Closed virial equations for hard parallel cubes and squares*
|
|
|
- Geraldine Jordan
- 6 years ago
- Views:
Transcription
1 Juiz de Fora WOODCOCK 011 Closed virial equations for hard parallel cubes and squares* SUMMARY Leslie V. Woodcock** Instituto Ciệnce Exacte Departamento de Fisica Universidade Federal de Juiz de Fora Brazil A correlation between maxima in virial coefficients (B n ), and kissing numbers for hard hyper-spheres up to dimension D=5, indicates a virial equation and close-packing relationship. Known virial coefficients up to B 7, both for hard parallel cubes and squares, indicate that the limiting differences B n B (n-1) behave similar to spheres and disks, in the respective expansions relative to maximum close packing. In all cases, the increment B n B (n-1) will approach a negative constant with similar functional form in each dimension. This observation enables closed-virial equations-of-state for cubes and squares to be obtained. In both the 3D and D cases, the virial pressures begin to deviate from MD thermodynamic pressures at densities well-below crystallization. These results consolidate the general conclusion, from previous papers on spheres and disks, that the Mayer cluster expansion cannot represent the thermodynamic fluid phases up to freezing as commonly assumed in statistical theories. CONTENT 1. Introduction. Kissing numbers and virial coefficients 3. Hard parallel cubes 4. Hard parallel squares 5. Conclusions Acknowledgement References APPENDIX 1 * Este papel e dedicadoa a memoria de Orozina Feliciano de Almeida ( ), de um povo popular advogada de Juiz de Fora; ela paciente determinada inspirada. ** les.woodcock@manchester.ac.uk permanent address: Manchester Interdisciplinary Biocentre, UK. 1
2 Juiz de Fora WOODCOCK Introduction An equation-of-state for the hard-sphere fluid pressure (p) was obtained by analytical closure of the virial expansion in powers of density relative to close-packing [1]. The virial series is simply a Maclaurin expansion of the pressure as a state function about zero density, and can be written in dimensionless form n n 1 0 n 1 Z B (1) where Z = pv/nk B T, T is absolute temperature, k B is Boltzmann s constant, B n are the dimensionless coefficients is the number density NV, and is the crystal close packing density. The theory of the coefficients B n is well-established; exact statistical expressions for B n are available as the cluster integrals that are calculated analytically up to B 4 and presently available numerically up to B 10. A closed form of equation (1) based only upon known coefficients, B 1 to B 10, has been shown to be everywhere convergent up to its first pole at 0. The equation-of-state is accurate for the equilibrium hard-sphere fluid, yielding the same thermodynamic pressures as may now be obtained with 6-figure precision from MD simulations of up-to a million spheres []. A more refined scrutiny of the margins of difference, with due consideration of the uncertainties, however, shows that the virial equation begins to deviate from the thermodynamic pressure equation, albeit very slightly, at a density in the mid-fluid range close to the available volume percolation transition [3]. It has also been shown that the known virial series of the two-dimensional (D=) harddisk fluid is also amenable to closure [4]; an equation-of-state is obtained with 6-figure accuracy at low density. As in the D=3 case, but more pronounced however, there is a clearer deviation well-below the freezing transition. A subsequent more accurate analytical closed form for the pressure equation-of-state for hard disks was derived [5]. The precision of this revised version of the closed virial equation was such that we were able to conclude that the deviation from the thermodynamic pressure occurs at or near the D-percolation density of available, or excluded volume, pa = 0.4, determined previously by Hoover et al. [6]. Thus, for both disks (D=) and spheres (D=3) the virial equations-of-state do not represent the thermodynamic state functions for densities above the respective free volume percolation transitions. It is also worth noting that the known virial coefficients for D=4 hyper-spheres [7], in powers of density relative to close packing, also increase from B 1 -B 6, then decrease with B n -B (n-1) linearly with n, going negative at n=10. This indicates that the higher virial coefficients should eventually go negative, yielding a closed virial equation with a negative pressure pole at maximum packing in this case also. These empirical observations regarding the destiny of the virial equations-of-state at higher densities would appear to confirm that the percolation transitions in these hardcore model fluids may indeed be higher-order thermodynamic phase transitions that
3 Juiz de Fora WOODCOCK 011 signify the onset of the divergence of the virial pressure equation from the thermodynamic pressure. There are those who seem to religiously believe the Mayer virial series is equivalent to the thermodynamic fluid equation-of-state; this is a common misapprehension amongst physicists. The suggestion that the virial expansion may not represent the physical thermodynamic state for densities above the percolation transition is not new. For both hard parallel cubes and hard parallel squares, the virial coefficients are known up to B 7 [8-10] In fact, it has been known for 50 years that the virial coefficients for parallel cubes go negative [10]. MD calculations 5 years ago [11], of the thermodynamic and transport properties of hard parallel cubes, indicated that the hard-parallel-cube fluid virial equation deviates above the percolation transition, at a density well-below the freezing transition. Kratky [1] further elucidated upon the suggestion the percolation transitions in spheres and disks may be higher order thermodynamic phase transitions. Reference [1] also gives valuable definitions and discussion of the various percolation transitions in hard-core fluids generally. Table 1: Known virial coefficients of the parallel hard-cube fluid (D=3) and parallel hard square fluid (D=): also given in columns 3 and 5, for D= 3 and respectively, are the predicted values up to B 1 from the closed virial equations obtained here. n B n D Eqn. (3) n [D=] Eqn. (6) On revisiting the known virial coefficients for both fluid systems of parallel cubes and squares (Table 1), it can be seen that in both cases, now in the expansion of the packing fraction (y = 0) even from just the known coefficients up to B 7, the differences B n -B (n-1) are approaching negative constants with functional forms analogous to the D=3 spheres [1-3] and D= disks [4,5] cases respectively. Here we obtain and test the closed virial equations-of-state for the systems of parallel cubes and squares, which by analogy with the closed equations of spheres [1-3] and disks [4,5], and then compare with available MD data from the literature. 3
4 kissing number Juiz de Fora WOODCOCK 011. Kissing numbers and virial coefficients The first simple observation we make, about the B n values in Table 1, is that for both D=3 and D=, B n first increases with n, peaks at n=4 (3D) or 5 (D) and then begin to decrease, and in the case of the cubes go negative. This is just the same behavior that is seen generally in hyper-spheres of D > 1, i.e. D=, 3 and 4 and 5 The highest virial coefficient in all these cases is of the same order as a maximum coordination or contact numbers, known as kissing numbers to mathematicians [1], of contiguous spheres to a central sphere. This correlation provides salient evidence that the virial expansions about zero density, in powers of density relative to close packing, equation (1), reflects information about the close packed state, and is per se suggestive of the closed forms with the first poles at that maximum density. Kissing numbers of hard hyperspheres dimension (D) Figure 1: Kissing numbers for D-dimensional hyper-spheres (shown as red circles centered on the exact whole number) compared to the values of B n (max) + D (shown as smaller black circles); the maximum virial coefficients for D = to 5 are 4.43 (B 9 ), 8.864(B 8 ),.639(B 6 ) and (B 5 ) respectively. The kissing number is simply defined as the maximum number of hyper-spheres in a configuration of like spheres that may be contiguous with a central sphere at the same time. This number is not necessarily the lattice near-neighbor number, but for the lower dimensions up to D=5, it can represent the first co-ordination number in the most stable crystal structure at infinite pressure, or equivalently, at zero temperature. Kissing numbers for hyper-spheres are known up to D=8 [1] but here (Figure 1) we compare with D=1 to D=5. The correlation is convincing evidence that the virial expansion of non-space-filling hyper-spheres does not reflect the physically unreal packing (y=1), seen in many theoretical approximate virial equations, but rather the maximum crystal close-packed states ( 0 ). 4
5 Juiz de Fora WOODCOCK 011 Looking again at Table 1, the B n values for the cases D= 3 (cubes) and D= (squares) have similar maxima at B 4 (D=3) value and B 5 (D=) value 3.7. There are three different types of kisses in these systems, faces, edges and corners. Counting only faces and edges, we get for B max +D, 14.3 and 5.7, compared to 14 and 4 for the respective kissing numbers of cubes and squares respectively. In these cases, the density relative to close packing 0 = packing fraction y, and the maximum density corresponds to y=1. Below, we will use y to represent reduced number density, and anticipate a singularity in the closed virial equations at y=1. 3. Hard parallel cubes The values of the known virial coefficients for hard parallel cubes are listed in Table 1. Although presently limited to B 7, the values B 5 to B 7 are already indicative of a similar closed functional form to that obtained for hard-spheres [1,]. In Figure, when plotted against exp(-n), by analogy with spheres in reference [], the incremental values B n -B (n-1) beyond (B 5 -B 4 ) decrease exponentially as n according to Bn B(n-1) A0 A1exp(-n) Aexp(-n) (3) and approach a constant value (5.5) which B 7 -B 6 (= 4.6) is almost upon D cubes y = x x R = 1.00 Bn-B(n-1) exp(-n) Figure : Difference between successive coefficients (B n ) from n= 7 to n = 5 in the expansion in powers of the density relative to close packing: the difference B n - B (n-1) decreases exponentially, and approaches the constant -5.5 when n. 5
6 Z Juiz de Fora WOODCOCK 011 Neglecting the higher order term, once A 0 is obtained, the constant A 1 is determined from B m -B m-1 and can be used to predict all higher values B n from m+1 to infinity, thereby effecting an analytic closure to the virial expansion. As with hard spheres, interpolation of the known virial coefficients suggests that since the limiting value of B n -B n-1 is negative, the virial coefficients beyond B 7 will remain negative. The corresponding virial equation-of-state will be continuous in all its derivatives, eventually showing a negative pressure, with the first pole at o, i.e. y=1. The virial equation-of-state for hard parallel cubes would then take the form Z 1 n m B (n-1) -(m 1) (n 1) n y (Bm A0 A1 e ) y n m 1 (4) In which each term of the second summation can be closed separately as with spheres, [,3] to obtain an equation-of-state. Equation (4) enables the closure for any known n greater or equal to m=7. The last term is a negligible correction for finite m and disappears for large m. It would appear from the data in Figure 1 and Table 1 that m=7 is sufficiently large for accuracy of the same order as that of the available MD pressures in this case, which is about 4-figure precision. Accordingly, if we close the summation at m= 7 and put A o = - 5.5, equation (4) reduces to a form which is the same as the closed virial equation for hard spheres [1,] with A 0 for cubes fixed at -5.5 (Figure ). m m m (n 1) y y Z 1 B n y Bm A0 (1 y) (1 y) n (5) 10 EoS hard parallel cubes density( ) 6
7 Z Juiz de Fora WOODCOCK 011 Figure 3: Closed-virial equation-of-state (equation (4) with m= 7 and A 0 = -5.5 : solid line) compared with thermodynamic pressures (Z = pv/nk B T) obtained from MD simulations by Woodcock and van Swol [11] (red circles) and Hoover et al. [14] ( blue circles). This equation-of-state for parallel cubes (equation (5) with A 0 = -5.5) which can now be compared with the literature values of available thermodynamic pressures obtained from MD simulations [11,14]. The virial equation it accurate to within the uncertainty in the experimental data up to the density around 0. to 0.3 and then it begins to deviate. The pressure peaks, then decreases and goes negative, and eventually diverges with a negative pole at the density of maximum packing. The difference in pressure between the closed virial equation (5), with A0= -5.5, and the thermodynamic pressure from MD computations in the vicinity of melting is shown in Figure deviation : hard parallel cubes density ( ) Figure 4: Deviation of closed-virial equation-of-state (equation (5): m=7, A 0 =5.5) and thermodynamic pressures obtained from MD simulations by Woodcock and van Swol [11](red circles) and Hoover et al. [13] (blue circles): Z = Z MD Z (virial) Inspection of the discrepancy, between closed virial equation (5), and thermodynamic MD pressures, in Figure 4, suggests that the virial pressure begins to deviate from the thermodynamic pressure at a density on or below the available volume percolation density (y pa ) [11]. This deviation is statistically significant; it would appear from the forgoing analyses that it cannot be explained either by uncertainties in the thermodynamic pressures, or errors in the known virial coefficients, or any combination of both. To do so the virial coefficients B 8 and beyond would have to take an extraordinary unusual twist. As with spheres, however, we cannot say that within the 7
8 Juiz de Fora WOODCOCK 011 uncertainties, the deviation does not begin at an even lower density, perhaps at the lower percolation transition density (y pe ) associated with the excluded volume (see also references [3,1]). 4. Hard parallel squares Turning now to the -D case of hard parallel squares, inspection of incremental values of successive virial coefficients in Table 1 shows that squares are behaving similar to -D disks. Differences in successive B n, in powers of density relative to crystal close packing in equation (1), B (n) -B (n-1) are plotted in Figure 5. With only the three points available the graph that beyond (B 5 -B 4 ) the increment decrease varies according to B 1 n B(n-1) α0 α /n (6) Equation (6) is the same functional form that was obtained for hard disks where the virial coefficients are known up to B 10. As with disks, this interpolation of the known virial coefficients suggest that since the limiting constant 0 is negative, the virial coefficients will eventually become negative and the corresponding virial equation-of-state will eventually give a negative pressure, with the first pole at y=1, as with cubes. Equation (6) with the parameters obtained from the EXCEL trendline in Figure 5 predicts the first negative coefficient for squares will be B 8. In the corresponding closed-virial equation for D= disks B 31 is predicted to be the first negative coefficient [5]. D squares y = 4.877x R = B n -B (n-1) /n Figure 5: Difference between successive coefficients (B n ) from n= 7 to n = 5 in the expansion in powers of the density relative to close packing: the difference B n - B (n-1) is decreasing roughly as 1/n, and would approach the constant
9 Z Juiz de Fora WOODCOCK 011 By analogy with disks, then the analytic closed form virial equation-of-state for the hard parallel square fluid takes the same form as that of the hard-disk fluid as follows (the derivation is given in the APPENDIX of reference [5]). Z m n 1 B n α n(1 y) y (n 1) Bm (1 y) αo (1 y) y m α ln(1 y(1- y) y) (7) where B m is the highest known virial coefficient, presently B 7. Using only the known coefficients B 5 to B 7 as given in Table 1, the constants in the closed virial equation for parallel squares, from the limiting value of B n - B (n-1) Figure 5, are 0 = -4.90, and the slope α = 4.9. The closed virial equation-of-state for the pressure of the parallel-square fluid can then be compared with the thermodynamic pressure from the recent MD computations of Hoover et al. [14]. The virial pressure deviates from the thermodynamic pressure at a density well-below the freezing transition (around y=0.7) eventually gives an unrealistic negative pressure and diverges with a negative pole at maximum close packed density 0 corresponding to y=1. EoS hard parallel squares density (Y) Figure 6: Closed-virial equation-of-state (equation (4) with m= 7 and the parameters A and A 0 as given in Figure 5: solid line) compared with thermodynamic pressures (z = pv/nk B T) obtained from MD simulations by Hoover et al. [14] (blue circles). In Figure 7, the deviation of equation (7) for squares from MD pressures [14] are plotted as a function of density for all the MD data points above in Figure 3 except the data point at the density 0.35 which is an aberration that may contain an error. This plot suggests that the deviation is originating in the vicinity of a low density percolation transition. We have not seen a report of the determination of this percolation transition for squares, but the deviation is around the same value of / 0 (= y for squares) as that 9
10 Z Juiz de Fora WOODCOCK 011 found by Hoover et al. to be the onset of the free volume percolation transition for the D= hard-disk fluid [6]. deviation: hard parallel squares density Figure 7: Density dependence of pressure difference between closed-virial equation-ofstate (equation (4): m=10) and thermodynamic pressures obtained from MD simulations by Hoover et al. [14]. 5. Conclusions In this paper we have looked at the trend in B n -B n-1 for the known virial coefficients of cubes and squares in the Mayer virial expansion equation (1) and observed that the same closed virial equations exhibit the same functional forms, as has been obtained for spheres and disks respectively [1-5]. When the resultant closed vrial equations are compared with available thermodynamic pressures, as with the fluids of spheres and disks, the virial pressure begins deviating from the thermodynamic pressure at a low fluid density. The percolation transition associated with free volume has only been estimated for cubes, and has not yet been reported for squares. Nonetheless, all the evidence is that the onset of the deviations may be associated with higher-order thermodynamic phase transitions. The APPENDIX to this paper illustrates the belief that the virial expansion of Mayer [15] is actually equivalent to the fluid equation-of-state of these hard core models, at least up to freezing, is a widespread misapprehension amongst theoretical physicists. In response to the various suggestions that the empirical results of these closed-virial comparisons are speculative, it seems that what has been unduly speculative is the incorrect assumption that Mayer s cluster expansion represents the thermodynamic state functions of the fluid phases up to or beyond the freezing transition. In five cases we have so far looked at, first spheres and disks, now here squares and cubes, and also D=4 hyperspheres (unpublished), the virial equations are failing at a low equilibrium-fluid density. 10
11 Juiz de Fora WOODCOCK 011 Many statistical theories are based upon the belief that, if all the terms in the Mayer cluster expansion could be approximated accurately, it would be a theory of liquids. We now see that all these simple hard-core models have two fluid phases, the low density gas phase where the Mayer virial expansion represents the thermodynamic state functions, and the high density fluid phase where it is invalid. Looking again at the theory of simple liquids, we may now conjecture that the high density fluid phase belongs to the same phase as the supercooled liquid phase, by definition. For hard-spheres this is the metastable branch that is a continuous extrapolation of the equilibrium high-density fluid at freezing, and which terminates at the random close packed (RCP) state. Perhaps we should now take another look at the RCP state as a starting point for the general theory of liquids. Standard treatises on simple liquids deal largely with theories of the liquid state based upon configurational surgery of Mayer virial cluster diagrams [15,16]. We now see that the Mayer cluster expansion whilst being an essentially exact theory of low density gases, may not be a starting point for theories of the liquid state. An analytical theory with all the virial coefficients correct would not still represent the high density equilibrium fluid. The title of Hansen and McDonald [16], when the 4 th Edition comes to be published, might be retitled The Theory of Simple Gases! Acknowledgements I wish to thank the Department of Physics at the Federal University of Juiz de Fora for a temporary visiting facility, and my host Professors, Socrates Danas and Bernhard Lesche, for their support and kind hospitality. References [1] Woodcock, L.V., Virial equation-of-state for hard spheres, LANL Ar. Xiv condmat [pdf] (008). [] Bannerman M., Lue L. and Woodcock L. V., Thermodynamic pressures for hard spheres and closed virial equation-of-state, J. Chem. Phys (010). [3] Woodcock, L. V., Percolation transitions in the hard-sphere fluid, AIChE Journal (accepted and published online April 011). [4] Woodcock, L.V., Virial equation-of-state for the hard disk fluid, 008 LANL Ar. Xiv cond-mat [pdf](008) [5] Beris, A. and Woodcock L.V. Closed virial equation-of-state for the hard-disk fluid, LANL Cond. Mat. Stat. Mech. arxiv: [pdf] (010). 11
12 Juiz de Fora WOODCOCK 011 [6] W. G. Hoover, N. E. Hoover and K. Hanson, Exact hard-disk free volumes, J. Chem. Phys. 70 (04), (1979). [7] N. Clisby and B. N. McCoy, Ninth and tenth virial coefficients for hard hyper-spheres in D-dimensions. J Statistical Physics, 1, (006). [8] R. W. Zwanzig, Virial coefficients of parallel square and parallel cube gases, J. Chem. Phys. 4, (1956). [9] W. G. Hoover and A. G. De Rocco, Sixth virial coefficients for gases of parallel hard lines, squares, and cubes, Journal of Chemical Physics 34, (1961). [10] W. G. Hoover and A. G. De Rocco, Sixth and seventh virial coefficients for the parallel hard cube model, J. Chem. Phys., 36, (196). [11] van Swol F. and Woodcock L. V., Percolation transition in the parallel hard cube model fluid, Molecular Simulation; 1 (1), (1987) [1] Kratky, K. W., Is the percolation transition of hard spheres a thermodynamic phase transition? J. Statistical Physics. 5, (1988) [13] see e.g. www. [14] W. G. Hoover, C. Hoover and M. Bannerman, Single speed molecular dynamics of hard parallel squares and cubes, J. Statistical Physics 136 (4) (009) [15] Mayer J.E. and Mayer, M.G., Statistical Mechanics (John Wiley, New York: 1940) [16] Hansen J-P. and McDonald I. R., The Theory of Simple Liquids, 1 st Edition (1976), nd Edition (1989) 3 rd Edition (006) (Academic Press: Oxford) 1
Fig. 3.1? Hard core potential
 6 Hard Sphere Gas The interactions between the atoms or molecules of a real gas comprise a strong repulsion at short distances and a weak attraction at long distances Both of these are important in determining
6 Hard Sphere Gas The interactions between the atoms or molecules of a real gas comprise a strong repulsion at short distances and a weak attraction at long distances Both of these are important in determining
New Closed Virial Equation of State for Hard-Sphere Fluids
 New Closed Virial Equation of State for Hard-Sphere Fluids Jianxiang Tian 1, 2, 4, Yuanxing Gui 2, Angel Mulero 3 1 Shandong Provincial Key Laboratory of Laser Polarization and Information Technology Department
New Closed Virial Equation of State for Hard-Sphere Fluids Jianxiang Tian 1, 2, 4, Yuanxing Gui 2, Angel Mulero 3 1 Shandong Provincial Key Laboratory of Laser Polarization and Information Technology Department
From the ideal gas to an ideal glass: a thermodynamic route to random close packing.
 Leslie V. Woodcock From the ideal gas to an ideal glass: a thermodynamic route to random close packing. Department of Chemical and Biomolecular Engineering National University of Singapore, Singapore 117576
Leslie V. Woodcock From the ideal gas to an ideal glass: a thermodynamic route to random close packing. Department of Chemical and Biomolecular Engineering National University of Singapore, Singapore 117576
Equations of State for Hard Spheres and Hard Disks
 3 Equations of State for Hard Spheres and Hard Disks A. Mulero 1, C.A. Galán 1, M.I. Parra 2, and F. Cuadros 1 1 Departamento de Física Aplicada, Universidad de Extremadura, 06071 Badajoz, Spain mulero@unex.es,
3 Equations of State for Hard Spheres and Hard Disks A. Mulero 1, C.A. Galán 1, M.I. Parra 2, and F. Cuadros 1 1 Departamento de Física Aplicada, Universidad de Extremadura, 06071 Badajoz, Spain mulero@unex.es,
Monte Carlo Simulations for a Soft Sphere Fluid
 Monte Carlo Simulations for a Soft Sphere Fluid Patrick Kreitzberg Advisor: Dr. David Roundy May 8 th, 2015 1 Contents 1 2 3 5 7 Abstract Introduction Methods Results and Discussion Conclusion Acknowledgments
Monte Carlo Simulations for a Soft Sphere Fluid Patrick Kreitzberg Advisor: Dr. David Roundy May 8 th, 2015 1 Contents 1 2 3 5 7 Abstract Introduction Methods Results and Discussion Conclusion Acknowledgments
II. Equilibrium Thermodynamics Lecture 7: Statistical Thermodynamics
 II. Equilibrium Thermodynamics Lecture 7: Statistical Thermodynamics Notes by ChangHoon Lim (and MZB) Open circuit voltage of galvanic cell is To understand compositional effects on, we need to consider
II. Equilibrium Thermodynamics Lecture 7: Statistical Thermodynamics Notes by ChangHoon Lim (and MZB) Open circuit voltage of galvanic cell is To understand compositional effects on, we need to consider
Equation of state of additive hard-disk fluid mixtures: A critical analysis of two recent proposals
 PHYSICAL REVIEW E 66, 0310 00 Equation of state of additive hard-disk fluid mixtures: A critical analysis of two recent proposals M. López de Haro* Centro de Investigación en Energía, UNAM, Temixco, Morelos
PHYSICAL REVIEW E 66, 0310 00 Equation of state of additive hard-disk fluid mixtures: A critical analysis of two recent proposals M. López de Haro* Centro de Investigación en Energía, UNAM, Temixco, Morelos
Dilatancy Transition in a Granular Model. David Aristoff and Charles Radin * Mathematics Department, University of Texas, Austin, TX 78712
 Dilatancy Transition in a Granular Model by David Aristoff and Charles Radin * Mathematics Department, University of Texas, Austin, TX 78712 Abstract We introduce a model of granular matter and use a stress
Dilatancy Transition in a Granular Model by David Aristoff and Charles Radin * Mathematics Department, University of Texas, Austin, TX 78712 Abstract We introduce a model of granular matter and use a stress
Packing hyperspheres in high-dimensional Euclidean spaces
 Packing hyperspheres in high-dimensional Euclidean spaces Monica Skoge, 1 Aleksandar Donev, 2,3 Frank H. Stillinger, 4 and Salvatore Torquato 2,3,4,5, * 1 Department of Physics, Princeton University, Princeton,
Packing hyperspheres in high-dimensional Euclidean spaces Monica Skoge, 1 Aleksandar Donev, 2,3 Frank H. Stillinger, 4 and Salvatore Torquato 2,3,4,5, * 1 Department of Physics, Princeton University, Princeton,
Glasses and Jamming: lessons from hard disks in a narrow channel
 Glasses and Jamming: lessons from hard disks in a narrow channel Mike Moore School of Physics and Astronomy, University of Manchester, UK June 2014 Co-Author: Mike Godfrey, University of Manchester Mike
Glasses and Jamming: lessons from hard disks in a narrow channel Mike Moore School of Physics and Astronomy, University of Manchester, UK June 2014 Co-Author: Mike Godfrey, University of Manchester Mike
Scaled particle theory for hard sphere pairs. II. Numerical analysis
 THE JOURNAL OF CHEMICAL PHYSICS 125, 204505 2006 Scaled particle theory for hard sphere pairs. II. Numerical analysis Swaroop Chatterjee and Pablo G. Debenedetti a Department of Chemical Engineering, Princeton
THE JOURNAL OF CHEMICAL PHYSICS 125, 204505 2006 Scaled particle theory for hard sphere pairs. II. Numerical analysis Swaroop Chatterjee and Pablo G. Debenedetti a Department of Chemical Engineering, Princeton
Phase Transitions in Networks: Giant Components, Dynamic Networks, Combinatoric Solvability
 in Networks: Giant Components, Dynamic Networks, Combinatoric Solvability Department of Physics UC Davis April 27, 2009 Outline Historical Prospective Old School New School Non-Physics 1 Historical Prospective
in Networks: Giant Components, Dynamic Networks, Combinatoric Solvability Department of Physics UC Davis April 27, 2009 Outline Historical Prospective Old School New School Non-Physics 1 Historical Prospective
arxiv:cond-mat/ v1 [cond-mat.soft] 16 Aug 2006
![arxiv:cond-mat/ v1 [cond-mat.soft] 16 Aug 2006 arxiv:cond-mat/ v1 [cond-mat.soft] 16 Aug 2006](/thumbs/92/108008631.jpg) Molecular Physics, Vol. 00, No. 00, DD Month 200x, 1 9 arxiv:cond-mat/0608356v1 [cond-mat.soft] 16 Aug 2006 Simulation-based equation of state of the hard disk fluid and prediction of higher-order virial
Molecular Physics, Vol. 00, No. 00, DD Month 200x, 1 9 arxiv:cond-mat/0608356v1 [cond-mat.soft] 16 Aug 2006 Simulation-based equation of state of the hard disk fluid and prediction of higher-order virial
Specific heat of the S= 1 2 expansion analysis
 PHYSICAL REVIEW B 71, 014417 2005 Specific heat of the S= 1 2 Heisenberg model on the kagome lattice: expansion analysis High-temperature series G. Misguich* Service de Physique Théorique, URA 2306 of
PHYSICAL REVIEW B 71, 014417 2005 Specific heat of the S= 1 2 Heisenberg model on the kagome lattice: expansion analysis High-temperature series G. Misguich* Service de Physique Théorique, URA 2306 of
The Equation of State of the Hard-Disk Fluid Revisited
 The Equation of State of the Hard-Disk Fluid Revisited J. Ramon Solana, Angel Mulero, Isidro Cachadina To cite this version: J. Ramon Solana, Angel Mulero, Isidro Cachadina. The Equation of State of the
The Equation of State of the Hard-Disk Fluid Revisited J. Ramon Solana, Angel Mulero, Isidro Cachadina To cite this version: J. Ramon Solana, Angel Mulero, Isidro Cachadina. The Equation of State of the
Approximate Linear Relationships
 Approximate Linear Relationships In the real world, rarely do things follow trends perfectly. When the trend is expected to behave linearly, or when inspection suggests the trend is behaving linearly,
Approximate Linear Relationships In the real world, rarely do things follow trends perfectly. When the trend is expected to behave linearly, or when inspection suggests the trend is behaving linearly,
Melting line of the Lennard-Jones system, infinite size, and full potential
 THE JOURNAL OF CHEMICAL PHYSICS 127, 104504 2007 Melting line of the Lennard-Jones system, infinite size, and full potential Ethan A. Mastny a and Juan J. de Pablo b Chemical and Biological Engineering
THE JOURNAL OF CHEMICAL PHYSICS 127, 104504 2007 Melting line of the Lennard-Jones system, infinite size, and full potential Ethan A. Mastny a and Juan J. de Pablo b Chemical and Biological Engineering
Thermodynamics of Three-phase Equilibrium in Lennard Jones System with a Simplified Equation of State
 23 Bulletin of Research Center for Computing and Multimedia Studies, Hosei University, 28 (2014) Thermodynamics of Three-phase Equilibrium in Lennard Jones System with a Simplified Equation of State Yosuke
23 Bulletin of Research Center for Computing and Multimedia Studies, Hosei University, 28 (2014) Thermodynamics of Three-phase Equilibrium in Lennard Jones System with a Simplified Equation of State Yosuke
V.E Mean Field Theory of Condensation
 V.E Mean Field heory of Condensation In principle, all properties of the interacting system, including phase separation, are contained within the thermodynamic potentials that can be obtained by evaluating
V.E Mean Field heory of Condensation In principle, all properties of the interacting system, including phase separation, are contained within the thermodynamic potentials that can be obtained by evaluating
arxiv:cond-mat/ v1 [cond-mat.stat-mech] 16 Aug 2006
![arxiv:cond-mat/ v1 [cond-mat.stat-mech] 16 Aug 2006 arxiv:cond-mat/ v1 [cond-mat.stat-mech] 16 Aug 2006](/thumbs/92/108837051.jpg) Packing Hyperspheres in High-Dimensional Euclidean Spaces arxiv:cond-mat/0608362v1 [cond-mat.stat-mech] 16 Aug 2006 Monica Skoge, 1 Aleksandar Donev, 2,3 Frank H. Stillinger, 4 and Salvatore Torquato 2,3,4,5,
Packing Hyperspheres in High-Dimensional Euclidean Spaces arxiv:cond-mat/0608362v1 [cond-mat.stat-mech] 16 Aug 2006 Monica Skoge, 1 Aleksandar Donev, 2,3 Frank H. Stillinger, 4 and Salvatore Torquato 2,3,4,5,
Intermolecular Potentials and The Second Virial Coefficient
 Intermolecular Potentials and The Second Virial Coefficient Patrick L. Holt Department of Chemistry & Physics Bellarmine University Louisville, Kentucky 40205 pholt@bellarmine.edu Copyright 2004 by the
Intermolecular Potentials and The Second Virial Coefficient Patrick L. Holt Department of Chemistry & Physics Bellarmine University Louisville, Kentucky 40205 pholt@bellarmine.edu Copyright 2004 by the
A student-oriented derivation of a reliable equation of state for a hard-disc fluid
 Eur. J. Phys. 19 (1998) 281 286. Printed in the UK PII: S0143-0807(98)90372-5 A student-oriented derivation of a reliable equation of state for a hard-disc fluid Mariano López de Haro, Andrés Santos and
Eur. J. Phys. 19 (1998) 281 286. Printed in the UK PII: S0143-0807(98)90372-5 A student-oriented derivation of a reliable equation of state for a hard-disc fluid Mariano López de Haro, Andrés Santos and
ADSORPTION IN MICROPOROUS MATERIALS: ANALYTICAL EQUATIONS FOR TYPE I ISOTHERMS AT HIGH PRESSURE
 ADSORPTION IN MICROPOROUS MATERIALS: ANALYTICAL EQUATIONS FOR TYPE I ISOTHERMS AT HIGH PRESSURE A. L. MYERS Department of Chemical and Biomolecular Engineering University of Pennsylvania, Philadelphia
ADSORPTION IN MICROPOROUS MATERIALS: ANALYTICAL EQUATIONS FOR TYPE I ISOTHERMS AT HIGH PRESSURE A. L. MYERS Department of Chemical and Biomolecular Engineering University of Pennsylvania, Philadelphia
VII. Porous Media Lecture 32: Percolation
 VII. Porous Media Lecture 32: Percolation April 25 th, 2011 Notes by John Casey (and MZB) References: S. Torquato, Random Heterogeneous Materials (Springer 2002) D. Stauffer, Introduction to Percolation
VII. Porous Media Lecture 32: Percolation April 25 th, 2011 Notes by John Casey (and MZB) References: S. Torquato, Random Heterogeneous Materials (Springer 2002) D. Stauffer, Introduction to Percolation
CHEM-UA 652: Thermodynamics and Kinetics
 1 CHEM-UA 652: Thermodynamics and Kinetics Notes for Lecture 13 I. PHASE DIAGRAMS The different phases of substances are characterized by different ranges of thermodynamic variables in which these phasesarethestablephases.
1 CHEM-UA 652: Thermodynamics and Kinetics Notes for Lecture 13 I. PHASE DIAGRAMS The different phases of substances are characterized by different ranges of thermodynamic variables in which these phasesarethestablephases.
Comparative study on methodology in molecular dynamics simulation of nucleation
 THE JOURNAL OF CHEMICAL PHYSICS 126, 224517 2007 Comparative study on methodology in molecular dynamics simulation of nucleation Jan Julin, Ismo Napari, and Hanna Vehkamäki Department of Physical Sciences,
THE JOURNAL OF CHEMICAL PHYSICS 126, 224517 2007 Comparative study on methodology in molecular dynamics simulation of nucleation Jan Julin, Ismo Napari, and Hanna Vehkamäki Department of Physical Sciences,
The Boundary Conditions Geometry in Lattice-Ising model
 The Boundary Conditions Geometry in Lattice-Ising model You-gang Feng Department of Basic Science, College of Science, Gui Zhou University. Cai Jia Guan, Guiyang 550003, China E-mail: ygfeng45@yahoo.com.cn
The Boundary Conditions Geometry in Lattice-Ising model You-gang Feng Department of Basic Science, College of Science, Gui Zhou University. Cai Jia Guan, Guiyang 550003, China E-mail: ygfeng45@yahoo.com.cn
Gas-liquid phase separation in oppositely charged colloids: stability and interfacial tension
 7 Gas-liquid phase separation in oppositely charged colloids: stability and interfacial tension We study the phase behaviour and the interfacial tension of the screened Coulomb (Yukawa) restricted primitive
7 Gas-liquid phase separation in oppositely charged colloids: stability and interfacial tension We study the phase behaviour and the interfacial tension of the screened Coulomb (Yukawa) restricted primitive
Phase behaviour of very asymmetric binary mixtures
 J. Phys.: Condens. Matter 12 (2000) A109 A114. Printed in the UK PII: S0953-8984(00)06914-9 Phase behaviour of very asymmetric binary mixtures José A Cuesta and Yuri Martínez-Ratón Grupo Interdisciplinar
J. Phys.: Condens. Matter 12 (2000) A109 A114. Printed in the UK PII: S0953-8984(00)06914-9 Phase behaviour of very asymmetric binary mixtures José A Cuesta and Yuri Martínez-Ratón Grupo Interdisciplinar
Local persistense and blocking in the two dimensional Blume-Capel Model arxiv:cond-mat/ v1 [cond-mat.stat-mech] 4 Apr 2004.
![Local persistense and blocking in the two dimensional Blume-Capel Model arxiv:cond-mat/ v1 [cond-mat.stat-mech] 4 Apr 2004. Local persistense and blocking in the two dimensional Blume-Capel Model arxiv:cond-mat/ v1 [cond-mat.stat-mech] 4 Apr 2004.](/thumbs/90/103880677.jpg) Local persistense and blocking in the two dimensional Blume-Capel Model arxiv:cond-mat/0404082v1 [cond-mat.stat-mech] 4 Apr 2004 Roberto da Silva Departamento de Informática Teórica, Instituto de Informática,
Local persistense and blocking in the two dimensional Blume-Capel Model arxiv:cond-mat/0404082v1 [cond-mat.stat-mech] 4 Apr 2004 Roberto da Silva Departamento de Informática Teórica, Instituto de Informática,
PAPER No.6: PHYSICAL CHEMISTRY-II (Statistical
 Subject PHYSICAL Paper No and Title Module No and Title Module Tag 6, PHYSICAL -II (Statistical 34, Method for determining molar mass - I CHE_P6_M34 Table of Contents 1. Learning Outcomes 2. Introduction
Subject PHYSICAL Paper No and Title Module No and Title Module Tag 6, PHYSICAL -II (Statistical 34, Method for determining molar mass - I CHE_P6_M34 Table of Contents 1. Learning Outcomes 2. Introduction
Generalized Manna Sandpile Model with Height Restrictions
 75 Brazilian Journal of Physics, vol. 36, no. 3A, September, 26 Generalized Manna Sandpile Model with Height Restrictions Wellington Gomes Dantas and Jürgen F. Stilck Instituto de Física, Universidade
75 Brazilian Journal of Physics, vol. 36, no. 3A, September, 26 Generalized Manna Sandpile Model with Height Restrictions Wellington Gomes Dantas and Jürgen F. Stilck Instituto de Física, Universidade
Suggestions for Further Reading
 Contents Preface viii 1 From Microscopic to Macroscopic Behavior 1 1.1 Introduction........................................ 1 1.2 Some Qualitative Observations............................. 2 1.3 Doing
Contents Preface viii 1 From Microscopic to Macroscopic Behavior 1 1.1 Introduction........................................ 1 1.2 Some Qualitative Observations............................. 2 1.3 Doing
Chapter 5. On-line resource
 Chapter 5 The water-air heterogeneous system On-line resource on-line analytical system that portrays the thermodynamic properties of water vapor and many other gases http://webbook.nist.gov/chemistry/fluid/
Chapter 5 The water-air heterogeneous system On-line resource on-line analytical system that portrays the thermodynamic properties of water vapor and many other gases http://webbook.nist.gov/chemistry/fluid/
Computer generation of dense polydisperse sphere packings
 JOURNAL OF CHEMICAL PHYSICS VOLUME 117, NUMBER 18 8 NOVEMBER 2002 Computer generation of dense polydisperse sphere packings Anuraag R. Kansal Department of Chemical Engineering, Princeton University, Princeton,
JOURNAL OF CHEMICAL PHYSICS VOLUME 117, NUMBER 18 8 NOVEMBER 2002 Computer generation of dense polydisperse sphere packings Anuraag R. Kansal Department of Chemical Engineering, Princeton University, Princeton,
Renormalization Group for the Two-Dimensional Ising Model
 Chapter 8 Renormalization Group for the Two-Dimensional Ising Model The two-dimensional (2D) Ising model is arguably the most important in statistical physics. This special status is due to Lars Onsager
Chapter 8 Renormalization Group for the Two-Dimensional Ising Model The two-dimensional (2D) Ising model is arguably the most important in statistical physics. This special status is due to Lars Onsager
Physics Nov Phase Transitions
 Physics 301 11-Nov-1999 15-1 Phase Transitions Phase transitions occur throughout physics. We are all familiar with melting ice and boiling water. But other kinds of phase transitions occur as well. Some
Physics 301 11-Nov-1999 15-1 Phase Transitions Phase transitions occur throughout physics. We are all familiar with melting ice and boiling water. But other kinds of phase transitions occur as well. Some
Comment on Conjectures on exact solution of three-dimensional (3D) simple orthorhombic Ising lattices
 arxiv:0811.1802v2 [cond-mat.stat-mech] 22 Nov 2008 Comment on Conectures on exact solution of three-dimensional (3D) simple orthorhombic Ising lattices Jacques H.H. Perk 145 Physical Sciences, Oklahoma
arxiv:0811.1802v2 [cond-mat.stat-mech] 22 Nov 2008 Comment on Conectures on exact solution of three-dimensional (3D) simple orthorhombic Ising lattices Jacques H.H. Perk 145 Physical Sciences, Oklahoma
Nucleation rate (m -3 s -1 ) Radius of water nano droplet (Å) 1e+00 1e-64 1e-128 1e-192 1e-256
 Supplementary Figures Nucleation rate (m -3 s -1 ) 1e+00 1e-64 1e-128 1e-192 1e-256 Calculated R in bulk water Calculated R in droplet Modified CNT 20 30 40 50 60 70 Radius of water nano droplet (Å) Supplementary
Supplementary Figures Nucleation rate (m -3 s -1 ) 1e+00 1e-64 1e-128 1e-192 1e-256 Calculated R in bulk water Calculated R in droplet Modified CNT 20 30 40 50 60 70 Radius of water nano droplet (Å) Supplementary
High-order virial coefficients and equation of state for hard sphere and hard disk systems
 PAPER www.rsc.org/pccp Physical Chemistry Chemical Physics High-order virial coefficients and equation of state for hard sphere and hard disk systems Jiawen Hu ab and Yang-Xin Yu* b Received 17th June
PAPER www.rsc.org/pccp Physical Chemistry Chemical Physics High-order virial coefficients and equation of state for hard sphere and hard disk systems Jiawen Hu ab and Yang-Xin Yu* b Received 17th June
Chapter 2 Experimental sources of intermolecular potentials
 Chapter 2 Experimental sources of intermolecular potentials 2.1 Overview thermodynamical properties: heat of vaporization (Trouton s rule) crystal structures ionic crystals rare gas solids physico-chemical
Chapter 2 Experimental sources of intermolecular potentials 2.1 Overview thermodynamical properties: heat of vaporization (Trouton s rule) crystal structures ionic crystals rare gas solids physico-chemical
Physical Modeling of Multiphase flow. Boltzmann method
 with lattice Boltzmann method Exa Corp., Burlington, MA, USA Feburary, 2011 Scope Scope Re-examine the non-ideal gas model in [Shan & Chen, Phys. Rev. E, (1993)] from the perspective of kinetic theory
with lattice Boltzmann method Exa Corp., Burlington, MA, USA Feburary, 2011 Scope Scope Re-examine the non-ideal gas model in [Shan & Chen, Phys. Rev. E, (1993)] from the perspective of kinetic theory
Comparison of the DSMC Method with an Exact Solution of the Boltzmann Equation
 Comparison of the DSMC Method with an Exact Solution of the Boltzmann Equation J M Montanero and A Santos Departamento de Fisica, Universidad de Extremadura, Spain Abstract Results obtained from the Direct
Comparison of the DSMC Method with an Exact Solution of the Boltzmann Equation J M Montanero and A Santos Departamento de Fisica, Universidad de Extremadura, Spain Abstract Results obtained from the Direct
An Extended van der Waals Equation of State Based on Molecular Dynamics Simulation
 J. Comput. Chem. Jpn., Vol. 8, o. 3, pp. 97 14 (9) c 9 Society of Computer Chemistry, Japan An Extended van der Waals Equation of State Based on Molecular Dynamics Simulation Yosuke KATAOKA* and Yuri YAMADA
J. Comput. Chem. Jpn., Vol. 8, o. 3, pp. 97 14 (9) c 9 Society of Computer Chemistry, Japan An Extended van der Waals Equation of State Based on Molecular Dynamics Simulation Yosuke KATAOKA* and Yuri YAMADA
arxiv:cond-mat/ v2 [cond-mat.dis-nn] 3 Oct 2002
![arxiv:cond-mat/ v2 [cond-mat.dis-nn] 3 Oct 2002 arxiv:cond-mat/ v2 [cond-mat.dis-nn] 3 Oct 2002](/thumbs/73/68112465.jpg) Discrepancy between Monte-Carlo Results and Analytic Values for the Average Excluded Volume of Rectangular Prisms arxiv:cond-mat/0208145v2 [cond-mat.dis-nn] 3 Oct 2002 Sameet Sreenivasan, Don R. Baker,
Discrepancy between Monte-Carlo Results and Analytic Values for the Average Excluded Volume of Rectangular Prisms arxiv:cond-mat/0208145v2 [cond-mat.dis-nn] 3 Oct 2002 Sameet Sreenivasan, Don R. Baker,
Finite-size analysis via the critical energy -subspace method in the Ising models
 Materials Science-Poland, Vol. 23, No. 4, 25 Finite-size analysis via the critical energy -subspace method in the Ising models A. C. MAAKIS *, I. A. HADJIAGAPIOU, S. S. MARTINOS, N. G. FYTAS Faculty of
Materials Science-Poland, Vol. 23, No. 4, 25 Finite-size analysis via the critical energy -subspace method in the Ising models A. C. MAAKIS *, I. A. HADJIAGAPIOU, S. S. MARTINOS, N. G. FYTAS Faculty of
Effect of Diffusing Disorder on an. Absorbing-State Phase Transition
 Effect of Diffusing Disorder on an Absorbing-State Phase Transition Ronald Dickman Universidade Federal de Minas Gerais, Brazil Support: CNPq & Fapemig, Brazil OUTLINE Introduction: absorbing-state phase
Effect of Diffusing Disorder on an Absorbing-State Phase Transition Ronald Dickman Universidade Federal de Minas Gerais, Brazil Support: CNPq & Fapemig, Brazil OUTLINE Introduction: absorbing-state phase
Uniformization and percolation
 Uniformization and percolation Itai Benjamini October 2015 Conformal maps A conformal map, between planar domains, is a function that infinitesimally preserves angles. The derivative of a conformal map
Uniformization and percolation Itai Benjamini October 2015 Conformal maps A conformal map, between planar domains, is a function that infinitesimally preserves angles. The derivative of a conformal map
3. General properties of phase transitions and the Landau theory
 3. General properties of phase transitions and the Landau theory In this Section we review the general properties and the terminology used to characterise phase transitions, which you will have already
3. General properties of phase transitions and the Landau theory In this Section we review the general properties and the terminology used to characterise phase transitions, which you will have already
Functions, Graphs, Equations and Inequalities
 CAEM DPP Learning Outcomes per Module Module Functions, Graphs, Equations and Inequalities Learning Outcomes 1. Functions, inverse functions and composite functions 1.1. concepts of function, domain and
CAEM DPP Learning Outcomes per Module Module Functions, Graphs, Equations and Inequalities Learning Outcomes 1. Functions, inverse functions and composite functions 1.1. concepts of function, domain and
Phase Transitions in Artificial Intelligence
 Phase Transitions in Artificial Intelligence Alan M. Luu May 8, 2017 Abstract Artificial intelligence often involves search and computation over large networks. This essay discusses a family of closely
Phase Transitions in Artificial Intelligence Alan M. Luu May 8, 2017 Abstract Artificial intelligence often involves search and computation over large networks. This essay discusses a family of closely
Competition between face-centered cubic and icosahedral cluster structures
 Competition between face-centered cubic and icosahedral cluster structures R. S. Berry University of Chicago, Chicago, IL 60637, USA B. M. Smyrnov, and A. Yu. Strizhev High-Temperatures Institute, Russian
Competition between face-centered cubic and icosahedral cluster structures R. S. Berry University of Chicago, Chicago, IL 60637, USA B. M. Smyrnov, and A. Yu. Strizhev High-Temperatures Institute, Russian
Paper No. : 04 Paper Title: Unit Operations in Food Processing Module- 18: Circulation of fluids through porous bed
 Paper No. : 04 Paper Title: Unit Operations in Food Processing Module- 18: Circulation of fluids through porous bed 18.1 Introduction A typical packed bed is a cylindrical column that is filled with a
Paper No. : 04 Paper Title: Unit Operations in Food Processing Module- 18: Circulation of fluids through porous bed 18.1 Introduction A typical packed bed is a cylindrical column that is filled with a
Disordered Hyperuniformity: Liquid-like Behaviour in Structural Solids, A New Phase of Matter?
 Disordered Hyperuniformity: Liquid-like Behaviour in Structural Solids, A New Phase of Matter? Kabir Ramola Martin Fisher School of Physics, Brandeis University August 19, 2016 Kabir Ramola Disordered
Disordered Hyperuniformity: Liquid-like Behaviour in Structural Solids, A New Phase of Matter? Kabir Ramola Martin Fisher School of Physics, Brandeis University August 19, 2016 Kabir Ramola Disordered
EQUATION OF STATE DEVELOPMENT
 EQUATION OF STATE DEVELOPMENT I. Nieuwoudt* & M du Rand Institute for Thermal Separation Technology, Department of Chemical Engineering, University of Stellenbosch, Private bag X1, Matieland, 760, South
EQUATION OF STATE DEVELOPMENT I. Nieuwoudt* & M du Rand Institute for Thermal Separation Technology, Department of Chemical Engineering, University of Stellenbosch, Private bag X1, Matieland, 760, South
Phase Transitions in Physics and Computer Science. Cristopher Moore University of New Mexico and the Santa Fe Institute
 Phase Transitions in Physics and Computer Science Cristopher Moore University of New Mexico and the Santa Fe Institute Magnetism When cold enough, Iron will stay magnetized, and even magnetize spontaneously
Phase Transitions in Physics and Computer Science Cristopher Moore University of New Mexico and the Santa Fe Institute Magnetism When cold enough, Iron will stay magnetized, and even magnetize spontaneously
Interface tension of the 3d 4-state Potts model using the Wang-Landau algorithm
 Interface tension of the 3d 4-state Potts model using the Wang-Landau algorithm CP3-Origins and the Danish Institute for Advanced Study DIAS, University of Southern Denmark, Campusvej 55, DK-5230 Odense
Interface tension of the 3d 4-state Potts model using the Wang-Landau algorithm CP3-Origins and the Danish Institute for Advanced Study DIAS, University of Southern Denmark, Campusvej 55, DK-5230 Odense
On the local and nonlocal components of solvation thermodynamics and their relation to solvation shell models
 JOURNAL OF CHEMICAL PHYSICS VOLUME 109, NUMBER 12 22 SEPTEMBER 1998 On the local and nonlocal components of solvation thermodynamics and their relation to solvation shell models Nobuyuki Matubayasi Institute
JOURNAL OF CHEMICAL PHYSICS VOLUME 109, NUMBER 12 22 SEPTEMBER 1998 On the local and nonlocal components of solvation thermodynamics and their relation to solvation shell models Nobuyuki Matubayasi Institute
Liquids and Solids. H fus (Heat of fusion) H vap (Heat of vaporization) H sub (Heat of sublimation)
 Liquids and Solids Phase Transitions All elements and compounds undergo some sort of phase transition as their temperature is increase from 0 K. The points at which these phase transitions occur depend
Liquids and Solids Phase Transitions All elements and compounds undergo some sort of phase transition as their temperature is increase from 0 K. The points at which these phase transitions occur depend
Uniformization and percolation
 Uniformization and percolation Itai Benjamini May 2016 Conformal maps A conformal map, between planar domains, is a function that infinitesimally preserves angles. The derivative of a conformal map is
Uniformization and percolation Itai Benjamini May 2016 Conformal maps A conformal map, between planar domains, is a function that infinitesimally preserves angles. The derivative of a conformal map is
The propagation of chaos for a rarefied gas of hard spheres
 The propagation of chaos for a rarefied gas of hard spheres Ryan Denlinger 1 1 University of Texas at Austin 35th Annual Western States Mathematical Physics Meeting Caltech February 13, 2017 Ryan Denlinger
The propagation of chaos for a rarefied gas of hard spheres Ryan Denlinger 1 1 University of Texas at Austin 35th Annual Western States Mathematical Physics Meeting Caltech February 13, 2017 Ryan Denlinger
Geometry explains the large difference in the elastic properties of fcc and hcp crystals of hard spheres Sushko, N.; van der Schoot, P.P.A.M.
 Geometry explains the large difference in the elastic properties of fcc and hcp crystals of hard spheres Sushko, N.; van der Schoot, P.P.A.M. Published in: Physical Review E DOI: 10.1103/PhysRevE.72.067104
Geometry explains the large difference in the elastic properties of fcc and hcp crystals of hard spheres Sushko, N.; van der Schoot, P.P.A.M. Published in: Physical Review E DOI: 10.1103/PhysRevE.72.067104
Access from the University of Nottingham repository: %20%28002%29.
 Do, Hainam and Feng, Chao and Schultz, Andrew J. and Kofke, David A. and Wheatley, Richard J. (2016) Calculation of high-order virial coefficients for the square-well potential. Physical Review E, 94 (1).
Do, Hainam and Feng, Chao and Schultz, Andrew J. and Kofke, David A. and Wheatley, Richard J. (2016) Calculation of high-order virial coefficients for the square-well potential. Physical Review E, 94 (1).
6.207/14.15: Networks Lecture 12: Generalized Random Graphs
 6.207/14.15: Networks Lecture 12: Generalized Random Graphs 1 Outline Small-world model Growing random networks Power-law degree distributions: Rich-Get-Richer effects Models: Uniform attachment model
6.207/14.15: Networks Lecture 12: Generalized Random Graphs 1 Outline Small-world model Growing random networks Power-law degree distributions: Rich-Get-Richer effects Models: Uniform attachment model
5.60 Thermodynamics & Kinetics Spring 2008
 MIT OpenCourseWare http://ocw.mit.edu 5.60 Thermodynamics & Kinetics Spring 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 5.60 Spring 2008 Lecture
MIT OpenCourseWare http://ocw.mit.edu 5.60 Thermodynamics & Kinetics Spring 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 5.60 Spring 2008 Lecture
Virial Expansion. Silicon Valley FIG March 24, Chen-Hanson Ting
 Virial Expansion Silicon Valley FIG March 24, 2018 Chen-Hanson Ting Virial Expansion This paper was uploaded to Wikipedia. Google virial expansion and you will find it. Let s first scan through it on Wiki.
Virial Expansion Silicon Valley FIG March 24, 2018 Chen-Hanson Ting Virial Expansion This paper was uploaded to Wikipedia. Google virial expansion and you will find it. Let s first scan through it on Wiki.
X B1 X B Quiz Fall points total. 1. Thermodynamics. (50 points)
 3.012 Quiz 4 3.012 12.15.03 Fall 2003 100 points total 1. Thermodynamics. (50 points) a. Shown below is a portion of the nickel-titanium binary phase diagram. Using the diagram, answer the questions below.
3.012 Quiz 4 3.012 12.15.03 Fall 2003 100 points total 1. Thermodynamics. (50 points) a. Shown below is a portion of the nickel-titanium binary phase diagram. Using the diagram, answer the questions below.
Critical Phenomena and Percolation Theory: II
 Critical Phenomena and Percolation Theory: II Kim Christensen Complexity & Networks Group Imperial College London Joint CRM-Imperial College School and Workshop Complex Systems Barcelona 8-13 April 2013
Critical Phenomena and Percolation Theory: II Kim Christensen Complexity & Networks Group Imperial College London Joint CRM-Imperial College School and Workshop Complex Systems Barcelona 8-13 April 2013
The Boguslawski Melting Model
 World Journal of Condensed Matter Physics, 6, 6, 45-55 Published Online February 6 in SciRes. http://www.scirp.org/journal/wjcmp http://dx.doi.org/.46/wjcmp.6.67 The Boguslawski Melting Model Vladimir
World Journal of Condensed Matter Physics, 6, 6, 45-55 Published Online February 6 in SciRes. http://www.scirp.org/journal/wjcmp http://dx.doi.org/.46/wjcmp.6.67 The Boguslawski Melting Model Vladimir
Precursors of a phase transition in a simple model system
 Precursors of a phase transition in a simple model system V. Halpern Department of Physics, Bar-Ilan University, Ramat-Gan 52900, Israel halpeh@mail.biu.ac.il Abstract Most theoretical and numerical studies
Precursors of a phase transition in a simple model system V. Halpern Department of Physics, Bar-Ilan University, Ramat-Gan 52900, Israel halpeh@mail.biu.ac.il Abstract Most theoretical and numerical studies
Thermodynamic Properties of Polymer Solutions
 @@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@?e?@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@
@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@?e?@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@
Multicanonical distribution and the origin of power laws. Abstract
 Multicanonical distribution and the origin of power laws G. L. Vasconcelos and D. S. P. Salazar Departamento de Física, Universidade Federal de Pernambuco, Brazil. and arxiv:128.5624v1 [cond-mat.stat-mech]
Multicanonical distribution and the origin of power laws G. L. Vasconcelos and D. S. P. Salazar Departamento de Física, Universidade Federal de Pernambuco, Brazil. and arxiv:128.5624v1 [cond-mat.stat-mech]
Daan Frenkel, Frank E. Hanson and John P. McTague
 PHASE TRANSITIONS AND ORIENTATIONAL ORDER IN A TWO DIMENSIONAL LENNARD-JONES SYSTEM Daan Frenkel, Frank E. Hanson and John P. McTague University of California Department of Chemistry Los Angeles, CA 90024
PHASE TRANSITIONS AND ORIENTATIONAL ORDER IN A TWO DIMENSIONAL LENNARD-JONES SYSTEM Daan Frenkel, Frank E. Hanson and John P. McTague University of California Department of Chemistry Los Angeles, CA 90024
A RANDOM VARIANT OF THE GAME OF PLATES AND OLIVES
 A RANDOM VARIANT OF THE GAME OF PLATES AND OLIVES ANDRZEJ DUDEK, SEAN ENGLISH, AND ALAN FRIEZE Abstract The game of plates and olives was originally formulated by Nicolaescu and encodes the evolution of
A RANDOM VARIANT OF THE GAME OF PLATES AND OLIVES ANDRZEJ DUDEK, SEAN ENGLISH, AND ALAN FRIEZE Abstract The game of plates and olives was originally formulated by Nicolaescu and encodes the evolution of
The 6-vertex model of hydrogen-bonded crystals with bond defects
 arxiv:cond-mat/9908379v [cond-mat.stat-mech] Oct 999 The 6-vertex model of hydrogen-bonded crystals with bond defects N. Sh. Izmailian, Chin-Kun Hu and F. Y. Wu Institute of Physics, Academia Sinica, Nankang,
arxiv:cond-mat/9908379v [cond-mat.stat-mech] Oct 999 The 6-vertex model of hydrogen-bonded crystals with bond defects N. Sh. Izmailian, Chin-Kun Hu and F. Y. Wu Institute of Physics, Academia Sinica, Nankang,
Exact solution of site and bond percolation. on small-world networks. Abstract
 Exact solution of site and bond percolation on small-world networks Cristopher Moore 1,2 and M. E. J. Newman 2 1 Departments of Computer Science and Physics, University of New Mexico, Albuquerque, New
Exact solution of site and bond percolation on small-world networks Cristopher Moore 1,2 and M. E. J. Newman 2 1 Departments of Computer Science and Physics, University of New Mexico, Albuquerque, New
arxiv: v1 [cond-mat.stat-mech] 26 Jan 2008
![arxiv: v1 [cond-mat.stat-mech] 26 Jan 2008 arxiv: v1 [cond-mat.stat-mech] 26 Jan 2008](/thumbs/82/85720984.jpg) Long Time Tail of the Velocity Autocorrelation Function in a Two-Dimensional Moderately Dense Hard Disk Fluid Masaharu Isobe Graduate School of Engineering, Nagoya Institute of Technology, Nagoya 466-8555,
Long Time Tail of the Velocity Autocorrelation Function in a Two-Dimensional Moderately Dense Hard Disk Fluid Masaharu Isobe Graduate School of Engineering, Nagoya Institute of Technology, Nagoya 466-8555,
Improving Dense Packings of Equal Disks in a Square
 Improving Dense Packings of Equal Disks in a Square David W. Boll Jerry Donovan Ronald L. Graham Boris D. Lubachevsky Hewlett-Packard Hewlett-Packard University of California Lucent Technologies 00 st
Improving Dense Packings of Equal Disks in a Square David W. Boll Jerry Donovan Ronald L. Graham Boris D. Lubachevsky Hewlett-Packard Hewlett-Packard University of California Lucent Technologies 00 st
On Exponential Decay and the Riemann Hypothesis
 On Exponential Decay and the Riemann Hypothesis JEFFREY N. COOK ABSTRACT. A Riemann operator is constructed in which sequential elements are removed from a decaying set by means of prime factorization,
On Exponential Decay and the Riemann Hypothesis JEFFREY N. COOK ABSTRACT. A Riemann operator is constructed in which sequential elements are removed from a decaying set by means of prime factorization,
12. Heat of melting and evaporation of water
 VS 12. Heat of melting and evaporation of water 12.1 Introduction The change of the physical state of a substance in general requires the absorption or release of heat. In this case, one speaks of a first
VS 12. Heat of melting and evaporation of water 12.1 Introduction The change of the physical state of a substance in general requires the absorption or release of heat. In this case, one speaks of a first
Chapter 4 Sequences and Series
 Chapter 4 Sequences and Series 4.1 Sequence Review Sequence: a set of elements (numbers or letters or a combination of both). The elements of the set all follow the same rule (logical progression). The
Chapter 4 Sequences and Series 4.1 Sequence Review Sequence: a set of elements (numbers or letters or a combination of both). The elements of the set all follow the same rule (logical progression). The
Avalanches, transport, and local equilibrium in self-organized criticality
 PHYSICAL REVIEW E VOLUME 58, NUMBER 5 NOVEMBER 998 Avalanches, transport, and local equilibrium in self-organized criticality Afshin Montakhab and J. M. Carlson Department of Physics, University of California,
PHYSICAL REVIEW E VOLUME 58, NUMBER 5 NOVEMBER 998 Avalanches, transport, and local equilibrium in self-organized criticality Afshin Montakhab and J. M. Carlson Department of Physics, University of California,
Free Energy Estimation in Simulations
 Physics 363/MatSE 38/ CSE 36 Free Energy Estimation in Simulations This is a brief overview of free energy estimation, much of which can be found in the article by D. Frenkel Free-Energy Computation and
Physics 363/MatSE 38/ CSE 36 Free Energy Estimation in Simulations This is a brief overview of free energy estimation, much of which can be found in the article by D. Frenkel Free-Energy Computation and
Intermolecular Model Potentials and Virial Coefficients from Acoustic Data
 JASEM ISSN 1119-8362 All rights reserved Full-text Available Online at https://www.ajol.info/index.php/jasem http://www.bioline.org.br/ja J. Appl. Sci. Environ. Manage. Vol.22 (2) 246-251. February 2018
JASEM ISSN 1119-8362 All rights reserved Full-text Available Online at https://www.ajol.info/index.php/jasem http://www.bioline.org.br/ja J. Appl. Sci. Environ. Manage. Vol.22 (2) 246-251. February 2018
Thermodynamic Functions at Isobaric Process of van der Waals Gases
 Thermodynamic Functions at Isobaric Process of van der Waals Gases Akira Matsumoto Department of Material Sciences, College of Integrated Arts Sciences, Osaka Prefecture University, Sakai, Osaka, 599-853,
Thermodynamic Functions at Isobaric Process of van der Waals Gases Akira Matsumoto Department of Material Sciences, College of Integrated Arts Sciences, Osaka Prefecture University, Sakai, Osaka, 599-853,
Chapter 6. Phase transitions. 6.1 Concept of phase
 Chapter 6 hase transitions 6.1 Concept of phase hases are states of matter characterized by distinct macroscopic properties. ypical phases we will discuss in this chapter are liquid, solid and gas. Other
Chapter 6 hase transitions 6.1 Concept of phase hases are states of matter characterized by distinct macroscopic properties. ypical phases we will discuss in this chapter are liquid, solid and gas. Other
Notes for Expansions/Series and Differential Equations
 Notes for Expansions/Series and Differential Equations In the last discussion, we considered perturbation methods for constructing solutions/roots of algebraic equations. Three types of problems were illustrated
Notes for Expansions/Series and Differential Equations In the last discussion, we considered perturbation methods for constructing solutions/roots of algebraic equations. Three types of problems were illustrated
The Failure of Classical Mechanics
 Chapter 1 The Failure of Classical Mechanics Classical mechanics, erected by Galileo and Newton, with enormous contributions from many others, is remarkably successful. It enables us to calculate celestial
Chapter 1 The Failure of Classical Mechanics Classical mechanics, erected by Galileo and Newton, with enormous contributions from many others, is remarkably successful. It enables us to calculate celestial
Power, Taylor, & Maclaurin Series Page 1
 Power, Taylor, & Maclaurin Series Page 1 Directions: Solve the following problems using the available space for scratchwork. Indicate your answers on the front page. Do not spend too much time on any one
Power, Taylor, & Maclaurin Series Page 1 Directions: Solve the following problems using the available space for scratchwork. Indicate your answers on the front page. Do not spend too much time on any one
Mini course on Complex Networks
 Mini course on Complex Networks Massimo Ostilli 1 1 UFSC, Florianopolis, Brazil September 2017 Dep. de Fisica Organization of The Mini Course Day 1: Basic Topology of Equilibrium Networks Day 2: Percolation
Mini course on Complex Networks Massimo Ostilli 1 1 UFSC, Florianopolis, Brazil September 2017 Dep. de Fisica Organization of The Mini Course Day 1: Basic Topology of Equilibrium Networks Day 2: Percolation
A New Uniform Phase Bridge Functional: Test and Its Application to Non-uniform Phase Fluid
 Commun. Theor. Phys. (Beijing, China) 39 (2003) pp. 231 237 c International Academic Publishers Vol. 39, No. 2, February 15, 2003 A New Uniform Phase Bridge Functional: Test and Its Application to Non-uniform
Commun. Theor. Phys. (Beijing, China) 39 (2003) pp. 231 237 c International Academic Publishers Vol. 39, No. 2, February 15, 2003 A New Uniform Phase Bridge Functional: Test and Its Application to Non-uniform
the expansion for the Helmholtz energy derived in Appendix A, part 2, the expression for the surface tension becomes: σ = ( a + ½ k(ρ) ρ 2 x ) dx
 Motivation The surface tension plays a major role in interfacial phenomena. It is the fundamental quantity that determines the pressure change across a surface due to curvature. This in turn is the basis
Motivation The surface tension plays a major role in interfacial phenomena. It is the fundamental quantity that determines the pressure change across a surface due to curvature. This in turn is the basis
4th Quarter Pacing Guide LESSON PLANNING
 Domain: Reasoning with Equations and Inequalities Cluster: Solve equations and inequalities in one variable KCAS: A.REI.4a: Solve quadratic equations in one variable. a. Use the method of completing the
Domain: Reasoning with Equations and Inequalities Cluster: Solve equations and inequalities in one variable KCAS: A.REI.4a: Solve quadratic equations in one variable. a. Use the method of completing the
Quasi-Stationary Simulation: the Subcritical Contact Process
 Brazilian Journal of Physics, vol. 36, no. 3A, September, 6 685 Quasi-Stationary Simulation: the Subcritical Contact Process Marcelo Martins de Oliveira and Ronald Dickman Departamento de Física, ICEx,
Brazilian Journal of Physics, vol. 36, no. 3A, September, 6 685 Quasi-Stationary Simulation: the Subcritical Contact Process Marcelo Martins de Oliveira and Ronald Dickman Departamento de Física, ICEx,
19 th INTERNATIONAL CONGRESS ON ACOUSTICS MADRID, 2-7 SEPTEMBER 2007
 19 th INTERNATIONAL CONGRESS ON ACOUSTICS MADRID, -7 SEPTEMBER 007 INVESTIGATION OF AMPLITUDE DEPENDENCE ON NONLINEAR ACOUSTICS USING THE DIRECT SIMULATION MONTE CARLO METHOD PACS: 43.5.Ed Hanford, Amanda
19 th INTERNATIONAL CONGRESS ON ACOUSTICS MADRID, -7 SEPTEMBER 007 INVESTIGATION OF AMPLITUDE DEPENDENCE ON NONLINEAR ACOUSTICS USING THE DIRECT SIMULATION MONTE CARLO METHOD PACS: 43.5.Ed Hanford, Amanda
Imperfect Gases. NC State University
 Chemistry 431 Lecture 3 Imperfect Gases NC State University The Compression Factor One way to represent the relationship between ideal and real gases is to plot the deviation from ideality as the gas is
Chemistry 431 Lecture 3 Imperfect Gases NC State University The Compression Factor One way to represent the relationship between ideal and real gases is to plot the deviation from ideality as the gas is
1 Solutions in cylindrical coordinates: Bessel functions
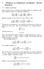 1 Solutions in cylindrical coordinates: Bessel functions 1.1 Bessel functions Bessel functions arise as solutions of potential problems in cylindrical coordinates. Laplace s equation in cylindrical coordinates
1 Solutions in cylindrical coordinates: Bessel functions 1.1 Bessel functions Bessel functions arise as solutions of potential problems in cylindrical coordinates. Laplace s equation in cylindrical coordinates
Crystal nucleation for a model of globular proteins
 JOURNAL OF CHEMICAL PHYSICS VOLUME 120, NUMBER 17 1 MAY 2004 Crystal nucleation for a model of globular proteins Andrey Shiryayev and James D. Gunton Department of Physics, Lehigh University, Bethlehem,
JOURNAL OF CHEMICAL PHYSICS VOLUME 120, NUMBER 17 1 MAY 2004 Crystal nucleation for a model of globular proteins Andrey Shiryayev and James D. Gunton Department of Physics, Lehigh University, Bethlehem,
Linear Theory of Evolution to an Unstable State
 Chapter 2 Linear Theory of Evolution to an Unstable State c 2012 by William Klein, Harvey Gould, and Jan Tobochnik 1 October 2012 2.1 Introduction The simple theory of nucleation that we introduced in
Chapter 2 Linear Theory of Evolution to an Unstable State c 2012 by William Klein, Harvey Gould, and Jan Tobochnik 1 October 2012 2.1 Introduction The simple theory of nucleation that we introduced in
Introduction to Thermodynamic States Gases
 Chapter 1 Introduction to Thermodynamic States Gases We begin our study in thermodynamics with a survey of the properties of gases. Gases are one of the first things students study in general chemistry.
Chapter 1 Introduction to Thermodynamic States Gases We begin our study in thermodynamics with a survey of the properties of gases. Gases are one of the first things students study in general chemistry.
