Lesson 8: Introduction to the Atom Introduction
|
|
|
- Deborah Logan
- 6 years ago
- Views:
Transcription
1 Lesson 8: Introduction to the Atom Introduction This document is Lesson 1 of the SEAChem2020 open source chemistry curriculum program for secular homeschoolers. This version was current as of 2 October To if there is a more current version of this document, visit Table of Contents: Text 2 Glossary 7 Practice Sheet 8 Lab 10 Assessment 15 References for further study 17 We welcome any help you are willing to provide in supporting this project. Even if you're not a chemist or a professional educator, you can help in the following ways: Let us know about minor fixes. If you find anything from a factual to a stylistic error, or even a typo, let us know by using this form. Let us know if you find big problems. Does something need rewriting? Let us know by contacting us using this form. Give us your resources. If you've done one of these lessons and have put something cool together, us at misterguch@chemfiesta.com so we can include it! Volunteer. Do you want to edit this curriculum? Do you have things to add? Would you like to write some of these lessons? Let us know! Publicity. Tell your friends about these resources. When the community grows, the project keeps growing! Thank you for using this resource, and please consider helping out! 1
2 Lesson 8 An Introduction to the Atom You've heard the term atom before and doubtlessly have a pretty good idea of what it is. However, what you may not know is how we came to our current understanding of the atom. Not surprisingly, this required a great deal of insight, as well as a fair amount of trial and error. What is an Atom? The definition that's most relevant to chemists states that atoms are the smallest unit of matter that have the properties of an element. Everything that you would normally think of as a thing is made of atoms. 1 Your dog is made of atoms, as are disposable razors, air, and the Eiffel Tower. The logo of the International Atomic Energy Agency, a department of the United Nations, shows a common, but incorrect, depiction of an atom. 2 Though the definition above is a good first draft, it doesn't really explain what atoms are like. In order to understand how atoms work, we'll explore how people have thought about them through history. Why explore the history of the atom? You may wonder why anybody would feel the need to read about the history of the atom rather than just learning about our current understanding. The reason we do this is not just to force you to learn irrelevant information, but to help you to better understand the atom. As we'll see, atoms are not quite as easily understood as you may have been led to believe! 1 See lesson 7 for a description of what we mean by thing. Incidentally, this definition of the atom is based on the one on Wikipedia. 2 By IAEA (Flag code: [1]) [Public domain], via Wikimedia Commons. 2
3 The Early Greek Model of the Atom For most of man's history, we didn't have any idea what matter what made of. For that matter, we didn't really care, either. After all, you don't need to know what wood is in order to use it to build a home. The first person to come up with a good understanding of the nature of matter was Leucippus (early 5 th century BCE). 3 He believed that all matter was made up of indivisible atoms, and that the properties of matter could be attributed to the different ways that they were stacked together. This belief was referred to as atomism. Plato ( BCE) refined this idea somewhat. He believed that there were four elements (earth, air, fire, water) and that teach element was made of atoms that that were differently-shaped from each other. Thus, he believed that earth was made of cubic atoms, air was made of octahedral atoms, fire was made of tetrahedral atoms, and water was made of icosahedral atoms. Right to left: Tetrahedron, octahedron, cube, icosahedron. Aristotle ( BCE) rejected the theory of the atom. He believed that objects weren't made of smaller atoms, but were instead continuous structures. Unlike atomists, who believed that things changed because the arrangements of atoms changed, Aristotle taught that things changed because they had a pre-existing ability to change from one thing to another, and that changes occurred because the matter simply actualized its earlier potential. For approximately 2,000 years, Aristotle's view was the most widely-held. This may not make sense to us, but consider that the technology to test atomic views was still very far in the future. Because ancient philosophers believed in reason, it's not hard to understand why they would believe in a concept that made sense over one that couldn't be verified. Discoveries of the Late 18 th Century For a long time, there was no way to test the various theories of the nature of matter. However, in the late 18 th century, there were some interesting scientific discoveries that again got people thinking about atoms. 3 The earliest atomic theory is frequently attributed to Democritus, one of Leucippus' students. Because none of Leucippus' works have survived, little is known about him. 3
4 In 1789, Antoine Lavoisier devised the law of conservation of mass, which states that mass is neither created nor destroyed in any process. This seems obvious to us. After all, if you mix salt with water, you'd imagine the salt water would weigh the same as the weight of the salt plus the weight of the water. However, consider that, in many processes such as combustion reactions, some of the products are gases. If you burn a piece of wood, you may believe that, because the ash weighs less than the wood, the mass has changed over the reaction. For Lavoisier to determine otherwise was quite a discovery. The next big discovery came in 1794, when Joseph Proust described the law of definite proportions 4, which states that chemical compounds consist of atoms combined in wholenumber proportions. For example, water has the formula H 2 O, never H 1.7 O or H 2.3 O. Again, this may seem obvious to us with our modern technology, but it's only been relatively recently that we've been able to find the compositions of chemical compounds to a high degree of precision. A scientist of three hundred years ago may very well have found that the formula of water is H 1.7 O rather than H 2 O, simply because of experimental error. Proust vs. Proust Joseph Proust, analytical chemist For some reason, students tend to mix up the chemist Joseph Proust ( ) with the writer Marcel Proust ( ). Seriously, they're not the same guy. Marcel Proust, author of À la recherche du temps perdu. Dalton's Atomic Theory In 1803, an English scientist named John Dalton assembled a lot of ideas that were going around the scientific community into a new model of the atom. In this theory, he stated that atoms have the following properties: Matter is made of tiny, indestructible atoms. All atoms of the same element have the same chemical and physical properties, different from those of other elements. Atoms of elements combine in whole-number ratios to form chemical compounds. 5 Atoms obey the law of conservation of mass. Chemical reactions occur when atoms are rearranged. 4 Also called the law of constant composition or Proust's Law. 5 Basically, the law of definite proportions, along with the law of multiple proportions, which we won't discuss because it doesn't really add much to the conversation. 4
5 All of these laws probably seem reasonable to you, and it may seem as if they're all correct. Let's take a look at each of them and see if this is the case. Matter is made of tiny indestructible atoms: Partially true Atoms are, indeed, very tiny. Hydrogen atoms are about 1.2 x meters across, which means that you could stretch 630,000 of them across the length of a human hair. As to whether they're indestructible, the answer is sort of. For us chemists, atoms are, for all intents and purposes, indestructible. However, when particle physicsts put enough energy into an atom, they can break it apart into much smaller pieces. A better statement would be to say that atoms are tiny particles that are indestructible during chemical reactions. All atoms of the same element have the same chemical and physical properties, apart from those of other elements. Partially true Atoms of different elements do, in fact, have different chemical and physical properties. Though some properties may be similar to one another, no two elements have the same properties. However, when it comes to atoms of the same element, there can be small differences between isotopes of the element, which have different numbers of neutrons. We will talk about this in more detail in a later lesson. Atoms of elements combine in whole-number ratios to form chemical compounds: Mostly true We understand that carbon dioxide has the formula CO 2, and don't expect anything like CO 2.1 to show up in a reaction. However, there is a class of materials called nonstoichiometric compounds, in which defects in their structure causes the ratios of elements to be something other than whole numbers. An important example of this are the palladium hydrides, which have the formula PdH x, where x is somewhere between 0.02 and 0.58, depending on conditions. Superconductors Many superconductors are also nonstoichiometric compounds. The superconducting material YBCO (YBa 2Cu 3O 7 x) was the first high temperature material that retained its superconductive properties warmer than the temperature of liquid nitrogen (77 K, -196 C). Superconductors are used in both medicine and science because they have no electrical resistance. 6 6 By SPat (Own work) [CC BY-SA 3.0 ( via Wikimedia Commons. 5
6 Atoms obey the law of conservation of mass: True When you form a chemical compound, the mass of your products (i.e. what you make) will be equal to the mass of the reagents (i.e. what you started with). No problems here. 7 Chemical reactions occur when atoms are rearranged: True All of chemistry is built around the fact that when you rearrange atoms, you get new chemical compounds that have different properties. This one is absolutely true! For nearly a hundred years, Dalton's model of the atom was considered to be the final model of the atom. However, as we shall see shortly, things were to change. The main ideas in this lesson: Atoms are the smallest units of matter that have the properties of elements. The law of conservation of mass states that matter is neither created nor destroyed during any process. The law of definite proportions states that compounds consist of atoms of elements combined in whole-number ratios. Dalton's model of the atom was the first really comprehensive one that described their properties and behavior. 7 Read Lesson 7 for an explanation of why there are problems here. 6
7 Lesson 8: Glossary atom: The smallest unit of matter that has the properties of an element. atomism: The belief that matter consists of small particles called atoms. The theory of atomism originated with ancient Greek philosophers. law of conservation of mass: Matter can be neither created nor destroyed. law of definite proportions: Chemical compounds are formed through the combination of atoms in whole number ratios. superconductor: A material that conducts electricity without resistance. 7
8 Lesson 8: Practice Sheet 1) Though atomic theory was devised over two thousand years ago, it wasn't until relatively recently that was generally accepted by scientists. Given that we have definitively established the existence of atoms, why do you believe that it took so long for people to realize the truth? 2) Why is it generally OK for us to treat atoms as if they were indestructible particles? 3) Why do you think Plato and some other Greek philosophers believed that the atoms of each element had different shapes? Is this a reasonable belief for them to have had? 8
9 Lesson 8: Practice Sheet Answers 1) Though atomic theory was devised over two thousand years ago, it wasn't until relatively recently that was generally accepted by scientists. Given that we have definitively established the existence of atoms, why do you believe that it took so long for people to realize the truth? Without evidence, philosophers and scientists had no way of knowing what matter was made of. The naked eye can't see objects smaller than around 0.1 mm. Given that atoms are a million times smaller than this, any guesses about the structure of atoms had to be just that: guesses. It wasn't until experimental evidence was gained through more careful measurements that people such as Dalton were able to infer the existence of atoms, and not until the 1970's when people were first able to see them at the Lawrence Berkeley Laboratories with an electron microscope. 2) Why is it generally OK for us to treat atoms as if they were indestructible particles? For our purposes, they are indestructible particles. Though huge amounts of energy can be used to break atoms, these quantities of energy just aren't present when performing chemical reactions. 3) Why do you think Plato and some other Greek philosophers believed that the atoms of each element had different shapes? Is this a reasonable belief for them to have had? Though the Greek philosophers were passionate about learning about the world, they had very little information from which to work. Given that they had given a great deal of thought to geometry, it makes sense that they believed the main distinguishing characteristic between the elements were their shapes. Though they could have just as easily picked another characteristic, their interests led them to believe that shape was the primary determination of atomic makeup. 9
10 Lesson 8 Activity: Law of Conservation of Mass Lab Parent Information Goal: To teach your child how the law of conservation of mass works Home destruction factor: 2/10. There might be a little mess, but nothing too bad. Safety: Though there are no major safety factors involved in doing this lab, it is probably best to have your child wear goggles. Materials (do-it-yourself version): This will depend on what your child comes up with. However, a scale or balance will be required. Materials (baking soda and vinegar version): 1 box of baking soda 1 bottle of 5% vinegar 1 scale or balance Clean-up: This will vary depending on which version of the lab your child tries and what they've done. If baking soda and vinegar are used, the waste can be put down the sink. How to perform this lab: In this lab, your child will be asked to demonstrate the law of conservation of mass, which states that mass is neither created nor destroyed during any change. Depending on which version of this lab is used, this can be done in a couple of ways: Do-it-yourself version: Your child will simply be asked to demonstrate the law of conservation of mass. How they do this will be up to them, with your approval. Baking soda and vinegar version: Your child will combine baking soda and vinegar, measuring and comparing the masses of the reagents and products. The do-it-yourself version of this lab requires nothing in the way of preparation. When you tell your child to demonstrate the law of conservation of mass, they'll end up doing any one of a thousand different things to prove that mass remains constant during some process. Your child will have to get your approval before they can do this experiment, so you can make this lab as straightforward or as challenging as you'd like. Some good ways of demonstrating the law of conservation of mass: Bake something or otherwise prepare food items Mix baking soda and vinegar. This is the basis for the other version of this experiment, but it would not be surprising if your child came up with it on his/her own. Dissolving sugar or salt in water. Weighing two objects separately, then weighing them together. This may seem like a trivial example, but it does the job. 10
11 The baking soda and vinegar version of this lab requires that your child first weigh baking soda and vinegar, and then weigh the resulting solution formed when they are combined. Your child will come up with the experimental procedure on their own, but as this really consists only of combining baking soda and vinegar, this is a simple lab to run. The surprise: Unlike most other labs, this one has a surprise: It's nearly impossible to demonstrate the law of conservation of mass with the equipment we have at home. Sure, with trivial examples such as stacking books, we shouldn't have a problem. However, when you consider other examples, you can see where the problem starts: When you cook food, some of it splashes or evaporates out, resulting in a lower than expected mass. If dissolving things, you will frequently find that a small amount of either the thing being dissolved or the water splashes out, causing a lower than expected mass. Most importantly for this lab, you'll find that when you combine baking soda and vinegar that the mass decreases significantly after the reaction occurs. This takes place because the bubbles of carbon dioxide given off have mass of their own, and this mass is equivalent to 30% of the mass of the reaction product! As a result, if you performed this reaction with pure acetic acid and pure sodium bicarbonate, 100 grams of reagent would turn into 70 grams of product. In practice, the change will not be this drastic. We are not using pure acetic acid, so the actual percent change will be closer to 1.5%, as the water in the vinegar will not react. However, this change is unavoidable when performing this lab, regardless of any steps your child takes to avoid it. The reason that this error is built into the lab is that we're trying to prove two main points: Even simple ideas are hard to devise: In this lab, we're essentially trying to prove something that's intuitively obvious to us. We know that the basic premise is true and that we can prove it. However, even with these advantages, we're unable to prove that the law of conservation of mass works! This makes it easier to understand that, while this idea is simple from a conceptual standpoint, it would have been much harder to learn from the historical perspective of the people doing the experiments. Screwing up is normal: You may think it's a little cruel to set up a lab where your child is unable to get the desired result. When you think about it, you've really set him or her up to fail. And failing is a terrible thing, right? Actually, it's not. When performing scientific experiments, failure is not just possible but inevitable. There's no such thing as a perfect experiment because the world is not a perfect place. By giving your child a lab in which you know they'll be unable to get a perfect answer, you'll be able to control the way in which they learn this lesson. Some kids are just fine with the idea that the world isn't a perfect place, while others will feel that anything less than perfection is abject failure. In this lab, you have the chance to be there when things go wrong and to tell them that everything is just fine. Assessment: 11
12 Because there are many different ways to do this lab, there are several different criteria you should use in your assessment: Did your child's conclusion support the law of conservation of mass? Depending on how the experiment went, the answer could either be yes or no both answers are fine, depending on the experimental results obtained by your child in the lab. However, if the answer is no, it should be clear from your child's lab that they understand that the law of conservation of mass is actually true and that its apparent invalidity is a function of normal experimental error. Does your child understand why their experiment supports/doesn't support the law of conservation of mass? It is sometimes the case that kids will say their conclusions are in line with the law of conservation of mass, simply because they know the law of conservation of mass is correct. Depending on the experimental data, your child should understand precisely why their data supports or refutes the law of conservation of mass. Does your child understand the concept of experimental error? Given that this lab is nearly impossible to do under any but the most trivial cases, it is important for your child to realize that experimental error is inevitable whenever performing an experiment. Furthermore, your child should understand that you will not expect perfection from him/her, so long as they show that they understand why the lab either did or did not work. Does your child have any ideas about how their procedure can be improved? Though experimental error is inevitable in the lab, it's also the case that there are always ways to minimize its impact. Your child should have at least two concrete ideas about how the lab can be better performed. 12
13 Do-It-Yourself Law of Conservation of Mass Lab The goal of this lab is simple: Demonstrate the validity of the law of conservation of mass. You may do this using whatever equipment or supplies you believe are necessary, provided that the person supervising the experiment has given it his/her OK. For the lab write-up, please do the following: 1. Write a procedure explaining what you did. 2. Record whatever data you collected during the lab. 3. Analyze your data and determine whether it supported or disproved the law of conservation of mass. 4. If your experiment did not support the law of conservation of mass, explain why you believe this to be the case. 5. List two specific sources of experimental error that may have been present when you performed the lab. 6. Describe two specific improvements you could make to your lab to minimize the effect of experimental error. Good luck! 13
14 Baking Soda and Vinegar Law of Conservation of Mass Lab The goal of this lab is simple: Demonstrate the validity of the law of conservation of mass this must be done through the combination of baking soda and vinegar. You may set up your experiment in whatever way you'd like, provided that the person supervising the experiment has given it his/her OK. For the lab write-up, please do the following: 1. Write a procedure explaining what you did. 2. Record whatever data you collected during the lab. 3. Analyze your data and determine whether it supported or disproved the law of conservation of mass. 4. If your experiment did not support the law of conservation of mass, explain why you believe this to be the case. 5. List two specific sources of experimental error that may have been present when you performed the lab. 6. Describe two specific improvements you could make to your lab to minimize the effect of experimental error. Good luck! 14
15 Lesson 8 Assessment 1) Explain how the Greek model of the atom differed from Dalton's model. 2) Dalton stated that all atoms of the same element have the same chemical and physical properties. Given that we now know that this isn't true, is this a reasonable assumption for him to have made? Explain why or why not. 3) It has been said that experimental error is inevitable. However, the author of this exam can state definitively that he has observed experimental results that showed no error whatsoever. How can we reconcile the idea that experimental error is inevitable with experiments observed to have no error? 15
16 Lesson 8 Assessment Answers 1) Explain how the Greek model of the atom differed from Dalton's model. Dalton's laws are essentially the Greek model of the atom with some other stuff added to it. Dalton and early Greek philosophers agreed that atoms are very tiny particles that make up all of matter, and that the way in which they're combined determines what substance is present. However, Dalton had some additional ideas based on data unavailable to the Greeks: Atoms combine in whole-number ratios to form chemical compounds: The concept of chemical compounds wasn't really something the Greeks were familiar with, so they never came up with this. Atoms obey the law of conservation of mass: Lack of experimental data kept the ancient Greeks from even conceiving of this idea. Ideas such as atoms of the same element are all identical and atoms of different elements are all identical are difficult to assess, based on the Greeks' limited understanding of how the world came together. Based on Plato's concept of the atom, however, it seems reasonable to assume that his model of the atom would support this. For parents: This question is really asking two things: 1) Do you know how the Greek and Dalton models of the atom differ; and 2) Have you spent some time thinking about how they fit together? The specific answer your child gives doesn't necessarily have to be as in-depth as the one I gave above, so long as they've answered the two main parts of the question. 2) Dalton stated that all atoms of the same element have the same chemical and physical properties. Given that we now know that this isn't true, is this a reasonable assumption for him to have made? Explain why or why not. It is entirely reasonable for him to have believed this. Not only was it difficult to perform precision experiments that would disprove this idea, but the very concept that the atom had internal structure that could vary was completely unknown. 3) It has been said that experimental error is inevitable. However, the author of this exam can state definitively that he has observed experimental results that showed no error whatsoever. How can we reconcile the idea that experimental error is inevitable with experiments observed to have no error? A result of this is caused because of offsetting errors. Let's say, for example, that you attempt to verify the law of conservation of mass by weighing ice cream both before and after you transfer it from one place to another. You undoubtedly understand that this would cause errors, because some of the ice cream would be left behind in the original container. However, it may look as if the law of conservation of mass was verified if, for example, atmospheric moisture condensed on the ice cream in its new container. If the amount of mass lost by moving the ice cream was offset with an identical amount of condensation, it may look as if no ice cream was lost. 16
17 Lesson 8: References for Further Study Everything you've ever wanted to know about Greek atomism: About Aristotle's refutation of atomism: Lavoisier and the discovery of the law of conservation of mass: %20of%20Mass.htm. Additionally, the last section is a very brief (but very good) discussion both of the apparent failings of the law of conservation of mass due to experimental error, as well as the law of conservation of mass-energy. Defects and Nonstoichiometry: This is a good, though technically-challenging, discussion of nonstoichiometric compounds (i.e. compounds that don't contain atoms in whole-number ratios). Just How Small is an Atom? An interesting YouTube video that covers a lot of ground regarding the sizes of atoms. 17
Lesson 10: Isotopes Introduction
 Lesson 10: Isotopes Introduction This document is Lesson 10 of the SEAChem2020 open source chemistry curriculum program for secular homeschoolers. This version was current as of 18 October 2017. To if
Lesson 10: Isotopes Introduction This document is Lesson 10 of the SEAChem2020 open source chemistry curriculum program for secular homeschoolers. This version was current as of 18 October 2017. To if
Atomic Theory. Introducing the Atomic Theory:
 Atomic Theory Chemistry is the science of matter. Matter is made up of things called atoms, elements, and molecules. But have you ever wondered if atoms and molecules are real? Would you be surprised to
Atomic Theory Chemistry is the science of matter. Matter is made up of things called atoms, elements, and molecules. But have you ever wondered if atoms and molecules are real? Would you be surprised to
Vocabulary atom atomos Dalton's atomic theory law of constant composition law of definite proportions law of multiple proportions matter.
 1.3 Early Atomic Theory Lesson Objectives The student will: define matter and explain how it is composed of building blocks known as atoms. give a short history of how the concept of the atom developed.
1.3 Early Atomic Theory Lesson Objectives The student will: define matter and explain how it is composed of building blocks known as atoms. give a short history of how the concept of the atom developed.
What Do You Think? Investigate GOALS
 Activity 3 Atoms and Their Masses GOALS In this activity you will: Explore the idea of atoms by trying to isolate a single atom. Measure how many times greater the mass of a copper atom is than a magnesium
Activity 3 Atoms and Their Masses GOALS In this activity you will: Explore the idea of atoms by trying to isolate a single atom. Measure how many times greater the mass of a copper atom is than a magnesium
MITOCW ocw f99-lec09_300k
 MITOCW ocw-18.06-f99-lec09_300k OK, this is linear algebra lecture nine. And this is a key lecture, this is where we get these ideas of linear independence, when a bunch of vectors are independent -- or
MITOCW ocw-18.06-f99-lec09_300k OK, this is linear algebra lecture nine. And this is a key lecture, this is where we get these ideas of linear independence, when a bunch of vectors are independent -- or
5th Grade. Slide 1 / 67. Slide 2 / 67. Slide 3 / 67. Matter and Its Interactions. Table of Contents: Matter and Its Interactions
 Slide 1 / 67 Slide 2 / 67 5th Grade Matter and Its Interactions 2015-11-02 www.njctl.org Table of Contents: Matter and Its Interactions Slide 3 / 67 Click on the topic to go to that section What Is Matter?
Slide 1 / 67 Slide 2 / 67 5th Grade Matter and Its Interactions 2015-11-02 www.njctl.org Table of Contents: Matter and Its Interactions Slide 3 / 67 Click on the topic to go to that section What Is Matter?
Amount of Substance and Its Unit Mole- Connecting the Invisible Micro World to the Observable Macro World Part 2 (English, mp4)
 Amount of Substance and Its Unit Mole- Connecting the Invisible Micro World to the Observable Macro World Part 2 (English, mp4) [MUSIC PLAYING] Instructor: Hi, everyone. Welcome back. I hope you had some
Amount of Substance and Its Unit Mole- Connecting the Invisible Micro World to the Observable Macro World Part 2 (English, mp4) [MUSIC PLAYING] Instructor: Hi, everyone. Welcome back. I hope you had some
MITOCW ocw f99-lec17_300k
 MITOCW ocw-18.06-f99-lec17_300k OK, here's the last lecture in the chapter on orthogonality. So we met orthogonal vectors, two vectors, we met orthogonal subspaces, like the row space and null space. Now
MITOCW ocw-18.06-f99-lec17_300k OK, here's the last lecture in the chapter on orthogonality. So we met orthogonal vectors, two vectors, we met orthogonal subspaces, like the row space and null space. Now
Talk Science Professional Development
 Talk Science Professional Development Transcript for Grade 5 Scientist Case: The Water to Ice Investigations 1. The Water to Ice Investigations Through the Eyes of a Scientist We met Dr. Hugh Gallagher
Talk Science Professional Development Transcript for Grade 5 Scientist Case: The Water to Ice Investigations 1. The Water to Ice Investigations Through the Eyes of a Scientist We met Dr. Hugh Gallagher
Atomic Origins. Slide 1 / 20. Slide 2 / 20. Slide 3 / 20. Observing Chemical Reactions Lab. Thoughts on the Nature of Matter
 Slide 1 / 20 Slide 2 / 20 Atomic Origins Observing Chemical Reactions Lab 2015-10-27 www.njctl.org Thoughts on the Nature of Matter Slide 3 / 20 Since ancient times, humans have pondered the nature of
Slide 1 / 20 Slide 2 / 20 Atomic Origins Observing Chemical Reactions Lab 2015-10-27 www.njctl.org Thoughts on the Nature of Matter Slide 3 / 20 Since ancient times, humans have pondered the nature of
MITOCW watch?v=ed_xr1bzuqs
 MITOCW watch?v=ed_xr1bzuqs The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
MITOCW watch?v=ed_xr1bzuqs The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
MITOCW watch?v=ko0vmalkgj8
 MITOCW watch?v=ko0vmalkgj8 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
MITOCW watch?v=ko0vmalkgj8 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
Recognizing Chemical_Reactions By Dr. Kathleen Vandiver
 Recognizing Chemical_Reactions By Dr. Kathleen Vandiver Hello. My name is Dr. Kathy Vandiver. And we're here at the Edgerton Center at MIT, a university in the United States. And the Edgerton Center is
Recognizing Chemical_Reactions By Dr. Kathleen Vandiver Hello. My name is Dr. Kathy Vandiver. And we're here at the Edgerton Center at MIT, a university in the United States. And the Edgerton Center is
MITOCW MITRES18_005S10_DerivOfSinXCosX_300k_512kb-mp4
 MITOCW MITRES18_005S10_DerivOfSinXCosX_300k_512kb-mp4 PROFESSOR: OK, this lecture is about the slopes, the derivatives, of two of the great functions of mathematics: sine x and cosine x. Why do I say great
MITOCW MITRES18_005S10_DerivOfSinXCosX_300k_512kb-mp4 PROFESSOR: OK, this lecture is about the slopes, the derivatives, of two of the great functions of mathematics: sine x and cosine x. Why do I say great
Chemistry and Atoms! 8 th grade history information to help you understand the background of how our knowledge grew through the years.
 Chemistry and Atoms! 8 th grade history information to help you understand the background of how our knowledge grew through the years. Chemistry lies at the roots of civilization! Chemical reactions are
Chemistry and Atoms! 8 th grade history information to help you understand the background of how our knowledge grew through the years. Chemistry lies at the roots of civilization! Chemical reactions are
The History of Atomic Theory Chapter 3--Chemistry
 The History of Atomic Theory Chapter 3--Chemistry In this lesson, we ll learn about the men whose quests for knowledge about the fundamental nature of the universe helped define our views. The atomic model
The History of Atomic Theory Chapter 3--Chemistry In this lesson, we ll learn about the men whose quests for knowledge about the fundamental nature of the universe helped define our views. The atomic model
UNIT 2 Atomic Structure
 UNIT 2 Atomic Structure Section 1: History & Development of Atomic Theory (Chapter 3) History of the Atom Video The Greeks Democritus World made of empty space and tiny particles ( atoms ) Thought there
UNIT 2 Atomic Structure Section 1: History & Development of Atomic Theory (Chapter 3) History of the Atom Video The Greeks Democritus World made of empty space and tiny particles ( atoms ) Thought there
MITOCW R11. Double Pendulum System
 MITOCW R11. Double Pendulum System The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for
MITOCW R11. Double Pendulum System The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for
MITOCW Investigation 4, Part 3
 MITOCW Investigation 4, Part 3 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free.
MITOCW Investigation 4, Part 3 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free.
1.02 Scientific Method
 1.02 Scientific Method Dr. Fred O. Garces Chemistry 100 Miramar College 1 Science and the meaning of life Ever since humans had the ability to think and reason, they have been trying to answer some basic
1.02 Scientific Method Dr. Fred O. Garces Chemistry 100 Miramar College 1 Science and the meaning of life Ever since humans had the ability to think and reason, they have been trying to answer some basic
2 History of the Atomic
 www.ck12.org CHAPTER 2 History of the Atomic Theory Chapter Outline 2.1 DEMOCRITUS IDEA OF THE ATOM 2.2 DALTON S ATOMIC THEORY 2.3 THOMSON S ATOMIC MODEL 2.4 RUTHERFORD S ATOMIC MODEL 2.5 BOHR S ATOMIC
www.ck12.org CHAPTER 2 History of the Atomic Theory Chapter Outline 2.1 DEMOCRITUS IDEA OF THE ATOM 2.2 DALTON S ATOMIC THEORY 2.3 THOMSON S ATOMIC MODEL 2.4 RUTHERFORD S ATOMIC MODEL 2.5 BOHR S ATOMIC
MITOCW ocw f99-lec30_300k
 MITOCW ocw-18.06-f99-lec30_300k OK, this is the lecture on linear transformations. Actually, linear algebra courses used to begin with this lecture, so you could say I'm beginning this course again by
MITOCW ocw-18.06-f99-lec30_300k OK, this is the lecture on linear transformations. Actually, linear algebra courses used to begin with this lecture, so you could say I'm beginning this course again by
Chemistry and Atoms! 8 th grade history information to help you understand the background of how our knowledge grew through the years.
 Chemistry and Atoms! 8 th grade history information to help you understand the background of how our knowledge grew through the years. Chemistry lies at the roots of civilization! Chemical reactions are
Chemistry and Atoms! 8 th grade history information to help you understand the background of how our knowledge grew through the years. Chemistry lies at the roots of civilization! Chemical reactions are
PROFESSOR: WELCOME BACK TO THE LAST LECTURE OF THE SEMESTER. PLANNING TO DO TODAY WAS FINISH THE BOOK. FINISH SECTION 6.5
 1 MATH 16A LECTURE. DECEMBER 9, 2008. PROFESSOR: WELCOME BACK TO THE LAST LECTURE OF THE SEMESTER. I HOPE YOU ALL WILL MISS IT AS MUCH AS I DO. SO WHAT I WAS PLANNING TO DO TODAY WAS FINISH THE BOOK. FINISH
1 MATH 16A LECTURE. DECEMBER 9, 2008. PROFESSOR: WELCOME BACK TO THE LAST LECTURE OF THE SEMESTER. I HOPE YOU ALL WILL MISS IT AS MUCH AS I DO. SO WHAT I WAS PLANNING TO DO TODAY WAS FINISH THE BOOK. FINISH
The Story of the Atom. A history of atomic theory over many years
 The Story of the Atom A history of atomic theory over many years Democritus Many years ago, between 460BC and 370BC the Greek philosophers wondered what we were made of. Leucippus and Democritus came up
The Story of the Atom A history of atomic theory over many years Democritus Many years ago, between 460BC and 370BC the Greek philosophers wondered what we were made of. Leucippus and Democritus came up
MITOCW ocw f99-lec01_300k
 MITOCW ocw-18.06-f99-lec01_300k Hi. This is the first lecture in MIT's course 18.06, linear algebra, and I'm Gilbert Strang. The text for the course is this book, Introduction to Linear Algebra. And the
MITOCW ocw-18.06-f99-lec01_300k Hi. This is the first lecture in MIT's course 18.06, linear algebra, and I'm Gilbert Strang. The text for the course is this book, Introduction to Linear Algebra. And the
MITOCW ocw-5_60-s08-lec07_300k
 MITOCW ocw-5_60-s08-lec07_300k The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality educational resources for free.
MITOCW ocw-5_60-s08-lec07_300k The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality educational resources for free.
MITOCW MITRES18_005S10_DiffEqnsGrowth_300k_512kb-mp4
 MITOCW MITRES18_005S10_DiffEqnsGrowth_300k_512kb-mp4 GILBERT STRANG: OK, today is about differential equations. That's where calculus really is applied. And these will be equations that describe growth.
MITOCW MITRES18_005S10_DiffEqnsGrowth_300k_512kb-mp4 GILBERT STRANG: OK, today is about differential equations. That's where calculus really is applied. And these will be equations that describe growth.
Note: Please use the actual date you accessed this material in your citation.
 MIT OpenCourseWare http://ocw.mit.edu 18.06 Linear Algebra, Spring 2005 Please use the following citation format: Gilbert Strang, 18.06 Linear Algebra, Spring 2005. (Massachusetts Institute of Technology:
MIT OpenCourseWare http://ocw.mit.edu 18.06 Linear Algebra, Spring 2005 Please use the following citation format: Gilbert Strang, 18.06 Linear Algebra, Spring 2005. (Massachusetts Institute of Technology:
MITOCW Lec 15 MIT 6.042J Mathematics for Computer Science, Fall 2010
 MITOCW Lec 15 MIT 6.042J Mathematics for Computer Science, Fall 2010 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality
MITOCW Lec 15 MIT 6.042J Mathematics for Computer Science, Fall 2010 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality
Lesson 6: Units and Unit Conversions Introduction
 Lesson 6: Units and Unit Conversions Introduction This document is Lesson 6 of the SEAChem2020 open source chemistry curriculum program for secular homeschoolers. This version was current as of 8/30/2017.
Lesson 6: Units and Unit Conversions Introduction This document is Lesson 6 of the SEAChem2020 open source chemistry curriculum program for secular homeschoolers. This version was current as of 8/30/2017.
Lesson 9: The History of the Atom, Part 2 Introduction
 Lesson 9: The History of the Atom, Part 2 Introduction This document is Lesson 9 of the SEAChem2020 open source chemistry curriculum program for secular homeschoolers. This version was current as of 8
Lesson 9: The History of the Atom, Part 2 Introduction This document is Lesson 9 of the SEAChem2020 open source chemistry curriculum program for secular homeschoolers. This version was current as of 8
STATES OF MATTER NOTES..
 STATES OF MATTER NOTES.. While you are reading, answer the following which will help you with the States of Matter Project. What is matter (definition): What are the states of matter and what are the characteristics/properties
STATES OF MATTER NOTES.. While you are reading, answer the following which will help you with the States of Matter Project. What is matter (definition): What are the states of matter and what are the characteristics/properties
- measures the center of our distribution. In the case of a sample, it s given by: y i. y = where n = sample size.
 Descriptive Statistics: One of the most important things we can do is to describe our data. Some of this can be done graphically (you should be familiar with histograms, boxplots, scatter plots and so
Descriptive Statistics: One of the most important things we can do is to describe our data. Some of this can be done graphically (you should be familiar with histograms, boxplots, scatter plots and so
Activity 2 Elements and Their Properties
 Activity 2 Elements and Their Properties Activity 2 Elements and Their Properties GOALS In this activity you will: Apply ancient definitions of elements to materials you believe are elements. Test some
Activity 2 Elements and Their Properties Activity 2 Elements and Their Properties GOALS In this activity you will: Apply ancient definitions of elements to materials you believe are elements. Test some
MITOCW Colorful Indicators MIT Chemistry Behind the Magic
 MITOCW Colorful Indicators MIT Chemistry Behind the Magic [MUSIC PLAYING] Hi, I'm Jessica and today I'm going to talk to you about a chemical demonstration I like to call colorful indicators. Indicators
MITOCW Colorful Indicators MIT Chemistry Behind the Magic [MUSIC PLAYING] Hi, I'm Jessica and today I'm going to talk to you about a chemical demonstration I like to call colorful indicators. Indicators
MITOCW ocw f99-lec16_300k
 MITOCW ocw-18.06-f99-lec16_300k OK. Here's lecture sixteen and if you remember I ended up the last lecture with this formula for what I called a projection matrix. And maybe I could just recap for a minute
MITOCW ocw-18.06-f99-lec16_300k OK. Here's lecture sixteen and if you remember I ended up the last lecture with this formula for what I called a projection matrix. And maybe I could just recap for a minute
MITOCW ocw f99-lec05_300k
 MITOCW ocw-18.06-f99-lec05_300k This is lecture five in linear algebra. And, it will complete this chapter of the book. So the last section of this chapter is two point seven that talks about permutations,
MITOCW ocw-18.06-f99-lec05_300k This is lecture five in linear algebra. And, it will complete this chapter of the book. So the last section of this chapter is two point seven that talks about permutations,
MITOCW MIT18_02SCF10Rec_61_300k
 MITOCW MIT18_02SCF10Rec_61_300k JOEL LEWIS: Hi. Welcome back to recitation. In lecture, you've been learning about the divergence theorem, also known as Gauss's theorem, and flux, and all that good stuff.
MITOCW MIT18_02SCF10Rec_61_300k JOEL LEWIS: Hi. Welcome back to recitation. In lecture, you've been learning about the divergence theorem, also known as Gauss's theorem, and flux, and all that good stuff.
Atoms, Elements, and the Periodic Table
 chapter 00 3 3 Atoms, Elements, and the Periodic Table section 1 Structure of Matter Before You Read Take a deep breath. What fills your lungs? Can you see it or hold it in your hand? What You ll Learn
chapter 00 3 3 Atoms, Elements, and the Periodic Table section 1 Structure of Matter Before You Read Take a deep breath. What fills your lungs? Can you see it or hold it in your hand? What You ll Learn
Early Atomic Theory. Alchemy. The atom
 Early Atomic Theory Chapter 3 Democritus 460 BC- ~ 370 BC Nothing exists except atoms and empty space; everything else is opinion. Matter is composed of small indivisible particles, atomos meaning Indivisible
Early Atomic Theory Chapter 3 Democritus 460 BC- ~ 370 BC Nothing exists except atoms and empty space; everything else is opinion. Matter is composed of small indivisible particles, atomos meaning Indivisible
Chapter 2: An Evolving Model
 Physical Science 4010 Nuclear Technology Chapter 2: An Evolving Model Introduction The universe is made up of two things: Matter (physical, tangible stuff) Energy (more than just the ability to do work)
Physical Science 4010 Nuclear Technology Chapter 2: An Evolving Model Introduction The universe is made up of two things: Matter (physical, tangible stuff) Energy (more than just the ability to do work)
3.01 Understanding Atoms
 3.01 Understanding Atoms The Events Leading to the Discovery of the Building Block of Matter Dr. Fred Omega Garces Chemistry 111 Miramar College 1 3.02 Atomic Evolution Environmental Problems in our Lifetime
3.01 Understanding Atoms The Events Leading to the Discovery of the Building Block of Matter Dr. Fred Omega Garces Chemistry 111 Miramar College 1 3.02 Atomic Evolution Environmental Problems in our Lifetime
Fog Chamber Testing the Label: Photo of Fog. Joshua Gutwill 10/29/1999
 Fog Chamber Testing the Label: Photo of Fog Joshua Gutwill 10/29/1999 Keywords: < > formative heat&temp exhibit interview 1 Goals/Context Fog Chamber Interview Results Testing Label: Photo of fog on Golden
Fog Chamber Testing the Label: Photo of Fog Joshua Gutwill 10/29/1999 Keywords: < > formative heat&temp exhibit interview 1 Goals/Context Fog Chamber Interview Results Testing Label: Photo of fog on Golden
UNIT 2 The Particulate Nature of Matter
 UNIT 2 The Particulate Nature of Matter Take a moment to think about all of the different properties which can be exhibited by matter. The list seems endless. Matter can be found in a variety of forms,
UNIT 2 The Particulate Nature of Matter Take a moment to think about all of the different properties which can be exhibited by matter. The list seems endless. Matter can be found in a variety of forms,
Atomic Structure. For thousands of years, people had many ideas about matter Ancient Greeks believed that everything was made up of the four elements
 An atom is the smallest particle of an element that retains its identity in a chemical reaction. Although early philosophers and scientists could not observe individual atoms, they were still able to propose
An atom is the smallest particle of an element that retains its identity in a chemical reaction. Although early philosophers and scientists could not observe individual atoms, they were still able to propose
The Cycloid. and the Kinematic Circumference. by Miles Mathis
 return to updates The Cycloid and the Kinematic Circumference First published August 31, 2016 by Miles Mathis Those of you who have read my papers on π=4 will know I have explained that problem using many
return to updates The Cycloid and the Kinematic Circumference First published August 31, 2016 by Miles Mathis Those of you who have read my papers on π=4 will know I have explained that problem using many
6.041SC Probabilistic Systems Analysis and Applied Probability, Fall 2013 Transcript Tutorial:A Random Number of Coin Flips
 6.041SC Probabilistic Systems Analysis and Applied Probability, Fall 2013 Transcript Tutorial:A Random Number of Coin Flips Hey, everyone. Welcome back. Today, we're going to do another fun problem that
6.041SC Probabilistic Systems Analysis and Applied Probability, Fall 2013 Transcript Tutorial:A Random Number of Coin Flips Hey, everyone. Welcome back. Today, we're going to do another fun problem that
Major upsetting discoveries: Today s Objectives/Agenda. Notice: New Unit: with Ms. V. after school Before Friday 9/22.
 Bellwork Monday 9 18 17 Tuesday 9 19 17 *This should be the last box on bellwork: 1. What are some major points in history where common knowledge was upset by a new discovery? 2. Draw what you think an
Bellwork Monday 9 18 17 Tuesday 9 19 17 *This should be the last box on bellwork: 1. What are some major points in history where common knowledge was upset by a new discovery? 2. Draw what you think an
MITOCW MITRES18_006F10_26_0501_300k-mp4
 MITOCW MITRES18_006F10_26_0501_300k-mp4 ANNOUNCER: The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational
MITOCW MITRES18_006F10_26_0501_300k-mp4 ANNOUNCER: The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational
CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED)
 CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED) Overview: Students investigate chemical reactions using given substances. Students identify chemical reactions in their daily lives. Objectives: The student
CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED) Overview: Students investigate chemical reactions using given substances. Students identify chemical reactions in their daily lives. Objectives: The student
2/22/2019 NEW UNIT! Chemical Interactions. Atomic Basics #19
 NEW UNIT! Chemical Interactions Atomic Basics #19 1 Vocabulary: Matter: Anything that has mass and takes up space. Atom: the smallest particle of matter. Element: A pure substance made up of only one type
NEW UNIT! Chemical Interactions Atomic Basics #19 1 Vocabulary: Matter: Anything that has mass and takes up space. Atom: the smallest particle of matter. Element: A pure substance made up of only one type
Instructor (Brad Osgood)
 TheFourierTransformAndItsApplications-Lecture26 Instructor (Brad Osgood): Relax, but no, no, no, the TV is on. It's time to hit the road. Time to rock and roll. We're going to now turn to our last topic
TheFourierTransformAndItsApplications-Lecture26 Instructor (Brad Osgood): Relax, but no, no, no, the TV is on. It's time to hit the road. Time to rock and roll. We're going to now turn to our last topic
Topic 2 Atomic Structure. IB Chemistry SL Coral Gables Senior High School Ms. Kiely
 Topic 2 Atomic Structure IB Chemistry SL Coral Gables Senior High School Ms. Kiely Prepare for Lab Quiz: Put everything away except for a pen (no pencil) and a calculator. Bell-Ringer This is an example
Topic 2 Atomic Structure IB Chemistry SL Coral Gables Senior High School Ms. Kiely Prepare for Lab Quiz: Put everything away except for a pen (no pencil) and a calculator. Bell-Ringer This is an example
Electricity Show Outline
 Electricity Show Outline Idea Who Slide Demos Intro. All Terms Voltage V Voltage Balls, balloons Current C Current Ladder, tennis ball, foam peanuts, Jacob s ladder Resistance R Resistance Balloons, 1
Electricity Show Outline Idea Who Slide Demos Intro. All Terms Voltage V Voltage Balls, balloons Current C Current Ladder, tennis ball, foam peanuts, Jacob s ladder Resistance R Resistance Balloons, 1
Chemistry Chapter 3. Atoms: The Building Blocks of Matter
 Chemistry Chapter 3 Atoms: The Building Blocks of Matter I. From Philosophical Idea to Scientific Theory History of the Atom The Ancient Greeks were the first to come up with the idea of the atom. Democritus
Chemistry Chapter 3 Atoms: The Building Blocks of Matter I. From Philosophical Idea to Scientific Theory History of the Atom The Ancient Greeks were the first to come up with the idea of the atom. Democritus
Uncertainty: A Reading Guide and Self-Paced Tutorial
 Uncertainty: A Reading Guide and Self-Paced Tutorial First, read the description of uncertainty at the Experimental Uncertainty Review link on the Physics 108 web page, up to and including Rule 6, making
Uncertainty: A Reading Guide and Self-Paced Tutorial First, read the description of uncertainty at the Experimental Uncertainty Review link on the Physics 108 web page, up to and including Rule 6, making
The Inductive Proof Template
 CS103 Handout 24 Winter 2016 February 5, 2016 Guide to Inductive Proofs Induction gives a new way to prove results about natural numbers and discrete structures like games, puzzles, and graphs. All of
CS103 Handout 24 Winter 2016 February 5, 2016 Guide to Inductive Proofs Induction gives a new way to prove results about natural numbers and discrete structures like games, puzzles, and graphs. All of
MITOCW MIT18_01SCF10Rec_24_300k
 MITOCW MIT18_01SCF10Rec_24_300k JOEL LEWIS: Hi. Welcome back to recitation. In lecture, you've been doing related rates problems. I've got another example for you, here. So this one's a really tricky one.
MITOCW MIT18_01SCF10Rec_24_300k JOEL LEWIS: Hi. Welcome back to recitation. In lecture, you've been doing related rates problems. I've got another example for you, here. So this one's a really tricky one.
HIGH SCHOOL SCIENCE. Physical Science 9: Atomic Structure
 HIGH SCHOOL SCIENCE Physical Science 9: Atomic Structure WILLMAR PUBLIC SCHOOL 2013-2014 EDITION CHAPTER 9 Atomic Structure In this chapter you will: 1. Compare and contrast quarks, leptons, and bosons.
HIGH SCHOOL SCIENCE Physical Science 9: Atomic Structure WILLMAR PUBLIC SCHOOL 2013-2014 EDITION CHAPTER 9 Atomic Structure In this chapter you will: 1. Compare and contrast quarks, leptons, and bosons.
MITOCW ocw f99-lec23_300k
 MITOCW ocw-18.06-f99-lec23_300k -- and lift-off on differential equations. So, this section is about how to solve a system of first order, first derivative, constant coefficient linear equations. And if
MITOCW ocw-18.06-f99-lec23_300k -- and lift-off on differential equations. So, this section is about how to solve a system of first order, first derivative, constant coefficient linear equations. And if
MITOCW MIT7_01SCF11_track24_300k.mp4
 MITOCW MIT7_01SCF11_track24_300k.mp4 PROFESSOR: OK. So I need to move on a little bit now, and I want to talk about in fact, the earlier way that nature developed to make energy using proton gradients.
MITOCW MIT7_01SCF11_track24_300k.mp4 PROFESSOR: OK. So I need to move on a little bit now, and I want to talk about in fact, the earlier way that nature developed to make energy using proton gradients.
MITOCW MITRES_18-007_Part5_lec3_300k.mp4
 MITOCW MITRES_18-007_Part5_lec3_300k.mp4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality educational resources
MITOCW MITRES_18-007_Part5_lec3_300k.mp4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality educational resources
MITOCW ocw feb k
 MITOCW ocw-18-086-13feb2006-220k INTRODUCTION: The following content is provided by MIT OpenCourseWare under a Creative Commons license. Additional information about our license and MIT OpenCourseWare
MITOCW ocw-18-086-13feb2006-220k INTRODUCTION: The following content is provided by MIT OpenCourseWare under a Creative Commons license. Additional information about our license and MIT OpenCourseWare
MITOCW ocw f08-lec19_300k
 MITOCW ocw-5-111-f08-lec19_300k The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free.
MITOCW ocw-5-111-f08-lec19_300k The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free.
MITOCW 8. Electromagnetic Waves in a Vacuum
 MITOCW 8. Electromagnetic Waves in a Vacuum The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality educational resources
MITOCW 8. Electromagnetic Waves in a Vacuum The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality educational resources
4.1 Defining the Atom > Chapter 4 Atomic Structure. 4.1 Defining the Atom. 4.2 Structure of the Nuclear Atom. 4.3 Distinguishing Among Atoms
 Chapter 4 Atomic Structure 4.1 Defining the Atom 4.2 Structure of the Nuclear Atom 4.3 Distinguishing Among Atoms 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY
Chapter 4 Atomic Structure 4.1 Defining the Atom 4.2 Structure of the Nuclear Atom 4.3 Distinguishing Among Atoms 1 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved. CHEMISTRY
MITOCW ocw-5_60-s08-lec19_300k
 MITOCW ocw-5_60-s08-lec19_300k The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free.
MITOCW ocw-5_60-s08-lec19_300k The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free.
MITOCW ocw f07-lec39_300k
 MITOCW ocw-18-01-f07-lec39_300k The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free.
MITOCW ocw-18-01-f07-lec39_300k The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free.
MITOCW watch?v=pqkyqu11eta
 MITOCW watch?v=pqkyqu11eta The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
MITOCW watch?v=pqkyqu11eta The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
Post-Show. Chemistry. Periodic Table of the Elements. After the Show. Traveling Science Shows
 Traveling Science Shows Post-Show Chemistry After the Show We recently presented a Chemistry show at your school, and thought you and your students might like to continue investigating this topic. The
Traveling Science Shows Post-Show Chemistry After the Show We recently presented a Chemistry show at your school, and thought you and your students might like to continue investigating this topic. The
MITOCW MITRES18_006F10_26_0602_300k-mp4
 MITOCW MITRES18_006F10_26_0602_300k-mp4 FEMALE VOICE: The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational
MITOCW MITRES18_006F10_26_0602_300k-mp4 FEMALE VOICE: The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational
MITOCW 6. Standing Waves Part I
 MITOCW 6. Standing Waves Part I The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free.
MITOCW 6. Standing Waves Part I The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free.
MITOCW ocw nov2005-pt1-220k_512kb.mp4
 MITOCW ocw-3.60-03nov2005-pt1-220k_512kb.mp4 PROFESSOR: All right, I would like to then get back to a discussion of some of the basic relations that we have been discussing. We didn't get terribly far,
MITOCW ocw-3.60-03nov2005-pt1-220k_512kb.mp4 PROFESSOR: All right, I would like to then get back to a discussion of some of the basic relations that we have been discussing. We didn't get terribly far,
CHM Units and Dimensional Analysis (r14) Charles Taylor 1/6
 CHM 110 - Units and Dimensional Analysis (r14) - 2014 Charles Taylor 1/6 Introduction Units are critical in all measurements. If you don't tell someone a unit when you tell them a number, they're not likely
CHM 110 - Units and Dimensional Analysis (r14) - 2014 Charles Taylor 1/6 Introduction Units are critical in all measurements. If you don't tell someone a unit when you tell them a number, they're not likely
Chemistry. Robert Taggart
 Chemistry Robert Taggart Table of Contents To the Student..................................................v Unit 1: Matter and Measurement Lesson 1: Chemistry and the Scientific Method...................3
Chemistry Robert Taggart Table of Contents To the Student..................................................v Unit 1: Matter and Measurement Lesson 1: Chemistry and the Scientific Method...................3
Atomic theory. Atoms: The Building Blocks of Matter
 Atomic theory Atoms: The Building Blocks of Matter First, there was Democritus Democritus was a Greek philosopher atomos He came up with the idea of the atom around 400BCE He had no evidence, he just thought
Atomic theory Atoms: The Building Blocks of Matter First, there was Democritus Democritus was a Greek philosopher atomos He came up with the idea of the atom around 400BCE He had no evidence, he just thought
Atoms Around Us. Common Elements
 Atoms Around Us Atoms are building blocks. If you want to create a language, you'll need an alphabet. If you want to build molecules, you will need atoms of different elements. Elements are the alphabet
Atoms Around Us Atoms are building blocks. If you want to create a language, you'll need an alphabet. If you want to build molecules, you will need atoms of different elements. Elements are the alphabet
Atomic Pudding Models of the Atom
 Atomic Pudding Models of the Atom Think About It The drawing depicts a very tiny sample of gold taken from a gold ring. The spheres in the cube of gold are so small that they cannot be seen. What are the
Atomic Pudding Models of the Atom Think About It The drawing depicts a very tiny sample of gold taken from a gold ring. The spheres in the cube of gold are so small that they cannot be seen. What are the
Module 3 Study Guide. GCF Method: Notice that a polynomial like 2x 2 8 xy+9 y 2 can't be factored by this method.
 Module 3 Study Guide The second module covers the following sections of the textbook: 5.4-5.8 and 6.1-6.5. Most people would consider this the hardest module of the semester. Really, it boils down to your
Module 3 Study Guide The second module covers the following sections of the textbook: 5.4-5.8 and 6.1-6.5. Most people would consider this the hardest module of the semester. Really, it boils down to your
Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na
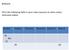 Bellwork: Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na + 11 29 63 Bellwork: Fill in the Following Table in
Bellwork: Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na + 11 29 63 Bellwork: Fill in the Following Table in
Atoms and Bonding Study Guide
 Parts of an atom Atoms and Bonding Study Guide All matter in the universe, including stars, buildings, people, and ipods is made of tiny particles called atoms. The behavior of atoms is chemistry's main
Parts of an atom Atoms and Bonding Study Guide All matter in the universe, including stars, buildings, people, and ipods is made of tiny particles called atoms. The behavior of atoms is chemistry's main
What s the Matter? An in depth look at matter.
 What s the Matter? An in depth look at matter. What is a mixture? Examine the objects. Then sort them into at least three groups. Each item should be grouped with similar items. Think about each objects
What s the Matter? An in depth look at matter. What is a mixture? Examine the objects. Then sort them into at least three groups. Each item should be grouped with similar items. Think about each objects
MITOCW 2. Harmonic Oscillators with Damping
 MITOCW 2. Harmonic Oscillators with Damping The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources
MITOCW 2. Harmonic Oscillators with Damping The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources
MITOCW 5. Quantum Mechanics: Free Particle and Particle in 1D Box
 MITOCW 5. Quantum Mechanics: Free Particle and Particle in 1D Box The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality
MITOCW 5. Quantum Mechanics: Free Particle and Particle in 1D Box The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality
Name: Packet Due Date: Tuesday, 9/18. Science
 Name: Packet Due Date: Tuesday, 9/18 Science Module 2 Chapter 1 Phase Change Describing Phase Change at Two Scales What happened to the liquid in Titan s Lake? (NGSS Performance Expectations: MS-PS1-1;
Name: Packet Due Date: Tuesday, 9/18 Science Module 2 Chapter 1 Phase Change Describing Phase Change at Two Scales What happened to the liquid in Titan s Lake? (NGSS Performance Expectations: MS-PS1-1;
Today is Thursday, September 7 th, 2017
 In This Lesson: Unit 1 Matter and Change (Lesson 1 of 6) Today is Thursday, September 7 th, 2017 Pre-Class: Identify the chemistry in this scene (and I don t mean between the people). Hint: There are chemical
In This Lesson: Unit 1 Matter and Change (Lesson 1 of 6) Today is Thursday, September 7 th, 2017 Pre-Class: Identify the chemistry in this scene (and I don t mean between the people). Hint: There are chemical
Talk Science Professional Development
 Talk Science Professional Development Transcript for Grade 4 Scientist Case: The Heavy for Size Investigations 1. The Heavy for Size Investigations, Through the Eyes of a Scientist We met Associate Professor
Talk Science Professional Development Transcript for Grade 4 Scientist Case: The Heavy for Size Investigations 1. The Heavy for Size Investigations, Through the Eyes of a Scientist We met Associate Professor
Changes of State. Thermal Energy: Changes of State 1
 Changes of State Last lesson we learned about thermal energy and temperature. Objects whose molecules are moving very quickly are said to have high thermal energy or high temperature. The higher the temperature,
Changes of State Last lesson we learned about thermal energy and temperature. Objects whose molecules are moving very quickly are said to have high thermal energy or high temperature. The higher the temperature,
Particle Theory of Matter. By the late 1700s, scientists had adopted the Particle Theory of Matter. This theory states that:
 Particle Theory of Matter By the late 1700s, scientists had adopted the Particle Theory of Matter. This theory states that: all matter is made up of very tiny particles each pure substance has its own
Particle Theory of Matter By the late 1700s, scientists had adopted the Particle Theory of Matter. This theory states that: all matter is made up of very tiny particles each pure substance has its own
But, there is always a certain amount of mystery that hangs around it. People scratch their heads and can't figure
 MITOCW 18-03_L19 Today, and for the next two weeks, we are going to be studying what, for many engineers and a few scientists is the most popular method of solving any differential equation of the kind
MITOCW 18-03_L19 Today, and for the next two weeks, we are going to be studying what, for many engineers and a few scientists is the most popular method of solving any differential equation of the kind
Guide to Negating Formulas
 Guide to Negating Formulas Hi everybody! We spent a little bit of time in class talking about how to negate formulas in propositional or frst-order logic. This is a really valuable skill! If you ever need
Guide to Negating Formulas Hi everybody! We spent a little bit of time in class talking about how to negate formulas in propositional or frst-order logic. This is a really valuable skill! If you ever need
1 Development of the Atomic Theory
 CHAPTER 4 1 Development of the Atomic Theory SECTION Introduction to Atoms BEFORE YOU READ After you read this section, you should be able to answer these questions: What is the atomic theory? How has
CHAPTER 4 1 Development of the Atomic Theory SECTION Introduction to Atoms BEFORE YOU READ After you read this section, you should be able to answer these questions: What is the atomic theory? How has
Acids, Bases, and Buffers
 Print Presentation Acids, Bases, and Buffers OVERVIEW You're probably familiar with acids and bases in the products you use at home. Rust removers often contain phosphoric acid. Muriatic acid (a common
Print Presentation Acids, Bases, and Buffers OVERVIEW You're probably familiar with acids and bases in the products you use at home. Rust removers often contain phosphoric acid. Muriatic acid (a common
Chemistry of Our Environment Notes. Chapter 1
 Chemistry of Our Environment Notes Introduction: Purpose of Course: 1. Meet Lib Ed Requirement 2. Fire Science & Dental Hygiene How we will meet both needs. Exams & Grading Homework Assignments Chapter
Chemistry of Our Environment Notes Introduction: Purpose of Course: 1. Meet Lib Ed Requirement 2. Fire Science & Dental Hygiene How we will meet both needs. Exams & Grading Homework Assignments Chapter
MITOCW MITRES_18-007_Part1_lec5_300k.mp4
 MITOCW MITRES_18-007_Part1_lec5_300k.mp4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality educational resources
MITOCW MITRES_18-007_Part1_lec5_300k.mp4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high-quality educational resources
Bellevue College CHEM& 121 Experiment: Stoichiometric Analysis of an Antacid 1
 Experiment: Stoichiometric Analysis of an Antacid 1 Introduction In this lab, you will use the concept of stoichiometry to solve two sequential problems. First, you will try to determine the products of
Experiment: Stoichiometric Analysis of an Antacid 1 Introduction In this lab, you will use the concept of stoichiometry to solve two sequential problems. First, you will try to determine the products of
Investigating Atoms and Atomic Theory
 Investigating Atoms and Atomic Theory Students should be able to: Describe the particle theory of matter. PS.2a Use the Bohr model to differentiate among the three basic particles in the atom (proton,
Investigating Atoms and Atomic Theory Students should be able to: Describe the particle theory of matter. PS.2a Use the Bohr model to differentiate among the three basic particles in the atom (proton,
Chemistry for Changing Times, 13e (Hill) Chapter 2 Atoms. 2.1 Multiple Choice Questions
 Chemistry for Changing Times 13th Edition Hill Test Bank Full Download: http://testbanklive.com/download/chemistry-for-changing-times-13th-edition-hill-test-bank/ Chemistry for Changing Times, 13e (Hill)
Chemistry for Changing Times 13th Edition Hill Test Bank Full Download: http://testbanklive.com/download/chemistry-for-changing-times-13th-edition-hill-test-bank/ Chemistry for Changing Times, 13e (Hill)
CHM 105 & 106 UNIT TWO, LECTURE EIGHT 1 IN OUR PREVIOUS LECTURE WE WERE LOOKING AT CONCENTRATION UNITS FOR SOLUTIONS
 CHM 105 & 106 UNIT TWO, LECTURE EIGHT 1 CHM 105/106 Program 15: Unit 2 Lecture 8 IN OUR PREVIOUS LECTURE WE WERE LOOKING AT CONCENTRATION UNITS FOR SOLUTIONS AND WE HAD LOOKED AT PERCENT BY MASS AND PERCENT
CHM 105 & 106 UNIT TWO, LECTURE EIGHT 1 CHM 105/106 Program 15: Unit 2 Lecture 8 IN OUR PREVIOUS LECTURE WE WERE LOOKING AT CONCENTRATION UNITS FOR SOLUTIONS AND WE HAD LOOKED AT PERCENT BY MASS AND PERCENT
