THE ATOMIC SPECTRUM OF HYDROGEN
|
|
|
- Stanley Hall
- 6 years ago
- Views:
Transcription
1 THE ATOMIC SPECTRUM OF HYDROGEN When atoms are excited, either in an electric discharge or with heat, they tend to give off light. The light is emitted only at certain wavelengths that are characteristic of the atoms in the sample. These wavelengths constitute what is called the atomic spectrum of the excited element and reveal much of the detailed information we have regarding the electronic structure of atoms. Atomic spectra are interpreted in terms of quantum theory, which states that atoms can exist only at certain states that correspond to fixed energy levels. When an atom changes its state, it must absorb or emit an amount of energy that is just equal to the difference between the energies of the initial and final states. This energy may be absorbed or emitted in the form of light. The emission spectrum of an atom is obtained when excited atoms fall from a higher to a lower energy level. Since there are many such levels, the atomic spectra of most elements are very complex. Light is absorbed or emitted by atoms in the form of photons, each with a specific amount of energy, E. This energy is related to the frequency (ν) and wavelength (l) of light by the following equation: E photon = hν = hc λ where h = Planck's constant = x J s and c = speed of light = x 10 8 m / s The law of conservation of energy states that total energy is conserved. Thus, the change in energy of the atom must equal the energy change of the photon emitted. The energy change of the atom is equal to the energy of the upper energy level minus the energy of the lower level. ΔE atom = (E final - E initial ) = E photon = hc λ The amount of energy in a photon given off when an atom changes from one level to another is very small, of the order of joules. To avoid working with such small numbers, we will work with one mole of atoms. The above equation is multiplied by Avogadro's number, N: The above equation is useful in the interpretation of atomic spectra. For example, in the study of the atomic spectrum of sodium, a strong yellow line of wavelength nm is observed. The above equation can be used to determine the change in energy. This in turn is corresponded to the transition in energy levels. The simplest of all atomic spectra is that of the hydrogen atom. In 1886 Balmer showed that the lines in the spectrum of the hydrogen atom had wavelengths that could be expressed by a rather simple equation. In 1913, Bohr explained the spectrum on a theoretical basis with his model of the hydrogen atom. According to Bohr's theory, the energies allowed to a hydrogen atom are given by the so-called Bohr's Equation: E n = B n = n 2 where B = a constant ( kj/mol) and n = the quantum number (1, 2, 3,...) Bohr's equation allows you to calculate quite accurately the energy levels for hydrogen. Transitions between these levels give rise to the wavelengths in the atomic spectrum of hydrogen. These wavelengths are also known very accurately. Page I-55 / The Atomic Spectrum of Hydrogen
2 Given both the energy levels and the wavelengths, it is possible to determine the actual levels associated with each wavelength. In this experiment, your task will be to measure the wavelengths of the hydrogen spectrum and then determine the transition in energy levels associated with each wavelength. This lab consists of a worksheet that needs to be completed for credit; no formal typed laboratory report is due this week. PROCEDURE: Part A: Visual Observation of a Hydrogen Discharge Tube Using a Spectroscope The instructor will set up a spectroscope with a hydrogen discharge tube. Note the color of the emitted light without using the spectroscope. Now view the hydrogen tube using the spectroscope. How many lines do you see? What color does each line have? Now we shall use the Vernier system and an emission spectrometer to determine the wavelengths of the hydrogen lines you viewed in the spectroscope. Part B: The Emission Spectrum of Hydrogen Using the Vernier LabQuest 2 Assemble the emission spectrometer and Lab Quest 2 per your instructor s instructions. In the Lab Quest program, you should see USB: Intensity rel if everything is connected correctly. Place the close to (but not touching!) the middle of a hydrogen discharge tube using a LabJack. Start the data collection by pushing the green start button (a green triangle) in the lower left of the Vernier LabQuest 2. You should see at least three distinct peaks to perform the analysis: the main central peak (656.3 nm) and at least two to the left of the main nm peak (at and nm.) Ignore any peaks to the right (i.e. larger than 700 nm) these are due to impurities in the hydrogen tube Once you have at least three peaks visible, stop your experiment and turn off the hydrogen discharge tube. Use the LabQuest 2 tools to find the wavelengths of the four emission peaks for hydrogen. Once the experiment is stopped, go Graph Graph Options, then change the following: Left to 400, Right to 700 and Top to 0.400, then press OK. Your data points will be visible, and a fourth point should be apparent at about 410 nm. Determine the four experimental emission wavelengths using the left and right buttons (which control the cursor; alternatively, use the pointer to select the exact point.) Record these values and compare them to the theoretical wavelengths for hydrogen to calculate the percent error for each line on the next page. Part C: Calculations for the Energy Levels of Hydrogen Atom Next, you can use the hydrogen wavelengths to calculate the energy change for each line in the observed hydrogen spectrum. Using Bohr's equation, calculate the energy levels (e n ) in kj/mole for each of the eight lowest allowed levels of the hydrogen atom starting with n=1 to n=8. Note that all the energies are negative, so that the lowest energy will have the largest allowed negative value. The energy levels will allow you to determine the energy transition (DE) that corresponds to the observed wavelengths. Determine the quantum numbers for the initial (n hi ) and final (n low ) states for these transitions. Page I-56 / The Atomic Spectrum of Hydrogen
3 The Atomic Spectrum of Hydrogen: Worksheet Complete the following worksheet using the instructions provided. Name: Lab Partner(s): Part A: Visual Observations What color was the hydrogen discharge tube when you looked directly at it? What four colors did you observe through the spectroscope? Part B: The Emission Spectrum of Hydrogen Use the Vernier system to find the hydrogen wavelengths. Colorl l (Vernier, nm) l (theoretical, nm) Percent Errorl Red Blue Violet Violet Recall: Percent Error = absolute value (theoretical value - experimental value) theoretical value * 100% Part C: Calculations for the Energy Levels of Hydrogen Atom Find the energy level e n (in kj/mol) for each quantum number from 1 through 8 using the following equation: ε n = (kj/mol) 2 n Example: The n=1 energy level can be calculated as follows: e n = ( /1 2 ) = kj/mol Example: The n=2 energy level can be calculated as follows: e n = ( /2 2 ) = kj/mol n value e n (kj/mol) n value e n (kj/mol) Page I-57 / The Atomic Spectrum of Hydrogen
4 Now complete the table below. The first row has been partially completed for you. Colorl l (actual, nm) n (s -1 ) DE (J/photon) DE (kj/mol) n hi l n low l red _ x x blue _486.1 violet _434.1 violet _410.2 Convert the wavelength values into DE (in kj/mol) using the following equation: The calculated DE values correspond to a transition between the various energy levels, e n, calculated previously. Determine which transition they correspond to by finding the change in energy (i.e. DE) between levels. Example: Find the change in energy in a transition of hydrogen between the n=2 and n=1 energy levels. The energy level, e n, for n=2 is kj/mol, and the energy level, e n, for n=1 is kj/mol. A change in energy, DE, corresponds to the final energy state minus the initial energy state, or: DE = e final - e initial = e 1 - e 2 = ( ) = kj/mol If your calculated value of DE is about kj/mol, then your n hi would be 2 (the higher value of n) and your n low value would be 1 (the lower value of n). Show the frequency calculation for the red line below: Show the ΔE (J/photon) calculation for the red line below: Show the ΔE (kj/mol) calculation for the red line below: Page I-58 / The Atomic Spectrum of Hydrogen
5 Post Lab Questions: 1. When Balmer found his famous series for hydrogen in 1886, he was limited experimentally to wavelengths in the visible and near ultraviolet regions from 250 nm to 700 nm, as in your experiment. What common characteristic do the lines in the Balmer series have? 2. In the hydrogen atom, the electron is in its lowest energy state, n=1. The maximum electron energy that a hydrogen atom can have is 0 kj/mole, at which point the electron would essentially be removed from the atom and it would become a H + ion. How much energy does it take to ionize one hydrogen atom in kilojoules per mole and in Joules per atom? (Hint: calculate DE where e final is zero and e initial is kj/mol.) Questions #3 through #5 will use the equation below for the helium ion. The helium ion, He +, has energy levels similar to those of the hydrogen atom, since both species have only one electron. The energy levels of the helium ion are given by the following equation: E n = n 2 kj/mol where n = 1, 2, Calculate the energies in kj/mole for the four lowest energy levels of the helium ion using the equation above. e 1 e 3 e 2 e 4 4. One of the most important transitions for the helium ion involves a jump from the n = 2 to the n = 1 level. Calculate the change in energy in kj/mole for this transition. (Hint: DE = e 1 - e 2 ). Use the equation found in Part C of the worksheet to calculate the wavelength (in nm) of this transition. 5. Three of the strongest lines in the helium ion spectrum are observed at the following wavelengths. Find the quantum numbers of the initial and final energy states for the transitions that give rise to these three lines: l DE (kj/mol) n hi n low nm nm nm Page I-59 / The Atomic Spectrum of Hydrogen
6 Page I-60 / The Atomic Spectrum of Hydrogen
CSUS Department of Chemistry Experiment 9 Chem. 1A
 CSUS Department of Chemistry xperiment 9 Chem. 1A xp. 9 PR-Lab ASSIGNMNT Name: Lab Section (1) Use equation (2) [see the discussion on the next page] to calculate the energies of the ten lowest states
CSUS Department of Chemistry xperiment 9 Chem. 1A xp. 9 PR-Lab ASSIGNMNT Name: Lab Section (1) Use equation (2) [see the discussion on the next page] to calculate the energies of the ten lowest states
Experiment #9. Atomic Emission Spectroscopy
 Introduction Experiment #9. Atomic Emission Spectroscopy Spectroscopy is the study of the interaction of light with matter. This interaction can be in the form of the absorption or the emission of electromagnetic
Introduction Experiment #9. Atomic Emission Spectroscopy Spectroscopy is the study of the interaction of light with matter. This interaction can be in the form of the absorption or the emission of electromagnetic
Atomic Spectroscopy and Energy Levels
 Activity 18 Atomic Spectroscopy and Energy Levels Why? The emission of light by the hydrogen atom and other atoms played a key role in helping scientists to understand the electronic structure of atoms.
Activity 18 Atomic Spectroscopy and Energy Levels Why? The emission of light by the hydrogen atom and other atoms played a key role in helping scientists to understand the electronic structure of atoms.
What are the energies (J) and wavelengths (in nm) for these colors? Color Energy wavelength. Rev. F11 Page 1 of 5
 Exp. 8 Pre Lab ASSIGNMENT Name: Lab Section Score: / 10 (1) Use the equations [see the discussion on the next page] to calculate the energies of the 6 lowest states for the hydrogen atom, and enter your
Exp. 8 Pre Lab ASSIGNMENT Name: Lab Section Score: / 10 (1) Use the equations [see the discussion on the next page] to calculate the energies of the 6 lowest states for the hydrogen atom, and enter your
PHYS General Physics II Lab The Balmer Series for Hydrogen Source. c = speed of light = 3 x 10 8 m/s
 PHYS 1040 - General Physics II Lab The Balmer Series for Hydrogen Source Purpose: The purpose of this experiment is to analyze the emission of light from a hydrogen source and measure and the wavelengths
PHYS 1040 - General Physics II Lab The Balmer Series for Hydrogen Source Purpose: The purpose of this experiment is to analyze the emission of light from a hydrogen source and measure and the wavelengths
high energy state for the electron in the atom low energy state for the electron in the atom
 Atomic Spectra Objectives The objectives of this experiment are to: 1) Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. 2) Measure several wavelengths of light
Atomic Spectra Objectives The objectives of this experiment are to: 1) Build and calibrate a simple spectroscope capable of measuring wavelengths of visible light. 2) Measure several wavelengths of light
Observation of Atomic Spectra
 Observation of Atomic Spectra Introduction In this experiment you will observe and measure the wavelengths of different colors of light emitted by atoms. You will first observe light emitted from excited
Observation of Atomic Spectra Introduction In this experiment you will observe and measure the wavelengths of different colors of light emitted by atoms. You will first observe light emitted from excited
The relationship between these aspects is described by the following equation: E = hν =
 1 Learning Outcomes EXPERIMENT A10: LINE SPECTRUM Upon completion of this lab, the student will be able to: 1) Examine the line spectrum of the hydrogen atom. 2) Calculate the frequency and energy of the
1 Learning Outcomes EXPERIMENT A10: LINE SPECTRUM Upon completion of this lab, the student will be able to: 1) Examine the line spectrum of the hydrogen atom. 2) Calculate the frequency and energy of the
( J s)( m/s)
 Ch100: Fundamentals for Chemistry 1 LAB: Spectroscopy Neon lights are orange. Sodium lamps are yellow. Mercury lights are bluish. Electricity is doing something to the electrons of these elements to produce
Ch100: Fundamentals for Chemistry 1 LAB: Spectroscopy Neon lights are orange. Sodium lamps are yellow. Mercury lights are bluish. Electricity is doing something to the electrons of these elements to produce
Emission Spectroscopy
 Objectives Emission Spectroscopy Observe spectral lines from a hydrogen gas discharge tube Determine the initial and final energy levels for the electronic transitions associated with the visible portion
Objectives Emission Spectroscopy Observe spectral lines from a hydrogen gas discharge tube Determine the initial and final energy levels for the electronic transitions associated with the visible portion
Atomic Spectra for Atoms and Ions. Light is made up of different wavelengths
 Atomic Spectra for Atoms and Ions What will you be doing in lab next week? Recording the line spectra of several different substances in discharge tubes. Recording the line spectra of several ions from
Atomic Spectra for Atoms and Ions What will you be doing in lab next week? Recording the line spectra of several different substances in discharge tubes. Recording the line spectra of several ions from
Atomic Emission Spectra
 Atomic Emission Spectra Objectives The objectives of this laboratory are as follows: To build and calibrate a simple meter-stick spectroscope that is capable of measuring wavelengths of visible light.
Atomic Emission Spectra Objectives The objectives of this laboratory are as follows: To build and calibrate a simple meter-stick spectroscope that is capable of measuring wavelengths of visible light.
I understand the relationship between energy and a quanta I understand the difference between an electron s ground state and an electron s excited
 NCCS 1.1.2 & 1.1.3 I understand the relationship between energy and a quanta I understand the difference between an electron s ground state and an electron s excited state I will describe how an electron
NCCS 1.1.2 & 1.1.3 I understand the relationship between energy and a quanta I understand the difference between an electron s ground state and an electron s excited state I will describe how an electron
Chapter 28 Assignment Solutions
 Chapter 28 Assignment Solutions Page 770 #23-26, 29-30, 43-48, 55 23) Complete the following concept map using these terms: energy levels, fixed electron radii, Bohr model, photon emission and absorption,
Chapter 28 Assignment Solutions Page 770 #23-26, 29-30, 43-48, 55 23) Complete the following concept map using these terms: energy levels, fixed electron radii, Bohr model, photon emission and absorption,
Atomic Spectra. d sin θ = mλ (1)
 Atomic Spectra Objectives: To measure the wavelengths of visible light emitted by atomic hydrogen and verify that the measured wavelengths obey the empirical Rydberg formula. To observe emission spectra
Atomic Spectra Objectives: To measure the wavelengths of visible light emitted by atomic hydrogen and verify that the measured wavelengths obey the empirical Rydberg formula. To observe emission spectra
Physics 23 Fall 1998 Lab 4 - The Hydrogen Spectrum
 Physics 3 Fall 998 Lab 4 - The Hydrogen Spectrum Theory In the late 800's, it was known that when a gas is excited by means of an electric discharge and the light emitted is viewed through a diffraction
Physics 3 Fall 998 Lab 4 - The Hydrogen Spectrum Theory In the late 800's, it was known that when a gas is excited by means of an electric discharge and the light emitted is viewed through a diffraction
Atomic Physics Worksheet. between 4000 and 5000 Angstroms ( nanometers): between 6500 and 7500 Angstroms ( nanometers):
 Atomic Physics Worksheet 1. Which of the gas samples shows an emission line with a wavelength between 4000 and 5000 Angstroms (400-500 nanometers): between 6500 and 7500 Angstroms (650-750 nanometers):
Atomic Physics Worksheet 1. Which of the gas samples shows an emission line with a wavelength between 4000 and 5000 Angstroms (400-500 nanometers): between 6500 and 7500 Angstroms (650-750 nanometers):
Spectrum of Hydrogen. Physics 227 Lab
 Introduction In today's lab you will be dealing with an area of physics called quantum mechanics. The only quantum mechanical idea that you will be using today is that electrons in an atom can exist only
Introduction In today's lab you will be dealing with an area of physics called quantum mechanics. The only quantum mechanical idea that you will be using today is that electrons in an atom can exist only
Laboratory Exercise. Quantum Mechanics
 Laboratory Exercise Quantum Mechanics Exercise 1 Atomic Spectrum of Hydrogen INTRODUCTION You have no doubt been exposed many times to the Bohr model of the atom. You may have even learned of the connection
Laboratory Exercise Quantum Mechanics Exercise 1 Atomic Spectrum of Hydrogen INTRODUCTION You have no doubt been exposed many times to the Bohr model of the atom. You may have even learned of the connection
The Emission Spectra of Light
 The Emission Spectra of Light Objectives: Theory: 1.... measured the wavelength limits of the color bands in the visible spectrum, 2.... measured the wavelengths of the emission lines of the hydrogen Balmer
The Emission Spectra of Light Objectives: Theory: 1.... measured the wavelength limits of the color bands in the visible spectrum, 2.... measured the wavelengths of the emission lines of the hydrogen Balmer
EXPERIMENT 17: Atomic Emission
 EXPERIMENT 17: Atomic Emission PURPOSE: To construct an energy level diagram of the hydrogen atom To identify an element from its line spectrum. PRINCIPLES: White light, such as emitted by the sun or an
EXPERIMENT 17: Atomic Emission PURPOSE: To construct an energy level diagram of the hydrogen atom To identify an element from its line spectrum. PRINCIPLES: White light, such as emitted by the sun or an
WEEK 2: 4 SEP THRU 10 SEP; LECTURES 4-6
 Learning Objectives Energy: Light as energy Describe the wave nature of light, wavelength, and frequency using the equation c = λν What is meant by the particle nature of light? Calculate the energy of
Learning Objectives Energy: Light as energy Describe the wave nature of light, wavelength, and frequency using the equation c = λν What is meant by the particle nature of light? Calculate the energy of
5.111 Lecture Summary #5 Friday, September 12, 2014
 5.111 Lecture Summary #5 Friday, September 12, 2014 Readings for today: Section 1.3 Atomic Spectra, Section 1.7 up to equation 9b Wavefunctions and Energy Levels, Section 1.8 The Principle Quantum Number.
5.111 Lecture Summary #5 Friday, September 12, 2014 Readings for today: Section 1.3 Atomic Spectra, Section 1.7 up to equation 9b Wavefunctions and Energy Levels, Section 1.8 The Principle Quantum Number.
The Bohr Model of the Atom
 Unit 4: The Bohr Model of the Atom Properties of light Before the 1900 s, light was thought to behave only as a wave. Light is a type of electromagnetic radiation - a form of energy that exhibits wave
Unit 4: The Bohr Model of the Atom Properties of light Before the 1900 s, light was thought to behave only as a wave. Light is a type of electromagnetic radiation - a form of energy that exhibits wave
Atomic Spectra & Electron Energy Levels
 CHM151LL: ATOMIC SPECTRA & ELECTRON ENERGY LEVELS 1 Atomic Spectra & Electron Energy Levels OBJECTIVES: To measure the wavelength of visible light emitted by excited atoms to calculate the energy of that
CHM151LL: ATOMIC SPECTRA & ELECTRON ENERGY LEVELS 1 Atomic Spectra & Electron Energy Levels OBJECTIVES: To measure the wavelength of visible light emitted by excited atoms to calculate the energy of that
Analyzing Line Emission Spectra viewed through a Spectroscope using a Smartphone
 Energy (ev) Analyzing Line Emission Spectra viewed through a Spectroscope using a Smartphone Eugene T. Smith, PhD Goals: 1. Calibrate spectroscope using mercury emission source or fluorescent bulb. 2.
Energy (ev) Analyzing Line Emission Spectra viewed through a Spectroscope using a Smartphone Eugene T. Smith, PhD Goals: 1. Calibrate spectroscope using mercury emission source or fluorescent bulb. 2.
Experiment 7: Spectrum of the Hydrogen Atom
 Experiment 7: Spectrum of the Hydrogen Nate Saffold nas2173@columbia.edu Office Hour: Mondays, 5:30-6:30PM INTRO TO EXPERIMENTAL PHYS-LAB 1493/1494/2699 Introduction The physics behind: The spectrum of
Experiment 7: Spectrum of the Hydrogen Nate Saffold nas2173@columbia.edu Office Hour: Mondays, 5:30-6:30PM INTRO TO EXPERIMENTAL PHYS-LAB 1493/1494/2699 Introduction The physics behind: The spectrum of
Physics 197 Lab 11: Spectrometer
 Physics 197 Lab 11: Spectrometer Equipment: Item Part # Qty per Team # of Teams Red Tide Spectrometer Vernier V-Spec 1 7 7 Computer with Logger Pro 1 7 7 Optical Fiber Assembly For Red Tide 1 7 7 Ring
Physics 197 Lab 11: Spectrometer Equipment: Item Part # Qty per Team # of Teams Red Tide Spectrometer Vernier V-Spec 1 7 7 Computer with Logger Pro 1 7 7 Optical Fiber Assembly For Red Tide 1 7 7 Ring
UNIT : QUANTUM THEORY AND THE ATOM
 Name St.No. Date(YY/MM/DD) / / Section UNIT 102-10: QUANTUM THEORY AND THE ATOM OBJECTIVES Atomic Spectra for Hydrogen, Mercury and Neon. 1. To observe various atomic spectra with a diffraction grating
Name St.No. Date(YY/MM/DD) / / Section UNIT 102-10: QUANTUM THEORY AND THE ATOM OBJECTIVES Atomic Spectra for Hydrogen, Mercury and Neon. 1. To observe various atomic spectra with a diffraction grating
Lab: Excited Electrons
 Part A: EMISSION SPECTROSCOPY Lab: Excited Electrons According to the Bohr atomic model, electrons orbit the nucleus within specific energy levels. These levels are defined by unique amounts of energy.
Part A: EMISSION SPECTROSCOPY Lab: Excited Electrons According to the Bohr atomic model, electrons orbit the nucleus within specific energy levels. These levels are defined by unique amounts of energy.
Experiment 9. Emission Spectra. measure the emission spectrum of a source of light using the digital spectrometer.
 Experiment 9 Emission Spectra 9.1 Objectives By the end of this experiment, you will be able to: measure the emission spectrum of a source of light using the digital spectrometer. find the wavelength of
Experiment 9 Emission Spectra 9.1 Objectives By the end of this experiment, you will be able to: measure the emission spectrum of a source of light using the digital spectrometer. find the wavelength of
Atomic Spectroscopy. Objectives
 Atomic Spectroscopy Name Objectives explain the difference between emission and absorption spectra calculate the energy of orbits in the Bohr model of hydrogen calculate E for energy transitions in the
Atomic Spectroscopy Name Objectives explain the difference between emission and absorption spectra calculate the energy of orbits in the Bohr model of hydrogen calculate E for energy transitions in the
Chemistry 212 ATOMIC SPECTROSCOPY
 Chemistry 212 ATOMIC SPECTROSCOPY The emission and absorption of light energy of particular wavelengths by atoms and molecules is a common phenomenon. The emissions/absorptions are characteristic for each
Chemistry 212 ATOMIC SPECTROSCOPY The emission and absorption of light energy of particular wavelengths by atoms and molecules is a common phenomenon. The emissions/absorptions are characteristic for each
Lecture 11 Atomic Structure
 Lecture 11 Atomic Structure Earlier in the semester, you read about the discoveries that lead to the proposal of the nuclear atom, an atom of atomic number Z, composed of a positively charged nucleus surrounded
Lecture 11 Atomic Structure Earlier in the semester, you read about the discoveries that lead to the proposal of the nuclear atom, an atom of atomic number Z, composed of a positively charged nucleus surrounded
Pre-Lab Exercises Lab 2: Spectroscopy
 Pre-Lab Exercises Lab 2: Spectroscopy 1. Which color of visible light has the longest wavelength? Name Date Section 2. List the colors of visible light from highest frequency to lowest frequency. 3. Does
Pre-Lab Exercises Lab 2: Spectroscopy 1. Which color of visible light has the longest wavelength? Name Date Section 2. List the colors of visible light from highest frequency to lowest frequency. 3. Does
Einstein. Quantum Physics at a glance. Planck s Hypothesis (blackbody radiation) (ultraviolet catastrophe) Quantized Energy
 Quantum Physics at a glance Quantum Physics deals with the study of light and particles at atomic and smaller levels. Planck s Hypothesis (blackbody radiation) (ultraviolet catastrophe) Quantized Energy
Quantum Physics at a glance Quantum Physics deals with the study of light and particles at atomic and smaller levels. Planck s Hypothesis (blackbody radiation) (ultraviolet catastrophe) Quantized Energy
Atomic Emission and Molecular Absorption Spectra
 Atomic Emission and Molecular Absorption Spectra v062513_6pm Objective: The student will observe the atomic emission spectra of hydrogen using a spectroscope, determine the identity of an unknown metal
Atomic Emission and Molecular Absorption Spectra v062513_6pm Objective: The student will observe the atomic emission spectra of hydrogen using a spectroscope, determine the identity of an unknown metal
Chapter 8. Spectroscopy. 8.1 Purpose. 8.2 Introduction
 Chapter 8 Spectroscopy 8.1 Purpose In the experiment atomic spectra will be investigated. The spectra of three know materials will be observed. The composition of an unknown material will be determined.
Chapter 8 Spectroscopy 8.1 Purpose In the experiment atomic spectra will be investigated. The spectra of three know materials will be observed. The composition of an unknown material will be determined.
HYDROGEN SPECTRUM. Figure 1 shows the energy level scheme for the hydrogen atom as calculated from equation. Figure 1 Figure 2
 15 Jul 04 Hydrogen.1 HYDROGEN SPECTRUM In this experiment the wavelengths of the visible emission lines of hydrogen (Balmer series) will be measured and compared to the values predicted by Bohr s quantum
15 Jul 04 Hydrogen.1 HYDROGEN SPECTRUM In this experiment the wavelengths of the visible emission lines of hydrogen (Balmer series) will be measured and compared to the values predicted by Bohr s quantum
Chapter 7. Quantum Theory and Atomic Structure
 Chapter 7 Quantum Theory and Atomic Structure Outline 1. The Nature of Light 2. Atomic Spectra 3. The Wave-Particle Duality of Matter and Energy 4. The Quantum-Mechanical Model of the Atom 3 September
Chapter 7 Quantum Theory and Atomic Structure Outline 1. The Nature of Light 2. Atomic Spectra 3. The Wave-Particle Duality of Matter and Energy 4. The Quantum-Mechanical Model of the Atom 3 September
Emission of Light: Discharge Lamps & Flame Tests 1
 Emission of Light: Discharge Lamps & Flame Tests 1 Objectives At the end of this activity you should be able to: o Describe how discharge lamps emit photons following electrical excitation of gaseous atoms.
Emission of Light: Discharge Lamps & Flame Tests 1 Objectives At the end of this activity you should be able to: o Describe how discharge lamps emit photons following electrical excitation of gaseous atoms.
Unit 7. Atomic Structure
 Unit 7. Atomic Structure Upon successful completion of this unit, the students should be able to: 7.1 List the eight regions of the electromagnetic spectrum in the designated order and perform calculations
Unit 7. Atomic Structure Upon successful completion of this unit, the students should be able to: 7.1 List the eight regions of the electromagnetic spectrum in the designated order and perform calculations
Previewer Tools Show All In View: Hide All Description Atomic Structure
 Assignment Previewer Chapter 7 (397929) About this Assignment Previewer Tools Show All In View: Hide All Close this window Hidden: Assignment Score Mark Help/Hints Key Solution Show New Randomization Reload
Assignment Previewer Chapter 7 (397929) About this Assignment Previewer Tools Show All In View: Hide All Close this window Hidden: Assignment Score Mark Help/Hints Key Solution Show New Randomization Reload
Chapter 7. Part I Dr. Stone Stan State
 Chapter 7 Part I Dr. Stone Stan State 1 2 Electromagnetic Radiation Perpendicular oscillating fields: Electric: PET scan: gamma rays X-rays Visible light Infrared (heat) Microwaves Magnetic MRI = magnetic
Chapter 7 Part I Dr. Stone Stan State 1 2 Electromagnetic Radiation Perpendicular oscillating fields: Electric: PET scan: gamma rays X-rays Visible light Infrared (heat) Microwaves Magnetic MRI = magnetic
Fingerprinting the Stars Lab
 Name: Block: Fingerprinting the Stars Lab Background: Every element produces a unique fingerprint of spectral lines. By identifying the spectral features in stellar spectra, we can determine the composition
Name: Block: Fingerprinting the Stars Lab Background: Every element produces a unique fingerprint of spectral lines. By identifying the spectral features in stellar spectra, we can determine the composition
Honors Unit 6 Notes - Atomic Structure
 Name: Honors Unit 6 Notes - Atomic Structure Objectives: 1. Students will have a general understanding of the wave nature of light and the interrelationship between frequency, wavelength, and speed of
Name: Honors Unit 6 Notes - Atomic Structure Objectives: 1. Students will have a general understanding of the wave nature of light and the interrelationship between frequency, wavelength, and speed of
How Do We Get Light from Matter: The Origin of Emission
 1 How Do We Get Light from Matter: The Origin of Emission Lines ORGANIZATION Pre-Lab: Origins of Lines Mode: inquiry, groups of 2 Grading: lab notes and post-lab questions Safety: no special requirements
1 How Do We Get Light from Matter: The Origin of Emission Lines ORGANIZATION Pre-Lab: Origins of Lines Mode: inquiry, groups of 2 Grading: lab notes and post-lab questions Safety: no special requirements
The Spectrophotometer and Atomic Spectra of Hydrogen Physics 246
 The Spectrophotometer and Atomic Spectra of Hydrogen Physics 46 Introduction: When heated sufficiently, most elements emit light. With a spectrometer, the emitted light can be broken down into its various
The Spectrophotometer and Atomic Spectra of Hydrogen Physics 46 Introduction: When heated sufficiently, most elements emit light. With a spectrometer, the emitted light can be broken down into its various
Laboratory Atomic Emission Spectrum
 Laboratory Atomic Emission Spectrum Pre-Lab Questions: Answer the following questions in complete sentences by reading through the Overview and Background sections below. 1. What is the purpose of the
Laboratory Atomic Emission Spectrum Pre-Lab Questions: Answer the following questions in complete sentences by reading through the Overview and Background sections below. 1. What is the purpose of the
10. Wavelength measurement using prism spectroscopy
 Spk 0. Wavelength measurement using prism spectroscopy 0. Introduction The study of emitted spectra of electromagnetic waves by excited atoms makes for one of the most important methods to investigate
Spk 0. Wavelength measurement using prism spectroscopy 0. Introduction The study of emitted spectra of electromagnetic waves by excited atoms makes for one of the most important methods to investigate
Chem 115 POGIL Worksheet - Week 8 - Answers Thermochemistry (Continued), Electromagnetic Radiation, and Line Spectra. ΔH o = 285.
 Chem 115 POGIL Worksheet - Week 8 - Answers Thermochemistry (Continued), Electromagnetic Radiation, and Line Spectra Key Questions & Exercises 1. Calculate ΔH o for the reaction, Given: C 2 H 2 (g) + H
Chem 115 POGIL Worksheet - Week 8 - Answers Thermochemistry (Continued), Electromagnetic Radiation, and Line Spectra Key Questions & Exercises 1. Calculate ΔH o for the reaction, Given: C 2 H 2 (g) + H
Electrons! Chapter 5
 Electrons! Chapter 5 I.Light & Quantized Energy A.Background 1. Rutherford s nuclear model: nucleus surrounded by fast-moving electrons; no info on how electrons move, how they re arranged, or differences
Electrons! Chapter 5 I.Light & Quantized Energy A.Background 1. Rutherford s nuclear model: nucleus surrounded by fast-moving electrons; no info on how electrons move, how they re arranged, or differences
Homework Week 1. electronic excited state. electronic ground state
 Statistical Molecular Thermodynamics University of Minnesota Homework Week. The diagram below shows the energy of a diatomic molecule as a function of the separation between the atoms, R. A electronic
Statistical Molecular Thermodynamics University of Minnesota Homework Week. The diagram below shows the energy of a diatomic molecule as a function of the separation between the atoms, R. A electronic
DETERMINATION OF AN EQUILIBRIUM CONSTANT
 DETERMINATION OF AN EQUILIBRIUM CONSTANT In this experiment the equilibrium properties of the reaction between the iron(iii) ion and the thiocyanate ion will be studied. The relevant chemical equation
DETERMINATION OF AN EQUILIBRIUM CONSTANT In this experiment the equilibrium properties of the reaction between the iron(iii) ion and the thiocyanate ion will be studied. The relevant chemical equation
Physics P202, Lab #12. Rydberg s Constant
 Physics P0, Lab #1 Rydberg s Constant The light you see when you plug in a hydrogen gas discharge tube is a shade of lavender, with some pinkish tint at a higher current. If you observe the light through
Physics P0, Lab #1 Rydberg s Constant The light you see when you plug in a hydrogen gas discharge tube is a shade of lavender, with some pinkish tint at a higher current. If you observe the light through
ATOMIC PHYSICS. history/cosmology/tools/ tools-spectroscopy.htm CHAPTER 9 - FROM SPECTROSCOPY TO ATOMS
 ATOMIC PHYSICS http://www.aip.org/ history/cosmology/tools/ tools-spectroscopy.htm CHAPTER 9 - FROM SPECTROSCOPY TO ATOMS What We Will Study Basics of electromagnetic radiation - The AC generator, again
ATOMIC PHYSICS http://www.aip.org/ history/cosmology/tools/ tools-spectroscopy.htm CHAPTER 9 - FROM SPECTROSCOPY TO ATOMS What We Will Study Basics of electromagnetic radiation - The AC generator, again
Different energy levels
 Different energy levels In the microscopic world energy is discrete www.cgrahamphysics.com Review Atomic electrons can only exist in certain discrete energy levels Light is made of photons When e s lose
Different energy levels In the microscopic world energy is discrete www.cgrahamphysics.com Review Atomic electrons can only exist in certain discrete energy levels Light is made of photons When e s lose
Physics Lab #2: Spectroscopy
 Physics 10263 Lab #2: Spectroscopy Introduction This lab is meant to serve as an introduction to the science of spectroscopy. In this lab, we ll learn about how emission and absorption works, and we ll
Physics 10263 Lab #2: Spectroscopy Introduction This lab is meant to serve as an introduction to the science of spectroscopy. In this lab, we ll learn about how emission and absorption works, and we ll
The Quantum Model of the Hydrogen Atom
 Physics 109 Science 1 Experiment 1 1 The Quantum Model of the Hydrogen Atom In this experiment you will use a spectrometer to determine the wavelengths of the visible lines of atomic hydrogen. The goal
Physics 109 Science 1 Experiment 1 1 The Quantum Model of the Hydrogen Atom In this experiment you will use a spectrometer to determine the wavelengths of the visible lines of atomic hydrogen. The goal
The Hydrogen Spectrum
 1 The Hydrogen Spectrum PHYS 1301 F98 Prof. T.E. Coan Last edit: 6 Aug 98 Introduction In last week's laboratory experiment on diffraction, you should have noticed that the light from the mercury discharge
1 The Hydrogen Spectrum PHYS 1301 F98 Prof. T.E. Coan Last edit: 6 Aug 98 Introduction In last week's laboratory experiment on diffraction, you should have noticed that the light from the mercury discharge
ACTIVITY 1. Exploring Light from Gases
 Name: WAVES of matter Class: Visual Quantum Mechanics ACTIVITY 1 Exploring Light from Gases Goal We will view the colors of light which are emitted by different gases. From these patterns of light we gain
Name: WAVES of matter Class: Visual Quantum Mechanics ACTIVITY 1 Exploring Light from Gases Goal We will view the colors of light which are emitted by different gases. From these patterns of light we gain
Quantum Theory of the Atom
 The Wave Nature of Light Quantum Theory of the Atom Electromagnetic radiation carries energy = radiant energy some forms are visible light, x rays, and radio waves Wavelength ( λ) is the distance between
The Wave Nature of Light Quantum Theory of the Atom Electromagnetic radiation carries energy = radiant energy some forms are visible light, x rays, and radio waves Wavelength ( λ) is the distance between
where c m s (1)
 General Physics Experiment 6 Spectrum of Hydrogen s Emission Lines Objectives: < To determine wave lengths of the bright emission lines of hydrogen. < To test the relationship between wavelength and energy
General Physics Experiment 6 Spectrum of Hydrogen s Emission Lines Objectives: < To determine wave lengths of the bright emission lines of hydrogen. < To test the relationship between wavelength and energy
Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 The Bohr Atom Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the energy of the emitted photon when an electron drops from the third
The Bohr Atom Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. What is the energy of the emitted photon when an electron drops from the third
An element Is a substance that cannot be split into simpler substance. It is composed of discrete particles called atoms.
 digitalteachers.co.ug Atomic structure Inorganic chemistry deals with the physical and chemical properties of the elements of the the periodic table. An element Is a substance that cannot be split into
digitalteachers.co.ug Atomic structure Inorganic chemistry deals with the physical and chemical properties of the elements of the the periodic table. An element Is a substance that cannot be split into
CHEMISTRY SEMESTER ONE
 EMISSION SPECTROSCOPY Lab format: this lab is a remote lab activity Relationship to theory: This activity covers the relationship between colors and absorbed/emitted light, as well as the relationship
EMISSION SPECTROSCOPY Lab format: this lab is a remote lab activity Relationship to theory: This activity covers the relationship between colors and absorbed/emitted light, as well as the relationship
Atomic Structure. Standing Waves x10 8 m/s. (or Hz or 1/s) λ Node
 Atomic Structure Topics: 7.1 Electromagnetic Radiation 7.2 Planck, Einstein, Energy, and Photons 7.3 Atomic Line Spectra and Niels Bohr 7.4 The Wave Properties of the Electron 7.5 Quantum Mechanical View
Atomic Structure Topics: 7.1 Electromagnetic Radiation 7.2 Planck, Einstein, Energy, and Photons 7.3 Atomic Line Spectra and Niels Bohr 7.4 The Wave Properties of the Electron 7.5 Quantum Mechanical View
University Chemistry
 University Chemistry 1 Electromagnetic Radiation Electromagnetic radiation has some of the properties of both a particle and a wave. Particles: have a definite mass and they occupy space. Wave have no
University Chemistry 1 Electromagnetic Radiation Electromagnetic radiation has some of the properties of both a particle and a wave. Particles: have a definite mass and they occupy space. Wave have no
Electronic Structure of Atoms
 Electronic Structure of Atoms Electrons inhabit regions of space known as orbitals. Heisenberg Uncertainty Principle NOTE Impossible to define with absolute precision, at the same time, both the position
Electronic Structure of Atoms Electrons inhabit regions of space known as orbitals. Heisenberg Uncertainty Principle NOTE Impossible to define with absolute precision, at the same time, both the position
Experiment 4 Radiation in the Visible Spectrum
 Experiment 4 Radiation in the Visible Spectrum Emission spectra can be a unique fingerprint of an atom or molecule. The photon energies and wavelengths are directly related to the allowed quantum energy
Experiment 4 Radiation in the Visible Spectrum Emission spectra can be a unique fingerprint of an atom or molecule. The photon energies and wavelengths are directly related to the allowed quantum energy
The Nature of Energy
 The Nature of Energy For atoms and molecules, one does not observe a continuous spectrum, as one gets from a white light source.? Only a line spectrum of discrete wavelengths is observed. 2012 Pearson
The Nature of Energy For atoms and molecules, one does not observe a continuous spectrum, as one gets from a white light source.? Only a line spectrum of discrete wavelengths is observed. 2012 Pearson
ACTIVITY 2 Exploring Light Patterns
 Name: Class: SOLIDS & Visual Quantum Mechanics LIGHT ACTIVITY 2 Exploring Light Patterns Goal We will continue to investigate the properties of LEDs and the incandescent lamp by observing and exploring
Name: Class: SOLIDS & Visual Quantum Mechanics LIGHT ACTIVITY 2 Exploring Light Patterns Goal We will continue to investigate the properties of LEDs and the incandescent lamp by observing and exploring
ASTRO Fall 2012 LAB #7: The Electromagnetic Spectrum
 ASTRO 1050 - Fall 2012 LAB #7: The Electromagnetic Spectrum ABSTRACT Astronomers rely on light to convey almost all of the information we have on distant astronomical objects. In addition to measuring
ASTRO 1050 - Fall 2012 LAB #7: The Electromagnetic Spectrum ABSTRACT Astronomers rely on light to convey almost all of the information we have on distant astronomical objects. In addition to measuring
Emission of Light & Atomic Models 1
 Emission of Light & Atomic Models 1 Objective At the end of this activity you should be able to: o Explain what photons are, and be able to calculate their energies given either their frequency or wavelength.
Emission of Light & Atomic Models 1 Objective At the end of this activity you should be able to: o Explain what photons are, and be able to calculate their energies given either their frequency or wavelength.
EXPERIMENT 12 THE GRATING SPECTROMETER AND ATOMIC SPECTRA
 OBJECTIVES Learn the theory of the grating spectrometer Observe the spectrum of mercury and hydrogen Measure the grating constant of a diffraction grating Measure the Rydberg Constant EXPERIMENT THE GRATING
OBJECTIVES Learn the theory of the grating spectrometer Observe the spectrum of mercury and hydrogen Measure the grating constant of a diffraction grating Measure the Rydberg Constant EXPERIMENT THE GRATING
E n = n h ν. The oscillators must absorb or emit energy in discrete multiples of the fundamental quantum of energy given by.
 Planck s s Radiation Law Planck made two modifications to the classical theory The oscillators (of electromagnetic origin) can only have certain discrete energies determined by E n = n h ν with n is an
Planck s s Radiation Law Planck made two modifications to the classical theory The oscillators (of electromagnetic origin) can only have certain discrete energies determined by E n = n h ν with n is an
Exercise 5: The electromagnetic spectrum and spectroscopy
 Physics 223 Name: Exercise 5: The electromagnetic spectrum and spectroscopy Objectives: Experience an example of a discovery exercise Predict and confirm the relationship between measured quantities Using
Physics 223 Name: Exercise 5: The electromagnetic spectrum and spectroscopy Objectives: Experience an example of a discovery exercise Predict and confirm the relationship between measured quantities Using
ATOMIC SPECTRA. To identify elements through their emission spectra. Apparatus: spectrometer, spectral tubes, power supply, incandescent lamp.
 ATOMIC SPECTRA Objective: To measure the wavelengths of visible light emitted by atomic hydrogen and verify the measured wavelengths against those predicted by quantum theory. To identify elements through
ATOMIC SPECTRA Objective: To measure the wavelengths of visible light emitted by atomic hydrogen and verify the measured wavelengths against those predicted by quantum theory. To identify elements through
Information in Radio Waves
 Summative Assessment: Natural Sources of Radio Performance expectation: Develop and use a model of two objects interacting through electric or magnetic fields to illustrate the forces between objects and
Summative Assessment: Natural Sources of Radio Performance expectation: Develop and use a model of two objects interacting through electric or magnetic fields to illustrate the forces between objects and
Electrons, Energy, & the Electromagnetic Spectrum Notes
 Electrons, Energy, & the Electromagnetic Spectrum Notes Bohr Model Diagram Interpretation What form of EM radiation is released when an electron in a hydrogen atom falls from the 5 th energy level to the
Electrons, Energy, & the Electromagnetic Spectrum Notes Bohr Model Diagram Interpretation What form of EM radiation is released when an electron in a hydrogen atom falls from the 5 th energy level to the
Chapter Test B. Chapter: Arrangement of Electrons in Atoms. possible angular momentum quantum numbers? energy level? a. 4 b. 8 c. 16 d.
 Assessment Chapter Test B Chapter: Arrangement of Electrons in Atoms PART I In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question
Assessment Chapter Test B Chapter: Arrangement of Electrons in Atoms PART I In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question
Bright line spectrum questions
 Base your answers to questions 1 and 2 on the information below and on your knowledge of chemistry. The bright-line spectra for four elements and a mixture of elements are shown in the diagram below. 1.
Base your answers to questions 1 and 2 on the information below and on your knowledge of chemistry. The bright-line spectra for four elements and a mixture of elements are shown in the diagram below. 1.
Quantum Physics Objective: Apparatus:
 1 Quantum Physics Objective: 1. To measure the wavelengths of visible light emitted by atomic hydrogen and verify the measured wavelengths against those predicted by quantum theory. To identify an unknown
1 Quantum Physics Objective: 1. To measure the wavelengths of visible light emitted by atomic hydrogen and verify the measured wavelengths against those predicted by quantum theory. To identify an unknown
Experiment 24: Spectroscopy
 Experiment 24: Spectroscopy Figure 24.1: Spectroscopy EQUIPMENT High Voltage Power Supply Incandescent Light Source (3) Gas Discharge Tubes: 1. Helium 2. Hydrogen 3. Unknown Element Spectrometer Felt (1)
Experiment 24: Spectroscopy Figure 24.1: Spectroscopy EQUIPMENT High Voltage Power Supply Incandescent Light Source (3) Gas Discharge Tubes: 1. Helium 2. Hydrogen 3. Unknown Element Spectrometer Felt (1)
CHEM Chapter 6. Basic Quantum Chemistry (Homework). WL36
 CHEM 1411. Chapter 6. Basic Quantum Chemistry (Homework). WL36 1. The Bohr model of the hydrogen atom found its greatest support in experimental work on the photoelectric effect. A) True B) False 2. A
CHEM 1411. Chapter 6. Basic Quantum Chemistry (Homework). WL36 1. The Bohr model of the hydrogen atom found its greatest support in experimental work on the photoelectric effect. A) True B) False 2. A
A Determination of Planck s Constant with LED s written by Mark Langella
 A Determination of Planck s Constant with LED s written by Mark Langella The purpose of this experiment is to measure Planck s constant, a fundamental physical constant in nature, by studying the energy
A Determination of Planck s Constant with LED s written by Mark Langella The purpose of this experiment is to measure Planck s constant, a fundamental physical constant in nature, by studying the energy
Radiation - Electromagnetic Waves (EMR): wave consisting of oscillating electric and magnetic fields that move at the speed of light through space.
 Radiation - Electromagnetic Waves (EMR): wave consisting of oscillating electric and magnetic fields that move at the speed of light through space. Photon: a quantum of light or electromagnetic wave. Quantum:
Radiation - Electromagnetic Waves (EMR): wave consisting of oscillating electric and magnetic fields that move at the speed of light through space. Photon: a quantum of light or electromagnetic wave. Quantum:
The energy of the emitted light (photons) is given by the difference in energy between the initial and final states of hydrogen atom.
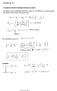 Lecture 20-21 Page 1 Lectures 20-21 Transitions between hydrogen stationary states The energy of the emitted light (photons) is given by the difference in energy between the initial and final states of
Lecture 20-21 Page 1 Lectures 20-21 Transitions between hydrogen stationary states The energy of the emitted light (photons) is given by the difference in energy between the initial and final states of
Physics 1CL OPTICAL SPECTROSCOPY Spring 2010
 Introduction In this lab, you will use a diffraction grating to split up light into the various colors which make up the different wavelengths of the visible electromagnetic spectrum. You will assemble
Introduction In this lab, you will use a diffraction grating to split up light into the various colors which make up the different wavelengths of the visible electromagnetic spectrum. You will assemble
Bohr Diagram, Lewis Structures, Valence Electrons Review 1. What is the maximum number of electrons you can fit in each energy level or shell?
 AP Chemistry Ms. Ye Name Date Block Bohr Diagram, Lewis Structures, Valence Electrons Review 1. What is the maximum number of electrons you can fit in each energy level or shell? 1 st shell 2 nd shell
AP Chemistry Ms. Ye Name Date Block Bohr Diagram, Lewis Structures, Valence Electrons Review 1. What is the maximum number of electrons you can fit in each energy level or shell? 1 st shell 2 nd shell
The Development of Atomic Theory
 The Development of Atomic Theory Democritus (400 BC) John Dalton (1803) J.J. Thomson (1897) Ernest Rutherford (1911) James Chadwick (1932) - suggested that matter is composed of indivisible particles called
The Development of Atomic Theory Democritus (400 BC) John Dalton (1803) J.J. Thomson (1897) Ernest Rutherford (1911) James Chadwick (1932) - suggested that matter is composed of indivisible particles called
Sample Exercise 6.1 Concepts of Wavelength and Frequency
 Sample Exercise 6.1 Concepts of Wavelength and Frequency Two electromagnetic waves are represented in the margin. (a) Which wave has the higher frequency? (b) If one wave represents visible light and the
Sample Exercise 6.1 Concepts of Wavelength and Frequency Two electromagnetic waves are represented in the margin. (a) Which wave has the higher frequency? (b) If one wave represents visible light and the
Bohr. Electronic Structure. Spectroscope. Spectroscope
 Bohr Electronic Structure Bohr proposed that the atom has only certain allowable energy states. Spectroscope Using a device called a it was found that gaseous elements emitted electromagnetic radiation
Bohr Electronic Structure Bohr proposed that the atom has only certain allowable energy states. Spectroscope Using a device called a it was found that gaseous elements emitted electromagnetic radiation
Quantum and Atomic Physics - Multiple Choice
 PSI AP Physics 2 Name 1. The Cathode Ray Tube experiment is associated with: (A) J. J. Thomson (B) J. S. Townsend (C) M. Plank (D) A. H. Compton 2. The electron charge was measured the first time in: (A)
PSI AP Physics 2 Name 1. The Cathode Ray Tube experiment is associated with: (A) J. J. Thomson (B) J. S. Townsend (C) M. Plank (D) A. H. Compton 2. The electron charge was measured the first time in: (A)
Hint: unit of energy transferred is equal to: hν = hc
 CH 0 Fall 08 Discussion # Chapter 8 Your name: TF s name Discussion Day/Time: Things you should know when you leave Discussion today: Atomic (matter) Emission and absorption of light. Energy Conservation
CH 0 Fall 08 Discussion # Chapter 8 Your name: TF s name Discussion Day/Time: Things you should know when you leave Discussion today: Atomic (matter) Emission and absorption of light. Energy Conservation
University of Massachusetts, Amherst
 PHYSICS 286: Modern Physics Laboratory SPRING 2010 (A. Dinsmore and K. Kumar) Feb 2009 Experiment 4: THE FRANCK HERTZ EXPERIMENT Electronic Excitations of a Gas, and Evidence for the Quantization of Atomic
PHYSICS 286: Modern Physics Laboratory SPRING 2010 (A. Dinsmore and K. Kumar) Feb 2009 Experiment 4: THE FRANCK HERTZ EXPERIMENT Electronic Excitations of a Gas, and Evidence for the Quantization of Atomic
Student Exploration: Bohr Model: Introduction
 Name: Date: Student Exploration: Bohr Model: Introduction Vocabulary: absorption spectrum, Bohr model, electron volt, energy level, laser, orbital, photon Prior Knowledge Questions (Do these BEFORE using
Name: Date: Student Exploration: Bohr Model: Introduction Vocabulary: absorption spectrum, Bohr model, electron volt, energy level, laser, orbital, photon Prior Knowledge Questions (Do these BEFORE using
Duncan. Electrons, Energy, & the Electromagnetic Spectrum Notes Simplified, 2-D Bohr Model: Figure 1. Figure 2. Figure 3
 Electrons, Energy, & the Electromagnetic Spectrum Notes Simplified, 2-D Bohr Model: Figure 1 Figure 2 Figure 3 Light Calculation Notes Here s how the type/form of EM radiation can be determined The amount
Electrons, Energy, & the Electromagnetic Spectrum Notes Simplified, 2-D Bohr Model: Figure 1 Figure 2 Figure 3 Light Calculation Notes Here s how the type/form of EM radiation can be determined The amount
Electrons, Energy, & the Electromagnetic Spectrum Notes Simplified, 2-D Bohr Model: Figure 2. Figure 3 UNIT 4 - ELECTRONS & ELECTRON ARRANGEMENT
 Electrons, Energy, & the Electromagnetic Spectrum Notes Simplified, 2-D Bohr Model: Figure 1 UNIT 4 - ELECTRONS & ELECTRON ARRANGEMENT Figure 2 Figure 3 The energy is released as electromagnetic radiation.
Electrons, Energy, & the Electromagnetic Spectrum Notes Simplified, 2-D Bohr Model: Figure 1 UNIT 4 - ELECTRONS & ELECTRON ARRANGEMENT Figure 2 Figure 3 The energy is released as electromagnetic radiation.
Unit 1. Electronic Structure page 1
 Unit 1 Electronic Structure Section 1. Learning Outcomes Practice Questions Answers Electronic Structure Electromagnetic spectrum / calculations Electron configuration / Periodic Table Electronic Structure
Unit 1 Electronic Structure Section 1. Learning Outcomes Practice Questions Answers Electronic Structure Electromagnetic spectrum / calculations Electron configuration / Periodic Table Electronic Structure
