Chapter 5-7. Exam 2. Including some materials from lectures by Gregory Ahearn University of North Florida Amended by John Crocker
|
|
|
- Brandon Sherman
- 6 years ago
- Views:
Transcription
1 Chapter 5-7 Exam 2 Including some materials from lectures by Gregory Ahearn University of North Florida Amended by John Crocker Copyright 2009 Pearson Education, Inc..
2 Review Questions Chapter 5-7 These questions need full and complete answers. Typically this will require a paragraph or two. If you are not certain if your answer is complete, ASK. 1. Define Energy, Chemical Energy, and Work. 2. What are the First and Second Laws of Thermodynamics? How do they impact growing complexity and decreasing entropy in living things? 3. Describe the process of photosynthesis. What is happening at a molecular and atomic level? 4. Compare and contrast exergonic and endergonic reactions and explain how they are related in coupled reactions. 5. Detail two coupled reactions involving ATP. 6. What are enzymes and how do they function? 7. What environmental factors effect enzyme function? How do they effect enzyme function? 8. Describe allosteric regulation and feedback inhibition. 9. How does photosynthesis convert solar energy into energy usable by cells? Be specific. What are the chemical reactions? 10. Describe the structure and location of chloroplasts within a leaf? 11. Describe PSI and PSII. How are they coupled? 12. What happens in the light reactions of photosynthesis? What happens in the dark reactions? How are light and dark reactions coupled? 13. What role does the color of photosynthetic pigments play in photosynthesis? 14. What is photorespiration? Why is it undesirable? 15. Compare and contrast photosynthesis and cellular respiration. Again be specific about reactions energy requirements etc. 16. How is cellular energy stored? 17. Describe in detail the processes of cellular metabolism. (glycolysis and cellular respiration) 18. Compare and contrast cellular respiration and fermentation. Once again be specific. What chemical processes are occurring in each and how are those similar and/or different?
3 5.1 What Is Energy? Energy is the capacity to do work. Synthesizing molecules Moving objects Generating heat and light Chemical energy is the energy that powers life The objects that move are electrons, which reposition during chemical reactions Work is force acting over distance. Change in kinetic energy
4 What Is Energy? Two fundamental types of energy Kinetic energy is the energy of movement e.g. light, heat, electricity, moving objects Potential energy is stored energy e.g. chemical energy in bonds, electrical charge in a battery, a rock at the top of a hill The laws of thermodynamics describe the availability and usefulness of energy
5 The Laws of Thermodynamics First Law of Thermodynamics Energy cannot be created nor destroyed, but it can change its form. (conservation of energy) The total amount of energy within a given system remains constant unless energy is added or removed from the system Example: potential energy in gasoline can be converted to kinetic energy (and heat energy) in a car, but the energy is not lost
6 The Laws of Thermodynamics Second Law of Thermodynamics The amount of useful energy decreases when energy is converted from one form to another. No process is 100% efficient, so no perpetual motion is possible. The remaining energy is released in a less useful form as heat, but the total energy is constant.
7 The Laws of Thermodynamics Matter tends to become less organized. Entropy: the spontaneous reduction in ordered forms of energy, and an increase in randomness and disorder Useful energy decreases as heat and other non-useful forms of energy increases. Example: gasoline is made up of an eight-carbon molecule that is highly ordered, when broken down to single carbons in CO 2, it is less ordered and more random.
8 Energy of Sunlight In order to keep useful energy flowing in ecosystems where the plants and animals produce more random forms of energy, new energy must be brought in. Sunlight provides an continuous supply of new energy to power all molecular reactions in living organisms. Photosynthetic organisms use external solar energy to maintain orderly structure Non-photosynthetic organisms use the stored chemical energy in other living things to counter increasing entropy
9 Chemical Reactions Chemical reactions are processes that form or break chemical bonds between atoms Chemical reactions convert reactants to products Reactants Products
10 Chemical Reactions All chemical reactions require an initial energy input (activation energy) to begin Molecules need to be moving with sufficient collision speed The source of activation energy is the kinetic energy of movement when molecules collide. The electrons of an atom repel other atoms and inhibit bond formation Molecular collisions force electron shells of atoms to mingle and interact, resulting in chemical reactions.
11
12 Chemical Reactions Reactions can be categorized as exergonic or endergonic based on energy gain or loss
13 Exergonic Reactions Exergonic reactions release energy Reactants contain more energy than products in exergonic reactions + energy released reactants + products
14 Exergonic Reaction Example Burning of glucose Sugar and oxygen contain more energy than the molecules of CO 2 and water. The extra energy is released as heat.
15 Endergonic Reactions Endergonic reactions require an input of energy to begin and more energy to continue Products contain more energy than reactants in endergonic reactions energy used (b) + reactants Endergonic reaction + products
16 Endergonic Reaction Example Photosynthesis sunlight energy + CO 2 + H 2 O sugar and O 2 The sugar contains far more energy than the CO 2 and water used to form it.
17 Chemical Reactions Glucose respiration and photosynthesis are complementary exergonic and endergonic reactions high Activation energy needed to ignite glucose Energy level of reactants Activation energy captured from sunlight glucose energy content of molecules glucose + O 2 CO 2 + H 2 O CO 2 + H 2 O Energy level of reactants low (a) progress of reaction Burning glucose (sugar): an exergonic reaction (b) progress of reaction Photosynthesis: an endergonic reaction Fig. 5-6
18 Coupled Reactions Exergonic reactions drive endergonic reactions The product of an energy-yielding reaction fuels an energy-requiring reaction in a coupled reaction The exergonic and endergonic parts of coupled reactions often occur at different places within the cell Energy-carrier molecules are used to transfer the energy within cells
19 Coupled Reactions Exergonic reactions may be linked with endergonic reactions. Endergonic reactions obtain energy from energy-releasing exergonic reactions in coupled reactions. Example 1: the exergonic reaction of burning gasoline in a car provides the endergonic reaction of moving the car Example 2: exergonic reactions in the sun release light energy used to drive endergonic sugar-making reactions in plants
20 5.3 How Is Energy Carried Between Coupled Reactions? Food energy cannot be used directly to power energy-requiring reactions Energy carrier molecules act as intermediates to carry energy between exergonic and endergonic reactions Energy carrier molecules are only used within cells because they are unstable
21 ATP Adenosine triphosphate (ATP) is the primary energy carrying molecule within cells ATP is composed of an adenosine molecule and three phosphates
22 ATP Energy is stored in the high-energy bond extending to the last phosphate Heat is given off when ATP breaks into ADP (adenosine diphosphate) and P (phosphate)
23 ATP The energy released when ATP is broken down into ADP + P is transferred to endergonic reactions through coupling
24 Energy Transfer in Coupled Reactions To summarize: Exergonic reactions drive endergonic reactions ATP moves to different parts of the cell and is broken down exergonically to liberate its energy to drive endergonic reactions. A biological example Muscle contraction (an endergonic reaction) is powered by the exergonic breakdown of ATP. During energy transfer in this coupled reaction, heat is given off, with overall loss of usable energy.
25 Energy Transfer in Coupled Reactions ATP breakdown is coupled with muscle contraction. Exergonic reaction: Endergonic reaction: relaxed muscle + ATP 20 units energy contracted muscle 100 units + ADP + P energy released Energy released from ATP breakdown exceeds the energy used for muscle contraction, so the overall coupled reaction is exergonic Coupled reaction: relaxed muscle + ATP contracted muscle + 80 units energy released as heat + ADP + P Fig. 5-10
26 Electron Carriers Electron carriers also transport energy within cells. Carrier molecules other than ATP also transport energy within a cell. Energy can be transferred to electrons in glucose metabolism and photosynthesis Electron carriers capture energetic electrons transferred by some exergonic reaction. Energized electron carriers then donate these energy-containing electrons to endergonic reactions. Energy can be transferred to electrons in glucose metabolism and photosynthesis
27 Electron Carriers Two common electron carriers 1. Nicotinamide adenine dinucleotide (NAD + ) 2. Flavin adenine dinucleotide (FAD)
28 5.4 How Do Cells Control Their Metabolic Reactions? Cellular metabolism: the multitude of chemical reactions going on at any specific time in a cell Metabolic pathways: the sequence of cellular reactions (e.g., photosynthesis and glycolysis)
29 Overview of Metabolism Metabolic pathways proceed smoothly for three reasons: 1. Endergonic reactions are coupled with exergonic reactions 2. Energy-carrier molecules capture energy and transfer it between endergonic and exergonic reactions 3. Chemical reactions are regulated through protein enzymes
30 Control of Metabolic Reactions At body temperature, many spontaneous reactions proceed too slowly to sustain life. A reaction can be controlled by controlling its activation energy, the energy needed to start the reaction. At body temperature, reactions occur too slowly because their activation energies are too high.
31 Spontaneous Reactions Reaction speed is generally determined by the activation energy required Reactions with low activation energies proceed rapidly at body temperature Reactions with high activation energies (e.g. sugar breakdown) move very slowly at body temperature, even if exergonic overall
32 Spontaneous Reactions Enzyme molecules are employed to catalyze (speed up) chemical reactions in cells Catalysts speed up the rate of a chemical reaction without themselves being used up Catalysts speed up spontaneous reactions by reducing activation energy
33
34 Catalyst Summary Four important principles about all catalysts They speed up reactions that would occur anyway, if their activation energy could be surmounted. Catalysts lower activation energy. The lowered activation energy allows reactions to move forward more quickly. Catalysts are not altered by the reaction.
35 Enzymes Are Biological Catalysts Almost all enzymes are proteins. Enzymes are highly specialized, generally catalyzing only a single reaction. In metabolic pathways each reaction is catalyzed by a different enzyme. Enzymes orient, distort, and reconfigure molecules in the process of lowering activation energy Enzyme activity is often enhanced or suppressed by its reactants or product Some enzymes require helper coenzymes (ex/ certain B vitamins)
36 Enzymes Are Biological Catalysts How does an enzyme catalyze a reaction? Both substrates enter the enzyme s active site, which is shaped specifically for those substrate molecules. The shape of the active site makes it specific to one set of substrates The substrates in an enzyme s active site change shape and conformation. The chemical bonds are altered in the substrates, promoting the reaction. The substrates change into a new form that no longer fits the active site, and so are released.
37 Enzyme Structure Enzymes have a pocket called an active site Reactants (substrates) bind to the active site Distinctive shape of active site is complementary and specific to the substrate Active site amino acids binds the substrate in a specific orientation Upon binding, the substrates and enzyme change shape and distort bonds to facilitate a reaction Products of the reaction leave the active site, leaving the enzyme ready for another catalysis
38
39 Control of Metabolic Reactions Metabolic pathways are controlled in several ways 1. Control of enzyme synthesis regulates availability 2. Some enzymes are inactive when created and must be turned on to be active 3. Small organic molecules can bind to enzymes and enhance/inhibit activity (allosteric regulation) 4. Adequate amounts of formed product inhibit enzyme activity (feedback inhibition)
40 Allosteric Regulation Allosteric regulation can increase or decrease enzyme activity. In allosteric regulation, an enzyme s activity is modified by a regulator molecule. The regulator molecule binds to a special regulatory site on the enzyme separate from the enzyme s active site. Binding of the regulator molecule modifies the active site on the enzyme, causing the enzyme to become more or less able to bind substrate. Thus, allosteric regulation can either promote or inhibit enzyme activity.
41
42 Competitive Inhibition Competitive inhibition can be temporary or permanent. Some regulatory molecules temporarily bind directly to an enzyme s active site, preventing the substrate molecules from binding. These molecules compete with the substrate for access to the active site, and control the enzyme by competitive inhibition.
43 Competetive Inhibition A competitive inhibitor molecule occupies the active site and blocks entry of the substrate Fig. 5-16
44 Feedback Inhibition
45 Drugs and Poisons Drugs and poisons often inhibit enzymes by competing with the natural substrate for the active site (competitive inhibition) Some inhibitors bind permanently to the enzyme
46 Environmental Conditions The three-dimensional structure of an enzyme (protein) is sensitive to environmental conditions Most enzymes function optimally only within a very narrow range of environmental conditions Enzyme structure is distorted and function is destroyed when ph is too high or low Salts in an enzyme s environment can also destroy function by altering structure Temperature also affects enzyme activity Low temperatures slow down molecular movement High temperatures cause enzyme shape to be altered, destroying function
47
48 6.1 What Is Photosynthesis? Life on earth depends on photosynthesis. Photosynthesis is the capturing and conversion of sunlight into chemical energy. Virtually all life depends directly or indirectly on the energy captured by plants and stored as sugars. Before photosynthesis, there was little oxygen on Earth, and therefore, no organisms that used oxygen. All present-day organisms that use oxygen as their respiratory gas depend upon photosynthesis to generate new oxygen.
49 6.1 What Is Photosynthesis? Photosynthesis converts carbon dioxide and water to glucose. The chemical reaction for photosynthesis: 6 CO H light energy C 6 H 12 O O 2 Plants, seaweeds, and single-celled organisms all show the basic aspects of photosynthesis.
50 6.1 What Is Photosynthesis? Plant photosynthesis takes place in leaves. Leaves are the main location of photosynthesis. Plants have thin leaves so sunlight can penetrate. Plant leaves have a large surface area to expose them to the sun. Plant leaves have pores to admit CO 2 and expel O 2, called stomata (singular, stoma).
51 6.1 What Is Photosynthesis? Leaf cells contain chloroplasts. Photosynthesis occurs in chloroplasts, in layers of cells called the mesophyll. The stroma contains sacs called thylakoids within which photosynthesis occurs. mesophyll cells (a) Leaves outer membrane inner membrane thylakoid stroma (b) vein Internal leaf structure chloroplasts stoma (c) Chloroplast in mesophyll cell
52 6.1 What Is Photosynthesis? Photosynthesis consists of light-dependent and light-independent (dark) reactions. These reactions occur at different locations in the chloroplast. The two types of reactions are linked by the energy-carrier molecules adenosine triphosphatase (ATP) and nicotinamide adenine dinucleotide phosphate (NADPH).
53 6.1 What Is Photosynthesis? Light-dependent reactions Occur in the membranes of the thylakoids Light is captured here and stored in ATP and NADPH. H 2 O is consumed and O 2 is given off.
54 6.1 What Is Photosynthesis? Light-independent reactions Enzymes in the stroma use ATP and NADPH produced by light-dependent reactions to make glucose and other molecules. CO 2 is consumed in the process. ATP and NADPH are converted to low-energy ADP and NADP +. These low-energy molecules are re-charged to ATP and NADPH when recycled in lightdependent reactions.
55 6.1 What Is Photosynthesis? An overview of photosynthesis: lightdependent and light-independent reactions H 2 O LIGHT-DEPENDENT REACTIONS O 2 (thylakoids) depleted carriers (ADP, NADP + ) energized carriers (ATP, NADPH) CO 2 LIGHT-INDEPENDENT REACTIONS (stroma) glucose Fig. 6-2
56 The Energy in Visible Light The sun radiates electromagnetic energy Visible light is radiation falling between nanometers of wavelength Packets of energy called photons have different energy levels depending on wavelength Short-wavelength photons (indigo, UV, x-rays) are very energetic Longer-wavelength photons have lower energies (red, infrared)
57 Light Captured by Pigments Action of light-capturing pigments Absorption of certain wavelengths light is trapped trapped light it is no longer visible Reflection of certain wavelengths light bounces back This light is visible on the same side as the light Transmission of certain wavelengths light passes through This light is visible on the opposite side from the light source
58 Light Captured by Pigments Absorbed light is converted to chemical energy and drives biological processes Common pigments found in chloroplasts: Chlorophyll a and b absorb violet, blue, and red light reflect green light (hence they appear green) Accessory pigments such as carotenoids Carotenoids absorb blue and green light reflect yellow, orange, or red (hence they appear yellow-orange)
59
60 Why Autumn Leaves Turn Color Both chlorophylls and carotenoids are present in leaves Chlorophyll breaks down before carotenoids in dying autumn leaves revealing yellow colors Red (anthocyanin) pigments are synthesized by some autumn leaves, producing red colors
61 6.2 How Is Light Energy Converted To Chemical Energy? Light is first captured by pigments in chloroplasts in light dependent reactions. Membranes of choroplast thylakoids contain pigments (light-absorbing molecules). Captured sunlight energy is stored as chemical energy in two carrier molecules in light-independent reactions Adenosine triphosphate (ATP) Nicotinamide adenine dinucleotide phosphate (NADPH)
62 6.2 How Is Light Energy Converted To Chemical Energy? The light-dependent reactions generate energy-carrier molecules. Light-dependent reactions take place in two photosystems (PSI & PSII) found in the thylakoid membranes. Each photosystem consists of an assemblage of proteins, chlorophyll, accessory pigment molecules, and a chain of electron-carriers.
63 6.2 How Is Light Energy Converted To Chemical Energy? Each photosystem consists of two major subsystems. A light-harvesting complex collects light energy and passes it on to a specific chlorophyll molecule called the reaction center. An electron transport system (ETS) transports energized electrons from one molecule to another.
64 6.2 How Is Light Energy Converted To Chemical Energy? Structures associated with the lightdependent reactions thylakoids chloroplast within thylakoid membrane PS II ETC reaction centers PS I ETC Fig. 6-4
65 6.2 How Is Light Energy Converted To Chemical Energy? Photosystem II generates ATP. Step 1: The light-harvesting complex passes light to the reaction center. Step 2: Electrons of the reaction center become energized. Step 3: The energized electrons jump to the ETS and jump from molecule to molecule, releasing energy at each step. Step 4: The released energy powers reactions that synthesize ATP.
66 6.2 How Is Light Energy Converted To Chemical Energy? Photosystem I generates NADPH. Step 5: The light-harvesting complex passes light to the reaction center. Step 6: Activated electrons from the reaction center are passed to the ETS and are replaced by electrons coming from the ETS of photosystem II. Step 7: Electrons jump from one molecule of the ETS to another, until they reach NADP +. Step 8: Each molecule of NADP + picks up two electrons, forming NADPH.
67 6.2 How Is Light Energy Converted To Chemical Energy? Step 9: The breakdown of H 2 O provides the replacement electrons to keep the process continuing, through the reaction: H 2 O ½ O 2 + 2H + + 2e The two electrons are donated to photosystem II. The hydrogen ions are used to convert NADP + to NADPH. Oxygen atoms combine to form a molecule of oxygen gas (O 2 ), which is given off to the atmosphere.
68 sunlight 7 e NADPH 8 energy level of electrons electron transport chain e 4 reaction center energy to drive ATP synthesis 6 e 5 photosystem I NADP + + H + within thylakoid membrane e photosystem II H 2 O 9 2 H + 1/2 O 2 Fig. 6-5
69
70 Maintaining Electron Flow Redux Electrons leaving PS II replaced when H 2 O is split: H 2 O ½O 2 + 2H + + 2e - Two electrons from water replace those lost when 2 photons boost 2 electrons out of PSII Two hydrogen ions used to form NADPH Oxygen atoms combine to form O 2
71 6.3 How Is Chemical Energy Stored in Glucose Molecules? The ATP and NADPH generated in lightdependent reactions are used in lightindependent reactions to make molecules for long-term storage. These reactions occur in the fluid stroma that surrounds the thalakoids, and do not require light. In the stroma, ATP and NADPH are used with CO 2 and H 2 O to synthesize the storage form of energy glucose.
72 6.3 How Is Chemical Energy Stored in Glucose Molecules? The C 3 cycle captures carbon dioxide. Step 1: CO 2 from air combines with a five-carbon sugar, ribulose biphosphate (RuBP), and H 2 O to form phosphoglyceric acid (PGA). Step 2: PGA receives energy input from ATP and NADPH to form glyceraldehyde-3-phosphate (G 3 P). Step 3: Two G 3 P molecules (three carbons each) combine to form one molecule of glucose (six carbons). Step 4: 10 G 3 P molecules powered by ATP are used to regenerate six molecules of RuBP to restart the cycle.
73 6.3 How Is Chemical Energy Stored in Glucose Molecules? The C 3 cycle of carbon fixation 1 Carbon fixation combines CO 2 with RuBP 6 CO 2 C 6 4 RuBP synthesis uses energy and 10 G3Ps C C C C C RuBP C 3 cycle 12 C C C PGA 2 G3P synthesis uses energy ATP ADP 6 6 ADP ATP 12 C C C G3P 12 NADPH 12 NADP G3Ps available for synthesis of glucose C C C C C C glucose Fig. 6-6
74 6.4 What Is The Relationship Between Light-Dependent And Light-Independent Reactions? Photosynthesis includes two separate sets of reactions (light-dependent and lightindependent) that are closely linked.
75 Light-Dependent And Light-Independent Reactions Light-dependent reactions capture solar energy; light-independent reactions use captured energy to make glucose. Energy-carrier molecules provide the link between these two sets of reactions. Light-dependent reactions of thylakoids use light to charge ADP and NADP + to make ATP and NADPH. ATP and NADPH move to the stroma where they provide energy to synthesize glucose.
76 Light-Dependent And Light-Independent Reactions Two sets of reactions are connected in photosynthesis. energy from sunlight O 2 CO 2 ATP NADPH Light-dependent reactions occur in thylakoids ADP Lightindependent reactions (C 3 cycle) occur in stroma H 2 O NADP + chloroplast glucose Fig. 6-7
77 6.5 How Does the Need To Conserve Water Affect Photosynthesis? Photosynthesis requires carbon dioxide; porous leaves would allow the entry of CO 2, but would also result in the loss of H 2 O. Evolution of the stomata resulted in pores that could open, letting in CO 2, but also to close, to restrict H 2 O losses. Closing stomata to prevent H 2 O loss also restricts the release of O 2, produced by photosynthesis, to the atmosphere.
78 Water Conservation and Photosynthesis When stomata are closed to conserve water, wasteful photorespiration occurs. In hot, dry conditions, plant stomata are closed much of the time, reducing internal CO 2 concentrations and increasing O 2 concentrations. Increased O 2 reacts with RuBP (instead of CO 2 ) in a process called photorespiration. Photorespiration does not produce useful cellular energy, and prevents the C 3 synthesis of glucose.
79 Water Conservation and Photosynthesis Alternative pathways reduce photorespiration. Some plants have evolved metabolic pathways that reduce photorespiration. These plants can produce glucose even under hot and dry conditions. The two most important alternative pathways are: The C 4 pathway Crassulacean acid metabolism (CAM)
80 6.5 How Does the Need To Conserve Water Affect Photosynthesis? Plants capture carbon and synthesize glucose in different places. Typical plants (C 3 plants) fix carbon and synthesize glucose as a result of the C 3 cycle in mesophyll cells.
81 6.5 How Does the Need To Conserve Water Affect Photosynthesis The C 4 pathway includes two stages that take place in different parts of the leaf. In the first stage, CO 2 is captured in mesophyll cells in the presence of high O 2, producing a four-carbon molecule. The four-carbon molecule is transferred from mesophyll cells to the bundle-sheath cells where the four-carbon molecule is broken down to CO 2.
82 6.5 How Does the Need to Conserve Water Affect Photosynthesis C 4 plants capture carbon and synthesize glucose in different places. In the sheath-bundle cells, the released CO 2 proceeds to the second stage of the pathway the regular C 3 cycle without excess O 2 interfering with the process. Many C 4 plant species are grasses, and are agriculturally important species such as sugar cane, corn, and sorghum.
83 C3 vs C4 Carbon Capture (a) C3 plant In a C 3 plant, carbon capture and glucose synthesis are in mesophyll cells bundlesheath cell mesophyll cell In a C 4 plant, carbon capture is in mesophyll cells, but glucose is synthesized in bundle-sheath cells (b) C 4 plant bundlesheath cell mesophyll cell
84 6.5 How Does the Need to Conserve Water Affect Photosynthesis CAM plants capture carbon and synthesize glucose at different times. In CAM plants, photorespiration is reduced by fixing carbon in two stages that take place in the same cells but at different times of the day. At night, with open stoma, reactions in mesophyll cells incorporate CO 2 into the organic acid molecules that are stored in vacuoles. During the day, with stoma closed, the organic acids release their CO 2 and the regular C 3 cycle proceeds.
85 6.5 How Does the Need to Conserve Water Affect Photosynthesis Two ways to reduce photorespiration in different places and times mesophyll cell bundle-sheath cell CO 2 CO 2 night C C C C 1 CO 2 is incorporated into four-carbon molecules C C C C CO 2 2 Four-carbon CO 2 day molecules release CO 2 to C 3 C the C 3 cycle 3 cycle cycle mesophyll cell C 4 CAM (a) Steps in separate places (b) Steps at separate times Fig. 6-10
86 7.1 What Is The Source Of A Cell s Energy? The energy for cellular activities is stored in bonds of molecules such as carbohydrates and fats. Stored energy is transferred to the bonds of energy-carrier molecules including ATP. Glucose is a key energy-storage molecule. Nearly all cells metabolize glucose for energy Glucose metabolism is fairly simple Other organic molecules are converted to glucose for energy harvesting
87 Source Of Cellular Energy Photosynthesis is the ultimate source of cellular energy. Photosynthetic cells capture and store sunlight energy as glucose This energy is later used by cells. These cells can be the photosynthetic organisms, or can be other organisms that consume photosynthetic organisms.
88 Source Of Cellular Energy Glucose metabolism and photosynthesis are complementary processes. The products of each reaction provide reactants for the other. The symmetry is visible in the equations that describe each process. Photosynthesis: 6 CO 2 + 6H 2 O + sunlight energy C 6 H 12 O O 2 Glucose metabolism: C 6 H 12 O 6 + 6O 2 6 CO H ATP + heat energy
89 7.2 How Do Cells Harvest Energy From Glucose? Glucose metabolism occurs in stages 1st stage is glycolysis. 2nd stage, cellular respiration Under anaerobic (no O 2 ) conditions the 2nd stage of glucose metabolism is fermentation. Flash
90 C C C C C C glucose (cytoplasmic fluid) Glycolysis 2 ATP 2 C C C pyruvate Fermentation 2 C C C lactate or 2 C C + 2 C ethanol CO 2 Cellular respiration C 2 CO 2 C C 2 acetyl CoA 4 C CO 2 Krebs cycle 2 ATP electron carriers H 2 O intermembrane compartment Electron transport chain (mitochondrion) 32 or 34 ATP O 2 Fig. 7-1
91 Overview of Glucose Breakdown Stage 1: Glycolysis. Glycolysis occurs in the cytoplasm of cells. Does not require oxygen Glucose (6 C sugar) is split into two pyruvate molecules (3 C each). Yields two molecules of ATP per molecule of glucose.
92 Overview of Glucose Breakdown Stage 2: Cellular respiration Occurs in mitochondria (in eukaryotes) Requires oxygen (aerobic) Breaks down pyruvate into CO 2 and H 2 0 Produces an additional 32 or 34 ATP molecules, depending on the cell type
93 Overview of Glucose Breakdown If oxygen is absent fermentation occurs Pyruvate remains in the cytoplasm Pyruvate may be converted into either lactate, or ethanol and CO 2 No ATP is produced If oxygen is present cellular respiration occurs
94
95 7.3 What Happens During Glycolysis? Glycolysis splits one molecule of glucose into two molecules of pyruvate. During glycolysis, one molecule of glucose yields two ATP and two molecules of nicotinamide adenine dinucleotide (NADH) an electron carrier. Glycolysis involves two major steps: Glucose activation Energy harvest
96 Glycolysis 1. Glucose activation phase Glucose molecule converted into the highly reactive fructose bisphosphate Two enzyme-catalyzed reactions drive the conversion Yields 2 ATP molecules
97 Glycolysis Two ATP power the phosphorylation of glucose to form fructose bisphosphate.
98 Glycolysis 2. Energy harvesting phase Fructose bisphosphate is split into two three-carbon molecules of glyceraldehyde 3 phosphate (G3P) Each G3P molecule is converted into pyruvate, generating two ATPs per conversion (4 total ATPs) Two ATPs were used to activate the glucose molecule so there is a net gain of two ATPs per glucose molecule As each G3P is converted to pyruvate, two highenergy electrons and a hydrogen ion are added to an empty electron-carrier NAD+ to make the highenergy electron-carrier molecule NADH Because two G3P molecules are produced per glucose molecule, two NADH carrier molecules are formed
99 Glycolysis Summary Energy harvest from glycolysis : Each molecule of glucose is broken down to two molecules of pyruvate A net of two ATP molecules and two NADH (high-energy electron carriers) are formed
100 7.4 What Happens During Cellular Respiration? Cellular respiration is the second stage of glucose metabolism Only occurs in the presence of O 2 (aerobic). Occurs in the mitochondria. Converts pyruvate to CO 2 and H 2 O. Large amounts of ATP are produced
101 Cellular Respiration Steps of Cellular Respiration 1. Two molecules of pyruvate are transported into the matrix of a mitochondrion. 2. Each pyruvate is split into CO 2 and acetyl CoA, which enters the Krebs cycle. The Krebs cycle produces one ATP from each pyruvate (total 2 per glucose) and donates electrons to NADH and FADH NADH and FADH 2 donate energized electrons to the electron transport chain of the inner membrane.
102 Cellular Respiration Steps of cellular respiration (continued) 4. In the electron transport chain, electron energy is used to transport protons (H + ) from the matrix to the intermembrane compartment. 5. Electrons combine with O 2 and H + to form H 2 O. 6. Hydrogen ions in the intermembrane compartment diffuse across the inner membrane down their concentration gradient. 7. The flow of ions into the matrix provides the energy to produce ATP from ADP. 8. ATP moves out of mitochondrion into the cytoplasm.
103 intermembrane compartment matrix outer membrane inner mitochondrion membrane glucose cristae Glycolysis 2 pyruvate (cytoplasmic fluid) electron transport chain outer membrane intermembrane compartment inner membrane ATP ATP-synthesizing enzyme H + H + 8 H + H + H + 4 H + H + 2e 6 ADP 2 H + H + 1/2 O 2 5 H 2 O 2e ATP ATP CO 2 energized electron carriers: NADH, FADH 2 depleted carriers: NAD +, FAD coenzyme A 2 acetyl CoA Krebs cycle CO 2 (matrix) Fig. 7-3
104 Cellular Respiration The Krebs cycle breaks down pyruvate in the mitochondrial matrix. Pyruvate produced by glycolysis reaches the matrix and reacts with coenzyme A, forming acetyl CoA. During this reaction, two electrons and a H + are transferred to NAD + to form NADH. Acetyl CoA enters the Krebs cycle and produces one ATP, one FADH 2, and three NADH.
105 Cellular Respiration The reactions in the mitochondrial matrix 1 Formation of acetyl CoA 3 NAD + 3 NADH FAD coenzyme A C CO 2 coenzyme A FADH 2 C C C pyruvate C C CoA acetyl CoA 2 Krebs cycle 2 C CO 2 NAD + NADH ADP ATP Fig. 7-4
106 Cellular Respiration Energetic electrons are carried to the electron transport chain. 1. Energized carriers deposit their electrons in the electron transport chains (ETC) in the inner mitochondrial membrane. 2. Electrons in the ETC move from one molecule to the next, transferring energy that is used to pump H + out of the matrix and into the intermembrane compartment. 3. At the end of the ETC, oxygen atoms combine with two H + and two depleted electrons to form H 2 O.
107 Cellular Respiration Energetic electrons are carried to the electron transport chain (continued). 4. Oxygen accepts electrons after they have passed through the ETC and given up most of their energy. 5. If O 2 is not present, electrons accumulate in the ETC, H + pumping out of the matrix stops, and cellular respiration ceases.
108 Cellular Respiration The electron transport chain in the mitochondrial matrix (matrix) FADH 2 3 NADH 2e NAD + 1 1/2 O 2 + 2H + 2e FAD H 2 O electron carriers (inner membrane) H + H + H + 2 energy to drive ATP synthesis (intermembrane compartment) Fig. 7-5
109 Cellular Respiration Energy from a hydrogen-ion gradient is used to produce ATP. Hydrogen ions accumulate in the intermembrane compartment and diffuse back into the matrix. The energy released when hydrogen ions move down their concentration gradient is used to make ATP in a process called chemiosmosis. During chemiosmosis, 32 to 34 molecules of ATP are produced from each molecule of glucose. This ATP is transported from the matrix to the cytoplasm, where it is used to power metabolic reactions.
110 7.5 What Happens During Fermentation? Under anaerobic conditions (no O 2 ), glucose cannot be metabolized by cellular respiration; instead, fermentation takes place. Unlike cellular respiration, fermentation generates no ATP, but instead, regenerates NAD + that is used to generate ATP from glycolysis. Flash
111 Fermentation In fermentation, pyruvate acts as an electron acceptor from the NADH produced during glycolysis. When pyruvate accepts electrons from NADH, it recycles the NAD + so that more glucose can be converted to pyruvate, generating a small amount of ATP in the process. When no O 2 is present, glycolysis becomes the main source of ATP and NADH production.
112 Fermentation There are two types of fermentation: one converts pyruvate to ethanol and CO 2, and the other converts pyruvate to lactate. Alcoholic fermentation is the primary mode of metabolism in many microorganisms. The reactions use hydrogen ions and electrons from NADH, thereby regenerating NAD +. Alcoholic fermentation is responsible for the production of many economic products, such as wine, beer, and bread.
113 Fermentation Glycolysis followed by alcoholic fermentation NAD + NADH NADH NAD + C C C C C C 2 C C C 2 C C + 2 C (glycolysis) (fermentation) glucose pyruvate ethanol CO 2 2 ADP 2 ATP Fig. 7-6
114 Fermentation Other cells ferment pyruvate to lactate, and include microorganisms that produce yogurt, sour cream, and cheese. Lactate fermentation also occurs in aerobic organisms when cells are temporarily deprived of oxygen, such as muscle cells during vigorous exercise. These muscle cells ferment pyruvate to lactate, which uses H + and electrons from NADH to regenerate NAD +.
115 Fermentation Glycolysis followed by lactate fermentation NAD + NADH NADH NAD + C C C C C C 2 C C C 2 C C C (glycolysis) (fermentation) glucose pyruvate lactate 2 ADP 2 ATP Fig. 7-8
116 Fermentation Fermentation limits human muscle performance. During a sprint muscles use more ATP than can be delivered by cellular respiration because O 2 cannot be delivered to muscles fast enough. Glycolysis can deliver a small amount of ATP to rapidly contracting muscles, but toxic buildup of lactate will occur. Long distance runners must therefore pace themselves so that cellular respiration can power their muscles for most of the race.
117 Fermentation Some microbes ferment pyruvate to other acids (as seen in making of cheese, yogurt, sour cream) Some microbes perform fermentation exclusively (instead of aerobic respiration) Yeast cells perform alcoholic fermentation
118 Summary of Glucose Breakdown
119 Influence on How Organisms Function Metabolic processes in cells are heavily dependent on ATP generation (cyanide kills by preventing this) Muscle cells switch between fermentation and aerobic cell respiration depending on O 2 availability
Chapter 6. Capturing Solar Energy: Photosynthesis. Lectures by Gregory Ahearn. University of North Florida. Copyright 2009 Pearson Education, Inc.
 Chapter 6 Capturing Solar Energy: Photosynthesis Lectures by Gregory Ahearn University of North Florida Copyright 2009 Pearson Education, Inc. 6.1 What Is Photosynthesis? Life on earth depends on photosynthesis.
Chapter 6 Capturing Solar Energy: Photosynthesis Lectures by Gregory Ahearn University of North Florida Copyright 2009 Pearson Education, Inc. 6.1 What Is Photosynthesis? Life on earth depends on photosynthesis.
Chapter 5. Energy Flow in the Life of a Cell
 Chapter 5 Energy Flow in the Life of a Cell Including some materials from lectures by Gregory Ahearn University of North Florida Ammended by John Crocker Copyright 2009 Pearson Education, Inc.. Review
Chapter 5 Energy Flow in the Life of a Cell Including some materials from lectures by Gregory Ahearn University of North Florida Ammended by John Crocker Copyright 2009 Pearson Education, Inc.. Review
Photosynthesis and Cellular Respiration
 Photosynthesis and Cellular Respiration Photosynthesis and Cellular Respiration All cellular activities require energy. Directly or indirectly nearly all energy for life comes from the sun. Autotrophs:
Photosynthesis and Cellular Respiration Photosynthesis and Cellular Respiration All cellular activities require energy. Directly or indirectly nearly all energy for life comes from the sun. Autotrophs:
2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of October
 Name: Class: _ Date: _ 2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of 19-23 October Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which
Name: Class: _ Date: _ 2015 AP Biology PRETEST Unit 3: Cellular Energetics Week of 19-23 October Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which
Biology: Life on Earth
 Biology: Life on Earth Eighth Edition Lecture for Chapter 7 Capturing Solar Energy: Photosynthesis Chapter 7 Outline 7.1 What Is Photosynthesis? p. 118 7.2 Light-Dependent Reactions: How Is Light Energy
Biology: Life on Earth Eighth Edition Lecture for Chapter 7 Capturing Solar Energy: Photosynthesis Chapter 7 Outline 7.1 What Is Photosynthesis? p. 118 7.2 Light-Dependent Reactions: How Is Light Energy
Energy in the World of Life
 Cellular Energy Energy in the World of Life Sustaining life s organization requires ongoing energy inputs Assembly of the molecules of life starts with energy input into living cells Energy Conversion
Cellular Energy Energy in the World of Life Sustaining life s organization requires ongoing energy inputs Assembly of the molecules of life starts with energy input into living cells Energy Conversion
Chapter 5. Table of Contents. Section 1 Energy and Living Things. Section 2 Photosynthesis. Section 3 Cellular Respiration
 Photosynthesis and Cellular Respiration Table of Contents Section 1 Energy and Living Things Section 2 Photosynthesis Section 3 Cellular Respiration Section 1 Energy and Living Things Objectives Analyze
Photosynthesis and Cellular Respiration Table of Contents Section 1 Energy and Living Things Section 2 Photosynthesis Section 3 Cellular Respiration Section 1 Energy and Living Things Objectives Analyze
Photosynthesis and Cellular Respiration Unit
 Photosynthesis and Cellular Respiration Unit All cellular activities require energy. Directly or indirectly nearly all energy for life comes from the sun. Autotrophs: organisms that can make their own
Photosynthesis and Cellular Respiration Unit All cellular activities require energy. Directly or indirectly nearly all energy for life comes from the sun. Autotrophs: organisms that can make their own
METABOLISM. What is metabolism? Categories of metabolic reactions. Total of all chemical reactions occurring within the body
 METABOLISM What is metabolism? METABOLISM Total of all chemical reactions occurring within the body Categories of metabolic reactions Catabolic reactions Degradation pathways Anabolic reactions Synthesis
METABOLISM What is metabolism? METABOLISM Total of all chemical reactions occurring within the body Categories of metabolic reactions Catabolic reactions Degradation pathways Anabolic reactions Synthesis
6CO 2 + 6H 2 O C 6 H 12 O 6 + 6O 2. sun. Occurs in chloroplasts ATP. enzymes CO 2 O 2 H 2 O. sugars
 4.2 8.2 Overview Photosynthesis: of Photosynthesis An Overview Photosynthesis process by which plants make food using energy from the sun Plants are autotrophs that make their own source of chemical energy.
4.2 8.2 Overview Photosynthesis: of Photosynthesis An Overview Photosynthesis process by which plants make food using energy from the sun Plants are autotrophs that make their own source of chemical energy.
Cell Energy Notes ATP THE ENDOSYMBIOTIC THEORY. CELL ENERGY Cells usable source of is called ATP stands for. Name Per
 Cell Energy Notes Name Per THE ENDOSYMBIOTIC THEORY The Endosymbiotic theory is the idea that a long time ago, engulfed other prokaryotic cells by. This resulted in the first First proposed by Explains
Cell Energy Notes Name Per THE ENDOSYMBIOTIC THEORY The Endosymbiotic theory is the idea that a long time ago, engulfed other prokaryotic cells by. This resulted in the first First proposed by Explains
Unit 5 Cellular Energy
 Unit 5 Cellular Energy I. Enzymes (159) 1.Are CATALYSTS: Speed up chemical reactions that would otherwise happen too slowly to support life. Catalysts DO NOT make reactions happen that couldn t happen
Unit 5 Cellular Energy I. Enzymes (159) 1.Are CATALYSTS: Speed up chemical reactions that would otherwise happen too slowly to support life. Catalysts DO NOT make reactions happen that couldn t happen
Lecture 9: Photosynthesis
 Lecture 9: Photosynthesis I. Characteristics of Light A. Light is composed of particles that travel as waves 1. Comprises a small part of the electromagnetic spectrum B. Radiation varies in wavelength
Lecture 9: Photosynthesis I. Characteristics of Light A. Light is composed of particles that travel as waves 1. Comprises a small part of the electromagnetic spectrum B. Radiation varies in wavelength
Energy Metabolism exergonic reaction endergonic reaction Energy of activation
 Metabolism Energy Living things require energy to grow and reproduce Most energy used originates from the sun Plants capture 2% of solar energy Some captured energy is lost as metabolic heat All energy
Metabolism Energy Living things require energy to grow and reproduce Most energy used originates from the sun Plants capture 2% of solar energy Some captured energy is lost as metabolic heat All energy
4.1 Chemical Energy and ATP. KEY CONCEPT All cells need chemical energy.
 4.1 Chemical Energy and ATP KEY CONCEPT All cells need chemical energy. 4.1 Chemical Energy and ATP The chemical energy used for most cell processes is carried by ATP. Molecules in food store chemical
4.1 Chemical Energy and ATP KEY CONCEPT All cells need chemical energy. 4.1 Chemical Energy and ATP The chemical energy used for most cell processes is carried by ATP. Molecules in food store chemical
The Life of a Cell. The Chemistry of Life. A View of the Cell. Cellular Transport and the Cell Cycle. Energy in a Cell
 The Life of a Cell The Chemistry of Life A View of the Cell Cellular Transport and the Cell Cycle Energy in a Cell Chapter 9 Energy in a Cell 9.1: The Need for Energy 9.1: Section Check 9.2: Photosynthesis:
The Life of a Cell The Chemistry of Life A View of the Cell Cellular Transport and the Cell Cycle Energy in a Cell Chapter 9 Energy in a Cell 9.1: The Need for Energy 9.1: Section Check 9.2: Photosynthesis:
Ch. 6 & 7 Photosynthesis & Cellular Respiration
 Ch. 6 & 7 Photosynthesis & Cellular Respiration 6.1 Energy Reactions The Cycle of Energy Sun CO 2 H 2 O Photosynthesis (energy stored) Cellular Respiration (energy released) O 2 Glucose Obtaining Energy
Ch. 6 & 7 Photosynthesis & Cellular Respiration 6.1 Energy Reactions The Cycle of Energy Sun CO 2 H 2 O Photosynthesis (energy stored) Cellular Respiration (energy released) O 2 Glucose Obtaining Energy
2.A.2- Capture and Storage of Free Energy
 2.A.2- Capture and Storage of Free Energy Big Idea 2: Biological systems utilize free energy and molecular building blocks to grow, to reproduce, and to maintain dynamic homeostasis. EU 2.A- Growth, reproduction
2.A.2- Capture and Storage of Free Energy Big Idea 2: Biological systems utilize free energy and molecular building blocks to grow, to reproduce, and to maintain dynamic homeostasis. EU 2.A- Growth, reproduction
Photosynthesis
 Student Expectations: Cellular Energy Understand that cellular energy is temporarily stored in the nucleotide ATP (adenosine triphosphate) Describe how energy is released by ATP When the outer phosphate
Student Expectations: Cellular Energy Understand that cellular energy is temporarily stored in the nucleotide ATP (adenosine triphosphate) Describe how energy is released by ATP When the outer phosphate
Cellular Energetics. Photosynthesis, Cellular Respiration and Fermentation
 Cellular Energetics Photosynthesis, Cellular Respiration and Fermentation TEKS B.4 Science concepts. The student knows that cells are the basic structures of all living things with specialized parts that
Cellular Energetics Photosynthesis, Cellular Respiration and Fermentation TEKS B.4 Science concepts. The student knows that cells are the basic structures of all living things with specialized parts that
Cellular Energy. How Organisms Obtain Energy Section 2: Photosynthesis Section 3: Cellular Respiration. Click on a lesson name to select.
 Section 1: How Organisms Obtain Energy Section 2: Photosynthesis Section 3: Cellular Respiration Click on a lesson name to select. Section 1 How Organisms Obtain Energy Transformation of Energy Energy
Section 1: How Organisms Obtain Energy Section 2: Photosynthesis Section 3: Cellular Respiration Click on a lesson name to select. Section 1 How Organisms Obtain Energy Transformation of Energy Energy
Cellular Respiration: Harvesting Chemical Energy. 9.1 Catabolic pathways yield energy by oxidizing organic fuels
 Cellular Respiration: Harvesting Chemical Energy 9.1 Catabolic pathways yield energy by oxidizing organic fuels 9.2 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate 9.3 The citric acid
Cellular Respiration: Harvesting Chemical Energy 9.1 Catabolic pathways yield energy by oxidizing organic fuels 9.2 Glycolysis harvests chemical energy by oxidizing glucose to pyruvate 9.3 The citric acid
Cellular Energy. The cell will store energy in molecules like sugars and ATP
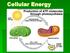 Cellular Energy Cellular Energy The cell will store energy in molecules like sugars and ATP Most cells have small stores of ATP that only last a few seconds, but cannot store energy there long-term. Cells
Cellular Energy Cellular Energy The cell will store energy in molecules like sugars and ATP Most cells have small stores of ATP that only last a few seconds, but cannot store energy there long-term. Cells
The summary equation of photosynthesis including the source and fate of the reactants and products. How leaf and chloroplast anatomy relates to
 1 The summary equation of photosynthesis including the source and fate of the reactants and products. How leaf and chloroplast anatomy relates to photosynthesis. How photosystems convert solar energy to
1 The summary equation of photosynthesis including the source and fate of the reactants and products. How leaf and chloroplast anatomy relates to photosynthesis. How photosystems convert solar energy to
Energy Exchanges Exam: What to Study
 Energy Exchanges Exam: What to Study Here s what you will need to make sure you understand in order to prepare for our exam: Free Energy Conceptual understanding of free energy as available energy in a
Energy Exchanges Exam: What to Study Here s what you will need to make sure you understand in order to prepare for our exam: Free Energy Conceptual understanding of free energy as available energy in a
Photosynthesis and Cellular Respiration Practice Test Name
 Photosynthesis and Cellular Respiration Practice Test Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which H+ has just passed through the
Photosynthesis and Cellular Respiration Practice Test Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which H+ has just passed through the
Respiration and Photosynthesis. The Ying and Yang of Life.
 Respiration and Photosynthesis The Ying and Yang of Life. Why? You ve always been told that you must eat and breathe. Why? In this unit we will attempt to answer those questions. 1 st Law of Thermodynamics
Respiration and Photosynthesis The Ying and Yang of Life. Why? You ve always been told that you must eat and breathe. Why? In this unit we will attempt to answer those questions. 1 st Law of Thermodynamics
PHOTOSYNTHESIS STARTS WITH
 Name Date Period PHOTOSYNTHESIS STARTS WITH 1. Molecules that collect light energy are called _P. 2. Chlorophyll a and b absorb _B -_V and _R wavelengths of light best. 3. _C is the main light absorbing
Name Date Period PHOTOSYNTHESIS STARTS WITH 1. Molecules that collect light energy are called _P. 2. Chlorophyll a and b absorb _B -_V and _R wavelengths of light best. 3. _C is the main light absorbing
Ev e ry living c e l l needs a source of
 12 Photosynthesis and Cellular Respiration Ev e ry living c e l l needs a source of energy. Without energy, metabolism all of the chemical reactions that occur within cells will not occur. In this activity,
12 Photosynthesis and Cellular Respiration Ev e ry living c e l l needs a source of energy. Without energy, metabolism all of the chemical reactions that occur within cells will not occur. In this activity,
Photosynthesis and Cellular Respiration
 Photosynthesis and Cellular Respiration Outline I. Energy and Carbon Cycle II. Photosynthesis A. Introduction B. Reactions II. Cellular Respiration A. Introduction B. Reactions Carbon Cycle All organisms
Photosynthesis and Cellular Respiration Outline I. Energy and Carbon Cycle II. Photosynthesis A. Introduction B. Reactions II. Cellular Respiration A. Introduction B. Reactions Carbon Cycle All organisms
What Is Energy? Energy is the capacity to do work. First Law of Thermodynamics. Types of energy
 What Is Energy? Energy is the capacity to do work. Synthesizing molecules Moving objects Generating heat and light Types of Kinetic: of movement otential: stored First Law of Thermodynamics Energy cannot
What Is Energy? Energy is the capacity to do work. Synthesizing molecules Moving objects Generating heat and light Types of Kinetic: of movement otential: stored First Law of Thermodynamics Energy cannot
Energy Conversions. Photosynthesis. Plants. Chloroplasts. Plant Pigments 10/13/2014. Chapter 10 Pg
 Energy Conversions Photosynthesis Chapter 10 Pg. 184 205 Life on Earth is solar-powered by autotrophs Autotrophs make their own food and have no need to consume other organisms. They are the ultimate source
Energy Conversions Photosynthesis Chapter 10 Pg. 184 205 Life on Earth is solar-powered by autotrophs Autotrophs make their own food and have no need to consume other organisms. They are the ultimate source
Chapter 5. The Chloroplast. 5.1 Matter and Energy Pathways in Living Systems. Photosynthesis & Cellular Respiration
 Chapter 5 Photosynthesis & Cellular Respiration 5.1 Matter and Energy Pathways in Living Systems Both cellular respiration and photosynthesis are examples of biological processes that involve matter &
Chapter 5 Photosynthesis & Cellular Respiration 5.1 Matter and Energy Pathways in Living Systems Both cellular respiration and photosynthesis are examples of biological processes that involve matter &
CP Biology Unit 5 Cell Energy Study Guide. Electron Carriers Electron Transport Chain Fermentation Glycolysis Krebs cycle Light-Dependent Reactions
 Name: KEY CP Biology Unit 5 Cell Energy Study Guide Vocabulary to know: ATP ADP Aerobic Anaerobic ATP Synthases Cellular Respiration Chlorophyll Chloroplast Electron Carriers Electron Transport Chain Fermentation
Name: KEY CP Biology Unit 5 Cell Energy Study Guide Vocabulary to know: ATP ADP Aerobic Anaerobic ATP Synthases Cellular Respiration Chlorophyll Chloroplast Electron Carriers Electron Transport Chain Fermentation
Chapter 10 Photosynthesis
 Chapter 10 Photosynthesis Overview: The Process That Feeds the Biosphere Photosynthesis is the process that converts solar energy into chemical energy Photosynthesis occurs in plants, algae, certain other
Chapter 10 Photosynthesis Overview: The Process That Feeds the Biosphere Photosynthesis is the process that converts solar energy into chemical energy Photosynthesis occurs in plants, algae, certain other
AP Biology. Chloroplasts: sites of photosynthesis in plants
 The summary equation of photosynthesis including the source and fate of the reactants and products. How leaf and chloroplast anatomy relates to photosynthesis. How photosystems convert solar energy to
The summary equation of photosynthesis including the source and fate of the reactants and products. How leaf and chloroplast anatomy relates to photosynthesis. How photosystems convert solar energy to
Unit 3: Cell Energy Guided Notes
 Enzymes Unit 3: Cell Energy Guided Notes 1 We get energy from the food we eat by breaking apart the chemical bonds where food is stored. energy is in the bonds, energy is the energy we use to do things.
Enzymes Unit 3: Cell Energy Guided Notes 1 We get energy from the food we eat by breaking apart the chemical bonds where food is stored. energy is in the bonds, energy is the energy we use to do things.
Photosynthesis. Nearly all of the usable energy on this planet came, at one time or another, from the sun by the process of photosynthesis
 Photosynthesis Nearly all of the usable energy on this planet came, at one time or another, from the sun by the process of photosynthesis Photosynthesis 6CO 2 + 12H 2 O C 6 H 12 O 6 + 6O 2 + 6H 2 O Pigments
Photosynthesis Nearly all of the usable energy on this planet came, at one time or another, from the sun by the process of photosynthesis Photosynthesis 6CO 2 + 12H 2 O C 6 H 12 O 6 + 6O 2 + 6H 2 O Pigments
AN OVERVIEW OF PHOTOSYNTHESIS. Copyright 2009 Pearson Education, Inc.
 AN OVERVIEW OF PHOTOSYNTHESIS Copyright 2009 Pearson Education, Inc. Introduction: Plant Power Plants use water and atmospheric carbon dioxide to produce a simple sugar and liberate oxygen Earth s plants
AN OVERVIEW OF PHOTOSYNTHESIS Copyright 2009 Pearson Education, Inc. Introduction: Plant Power Plants use water and atmospheric carbon dioxide to produce a simple sugar and liberate oxygen Earth s plants
Cell Energy: The Big Picture. So, What Exactly is ATP. Adenosine Triphosphate. Your turn to Practice converting ATP to ADP:
 Understanding How Living Things Obtain and Use Energy. Cell Energy: The Big Picture Most Autotrophs produce food (sugar) using light energy during Photosynthesis. Then, both Autotrophs and Heterotroph
Understanding How Living Things Obtain and Use Energy. Cell Energy: The Big Picture Most Autotrophs produce food (sugar) using light energy during Photosynthesis. Then, both Autotrophs and Heterotroph
Chapter 10. Photosynthesis
 Chapter 10 Photosynthesis Overview: The Process That Feeds the Biosphere Photosynthesis is the process that converts solar energy into chemical energy Directly or indirectly, photosynthesis nourishes almost
Chapter 10 Photosynthesis Overview: The Process That Feeds the Biosphere Photosynthesis is the process that converts solar energy into chemical energy Directly or indirectly, photosynthesis nourishes almost
Chapter 4: Cellular Metabolism (Sections 1,3,5,6) KEY CONCEPT All cells need chemical energy.
 KEY CONCEPT All cells need chemical energy. ! The chemical energy used for most cell processes is carried by ATP. Molecules in food store chemical energy in their bonds. Starch molecule Glucose molecule
KEY CONCEPT All cells need chemical energy. ! The chemical energy used for most cell processes is carried by ATP. Molecules in food store chemical energy in their bonds. Starch molecule Glucose molecule
Chapter Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow,
 Chapter 6 6.1 Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow, repair, reproduce, etc. 2. Kinetic energy is energy of motion;
Chapter 6 6.1 Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow, repair, reproduce, etc. 2. Kinetic energy is energy of motion;
Respiration and Photosynthesis
 Respiration and Photosynthesis Cellular Respiration Glycolysis The Krebs Cycle Electron Transport Chains Anabolic Pathway Photosynthesis Calvin Cycle Flow of Energy Energy is needed to support all forms
Respiration and Photosynthesis Cellular Respiration Glycolysis The Krebs Cycle Electron Transport Chains Anabolic Pathway Photosynthesis Calvin Cycle Flow of Energy Energy is needed to support all forms
Photosynthesis in Detail. 3/19/2014 Averett
 Photosynthesis in Detail 1 In photosynthesis many chemical reactions, enzymes and ions work together in a precise order. Enzymes Biological catalyst Substance that initiates or speeds up the rate of a
Photosynthesis in Detail 1 In photosynthesis many chemical reactions, enzymes and ions work together in a precise order. Enzymes Biological catalyst Substance that initiates or speeds up the rate of a
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy
 Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Lecture Series 9 Cellular Pathways That Harvest Chemical Energy Reading Assignments Review Chapter 3 Energy, Catalysis, & Biosynthesis Read Chapter 13 How Cells obtain Energy from Food Read Chapter 14
Chapter 7: Photosynthesis
 Chapter 7: Photosynthesis Electromagnetic Spectrum Shortest wavelength Longest wavelength Gamma rays X-rays UV radiation Visible light Infrared radiation Microwaves Radio waves Photons Packets of light
Chapter 7: Photosynthesis Electromagnetic Spectrum Shortest wavelength Longest wavelength Gamma rays X-rays UV radiation Visible light Infrared radiation Microwaves Radio waves Photons Packets of light
pigments AP BIOLOGY PHOTOSYNTHESIS Chapter 10 Light Reactions Visible light is part of electromagnetic spectrum
 AP BIOLOGY PHOTOSYNTHESIS Chapter 10 Light Reactions http://vilenski.org/science/safari/cellstructure/chloroplasts.html Sunlight is made up of many different wavelengths of light Your eyes see different
AP BIOLOGY PHOTOSYNTHESIS Chapter 10 Light Reactions http://vilenski.org/science/safari/cellstructure/chloroplasts.html Sunlight is made up of many different wavelengths of light Your eyes see different
Photosynthesis (Outline)
 Photosynthesis (Outline) 1. Overview of photosynthesis 2. Producers, consumers, and decomposers of the ecosystem (source of carbon and energy) 3. Plant structures: organ, tissue, cells, sub-cellular organelle,
Photosynthesis (Outline) 1. Overview of photosynthesis 2. Producers, consumers, and decomposers of the ecosystem (source of carbon and energy) 3. Plant structures: organ, tissue, cells, sub-cellular organelle,
Photosynthesis. Dr. Bertolotti
 Photosynthesis Dr. Bertolotti Photosynthesis: Life from Light and Air How do plants and other organisms capture energy from the sun? What is ATP and why is it useful in cells? Plants are energy producers
Photosynthesis Dr. Bertolotti Photosynthesis: Life from Light and Air How do plants and other organisms capture energy from the sun? What is ATP and why is it useful in cells? Plants are energy producers
The conversion of usable sunlight energy into chemical energy is associated with the action of the green pigment chlorophyll.
 Photosynthesis Photosynthesis is the process by which plants, some bacteria and some protistans use the energy from sunlight to produce glucose from carbon dioxide and water. This glucose can be converted
Photosynthesis Photosynthesis is the process by which plants, some bacteria and some protistans use the energy from sunlight to produce glucose from carbon dioxide and water. This glucose can be converted
1/25/2018. Bio 1101 Lec. 5, Part A Chapter 6: Cellular Respiration
 1 2 3 4 5 Bio 1101 Lec. 5, Part A Chapter 6: Cellular Respiration Energy is needed by cells to do work Chemical energy, a form of potential energy, is stored in bonds of food molecules (such as glucose)
1 2 3 4 5 Bio 1101 Lec. 5, Part A Chapter 6: Cellular Respiration Energy is needed by cells to do work Chemical energy, a form of potential energy, is stored in bonds of food molecules (such as glucose)
UNIT 3: Cell Energy What is energy? energy is a property of objects which can be transferred to other objects or converted into different forms.
 UNIT 3: Cell Energy What is energy? energy is a property of objects which can be transferred to other objects or converted into different forms. Energy can be found in a number of different forms. 1 Law
UNIT 3: Cell Energy What is energy? energy is a property of objects which can be transferred to other objects or converted into different forms. Energy can be found in a number of different forms. 1 Law
Outline. Metabolism: Energy and Enzymes. Forms of Energy. Chapter 6
 Metabolism: Energy and Enzymes Chapter 6 Forms of Energy Outline Laws of Thermodynamics Metabolic Reactions ATP Metabolic Pathways Energy of Activation Enzymes Photosynthesis Cellular Respiration 1 2 Forms
Metabolism: Energy and Enzymes Chapter 6 Forms of Energy Outline Laws of Thermodynamics Metabolic Reactions ATP Metabolic Pathways Energy of Activation Enzymes Photosynthesis Cellular Respiration 1 2 Forms
PHOTOSYNTHESIS CHAPTER 7. Where It Starts - Photosynthesis
 PHOTOSYNTHESIS CHAPTER 7 Where It Starts - Photosynthesis IMPACTS, ISSUES: SUNLIGHT AND SURVIVAL Plants are autotrophs, or self-nourishing organisms The first autotrophs filled Earth s atmosphere with
PHOTOSYNTHESIS CHAPTER 7 Where It Starts - Photosynthesis IMPACTS, ISSUES: SUNLIGHT AND SURVIVAL Plants are autotrophs, or self-nourishing organisms The first autotrophs filled Earth s atmosphere with
Transformation of Energy! Energy is the ability to do work.! Thermodynamics is the study of the flow and transformation of energy in the universe.
 Section 1 How Organisms Obtain Energy Transformation of Energy! Energy is the ability to do work.! Thermodynamics is the study of the flow and transformation of energy in the universe. Section 1 How Organisms
Section 1 How Organisms Obtain Energy Transformation of Energy! Energy is the ability to do work.! Thermodynamics is the study of the flow and transformation of energy in the universe. Section 1 How Organisms
Photosynthesis. Chapter 10. Biology Eighth Edition Neil Campbell and Jane Reece. PowerPoint Lecture Presentations for
 Chapter 10 Photosynthesis PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Copyright
Chapter 10 Photosynthesis PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Copyright
Pearson Biology Chapter 8 Class Notes
 Pearson Biology Chapter 8 Class Notes Photosynthesis Chemical Energy and ATP Why is ATP useful to cells? Energy is the Ability to do Work. Cells use Adenosine Triphosphate (ATP) to Store and Release Energy
Pearson Biology Chapter 8 Class Notes Photosynthesis Chemical Energy and ATP Why is ATP useful to cells? Energy is the Ability to do Work. Cells use Adenosine Triphosphate (ATP) to Store and Release Energy
Photosynthesis is the main route by which that energy enters the biosphere of the Earth.
 Chapter 5-Photosynthesis Photosynthesis is the main route by which that energy enters the biosphere of the Earth. To sustain and power life on Earth, the captured energy has to be released and used in
Chapter 5-Photosynthesis Photosynthesis is the main route by which that energy enters the biosphere of the Earth. To sustain and power life on Earth, the captured energy has to be released and used in
Edexcel (B) Biology A-level
 Edexcel (B) Biology A-level Topic 5: Energy for Biological Processes Notes Aerobic Respiration Aerobic respiration as splitting of the respiratory substrate, to release carbon dioxide as a waste product
Edexcel (B) Biology A-level Topic 5: Energy for Biological Processes Notes Aerobic Respiration Aerobic respiration as splitting of the respiratory substrate, to release carbon dioxide as a waste product
Photosynthesis and Cellular Respiration
 Photosynthesis and Cellular Respiration Outline I. Energy and Carbon Cycle II. Photosynthesis A. Introduction B. Reactions II. Cellular Respiration A. Introduction B. Reactions Carbon Cycle All organisms
Photosynthesis and Cellular Respiration Outline I. Energy and Carbon Cycle II. Photosynthesis A. Introduction B. Reactions II. Cellular Respiration A. Introduction B. Reactions Carbon Cycle All organisms
Harvesting energy: photosynthesis & cellular respiration part 1
 Harvesting energy: photosynthesis & cellular respiration part 1 Agenda I. Overview (Big Pictures) of Photosynthesis & Cellular Respiration II. Making Glucose - Photosynthesis III. Making ATP - Cellular
Harvesting energy: photosynthesis & cellular respiration part 1 Agenda I. Overview (Big Pictures) of Photosynthesis & Cellular Respiration II. Making Glucose - Photosynthesis III. Making ATP - Cellular
Be sure to understand:
 Learning Targets & Focus Questions for Unit 6: Bioenergetics Chapter 8: Thermodynamics Chapter 9: Cell Resp Focus Q Ch. 10: Photosynthesis Chapter 8 (141-150) 1. I can explain how living systems adhere
Learning Targets & Focus Questions for Unit 6: Bioenergetics Chapter 8: Thermodynamics Chapter 9: Cell Resp Focus Q Ch. 10: Photosynthesis Chapter 8 (141-150) 1. I can explain how living systems adhere
Photosynthesis 6CO 2 + 6H 2 O C 6 H 12 O 6 + 6O 2
 PHOTOSYNTHESIS Photosynthesis An anabolic, endergonic, carbon dioxide (CO 2 ) requiring process that uses light energy (photons) and water (H 2 O) to produce organic macromolecules (glucose). photons SUN
PHOTOSYNTHESIS Photosynthesis An anabolic, endergonic, carbon dioxide (CO 2 ) requiring process that uses light energy (photons) and water (H 2 O) to produce organic macromolecules (glucose). photons SUN
Chapter 6: Energy Flow in the Life of a Cell
 Chapter 6: Energy Flow in the Life of a Cell What is Energy? Answer: The Capacity to do Work Types of Energy: 1) Kinetic Energy = Energy of movement Light (movement of photons) Heat (movement of particles)
Chapter 6: Energy Flow in the Life of a Cell What is Energy? Answer: The Capacity to do Work Types of Energy: 1) Kinetic Energy = Energy of movement Light (movement of photons) Heat (movement of particles)
Chapter 10: PHOTOSYNTHESIS
 Chapter 10: PHOTOSYNTHESIS 1. Overview of Photosynthesis 2. Light Absorption 3. The Light Reactions 4. The Calvin Cycle 1. Overview of Photosynthesis Chapter Reading pp. 185-190, 206-207 What is Photosynthesis?
Chapter 10: PHOTOSYNTHESIS 1. Overview of Photosynthesis 2. Light Absorption 3. The Light Reactions 4. The Calvin Cycle 1. Overview of Photosynthesis Chapter Reading pp. 185-190, 206-207 What is Photosynthesis?
CHAPTER 8 PHOTOSYNTHESIS
 CHAPTER 8 PHOTOSYNTHESIS Con. 8.1 Photosynthesis process by which plants use light to make food molecules from carbon dioxide and water (chlorophyll) 6CO 2 + 12H 2 O + Light C 6 H 12 O 6 + 6O 2 + 6H 2
CHAPTER 8 PHOTOSYNTHESIS Con. 8.1 Photosynthesis process by which plants use light to make food molecules from carbon dioxide and water (chlorophyll) 6CO 2 + 12H 2 O + Light C 6 H 12 O 6 + 6O 2 + 6H 2
Lesson Overview. Photosynthesis: An Overview. Lesson Overview. 8.2 Photosynthesis: An Overview
 Lesson Overview 8.2 Photosynthesis: An Overview Light and pigments Energy from the sun travels to Earth in the form of light. Sunlight is a mixture of different wavelengths. The wavelengths we see is known
Lesson Overview 8.2 Photosynthesis: An Overview Light and pigments Energy from the sun travels to Earth in the form of light. Sunlight is a mixture of different wavelengths. The wavelengths we see is known
Energy for Life 12/11/14. Light Absorption in Chloroplasts
 Energy for Life Biochemical pathways A series of reactions where the products of one reaction is used in the next reaction Light Absorption in Chloroplasts Chloroplasts Two membranes Grana- layered stacks
Energy for Life Biochemical pathways A series of reactions where the products of one reaction is used in the next reaction Light Absorption in Chloroplasts Chloroplasts Two membranes Grana- layered stacks
Overview - the process that feeds the biosphere. Photosynthesis: transformation of solar energy into chemical energy.
 Chapter 7 Capturing Solar Energy: Photosynthesis Overview - the process that feeds the biosphere Photosynthesis: transformation of solar energy into chemical energy. Responsible for O 2 in our atmosphere
Chapter 7 Capturing Solar Energy: Photosynthesis Overview - the process that feeds the biosphere Photosynthesis: transformation of solar energy into chemical energy. Responsible for O 2 in our atmosphere
Photosynthesis. Chapter 10. Biology Eighth Edition Neil Campbell and Jane Reece. PowerPoint Lecture Presentations for
 Chapter 10 Photosynthesis PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Overview:
Chapter 10 Photosynthesis PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Overview:
PHOTOSYNTHESIS. Chapter 8
 PHOTOSYNTHESIS Chapter 8 ENERGY & LIFE ENERGY The ability to do work. Can be stored in chemical bonds. Cells need energy to do things like active transport, dividing, moving, and producing and storing
PHOTOSYNTHESIS Chapter 8 ENERGY & LIFE ENERGY The ability to do work. Can be stored in chemical bonds. Cells need energy to do things like active transport, dividing, moving, and producing and storing
Chapter 7 Capturing Solar Energy: Photosynthesis. Chapter 7: Photosynthesis. What is Photosynthesis?
 Chapter 7 Capturing Solar Energy: Photosynthesis What is Photosynthesis? Answer: The capture of sunlight energy and the subsequent storage of that energy in the chemical bonds (e.g., glucose) Chemical
Chapter 7 Capturing Solar Energy: Photosynthesis What is Photosynthesis? Answer: The capture of sunlight energy and the subsequent storage of that energy in the chemical bonds (e.g., glucose) Chemical
Energy and Life. Lesson Overview. Lesson Overview. 8.1 Energy and Life
 8.1 Chemical Energy and ATP Energy is the ability to do work. Your cells are busy using energy to build new molecules, contract muscles, and carry out active transport. Without the ability to obtain and
8.1 Chemical Energy and ATP Energy is the ability to do work. Your cells are busy using energy to build new molecules, contract muscles, and carry out active transport. Without the ability to obtain and
4 GETTING READY TO LEARN Preview Key Concepts 4.1 Chemical Energy and ATP All cells need chemical energy.
 CHAPTER 4 Cells and Energy GETTING READY TO LEARN Preview Key Concepts 4.1 Chemical Energy and ATP All cells need chemical energy. 4.2 Overview of Photosynthesis The overall process of photosynthesis produces
CHAPTER 4 Cells and Energy GETTING READY TO LEARN Preview Key Concepts 4.1 Chemical Energy and ATP All cells need chemical energy. 4.2 Overview of Photosynthesis The overall process of photosynthesis produces
Center for Academic Services & Advising
 March 2, 2017 Biology I CSI Worksheet 6 1. List the four components of cellular respiration, where it occurs in the cell, and list major products consumed and produced in each step. i. Hint: Think about
March 2, 2017 Biology I CSI Worksheet 6 1. List the four components of cellular respiration, where it occurs in the cell, and list major products consumed and produced in each step. i. Hint: Think about
AP Bio-Ms.Bell Unit#3 Cellular Energies Name
 AP Bio-Ms.Bell Unit#3 Cellular Energies Name 1. Base your answer to the following question on the image below. 7. Base your answer to the following question on Which of the following choices correctly
AP Bio-Ms.Bell Unit#3 Cellular Energies Name 1. Base your answer to the following question on the image below. 7. Base your answer to the following question on Which of the following choices correctly
Chapter 8 Photosynthesis Lecture Outline. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Chapter 8 Photosynthesis Lecture Outline Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 8.1 Overview of Photosynthesis Photosynthesis converts solar energy
Chapter 8 Photosynthesis Lecture Outline Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 8.1 Overview of Photosynthesis Photosynthesis converts solar energy
Chapter 8: Cellular Energy
 Chapter 8: Cellular Energy Section 1: How Organisms Obtain Energy Transformation of Energy All cellular activities require Energy!! ( The ability to do work). The study of flow and the transformation of
Chapter 8: Cellular Energy Section 1: How Organisms Obtain Energy Transformation of Energy All cellular activities require Energy!! ( The ability to do work). The study of flow and the transformation of
Cellular Energy (Photosynthesis & Cellular Respiration)
 (Photosynthesis & Cellular Respiration) Before You Read Before you read the chapter, respond to these statements. 1. Write an A if you agree with the statement. 2. Write a D if you disagree with the statement.
(Photosynthesis & Cellular Respiration) Before You Read Before you read the chapter, respond to these statements. 1. Write an A if you agree with the statement. 2. Write a D if you disagree with the statement.
Chapter 6: Energy Flow in the Life of a Cell
 Chapter 6: Energy Flow in the Life of a Cell What is Energy? Answer: The capacity to do work Types of Energy: 1) Potential Energy = Stored energy Positional (stored in location of object) Chemical (stored
Chapter 6: Energy Flow in the Life of a Cell What is Energy? Answer: The capacity to do work Types of Energy: 1) Potential Energy = Stored energy Positional (stored in location of object) Chemical (stored
Photosynthesis and Cellular Respiration Note-taking Guide
 Photosynthesis and Cellular Respiration Note-taking Guide Preview to Photosynthesis glucose, reactions, light-dependent, Calvin cycle, thylakoid, photosystem II, oxygen, light-harvesting, two, chloroplasts,
Photosynthesis and Cellular Respiration Note-taking Guide Preview to Photosynthesis glucose, reactions, light-dependent, Calvin cycle, thylakoid, photosystem II, oxygen, light-harvesting, two, chloroplasts,
Name Date Class. Photosynthesis and Respiration
 Concept Mapping Photosynthesis and Respiration Complete the Venn diagram about photosynthesis and respiration. These terms may be used more than once: absorbs, Calvin cycle, chlorophyll, CO 2, H 2 O, Krebs
Concept Mapping Photosynthesis and Respiration Complete the Venn diagram about photosynthesis and respiration. These terms may be used more than once: absorbs, Calvin cycle, chlorophyll, CO 2, H 2 O, Krebs
ATP, Cellular Respiration and Photosynthesis
 ATP, Cellular Respiration and Photosynthesis Energy for Cells Free Energy: the energy available to do work Types of Reactions Endergonic Reactions: require an input of energy Exergonic Reactions: release
ATP, Cellular Respiration and Photosynthesis Energy for Cells Free Energy: the energy available to do work Types of Reactions Endergonic Reactions: require an input of energy Exergonic Reactions: release
UNIT 2: CELLS Chapter 4: Cells and Energy
 CORNELL NOTES Directions: You must create a minimum of 5 questions in this column per page (average). Use these to study your notes and prepare for tests and quizzes. Notes will be stamped after each assigned
CORNELL NOTES Directions: You must create a minimum of 5 questions in this column per page (average). Use these to study your notes and prepare for tests and quizzes. Notes will be stamped after each assigned
Sunlight and Survival. Plants are photoautotrophs; they use sunlight and CO2 to produce sugar in the process of photosynthesis
 Photosynthesis Sunlight and Survival Plants are photoautotrophs; they use sunlight and CO2 to produce sugar in the process of photosynthesis Energy From The Sun Many kinds of energy Wavelengths of visible
Photosynthesis Sunlight and Survival Plants are photoautotrophs; they use sunlight and CO2 to produce sugar in the process of photosynthesis Energy From The Sun Many kinds of energy Wavelengths of visible
Metabolism 2 Photosynthesis
 Metabolism 2 Photosynthesis Light energy is trapped in the form of high energy electrons. High energy electrons are used to synthesize ATP and reduce CO 2 to form carbohydrates. Oxygen is produced as a
Metabolism 2 Photosynthesis Light energy is trapped in the form of high energy electrons. High energy electrons are used to synthesize ATP and reduce CO 2 to form carbohydrates. Oxygen is produced as a
In Cellular Respiration, are removed from sugar and transferred to
 1 2 3 4 5 Bio 1101 Lec. 5, Part A (Guided Notes) Chapter 6: Cellular Respiration Energy is needed by cells to do work Chemical energy, a form of potential energy, is stored in bonds of food molecules (such
1 2 3 4 5 Bio 1101 Lec. 5, Part A (Guided Notes) Chapter 6: Cellular Respiration Energy is needed by cells to do work Chemical energy, a form of potential energy, is stored in bonds of food molecules (such
1 Which of the following organisms do NOT carry on photosynthesis?
 1 Which of the following organisms do NOT carry on photosynthesis? plants algae some bacteria 2 3 animals The correct description of the relationship between photosynthesis and the living world is. herbivores,
1 Which of the following organisms do NOT carry on photosynthesis? plants algae some bacteria 2 3 animals The correct description of the relationship between photosynthesis and the living world is. herbivores,
Photosynthesis and Life
 7-1 Chapter 7 Photosynthesis and Life During photosynthesis Organisms use the energy of light to build highenergy organic molecules. Plants, algae, and some bacteria can do this. Can make their own food
7-1 Chapter 7 Photosynthesis and Life During photosynthesis Organisms use the energy of light to build highenergy organic molecules. Plants, algae, and some bacteria can do this. Can make their own food
A. Structures of PS. Site of PS in plants: mostly in leaves in chloroplasts. Leaf cross section. Vein. Mesophyll CO 2 O 2. Stomata
 PS Lecture Outline I. Introduction A. Structures B. Net Reaction II. Overview of PS A. Rxns in the chloroplast B. pigments III. Closer looks A. LD Rxns B. LI Rxns 1. non-cyclic e- flow 2. cyclic e- flow
PS Lecture Outline I. Introduction A. Structures B. Net Reaction II. Overview of PS A. Rxns in the chloroplast B. pigments III. Closer looks A. LD Rxns B. LI Rxns 1. non-cyclic e- flow 2. cyclic e- flow
Section 1 The Light Reactions. Section 2 The Calvin Cycle. Resources
 How to Use This Presentation To View the presentation as a slideshow with effects select View on the menu bar and click on Slide Show. To advance through the presentation, click the right-arrow key or
How to Use This Presentation To View the presentation as a slideshow with effects select View on the menu bar and click on Slide Show. To advance through the presentation, click the right-arrow key or
A + B = C C + D = E E + F = A
 Photosynthesis - Plants obtain energy directly from the sun - Organisms that do this are autotrophs (make their own food from inorganic forms) - Photosynthesis is a series of chemical reactions where the
Photosynthesis - Plants obtain energy directly from the sun - Organisms that do this are autotrophs (make their own food from inorganic forms) - Photosynthesis is a series of chemical reactions where the
Chapter 7. Introduction. Introduction. Photosynthesis: Using Light to Make Food. Plants, algae, and certain prokaryotes
 Chapter 7 hotosynthesis: Using to Make Food oweroint Lectures for Campbell Biology: Concepts & Connections, Seventh Edition Reece, Taylor, Simon, and Dickey Lecture by Edward J. Zalisko Introduction lants,
Chapter 7 hotosynthesis: Using to Make Food oweroint Lectures for Campbell Biology: Concepts & Connections, Seventh Edition Reece, Taylor, Simon, and Dickey Lecture by Edward J. Zalisko Introduction lants,
Photosynthesis: Life from Light and Air
 Photosynthesis: Life from Light and Air 2007-2008 Energy needs of life All life needs a constant input of energy consumers producers Heterotrophs (Animals) get their energy from eating others eat food
Photosynthesis: Life from Light and Air 2007-2008 Energy needs of life All life needs a constant input of energy consumers producers Heterotrophs (Animals) get their energy from eating others eat food
Just Like the Guy From Krypton Photosynthesis
 Just Like the Guy From Krypton Photosynthesis An Overview of Photosynthesis Most of the energy used by almost all living cells ultimately comes from the sun plants, algae, and some bacteria capture the
Just Like the Guy From Krypton Photosynthesis An Overview of Photosynthesis Most of the energy used by almost all living cells ultimately comes from the sun plants, algae, and some bacteria capture the
All Cells need energy. (Ability to perform work) What do cells use energy for? Mitosis. Repair. Active transport. Movement.
 Cell Energetics All Cells need energy. (Ability to perform work) What do cells use energy for? Mitosis. Repair. Active transport. Movement. What Is ATP? ATP adenosine triphosphate is a chemical molecule
Cell Energetics All Cells need energy. (Ability to perform work) What do cells use energy for? Mitosis. Repair. Active transport. Movement. What Is ATP? ATP adenosine triphosphate is a chemical molecule
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 AP Exam Chapters 9 and 10; Photosynthesis and Respiration Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Carbon dioxide (CO2) is released
AP Exam Chapters 9 and 10; Photosynthesis and Respiration Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Carbon dioxide (CO2) is released
ATP. Chapter 4. Photosynthesis. Cell Respiration. Energy of Life. All organisms need energy in order to survive
 ATP Chapter 4 Photosynthesis Energy of Life All organisms need energy in order to survive 2 Major groups of organisms: A. autotrophs make their own food Ex: plants B. heterotrophs must eat others living
ATP Chapter 4 Photosynthesis Energy of Life All organisms need energy in order to survive 2 Major groups of organisms: A. autotrophs make their own food Ex: plants B. heterotrophs must eat others living
ATP: Energy for Life ATP. Chapter 6. What Is ATP? What Does ATP Do for You? Photosynthesis. Cell Respiration. Chemical Structure of ATP
 Chapter 6 Photosynthesis : Energy for Life Cell Respiration What Is? Energy used by all Cells Chemical Structure of Adenine Base Adenosine Triphosphate Organic molecule containing highenergy Phosphate
Chapter 6 Photosynthesis : Energy for Life Cell Respiration What Is? Energy used by all Cells Chemical Structure of Adenine Base Adenosine Triphosphate Organic molecule containing highenergy Phosphate
