3.9. Thermoluminescence
|
|
|
- Opal Evans
- 6 years ago
- Views:
Transcription
1 3.9. Thermoluminescence Thermoluminescence (TL) dating is a technique that is based on the analysis of light release when heating crystalline material. TL-dating is used in mineralogy and geology, but is also increasingly being applied for dating of anthropological and archaeological samples. chlorophane mineral cool state heated state
2 Applicability of TL dating TL dating is mainly applicable for material with mineral or crystalline structure or with spurious crystalline contents. It is only usable of insulating material not for metallic artifacts. In archaeology TL is mainly used for pottery analysis. In anthropology the main use of TL is the dating of flint stone as early tool material for mankind.
3 The glow curve first heating second heating Typical phenomenon of thermoluminescence, when sample is heated above 200 o C, light emission in blue range is observed up to 400 o C. At higher temperature, material emits a red glow. For second heating no blue light emission is observed, only the red glow curve remains at the higher temperature range.
4 Origin of thermoluminescence Thermoluminescence (TL) originates from the temperature induced release of energy, stored in the lattice structure of the crystal following long-term internal and external exposure to nuclear radiation from natural sources. TL accumulates in the material with time depending on the radiation (and light) exposure. The TL is released by heating. The dating clock starts with the initial firing of the material, when originally accumulated TL is being driven out.
5 Age determination the amount of accumulated TL is proportional to the age of the sample and inverse proportional to the nuclear radiation exposure of the sample. Age Archaeological TL (Annual ose) (TL/unit dose) Annual ose: t m E t Amount of energy E deposited by radiation exposure into sample of mass m within one year. It depends on the content of radioactive nuclei in the sample material and on the exposure to external radioactivity.
6 reminder from Chapter 1.2: ose: E m Amount of energy E deposited by radiation into body of mass m. 1 Gray (Gy) 1 Joule/kg (Energy/mass) 1 rad 1 centigray 10 milligrays ( 1 rad 1cGy 10 mgy ) Nominal background radiation absorbed dose 100 mrad/year 1 mgy/yr. TL/unit dose is material specific. It describes the probability for a material to develop thermoluminescence (TL) under radiation exposure. This has to be determined by independent analysis typically by exposure to well defined radioactive sources. For metals: TL/unit dose 0
7 experimental set-up heater with temperature control; blue filter for red light glow absorption and photomultiplier with scintillator for TL photon measurement.
8 Example TL-cts/(s 0.1Gy) The TL curve shows TL-counts/s at an average annual dose of 1 mgy/y TL [10 3 cts/s] TL-cts/s Measurement of TL with an calibrated source gives TL-cts/s after an exposure to 0.1Gy radiation. TL/dose TL-cts/(s Gy) temperature o C -1 Age 21000[s ] [Gy/y] [s Gy 1 ] Age 4038y
9 The Plateau Test two portions of the sample are TL tested, one after additional exposure to radioactive source ( radiation) and one without additional exposure - paleodose. Ratio of the two TL curves should provide a plateau in the TL range. short-lived TL components
10 The plateau method The plateau method initial developed as an independent test provides a more reliable method since with this relative approach the systematic uncertainties in the absolute dose rates cancel out. Age paleodose annual dose The paleodose is the natural dose received by sample since its last heating (production); it can be determined from the plateau ratio R between the paleodose and the paleodose plus the source related dose from the sample irradiation. The annual dose is material dependent and must be measured.
11 Paleodose etermination N R N + N R ( N + N ) R N N( 1 N N ( R 1 1) N R ) If R0.5 (R -1 2) the sample received during the source irradiation the same dose as naturally over its life time. If R0.33 (R -1 3) the sample received during the source irradiation twice the natural life time dose.
12 Example 2 N 10 Gy N R 0. 47; N N ( 1/ ) with N 10 Gy; Paleodose : N Gy
13 Annual ose etermination For most pottery samples the TL is produced in roughly equal proportions by the nuclear radiation from potassium 40 K (, γ emitter), thorium (α-emitter), and uranium (α-emitter) plus its additional and γ radiation which is emitted along the decay chains. Minor contributions yield from rubidium contamination (-emission) and cosmic radiation bombardment. Annual dose : ' α γ cr α radiation saturates the TL traps due to its high ionization probability, therefore only 10-15% of the α leads to TL dose. ' α k k α α α
14 fine and coarse grain method the fine grain method seeks to maximize the α-dose in sample powder of 3-10 μm grain size! 0.5mm the coarse grain method is based only on and γ induced luminescence using a coarse powder with more than 500 μm grain size!
15 Annual doses for typical pottery fine grain technique
16 Example 3 (fine grain method) Annual dose for typical pottery and soil conditions can be estimated (neglecting Cosmic Ray ose): ( k α + + γ ) ( ) [Gy/y] [Gy/y] Comparison with the paleodose yields an age of: paleodose [Gy] Age 3 annual dose [Gy/y] 1710 [y]
17 Uncertainties & Problems If sample has been exposed to light for considerable time bleaching may occur, de-excitation of TL traps by photon interaction. TL drops, suggesting a lower paleodose & age. TL 0.72 TL a) natural TL curve b) after 1h light exposure c) after 24 h light exposure 0.15 TL since age is proportional to paleodose, significant errors can occur!
18 bleaching time dependence Since the light exposure causes photon induced de-excitation of intra-band states the TL drops exponentially with exposure time t. TL( t κ ) TL 0 material e κ t constant Quartz is more slowly bleached than Feldspar, if κ and light exposure time t is known, corrections can be applied.
19 bleached piece of pottery unbleached case: paleodose [Gy] Age 3 annual dose [Gy/y] 1710 [y] κ0.33: 1 h bleached case: paleodose is reduced to 72%: paleodose [Gy] Age 3 annual dose [Gy/y] 1231[y] sample appears considerably younger!
20 water uptake and water damage Pottery within a meter or so from ground level are typically wet! Water uptake has direct impact on annual dose since it acts as absorber material for the radiation. Wet pottery has lower annual dose suggesting a higher age of pottery sample. Reduction in dose rates for α,, and γ exposure is: k k k wet α wet wet γ kα dry f dry k dry kγ w f f w w water absorbs α-radiation 50% more efficiently than dry clay, water is 25% more efficient in absorbing -radiation but only 14% more efficient for γ-radiation. f: fraction of water retained in soil material (0.95 in UK, 0.6 in Italy) w: saturation of water uptake; obtained by weighting wet and dry sample (w )
21 wet piece of pottery in soaked soil, f0.95, soaked piece of pottery, w0.3. If sample has a paleodose of 8.86 Gy like a dry sample: ( k wet α ( α 3 + k wet 3 [Gy/y] + k wet γ γ ) about 73% reduction in annual dose ) [Gy/y] paleodose [Gy] Age 3 annual dose [Gy/y] 2356 [y]
Radioactivity. Lecture 7 Dosimetry and Exposure Limits
 Radioactivity Lecture 7 Dosimetry and Exposure Limits Radiation Exposure - Radiology The radiation impact on biological and genetic materials requires some protective measures! Units for scaling the decay
Radioactivity Lecture 7 Dosimetry and Exposure Limits Radiation Exposure - Radiology The radiation impact on biological and genetic materials requires some protective measures! Units for scaling the decay
Question. 1. Which natural source of background radiation do you consider as dominant?
 Question 1. Which natural source of background radiation do you consider as dominant? 2. Is the radiation background constant or does it change with time and location? 3. What is the level of anthropogenic
Question 1. Which natural source of background radiation do you consider as dominant? 2. Is the radiation background constant or does it change with time and location? 3. What is the level of anthropogenic
SCIENTIFIC DATING IN ARCHAEOLOGY
 SCIENTIFIC DATING IN ARCHAEOLOGY Tsuneto Nagatomo 1. AGE DETERMINATION IN ARCHAEOLOGY Relative Age: stratigraphy, typology Absolute Chronology: historical data Age Determination by (natural) Scientific
SCIENTIFIC DATING IN ARCHAEOLOGY Tsuneto Nagatomo 1. AGE DETERMINATION IN ARCHAEOLOGY Relative Age: stratigraphy, typology Absolute Chronology: historical data Age Determination by (natural) Scientific
Radioactivity. Lecture 7 Dosimetry and Exposure Limits
 Radioactivity Lecture 7 Dosimetry and Exposure Limits Radiation Exposure - Radiology The radiation impact on biological and genetic materials requires some protective measures! Units for scaling the decay
Radioactivity Lecture 7 Dosimetry and Exposure Limits Radiation Exposure - Radiology The radiation impact on biological and genetic materials requires some protective measures! Units for scaling the decay
Introduction to Archaeology: Notes 9 Chronology, part 2 Copyright Bruce Owen 2009 Trapped-charge dating methods Several different kinds, one basic
 Introduction to Archaeology: Notes 9 Chronology, part 2 Copyright Bruce Owen 2009 Trapped-charge dating methods Several different kinds, one basic principle All measure the accumulated damage (displaced
Introduction to Archaeology: Notes 9 Chronology, part 2 Copyright Bruce Owen 2009 Trapped-charge dating methods Several different kinds, one basic principle All measure the accumulated damage (displaced
Radiation Dose, Biology & Risk
 ENGG 167 MEDICAL IMAGING Lecture 2: Sept. 27 Radiation Dosimetry & Risk References: The Essential Physics of Medical Imaging, Bushberg et al, 2 nd ed. Radiation Detection and Measurement, Knoll, 2 nd Ed.
ENGG 167 MEDICAL IMAGING Lecture 2: Sept. 27 Radiation Dosimetry & Risk References: The Essential Physics of Medical Imaging, Bushberg et al, 2 nd ed. Radiation Detection and Measurement, Knoll, 2 nd Ed.
Complement: Natural sources of radiations
 Complement: Natural sources of radiations 1 Notions of dose Absorbed dose at 1 point (D): Mean value of the energy deposited by ionizing radiation to matter per mass unit (unit: J/kg = gray (Gy)) Equivalent
Complement: Natural sources of radiations 1 Notions of dose Absorbed dose at 1 point (D): Mean value of the energy deposited by ionizing radiation to matter per mass unit (unit: J/kg = gray (Gy)) Equivalent
11 Gamma Ray Energy and Absorption
 11 Gamma Ray Energy and Absorption Before starting this laboratory, we must review the physiological effects and the proper use of the radioactive samples you will be using during the experiment. Physiological
11 Gamma Ray Energy and Absorption Before starting this laboratory, we must review the physiological effects and the proper use of the radioactive samples you will be using during the experiment. Physiological
Dosimetry. Sanja Dolanski Babić May, 2018.
 Dosimetry Sanja Dolanski Babić May, 2018. What s the difference between radiation and radioactivity? Radiation - the process of emitting energy as waves or particles, and the radiated energy Radioactivity
Dosimetry Sanja Dolanski Babić May, 2018. What s the difference between radiation and radioactivity? Radiation - the process of emitting energy as waves or particles, and the radiated energy Radioactivity
NORM and TENORM: Occurrence, Characterizing, Handling and Disposal
 NORM and TENORM: Occurrence, Characterizing, Handling and Disposal Ionizing Radiation and Hazard Potential John R. Frazier, Ph.D. Certified Health Physicist May 12, 2014 Radiation Radiation is a word that
NORM and TENORM: Occurrence, Characterizing, Handling and Disposal Ionizing Radiation and Hazard Potential John R. Frazier, Ph.D. Certified Health Physicist May 12, 2014 Radiation Radiation is a word that
SOURCES of RADIOACTIVITY
 Section 9: SOURCES of RADIOACTIVITY This section briefly describes various sources of radioactive nuclei, both naturally occurring and those produced artificially (man-made) in, for example, reactors or
Section 9: SOURCES of RADIOACTIVITY This section briefly describes various sources of radioactive nuclei, both naturally occurring and those produced artificially (man-made) in, for example, reactors or
4/4/17. Dating Methods. Dating in Archaeology. These things date to 2500 B.C. (but there are no completion dates written anywhere on or near them)
 Dating in Archaeology These things date to 2500 B.C. (but there are no completion dates written anywhere on or near them) Dating Methods One of the biggest questions in archaeology is WHEN? Successfully
Dating in Archaeology These things date to 2500 B.C. (but there are no completion dates written anywhere on or near them) Dating Methods One of the biggest questions in archaeology is WHEN? Successfully
WHAT IS IONIZING RADIATION
 WHAT IS IONIZING RADIATION Margarita Saraví National Atomic Energy Commission - Argentina Workshop on Ionizing Radiation SIM Buenos Aires 10 November 2011 What is ionizing radiation? What is ionizing radiation?
WHAT IS IONIZING RADIATION Margarita Saraví National Atomic Energy Commission - Argentina Workshop on Ionizing Radiation SIM Buenos Aires 10 November 2011 What is ionizing radiation? What is ionizing radiation?
U (superscript is mass number, subscript atomic number) - radionuclides nuclei that are radioactive - radioisotopes atoms containing radionuclides
 Chapter : Nuclear Chemistry. Radioactivity nucleons neutron and proton all atoms of a given element have the same number of protons, atomic number isotopes atoms with the same atomic number but different
Chapter : Nuclear Chemistry. Radioactivity nucleons neutron and proton all atoms of a given element have the same number of protons, atomic number isotopes atoms with the same atomic number but different
Module 1. An Introduction to Radiation
 Module 1 An Introduction to Radiation General Definition of Radiation Ionizing radiation, for example, X-rays, gamma-rays, α particles Ionizing radiation is capable of removing an electron from the atom
Module 1 An Introduction to Radiation General Definition of Radiation Ionizing radiation, for example, X-rays, gamma-rays, α particles Ionizing radiation is capable of removing an electron from the atom
CHEMISTRY Topic #1: Atomic Structure and Nuclear Chemistry Fall 2017 Dr. Susan Findlay See Exercises 2.3 to 2.6
 CHEMISTRY 1000 Topic #1: Atomic Structure and Nuclear Chemistry Fall 2017 Dr. Susan Findlay See Exercises 2.3 to 2.6 Balancing Nuclear Reactions mass number (A) atomic number (Z) 12 6 C In an ordinary
CHEMISTRY 1000 Topic #1: Atomic Structure and Nuclear Chemistry Fall 2017 Dr. Susan Findlay See Exercises 2.3 to 2.6 Balancing Nuclear Reactions mass number (A) atomic number (Z) 12 6 C In an ordinary
Radiometric Dating and the Age of the Earth
 Radiometric Dating and the Age of the Earth How to tell time: Relative Time: putting events in time order. Law of Superposition Correlation of rock layers using fossils. There is a wonderful order and
Radiometric Dating and the Age of the Earth How to tell time: Relative Time: putting events in time order. Law of Superposition Correlation of rock layers using fossils. There is a wonderful order and
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,900 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,900 116,000 120M Open access books available International authors and editors Downloads Our
Pleistocene Terrace Deposits of the Crystal Geyser Area e. r G. P5 5o. M1/Qal. M3 3y M4 M5 M5. 5o M6y P6. M1/Qal
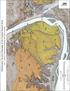 0 M6 C O N T O UR IN T E R V A L 4 0 F E E T NO R T H A ME R IC A N V E R T IC A L D A T UM O F 1 9 8 8 500 METERS KILOMETERS 0.5 S C A L E 1 : 1 2,0 0 0 r M1/Qal USU-780 P6 1000 1 5o M6y P6 y M4 M5 M5
0 M6 C O N T O UR IN T E R V A L 4 0 F E E T NO R T H A ME R IC A N V E R T IC A L D A T UM O F 1 9 8 8 500 METERS KILOMETERS 0.5 S C A L E 1 : 1 2,0 0 0 r M1/Qal USU-780 P6 1000 1 5o M6y P6 y M4 M5 M5
Nuclear Chemistry. Nuclear Terminology
 Nuclear Chemistry Up to now, we have been concerned mainly with the electrons in the elements the nucleus has just been a positively charged things that attracts electrons The nucleus may also undergo
Nuclear Chemistry Up to now, we have been concerned mainly with the electrons in the elements the nucleus has just been a positively charged things that attracts electrons The nucleus may also undergo
Interaction of the radiation with a molecule knocks an electron from the molecule. a. Molecule ¾ ¾ ¾ ion + e -
 Interaction of the radiation with a molecule knocks an electron from the molecule. radiation a. Molecule ¾ ¾ ¾ ion + e - This can destroy the delicate balance of chemical reactions in living cells. The
Interaction of the radiation with a molecule knocks an electron from the molecule. radiation a. Molecule ¾ ¾ ¾ ion + e - This can destroy the delicate balance of chemical reactions in living cells. The
Radionuclide Imaging MII Detection of Nuclear Emission
 Radionuclide Imaging MII 3073 Detection of Nuclear Emission Nuclear radiation detectors Detectors that are commonly used in nuclear medicine: 1. Gas-filled detectors 2. Scintillation detectors 3. Semiconductor
Radionuclide Imaging MII 3073 Detection of Nuclear Emission Nuclear radiation detectors Detectors that are commonly used in nuclear medicine: 1. Gas-filled detectors 2. Scintillation detectors 3. Semiconductor
SECTION 8 Part I Typical Questions
 SECTION 8 Part I Typical Questions 1. For a narrow beam of photons, the relaxation length is that thickness of absorber that will result in a reduction of in the initial beam intensity. 1. 1/10. 2. 1/2.
SECTION 8 Part I Typical Questions 1. For a narrow beam of photons, the relaxation length is that thickness of absorber that will result in a reduction of in the initial beam intensity. 1. 1/10. 2. 1/2.
Supplementary information (SI)
 Optical dating in a new light: A direct, non-destructive probe of trapped electrons Amit Kumar Prasad *, Nigel R. J. Poolton,, Myungho Kook, Mayank Jain Center for Nuclear Technologies, Technical University
Optical dating in a new light: A direct, non-destructive probe of trapped electrons Amit Kumar Prasad *, Nigel R. J. Poolton,, Myungho Kook, Mayank Jain Center for Nuclear Technologies, Technical University
Neutron activation analysis. Contents. Introduction
 Neutron activation analysis Contents Neutron activation analysis... 1 Introduction... 1 Principle of method... 2 Detection of radionuclides... 3 Kinetics of activation... 4 Choosing the appropriate procedure...
Neutron activation analysis Contents Neutron activation analysis... 1 Introduction... 1 Principle of method... 2 Detection of radionuclides... 3 Kinetics of activation... 4 Choosing the appropriate procedure...
Unit 08 Nuclear Structure. Unit 08 Nuclear Structure Slide 1
 Unit 08 Nuclear Structure Unit 08 Nuclear Structure Slide 1 The Plan Nuclear Structure Nuclear Decays Measuring Radiation Nuclear Power Plants Major Nuclear Power Accidents New Possibilities for Nuclear
Unit 08 Nuclear Structure Unit 08 Nuclear Structure Slide 1 The Plan Nuclear Structure Nuclear Decays Measuring Radiation Nuclear Power Plants Major Nuclear Power Accidents New Possibilities for Nuclear
Radiation Safety Talk. UC Santa Cruz Physics 133 Winter 2018
 Radiation Safety Talk UC Santa Cruz Physics 133 Winter 2018 Outline Types of radiation Sources of radiation Dose limits and risks ALARA principle Safety procedures Types of radiation Radiation is energy
Radiation Safety Talk UC Santa Cruz Physics 133 Winter 2018 Outline Types of radiation Sources of radiation Dose limits and risks ALARA principle Safety procedures Types of radiation Radiation is energy
Fundamentals of radiation protection
 Fundamentals of radiation protection Kamel ABBAS European Commission, Joint Research Centre Institute for Transuranium Elements, Nuclear Security Unit Via E. Fermi, 2749, I-21027 Ispra, Italy tel. +39-0332-785673,
Fundamentals of radiation protection Kamel ABBAS European Commission, Joint Research Centre Institute for Transuranium Elements, Nuclear Security Unit Via E. Fermi, 2749, I-21027 Ispra, Italy tel. +39-0332-785673,
INAYA MEDICAL COLLEGE (IMC) RAD LECTURE 1 RADIATION PHYSICS DR. MOHAMMED MOSTAFA EMAM
 INAYA MEDICAL COLLEGE (IMC) RAD 232 - LECTURE 1 RADIATION PHYSICS DR. MOHAMMED MOSTAFA EMAM Radiation: It is defined as the process by which energy is emitted from a source and propagated through the surrounding
INAYA MEDICAL COLLEGE (IMC) RAD 232 - LECTURE 1 RADIATION PHYSICS DR. MOHAMMED MOSTAFA EMAM Radiation: It is defined as the process by which energy is emitted from a source and propagated through the surrounding
INAYA MEDICAL COLLEGE (IMC) RAD LECTURE 1 RADIATION PHYSICS DR. MOHAMMED MOSTAFA EMAM
 INAYA MEDICAL COLLEGE (IMC) RAD 232 - LECTURE 1 RADIATION PHYSICS DR. MOHAMMED MOSTAFA EMAM LECTURES & CLASS ACTIVITIES https://inayacollegedrmohammedemam.wordpress.com/ Password: drmohammedemam 16-02-2015
INAYA MEDICAL COLLEGE (IMC) RAD 232 - LECTURE 1 RADIATION PHYSICS DR. MOHAMMED MOSTAFA EMAM LECTURES & CLASS ACTIVITIES https://inayacollegedrmohammedemam.wordpress.com/ Password: drmohammedemam 16-02-2015
GAMMA RAY SPECTROSCOPY FOR ARTIFICIAL CONTAMINATION EFFECTS EVALUATION IN LUMINISCENCE DATING OF ARTEFACTS FROM LOW DEPTH LAYERS IN SOUTHERN ROMANIA *
 Romanian Reports in Physics, Vol. 64, No. 2, P. 381 386, 2012 NUCLEAR PHYSICS GAMMA RAY SPECTROSCOPY FOR ARTIFICIAL CONTAMINATION EFFECTS EVALUATION IN LUMINISCENCE DATING OF ARTEFACTS FROM LOW DEPTH LAYERS
Romanian Reports in Physics, Vol. 64, No. 2, P. 381 386, 2012 NUCLEAR PHYSICS GAMMA RAY SPECTROSCOPY FOR ARTIFICIAL CONTAMINATION EFFECTS EVALUATION IN LUMINISCENCE DATING OF ARTEFACTS FROM LOW DEPTH LAYERS
Radiological Preparedness & Emergency Response. Session II. Objectives. Basic Radiation Physics
 Radiological Preparedness & Emergency Response Session II Basic Radiation Physics Objectives Discuss the difference between ionizing and non-ionizing radiation. Describe radioactive decay. Discuss the
Radiological Preparedness & Emergency Response Session II Basic Radiation Physics Objectives Discuss the difference between ionizing and non-ionizing radiation. Describe radioactive decay. Discuss the
UNIT 13: NUCLEAR CHEMISTRY
 UNIT 13: NUCLEAR CHEMISTRY REVIEW: ISOTOPE NOTATION An isotope notation is written as Z A X, where X is the element, A is the mass number (sum of protons and neutrons), and Z is the atomic number. For
UNIT 13: NUCLEAR CHEMISTRY REVIEW: ISOTOPE NOTATION An isotope notation is written as Z A X, where X is the element, A is the mass number (sum of protons and neutrons), and Z is the atomic number. For
BASIC OF RADIATION; ORIGIN AND UNITS
 INAYA MEDICAL COLLEGE (IMC) RAD 243 - LECTURE 2 BASIC OF RADIATION; ORIGIN AND UNITS DR. MOHAMMED MOSTAFA EMAM LECTURES & CLASS ACTIVITIES https://inayacollegedrmohammedemam.wordpress.com/ Password: drmohammedemam
INAYA MEDICAL COLLEGE (IMC) RAD 243 - LECTURE 2 BASIC OF RADIATION; ORIGIN AND UNITS DR. MOHAMMED MOSTAFA EMAM LECTURES & CLASS ACTIVITIES https://inayacollegedrmohammedemam.wordpress.com/ Password: drmohammedemam
Dating methods Stragraphic or geologic methods. Thomas INGICCO
 Dating methods Stragraphic or geologic methods Thomas INGICCO Electro-spin resonance (ESR) An electron can be represented by a negatively charged sphere animated of a rotational movement on itself. This
Dating methods Stragraphic or geologic methods Thomas INGICCO Electro-spin resonance (ESR) An electron can be represented by a negatively charged sphere animated of a rotational movement on itself. This
Chemistry 201: General Chemistry II - Lecture
 Chemistry 201: General Chemistry II - Lecture Dr. Namphol Sinkaset Chapter 21 Study Guide Concepts 1. There are several modes of radioactive decay: (1) alpha (α) decay, (2) beta (β) decay, (3) gamma (γ)
Chemistry 201: General Chemistry II - Lecture Dr. Namphol Sinkaset Chapter 21 Study Guide Concepts 1. There are several modes of radioactive decay: (1) alpha (α) decay, (2) beta (β) decay, (3) gamma (γ)
Nuclear Radiation. Natural Radioactivity. A person working with radioisotopes wears protective clothing and gloves and stands behind a shield.
 Nuclear Radiation Natural Radioactivity A person working with radioisotopes wears protective clothing and gloves and stands behind a shield. 1 Radioactive Isotopes A radioactive isotope has an unstable
Nuclear Radiation Natural Radioactivity A person working with radioisotopes wears protective clothing and gloves and stands behind a shield. 1 Radioactive Isotopes A radioactive isotope has an unstable
Atomic Structure Summary
 Atomic Structure Summary All atoms have: a positively charged nucleus and negatively charged electrons around it Atomic nucleus consists of: positively charged protons and neutrons that have no electric
Atomic Structure Summary All atoms have: a positively charged nucleus and negatively charged electrons around it Atomic nucleus consists of: positively charged protons and neutrons that have no electric
General Physics (PHY 2140)
 General Physics (PHY 2140) Lecture 19 Modern Physics Nuclear Physics Nuclear Reactions Medical Applications Radiation Detectors Chapter 29 http://www.physics.wayne.edu/~alan/2140website/main.htm 1 Lightning
General Physics (PHY 2140) Lecture 19 Modern Physics Nuclear Physics Nuclear Reactions Medical Applications Radiation Detectors Chapter 29 http://www.physics.wayne.edu/~alan/2140website/main.htm 1 Lightning
General Physics (PHY 2140)
 General Physics (PHY 2140) Lightning Review Lecture 19 Modern Physics Nuclear Physics Nuclear Reactions Medical Applications Radiation Detectors Chapter 29 http://www.physics.wayne.edu/~alan/2140website/main.htm
General Physics (PHY 2140) Lightning Review Lecture 19 Modern Physics Nuclear Physics Nuclear Reactions Medical Applications Radiation Detectors Chapter 29 http://www.physics.wayne.edu/~alan/2140website/main.htm
Radioactivity. The Nobel Prize in Physics 1903 for their work on radioactivity. Henri Becquerel Pierre Curie Marie Curie
 Radioactivity Toward the end of the 19 th century, minerals were found that would darken a photographic plate even in the absence of light. This phenomenon is now called radioactivity. Marie and Pierre
Radioactivity Toward the end of the 19 th century, minerals were found that would darken a photographic plate even in the absence of light. This phenomenon is now called radioactivity. Marie and Pierre
Lecture Presentation. Chapter 21. Nuclear Chemistry. James F. Kirby Quinnipiac University Hamden, CT Pearson Education, Inc.
 Lecture Presentation Chapter 21, Inc. James F. Kirby Quinnipiac University Hamden, CT Energy: Chemical vs. Chemical energy is associated with making and breaking chemical bonds. energy is enormous in comparison.
Lecture Presentation Chapter 21, Inc. James F. Kirby Quinnipiac University Hamden, CT Energy: Chemical vs. Chemical energy is associated with making and breaking chemical bonds. energy is enormous in comparison.
UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY
 UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY teacher version www.toppr.com Contents (a) Types of Radiation (b) Properties of Radiation (c) Dangers of Radiation (d) Rates of radioactive decay (e) Nuclear
UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY teacher version www.toppr.com Contents (a) Types of Radiation (b) Properties of Radiation (c) Dangers of Radiation (d) Rates of radioactive decay (e) Nuclear
INTERNAL RADIATION DOSIMETRY
 INTERNAL RADIATION DOSIMETRY Introduction to Internal Dosimetry Importance of External and Internal Exposures by Radiation Type Charged particle radiation (α, p, β) Generally important only for internal
INTERNAL RADIATION DOSIMETRY Introduction to Internal Dosimetry Importance of External and Internal Exposures by Radiation Type Charged particle radiation (α, p, β) Generally important only for internal
Radiation Detection and Measurement
 Radiation Detection and Measurement June 2008 Tom Lewellen Tkldog@u.washington.edu Types of radiation relevant to Nuclear Medicine Particle Symbol Mass (MeV/c 2 ) Charge Electron e-,! - 0.511-1 Positron
Radiation Detection and Measurement June 2008 Tom Lewellen Tkldog@u.washington.edu Types of radiation relevant to Nuclear Medicine Particle Symbol Mass (MeV/c 2 ) Charge Electron e-,! - 0.511-1 Positron
Higher -o-o-o- Past Paper questions o-o-o- 3.6 Radiation
 Higher -o-o-o- Past Paper questions 2000-2010 -o-o-o- 3.6 Radiation 2000 Q29 Radium (Ra) decays to radon (Rn) by the emission of an alpha particle. Some energy is also released by this decay. The decay
Higher -o-o-o- Past Paper questions 2000-2010 -o-o-o- 3.6 Radiation 2000 Q29 Radium (Ra) decays to radon (Rn) by the emission of an alpha particle. Some energy is also released by this decay. The decay
UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY
 UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY student version www.toppr.com Contents (a) Types of Radiation (b) Properties of Radiation (c) Dangers of Radiation (d) Rates of radioactive decay (e) Nuclear
UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY student version www.toppr.com Contents (a) Types of Radiation (b) Properties of Radiation (c) Dangers of Radiation (d) Rates of radioactive decay (e) Nuclear
ZX or X-A where X is chemical symbol of element. common unit: [unified mass unit = u] also known as [atomic mass unit = amu] or [Dalton = Da]
![ZX or X-A where X is chemical symbol of element. common unit: [unified mass unit = u] also known as [atomic mass unit = amu] or [Dalton = Da] ZX or X-A where X is chemical symbol of element. common unit: [unified mass unit = u] also known as [atomic mass unit = amu] or [Dalton = Da]](/thumbs/87/95084584.jpg) 1 Part 5: Nuclear Physics 5.1. The Nucleus = atomic number = number of protons N = neutron number = number of neutrons = mass number = + N Representations: X or X- where X is chemical symbol of element
1 Part 5: Nuclear Physics 5.1. The Nucleus = atomic number = number of protons N = neutron number = number of neutrons = mass number = + N Representations: X or X- where X is chemical symbol of element
Chapter 29. Nuclear Physics
 Chapter 29 Nuclear Physics Ernest Rutherford 1871 1937 Discovery that atoms could be broken apart Studied radioactivity Nobel prize in 1908 Some Properties of Nuclei All nuclei are composed of protons
Chapter 29 Nuclear Physics Ernest Rutherford 1871 1937 Discovery that atoms could be broken apart Studied radioactivity Nobel prize in 1908 Some Properties of Nuclei All nuclei are composed of protons
sample What happens when we are exposed to radiation? 1.1 Natural radiation Cosmic radiation
 1.1 Natural radiation 3 1 What happens when we are exposed to radiation? 1.1 Natural radiation For as long as humans have walked the earth, we have continually been exposed to naturally-occurring radiation.
1.1 Natural radiation 3 1 What happens when we are exposed to radiation? 1.1 Natural radiation For as long as humans have walked the earth, we have continually been exposed to naturally-occurring radiation.
Chapter 20 Nuclear Chemistry. 1. Nuclear Reactions and Their Characteristics
 Chapter 2 Nuclear Chemistry 1. Nuclear Reactions and Their Characteristics Nuclear reactions involve the particles located in the nucleus of the atom: nucleons:. An atom is characterized by its atomic
Chapter 2 Nuclear Chemistry 1. Nuclear Reactions and Their Characteristics Nuclear reactions involve the particles located in the nucleus of the atom: nucleons:. An atom is characterized by its atomic
Experiment Radioactive Decay of 220 Rn and 232 Th Physics 2150 Experiment No. 10 University of Colorado
 Experiment 10 1 Introduction Radioactive Decay of 220 Rn and 232 Th Physics 2150 Experiment No. 10 University of Colorado Some radioactive isotopes formed billions of years ago have half- lives so long
Experiment 10 1 Introduction Radioactive Decay of 220 Rn and 232 Th Physics 2150 Experiment No. 10 University of Colorado Some radioactive isotopes formed billions of years ago have half- lives so long
GLOSSARY OF BASIC RADIATION PROTECTION TERMINOLOGY
 GLOSSARY OF BASIC RADIATION PROTECTION TERMINOLOGY ABSORBED DOSE: The amount of energy absorbed, as a result of radiation passing through a material, per unit mass of material. Measured in rads (1 rad
GLOSSARY OF BASIC RADIATION PROTECTION TERMINOLOGY ABSORBED DOSE: The amount of energy absorbed, as a result of radiation passing through a material, per unit mass of material. Measured in rads (1 rad
Nuclear Chemistry AP Chemistry Lecture Outline
 Nuclear Chemistry AP Chemistry Lecture Outline Name: involve changes with electrons. involve changes in atomic nuclei. Spontaneously-changing nuclei emit and are said to be. Radioactivity nucleons: mass
Nuclear Chemistry AP Chemistry Lecture Outline Name: involve changes with electrons. involve changes in atomic nuclei. Spontaneously-changing nuclei emit and are said to be. Radioactivity nucleons: mass
The basic structure of an atom is a positively charged nucleus composed of both protons and neutrons surrounded by negatively charged electrons.
 4.4 Atomic structure Ionising radiation is hazardous but can be very useful. Although radioactivity was discovered over a century ago, it took many nuclear physicists several decades to understand the
4.4 Atomic structure Ionising radiation is hazardous but can be very useful. Although radioactivity was discovered over a century ago, it took many nuclear physicists several decades to understand the
Rad T 290 Worksheet 2
 Class: Date: Rad T 290 Worksheet 2 1. Projectile electrons travel from a. anode to cathode. c. target to patient. b. cathode to anode. d. inner shell to outer shell. 2. At the target, the projectile electrons
Class: Date: Rad T 290 Worksheet 2 1. Projectile electrons travel from a. anode to cathode. c. target to patient. b. cathode to anode. d. inner shell to outer shell. 2. At the target, the projectile electrons
Nuclear Spectroscopy: Radioactivity and Half Life
 Particle and Spectroscopy: and Half Life 02/08/2018 My Office Hours: Thursday 1:00-3:00 PM 212 Keen Building Outline 1 2 3 4 5 Some nuclei are unstable and decay spontaneously into two or more particles.
Particle and Spectroscopy: and Half Life 02/08/2018 My Office Hours: Thursday 1:00-3:00 PM 212 Keen Building Outline 1 2 3 4 5 Some nuclei are unstable and decay spontaneously into two or more particles.
Activity 11 Solutions: Ionizing Radiation II
 Activity 11 Solutions: Ionizing Radiation II 11.1 Additional Sources of Ionizing Radiation 1) Cosmic Rays Your instructor will show you radiation events in a cloud chamber. Look for vapor trails that do
Activity 11 Solutions: Ionizing Radiation II 11.1 Additional Sources of Ionizing Radiation 1) Cosmic Rays Your instructor will show you radiation events in a cloud chamber. Look for vapor trails that do
Radioactivity. General Physics II PHYS 111. King Saud University College of Applied Studies and Community Service Department of Natural Sciences
 King Saud University College of Applied Studies and Community Service Department of Natural Sciences Radioactivity General Physics II PHYS 111 Nouf Alkathran nalkathran@ksu.edu.sa Outline Radioactive Decay
King Saud University College of Applied Studies and Community Service Department of Natural Sciences Radioactivity General Physics II PHYS 111 Nouf Alkathran nalkathran@ksu.edu.sa Outline Radioactive Decay
NATO HANDBOOK ON THE MEDICAL ASPECTS OF NBC DEFENSIVE OPERATIONS AMedP-6(B) PART I - NUCLEAR ANNEX A RADIATION DETECTION AND MEASUREMENT
 NATO HANDBOOK ON THE MEDICAL ASPECTS OF NBC DEFENSIVE OPERATIONS AMedP-6(B) PART I - NUCLEAR RADIATION DETECTION AND MEASUREMENT 1 FEBRUARY 1996 NATO UNCLASSIFIED A ORIGINAL (Reverse Blank) TABLE OF CONTENTS
NATO HANDBOOK ON THE MEDICAL ASPECTS OF NBC DEFENSIVE OPERATIONS AMedP-6(B) PART I - NUCLEAR RADIATION DETECTION AND MEASUREMENT 1 FEBRUARY 1996 NATO UNCLASSIFIED A ORIGINAL (Reverse Blank) TABLE OF CONTENTS
Radioactivity. Lecture 6 Detectors and Instrumentation
 Radioactivity Lecture 6 Detectors and Instrumentation The human organs Neither humans nor animals have an organ for detecting radiation from radioactive decay! We can not hear it, smell it, feel it or
Radioactivity Lecture 6 Detectors and Instrumentation The human organs Neither humans nor animals have an organ for detecting radiation from radioactive decay! We can not hear it, smell it, feel it or
Number of protons. 2. What is the nuclear symbol for a radioactive isotope of copper with a mass number of 60? A) Cu
 Chapter 5 Nuclear Chemistry Practice Problems 1. Fill in the missing information in the chart: Medical Use Atomic Mass symbol number Heart imaging 201 Tl 81 Number of protons Number of neutrons Abdominal
Chapter 5 Nuclear Chemistry Practice Problems 1. Fill in the missing information in the chart: Medical Use Atomic Mass symbol number Heart imaging 201 Tl 81 Number of protons Number of neutrons Abdominal
4.4.1 Atoms and isotopes The structure of an atom Mass number, atomic number and isotopes. Content
 4.4 Atomic structure Ionising radiation is hazardous but can be very useful. Although radioactivity was discovered over a century ago, it took many nuclear physicists several decades to understand the
4.4 Atomic structure Ionising radiation is hazardous but can be very useful. Although radioactivity was discovered over a century ago, it took many nuclear physicists several decades to understand the
Radioactivity. Lecture 11 The radioactive Earth
 Radioactivity Lecture 11 The radioactive Earth The Violent Beginning Most of the planet s radioactivity was generated in neutron driven nucleosynthesis processes in previous star generations and implemented
Radioactivity Lecture 11 The radioactive Earth The Violent Beginning Most of the planet s radioactivity was generated in neutron driven nucleosynthesis processes in previous star generations and implemented
Examples for experiments that can be done at the T9 beam line
 Examples for experiments that can be done at the T9 beam line Example 1: Use muon tomography to look for hidden chambers in pyramids (2016 winning proposal, Pyramid hunters) You may know computer tomography
Examples for experiments that can be done at the T9 beam line Example 1: Use muon tomography to look for hidden chambers in pyramids (2016 winning proposal, Pyramid hunters) You may know computer tomography
Nuclear Properties. Thornton and Rex, Ch. 12
 Nuclear Properties Thornton and Rex, Ch. 12 A pre-history 1896 Radioactivity discovered - Becquerel a rays + (Helium) b rays - (electrons) g rays 0 (EM waves) 1902 Transmutation observed - Rutherford and
Nuclear Properties Thornton and Rex, Ch. 12 A pre-history 1896 Radioactivity discovered - Becquerel a rays + (Helium) b rays - (electrons) g rays 0 (EM waves) 1902 Transmutation observed - Rutherford and
SCINTILLATION DETECTORS AND PM TUBES
 SCINTILLATION DETECTORS AND PM TUBES General Characteristics Introduction Luminescence Light emission without heat generation Scintillation Luminescence by radiation Scintillation detector Radiation detector
SCINTILLATION DETECTORS AND PM TUBES General Characteristics Introduction Luminescence Light emission without heat generation Scintillation Luminescence by radiation Scintillation detector Radiation detector
Radioactivity measurements and risk assessments in soil samples at south and middle of Qatar
 Radioactivity measurements and risk assessments in soil samples at south and middle of Qatar A. T. Al-Kinani*, M. A. Amr**, K. A. Al-Saad**, A. I. Helal***, and M. M. Al Dosari* *Radiation and Chemical
Radioactivity measurements and risk assessments in soil samples at south and middle of Qatar A. T. Al-Kinani*, M. A. Amr**, K. A. Al-Saad**, A. I. Helal***, and M. M. Al Dosari* *Radiation and Chemical
Radiation Protection Fundamentals and Biological Effects: Session 1
 Radiation Protection Fundamentals and Biological Effects: Session 1 Reading assignment: LLE Radiological Controls Manual (LLEINST 6610): Part 1 UR Radiation Safety Training Manual and Resource Book: Parts
Radiation Protection Fundamentals and Biological Effects: Session 1 Reading assignment: LLE Radiological Controls Manual (LLEINST 6610): Part 1 UR Radiation Safety Training Manual and Resource Book: Parts
College Physics B - PHY2054C
 College - PHY2054C Physics - Radioactivity 11/24/2014 My Office Hours: Tuesday 10:00 AM - Noon 206 Keen Building Review Question 1 Isotopes of an element A have the same number of protons and electrons,
College - PHY2054C Physics - Radioactivity 11/24/2014 My Office Hours: Tuesday 10:00 AM - Noon 206 Keen Building Review Question 1 Isotopes of an element A have the same number of protons and electrons,
Radiation Safety Training Session 1: Radiation Protection Fundamentals and Biological Effects
 Radiation Safety Training Session 1: Radiation Protection Fundamentals and Biological Effects Reading Assignment: LLE Radiological Controls Manual (LLEINST 6610) Part 1 UR Radiation Safety Training Manual
Radiation Safety Training Session 1: Radiation Protection Fundamentals and Biological Effects Reading Assignment: LLE Radiological Controls Manual (LLEINST 6610) Part 1 UR Radiation Safety Training Manual
The sources include Am-241 which emits alpha radiation, Sr-90 which emits beta radiation and Co-60 which emits gamma radiation.
 1 The physics department in a college has a number of radioactive sources which are used to demonstrate the properties of ionising radiations. The sources include Am-241 which emits alpha radiation, Sr-90
1 The physics department in a college has a number of radioactive sources which are used to demonstrate the properties of ionising radiations. The sources include Am-241 which emits alpha radiation, Sr-90
Chapter 18. Nuclear Chemistry
 Chapter 18 Nuclear Chemistry The energy of the sun comes from nuclear reactions. Solar flares are an indication of fusion reactions occurring at a temperature of millions of degrees. Introduction to General,
Chapter 18 Nuclear Chemistry The energy of the sun comes from nuclear reactions. Solar flares are an indication of fusion reactions occurring at a temperature of millions of degrees. Introduction to General,
Radiation Detection. 15 th Annual OSC Readiness Training Program.
 Radiation Detection 15 th Annual OSC Readiness Training Program www.oscreadiness.org GM Detectors 15 th Annual OSC Readiness Training Program www.oscreadiness.org 1 A closer look 15 th Annual OSC Readiness
Radiation Detection 15 th Annual OSC Readiness Training Program www.oscreadiness.org GM Detectors 15 th Annual OSC Readiness Training Program www.oscreadiness.org 1 A closer look 15 th Annual OSC Readiness
TABLE DR1. THERMOLUMINESCENCE AGE DATA FROM THIS STUDY FOR LOESS UNITS IN THE LOWER MISSISSIPPI VALLEY. Th (ppm) T, PB. T, TB** ( o C) ED, TB (Gy±CV)
 DR2010216 Sample No. Depth* U TABLE DR1. THERMOLUMINESCENCE AGE DATA FROM THIS STUDY FOR LOESS UNITS IN THE LOWER MISSISSIPPI VALLEY. Th K (%) CR (Gy/ka) Dose rate # (Gy/ka ±CV) TB** ED, TB ED, PB TLage
DR2010216 Sample No. Depth* U TABLE DR1. THERMOLUMINESCENCE AGE DATA FROM THIS STUDY FOR LOESS UNITS IN THE LOWER MISSISSIPPI VALLEY. Th K (%) CR (Gy/ka) Dose rate # (Gy/ka ±CV) TB** ED, TB ED, PB TLage
PHYSICS A2 UNIT 2 SECTION 1: RADIOACTIVITY & NUCLEAR ENERGY
 PHYSICS A2 UNIT 2 SECTION 1: RADIOACTIVITY & NUCLEAR ENERGY THE ATOMIC NUCLEUS / NUCLEAR RADIUS & DENSITY / PROPERTIES OF NUCLEAR RADIATION / INTENSITY & BACKGROUND RADIATION / EXPONENTIAL LAW OF DECAY
PHYSICS A2 UNIT 2 SECTION 1: RADIOACTIVITY & NUCLEAR ENERGY THE ATOMIC NUCLEUS / NUCLEAR RADIUS & DENSITY / PROPERTIES OF NUCLEAR RADIATION / INTENSITY & BACKGROUND RADIATION / EXPONENTIAL LAW OF DECAY
Simulation of Radiation Effects on NGST. Bryan Fodness, Thomas Jordan, Jim Pickel, Robert Reed, Paul Marshall, Ray Ladbury
 Simulation of Radiation Effects on NGST Bryan Fodness, Thomas Jordan, Jim Pickel, Robert Reed, Paul Marshall, Ray Ladbury 1 Outline Introduction to Project Goals and Challenges Approach Preliminary Results
Simulation of Radiation Effects on NGST Bryan Fodness, Thomas Jordan, Jim Pickel, Robert Reed, Paul Marshall, Ray Ladbury 1 Outline Introduction to Project Goals and Challenges Approach Preliminary Results
The Atomic Nucleus & Radioactive Decay. Major Constituents of an Atom 4/28/2016. Student Learning Outcomes. Analyze radioactive decay and its results
 The Atomic Nucleus & Radioactive Decay ( Chapter 10) Student Learning Outcomes Analyze radioactive decay and its results Differentiate between nuclear fission and fusion Major Constituents of an Atom U=unified
The Atomic Nucleus & Radioactive Decay ( Chapter 10) Student Learning Outcomes Analyze radioactive decay and its results Differentiate between nuclear fission and fusion Major Constituents of an Atom U=unified
Chapter. Nuclear Chemistry
 Chapter Nuclear Chemistry Nuclear Reactions 01 Chapter 22 Slide 2 Chapter 22 Slide 3 Alpha Decay: Loss of an α-particle (a helium nucleus) 4 2 He 238 92 U 234 4 U He 90 + 2 Chapter 22 Slide 4 Beta Decay:
Chapter Nuclear Chemistry Nuclear Reactions 01 Chapter 22 Slide 2 Chapter 22 Slide 3 Alpha Decay: Loss of an α-particle (a helium nucleus) 4 2 He 238 92 U 234 4 U He 90 + 2 Chapter 22 Slide 4 Beta Decay:
Radiation Basics. Candace C. Davison, M.Engr. Mary Lou Dunzik-Gougar, Ph.D.
 Radiation Basics Candace C. Davison, M.Engr. Research & Education Specialist Pennsylvania State University Radiation Science and Engineering Center Mary Lou Dunzik-Gougar, Ph.D. Assistant Prof of Nuclear
Radiation Basics Candace C. Davison, M.Engr. Research & Education Specialist Pennsylvania State University Radiation Science and Engineering Center Mary Lou Dunzik-Gougar, Ph.D. Assistant Prof of Nuclear
SIMULATION OF COMPETING FADING AND IRRADIATION EFFECTS IN THERMOLUMINESCENCE (TL) DOSIMETRY: 1 st ORDER KINETICS
 IMULATION OF COMPETING FADING AND IRRADIATION EFFECT IN THERMOLUMINECENCE (TL) DOIMETRY: 1 st ORDER KINETIC C.Furetta 1, J.Azorin 1, T.Rivera 1, G.Kitis 2 1. Dep.to de Fisica, Edif. T, cub. 331 Universidad
IMULATION OF COMPETING FADING AND IRRADIATION EFFECT IN THERMOLUMINECENCE (TL) DOIMETRY: 1 st ORDER KINETIC C.Furetta 1, J.Azorin 1, T.Rivera 1, G.Kitis 2 1. Dep.to de Fisica, Edif. T, cub. 331 Universidad
Nicholas J. Giordano. Chapter 30. Nuclear Physics. Marilyn Akins, PhD Broome Community College
 Nicholas J. Giordano www.cengage.com/physics/giordano Chapter 30 Nuclear Physics Marilyn Akins, PhD Broome Community College Atomic Nuclei Rutherford s discovery of the atomic nucleus caused scientists
Nicholas J. Giordano www.cengage.com/physics/giordano Chapter 30 Nuclear Physics Marilyn Akins, PhD Broome Community College Atomic Nuclei Rutherford s discovery of the atomic nucleus caused scientists
Radiation and Radioactivity. PHYS 0219 Radiation and Radioactivity
 Radiation and Radioactivity 1 Radiation and Radioactivity This experiment has four parts: 1. Counting Statistics 2. Gamma (g) Ray Absorption Half-length and shielding 3. 137 Ba Decay Half-life 4. Dosimetry
Radiation and Radioactivity 1 Radiation and Radioactivity This experiment has four parts: 1. Counting Statistics 2. Gamma (g) Ray Absorption Half-length and shielding 3. 137 Ba Decay Half-life 4. Dosimetry
Some nuclei are unstable Become stable by ejecting excess energy and often a particle in the process Types of radiation particle - particle
 Radioactivity George Starkschall, Ph.D. Lecture Objectives Identify methods for making radioactive isotopes Recognize the various types of radioactive decay Interpret an energy level diagram for radioactive
Radioactivity George Starkschall, Ph.D. Lecture Objectives Identify methods for making radioactive isotopes Recognize the various types of radioactive decay Interpret an energy level diagram for radioactive
Name Date Class NUCLEAR CHEMISTRY
 25 NUCLEAR CHEMISTRY SECTION 25.1 NUCLEAR RADIATION (pages 799 802) This section describes the nature of radioactivity and the process of radioactive decay. It characterizes alpha, beta, and gamma radiation
25 NUCLEAR CHEMISTRY SECTION 25.1 NUCLEAR RADIATION (pages 799 802) This section describes the nature of radioactivity and the process of radioactive decay. It characterizes alpha, beta, and gamma radiation
Chapter 21. Preview. Lesson Starter Objectives Mass Defect and Nuclear Stability Nucleons and Nuclear Stability Nuclear Reactions
 Preview Lesson Starter Objectives Mass Defect and Nuclear Stability Nucleons and Nuclear Stability Nuclear Reactions Section 1 The Nucleus Lesson Starter Nuclear reactions result in much larger energy
Preview Lesson Starter Objectives Mass Defect and Nuclear Stability Nucleons and Nuclear Stability Nuclear Reactions Section 1 The Nucleus Lesson Starter Nuclear reactions result in much larger energy
Detection and measurement of gamma-radiation by gammaspectroscopy
 Detection and measurement of gamma-radiation by gammaspectroscopy Gamma-radiation is electromagnetic radiation having speed equal to the light in vacuum. As reaching a matter it interact with the different
Detection and measurement of gamma-radiation by gammaspectroscopy Gamma-radiation is electromagnetic radiation having speed equal to the light in vacuum. As reaching a matter it interact with the different
Atoms and Nuclear Chemistry. Atoms Isotopes Calculating Average Atomic Mass Radioactivity
 Atoms and Nuclear Chemistry Atoms Isotopes Calculating Average Atomic Mass Radioactivity Atoms An atom is the smallest particle of an element that has all of the properties of that element. Composition
Atoms and Nuclear Chemistry Atoms Isotopes Calculating Average Atomic Mass Radioactivity Atoms An atom is the smallest particle of an element that has all of the properties of that element. Composition
1, 2, 3, 4, 6, 14, 17 PS1.B
 Correlations to Next Generation Science Standards Physical Science Disciplinary Core Ideas PS-1 Matter and Its Interactions PS1.A Structure and Properties of Matter Each atom has a charged substructure
Correlations to Next Generation Science Standards Physical Science Disciplinary Core Ideas PS-1 Matter and Its Interactions PS1.A Structure and Properties of Matter Each atom has a charged substructure
Optically stimulated luminescence from quartz measured using the linear modulation technique
 Radiation Measurements 32 (2000) 407±411 www.elsevier.com/locate/radmeas Optically stimulated luminescence from quartz measured using the linear modulation technique E. Bulur a, L. Bùtter-Jensen a, *,
Radiation Measurements 32 (2000) 407±411 www.elsevier.com/locate/radmeas Optically stimulated luminescence from quartz measured using the linear modulation technique E. Bulur a, L. Bùtter-Jensen a, *,
LECTURE 4 PRINCIPLE OF IMAGE FORMATION KAMARUL AMIN BIN ABDULLAH
 LECTURE 4 PRINCIPLE OF IMAGE FORMATION KAMARUL AMIN BIN ABDULLAH Lesson Objectives At the end of the lesson, student should able to: Define attenuation Explain interactions between x-rays and matter in
LECTURE 4 PRINCIPLE OF IMAGE FORMATION KAMARUL AMIN BIN ABDULLAH Lesson Objectives At the end of the lesson, student should able to: Define attenuation Explain interactions between x-rays and matter in
Chapter 20: Phenomena. Chapter 20: The Nucleus: A Chemist s View. Nuclear Decay. Nuclear Decay. Nuclear Decay. Nuclear Decay
 Chapter 20: Phenomena Phenomena: Below is a list of stable isotopes of different elements. Examine the data and see what patterns you can identify. The mass of a electron is 0.00055 u, the mass of a proton
Chapter 20: Phenomena Phenomena: Below is a list of stable isotopes of different elements. Examine the data and see what patterns you can identify. The mass of a electron is 0.00055 u, the mass of a proton
Nuclear Instruments and Methods in Physics Research B
 Nuclear Instruments and Methods in Physics Research B 359 (2015) 89 98 Contents lists available at ScienceDirect Nuclear Instruments and Methods in Physics Research B journal homepage: www.elsevier.com/locate/nimb
Nuclear Instruments and Methods in Physics Research B 359 (2015) 89 98 Contents lists available at ScienceDirect Nuclear Instruments and Methods in Physics Research B journal homepage: www.elsevier.com/locate/nimb
Phys 243 Lab 7: Radioactive Half-life
 Phys 243 Lab 7: Radioactive Half-life Dr. Robert MacDonald The King s University College Winter 2013 Abstract In today s lab you ll be measuring the half-life of barium-137, a radioactive isotope of barium.
Phys 243 Lab 7: Radioactive Half-life Dr. Robert MacDonald The King s University College Winter 2013 Abstract In today s lab you ll be measuring the half-life of barium-137, a radioactive isotope of barium.
Nuclear forces and Radioactivity. Two forces are at work inside the nucleus of an atom
 Nuclear forces and Radioactivity Two forces are at work inside the nucleus of an atom Forces act in opposing directions Electrostatic repulsion: pushes protons apart Strong nuclear force: pulls protons
Nuclear forces and Radioactivity Two forces are at work inside the nucleus of an atom Forces act in opposing directions Electrostatic repulsion: pushes protons apart Strong nuclear force: pulls protons
1.1 ALPHA DECAY 1.2 BETA MINUS DECAY 1.3 GAMMA EMISSION 1.4 ELECTRON CAPTURE/BETA PLUS DECAY 1.5 NEUTRON EMISSION 1.6 SPONTANEOUS FISSION
 Chapter NP-3 Nuclear Physics Decay Modes and Decay Rates TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 RADIOACTIVE DECAY 1.1 ALPHA DECAY 1.2 BETA MINUS DECAY 1.3 GAMMA EMISSION 1.4 ELECTRON CAPTURE/BETA
Chapter NP-3 Nuclear Physics Decay Modes and Decay Rates TABLE OF CONTENTS INTRODUCTION OBJECTIVES 1.0 RADIOACTIVE DECAY 1.1 ALPHA DECAY 1.2 BETA MINUS DECAY 1.3 GAMMA EMISSION 1.4 ELECTRON CAPTURE/BETA
Lecture PowerPoint. Chapter 31 Physics: Principles with Applications, 6 th edition Giancoli
 Lecture PowerPoint Chapter 31 Physics: Principles with Applications, 6 th edition Giancoli 2005 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the
Lecture PowerPoint Chapter 31 Physics: Principles with Applications, 6 th edition Giancoli 2005 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the
Name Date Class. alpha particle radioactivity gamma ray radioisotope beta particles radiation X-ray radioactive decay
 Name Date _ Class _ Nuclear Chemistry Section.1 Nuclear Radiation In your textbook, read about the terms used to describe nuclear changes. Use each of the terms below just once to complete the passage.
Name Date _ Class _ Nuclear Chemistry Section.1 Nuclear Radiation In your textbook, read about the terms used to describe nuclear changes. Use each of the terms below just once to complete the passage.
Chapter IV: Radioactive decay
 Chapter IV: Radioactive decay 1 Summary 1. Law of radioactive decay 2. Decay chain/radioactive filiation 3. Quantum description 4. Types of radioactive decay 2 History Radioactivity was discover in 1896
Chapter IV: Radioactive decay 1 Summary 1. Law of radioactive decay 2. Decay chain/radioactive filiation 3. Quantum description 4. Types of radioactive decay 2 History Radioactivity was discover in 1896
Radioactivity. Lecture 11 The radioactive Earth
 Radioactivity Lecture 11 The radioactive Earth The Violent Beginning Most of the planet s radioactivity was generated in neutron driven nucleosynthesis processes in previous star generations and implemented
Radioactivity Lecture 11 The radioactive Earth The Violent Beginning Most of the planet s radioactivity was generated in neutron driven nucleosynthesis processes in previous star generations and implemented
