Jacob s Ladder Controlling Lightning
|
|
|
- Aileen Alexander
- 6 years ago
- Views:
Transcription
1 Host: Fusion specialist: Jacob s Ladder Controlling Lightning PART 1 Jacob s ladder demonstration Video Teacher resources Phil Dooley European Fusion Development Agreement Peter de Vries European Fusion Development Agreement The Jacob s ladder is an example of a sustained electrical breakdown between two electrodes in other words a plasma. The voltage between the two copper electrodes is high enough to ionize the air (i.e. to separate electrons from their atoms), and this cloud of ions and electrons being charged allows current to flow between the two electrodes, which air being neutral does not. Unlike a spark of static electricity which might zap between you and a door handle on a dry day, these electrodes have their charge replenished by the power supply and hence the current continues to flow through this area of low resistance. The initial breakdown occurs at the point where the electrodes are closest, which is designed to be at the bottom of the electrodes. Then the ions, which are heated by this process, rise, and so the discharge, which is taking the path of low resistance provided by these ions, travels up the electrodes. At the top, the ions rise so far that the pathway gets too long. This long pathway means the resistance increases, to a point at which the resistance of the neutral air at the small gap at the bottom of the electrodes becomes a preferential path for the current to flow. Dr de Vries points out that there is an additional mechanism in the breakdown process, an avalanche process. This occurs when charged species (electrons or ions which are usually present in small quantities) are accelerated and collide with other atoms, ionizing them, and creating more electrons which can be accelerated to create other ions and so on. Hence the path length and density (or mean-free path) are important quantities as well as the electric field. PART 2 Application to JET Dr de Vries explains that the electric field causing breakdown in JET is not a gap between two electrodes. Instead it is provided by the transformer effect the central solenoid in the middle of the doughnut acts as a primary coil of a transformer, and the gas in the torus vessel acts as the secondary. By using gas at the optimum pressure level (about one million times less than atmosphere) the required electric field to achieve breakdown is only a couple of volts (compared with the V/cm required in air.) At this pressure the distance an electron would travel before colliding with an atom (known as the mean-free path) is several metres, which is why it would have time to pick up enough momentum to ionise an atom
2 Pre-questions Does air conduct electricity? Why? No, it doesn t because it is made up of neutral atoms (for the most part; there are always trace amounts of free electrons and ions) If air doesn t conduct electricity, how does a spark form? If air experiences a electric field (voltage) large enough to remove an electron from an atom, then you now have two charged particles, an electron and an ion. These are then affected by the electric field, i.e. current flows from one electrode to the other. What makes up the atom, and what charge is each species? Protons (positive), neutrons (neutral), electrons (negative). What is the fourth state of matter, and describe it. Plasma a gas which has been ionized, and therefore is made up of charged particles (ions) and free electrons. Because it is charged it conducts electricity, and reacts to electric and magnetic fields. It can be hot or cold, at high or low pressure. (compare the hot dense sun, with a cold, low pressure nebula in space.) What are some examples of plasma: Stars, fluorescent tubes, flames, nebulae, plasma screen TVs. How do the particles of a plasma react to a magnetic field? Because they are charged they experience a force at right angles to both their direction of motion and the magnetic field. This leads to them following a helical path along the magnetic field lines, or, if they are initially traveling exactly at right angles to the magnetic field, to follow a circular path. Why is plasma required for fusion? Because fusion relies on nuclei colliding, for this to happen all the electrons around the nucleus must be removed. (Note, not all plasmas are completely ionized.) How does a transformer work? A changing current is put through a coil (known as the primary). A secondary coil, usually wound around the primary, has a current generated in it by the changing magnetic flux created by the primary.
3 Post-questions (on video content) What is the voltage between the electrodes in the Jacob s Ladder? V What are the two mechanisms for ion formation cited in the video? (1) Electric field/voltage, which separates electrons from the atoms. (2) Free electrons accelerated to high speeds by the electric field, which collide with neutral atoms and knock off other electrons Avalanche Process What is the shape of the magnetic field in JET? It is toroidal (doughnut shaped) although not perfectly so combined with a poloidal which gives a twist to the poloidal field. How big is the voltage inside JET that starts the ionisation? A few volts What generates the electric field inside the JET torus? The gas inside JET acts as the secondary coil of a transformer. The primary coil is a solenoid that runs through the centre of the doughnut a changing current is run through this solenoid, to generate the transformer action. Is this a step-up or step down transformer? Step down, as the plasma has only one turn. (Note: you are not told the number of turns in the central solenoid, so you have to estimate that it has more than one turn!) Why is a voltage so much lower than the Jacob s Ladder all that is required? Because the pressure is lower, the electrons travel further between each collision, and so pick up more energy. What is the pressure inside JET? A million times less than atmosphere. How far do the electrons travel on average? Several metres between collisions. Several kilometres before they hit the wall (due to the imperfections in the toroidal magnetic field.) What is the plasma current and temperature at the end of the avalanche process? A, o C What are the maximum plasma current and temperature for JET s fusion experiments? Several million amps, over a million degrees. JET s central solenoid has 710 turns, and the final current is 1 ka, driven by approximately 5 volts. Calculate the voltage and current in the central solenoid: The ratio of turns between the primary and secondary coils is 710:1. Therefore the voltage is 710 x 5 = 3550, and the current is 1000/710 = 1.41 A
4 Extension Questions How is transformer action of a tokamak like JET achieved, and how does it limit the experiment? What methods are addressing this limitation (e.g. stellarators, or heating systems)? Transformer action requires a changing current in the primary, so the central solenoid must begin the experiment with a very large current through it. This is then reduced, through zero, until it reaches a maximum value in the opposite direction. At this stage the tokamak has to stop. (Sweeping back in the opposite direction is not practicable as the zero current point confinement would be lost, and then subsequent reversed direction of the plasma would be problematic). Newly built tokamaks such as JT60SA in Japan and ITER use superconductors to increase the current and thereby extend the lifetime of the current-sweep, but it is still finite. Stellarators use a twisted magnetic field to drive current without the central solenoid, while other methods use microwave or beam injection systems to drive the current. There are two separate magnetic fields at play in JET, toroidal and poloidal. How are these different, how are they generated and what are they used for? The central solenoid generates a poloidal magnetic field, similar to the Earth s magnetic field. It has a changing current, which has the effect of driving the current in the plasma. The toroidal magnetic field is ring shaped, and follows the torus vessel. This is a constant field and is generated by 32 coils encircling the vessel. The combined vector sum of these two fields gives a helical path, which ensures that particles rotating around the outside of the vessel are constantly circulated back into the centre, where the toroidal field is stronger (because the inner parts of the coils are closer together). The heating from the transformer effect (known as ohmic heating) is limited to about one million degrees. Why is this and what other methods are used to heat the plasma to the required 150 million degrees? Ohmic heating loses efficacy once the plasma is mostly ionized because the resistance becomes very low, therefore the power drops because P = I 2 R. Heating is provided by microwave/rf electromagnetic radiation for two mechanisms (cyclotron motion, and lower hybrid) or neutral beam injection.
5 General Fusion Questions The fusion process turns hydrogen in helium. Compare with fission of uranium. Fission is splitting atom, to make smaller atoms. Small and very large atoms are both less stable, the most stable atoms are middle sized most stable of all is iron. Compare the process of burning hydrogen to fusing hydrogen. Burning is a chemical reaction requiring oxygen (oxidation), that produces H 2 O. Fusion is a nuclear process that produces helium, releasing more than a million times more energy per gram than burning releases. Most Fusion experiments fuse deuterium and tritium (isotopes of hydrogen). Why is this process used? Is this the same process as occurs in the sun? No the sun has a complex many step process. DT fusion is much more efficient and easier to achieve. Fusion reactors put out much more energy per volume than the sun. What happens to the nucleons (collective term for protons and neutrons) in DT fusion? They re-arrange themselves to a lower energy state: 3 (T) + 2 (D) rearrange to become 4(He) + 1(neutron) Compare the safety considerations for fusion versus a coal or uranium (fission)? Fusion: use of tritium, activation of vessel Coal: Carbon dioxide production, other fallout Uranium: long lived radioactive waste 28 Countries signed to an agreement to work on an energy source for the future. EFDA provides the framework, JET is the shared experiment, Fusion energy is the goal.
Plasma & Fusion on Earth: merging age-old natural phenomena into your present and future
 Plasma & Fusion on Earth: merging age-old natural phenomena into your present and future Presented by Rick Lee Chief Operator, DIII-D Operations Manager, Energy/Fusion Outreach Program General Atomics
Plasma & Fusion on Earth: merging age-old natural phenomena into your present and future Presented by Rick Lee Chief Operator, DIII-D Operations Manager, Energy/Fusion Outreach Program General Atomics
Nuclear Fusion 1 of 24 Boardworks Ltd 2011
 Nuclear Fusion 1 of 24 Boardworks Ltd 2011 2 of 24 Boardworks Ltd 2011 How do we get energy from atoms? 3 of 24 Boardworks Ltd 2011 Energy is produced from atoms in power stations using the process of
Nuclear Fusion 1 of 24 Boardworks Ltd 2011 2 of 24 Boardworks Ltd 2011 How do we get energy from atoms? 3 of 24 Boardworks Ltd 2011 Energy is produced from atoms in power stations using the process of
Chapter IX: Nuclear fusion
 Chapter IX: Nuclear fusion 1 Summary 1. General remarks 2. Basic processes 3. Characteristics of fusion 4. Solar fusion 5. Controlled fusion 2 General remarks (1) Maximum of binding energy per nucleon
Chapter IX: Nuclear fusion 1 Summary 1. General remarks 2. Basic processes 3. Characteristics of fusion 4. Solar fusion 5. Controlled fusion 2 General remarks (1) Maximum of binding energy per nucleon
Lecture 31 Chapter 22, Sections 3-5 Nuclear Reactions. Nuclear Decay Kinetics Fission Reactions Fusion Reactions
 Lecture Chapter, Sections -5 Nuclear Reactions Nuclear Decay Kinetics Fission Reactions Fusion Reactions Gamma Radiation Electromagnetic photons of very high energy Very penetrating can pass through the
Lecture Chapter, Sections -5 Nuclear Reactions Nuclear Decay Kinetics Fission Reactions Fusion Reactions Gamma Radiation Electromagnetic photons of very high energy Very penetrating can pass through the
Neutral beam plasma heating
 Seminar I b 1 st year, 2 nd cycle program Neutral beam plasma heating Author: Gabrijela Ikovic Advisor: prof.dr. Tomaž Gyergyek Ljubljana, May 2014 Abstract For plasma to be ignited, external heating is
Seminar I b 1 st year, 2 nd cycle program Neutral beam plasma heating Author: Gabrijela Ikovic Advisor: prof.dr. Tomaž Gyergyek Ljubljana, May 2014 Abstract For plasma to be ignited, external heating is
Atomic and Nuclear Physics. Topic 7.3 Nuclear Reactions
 Atomic and Nuclear Physics Topic 7.3 Nuclear Reactions Nuclear Reactions Rutherford conducted experiments bombarding nitrogen gas with alpha particles from bismuth-214. He discovered that fast-moving particles
Atomic and Nuclear Physics Topic 7.3 Nuclear Reactions Nuclear Reactions Rutherford conducted experiments bombarding nitrogen gas with alpha particles from bismuth-214. He discovered that fast-moving particles
Lecture 14, 8/9/2017. Nuclear Reactions and the Transmutation of Elements Nuclear Fission; Nuclear Reactors Nuclear Fusion
 Lecture 14, 8/9/2017 Nuclear Reactions and the Transmutation of Elements Nuclear Fission; Nuclear Reactors Nuclear Fusion Nuclear Reactions and the Transmutation of Elements A nuclear reaction takes place
Lecture 14, 8/9/2017 Nuclear Reactions and the Transmutation of Elements Nuclear Fission; Nuclear Reactors Nuclear Fusion Nuclear Reactions and the Transmutation of Elements A nuclear reaction takes place
Unit 3: Chemistry in Society Nuclear Chemistry Summary Notes
 St Ninian s High School Chemistry Department National 5 Chemistry Unit 3: Chemistry in Society Nuclear Chemistry Summary Notes Name Learning Outcomes After completing this topic you should be able to :
St Ninian s High School Chemistry Department National 5 Chemistry Unit 3: Chemistry in Society Nuclear Chemistry Summary Notes Name Learning Outcomes After completing this topic you should be able to :
10.4 Fission and Fusion
 This painting of an alchemist s laboratory was made around 1570. For centuries, these early scientists, known as alchemists, tried to use chemical reactions to make gold. The alchemists failed in their
This painting of an alchemist s laboratory was made around 1570. For centuries, these early scientists, known as alchemists, tried to use chemical reactions to make gold. The alchemists failed in their
INTRODUCTION TO MAGNETIC NUCLEAR FUSION
 INTRODUCTION TO MAGNETIC NUCLEAR FUSION S.E. Sharapov Euratom/CCFE Fusion Association, Culham Science Centre, Abingdon, Oxfordshire OX14 3DB, UK With acknowledgments to B.Alper for use of his transparencies
INTRODUCTION TO MAGNETIC NUCLEAR FUSION S.E. Sharapov Euratom/CCFE Fusion Association, Culham Science Centre, Abingdon, Oxfordshire OX14 3DB, UK With acknowledgments to B.Alper for use of his transparencies
Physics & Engineering Physics University of Saskatchewan. Supported by NSERC, CRC
 Fusion Energy Chijin Xiao and Akira Hirose Plasma Physics laboratory Physics & Engineering Physics University of Saskatchewan Supported by NSERC, CRC Trends in Nuclear & Medical Technologies April il6-7,
Fusion Energy Chijin Xiao and Akira Hirose Plasma Physics laboratory Physics & Engineering Physics University of Saskatchewan Supported by NSERC, CRC Trends in Nuclear & Medical Technologies April il6-7,
Toward the Realization of Fusion Energy
 Toward the Realization of Fusion Energy Nuclear fusion is the energy source of the sun and stars, in which light atomic nuclei fuse together, releasing a large amount of energy. Fusion power can be generated
Toward the Realization of Fusion Energy Nuclear fusion is the energy source of the sun and stars, in which light atomic nuclei fuse together, releasing a large amount of energy. Fusion power can be generated
Nuclear Energy. Nuclear Structure and Radioactivity
 Nuclear Energy Nuclear Structure and Radioactivity I. Review - Periodic Table A. Atomic Number: The number of protons in the nucleus of an atom B. Atomic Mass: The sum of the mass of protons, neutrons
Nuclear Energy Nuclear Structure and Radioactivity I. Review - Periodic Table A. Atomic Number: The number of protons in the nucleus of an atom B. Atomic Mass: The sum of the mass of protons, neutrons
Chapter 10 Section 4 Notes
 Chapter 10 Section 4 Notes This painting of an alchemist s laboratory was made around 1570. For centuries, these early scientists, known as alchemists, tried to use chemical reactions to make gold. The
Chapter 10 Section 4 Notes This painting of an alchemist s laboratory was made around 1570. For centuries, these early scientists, known as alchemists, tried to use chemical reactions to make gold. The
Term 3 Week 2 Nuclear Fusion & Nuclear Fission
 Term 3 Week 2 Nuclear Fusion & Nuclear Fission Tuesday, November 04, 2014 Nuclear Fusion To understand nuclear fusion & fission Nuclear Fusion Why do stars shine? Stars release energy as a result of fusing
Term 3 Week 2 Nuclear Fusion & Nuclear Fission Tuesday, November 04, 2014 Nuclear Fusion To understand nuclear fusion & fission Nuclear Fusion Why do stars shine? Stars release energy as a result of fusing
JET and Fusion Energy for the Next Millennia
 JET and Fusion Energy for the Next Millennia JET Joint Undertaking Abingdon, Oxfordshire OX14 3EA JG99.294/1 Talk Outline What is Nuclear Fusion? How can Fusion help our Energy needs? Progress with Magnetic
JET and Fusion Energy for the Next Millennia JET Joint Undertaking Abingdon, Oxfordshire OX14 3EA JG99.294/1 Talk Outline What is Nuclear Fusion? How can Fusion help our Energy needs? Progress with Magnetic
Lecture 35 Chapter 22, Sections 4-6 Nuclear Reactions. Fission Reactions Fusion Reactions Stellar Radiation Radiation Damage
 Lecture 35 Chapter, Sections 4-6 Nuclear Reactions Fission Reactions Fusion Reactions Stellar Radiation Radiation Damage Induced Nuclear Reactions Reactions in which a nuclear projectile collides and reacts
Lecture 35 Chapter, Sections 4-6 Nuclear Reactions Fission Reactions Fusion Reactions Stellar Radiation Radiation Damage Induced Nuclear Reactions Reactions in which a nuclear projectile collides and reacts
Unpressurized steam reactor. Controlled Fission Reactors. The Moderator. Global energy production 2000
 From last time Fission of heavy elements produces energy Only works with 235 U, 239 Pu Fission initiated by neutron absorption. Fission products are two lighter nuclei, plus individual neutrons. These
From last time Fission of heavy elements produces energy Only works with 235 U, 239 Pu Fission initiated by neutron absorption. Fission products are two lighter nuclei, plus individual neutrons. These
UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY
 UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY teacher version www.toppr.com Contents (a) Types of Radiation (b) Properties of Radiation (c) Dangers of Radiation (d) Rates of radioactive decay (e) Nuclear
UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY teacher version www.toppr.com Contents (a) Types of Radiation (b) Properties of Radiation (c) Dangers of Radiation (d) Rates of radioactive decay (e) Nuclear
Erasmus Plus INS Ramon Berenguer IV LESSON PLAN
 LESSON PLAN Subject: Chemistry Topic: Matter matters! Age of students: 17 Language level: B1/B2 Time: 45-60 minutes Content aims: After completing the lesson, the student will be able to: Understand the
LESSON PLAN Subject: Chemistry Topic: Matter matters! Age of students: 17 Language level: B1/B2 Time: 45-60 minutes Content aims: After completing the lesson, the student will be able to: Understand the
Card #1/28. Card #2/28. Science Revision P2. Science Revision P2. Science Revision P2. Card #4/28. Topic: F = ma. Topic: Resultant Forces
 Card #1/28 Card #2/28 Topic: Resultant Forces Topic: F = ma Topic: Distance-TIme Graphs Card #3/28 Card #4/28 Topic: Velocity-Time Graphs Card #2/28 Card #1/28 Card #4/28 Card #3/28 Card #5/28 Card #6/28
Card #1/28 Card #2/28 Topic: Resultant Forces Topic: F = ma Topic: Distance-TIme Graphs Card #3/28 Card #4/28 Topic: Velocity-Time Graphs Card #2/28 Card #1/28 Card #4/28 Card #3/28 Card #5/28 Card #6/28
UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY
 UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY student version www.toppr.com Contents (a) Types of Radiation (b) Properties of Radiation (c) Dangers of Radiation (d) Rates of radioactive decay (e) Nuclear
UNIT 10 RADIOACTIVITY AND NUCLEAR CHEMISTRY student version www.toppr.com Contents (a) Types of Radiation (b) Properties of Radiation (c) Dangers of Radiation (d) Rates of radioactive decay (e) Nuclear
A is called the mass number gives, roughly, the mass of the nucleus or atom in atomic mass units = amu = u
 5/5 A is called the mass number gives, roughly, the mass of the nucleus or atom in atomic mass units = amu = u The number of neutrons in the nucleus is given by the symbol N. Clearly, N = A Z. Isotope:
5/5 A is called the mass number gives, roughly, the mass of the nucleus or atom in atomic mass units = amu = u The number of neutrons in the nucleus is given by the symbol N. Clearly, N = A Z. Isotope:
Atoms and Nuclear Chemistry. Atoms Isotopes Calculating Average Atomic Mass Radioactivity
 Atoms and Nuclear Chemistry Atoms Isotopes Calculating Average Atomic Mass Radioactivity Atoms An atom is the smallest particle of an element that has all of the properties of that element. Composition
Atoms and Nuclear Chemistry Atoms Isotopes Calculating Average Atomic Mass Radioactivity Atoms An atom is the smallest particle of an element that has all of the properties of that element. Composition
The Path to Fusion Energy creating a star on earth. S. Prager Princeton Plasma Physics Laboratory
 The Path to Fusion Energy creating a star on earth S. Prager Princeton Plasma Physics Laboratory The need for fusion energy is strong and enduring Carbon production (Gton) And the need is time urgent Goal
The Path to Fusion Energy creating a star on earth S. Prager Princeton Plasma Physics Laboratory The need for fusion energy is strong and enduring Carbon production (Gton) And the need is time urgent Goal
The Sun = Typical Star
 The Sun = Typical Star Some Properties Diameter - 109 times Earth s Volume - about 1,000,000 times Earth s Mass - about 300,000 times Earth s 99.8% of Solar System Density = Mass/Volume = 1.4 g/cm 3 The
The Sun = Typical Star Some Properties Diameter - 109 times Earth s Volume - about 1,000,000 times Earth s Mass - about 300,000 times Earth s 99.8% of Solar System Density = Mass/Volume = 1.4 g/cm 3 The
Fundamental Forces of the Universe
 Fundamental Forces of the Universe There are four fundamental forces, or interactions in nature. Strong nuclear Electromagnetic Weak nuclear Gravitational Strongest Weakest Strong nuclear force Holds the
Fundamental Forces of the Universe There are four fundamental forces, or interactions in nature. Strong nuclear Electromagnetic Weak nuclear Gravitational Strongest Weakest Strong nuclear force Holds the
1. Four different processes are described in List A. The names of these processes are given in List B.
 Nuclear fission and nuclear fusion 1. Four different processes are described in List A. The names of these processes are given in List B. Draw a line to link each description in List A to its correct name
Nuclear fission and nuclear fusion 1. Four different processes are described in List A. The names of these processes are given in List B. Draw a line to link each description in List A to its correct name
Recap I Lecture 41 Matthias Liepe, 2012
 Recap I Lecture 41 Matthias Liepe, 01 Recap II Nuclear Physics The nucleus Radioactive decay Fission Fusion Particle Physics: What is the Higgs? Today: Nuclear Physics: The Nucleus Positive charge and
Recap I Lecture 41 Matthias Liepe, 01 Recap II Nuclear Physics The nucleus Radioactive decay Fission Fusion Particle Physics: What is the Higgs? Today: Nuclear Physics: The Nucleus Positive charge and
Astronomy 104: Second Exam
 Astronomy 104: Second Exam Stephen Lepp October 29, 2014 Each question is worth 2 points. Write your name on this exam and on the scantron. Short Answer A The Sun is powered by converting hydrogen to what?
Astronomy 104: Second Exam Stephen Lepp October 29, 2014 Each question is worth 2 points. Write your name on this exam and on the scantron. Short Answer A The Sun is powered by converting hydrogen to what?
Alta Chemistry CHAPTER 25. Nuclear Chemistry: Radiation, Radioactivity & its Applications
 CHAPTER 25 Nuclear Chemistry: Radiation, Radioactivity & its Applications Nuclear Chemistry Nuclear Chemistry deals with changes in the nucleus The nucleus of an atom contains Protons Positively Charged
CHAPTER 25 Nuclear Chemistry: Radiation, Radioactivity & its Applications Nuclear Chemistry Nuclear Chemistry deals with changes in the nucleus The nucleus of an atom contains Protons Positively Charged
Atoms and Star Formation
 Atoms and Star Formation What are the characteristics of an atom? Atoms have a nucleus of protons and neutrons about which electrons orbit. neutrons protons electrons 0 charge +1 charge 1 charge 1.67 x
Atoms and Star Formation What are the characteristics of an atom? Atoms have a nucleus of protons and neutrons about which electrons orbit. neutrons protons electrons 0 charge +1 charge 1 charge 1.67 x
PLASMA: WHAT IT IS, HOW TO MAKE IT AND HOW TO HOLD IT. Felix I. Parra Rudolf Peierls Centre for Theoretical Physics, University of Oxford
 1 PLASMA: WHAT IT IS, HOW TO MAKE IT AND HOW TO HOLD IT Felix I. Parra Rudolf Peierls Centre for Theoretical Physics, University of Oxford 2 Overview Why do we need plasmas? For fusion, among other things
1 PLASMA: WHAT IT IS, HOW TO MAKE IT AND HOW TO HOLD IT Felix I. Parra Rudolf Peierls Centre for Theoretical Physics, University of Oxford 2 Overview Why do we need plasmas? For fusion, among other things
Neutron Sources Fall, 2017 Kyoung-Jae Chung Department of Nuclear Engineering Seoul National University
 Neutron Sources Fall, 2017 Kyoung-Jae Chung Department of Nuclear Engineering Seoul National University Neutrons: discovery In 1920, Rutherford postulated that there were neutral, massive particles in
Neutron Sources Fall, 2017 Kyoung-Jae Chung Department of Nuclear Engineering Seoul National University Neutrons: discovery In 1920, Rutherford postulated that there were neutral, massive particles in
An introduction to Nuclear Physics
 An introduction to Nuclear Physics Jorge Pereira pereira@nscl.msu.edu National Superconducting Cyclotron Laboratory Joint Institute for Nuclear Astrophysics The Origin of Everything Layout The Nucleus.
An introduction to Nuclear Physics Jorge Pereira pereira@nscl.msu.edu National Superconducting Cyclotron Laboratory Joint Institute for Nuclear Astrophysics The Origin of Everything Layout The Nucleus.
Nuclear Physics and Nuclear Reactions
 Slide 1 / 33 Nuclear Physics and Nuclear Reactions The Nucleus Slide 2 / 33 Proton: The charge on a proton is +1.6x10-19 C. The mass of a proton is 1.6726x10-27 kg. Neutron: The neutron is neutral. The
Slide 1 / 33 Nuclear Physics and Nuclear Reactions The Nucleus Slide 2 / 33 Proton: The charge on a proton is +1.6x10-19 C. The mass of a proton is 1.6726x10-27 kg. Neutron: The neutron is neutral. The
Introduction to Fusion Physics
 Introduction to Fusion Physics Hartmut Zohm Max-Planck-Institut für Plasmaphysik 85748 Garching DPG Advanced Physics School The Physics of ITER Bad Honnef, 22.09.2014 Energy from nuclear fusion Reduction
Introduction to Fusion Physics Hartmut Zohm Max-Planck-Institut für Plasmaphysik 85748 Garching DPG Advanced Physics School The Physics of ITER Bad Honnef, 22.09.2014 Energy from nuclear fusion Reduction
GCSE OCR Revision Physics. GCSE OCR Revision Physics. GCSE OCR Revision Physics. GCSE OCR Revision Physics. Journeys. GCSE OCR Revision Physics
 Matter, Models and Density What is a typical size of an atom? Choose from the following. 10 15 m 10 12 m 10 10 m Matter, Models and Density The size of an atom is of the order of 10 10 m. 1 1 Temperature
Matter, Models and Density What is a typical size of an atom? Choose from the following. 10 15 m 10 12 m 10 10 m Matter, Models and Density The size of an atom is of the order of 10 10 m. 1 1 Temperature
Lecture PowerPoints. Chapter 31 Physics: Principles with Applications, 7th edition Giancoli
 Lecture PowerPoints Chapter 31 Physics: Principles with Applications, 7th edition Giancoli This work is protected by United States copyright laws and is provided solely for the use of instructors in teaching
Lecture PowerPoints Chapter 31 Physics: Principles with Applications, 7th edition Giancoli This work is protected by United States copyright laws and is provided solely for the use of instructors in teaching
[2] State in what form the energy is released in such a reaction.... [1]
![[2] State in what form the energy is released in such a reaction.... [1] [2] State in what form the energy is released in such a reaction.... [1]](/thumbs/87/96058923.jpg) (a) The following nuclear reaction occurs when a slow-moving neutron is absorbed by an isotope of uranium-35. 0n + 35 9 U 4 56 Ba + 9 36Kr + 3 0 n Explain how this reaction is able to produce energy....
(a) The following nuclear reaction occurs when a slow-moving neutron is absorbed by an isotope of uranium-35. 0n + 35 9 U 4 56 Ba + 9 36Kr + 3 0 n Explain how this reaction is able to produce energy....
Forces and Nuclear Processes
 Forces and Nuclear Processes To understand how stars generate the enormous amounts of light they produce will require us to delve into a wee bit of physics. First we will examine the forces that act at
Forces and Nuclear Processes To understand how stars generate the enormous amounts of light they produce will require us to delve into a wee bit of physics. First we will examine the forces that act at
Nuclear Reactions A Z. Radioactivity, Spontaneous Decay: Nuclear Reaction, Induced Process: x + X Y + y + Q Q > 0. Exothermic Endothermic
 Radioactivity, Spontaneous Decay: Nuclear Reactions A Z 4 P D+ He + Q A 4 Z 2 Q > 0 Nuclear Reaction, Induced Process: x + X Y + y + Q Q = ( m + m m m ) c 2 x X Y y Q > 0 Q < 0 Exothermic Endothermic 2
Radioactivity, Spontaneous Decay: Nuclear Reactions A Z 4 P D+ He + Q A 4 Z 2 Q > 0 Nuclear Reaction, Induced Process: x + X Y + y + Q Q = ( m + m m m ) c 2 x X Y y Q > 0 Q < 0 Exothermic Endothermic 2
Year 12 Notes Radioactivity 1/5
 Year Notes Radioactivity /5 Radioactivity Stable and Unstable Nuclei Radioactivity is the spontaneous disintegration of certain nuclei, a random process in which particles and/or high-energy photons are
Year Notes Radioactivity /5 Radioactivity Stable and Unstable Nuclei Radioactivity is the spontaneous disintegration of certain nuclei, a random process in which particles and/or high-energy photons are
The Sun Our Nearest Star The Sun is an average star in mass, lifetime, and energy output. We will look at in detail before studying stars in general
 The Sun Our Nearest Star The Sun is an average star in mass, lifetime, and energy output. We will look at in detail before studying stars in general Some Properties Diameter - 09 times Earth s Volume -
The Sun Our Nearest Star The Sun is an average star in mass, lifetime, and energy output. We will look at in detail before studying stars in general Some Properties Diameter - 09 times Earth s Volume -
Lecture PowerPoint. Chapter 31 Physics: Principles with Applications, 6 th edition Giancoli
 Lecture PowerPoint Chapter 31 Physics: Principles with Applications, 6 th edition Giancoli 2005 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the
Lecture PowerPoint Chapter 31 Physics: Principles with Applications, 6 th edition Giancoli 2005 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the
Potential, Kinetic Notes.notebook. April 16, 2012
 Kinetic Energy: Consider a baseball flying through the air. The ball is said to have "kinetic energy" by virtue of the fact that its in motion relative to the ground. You can see that it is has energy
Kinetic Energy: Consider a baseball flying through the air. The ball is said to have "kinetic energy" by virtue of the fact that its in motion relative to the ground. You can see that it is has energy
FUSION NEUTRON DEUTERIUM HELIUM TRITIUM.
 FUSION AND FISSION THE SUN Nuclear Fusion Nuclear fusion is the process by which multiple nuclei join together to form a heavier nucleus. It is accompanied by the release or absorption of energy depending
FUSION AND FISSION THE SUN Nuclear Fusion Nuclear fusion is the process by which multiple nuclei join together to form a heavier nucleus. It is accompanied by the release or absorption of energy depending
Aim: What are the two types of Nuclear. Reactions? Do Now: 1. Get into your groups and compare your answers to your homework.
 Aim: What are the two types of Nuclear Reactions? Do Now: 1. Get into your groups and compare your answers to your homework. Nuclear Energy In nuclear reaction, mass is converted into energy; there is
Aim: What are the two types of Nuclear Reactions? Do Now: 1. Get into your groups and compare your answers to your homework. Nuclear Energy In nuclear reaction, mass is converted into energy; there is
General Physics (PHY 2140)
 General Physics (PHY 2140) Lecture 20 Modern Physics Nuclear Energy and Elementary Particles Fission, Fusion and Reactors Elementary Particles Fundamental Forces Classification of Particles Conservation
General Physics (PHY 2140) Lecture 20 Modern Physics Nuclear Energy and Elementary Particles Fission, Fusion and Reactors Elementary Particles Fundamental Forces Classification of Particles Conservation
Nuclear Reactions. Fission Fusion
 Nuclear Reactions Fission Fusion Nuclear Reactions and the Transmutation of Elements A nuclear reaction takes place when a nucleus is struck by another nucleus or particle. Compare with chemical reactions!
Nuclear Reactions Fission Fusion Nuclear Reactions and the Transmutation of Elements A nuclear reaction takes place when a nucleus is struck by another nucleus or particle. Compare with chemical reactions!
PHYSICS A 2825/04. Nuclear and Particle Physics. OXFORD CAMBRIDGE AND RSA EXAMINATIONS Advanced GCE. 1 hour 30 minutes
 OXFORD CAMBRIDGE AND RSA EXAMINATIONS Advanced GCE PHYSICS A 2825/04 Nuclear and Particle Physics Monday 27 JUNE 2005 Afternoon 1 hour 30 minutes Candidates answer on the question paper. Additional materials:
OXFORD CAMBRIDGE AND RSA EXAMINATIONS Advanced GCE PHYSICS A 2825/04 Nuclear and Particle Physics Monday 27 JUNE 2005 Afternoon 1 hour 30 minutes Candidates answer on the question paper. Additional materials:
Question Bank. Nuclear Physics
 Nuclear Physics 1. State one difference between a chemical change and a nuclear change. Ans. A chemical change takes place due to transfer/sharing of orbital electrons of atoms of different elements, whereas
Nuclear Physics 1. State one difference between a chemical change and a nuclear change. Ans. A chemical change takes place due to transfer/sharing of orbital electrons of atoms of different elements, whereas
11/19/08. Gravitational equilibrium: The outward push of pressure balances the inward pull of gravity. Weight of upper layers compresses lower layers
 Gravitational equilibrium: The outward push of pressure balances the inward pull of gravity Weight of upper layers compresses lower layers Gravitational equilibrium: Energy provided by fusion maintains
Gravitational equilibrium: The outward push of pressure balances the inward pull of gravity Weight of upper layers compresses lower layers Gravitational equilibrium: Energy provided by fusion maintains
Chemistry: The Central Science. Chapter 21: Nuclear Chemistry
 Chemistry: The Central Science Chapter 21: Nuclear Chemistry A nuclear reaction involves changes in the nucleus of an atom Nuclear chemistry the study of nuclear reactions, with an emphasis in their uses
Chemistry: The Central Science Chapter 21: Nuclear Chemistry A nuclear reaction involves changes in the nucleus of an atom Nuclear chemistry the study of nuclear reactions, with an emphasis in their uses
Lecture 12: Making the Sun Shine Readings: Sections 18-1, 18-4 and Box 18-1
 Lecture 12: Making the Sun Shine Readings: Sections 18-1, 18-4 and Box 18-1 Key Ideas Stars shine because they are hot need an internal energy source to stay hot Kelvin-Helmholtz Mechanism Energy from
Lecture 12: Making the Sun Shine Readings: Sections 18-1, 18-4 and Box 18-1 Key Ideas Stars shine because they are hot need an internal energy source to stay hot Kelvin-Helmholtz Mechanism Energy from
Physics 30 Modern Physics Unit: Fission and Fusion
 Physics 30 Modern Physics Unit: Fission and Fusion Nuclear Energy For years and years scientists struggled to describe where energy came from. They could see the uses of energy and the results of energy
Physics 30 Modern Physics Unit: Fission and Fusion Nuclear Energy For years and years scientists struggled to describe where energy came from. They could see the uses of energy and the results of energy
Nuclear Physics and Radioactivity
 Nuclear Physics and Radioactivity Structure and Properties of the Nucleus Nucleus is made of protons and neutrons Proton has positive charge: Neutron is electrically neutral: Neutrons and protons are collectively
Nuclear Physics and Radioactivity Structure and Properties of the Nucleus Nucleus is made of protons and neutrons Proton has positive charge: Neutron is electrically neutral: Neutrons and protons are collectively
Nuclear Energy Learning Outcomes
 1 Nuclear Energy Learning Outcomes Describe the principles underlying fission and fusion. Interpret nuclear reactions. Discuss nuclear weapons. Describe the structure and operation of a nuclear reactor.
1 Nuclear Energy Learning Outcomes Describe the principles underlying fission and fusion. Interpret nuclear reactions. Discuss nuclear weapons. Describe the structure and operation of a nuclear reactor.
Nuclear Energy Learning Outcomes. Nuclear Fission. Chain Reaction
 by fastfission public domain by fastfission public domain 1 Nuclear Energy Learning Outcomes Describe the principles underlying fission and fusion. Interpret nuclear reactions. Discuss nuclear weapons.
by fastfission public domain by fastfission public domain 1 Nuclear Energy Learning Outcomes Describe the principles underlying fission and fusion. Interpret nuclear reactions. Discuss nuclear weapons.
Physics (B): Physics in Context
 Centre Number Surname Candidate Number For Examinerʼs Use Other Names Candidate Signature Examinerʼs Initials General Certificate of Education Advanced Level Examination June 2012 Question 1 2 Mark Physics
Centre Number Surname Candidate Number For Examinerʼs Use Other Names Candidate Signature Examinerʼs Initials General Certificate of Education Advanced Level Examination June 2012 Question 1 2 Mark Physics
Year 9 AQA GCSE Physics Revision Booklet
 Year 9 AQA GCSE Physics Revision Booklet Atomic Structure and Radioactivity Models of the atom know: Plum pudding model of the atom and Rutherford and Marsden s alpha experiments, being able to explain
Year 9 AQA GCSE Physics Revision Booklet Atomic Structure and Radioactivity Models of the atom know: Plum pudding model of the atom and Rutherford and Marsden s alpha experiments, being able to explain
GCSE 0247/02 SCIENCE HIGHER TIER PHYSICS 3
 Surname Centre Number Candidate Number Other Names GCSE 247/2 SCIENCE HIGHER TIER PHYSICS 3 A.M. WEDNESDAY, 3 January 213 45 minutes ADDITIONAL MATERIALS In addition to this paper you may require a calculator.
Surname Centre Number Candidate Number Other Names GCSE 247/2 SCIENCE HIGHER TIER PHYSICS 3 A.M. WEDNESDAY, 3 January 213 45 minutes ADDITIONAL MATERIALS In addition to this paper you may require a calculator.
Introducing nuclear fission The Fizzics Organization
 Nuclear Fission is the splitting of the nucleus of an atom into two or more parts by hitting it with a small particle, almost always a neutron (a proton would be repelled from the positive nucleus and
Nuclear Fission is the splitting of the nucleus of an atom into two or more parts by hitting it with a small particle, almost always a neutron (a proton would be repelled from the positive nucleus and
The Sun Closest star to Earth - only star that we can see details on surface - easily studied Assumption: The Sun is a typical star
 The Sun Closest star to Earth - only star that we can see details on surface - easily studied Assumption: The Sun is a typical star Why is the Sun hot and bright? Surface Temperature of the Sun: T =
The Sun Closest star to Earth - only star that we can see details on surface - easily studied Assumption: The Sun is a typical star Why is the Sun hot and bright? Surface Temperature of the Sun: T =
Nuclear Chemistry. Chapter 24
 Nuclear Chemistry Chapter 24 Radioactivity Radioisotopes are isotopes that have an unstable nucleus. They emit radiation to attain more stable atomic configurations in a process called radioactive decay.
Nuclear Chemistry Chapter 24 Radioactivity Radioisotopes are isotopes that have an unstable nucleus. They emit radiation to attain more stable atomic configurations in a process called radioactive decay.
Atomic physics in fusion development
 Atomic physics in fusion development The next step in fusion development imposes new requirements on atomic physics research by R.K. Janev In establishing the scientific and technological base of fusion
Atomic physics in fusion development The next step in fusion development imposes new requirements on atomic physics research by R.K. Janev In establishing the scientific and technological base of fusion
Radioactivity & Nuclear. Chemistry. Mr. Matthew Totaro Legacy High School. Chemistry
 Radioactivity & Nuclear Chemistry Mr. Matthew Totaro Legacy High School Chemistry The Discovery of Radioactivity Antoine-Henri Becquerel designed an experiment to determine if phosphorescent minerals also
Radioactivity & Nuclear Chemistry Mr. Matthew Totaro Legacy High School Chemistry The Discovery of Radioactivity Antoine-Henri Becquerel designed an experiment to determine if phosphorescent minerals also
Ch Radioactivity. Henry Becquerel, using U-238, discovered the radioactive nature of elements in 1896.
 Ch. 10 - Radioactivity Henry Becquerel, using U-238, discovered the radioactive nature of elements in 1896. Radioactivity the process in which an unstable atomic nucleus emits charged particles and energy
Ch. 10 - Radioactivity Henry Becquerel, using U-238, discovered the radioactive nature of elements in 1896. Radioactivity the process in which an unstable atomic nucleus emits charged particles and energy
1 A Solar System Is Born
 CHAPTER 16 1 A Solar System Is Born SECTION Our Solar System California Science Standards 8.2.g, 8.4.b, 8.4.c, 8.4.d BEFORE YOU READ After you read this section, you should be able to answer these questions:
CHAPTER 16 1 A Solar System Is Born SECTION Our Solar System California Science Standards 8.2.g, 8.4.b, 8.4.c, 8.4.d BEFORE YOU READ After you read this section, you should be able to answer these questions:
Thursday 19 June 2014 Morning
 Thursday 19 June 2014 Morning A2 GCE PHYSICS A G485/01 Fields, Particles and Frontiers of Physics *3270254576* Candidates answer on the Question Paper. OCR supplied materials: Data, Formulae and Relationships
Thursday 19 June 2014 Morning A2 GCE PHYSICS A G485/01 Fields, Particles and Frontiers of Physics *3270254576* Candidates answer on the Question Paper. OCR supplied materials: Data, Formulae and Relationships
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *5638748962* PHYSICS 0625/32 Paper 3 Extended May/June 2013 1 hour 15 minutes Candidates answer
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education *5638748962* PHYSICS 0625/32 Paper 3 Extended May/June 2013 1 hour 15 minutes Candidates answer
Spherical Torus Fusion Contributions and Game-Changing Issues
 Spherical Torus Fusion Contributions and Game-Changing Issues Spherical Torus (ST) research contributes to advancing fusion, and leverages on several game-changing issues 1) What is ST? 2) How does research
Spherical Torus Fusion Contributions and Game-Changing Issues Spherical Torus (ST) research contributes to advancing fusion, and leverages on several game-changing issues 1) What is ST? 2) How does research
Chapter 25. Nuclear Chemistry. Types of Radiation
 Chapter 25 Nuclear Chemistry Chemical Reactions 1. Bonds are broken and formed 2. Atoms may rearrange, but remain unchanged 3. Involve only valence electrons 4. Small energy changes 5. Reaction rate is
Chapter 25 Nuclear Chemistry Chemical Reactions 1. Bonds are broken and formed 2. Atoms may rearrange, but remain unchanged 3. Involve only valence electrons 4. Small energy changes 5. Reaction rate is
1ST SEM MT CHAP 22 REVIEW
 1ST SEM MT CHAP 22 REVIEW Multiple Choice Identify the choice that best completes the statement or answers the question. (CAPITAL LETTERS ONLY PLEASE) 1. Mass defect is the difference between the mass
1ST SEM MT CHAP 22 REVIEW Multiple Choice Identify the choice that best completes the statement or answers the question. (CAPITAL LETTERS ONLY PLEASE) 1. Mass defect is the difference between the mass
Electrocution (large quantities of charge flow through the body to earth) Insulating mats or shoes
 P4 in 30 minutes P4a: Sparks Like charges repel and unlike charges attract. a positive charge due to lack of electrons a negative charge due to an excess of electrons. Atoms or molecules that have become
P4 in 30 minutes P4a: Sparks Like charges repel and unlike charges attract. a positive charge due to lack of electrons a negative charge due to an excess of electrons. Atoms or molecules that have become
Role and Challenges of Fusion Nuclear Science and Technology (FNST) toward DEMO
 Role and Challenges of Fusion Nuclear Science and Technology (FNST) toward DEMO Mohamed Abdou Distinguished Professor of Engineering and Applied Science (UCLA) Director, Center for Energy Science & Technology
Role and Challenges of Fusion Nuclear Science and Technology (FNST) toward DEMO Mohamed Abdou Distinguished Professor of Engineering and Applied Science (UCLA) Director, Center for Energy Science & Technology
Sample Examination Questions
 Sample Examination Questions Contents NB. Material covered by the AS papers may also appear in A2 papers. Question Question type Question focus number (section A or B) 1 A Ideal transformer 2 A Induced
Sample Examination Questions Contents NB. Material covered by the AS papers may also appear in A2 papers. Question Question type Question focus number (section A or B) 1 A Ideal transformer 2 A Induced
Bi β + Po Bismuth-214 is radioactive. It has a half-life of 20 minutes. (a) The nuclide notation for bismuth-214 is Bi.
 1 Bismuth-214 is radioactive. It has a half-life of 20 minutes. (a) The nuclide notation for bismuth-214 is Bi. State the composition of the nucleus of bismuth-214. [2] (b) Bismuth-214 decays by β-decay
1 Bismuth-214 is radioactive. It has a half-life of 20 minutes. (a) The nuclide notation for bismuth-214 is Bi. State the composition of the nucleus of bismuth-214. [2] (b) Bismuth-214 decays by β-decay
= : K A
 Atoms and Nuclei. State two limitations of JJ Thomson s model of atom. 2. Write the SI unit for activity of a radioactive substance. 3. What observations led JJ Thomson to conclusion that all atoms have
Atoms and Nuclei. State two limitations of JJ Thomson s model of atom. 2. Write the SI unit for activity of a radioactive substance. 3. What observations led JJ Thomson to conclusion that all atoms have
purposes is highly encouraged.
 The following slide show is a compilation of slides from many previous similar slide shows that have been produced by different members of the fusion and plasma physics education community. We realize
The following slide show is a compilation of slides from many previous similar slide shows that have been produced by different members of the fusion and plasma physics education community. We realize
Today. Homework Due. Stars. Properties (Recap) Nuclear Reactions. proton-proton chain. CNO cycle. Stellar Lifetimes
 Today Stars Properties (Recap) Nuclear Reactions proton-proton chain CNO cycle Stellar Lifetimes Homework Due Stellar Properties Luminosity Surface Temperature Size Mass Composition Stellar Properties
Today Stars Properties (Recap) Nuclear Reactions proton-proton chain CNO cycle Stellar Lifetimes Homework Due Stellar Properties Luminosity Surface Temperature Size Mass Composition Stellar Properties
Solar Fusion. After Steele Hill, SOHO
 Solar Fusion After Steele Hill, SOHO by Michael Gallagher for the BDAS Astronomy Course 28 th April 2006 Sources Books Michael Zeilic, Astronomy, the Evolving Universe, 5 th Edition, John Wiley, 1988 Simon
Solar Fusion After Steele Hill, SOHO by Michael Gallagher for the BDAS Astronomy Course 28 th April 2006 Sources Books Michael Zeilic, Astronomy, the Evolving Universe, 5 th Edition, John Wiley, 1988 Simon
The Electromagnetic Spectrum. 7.1 Atomic Theory and Radioactive Decay. Isotopes. 19K, 19K, 19K Representing Isotopes
 7.1 Atomic Theory and Radioactive Decay Natural background radiation exists all around us. Radioactivity is the release of high energy particles or waves When atoms lose high energy particles and waves,
7.1 Atomic Theory and Radioactive Decay Natural background radiation exists all around us. Radioactivity is the release of high energy particles or waves When atoms lose high energy particles and waves,
The sources include Am-241 which emits alpha radiation, Sr-90 which emits beta radiation and Co-60 which emits gamma radiation.
 1 The physics department in a college has a number of radioactive sources which are used to demonstrate the properties of ionising radiations. The sources include Am-241 which emits alpha radiation, Sr-90
1 The physics department in a college has a number of radioactive sources which are used to demonstrate the properties of ionising radiations. The sources include Am-241 which emits alpha radiation, Sr-90
Fission and Chain Reactions
 The Harnessed Atom Lesson Five Fission and Chain Reactions What you need to know about Fission and Chain Reactions: Fission Chain reaction Uranium fuel Mining Milling Enrichment Fuel fabrication 2 Nuclear
The Harnessed Atom Lesson Five Fission and Chain Reactions What you need to know about Fission and Chain Reactions: Fission Chain reaction Uranium fuel Mining Milling Enrichment Fuel fabrication 2 Nuclear
Today The Sun. Events
 Today The Sun Events Last class! Homework due now - will count best 5 of 6 Final exam Dec. 20 @ 12:00 noon here Review this Course! www.case.edu/utech/course-evaluations/ The Sun the main show in the solar
Today The Sun Events Last class! Homework due now - will count best 5 of 6 Final exam Dec. 20 @ 12:00 noon here Review this Course! www.case.edu/utech/course-evaluations/ The Sun the main show in the solar
Comparison of plasma breakdown with a carbon and ITER-like wall
 Comparison of plasma breakdown with a carbon and ITER-like wall P.C. de Vries, A.C.C. Sips, H.T. Kim, P.J. Lomas, F. Maviglia, R. Albanese, I. Coffey, E. Joffrin, M. Lehnen, A. Manzanares, M. O Mulane,
Comparison of plasma breakdown with a carbon and ITER-like wall P.C. de Vries, A.C.C. Sips, H.T. Kim, P.J. Lomas, F. Maviglia, R. Albanese, I. Coffey, E. Joffrin, M. Lehnen, A. Manzanares, M. O Mulane,
A Technology Review of Electricity Generation from Nuclear Fusion Reaction in Future
 International OPEN ACCESS Journal Of Modern Engineering Research (IJMER) 3 A Technology Review of Electricity Generation from Nuclear Fusion Reaction in Future 1 Joydeep Sarkar, 2 Karishma P. Patil, 3
International OPEN ACCESS Journal Of Modern Engineering Research (IJMER) 3 A Technology Review of Electricity Generation from Nuclear Fusion Reaction in Future 1 Joydeep Sarkar, 2 Karishma P. Patil, 3
FXA Candidates should be able to :
 1 Candidates should be able to : INTRODUCTION Describe qualitatively the alpha-particle scattering experiment and the evidence this provides for the existence, charge and small size of the nucleus. Describe
1 Candidates should be able to : INTRODUCTION Describe qualitatively the alpha-particle scattering experiment and the evidence this provides for the existence, charge and small size of the nucleus. Describe
Explain how the sun converts matter into energy in its core. Describe the three layers of the sun s atmosphere.
 Chapter 29 and 30 Explain how the sun converts matter into energy in its core. Describe the three layers of the sun s atmosphere. Explain how sunspots are related to powerful magnetic fields on the sun.
Chapter 29 and 30 Explain how the sun converts matter into energy in its core. Describe the three layers of the sun s atmosphere. Explain how sunspots are related to powerful magnetic fields on the sun.
Introduction. Introduction. Forces An. Forces An. Forces in Action. Forces in Action. Pressure and Pressure. Pressure and Pressure.
 Forces An Introduction A force is a vector quantity. What does this mean? Forces An Introduction A vector quantity, such as force, has a direction as well as a magnitude. 1 1 Forces in Action The moment
Forces An Introduction A force is a vector quantity. What does this mean? Forces An Introduction A vector quantity, such as force, has a direction as well as a magnitude. 1 1 Forces in Action The moment
Matter mass space atoms solid, a liquid, a gas, or plasm elements compounds mixtures atoms Compounds chemically combined Mixtures not chemically
 SOL PS.2 THE NATURE OF MATTER Matter is anything that has mass and occupies space. All matter is made up of small particles called atoms. Matter can exist as a solid, a liquid, a gas, or plasma. Matter
SOL PS.2 THE NATURE OF MATTER Matter is anything that has mass and occupies space. All matter is made up of small particles called atoms. Matter can exist as a solid, a liquid, a gas, or plasma. Matter
2 Energy from the Nucleus
 CHAPTER 4 2 Energy from the Nucleus SECTION Atomic Energy BEFORE YOU READ After you read this section, you should be able to answer these questions: What is nuclear fission? What is nuclear fusion? What
CHAPTER 4 2 Energy from the Nucleus SECTION Atomic Energy BEFORE YOU READ After you read this section, you should be able to answer these questions: What is nuclear fission? What is nuclear fusion? What
PhysicsAndMathsTutor.com 1
 PhysicsAndMathsTutor.com 1 1. Describe briefly one scattering experiment to investigate the size of the nucleus of the atom. Include a description of the properties of the incident radiation which makes
PhysicsAndMathsTutor.com 1 1. Describe briefly one scattering experiment to investigate the size of the nucleus of the atom. Include a description of the properties of the incident radiation which makes
Nuclear Chemistry. Background Radiation. Three-fourths of all exposure to radiation comes from background radiation.
 Chapter 11 Nuclear Chemistry Background Radiation Three-fourths of all exposure to radiation comes from background radiation. Most of the remaining one-fourth comes from medical irradiation such as X-rays.
Chapter 11 Nuclear Chemistry Background Radiation Three-fourths of all exposure to radiation comes from background radiation. Most of the remaining one-fourth comes from medical irradiation such as X-rays.
Nuclear Energy; Effects and Uses of Radiation
 Nuclear Energy; Effects and Uses of Radiation Nuclear Reactions and the Transmutation of Elements A nuclear reaction takes place when a nucleus is struck by another nucleus or particle. Compare with chemical
Nuclear Energy; Effects and Uses of Radiation Nuclear Reactions and the Transmutation of Elements A nuclear reaction takes place when a nucleus is struck by another nucleus or particle. Compare with chemical
Structure of the Nuclear Atom
 Structure of the Nuclear Atom I. The II. A. The is the smallest particle of an element that retains its of the element. History of the Atom A. Democritus 1. Democritus (460 B.C. 370 B.C) was the first
Structure of the Nuclear Atom I. The II. A. The is the smallest particle of an element that retains its of the element. History of the Atom A. Democritus 1. Democritus (460 B.C. 370 B.C) was the first
MAGNETIC FUSION m DO NOT CIRCULATE RESEARCH
 L4SL &i?o-zf? MAGNETIC FUSION m DO NOT CIRCULATE RESEARCH i= PERMANENT RETENTION 1 I c1..- Post Office Box 1663 Los Alamos. New Mexico 87545 \ I \\.. Magnetic Fusion Research at the. Los Alamos Scientific
L4SL &i?o-zf? MAGNETIC FUSION m DO NOT CIRCULATE RESEARCH i= PERMANENT RETENTION 1 I c1..- Post Office Box 1663 Los Alamos. New Mexico 87545 \ I \\.. Magnetic Fusion Research at the. Los Alamos Scientific
Chapter 14 Lecture. The Cosmic Perspective Seventh Edition. Our Star Pearson Education, Inc.
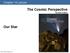 Chapter 14 Lecture The Cosmic Perspective Seventh Edition Our Star 14.1 A Closer Look at the Sun Our goals for learning: Why does the Sun shine? What is the Sun's structure? Why does the Sun shine? Is
Chapter 14 Lecture The Cosmic Perspective Seventh Edition Our Star 14.1 A Closer Look at the Sun Our goals for learning: Why does the Sun shine? What is the Sun's structure? Why does the Sun shine? Is
Sample Examination Questions
 Sample Examination Questions Contents NB. Material covered by the AS papers may also appear in A2 papers. Question Question type Question focus number (section A or B) 1 A Ideal transformer 2 A Induced
Sample Examination Questions Contents NB. Material covered by the AS papers may also appear in A2 papers. Question Question type Question focus number (section A or B) 1 A Ideal transformer 2 A Induced
