure 1 Figure 2 Figure 3 Light Calculation Notes Here s how the type/form of EM radiation can be determined The amount
Electrons, Energy, & the Electromagnetic Spectrum Notes Simplified, 2-D Bohr Model: Figure 1 Figure 2 Figure 3 Light Calculation Notes Here s how the type/form of EM radiation can be determined The amount
ASTRO Fall 2012 LAB #7: The Electromagnetic Spectrum
 ASTRO 1050 - Fall 2012 LAB #7: The Electromagnetic Spectrum ABSTRACT Astronomers rely on light to convey almost all of the information we have on distant astronomical objects. In addition to measuring
ASTRO 1050 - Fall 2012 LAB #7: The Electromagnetic Spectrum ABSTRACT Astronomers rely on light to convey almost all of the information we have on distant astronomical objects. In addition to measuring
The Structure of the Atom
 CHAPTER 5 The Structure of the Atom 5.4 Light and Spectroscopy 460 370 BC 1808 1870 1897 1910 1925 Today Democritus Atomism Dalton Modern atomic theory Crookes Cathode rays Thomson Discovery of the electron
CHAPTER 5 The Structure of the Atom 5.4 Light and Spectroscopy 460 370 BC 1808 1870 1897 1910 1925 Today Democritus Atomism Dalton Modern atomic theory Crookes Cathode rays Thomson Discovery of the electron
L 35 Modern Physics [1]
![L 35 Modern Physics [1] L 35 Modern Physics [1]](/thumbs/90/102858198.jpg) L 35 Modern Physics [1] Introduction- quantum physics Particles of light PHOTONS The photoelectric effect Photocells & intrusion detection devices The Bohr atom emission & absorption of radiation LASERS
L 35 Modern Physics [1] Introduction- quantum physics Particles of light PHOTONS The photoelectric effect Photocells & intrusion detection devices The Bohr atom emission & absorption of radiation LASERS
Atomic Emission Spectra
 Atomic Emission Spectra Objectives The objectives of this laboratory are as follows: To build and calibrate a simple meter-stick spectroscope that is capable of measuring wavelengths of visible light.
Atomic Emission Spectra Objectives The objectives of this laboratory are as follows: To build and calibrate a simple meter-stick spectroscope that is capable of measuring wavelengths of visible light.
29:006 FINAL EXAM FRIDAY MAY 11 3:00 5:00 PM IN LR1 VAN
 L 33 Modern Physics [1] 29:006 FINAL EXAM FRIDAY MAY 11 3:00 5:00 PM IN LR1 VAN Introduction- quantum physics Particles of light PHOTONS The photoelectric effect Photocells & intrusion detection devices
L 33 Modern Physics [1] 29:006 FINAL EXAM FRIDAY MAY 11 3:00 5:00 PM IN LR1 VAN Introduction- quantum physics Particles of light PHOTONS The photoelectric effect Photocells & intrusion detection devices
Introduction. Electromagnetic Waves. Electromagnetic Waves
 Introduction Much of the information we know about electrons comes from studies of interactions of light and matter. In the early 1900 s, scientists discovered that light has properties of both a wave
Introduction Much of the information we know about electrons comes from studies of interactions of light and matter. In the early 1900 s, scientists discovered that light has properties of both a wave
Chapter 6: The Electronic Structure of the Atom Electromagnetic Spectrum. All EM radiation travels at the speed of light, c = 3 x 10 8 m/s
 Chapter 6: The Electronic Structure of the Atom Electromagnetic Spectrum V I B G Y O R All EM radiation travels at the speed of light, c = 3 x 10 8 m/s Electromagnetic radiation is a wave with a wavelength
Chapter 6: The Electronic Structure of the Atom Electromagnetic Spectrum V I B G Y O R All EM radiation travels at the speed of light, c = 3 x 10 8 m/s Electromagnetic radiation is a wave with a wavelength
Observation of Atomic Spectra
 Observation of Atomic Spectra Introduction In this experiment you will observe and measure the wavelengths of different colors of light emitted by atoms. You will first observe light emitted from excited
Observation of Atomic Spectra Introduction In this experiment you will observe and measure the wavelengths of different colors of light emitted by atoms. You will first observe light emitted from excited
Ex: N has 5 valence electrons, so it s Lewis structure would look like: N
 Chemistry Ms. Ye Review: Bohr Model of the Atom Name Date Block Electrons are shown in concentric shells or energy levels around the nucleus o The first shell can hold up to o The second shell can hold
Chemistry Ms. Ye Review: Bohr Model of the Atom Name Date Block Electrons are shown in concentric shells or energy levels around the nucleus o The first shell can hold up to o The second shell can hold
Accounts for certain objects being colored. Used in medicine (examples?) Allows us to learn about structure of the atom
 1.1 Interaction of Light and Matter Accounts for certain objects being colored Used in medicine (examples?) 1.2 Wavelike Properties of Light Wavelength, : peak to peak distance Amplitude: height of the
1.1 Interaction of Light and Matter Accounts for certain objects being colored Used in medicine (examples?) 1.2 Wavelike Properties of Light Wavelength, : peak to peak distance Amplitude: height of the
Electrons, Energy, & the Electromagnetic Spectrum Notes Simplified, 2-D Bohr Model: Figure 2. Figure 3 UNIT 4 - ELECTRONS & ELECTRON ARRANGEMENT
 Electrons, Energy, & the Electromagnetic Spectrum Notes Simplified, 2-D Bohr Model: Figure 1 UNIT 4 - ELECTRONS & ELECTRON ARRANGEMENT Figure 2 Figure 3 The energy is released as electromagnetic radiation.
Electrons, Energy, & the Electromagnetic Spectrum Notes Simplified, 2-D Bohr Model: Figure 1 UNIT 4 - ELECTRONS & ELECTRON ARRANGEMENT Figure 2 Figure 3 The energy is released as electromagnetic radiation.
Atomic Theory C &03
 Atomic Theory Part One: Flame Tests Part Two: Atomic Spectra Part Three: Applications of Spectra (optional) C12-2-02 &03 This activity will focus on the visible portion of the electromagnetic spectrum.
Atomic Theory Part One: Flame Tests Part Two: Atomic Spectra Part Three: Applications of Spectra (optional) C12-2-02 &03 This activity will focus on the visible portion of the electromagnetic spectrum.
Atomic Theory: Spectroscopy and Flame Tests
 Atomic Theory: Spectroscopy and Flame Tests Introduction Light energy is also known as electromagnetic (EM) radiation. The light that we observe with our eyes, visible light, is just a small portion of
Atomic Theory: Spectroscopy and Flame Tests Introduction Light energy is also known as electromagnetic (EM) radiation. The light that we observe with our eyes, visible light, is just a small portion of
( J s)( m/s)
 Ch100: Fundamentals for Chemistry 1 LAB: Spectroscopy Neon lights are orange. Sodium lamps are yellow. Mercury lights are bluish. Electricity is doing something to the electrons of these elements to produce
Ch100: Fundamentals for Chemistry 1 LAB: Spectroscopy Neon lights are orange. Sodium lamps are yellow. Mercury lights are bluish. Electricity is doing something to the electrons of these elements to produce
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY
 ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
The Hydrogen Spectrum
 1 The Hydrogen Spectrum PHYS 1301 F98 Prof. T.E. Coan Last edit: 6 Aug 98 Introduction In last week's laboratory experiment on diffraction, you should have noticed that the light from the mercury discharge
1 The Hydrogen Spectrum PHYS 1301 F98 Prof. T.E. Coan Last edit: 6 Aug 98 Introduction In last week's laboratory experiment on diffraction, you should have noticed that the light from the mercury discharge
Introduction to light Light is a form of energy called electromagnetic radiation. A chart of the electromagnetic spectrum is shown below.
 Experiment: Spectroscopy Introduction to light Light is a form of energy called electromagnetic radiation. A chart of the electromagnetic spectrum is shown below. Radiowave Microwave Infrared Visible Ultraviolet
Experiment: Spectroscopy Introduction to light Light is a form of energy called electromagnetic radiation. A chart of the electromagnetic spectrum is shown below. Radiowave Microwave Infrared Visible Ultraviolet
Color. 3. Why are the color labels in the table above plural (i.e., Reds rather than Red )?
 NS D3 Electron Energy and Light Name From fireworks to stars, the color of light is useful in finding out what s in matter. The emission of light by hydrogen and other atoms has played a key role in understanding
NS D3 Electron Energy and Light Name From fireworks to stars, the color of light is useful in finding out what s in matter. The emission of light by hydrogen and other atoms has played a key role in understanding
Emission of Light & Atomic Models 1
 Emission of Light & Atomic Models 1 Objective At the end of this activity you should be able to: o Explain what photons are, and be able to calculate their energies given either their frequency or wavelength.
Emission of Light & Atomic Models 1 Objective At the end of this activity you should be able to: o Explain what photons are, and be able to calculate their energies given either their frequency or wavelength.
Chapter 5 Light and Matter
 Chapter 5 Light and Matter Stars and galaxies are too far for us to send a spacecraft or to visit (in our lifetimes). All we can receive from them is light But there is much we can learn (composition,
Chapter 5 Light and Matter Stars and galaxies are too far for us to send a spacecraft or to visit (in our lifetimes). All we can receive from them is light But there is much we can learn (composition,
An element Is a substance that cannot be split into simpler substance. It is composed of discrete particles called atoms.
 digitalteachers.co.ug Atomic structure Inorganic chemistry deals with the physical and chemical properties of the elements of the the periodic table. An element Is a substance that cannot be split into
digitalteachers.co.ug Atomic structure Inorganic chemistry deals with the physical and chemical properties of the elements of the the periodic table. An element Is a substance that cannot be split into
ELECTRONS LAB name: 80/1200
 EECTRONS B name: 80/1200 Objective: To use flame tests + bright line spectra to identify elements and compounds. Electrons once were in plum pudding, then flying randomly around the nucleus, until Niels
EECTRONS B name: 80/1200 Objective: To use flame tests + bright line spectra to identify elements and compounds. Electrons once were in plum pudding, then flying randomly around the nucleus, until Niels
Models of the Atom. Spencer Clelland & Katelyn Mason
 Models of the Atom Spencer Clelland & Katelyn Mason First Things First Electrons were accepted to be part of the atom structure by scientists in the1900 s. The first model of the atom was visualized as
Models of the Atom Spencer Clelland & Katelyn Mason First Things First Electrons were accepted to be part of the atom structure by scientists in the1900 s. The first model of the atom was visualized as
Atomic Theory: Spectroscopy and Flame Tests
 Atomic Theory: Spectroscopy and Flame Tests Introduction Light energy is also known as electromagnetic (EM) radiation. The light that we observe with our eyes, visible light, is just a small portion of
Atomic Theory: Spectroscopy and Flame Tests Introduction Light energy is also known as electromagnetic (EM) radiation. The light that we observe with our eyes, visible light, is just a small portion of
Lesmahagow High School AHChemistry Inorganic and Physical Chemistry Lesmahagow High School CfE Advanced Higher Chemistry
 Lesmahagow High School CfE Advanced Higher Chemistry Unit 1 Inorganic and Physical Chemistry Electromagnetic Radiation and Atomic Spectra 1 Electromagnetic Radiation Radiation such as light, microwaves,
Lesmahagow High School CfE Advanced Higher Chemistry Unit 1 Inorganic and Physical Chemistry Electromagnetic Radiation and Atomic Spectra 1 Electromagnetic Radiation Radiation such as light, microwaves,
Chapter 28 Assignment Solutions
 Chapter 28 Assignment Solutions Page 770 #23-26, 29-30, 43-48, 55 23) Complete the following concept map using these terms: energy levels, fixed electron radii, Bohr model, photon emission and absorption,
Chapter 28 Assignment Solutions Page 770 #23-26, 29-30, 43-48, 55 23) Complete the following concept map using these terms: energy levels, fixed electron radii, Bohr model, photon emission and absorption,
The Atom & Unanswered Questions:
 The Atom & Unanswered Questions: 1) Recall-Rutherford s model, that atom s mass is concentrated in the nucleus & electrons move around it. a) Doesn t explain how the electrons were arranged around the
The Atom & Unanswered Questions: 1) Recall-Rutherford s model, that atom s mass is concentrated in the nucleus & electrons move around it. a) Doesn t explain how the electrons were arranged around the
Atomic Theory: Spectroscopy and Flame Tests
 Atomic Theory: Spectroscopy and Flame Tests Introduction Light energy is also known as electromagnetic (EM) radiation. The light that we observe with our eyes, visible light, is just a small portion of
Atomic Theory: Spectroscopy and Flame Tests Introduction Light energy is also known as electromagnetic (EM) radiation. The light that we observe with our eyes, visible light, is just a small portion of
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY
 ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
ATOMIC STRUCTURE, ELECTRONS, AND PERIODICITY All matter is made of atoms. There are a limited number of types of atoms; these are the elements. (EU 1.A) Development of Atomic Theory Atoms are so small
Chapter 8. Spectroscopy. 8.1 Purpose. 8.2 Introduction
 Chapter 8 Spectroscopy 8.1 Purpose In the experiment atomic spectra will be investigated. The spectra of three know materials will be observed. The composition of an unknown material will be determined.
Chapter 8 Spectroscopy 8.1 Purpose In the experiment atomic spectra will be investigated. The spectra of three know materials will be observed. The composition of an unknown material will be determined.
Experiment 24: Spectroscopy
 Experiment 24: Spectroscopy Figure 24.1: Spectroscopy EQUIPMENT High Voltage Power Supply Incandescent Light Source (3) Gas Discharge Tubes: 1. Helium 2. Hydrogen 3. Unknown Element Spectrometer Felt (1)
Experiment 24: Spectroscopy Figure 24.1: Spectroscopy EQUIPMENT High Voltage Power Supply Incandescent Light Source (3) Gas Discharge Tubes: 1. Helium 2. Hydrogen 3. Unknown Element Spectrometer Felt (1)
Chapter 7 QUANTUM THEORY & ATOMIC STRUCTURE Brooks/Cole - Thomson
 Chapter 7 QUANTUM THEORY & ATOMIC STRUCTURE 1 7.1 The Nature of Light 2 Most subatomic particles behave as PARTICLES and obey the physics of waves. Light is a type of electromagnetic radiation Light consists
Chapter 7 QUANTUM THEORY & ATOMIC STRUCTURE 1 7.1 The Nature of Light 2 Most subatomic particles behave as PARTICLES and obey the physics of waves. Light is a type of electromagnetic radiation Light consists
Stellar Astrophysics: The Interaction of Light and Matter
 Stellar Astrophysics: The Interaction of Light and Matter The Photoelectric Effect Methods of electron emission Thermionic emission: Application of heat allows electrons to gain enough energy to escape
Stellar Astrophysics: The Interaction of Light and Matter The Photoelectric Effect Methods of electron emission Thermionic emission: Application of heat allows electrons to gain enough energy to escape
Chapter 4 Arrangement of Electrons in Atoms. 4.1 The Development of a New Atomic Model
 Chapter 4 Arrangement of Electrons in Atoms 4.1 The Development of a New Atomic Model Properties of Light Electromagnetic Radiation: EM radiation are forms of energy which move through space as waves There
Chapter 4 Arrangement of Electrons in Atoms 4.1 The Development of a New Atomic Model Properties of Light Electromagnetic Radiation: EM radiation are forms of energy which move through space as waves There
Chapter 28. Atomic Physics
 Chapter 28 Atomic Physics Sir Joseph John Thomson J. J. Thomson 1856-1940 Discovered the electron Did extensive work with cathode ray deflections 1906 Nobel Prize for discovery of electron Early Models
Chapter 28 Atomic Physics Sir Joseph John Thomson J. J. Thomson 1856-1940 Discovered the electron Did extensive work with cathode ray deflections 1906 Nobel Prize for discovery of electron Early Models
Physics 23 Fall 1998 Lab 4 - The Hydrogen Spectrum
 Physics 3 Fall 998 Lab 4 - The Hydrogen Spectrum Theory In the late 800's, it was known that when a gas is excited by means of an electric discharge and the light emitted is viewed through a diffraction
Physics 3 Fall 998 Lab 4 - The Hydrogen Spectrum Theory In the late 800's, it was known that when a gas is excited by means of an electric discharge and the light emitted is viewed through a diffraction
What are the energies (J) and wavelengths (in nm) for these colors? Color Energy wavelength. Rev. F11 Page 1 of 5
 Exp. 8 Pre Lab ASSIGNMENT Name: Lab Section Score: / 10 (1) Use the equations [see the discussion on the next page] to calculate the energies of the 6 lowest states for the hydrogen atom, and enter your
Exp. 8 Pre Lab ASSIGNMENT Name: Lab Section Score: / 10 (1) Use the equations [see the discussion on the next page] to calculate the energies of the 6 lowest states for the hydrogen atom, and enter your
Earlier we learned that hot, opaque objects produce continuous spectra of radiation of different wavelengths.
 Section7: The Bohr Atom Earlier we learned that hot, opaque objects produce continuous spectra of radiation of different wavelengths. Continuous Spectrum Everyone has seen the spectrum produced when white
Section7: The Bohr Atom Earlier we learned that hot, opaque objects produce continuous spectra of radiation of different wavelengths. Continuous Spectrum Everyone has seen the spectrum produced when white
5.3. Physics and the Quantum Mechanical Model
 Chemistry 5-3 Physics and the Quantum Mechanical Model Neon advertising signs are formed from glass tubes bent in various shapes. An electric current passing through the gas in each glass tube makes the
Chemistry 5-3 Physics and the Quantum Mechanical Model Neon advertising signs are formed from glass tubes bent in various shapes. An electric current passing through the gas in each glass tube makes the
Chapter 5 Light and Matter: Reading Messages from the Cosmos. What is light? Properties of Waves. Waves. The Electromagnetic Spectrum
 Chapter 5 Light and Matter: Reading Messages from the Cosmos What is light? Light is a form of radiant energy Light can act either like a wave or like a particle (photon) Spectrum of the Sun 1 2 Waves
Chapter 5 Light and Matter: Reading Messages from the Cosmos What is light? Light is a form of radiant energy Light can act either like a wave or like a particle (photon) Spectrum of the Sun 1 2 Waves
Atomic Emission and Molecular Absorption Spectra
 Atomic Emission and Molecular Absorption Spectra v062513_6pm Objective: The student will observe the atomic emission spectra of hydrogen using a spectroscope, determine the identity of an unknown metal
Atomic Emission and Molecular Absorption Spectra v062513_6pm Objective: The student will observe the atomic emission spectra of hydrogen using a spectroscope, determine the identity of an unknown metal
NOTES: 5.3 Light and Atomic Spectra (more Quantum Mechanics!)
 NOTES: 5.3 Light and Atomic Spectra (more Quantum Mechanics!) Light WAVE or PARTICLE? Electromagnetic Radiation Electromagnetic radiation includes: -radio waves -microwaves -infrared waves -visible light
NOTES: 5.3 Light and Atomic Spectra (more Quantum Mechanics!) Light WAVE or PARTICLE? Electromagnetic Radiation Electromagnetic radiation includes: -radio waves -microwaves -infrared waves -visible light
Chapter 9. Blimps, Balloons, and Models for the Atom. Electrons in Atoms and the Periodic Table. Hindenburg. Properties of Elements Hydrogen Atoms
 Chapter 9 Electrons in Atoms and the Periodic Table Blimps, Balloons, and Models for the Atom Hindenburg Blimps, Balloons, and Models for the Atom Properties of Elements Hydrogen Atoms Helium Atoms 1 Blimps,
Chapter 9 Electrons in Atoms and the Periodic Table Blimps, Balloons, and Models for the Atom Hindenburg Blimps, Balloons, and Models for the Atom Properties of Elements Hydrogen Atoms Helium Atoms 1 Blimps,
Chapter 5 Light and Matter: Reading Messages from the Cosmos. How do we experience light? Colors of Light. How do light and matter interact?
 Chapter 5 Light and Matter: Reading Messages from the Cosmos How do we experience light? The warmth of sunlight tells us that light is a form of energy We can measure the amount of energy emitted by a
Chapter 5 Light and Matter: Reading Messages from the Cosmos How do we experience light? The warmth of sunlight tells us that light is a form of energy We can measure the amount of energy emitted by a
WEEK 2: 4 SEP THRU 10 SEP; LECTURES 4-6
 Learning Objectives Energy: Light as energy Describe the wave nature of light, wavelength, and frequency using the equation c = λν What is meant by the particle nature of light? Calculate the energy of
Learning Objectives Energy: Light as energy Describe the wave nature of light, wavelength, and frequency using the equation c = λν What is meant by the particle nature of light? Calculate the energy of
Energy levels and atomic structures lectures chapter one
 Structure of Atom An atom is the smallest constituent unit of ordinary matter that has the properties of a element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are
Structure of Atom An atom is the smallest constituent unit of ordinary matter that has the properties of a element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are
Physics and the Quantum Mechanical Model
 chemistry 1 of 38 Mechanical Model Neon advertising signs are formed from glass tubes bent in various shapes. An electric current passing through the gas in each glass tube makes the gas glow with its
chemistry 1 of 38 Mechanical Model Neon advertising signs are formed from glass tubes bent in various shapes. An electric current passing through the gas in each glass tube makes the gas glow with its
EXPERIMENT 17: Atomic Emission
 EXPERIMENT 17: Atomic Emission PURPOSE: To construct an energy level diagram of the hydrogen atom To identify an element from its line spectrum. PRINCIPLES: White light, such as emitted by the sun or an
EXPERIMENT 17: Atomic Emission PURPOSE: To construct an energy level diagram of the hydrogen atom To identify an element from its line spectrum. PRINCIPLES: White light, such as emitted by the sun or an
E n = n h ν. The oscillators must absorb or emit energy in discrete multiples of the fundamental quantum of energy given by.
 Planck s s Radiation Law Planck made two modifications to the classical theory The oscillators (of electromagnetic origin) can only have certain discrete energies determined by E n = n h ν with n is an
Planck s s Radiation Law Planck made two modifications to the classical theory The oscillators (of electromagnetic origin) can only have certain discrete energies determined by E n = n h ν with n is an
Quantum and Atomic Physics - Multiple Choice
 PSI AP Physics 2 Name 1. The Cathode Ray Tube experiment is associated with: (A) J. J. Thomson (B) J. S. Townsend (C) M. Plank (D) A. H. Compton 2. The electron charge was measured the first time in: (A)
PSI AP Physics 2 Name 1. The Cathode Ray Tube experiment is associated with: (A) J. J. Thomson (B) J. S. Townsend (C) M. Plank (D) A. H. Compton 2. The electron charge was measured the first time in: (A)
10/27/2017 [pgs ]
![10/27/2017 [pgs ] 10/27/2017 [pgs ]](/thumbs/82/85139288.jpg) Objectives SWBAT explain the relationship between energy and frequency. SWBAT predict the behavior of and/or calculate quantum and photon energy from frequency. SWBAT explain how the quantization of energy
Objectives SWBAT explain the relationship between energy and frequency. SWBAT predict the behavior of and/or calculate quantum and photon energy from frequency. SWBAT explain how the quantization of energy
ATOMIC STRUCTURE ELECTRON CONFIGURATION 10/13/15 PROJECT DATE. Tuesday, October 13, 15
 PROJECT DATE ATOMIC STRUCTURE ELECTRON CONFIGURATION 10/13/15 Agenda Begin Topic 2.2 Electron Configuration Homework: Energy of waves calculations Due tomorrow! Yes - tomorrow Atomic Structure The nuclear
PROJECT DATE ATOMIC STRUCTURE ELECTRON CONFIGURATION 10/13/15 Agenda Begin Topic 2.2 Electron Configuration Homework: Energy of waves calculations Due tomorrow! Yes - tomorrow Atomic Structure The nuclear
The Wave Nature of Light Made up of. Waves of fields at right angles to each other. Wavelength = Frequency =, measured in
 Chapter 6 Electronic Structure of Atoms The Wave Nature of Light Made up of. Waves of fields at right angles to each other. Wavelength = Frequency =, measured in Kinds of EM Waves There are many different
Chapter 6 Electronic Structure of Atoms The Wave Nature of Light Made up of. Waves of fields at right angles to each other. Wavelength = Frequency =, measured in Kinds of EM Waves There are many different
Chapter 7. The Quantum Mechanical Model of the Atom
 Chapter 7 The Quantum Mechanical Model of the Atom The Nature of Light:Its Wave Nature Light is a form of electromagnetic radiation composed of perpendicular oscillating waves, one for the electric field
Chapter 7 The Quantum Mechanical Model of the Atom The Nature of Light:Its Wave Nature Light is a form of electromagnetic radiation composed of perpendicular oscillating waves, one for the electric field
Write the electron configuration for Chromium (Cr):
 Write the electron configuration for Chromium (Cr): Energy level Aufbau Principle Atomic orbital Quantum Hund s Rule Atomic number Electron Configuration Whole number Pauli Exlcusion Principle Quantum
Write the electron configuration for Chromium (Cr): Energy level Aufbau Principle Atomic orbital Quantum Hund s Rule Atomic number Electron Configuration Whole number Pauli Exlcusion Principle Quantum
Chapter 6 Electronic Structure of Atoms
 Chapter 6 Electronic Structure of Atoms What is the origin of color in matter? Demo: flame tests What does this have to do with the atom? Why are atomic properties periodic? 6.1 The Wave Nature of Light
Chapter 6 Electronic Structure of Atoms What is the origin of color in matter? Demo: flame tests What does this have to do with the atom? Why are atomic properties periodic? 6.1 The Wave Nature of Light
Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 The Bohr Atom Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the energy of the emitted photon when an electron drops from the third
The Bohr Atom Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the energy of the emitted photon when an electron drops from the third
2) The number of cycles that pass through a stationary point is called A) wavelength. B) amplitude. C) frequency. D) area. E) median.
 Chemistry Structure and Properties 2nd Edition Tro Test Bank Full Download: http://testbanklive.com/download/chemistry-structure-and-properties-2nd-edition-tro-test-bank/ Chemistry: Structure & Properties,
Chemistry Structure and Properties 2nd Edition Tro Test Bank Full Download: http://testbanklive.com/download/chemistry-structure-and-properties-2nd-edition-tro-test-bank/ Chemistry: Structure & Properties,
General Physics (PHY 2140)
 General Physics (PHY 140) Lecture 33 Modern Physics Atomic Physics Atomic spectra Bohr s theory of hydrogen http://www.physics.wayne.edu/~apetrov/phy140/ Chapter 8 1 Lightning Review Last lecture: 1. Atomic
General Physics (PHY 140) Lecture 33 Modern Physics Atomic Physics Atomic spectra Bohr s theory of hydrogen http://www.physics.wayne.edu/~apetrov/phy140/ Chapter 8 1 Lightning Review Last lecture: 1. Atomic
EMISSION AND ABSORPTION SPECTRUM
 EMISSION AND ABSORPTION SPECTRUM Topic 7: Atomic, nuclear and particle physics 7.1 Discrete energy and radioactivity Essential idea: In the microscopic world energy is discrete. Nature of science: Accidental
EMISSION AND ABSORPTION SPECTRUM Topic 7: Atomic, nuclear and particle physics 7.1 Discrete energy and radioactivity Essential idea: In the microscopic world energy is discrete. Nature of science: Accidental
Ch 9 Electrons in Atoms & the Periodic Table Study Sheet Acc. Chemistry SCANTRON. Name /99. 3) Light is a type of matter. 3)
 Ch 9 Electrons in Atoms & the Periodic Table Study Sheet Acc. Chemistry SCANTRON Name /99 TRUE/FALSE. Write 'T' if the statement is true and 'F' if the statement is false. 1) When the elements are arranged
Ch 9 Electrons in Atoms & the Periodic Table Study Sheet Acc. Chemistry SCANTRON Name /99 TRUE/FALSE. Write 'T' if the statement is true and 'F' if the statement is false. 1) When the elements are arranged
Einstein. Quantum Physics at a glance. Planck s Hypothesis (blackbody radiation) (ultraviolet catastrophe) Quantized Energy
 Quantum Physics at a glance Quantum Physics deals with the study of light and particles at atomic and smaller levels. Planck s Hypothesis (blackbody radiation) (ultraviolet catastrophe) Quantized Energy
Quantum Physics at a glance Quantum Physics deals with the study of light and particles at atomic and smaller levels. Planck s Hypothesis (blackbody radiation) (ultraviolet catastrophe) Quantized Energy
Atomic Structure and the Periodic Table
 Atomic Structure and the Periodic Table The electronic structure of an atom determines its characteristics Studying atoms by analyzing light emissions/absorptions Spectroscopy: analysis of light emitted
Atomic Structure and the Periodic Table The electronic structure of an atom determines its characteristics Studying atoms by analyzing light emissions/absorptions Spectroscopy: analysis of light emitted
The energy of the emitted light (photons) is given by the difference in energy between the initial and final states of hydrogen atom.
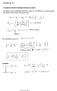 Lecture 20-21 Page 1 Lectures 20-21 Transitions between hydrogen stationary states The energy of the emitted light (photons) is given by the difference in energy between the initial and final states of
Lecture 20-21 Page 1 Lectures 20-21 Transitions between hydrogen stationary states The energy of the emitted light (photons) is given by the difference in energy between the initial and final states of
Electrons in Atoms. Section 5.1 Light and Quantized Energy
 Name Date Class 5 Electrons in Atoms Section 5.1 Light and Quantized Energy In your textbook, read about the wave nature of light. Use each of the terms below just once to complete the passage. amplitude
Name Date Class 5 Electrons in Atoms Section 5.1 Light and Quantized Energy In your textbook, read about the wave nature of light. Use each of the terms below just once to complete the passage. amplitude
Bellwork: Calculate the atomic mass of potassium and magnesium
 Bellwork: Calculate the atomic mass of potassium and magnesium Chapter 5 - electrons in atoms Section 5.1: Revising the atomic model What did Ernest Rutherford think about electrons? In Rutherford s model,
Bellwork: Calculate the atomic mass of potassium and magnesium Chapter 5 - electrons in atoms Section 5.1: Revising the atomic model What did Ernest Rutherford think about electrons? In Rutherford s model,
CHM 111 Unit 7 Sample Questions
 Name: Class: Date: As you work these problems, consider and explain: A. What type of question is it? B. How do you know what type of question it is? C. What information are you looking for? D. What information
Name: Class: Date: As you work these problems, consider and explain: A. What type of question is it? B. How do you know what type of question it is? C. What information are you looking for? D. What information
THE ATOMIC SPECTRUM OF HYDROGEN
 THE ATOMIC SPECTRUM OF HYDROGEN When atoms are excited, either in an electric discharge or with heat, they tend to give off light. The light is emitted only at certain wavelengths that are characteristic
THE ATOMIC SPECTRUM OF HYDROGEN When atoms are excited, either in an electric discharge or with heat, they tend to give off light. The light is emitted only at certain wavelengths that are characteristic
Particle nature of light & Quantization
 Particle nature of light & Quantization A quantity is quantized if its possible values are limited to a discrete set. An example from classical physics is the allowed frequencies of standing waves on a
Particle nature of light & Quantization A quantity is quantized if its possible values are limited to a discrete set. An example from classical physics is the allowed frequencies of standing waves on a
Ch 7 Quantum Theory of the Atom (light and atomic structure)
 Ch 7 Quantum Theory of the Atom (light and atomic structure) Electromagnetic Radiation - Electromagnetic radiation consists of oscillations in electric and magnetic fields. The oscillations can be described
Ch 7 Quantum Theory of the Atom (light and atomic structure) Electromagnetic Radiation - Electromagnetic radiation consists of oscillations in electric and magnetic fields. The oscillations can be described
Electron Energy and Light
 Why? Electron Energy and Light How does light reveal the behavior of electrons in an atom? From fireworks to stars, the color of light is useful in finding out what s in matter. The emission of light by
Why? Electron Energy and Light How does light reveal the behavior of electrons in an atom? From fireworks to stars, the color of light is useful in finding out what s in matter. The emission of light by
Chapter 5 Models of the Atom
 Chapter 5 Models of the Atom Atomic Models Rutherford used existing ideas about the atom and proposed an atomic model in which the electrons move around the nucleus. However, Rutherford s atomic model
Chapter 5 Models of the Atom Atomic Models Rutherford used existing ideas about the atom and proposed an atomic model in which the electrons move around the nucleus. However, Rutherford s atomic model
