The response made by part of a plant, by which it grow towards
|
|
|
- Abel Payne
- 6 years ago
- Views:
Transcription
1 Word/phrase Biology Nutrition Excretion Sensitivity Growth Reproduction Movement Digestion Enzyme Partially permeable Membrane Egestion Ingestion Photosynthesis Absorbtion Transpiration Translocation Respiration Aerobic respiration Anaerobic respiration Hormone Geotropism Phototropism Homeostasis Definition The taking in of nutrients which are organic substances or mineral ions, containing raw materials, or energy for growth and tissue repair, absorbing and assimilating them. The removal from organisms of toxic waste, the waste products of metabolism and substances in excess to requirements. The ability to detect or sense changes in the environment and to make responses. The permanent increase in size or dry mass by an increase in cell size, or number, or both. The process that makes more of the same kind of organism. An action by an organism or part of an organism causing a change in position or place. The break down of food molecules into small water soluble molecules using mechanical and chemical processes. A protein that functions as a biological catalyst. A membrane that is permeable to some substances and impermeable to others (all cell membranes are partially permeable). The passing out of undigested food as faeces, through the anus. Taking in substances into the body through the mouth. The fundamental process by which plants manufacture carbohydrates from raw materials using energy from light. The movement of digested food molecules though the wall of the small intestine into the blood. The evaporation of water from the surfaces of mesophyll cells followed by a loss of water vapour from plant leaves through stomata. The movement of sucrose and amino acids in phloem, from regions of production to regions of storage or utilisation in respiration and growth. The chemical reactions that break down nutrient molecules in living cells to release energy. The release of a relatively large amount of energy in cells by the break down of food substances in the presence of oxygen. The release of relatively small amounts of energy by the breakdown of food substance in the absence of oxygen. A chemical substance made in a gland, carried by the blood, which alters the activity of one or more specific target organs and is then destroyed by the liver. The response made by part of a plant in which it grows towards or away from gravity. The response made by part of a plant, by which it grow towards or away from the direction of light. The maintenance of a constant internal environment.
2 Sexual reproduction Asexual reproduction Pollination Inheritance Chromosome Gene Allele Haploid nucleus Diploid nucleus Mitosis Meiosis Genotype Phenotype Homozygous Heterozygous Dominant Recessive Mutation Natural selection Food chain Food web Ecosystem Trophic level Decomposer The process that involves the fusion of haploid nuclei to form a diploid zygote and the production of genetically dissimilar offspring. The process resulting in genetically identical offspring from one parent. The transfer of pollen from the male part of the plant (anther of stamen) to the female part (the stigma). The transmission of genetic information from generation to generation. A thread of DNA made up of a string of genes. A length of DNA that is a unit of heredity and codes for a specific protein. a gene may be copied and passed onto the next generation. Any two or more alternative forms for a gene. A nucleus containing only a single set of unpaired chromosomes. A nucleus containing two sets of chromosomes, one from each parent. A nuclear division giving rise to identical cells in which the chromosome number is maintained by the exact duplication of chromosomes. A reduction division in which the chromosome number is halved from diploid to haploid. The genetic make up of organism in terms of the alleles present. The physical or other features of an organism due to both its genotype and its environment. Having two identical alleles of a particular gene. Two identical homozygous individuals that breed together will be pure breeding. Having two different alleles of a particular gene and will not be pure breeding. An allele that is expressed when present. An allele that is only expressed if there is no dominant allele of that gene present. A change in a gene or chromosome. The greater chance of passing on of genes by the best adapted organisms. A chart showing the flow of energy from one organism to the next, beginning with a producer. A network of interconnected food chains showing the energy flow through part of an ecosystem. A unit containing all of the organisms and their environment interacting together, in a given area. The position in a food chain occupied by an organism. An organism that gets its energy from dead or waste organic matter.
3 Producer Consumer Herbivore Carnivore Omnivore Habitat Osmosis Diffusion Chemistry Atom Element Compound Mixture Molecule Diatomic molecule Proton number Nucleon number Isotope Relative Atomic Mass (RAM) Relative Molecular Mass (RMM) Relative Formula Mass (RFM) Mole Exothermic Endothermic Oxidation An organism that makes its own organic nutrients, usually using energy from sunlight through photosynthesis. An organism that gets its energy from feeding on other organisms. An animal that gets its energy by eating only plants. An animal that gets its energy by eating only other animals. An animal that gets its energy by eating both plants and other animals. The place where an organism lives to obtain its food and reproduces. The diffusion of water molecules from their region of higher concentration to a region of their lower concentration through a partially permeable membrane. The net movement of molecules from their region of higher concentration to a region of their lower concentration, down a concentration gradient as a result of their random movement. The smallest unit of an element. Atoms with the same number of protons in their nuclei. An element is a substance that cannot be broken down further by chemical means. Two or more elements chemically joined/bonded. Two or more substances physically mixed (easily separated). Two or more atoms joined together by a covalent bond. molecules can be elements (e.g. H2, O2 etc.) or compounds (e.g. CH4, H2SO4). Two atoms of the same element joined by a covalent bond (e.g. H2, N2, O2, F2, Cl2, Br2, I2, At2). This is the number of protons in the nucleus of an element (same as atomic number). This is the number of protons and neutrons in the nucleus of an element (slightly different from relative atomic mass). A form of an element with a different number of neutrons. The mass of an atom relative to one atom of carbon-12. The sum of all the relative atomic masses of the atoms in a molecule (e.g. RMM of H2O is =18). The same as above but for an ionic compound (e.g. NaCl or CaCO3). The number of atoms in exactly 12 grams of carbon-12. The number is called Avogadro's number. A reaction that releases heat energy (temperature of surroundings increases). A reaction that takes in heat energy (temperature of surroundings decreases). When a substances gains oxygen/when a substance loses
4 Reduction Redox Valency Hydrocarbon Unsaturated hydrocarbon Saturated hydrocarbon Monomer Polymer Electrolysis Electrode Anode Cathode Electrolyte Ion Anion Cation Balanced equation Group Period Catalyst Physics Speed Velocity Scalar quantity Vector quantity Mass Weight electrons. When a substance loses oxygen/when a substance gains electrons. Oxidation and reduction always occur at the same time and they are collectively known as redox reactions. Relating to outer shell electrons. A molecule that contains only hydrogen and carbon. A hydrocarbon with double bonds present. A hydrocarbon with no double bonds present (all bond spaces are saturated with hydrogen atoms). A simple compound whose molecules can join together to form polymers. A large molecule made up of chains of linked monomer units. The splitting of an ionic compound using electricity. A solid electrical conductor through which an electric current enters and leaves an electrolytic cell. The positive electrode. The negative electrode. A compound that conducts electricity when molten or when in solution. An ionic substance. A charged particle / An atom that has either gained or lost electrons. A negatively charged ion. A positively charged ion. The same number of atoms of each element on both sides of the equation. The columns in the periodic table. The group number equals the number of electrons in the outer shell. The rows in the periodic table. The row number equals the number of electron shells in the atom. A substance that speeds up a reaction but does not take part in the reaction (same mass of catalyst at the end of the reaction as there was at the beginning). The rate of change of distance of an object, or how quickly it moves through a given distance (usually measured in m/s). The speed of an object in a stated direction. A quantity that does not have a directional component (e.g. speed). A quantity that has a directional component (e.g. velocity). The amount of matter in a substance (measured in kilograms, kg). The force of gravity acting on an object (measured in newtons, N).
5 Density Force Renewable Non-renewable Fusion Fission Evaporation Boiling Freezing Condensing Melting Thermal capacity Specific heat capacity (SHC) Latent heat Latent heat of vaporisation Latent heat of fusion Conduction Convection Radiation Frequency Wavelength A measure of how much mass is contained in a given unit volume (density = mass/volume, usually measured in g/cm 3, or kg/m 3 ). A push or a pull. It is measured in newtons (N). A force can change the speed, direction and shape of an object. Examples include gravity, friction, upthrust, air resistance, magnetism, electrostatic force. A resource that can be created again in a human time scale (e.g. wood biomass), or one that will never run out (e.g. solar energy). A resource that will one day run out (e.g. coal, uranium). The joining of atomic nuclei to form a larger nucleus. Fusion is how energy is released from stars like our sun. The splitting of an unstable nucleus to produce smaller nuclei. The release of energy from these reactions is harnessed in nuclear power stations. A change of state from liquid to gas below the boiling point of that liquid. Evaporation occurs at the surface of the liquid. A change of state from a liquid to a gas. This occurs only at the boiling point of that liquid. Boiling occurs throughout the volume of the liquid. A change of state from a liquid to a solid. A change of state from a gas to a liquid. A change of state from a solid to a liquid. The amount of heat required to change a substance's temperature by a given amount. The specific heat capacity of a substance is the heat energy required to raise 1kg of the substance by 1 degree Celsius (measured in J/kg C). The heat released or absorbed by a body as it changes state without a change in temperature. The heat released by a body as it changes from a gas to a liquid, or absorbed by a body as it changes from a liquid to a gas. The heat released by a body as it changes from a liquid to a solid, or absorbed by a body as it changes from a solid to a liquid. The transfer of heat energy through matter from high temperature to low temperature. This is achieved by the transfer of kinetic energy from one particle to the next. It can occur in solids, liquids and gases although the latter two are very poor conductors. The transfer of heat energy through a fluid (gas or liquid) as a result of movement of the fluid itself. The transfer of heat energy by electromagnetic radiation (infrared radiation in particular). This requires no particles so can occur through the vacuum of space. The number of wavelengths per second (1 hertz = 1 wavelength per second). The distance from one point on a wave to the same point on the
6 next wave. Amplitude The maximum displacement of a wave from its equilibrium position. Longitudinal The oscillations of the wave are parallel to the motion of the wave (e.g. sound waves). Transverse The oscillations of the wave are perpendicular to the motion of the wave (e.g. light waves). Refraction The speeding up or slowing down of a wave as it passes from one medium to another. This is often accompanied by a change in direction as well. Critical angle The angle of incidence at which maximum refraction occurs. Any angle larger than this will result in total internal reflection. Total internal reflection For waves travelling from a more dense medium to a less dense (TIR) one, any angle larger than the critical angle will result in all of waves being reflected back into the medium. Principal focus Also called the focal point, it is the point on the axis of a lens or mirror to which parallel rays of light converge, or from which they appear to diverge after refraction or reflection. Focal length The distance from the centre of a lens, or the reflecting surface of a mirror, to the principal focus. Audible range The range of frequencies that an animal can detect, from the lowest pitch to the highest pitch. For humans this is 20Hz to 20,000Hz. Permanent magnet A material that is hard to magnetise but retains its magnetic properties once magnetised. Made from hard magnetic materials like steel. Temporary magnet A material that gains and loses its magnetic properties easily. These form the basis of electromagnets as they quickly demagnetise once the current is switched off. Made from soft magnetic materials like iron. Current A flow of electricity through a conductor, measured in amps, A. Potential difference The difference, measured in volts, in electric potential between (p.d.) two points. Electromotive force (emf) Refers to the voltage generated by a battery, measured in volts. Transformer A device that transfers an alternating current from one circuit to another circuit, usually with an increase (step-up transformer) or decrease (step-down transformer) of voltage. Generator A device for converting mechanical energy into electrical energy by electromagnetic induction. Motor A device that converts electrical energy into mechanical energy. Alternating current (ac) An electric current that reverses direction in a circuit at regular intervals. Direct current (dc) An electric current that flows in one direction only. Electro-magnetic When a wire is moved in relation to a magnetic field a current is induction induced in the wire.
7 Radioactive decay Half life Ionising radiation Electricity Occurs when an unstable nucleus emits ionising radiation and decays into another nuclide. The time taken for half of a radioactive material to decay into another substance. Radiation that has enough energy to strip electrons off atoms (thus creating ions). A flow of charge/electrons. Physics Formulae K.E. = ½ mv 2 P.E. = mgh Work done (energy) = force x distance travelled Force = mass x acceleration Speed = distance / time Acceleration = speed / time Power = work done (energy) / time I = Q/t V=IR P = IV E = IVt RT = R1 + R2 + R3... 1/RT = 1/R1 + 1/R2 + 1/R3... Efficiency = useful energy/total energy x 100% Moles = mass/molar mass Moles = volume of gas (in dm 3 )/24 Moles = volume of solution (in cm 3 )/1000 Density = mass/volume Hooke's Law force = constant x extension (F=kx) V = f λ
0654 CO-ORDINATED SCIENCES
 CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2013 series 0654 CO-DINATED SCIENCES 0654/32 Paper 3 (Extended Theory), maximum
CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2013 series 0654 CO-DINATED SCIENCES 0654/32 Paper 3 (Extended Theory), maximum
0654 CO-ORDINATED SCIENCES
 CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2013 series 0654 CO-ORDINATED SCIENCES 0654/22 Paper 2 (Core Theory), maximum
CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2013 series 0654 CO-ORDINATED SCIENCES 0654/22 Paper 2 (Core Theory), maximum
Matter mass space atoms solid, a liquid, a gas, or plasm elements compounds mixtures atoms Compounds chemically combined Mixtures not chemically
 SOL PS.2 THE NATURE OF MATTER Matter is anything that has mass and occupies space. All matter is made up of small particles called atoms. Matter can exist as a solid, a liquid, a gas, or plasma. Matter
SOL PS.2 THE NATURE OF MATTER Matter is anything that has mass and occupies space. All matter is made up of small particles called atoms. Matter can exist as a solid, a liquid, a gas, or plasma. Matter
GLOSSARY OF PHYSICS TERMS. v-u t. a =
 GLOSSARY OF PHYSICS TERMS Scalar: A quantity that has magnitude only. Vector: A quantity that has magnitude and direction. Speed is the distance travelled per unit time. OR the rate of change of distance.
GLOSSARY OF PHYSICS TERMS Scalar: A quantity that has magnitude only. Vector: A quantity that has magnitude and direction. Speed is the distance travelled per unit time. OR the rate of change of distance.
Optics Definitions. The apparent movement of one object relative to another due to the motion of the observer is called parallax.
 Optics Definitions Reflection is the bouncing of light off an object Laws of Reflection of Light: 1. The incident ray, the normal at the point of incidence and the reflected ray all lie in the same plane.
Optics Definitions Reflection is the bouncing of light off an object Laws of Reflection of Light: 1. The incident ray, the normal at the point of incidence and the reflected ray all lie in the same plane.
Cambridge IGCSE Science. Syllabus 0654 for 2016 Exam
 Cambridge IGCSE Science Syllabus 0654 for 2016 Exam What is in this revision guide? 1. A topic checklist: here you can find the names of all of the topics we cover. You can tick them off when we do them
Cambridge IGCSE Science Syllabus 0654 for 2016 Exam What is in this revision guide? 1. A topic checklist: here you can find the names of all of the topics we cover. You can tick them off when we do them
Biology Paper 1 1hr 15mins 70 marks
 Biology Paper 1 1hr 15mins 70 marks Cell Biology (Yr9) Cell Organisation (Yr9) Infection and Response (Yr10) Bioenergetics (Yr10) Cells Cell Organisation Competition Photosynthesis Microscopy Enzymes Abiotic
Biology Paper 1 1hr 15mins 70 marks Cell Biology (Yr9) Cell Organisation (Yr9) Infection and Response (Yr10) Bioenergetics (Yr10) Cells Cell Organisation Competition Photosynthesis Microscopy Enzymes Abiotic
Chemistry Terms. atomic number The atomic number of an element is the number of protons in the nucleus of each atom.
 Chemistry Terms atomic number The atomic number of an element is the number of protons in the nucleus of each atom. chemical reaction A process in which atoms and molecules interact, resulting in the alteration
Chemistry Terms atomic number The atomic number of an element is the number of protons in the nucleus of each atom. chemical reaction A process in which atoms and molecules interact, resulting in the alteration
Foundation Year Programme
 Foundation Year Programme Entrance Tests BIOLOGY SPECIFICATION Standard ATS sample material 2 3 Biology 1. Living organisms 1.1. Characteristics of living organisms a. List and define the main characteristics
Foundation Year Programme Entrance Tests BIOLOGY SPECIFICATION Standard ATS sample material 2 3 Biology 1. Living organisms 1.1. Characteristics of living organisms a. List and define the main characteristics
Science Year 10 Unit 1 Biology
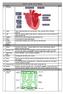 Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
Y8 Science Controlled Assessment Topics & Keywords
 Y8 Science Controlled Assessment Topics & Biology Respiration. Know that respiration in living organisms can be aerobic or anaerobic The word equation for aerobic respiration The process of anaerobic respiration
Y8 Science Controlled Assessment Topics & Biology Respiration. Know that respiration in living organisms can be aerobic or anaerobic The word equation for aerobic respiration The process of anaerobic respiration
CORE CONCEPTS & TERMINOLOGY FALL 2010
 CORE CONCEPTS & TERMINOLOGY FALL 2010 The following concepts and terms will be covered by all BIO 120 lecture instructors. Presentation of additional concepts is left to the discretion of the individual
CORE CONCEPTS & TERMINOLOGY FALL 2010 The following concepts and terms will be covered by all BIO 120 lecture instructors. Presentation of additional concepts is left to the discretion of the individual
Research Science Biology The study of living organisms (Study of life)
 Scientific method Why is there a hypothesis and prediction? If only prediction: then there is no way to finish the prediction and conclude whether the results support the hypothesis If surfaces are sampled
Scientific method Why is there a hypothesis and prediction? If only prediction: then there is no way to finish the prediction and conclude whether the results support the hypothesis If surfaces are sampled
Science Years 9 to 10
 Boardworks Contents Guide Boardworks Presentations: Acids and metal oxides 10 slides Reactions of metal oxides with acids. Adapting to changes 9 slides Ways that animals adapt to their habitats. Air pollution
Boardworks Contents Guide Boardworks Presentations: Acids and metal oxides 10 slides Reactions of metal oxides with acids. Adapting to changes 9 slides Ways that animals adapt to their habitats. Air pollution
This unit will help you define health, learn about some pathogens and the diseases they cause, medicines and about the immune system.
 YEAR 10 Biology Units 1-5 Biology Unit CB1 Key biological concepts [Paper 1 & CB2 Cells and Control CB3 Genetics CB4 Natural Selection and Genetic Modification CB5 Health, Disease and the Development of
YEAR 10 Biology Units 1-5 Biology Unit CB1 Key biological concepts [Paper 1 & CB2 Cells and Control CB3 Genetics CB4 Natural Selection and Genetic Modification CB5 Health, Disease and the Development of
Science 9-1 Grades Guidance
 U1 U2 U3 Science 9-1 Grades Guidance This guide is designed to help explain the level that a student is working at using examples of the curriculum that a student should be able to access. This is not
U1 U2 U3 Science 9-1 Grades Guidance This guide is designed to help explain the level that a student is working at using examples of the curriculum that a student should be able to access. This is not
Science. synthesis 2 Analysis and synthesis Using physics to make things work 3 Magnetic fields to keep things moving Energy calculations 4 Energy
 Year 11 (Triple science) 2016-17 Half-term Topic Week 1 Week 2 Week 3 Week 4 Week 5 Week 6 Week 7 1 Exchange of materials Keeping internal conditions constant Analysis and synthesis 2 Analysis and synthesis
Year 11 (Triple science) 2016-17 Half-term Topic Week 1 Week 2 Week 3 Week 4 Week 5 Week 6 Week 7 1 Exchange of materials Keeping internal conditions constant Analysis and synthesis 2 Analysis and synthesis
8 th Grade Integrated Science Curriculum
 Date Hobbs Science By being embedded throughout the curriculum, these Processing Skills will be addressed throughout the year. 8.1 Scientific Thinking and Practice 1. Use scientific methods to develop
Date Hobbs Science By being embedded throughout the curriculum, these Processing Skills will be addressed throughout the year. 8.1 Scientific Thinking and Practice 1. Use scientific methods to develop
Foundation Year Programme
 Foundation Year Programme Entrance Tests PHYSICS SPECIFICATION Standard ATS sample material 2 3 Physics 1. Electricity 1.1 Electrostatics: a. charging of insulators by friction b. object gaining electrons
Foundation Year Programme Entrance Tests PHYSICS SPECIFICATION Standard ATS sample material 2 3 Physics 1. Electricity 1.1 Electrostatics: a. charging of insulators by friction b. object gaining electrons
N5 Physics. Key Definitions. Name:
 N5 Physics Key s Name: Contents Page 3 Key s Electricity Equations Electricity Page 4 Key s Dynamics and Space Equations Dynamics and Space Page 5 Key s Properties of Matter Equations Properties of Matter
N5 Physics Key s Name: Contents Page 3 Key s Electricity Equations Electricity Page 4 Key s Dynamics and Space Equations Dynamics and Space Page 5 Key s Properties of Matter Equations Properties of Matter
8.L.5.1 Practice Questions
 Name: ate: 1. The diagram below represents a series of events that occur in living cells. Which molecule is indicated by X?. glucose. TP. carbon dioxide. protein 2. The diagram represents one metabolic
Name: ate: 1. The diagram below represents a series of events that occur in living cells. Which molecule is indicated by X?. glucose. TP. carbon dioxide. protein 2. The diagram represents one metabolic
Subject: GCSE Physics
 Subject: GCSE Physics Density States of matter SHC Atoms and radiation Discovery of the nucleus Changes in the nucleus Alpha, beta and gamma Activity and half life Nuclear radiation and medicine Nuclear
Subject: GCSE Physics Density States of matter SHC Atoms and radiation Discovery of the nucleus Changes in the nucleus Alpha, beta and gamma Activity and half life Nuclear radiation and medicine Nuclear
BIOMEDICAL ADMISSIONS TEST (BMAT Leiden) Specimen paper. Section 2 explained answers
 BIOMEDICAL ADMISSIONS TEST (BMAT Leiden) Specimen paper Section explained answers 1 Process 1 is nitrogen fixation which is the process of turning nitrogen gas from the air into nitrogen compounds in the
BIOMEDICAL ADMISSIONS TEST (BMAT Leiden) Specimen paper Section explained answers 1 Process 1 is nitrogen fixation which is the process of turning nitrogen gas from the air into nitrogen compounds in the
Curriculum Guide: Science Grades 6-8/
 6-ES-1 Nature of Science and Lab Safety 6-ES-1-1 Design and conduct scientific investigation using the Scientific Method 6-ES-1-2 Determine the dependent, independent and controlled variables of an experiment
6-ES-1 Nature of Science and Lab Safety 6-ES-1-1 Design and conduct scientific investigation using the Scientific Method 6-ES-1-2 Determine the dependent, independent and controlled variables of an experiment
Year 11 Science Learning Cycle 3 Overview
 Year 11 Science Learning Cycle 3 Overview Learning Cycle Overview: Biology Hypothesis 1 Hypothesis 2 Hypothesis 3 Hypothesis 4 Hypothesis 5 Hypothesis 6 Hypothesis 7 Hypothesis 8 Hypothesis 9 How does
Year 11 Science Learning Cycle 3 Overview Learning Cycle Overview: Biology Hypothesis 1 Hypothesis 2 Hypothesis 3 Hypothesis 4 Hypothesis 5 Hypothesis 6 Hypothesis 7 Hypothesis 8 Hypothesis 9 How does
Proficiency Review #1
 Proficiency Review #1 What is the best way to determine how two people are most closely DNA related? What best measures a liquid? Graduated Cylinder A hydro-electric generator converts mechanical energy
Proficiency Review #1 What is the best way to determine how two people are most closely DNA related? What best measures a liquid? Graduated Cylinder A hydro-electric generator converts mechanical energy
Colorado High School Physical Science Standards Foundations of Physical Science, 3rd Edition
 11/13/2014 Page 1 of 34 PS.1.A Physical Science 81 vectors and velocity 22 find speed of car Newton s laws of motion and gravitation describe the relationships among forces acting on and between objects,
11/13/2014 Page 1 of 34 PS.1.A Physical Science 81 vectors and velocity 22 find speed of car Newton s laws of motion and gravitation describe the relationships among forces acting on and between objects,
LONGMAN STUDY GUIDES. Science. Di Barton. SUB Q6ttlngen B1842 LONGMAN
 LONGMAN STUDY GUIDES Science Di Barton SUB Q6ttlngen 206 451 652 97B1842 LONGMAN CONTENTS Editors' Preface vii Acknowledgements vii Information about this book viii 1 Revision, examinations and coursework
LONGMAN STUDY GUIDES Science Di Barton SUB Q6ttlngen 206 451 652 97B1842 LONGMAN CONTENTS Editors' Preface vii Acknowledgements vii Information about this book viii 1 Revision, examinations and coursework
Ponce de Leon 8thGrade Winter Science Package '13-'14
 Name: Teacher: Period: Date: Ponce de Leon 8thGrade Winter Science Package '13-'14 Multiple Choice: Identify the choice that best completes the statement or answers the question. 1. Which of these phrases
Name: Teacher: Period: Date: Ponce de Leon 8thGrade Winter Science Package '13-'14 Multiple Choice: Identify the choice that best completes the statement or answers the question. 1. Which of these phrases
1.4 recall and use the relationship between acceleration, velocity and time: 1.6 determine acceleration from the gradient of a velocity-time graph
 Physics Section 1: Forces and motion b) Movement and position c) Forces, movement and shape d) Astronomy 1.1 use the following units: kilogram (kg), metre (m), metre/second (m/s), metre/second 2 (m/s 2
Physics Section 1: Forces and motion b) Movement and position c) Forces, movement and shape d) Astronomy 1.1 use the following units: kilogram (kg), metre (m), metre/second (m/s), metre/second 2 (m/s 2
Biology 5094 O Level 2013 Answers Scheme
 Biology 5094 O Level 2013 Answers Scheme Paper 1 1 2 3 4 5 6 7 8 9 10 B C C D D A D C D C 11 12 13 14 15 16 17 18 19 20 C D A A D B C C B D 21 22 23 24 25 26 27 28 29 30 D B A C B B B A B B 31 32 33 34
Biology 5094 O Level 2013 Answers Scheme Paper 1 1 2 3 4 5 6 7 8 9 10 B C C D D A D C D C 11 12 13 14 15 16 17 18 19 20 C D A A D B C C B D 21 22 23 24 25 26 27 28 29 30 D B A C B B B A B B 31 32 33 34
Chapter 03 Lecture Outline
 Chapter 03 Lecture Outline William P. Cunningham University of Minnesota Mary Ann Cunningham Vassar College Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1
Chapter 03 Lecture Outline William P. Cunningham University of Minnesota Mary Ann Cunningham Vassar College Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1
GCSE PHYSICS REVISION LIST
 GCSE PHYSICS REVISION LIST OCR Gateway Physics (J249) from 2016 Topic P1: Matter P1.1 Describe how and why the atomic model has changed over time Describe the structure of the atom and discuss the charges
GCSE PHYSICS REVISION LIST OCR Gateway Physics (J249) from 2016 Topic P1: Matter P1.1 Describe how and why the atomic model has changed over time Describe the structure of the atom and discuss the charges
Biology Final Review Ch pg Biology is the study of
 Biology Final Review Ch. 1 1-3 pg. 17-25 1. Biology is the study of Ch.2 2-3 pg. 45-49 2. All organic compounds contain. 3. Starch is an example of which type of organic compound? 4. What monomers make
Biology Final Review Ch. 1 1-3 pg. 17-25 1. Biology is the study of Ch.2 2-3 pg. 45-49 2. All organic compounds contain. 3. Starch is an example of which type of organic compound? 4. What monomers make
GCSE OCR Revision Physics. GCSE OCR Revision Physics. GCSE OCR Revision Physics. GCSE OCR Revision Physics. Journeys. GCSE OCR Revision Physics
 Matter, Models and Density What is a typical size of an atom? Choose from the following. 10 15 m 10 12 m 10 10 m Matter, Models and Density The size of an atom is of the order of 10 10 m. 1 1 Temperature
Matter, Models and Density What is a typical size of an atom? Choose from the following. 10 15 m 10 12 m 10 10 m Matter, Models and Density The size of an atom is of the order of 10 10 m. 1 1 Temperature
The Chemistry of Life
 The Chemistry of Life Things you should be able to do 1. Describe how the unique properties of water support life on Earth. 2. Explain how carbon is uniquely suited to form biological macromolecules. 3.
The Chemistry of Life Things you should be able to do 1. Describe how the unique properties of water support life on Earth. 2. Explain how carbon is uniquely suited to form biological macromolecules. 3.
Curriculum Map. Biology, Quarter 1 Big Ideas: From Molecules to Organisms: Structures and Processes (BIO1.LS1)
 1 Biology, Quarter 1 Big Ideas: From Molecules to Organisms: Structures and Processes (BIO1.LS1) Focus Standards BIO1.LS1.2 Evaluate comparative models of various cell types with a focus on organic molecules
1 Biology, Quarter 1 Big Ideas: From Molecules to Organisms: Structures and Processes (BIO1.LS1) Focus Standards BIO1.LS1.2 Evaluate comparative models of various cell types with a focus on organic molecules
PREFACE O-LEVEL TOPICAL SCIENCE (BIOLOGY)
 PREFACE O-LEVEL TOPICAL SCIENCE (BIOLOGY) provides a thorough revision for students taking the GCE O-Level Science (Biology) Examination. Past examination questions have been carefully classified into
PREFACE O-LEVEL TOPICAL SCIENCE (BIOLOGY) provides a thorough revision for students taking the GCE O-Level Science (Biology) Examination. Past examination questions have been carefully classified into
 1 of 5 9/18/2009 3:40 PM Map: Grade 5 Science Grade Level: 5 School Year: 2008-2009 Author: Jill Melvin District/Building: Minisink Valley CSD/Intermediate School Created: 11/03/2008 Last Updated: 11/04/2008
1 of 5 9/18/2009 3:40 PM Map: Grade 5 Science Grade Level: 5 School Year: 2008-2009 Author: Jill Melvin District/Building: Minisink Valley CSD/Intermediate School Created: 11/03/2008 Last Updated: 11/04/2008
KS3 Science Levelness Posters
 KS Science Levelness Posters Contents Year Year 8 Year 9 A. Cells 8A. Food and digestion 9A. Inheritance and selection B. Reproduction 8B. Respiration 9B. Fit and healthy C. Environment and feeding 8C.
KS Science Levelness Posters Contents Year Year 8 Year 9 A. Cells 8A. Food and digestion 9A. Inheritance and selection B. Reproduction 8B. Respiration 9B. Fit and healthy C. Environment and feeding 8C.
EH1008 : Biology for Public Health : Biomolecules and Metabolism
 EH1008 : Biology for Public Health : Biomolecules and Metabolism Biochemistry: The chemistry of living things What has this got to do with Epidemiology & Public Health? Aims of 'Epidemiology & Public Health:
EH1008 : Biology for Public Health : Biomolecules and Metabolism Biochemistry: The chemistry of living things What has this got to do with Epidemiology & Public Health? Aims of 'Epidemiology & Public Health:
Performance Level Descriptors. Science
 Performance Level Descriptors Science Grade 5 Content Summary Nature and Application of Science and Technology Distinguish well designed fair tests from flawed fair tests. Distinguish questions that can
Performance Level Descriptors Science Grade 5 Content Summary Nature and Application of Science and Technology Distinguish well designed fair tests from flawed fair tests. Distinguish questions that can
BioMedical Admissions Test. Specimen Section 2 answers
 BioMedical Admissions Test Specimen Section answers UCLES 017 1 The correct answer is option H. The removal of nitrogen gas from the air, process 1, into the soil is nitrogen fixation. The release of nitrogen
BioMedical Admissions Test Specimen Section answers UCLES 017 1 The correct answer is option H. The removal of nitrogen gas from the air, process 1, into the soil is nitrogen fixation. The release of nitrogen
MARK SCHEME for the October/November 2015 series 0610 BIOLOGY. 0610/21 Paper 2 (Core), maximum raw mark 80
 CAMBRIDGE INTERNATIONAL EXAMINATIONS Cambridge International General Certificate of Secondary Education MARK SCHEME for the October/November 2015 series 0610 BIOLOGY 0610/21 Paper 2 (Core), maximum raw
CAMBRIDGE INTERNATIONAL EXAMINATIONS Cambridge International General Certificate of Secondary Education MARK SCHEME for the October/November 2015 series 0610 BIOLOGY 0610/21 Paper 2 (Core), maximum raw
Biology Spring Final Exam Study Guide
 Name: Hour: Basic Biology Skills Graphing Know the keys to creating a graph Know how to interpret a graph Independent variable Dependent variable Biology Spring Final Exam Study Guide Levels of Organization
Name: Hour: Basic Biology Skills Graphing Know the keys to creating a graph Know how to interpret a graph Independent variable Dependent variable Biology Spring Final Exam Study Guide Levels of Organization
Year 11 IGCSE Physics Revision Checklist
 Year 11 IGCSE Physics Revision Checklist Use this booklet to help you with your revision in preparation for your year 11 Physics prelim and final examinations. This is the work that you will have covered
Year 11 IGCSE Physics Revision Checklist Use this booklet to help you with your revision in preparation for your year 11 Physics prelim and final examinations. This is the work that you will have covered
Name Date Period Unit 1 Basic Biological Principles 1. What are the 7 characteristics of life?
 Unit 1 Basic Biological Principles 1. What are the 7 characteristics of life? Eukaryotic cell parts you should be able a. to identify and label: Nucleus b. Nucleolus c. Rough/smooth ER Ribosomes d. Golgi
Unit 1 Basic Biological Principles 1. What are the 7 characteristics of life? Eukaryotic cell parts you should be able a. to identify and label: Nucleus b. Nucleolus c. Rough/smooth ER Ribosomes d. Golgi
9 th grade physical/earth Science
 9 th grade physical/earth Science How can one explain the structure and properties of matter? HS-PS1-1: Use the periodic table as a model to predict the relative properties of elements based on the patterns
9 th grade physical/earth Science How can one explain the structure and properties of matter? HS-PS1-1: Use the periodic table as a model to predict the relative properties of elements based on the patterns
Genetics word list. the molecule which contains genes. This will be looked at in more detail. it is shaped in a double helix (spiral)
 Genetics word list DNA the molecule which contains genes. This will be looked at in more detail. it is shaped in a double helix (spiral) Chromosomes X-shaped objects found in the nucleus of a cell. The
Genetics word list DNA the molecule which contains genes. This will be looked at in more detail. it is shaped in a double helix (spiral) Chromosomes X-shaped objects found in the nucleus of a cell. The
Do all living things grow, move, and breathe? All living things are made of what?
 All living things are made of what? Do all living things grow, move, and breathe? All living things respond to external conditions. This is called what? Which of the 7 traits of life is defined as the
All living things are made of what? Do all living things grow, move, and breathe? All living things respond to external conditions. This is called what? Which of the 7 traits of life is defined as the
Jamaica_Science_Grade 09 Table of Content with Listing of Activities
 Jamaica_Science_Grade 09 Table of Content with Listing of Activities 1. Atoms and Molecules 3.1 Tutorial : Law of Chemical Combination 3.2 Tutorial : Atoms and Atomic Theory of Matter 3.2 Review : Dolton's
Jamaica_Science_Grade 09 Table of Content with Listing of Activities 1. Atoms and Molecules 3.1 Tutorial : Law of Chemical Combination 3.2 Tutorial : Atoms and Atomic Theory of Matter 3.2 Review : Dolton's
TEST DESIGN. Science
 TEST DESIGN AND FRAMEWORK TEST DESIGN Science The Science assessment consists of two tests. Each test contains a section with selectedresponse questions and a section with constructed-response assignments.
TEST DESIGN AND FRAMEWORK TEST DESIGN Science The Science assessment consists of two tests. Each test contains a section with selectedresponse questions and a section with constructed-response assignments.
Characteristics of Life
 Characteristics of Life All living things share some basic characteristics: 1. Organization 2. Movement 3. Made up of cells 4. Reproduce 5. Grow and / or develop 6. Obtain and use energy 7. Respond to
Characteristics of Life All living things share some basic characteristics: 1. Organization 2. Movement 3. Made up of cells 4. Reproduce 5. Grow and / or develop 6. Obtain and use energy 7. Respond to
Cambridge International Examinations Cambridge International General Certificate of Secondary Education. Published
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education CO-ORDINATED SCIENCES 0654/4 Paper 4 Theory (Extended) May/June 017 MARK SCHEME Maximum Mark: 10
Cambridge International Examinations Cambridge International General Certificate of Secondary Education CO-ORDINATED SCIENCES 0654/4 Paper 4 Theory (Extended) May/June 017 MARK SCHEME Maximum Mark: 10
SUBJECT: Science Grade Level: 8. Unit: Technology & Engineering (1 week)
 Grade 8 Science Curriculum Map - Norwell Middle School SUBJECT: Science Grade Level: 8 Unit: Technology & Engineering (1 week) Standard 2: Engineering Design 2.1- Identify and explain the steps of the
Grade 8 Science Curriculum Map - Norwell Middle School SUBJECT: Science Grade Level: 8 Unit: Technology & Engineering (1 week) Standard 2: Engineering Design 2.1- Identify and explain the steps of the
What Is Biology? Biologists Study? The study of living things. Characteristics Classifications Interactions between organisms Health & Disease
 What Is Biology? The study of living things. Biologists Study? Characteristics Classifications Interactions between organisms Health & Disease Goal of Science To investigate To understand To explain To
What Is Biology? The study of living things. Biologists Study? Characteristics Classifications Interactions between organisms Health & Disease Goal of Science To investigate To understand To explain To
What is the role of the nucleus? What is the role of the cytoplasm? What is the role of the mitochondria? What is the role of the cell wall. membrane?
 Page 1 What is the role of the nucleus? What is the role of the cytoplasm? What is the role of the cell membrane? What is the role of the mitochondria? What is the role of ribosomes? What is the role of
Page 1 What is the role of the nucleus? What is the role of the cytoplasm? What is the role of the cell membrane? What is the role of the mitochondria? What is the role of ribosomes? What is the role of
Biology Mid-Term Study Guide
 Name: Date: Chapter 1: The Science of Biology 1. List the 8 characteristics of all living things: 1) 2) 3) 4) 5) 6) 7) 8) 2. What is biology? 3. What is homeostasis? 4. Define sexual and asexual reproduction.
Name: Date: Chapter 1: The Science of Biology 1. List the 8 characteristics of all living things: 1) 2) 3) 4) 5) 6) 7) 8) 2. What is biology? 3. What is homeostasis? 4. Define sexual and asexual reproduction.
Behavioral and Structural Adaptations PPT Guided Notes
 A Essential Standard 2.1.2 Analyze how various organisms accomplish the following life functions through adaptations with particular environments and that these adaptations have evolved to ensure survival
A Essential Standard 2.1.2 Analyze how various organisms accomplish the following life functions through adaptations with particular environments and that these adaptations have evolved to ensure survival
Name Period. Final Exam Study Guide
 Name Period Chapter 6-1 Chromosomes Final Exam Study Guide 1. What is the structure of chromosomes(what are they made of and what is on them)? How many do we have? When are they copied? 2. What is an autosome
Name Period Chapter 6-1 Chromosomes Final Exam Study Guide 1. What is the structure of chromosomes(what are they made of and what is on them)? How many do we have? When are they copied? 2. What is an autosome
10/4/2016. Matter, Energy, and Life
 DISCLAIMER: Principles and concepts on atomic structure, the Periodic Table, atoms, ions, ionic and covalent compounds, metals, and nonmetals will not be covered in this course. You are expected to know
DISCLAIMER: Principles and concepts on atomic structure, the Periodic Table, atoms, ions, ionic and covalent compounds, metals, and nonmetals will not be covered in this course. You are expected to know
K.P.2 Understand how objects are described based on their physical properties and how they are used.
 Grade 2 Grade 1 Kindergarten K.P.1 Understand the positions and motions of objects and organisms observed in the environment. K.P.2 Understand how objects are described based on their physical properties
Grade 2 Grade 1 Kindergarten K.P.1 Understand the positions and motions of objects and organisms observed in the environment. K.P.2 Understand how objects are described based on their physical properties
DEFINITIONS. Linear Motion. Conservation of Momentum. Vectors and Scalars. Circular Motion. Newton s Laws of Motion
 DEFINITIONS Linear Motion Mass: The mass of a body is the amount of matter in it. Displacement: The displacement of a body from a point is its distance from a point in a given direction. Velocity: The
DEFINITIONS Linear Motion Mass: The mass of a body is the amount of matter in it. Displacement: The displacement of a body from a point is its distance from a point in a given direction. Velocity: The
Cambridge Assessment International Education Cambridge International General Certificate of Secondary Education. Published
 Cambridge Assessment International Education Cambridge International General Certificate of Secondary Education CO-ORDINATED SCIENCES 0654/41 Paper 4 Theory (Extended) 017 MARK SCHEME Maximum Mark: 10
Cambridge Assessment International Education Cambridge International General Certificate of Secondary Education CO-ORDINATED SCIENCES 0654/41 Paper 4 Theory (Extended) 017 MARK SCHEME Maximum Mark: 10
This document includes the following supporting documentation to accompany the NGSS Formative Assessments for Chemistry in the Earth System:
 Fluence Learning High School NGSS Formative Assessments - New York Supporting Documents This document includes the following supporting documentation to accompany the NGSS Formative Assessments for Chemistry
Fluence Learning High School NGSS Formative Assessments - New York Supporting Documents This document includes the following supporting documentation to accompany the NGSS Formative Assessments for Chemistry
Unit 6 Forces in Nature gravity; Law of Universal Gravitation; current; series/parallel circuits; magnets; electromagnets
 8 th grade Physical Science comprehensive study guide Unit 2 Nature of Matter atoms/molecules; atomic models; physical/chemical properties; physical/chemical changes; types of bonds; periodic table; states
8 th grade Physical Science comprehensive study guide Unit 2 Nature of Matter atoms/molecules; atomic models; physical/chemical properties; physical/chemical changes; types of bonds; periodic table; states
Physics Important Terms and their Definitions
 Physics Important Terms and their S.No Word Meaning 1 Acceleration The rate of change of velocity of an object with respect to time 2 Angular Momentum A measure of the momentum of a body in rotational
Physics Important Terms and their S.No Word Meaning 1 Acceleration The rate of change of velocity of an object with respect to time 2 Angular Momentum A measure of the momentum of a body in rotational
Cell Structure. Cell Structure. Investigating Cells. Investigating Cells. Cell Division. Cell Division. Transport In and Out of Cells
 Cell Structure What are the two main types of cell? Cell Structure The two main types of cell are prokaryotic and eukaryotic. 1 1 Investigating Cells How is magnification calculated? 2 Investigating Cells
Cell Structure What are the two main types of cell? Cell Structure The two main types of cell are prokaryotic and eukaryotic. 1 1 Investigating Cells How is magnification calculated? 2 Investigating Cells
Mitosis and Meiosis. 2. The distribution of chromosomes in one type of cell division is shown in the diagram below.
 Name: Date: 1. Jack bought a small turtle. Three months later, the turtle had grown to twice its original size. Which of the following statements best describes why Jack s turtle got bigger? A. Parts of
Name: Date: 1. Jack bought a small turtle. Three months later, the turtle had grown to twice its original size. Which of the following statements best describes why Jack s turtle got bigger? A. Parts of
Level 789 Pathway: Combined Science Double Award
 Level 789 Pathway: Combined Science Double Award Yr Combined Science Targets: Pathway 8 11 Biology a) Explain in detail the role of the hormone ADH on the nephron b) Explain in detail the process by which
Level 789 Pathway: Combined Science Double Award Yr Combined Science Targets: Pathway 8 11 Biology a) Explain in detail the role of the hormone ADH on the nephron b) Explain in detail the process by which
Introduction. Introduction. Forces An. Forces An. Forces in Action. Forces in Action. Pressure and Pressure. Pressure and Pressure.
 Forces An Introduction A force is a vector quantity. What does this mean? Forces An Introduction A vector quantity, such as force, has a direction as well as a magnitude. 1 1 Forces in Action The moment
Forces An Introduction A force is a vector quantity. What does this mean? Forces An Introduction A vector quantity, such as force, has a direction as well as a magnitude. 1 1 Forces in Action The moment
Cambridge International Examinations Cambridge International General Certificate of Secondary Education. Published
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education CO-ORDINATED SCIENCES 0654/33 Paper 3 Extended Theory May/June 2016 MARK SCHEME Maximum Mark: 120
Cambridge International Examinations Cambridge International General Certificate of Secondary Education CO-ORDINATED SCIENCES 0654/33 Paper 3 Extended Theory May/June 2016 MARK SCHEME Maximum Mark: 120
Combined GCSE OCR Revision Science magnification tur tur magnification ell Struc ell Struc ells ells Combined GCSE OCR Revision Science
 Cell Structures How is the magnification of a light microscope calculated? Cell Structures Total magnification is calculated by multiplying the magnification of the eyepiece lens by the magnification of
Cell Structures How is the magnification of a light microscope calculated? Cell Structures Total magnification is calculated by multiplying the magnification of the eyepiece lens by the magnification of
Reproduction Chemical Reactions. 8J Light 8G Metals & Their Uses 8C Breathing & Respiration 8D Unicellular Organisms
 Science: Key Stage 3 Based on the Exploring Science Scheme of Learning Term 1 & 2 Term 3 & 4 Term 5 & 6 Year 7 Cells, Tissues & Organs Particles Forces & Motion Reproduction Chemical Reactions Chemical
Science: Key Stage 3 Based on the Exploring Science Scheme of Learning Term 1 & 2 Term 3 & 4 Term 5 & 6 Year 7 Cells, Tissues & Organs Particles Forces & Motion Reproduction Chemical Reactions Chemical
1 st Semester Vocabulary
 1 st Semester Vocabulary 1. Qualitative Observation Observation that involves descriptions and characteristics. Example: Color and appearance The cat looks scared. The cat has green eyes. The cat has long
1 st Semester Vocabulary 1. Qualitative Observation Observation that involves descriptions and characteristics. Example: Color and appearance The cat looks scared. The cat has green eyes. The cat has long
CHAPTER 2. Life s Chemical Basis
 CHAPTER 2 Life s Chemical Basis The Chemistry of Life We are made up of elements. Atoms of one kind make up an element. Atoms are the smallest unit of an element still maintaing the element s properties.
CHAPTER 2 Life s Chemical Basis The Chemistry of Life We are made up of elements. Atoms of one kind make up an element. Atoms are the smallest unit of an element still maintaing the element s properties.
Environmental Science and Technology
 Environmental Science and Technology Are You Ready for the June Exam? THE MATERIAL WORLD Properties of solutions: Concentration I can determine the concentration of an aqueous solution (g/l, percentage,
Environmental Science and Technology Are You Ready for the June Exam? THE MATERIAL WORLD Properties of solutions: Concentration I can determine the concentration of an aqueous solution (g/l, percentage,
YEAR 11- Physics Term 1 plan
 YEAR 11- Physics Term 1 plan 2016-2017 Week Topic Learning outcomes Week 1 5.1.2 Nucleus of the Atom Describe the composition of the nucleus in terms of protons and neutrons State the charges of protons
YEAR 11- Physics Term 1 plan 2016-2017 Week Topic Learning outcomes Week 1 5.1.2 Nucleus of the Atom Describe the composition of the nucleus in terms of protons and neutrons State the charges of protons
Name # Class Date Regents Review: Cells & Cell Transport
 Name # Class Date Regents Review: Cells & Cell Transport 1. All of the following are true regarding cells except? A) All cells have genetic material B) All cells have cell walls C) All cells have plasma
Name # Class Date Regents Review: Cells & Cell Transport 1. All of the following are true regarding cells except? A) All cells have genetic material B) All cells have cell walls C) All cells have plasma
Record your answers to Part A and Part B 1 on this answer sheet. Part A. Part A Score
 Tear Here The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION LIVING ENVIRONMENT Wednesday, June 20, 2007 9:15 a.m. to 12:15 p.m., only ANSWER SHEET Female Student........................................
Tear Here The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION LIVING ENVIRONMENT Wednesday, June 20, 2007 9:15 a.m. to 12:15 p.m., only ANSWER SHEET Female Student........................................
THINGS I NEED TO KNOW:
 THINGS I NEED TO KNOW: 1. Prokaryotic and Eukaryotic Cells Prokaryotic cells do not have a true nucleus. In eukaryotic cells, the DNA is surrounded by a membrane. Both types of cells have ribosomes. Some
THINGS I NEED TO KNOW: 1. Prokaryotic and Eukaryotic Cells Prokaryotic cells do not have a true nucleus. In eukaryotic cells, the DNA is surrounded by a membrane. Both types of cells have ribosomes. Some
Biology EOCT Review. Milton High School
 Biology EOCT Review Milton High School Cell Organelles Nucleus holds DNA Cell membrane what comes in and goes out Mitochondria powerhouse of the cell Ribosomes protein synthesis Lysosomes digestion Cell
Biology EOCT Review Milton High School Cell Organelles Nucleus holds DNA Cell membrane what comes in and goes out Mitochondria powerhouse of the cell Ribosomes protein synthesis Lysosomes digestion Cell
ESSENTIAL PHYSICAL SCIENCE VOCABULARY
 ESSENTIAL PHYSICAL SCIENCE VOCABULARY I. MATTER: ANYTHING THAT HAS MASS AND VOLUME A. mass 1. amount of matter in an object 2. measured in grams B. volume 1. amount of space 2. measured in Liters for liquid
ESSENTIAL PHYSICAL SCIENCE VOCABULARY I. MATTER: ANYTHING THAT HAS MASS AND VOLUME A. mass 1. amount of matter in an object 2. measured in grams B. volume 1. amount of space 2. measured in Liters for liquid
Iowa End-of-Course Assessment Programs Spring 2008 Results. Math & Science
 Iowa End-of-Course Assessment Programs Spring 2008 Results Math & Science Prepared at The University of Iowa by Iowa Testing Programs Copyright 2008 by The University of Iowa. All rights reserved. No part
Iowa End-of-Course Assessment Programs Spring 2008 Results Math & Science Prepared at The University of Iowa by Iowa Testing Programs Copyright 2008 by The University of Iowa. All rights reserved. No part
Ch(3)Matter & Change. John Dalton
 Ch(3)Matter & Change John Dalton What is Matter? Matter is anything that contains mass & volume (takes up space) Energy, such as light, heat, and sound, is NOT matter. The Particle Theory of Matter 1.
Ch(3)Matter & Change John Dalton What is Matter? Matter is anything that contains mass & volume (takes up space) Energy, such as light, heat, and sound, is NOT matter. The Particle Theory of Matter 1.
0654 CO-ORDINATED SCIENCES
 CAMBRIDGE INTERNATIONAL EXAMINATIONS Cambridge International General Certificate of Secondary Education MARK SCHEME for the May/June 2015 series 0654 CO-ORDINATED SCIENCES 0654/31 Paper 3 (Extended Theory),
CAMBRIDGE INTERNATIONAL EXAMINATIONS Cambridge International General Certificate of Secondary Education MARK SCHEME for the May/June 2015 series 0654 CO-ORDINATED SCIENCES 0654/31 Paper 3 (Extended Theory),
PHYSICAL SCIENCE: FINAL EXAM REVIEW
 UNIT 1: PHYSICAL SCIENCE: FINAL EXAM REVIEW Use appropriate laboratory apparatuses, technology, and techniques safely and accurately when conducting a scientific investigation. Use scientific instruments
UNIT 1: PHYSICAL SCIENCE: FINAL EXAM REVIEW Use appropriate laboratory apparatuses, technology, and techniques safely and accurately when conducting a scientific investigation. Use scientific instruments
AQA Physics Checklist
 Topic 1. Energy Video: Energy changes in a system To understand the ways in which energy can be stored in a system and can be transferred from one energy store to another within a system To understand
Topic 1. Energy Video: Energy changes in a system To understand the ways in which energy can be stored in a system and can be transferred from one energy store to another within a system To understand
Copy into Note Packet and Return to Teacher
 Copy into Note Packet and Return to Teacher Section 1: Nature of Matter Objectives: Differentiate between atoms and elements. Analyze how compounds are formed. Distinguish between covalent bonds, hydrogen
Copy into Note Packet and Return to Teacher Section 1: Nature of Matter Objectives: Differentiate between atoms and elements. Analyze how compounds are formed. Distinguish between covalent bonds, hydrogen
Foundation Year Programme. Entrance Tests PHYSICS SPECIFICATION. For NUFYP SET 2018
 Foundation Year Programme Entrance Tests PHYSICS SPECIFICATION For NUFYP SET 2018 V1.0 October 2017 2 Standard AT Sample Material Physics 1. Electricity 1.1 Electrostatics: a. charging of insulators by
Foundation Year Programme Entrance Tests PHYSICS SPECIFICATION For NUFYP SET 2018 V1.0 October 2017 2 Standard AT Sample Material Physics 1. Electricity 1.1 Electrostatics: a. charging of insulators by
Atoms. Atoms 9/9/2015
 The Chemistry of Life The Nature of Matter, Water,Carbon Compounds, Chemical Reactions and Enzymes The Nature of Matter B.1.9 Both living and nonliving things are composed of compounds, which are themselves
The Chemistry of Life The Nature of Matter, Water,Carbon Compounds, Chemical Reactions and Enzymes The Nature of Matter B.1.9 Both living and nonliving things are composed of compounds, which are themselves
100 Physics Facts. 1. The standard international unit (SI unit) for mass (m) is. kg (kilograms) s (seconds)
 100 Physics Facts 1. The standard international unit (SI unit) for mass (m) is. kg (kilograms) 2. The standard international unit (SI unit) for time (t) is. s (seconds) 3. The standard international unit
100 Physics Facts 1. The standard international unit (SI unit) for mass (m) is. kg (kilograms) 2. The standard international unit (SI unit) for time (t) is. s (seconds) 3. The standard international unit
1. Cell Theory Organelle containing the genetic information of the cell.
 GLOSSARY MATCHING GAME The words and definitions are all mixed up. Cut out each word and definition and glue the correct matches into your workbook. Word Definition 1. Cell Theory Organelle containing
GLOSSARY MATCHING GAME The words and definitions are all mixed up. Cut out each word and definition and glue the correct matches into your workbook. Word Definition 1. Cell Theory Organelle containing
Astronomy Forensic Science. Chemistry Honors Chemistry AP Chemistry. Biology AP Biology. Physics. Honors Physiology & Anatomy.
 HS PS1 1 HS PS1 2 HS PS1 3 HS PS1 4 HS PS1 5 HS PS1 6 HS PS1 7 HS PS1 8 HS PS2 1 HS PS2 2 HS PS2 3 HS PS2 4 Physical Science Use the periodic table as a model to predict the relative properties of elements
HS PS1 1 HS PS1 2 HS PS1 3 HS PS1 4 HS PS1 5 HS PS1 6 HS PS1 7 HS PS1 8 HS PS2 1 HS PS2 2 HS PS2 3 HS PS2 4 Physical Science Use the periodic table as a model to predict the relative properties of elements
Smart Science Lessons and High School Next Generation Science Standards
 Smart Science Lessons and High School Next Generation Science Standards You have chosen the right place to find great science learning and, beyond learning, how to think. The NGSS emphasize thinking and
Smart Science Lessons and High School Next Generation Science Standards You have chosen the right place to find great science learning and, beyond learning, how to think. The NGSS emphasize thinking and
Chemistry Review - Vocabulary
 Name Topic 1 - Atomic Concepts atom atomic number atomic mass electron valence electrons excited state ground state isotope mass number neutron orbital proton shell wave-mechanical model quanta spectra
Name Topic 1 - Atomic Concepts atom atomic number atomic mass electron valence electrons excited state ground state isotope mass number neutron orbital proton shell wave-mechanical model quanta spectra
Grade 7 Science Curriculum Maps
 Grade 7 Science Curriculum Maps Unit 1: Cells The Basic Unit of Life Unit 2: The Cell in Action Unit 3: Genes and DNA Unit 4: Heredity Unit 5: Evolution Unit 6: It s Alive! Or is it?! Unit 7: Bacteria
Grade 7 Science Curriculum Maps Unit 1: Cells The Basic Unit of Life Unit 2: The Cell in Action Unit 3: Genes and DNA Unit 4: Heredity Unit 5: Evolution Unit 6: It s Alive! Or is it?! Unit 7: Bacteria
2. The development of revolutionized the of life.
 Science 10 Unit 7 Worksheet Chapter 15, Part 1. 1. Briefly describe the three main parts of cell theory: 2. The development of revolutionized the of life. 3. Individual cells need to take in to build and
Science 10 Unit 7 Worksheet Chapter 15, Part 1. 1. Briefly describe the three main parts of cell theory: 2. The development of revolutionized the of life. 3. Individual cells need to take in to build and
Physics Standard level Paper 1
 Physics Standard level Paper 1 Friday 8 May 215 (morning) 45 minutes Instructions to candidates ydo not open this examination paper until instructed to do so. yanswer all the questions. yfor each question,
Physics Standard level Paper 1 Friday 8 May 215 (morning) 45 minutes Instructions to candidates ydo not open this examination paper until instructed to do so. yanswer all the questions. yfor each question,
K-12 Science Content Areas
 K-12 Science Content Areas 100 Nature of Science 2000 Earth Systems 200 Science & Technology 2100 Astronomy 300 Science, Health & Environment 2200 Meteorology 400 Measurement & Calculation in Science 2300
K-12 Science Content Areas 100 Nature of Science 2000 Earth Systems 200 Science & Technology 2100 Astronomy 300 Science, Health & Environment 2200 Meteorology 400 Measurement & Calculation in Science 2300
