ELECTROCHEMICAL CELLS
|
|
|
- Leonard Allen
- 6 years ago
- Views:
Transcription
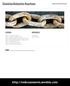
1 Experiment 11 ELECTROCHEMICAL CELLS Prepared by Ross S. Nord, Masanobu M. Yamauchi, and Stephen E. Schullery, Eastern Michigan University PURPOSE You will construct a table of reduction potentials and use it to predict cell potentials. You will learn about the accuracy of electrochemical measurements. You will practice writing simple redox reactions and calculating G and G for these reactions. CELL CONSTRUCTION AND POTENTIALS In principle, any spontaneous redox (oxidationreduction) reaction can be used to construct an electrochemical cell. Cells will be constructed as shown in Figure 1. First, it is necessary to physically separate the chemicals involved in the oxidation and reduction half-reactions. We will place aqueous solutions of two different metal salts in separate beakers. Then, suitable electrodes must be found. We will use a piece of each metal. The oxidation and reduction electrodes are commonly referred to as the anode and cathode, respectively. These terms derive from the fact that, when a voltaic cell reaction is running, anions migrate to the anode (to cast off their electrons and make it negative), and cations migrate to the cathode (to grab some electrons and leave it positive). Figure 1. A sample setup for construction and voltage measurement of electrochemical cells. 11-1
2 Next, wires are used to connect the electrodes. We will place a voltmeter in the circuit to measure the cell potential. When using a cell to power an electrical device (motor, light bulb, etc.), the device would be inserted in place of the voltmeter. Finally, provision must be made for ions to flow between the half-cells, to complete the "internal" circuit but yet not allow gross mixing of the reactants. A salt bridge composed of an absorptive material impregnated with an inert electrolyte is commonly used. A strip of filter paper soaked in 0.10 M KNO3 makes a surprisingly adequate salt bridge. The voltage of a cell (Ecell) is related to the potential energy difference between electrons at the two electrodes, in units of joules/coulomb. This can be thought of as the difference between the two half-cell potentials. E cell = E cathode E anode (1a) By convention, it was chosen to use reduction potentials for both half-cells. Since oxidation is the opposite of reduction, an oxidation potential is equal in magnitude, but opposite in sign, to the corresponding reduction potential. While reduction is actually occurring at the cathode, oxidation is occurring at the anode. So, the reduction potential corresponding to the anode half-reaction is subtracted in equation (1a). HALF-CELL REDUCTION POTENTIALS The voltage that we measure is related to the difference in potential energy between the two half-cells. Thus, we cannot measure the potential of a single half-cell by itself. So, the value for one half-cell is arbitrarily chosen and all other values are measured relative to it. By standard convention, the reduction potential of the standard hydrogen electrode, SHE, is chosen to be 0.00 V. 2 H + (aq, 1 M) + 2 e H 2 (g, 1 bar) (2) So, for example, when a SHE is connected to a half-cell containing Ag + Ag, the silver electrode is found to be the positive electrode (meaning it is the cathode and that it is easier to reduce the Ag + than the H + ) and the measured cell potential is V. Therefore, the Ag + Ag half-cell is assigned a reduction potential of V. Similarly, when the SHE is connected to a Pb 2+ Pb half-cell, the SHE is found to be the positive electrode (so it is easier to reduce the H + than the Pb 2+ ) and the measured cell potential is V. So, the Pb 2+ Pb electrode is assigned a reduction potential of V. We can use the half-cell reduction potentials to predict the cell potential for a Ag/Pb cell. Ag + (aq) + e Ag(s) Pb 2+ (aq) + 2 e Pb(s) E = V E = V As we shall see, since the Ag + Ag half-cell has the greater reduction potential, it is the cathode reaction and the Pb will be oxidized. The complete cell reaction can now be obtained by reversing the Pb half-cell reaction and then adding the two half-reactions together. However, since the number of electrons must cancel, we need to multiply the Ag half-cell reaction by 2 to yield: Pb(s) Pb 2+ (aq) + 2 e 2 Ag + (aq) + 2 e 2 Ag(s) 2 Ag + (aq) + Pb(s) 2 Ag(s) + Pb 2+ (aq) and E cell = V V = V Now, let s say that the Pb 2+ Pb half-cell had been chosen to have a reduction potential of 0.00 V (instead of the SHE). Then, all of the other reduction potentials would also increase by V (so Ag + Ag would have a reduction potential of V). However, the difference between any two potentials would be unchanged (and still equal to the experimental value). So, we could have chosen any half-cell as the starting point to construct a table of reduction potentials. When a measurement is made under standard conditions (e.g., the concentrations of the ions in each half-cell are exactly 1 M), the result is the standard cell potential E O cell O = E cathode O E anode (1b) where E O for each half-cell may be obtained from a table of standard reduction potentials. 11-2
3 VOLTAGE AND G The voltage is also related to the Gibbs free energy of the chemical reaction G = nfe (3a) where n is the number of electrons transferred in the balanced equation (n=2 in our Ag/Pb example), E is the cell's voltage, and F is the Faraday constant (96,500 C/mol or 96.5 kj/vmol). If the cell is a standard cell, then G = nfe (3b) Notice that a negative G, which corresponds to a spontaneous reaction, results in a positive voltage. Thus, the direction of spontaneous reaction can be deduced by noting how the electrodes must be connected to a voltmeter to give a positive reading. VOLTMETER READINGS The available multimeters in lab are capable of measuring resistance, current or voltage. All three functions share one terminal in common, on our meters labeled "COM." By convention, this terminal is to be connected with negative polarity. The other voltage terminal, although perhaps labeled only "V," must be the "positive" or "+" terminal. A voltmeter gives a positive reading when the positive terminal is in fact more positive than the common or negative terminal. Thus, we can deduce that a negative terminal must be connected to an electrode where electrons are being produced, i.e., where oxidation occurs. Similarly, a positive terminal must be connected to an electrode from which electrons are being removed, i.e., where reduction spontaneously occurs. Therefore, a positive voltmeter reading means that the half-cell with the higher reduction potential is connected to the positive terminal. IN THIS EXPERIMENT Five half-cells will be constructed. One of them, the Fe 2+ Fe half-cell will be arbitrarily assigned a reduction potential of zero. Fe 2+ (aq) + 2 e Fe(s) The reduction potentials of the other four halfcells will then be measured by connecting them to the Fe 2+ Fe half-cell. This will allow for the construction of a table of reduction potentials. Next, six different electrochemical cells will be constructed by pairing all possible combinations of the remaining four halfreactions: Zn 2+ (aq) + 2 e Zn(s) Ni 2+ (aq) + 2 e Ni(s) Cu 2+ (aq) + 2 e Cu(s) Al 3+ (aq) + 3 e Al(s) For each pair, one of these reduction halfreactions must be reversed to provide the oxidation necessary for a balanced redox reaction. Although the molecules will decide which half-reaction is to be reversed, you can make a good prediction by comparing the measured reduction potentials. The half-cell with the most positive reduction potential should remain as a reduction. The measured voltage of each cell will be compared with the expected voltage calculated from (1) the measured reduction potentials; and (2) the standard reduction potentials from your textbook. Note that the measured cell voltages are expected to roughly, but not exactly, match voltages calculated using textbook standard half-reaction E O values. The latter correspond to reactions carried out in hypothetical, ideal standard states, which your setups are not. Finally, you will write balanced cell reactions and then calculate G and G O for each reaction. 11-3
4 PRE-LABORATORY PREPARATION 1. Read the procedure and look over the Data Sheet and Data Analysis section of the experiment. 2. Complete the computer-generated prelab for this experiment. 3. You will need to look up the standard reduction potential for each of the five half-cells used. EXPERIMENTAL SECTION REAGENTS PROVIDED 0.10 M solutions of KNO 3, CuSO 4, ZnSO 4, NiSO 4, and FeSO M Al 2(SO 4) 3. (which is 0.10 M in Al 3+ ) Solid metal electrodes Filter paper for construction of salt bridges Hazardous Chemicals NiSO 4 possible carcinogen and poison. Do not swallow it and avoid skin contact. Wash off immediately with copious amounts of water if your skin comes in contact with it. WASTE DISPOSAL The Ni and Cu solutions should be disposed of in the designated waste containers. Other solutions may be safely washed down the sink. PROCEDURE Unless told otherwise, you will do this experiment with a partner (or two, if necessary). 1. Construct separate half-cells for each of the five half-reactions: Zn 2+ (aq) + 2 e Zn(s) Fe 2+ (aq) + 2 e Fe(s) Cu 2+ (aq) + 2 e Cu(s) Ni 2+ (aq) + 2 e Ni(s) Al 3+ (aq) + 3 e Al(s) (a) Add approximately 40 ml of each of the five metal salt solutions to separate 100- ml beakers. (If you do not have enough 100-mL beakers, you can use 50-mL beakers instead, in which case you should only use 30 ml of salt solution, so that the menisci of all of the solutions are at the same level). The Al 3+ and Zn 2+ solutions are both colorless, so label them. (b) Obtain one metal electrode of each of the five metals. Note that the identity of the metal is stamped on each electrode. (c) If necessary, very lightly sandpaper the metal electrodes to remove corrosion products and ensure good contact. This is not likely to be necessary except for possibly iron and zinc. If you sand an electrode, be sure to wipe it off with a paper towel to remove the sanding debris. (d) Stand the clean electrode in the corresponding solution. Please do NOT bend the electrodes. 2. Add about 100 ml of 0.10M KNO 3(aq) to a 250 ml beaker. This bath will be used for making salt bridges. Two groups could share a single bath. 11-4
5 3. Measure the voltage of each of the four cells it is possible to construct by pairing the Fe 2+ Fe half-cell with the other four half-cells: (a) Soak a ¾ in by 8 in piece of filter paper in 0.10 M KNO3. Pick up the strip by the middle, and drain excess solution by touching the ends to a paper towel. Position the paper-strip salt bridge so that it makes contact with both solutions, but is not touching the electrodes. See Figure 1. Use a new salt bridge for every voltage measurement. (b) Connect the multimeter leads to the two electrodes and set the multimeter to the 2V scale. (On some of the voltmeters this is the 2000 mv scale. If you have one of these voltmeters, you will need to divide the mv by 1000 to get the reading in volts. For example, 347 mv = V.) If the voltage is negative, reverse the leads to obtain a positive voltage reading. Do not allow the electrodes to come into contact with each other, or the salt bridge. (c) Watch the voltage for a few seconds and then take a reading. Some readings stabilize quickly, others will drift for as long as you want to watch. (Swirling the solution with the electrode can also change the reading.) Typically, waiting a few seconds after connecting the cell yields the most reliable reading. Record on your Data Sheet your best estimate of the cell voltage to the nearest V (in the First Voltage column of Data Table I), and note which metal electrode was positive. 4. After completing all four measurements, determine the technique s reproducibility by repeating each of these four measurements. (a) Remove and dry the electrodes, (b) Dispose of the three colorless solutions, Fe 2+, Zn 2+, and Al 3+, down the drain and get fresh samples. However, use the same Cu 2+ and Ni 2+ solutions to minimize waste, (c) Place the metal electrodes in the solutions, (d) Repeat the measurements using the same procedure as in step 3. Enter these voltage readings, along with your original readings, 11-5 on the Data Sheet. (These are the Second Voltages in Data Table I.) (e) If the two voltage readings for the same cell differ by more than 0.1 V, get a fresh solution of Fe 2+ (and Zn 2+ or Al 3+, if relevant) and then repeat the measurement a third time to see which trial was more accurate. (f) Average your two readings (or your two closest readings if you took three readings). 5. Measure the voltage of each of the six cells it is possible to construct by pairing the four half-cells other than the Fe 2+ Fe half-cell. Follow the same procedure as in step 3. You can use the same half-cells that you constructed in step 4 and do not need to prepare new halfcells. Record the voltages (and note which electrode was positive) in Data Table II. 6. Re-measure the voltage for the cell constructed using the Al 3+ Al and Zn 2+ Zn half-cells, as follows: (a) Attach the negative lead to the zinc electrode. (b) The positive lead should not be attached to any electrode, but should be ready. (c) Make sure the multimeter is on and still set to 2V. (d) Place a salt bridge, as normal, between the Al 3+ and Zn 2+ solutions. (e) Remove the Al metal electrode from its solution and dry it. The next several steps need to be done quickly. (f) Sand the bottom on one side of the Al electrode for a few (3-5) seconds. (g) Attach the positive lead to the Al electrode. (h) Insert the Al electrode into the Al solution and record the very first voltage reading you see (in Data Table III). Do NOT wait for the voltage to stabilize before taking the reading, (i) Watch the voltage reading for about one minute. Use the electrode to gently swirl the solution every seconds. Record the final voltage reading. 7. Wash and store your glassware. Wash your hands thoroughly before leaving lab.
6 Name Station Used Instructor/Day/Time Partner Partner Station Checked & Approved DATA SHEET AND DATA ANALYSIS Record and calculate all values to the proper number of significant figures. All measured cell potentials should be positive (although half-cell reduction potentials can be positive or negative). DATA TABLE I. Voltage Measured with the Fe 2+ Fe Half-Cell Positive Electrode First Voltage (V) Second Voltage (V) Third Voltage (V) (if necessary) Average Voltage (V) Measured Reduction Potential (V) Al 3+ Al Cu 2+ Cu Ni 2+ Ni Zn 2+ Zn 1. We can define a measured reduction potential for each electrode by simply equating it to the average voltage measured (since they were all measured relative to Fe 2+ Fe which we defined to have a reduction potential of zero), but with the proper sign. The electrodes that were positive compared to Fe, will have positive reduction potentials, while the electrodes that were negative compared to Fe (i.e., Fe was the positive electrode) will have negative reduction potentials. Given this information, you should be able to simply fill in the column of measured reduction potentials in the table above. 2. Create a table of reduction potentials for the five half-cells (including the Fe 2+ Fe half-cell, to which we have assigned to have a reduction potential of 0.00 V). List them from the most positive (at the top) to the most negative (at the bottom). Half-Cell Measured Reduction Potential, E (V) Standard Reduction Potential, E (V) 3. Complete the above table by inserting the standard reduction potential (as found in your textbook) for each half-cell. Note: you should see that (with the exception of Al) most of the measured reduction potentials are approximately 0.3 to 0.5 V larger than the standard ones. This is consistent with our alternate definition of 0.00 V for the Fe 2+ Fe half-cell, instead of the SHE. 11-6
7 DATA TABLE II. Measured Voltages for Cells (not containing the Fe 2+ Fe half-cell) Metal Electrodes Positive Electrode Measured Voltage, Ecell (V) Calculated Voltage, Ecell (V) Calculated Standard Voltage, E cell (V) Ni / Zn Cu / Zn Cu / Ni Al / Zn Al / Ni Al / Cu 4. Use equation (1a) and your Measured Reduction Potentials to determine and fill in the column of Calculated Voltages. Remember that the positive electrode is the cathode (in a galvanic cell). You should see good agreement (within 0.2 V) between measured and calculated voltages. 5. Use the Standard Reduction Potentials to complete the Calculated Standard Voltage column. You should see good agreement between the two sets of calculated voltages (except for cells containing Al), demonstrating that the choice of zero point, either the Fe 2+ Fe half-cell or the SHE, does not matter. 6. Combine the two relevant half-reactions to write the balanced (net ionic) reaction observed in each cell. Write the direction of the reaction based upon which electrode you observed to be positive. Ni / Zn Cu / Zn Cu / Ni Al / Zn Al / Ni Al / Cu 7. Use the balanced reactions to determine n and then use the Measured Voltages and the Calculated Standard Voltages to calculate the free energy changes and complete the table below. Ni / Zn Cu / Zn Cu / Ni Al / Zn Al / Ni n Calculated G (kj/mol) Calculated G (kj/mol) Show a sample calculation for the Ni/Zn reaction in the space below. Al / Cu 11-7
8 8. Compare your measured voltages with the calculated standard voltages for the cells. A) Are there any cells with measured voltages that differ from the expected standard voltage by more than V? If so list them: B) Do the cells listed in part (A) have any half-cells in common? If so, which one(s)? DATA TABLE III. Voltages at different times for the Al / Zn cell. Initial Voltage (V) Final Voltage (V) Previously Measured Voltage (V) (from Table II) 9. It is known that Al metal does not corrode easily because Al metal will form a stable coating of aluminum oxide that protects the metal underneath so it will not oxidize. Given this information, answer the following questions for the Al / Zn cell: A) Circle your choice from each pair: After sanding the Al electrode, the initial voltage was (higher, lower) than the previously measured voltage. Since the zinc half-cell potential does not change, that means the measured half-cell potential for Al 3+ Al must (increase, decrease) which means it is (closer to, farther from) its standard value. B) Why do you think sanding the electrode would cause this change (relative to its standard value)? C) You should have observed the cell voltage change during the minute that you observed it. Explain what is occurring on the electrode that causes the observed voltage to change. 11-8
ELECTROCHEMICAL CELLS
 Experiment 11 ELECTROCHEMICAL CELLS Prepared by Ross S. Nord, Masanobu M. Yamauchi, and Stephen E. Schullery, Eastern Michigan University PURPOSE You will construct a table of reduction potentials and
Experiment 11 ELECTROCHEMICAL CELLS Prepared by Ross S. Nord, Masanobu M. Yamauchi, and Stephen E. Schullery, Eastern Michigan University PURPOSE You will construct a table of reduction potentials and
ELECTROCHEMICAL CELLS
 Experiment 11 ELECTROCHEMICAL CELLS Prepared by Ross S. Nord, Masanobu M. Yamauchi, and Stephen E. Schullery, Eastern Michigan University PURPOSE You will construct a series of electrochemical cells. For
Experiment 11 ELECTROCHEMICAL CELLS Prepared by Ross S. Nord, Masanobu M. Yamauchi, and Stephen E. Schullery, Eastern Michigan University PURPOSE You will construct a series of electrochemical cells. For
Electrochemistry and the Nernst Equation
 Electrochemistry and the Nernst Equation LEARNING OBJECTIVES The objectives of this experiment are to... construct galvanic cells and develop an electrochemical series based on potential differences between
Electrochemistry and the Nernst Equation LEARNING OBJECTIVES The objectives of this experiment are to... construct galvanic cells and develop an electrochemical series based on potential differences between
Voltaic Cells. 100 ml graduated cylinder Emery cloth 150 ml beakers, 3 Salt bridge Voltmeter Wires with alligator clips, 2
 Skills Practice Voltaic Cells DATASHEET FOR IN-TEXT LAB In voltaic cells, oxidation and reduction half-reactions take place in separate halfcells, which can consist of a metal electrode immersed in a solution
Skills Practice Voltaic Cells DATASHEET FOR IN-TEXT LAB In voltaic cells, oxidation and reduction half-reactions take place in separate halfcells, which can consist of a metal electrode immersed in a solution
Electrochemistry. Part I: Electrochemical Activity from Chemical Reactions. Part II. Electrochemical activity from cell potentials.
 Electrochemistry Introduction: Redox (oxidation-reduction) reactions will be used to determine the relative electrochemical reactivity of 5 metals. In Part I of the experiment, you will determine the activity
Electrochemistry Introduction: Redox (oxidation-reduction) reactions will be used to determine the relative electrochemical reactivity of 5 metals. In Part I of the experiment, you will determine the activity
EXPERIMENT 16 Electrochemical Cells: A Discovery Exercise 1. Introduction. Discussion
 EXPERIMENT 16 Electrochemical Cells: A Discovery Exercise 1 Introduction This lab is designed for you to discover the properties of electrochemical cells. It requires little previous knowledge of electrochemical
EXPERIMENT 16 Electrochemical Cells: A Discovery Exercise 1 Introduction This lab is designed for you to discover the properties of electrochemical cells. It requires little previous knowledge of electrochemical
Introduction to electrochemistry
 Introduction to electrochemistry Oxidation reduction reactions involve energy changes. Because these reactions involve electronic transfer, the net release or net absorption of energy can occur in the
Introduction to electrochemistry Oxidation reduction reactions involve energy changes. Because these reactions involve electronic transfer, the net release or net absorption of energy can occur in the
Experiment 18: Galvanic Cells
 Chem 1B Dr. White 131 Experiment 18: Galvanic Cells Objectives Introduction To construct galvanic cells To learn how reduction potentials can be used to predict the relative reactivity of metals In a redox
Chem 1B Dr. White 131 Experiment 18: Galvanic Cells Objectives Introduction To construct galvanic cells To learn how reduction potentials can be used to predict the relative reactivity of metals In a redox
Lab #14: Electrochemical Cells
 Lab #14: Electrochemical Cells Objectives: 1. To understand the nature of electrochemical cells. 2. To construct a table listing the reduction potentials of a series of metal ions, in order of ease of
Lab #14: Electrochemical Cells Objectives: 1. To understand the nature of electrochemical cells. 2. To construct a table listing the reduction potentials of a series of metal ions, in order of ease of
To determine relative oxidizing and reducing strengths of a series of metals and ions.
 Redox Reactions PURPOSE To determine relative oxidizing and reducing strengths of a series of metals and ions. GOALS 1 To explore the relative oxidizing and reducing strengths of different metals. 2 To
Redox Reactions PURPOSE To determine relative oxidizing and reducing strengths of a series of metals and ions. GOALS 1 To explore the relative oxidizing and reducing strengths of different metals. 2 To
AP Chemistry Laboratory #21: Voltaic Cells. Lab day: Monday, April 21, 2014 Lab due: Wednesday, April 23, 2014
 AP Chemistry Laboratory #21: Voltaic Cells Lab day: Monday, April 21, 2014 Lab due: Wednesday, April 23, 2014 Goal (list in your lab book): The goal of this lab is to determine what factors affect the
AP Chemistry Laboratory #21: Voltaic Cells Lab day: Monday, April 21, 2014 Lab due: Wednesday, April 23, 2014 Goal (list in your lab book): The goal of this lab is to determine what factors affect the
3. ELECTROCHEMISTRY - GALVANIC CELLS
 1 3. ELECTROCHEMISTRY - GALVANIC CELLS JHU INTRO. CHEM. LAB SUMMER2013 You will have one lab period for this experiment. As part of the prelab homework, you will have calculated the amounts of the salts
1 3. ELECTROCHEMISTRY - GALVANIC CELLS JHU INTRO. CHEM. LAB SUMMER2013 You will have one lab period for this experiment. As part of the prelab homework, you will have calculated the amounts of the salts
Galvanic Cells Spontaneous Electrochemistry. Electrolytic Cells Backwards Electrochemistry
 Today Galvanic Cells Spontaneous Electrochemistry Electrolytic Cells Backwards Electrochemistry Balancing Redox Reactions There is a method (actually several) Learn one (4.10-4.12) Practice (worksheet)
Today Galvanic Cells Spontaneous Electrochemistry Electrolytic Cells Backwards Electrochemistry Balancing Redox Reactions There is a method (actually several) Learn one (4.10-4.12) Practice (worksheet)
Chemistry 212 Lab, Spring Design of the Experiment: Standard and Non-Standard Reduction Potentials For Metal/Metal Ion Half-Cells
 Chemistry 212 Lab, Spring 2009 Electrochemical Cells: Determination of Reduction Potentials for a Series of Metal/Metal Ion Systems, Verification of Nernst Equation, and Determination of Formation Constant
Chemistry 212 Lab, Spring 2009 Electrochemical Cells: Determination of Reduction Potentials for a Series of Metal/Metal Ion Systems, Verification of Nernst Equation, and Determination of Formation Constant
Chemistry 1B Experiment 14 65
 Chemistry 1B Experiment 14 65 14 Electrochemistry Introduction In this experiment you will observe some spontaneous and non-spontaneous oxidation-reduction reactions, and see how the spontaneous reactions
Chemistry 1B Experiment 14 65 14 Electrochemistry Introduction In this experiment you will observe some spontaneous and non-spontaneous oxidation-reduction reactions, and see how the spontaneous reactions
Instructors Guide: Introduction to Voltaic Cells
 Instructors Guide: Introduction to Voltaic Cells Standards Connections: Connections to NSTA Standards for Science Teacher Preparation C.3.a.8 Oxidation reduction chemistry. Connections to the National
Instructors Guide: Introduction to Voltaic Cells Standards Connections: Connections to NSTA Standards for Science Teacher Preparation C.3.a.8 Oxidation reduction chemistry. Connections to the National
Chemistry: The Central Science. Chapter 20: Electrochemistry
 Chemistry: The Central Science Chapter 20: Electrochemistry Redox reaction power batteries Electrochemistry is the study of the relationships between electricity and chemical reactions o It includes the
Chemistry: The Central Science Chapter 20: Electrochemistry Redox reaction power batteries Electrochemistry is the study of the relationships between electricity and chemical reactions o It includes the
Chapter Objectives. Chapter 13 Electrochemistry. Corrosion. Chapter Objectives. Corrosion. Corrosion
 Chapter Objectives Larry Brown Tom Holme Describe at least three types of corrosion and identify chemical reactions responsible for corrosion. www.cengage.com/chemistry/brown Chapter 13 Electrochemistry
Chapter Objectives Larry Brown Tom Holme Describe at least three types of corrosion and identify chemical reactions responsible for corrosion. www.cengage.com/chemistry/brown Chapter 13 Electrochemistry
ElectroChemistry * Section I: Reactivity of Metals and Metal Ions PRELAB
 ElectroChemistry * Name Section Instructions: You and your partner(s) will be working with a computer simulation that covers electrochemistry, please discuss each question with your partner(s) and write
ElectroChemistry * Name Section Instructions: You and your partner(s) will be working with a computer simulation that covers electrochemistry, please discuss each question with your partner(s) and write
EXPERIMENT C4: ELECTROCHEMISTRY. Learning Outcomes. Introduction. Upon completion of this lab, the student will be able to:
 1 Learning Outcomes EXPERIMENT C4: ELECTROCHEMISTRY Upon completion of this lab, the student will be able to: 1) Construct an electrochemical cell. 2) Measure the cell potential for an electrochemical
1 Learning Outcomes EXPERIMENT C4: ELECTROCHEMISTRY Upon completion of this lab, the student will be able to: 1) Construct an electrochemical cell. 2) Measure the cell potential for an electrochemical
Electrochemistry and the Nernst Equation
 Experiment Electrochemistry and the Nernst Equation The CCLI Initiative Computers in Chemistry Laboratory Instruction The objectives of this experiment are to... LEARNING OBJECTIVES construct galvanic
Experiment Electrochemistry and the Nernst Equation The CCLI Initiative Computers in Chemistry Laboratory Instruction The objectives of this experiment are to... LEARNING OBJECTIVES construct galvanic
A Study of Electrochemistry Prelab
 1. What is the purpose of this experiment? A Study of Electrochemistry Prelab 2. a. Calculate the standard cell potential of a cell constructed from Mg 2+ /Mg and Ni 2+ /Ni (Table I). Which is the anode
1. What is the purpose of this experiment? A Study of Electrochemistry Prelab 2. a. Calculate the standard cell potential of a cell constructed from Mg 2+ /Mg and Ni 2+ /Ni (Table I). Which is the anode
#13 Electrochemical Cells
 #13 Electrochemical Cells If a copper strip is placed in a solution of copper ions, one of the following reactions may occur: Cu 2+ + 2e - Cu Cu Cu 2+ + 2e - The electrical potential that would be developed
#13 Electrochemical Cells If a copper strip is placed in a solution of copper ions, one of the following reactions may occur: Cu 2+ + 2e - Cu Cu Cu 2+ + 2e - The electrical potential that would be developed
Electrochemical Cells
 Electrochemical Cells PURPOSE To see how changes in concentration and ph affect the potential in an electrochemical cell, and confirm the Nernst equation. GOALS To examine how standard reduction potentials
Electrochemical Cells PURPOSE To see how changes in concentration and ph affect the potential in an electrochemical cell, and confirm the Nernst equation. GOALS To examine how standard reduction potentials
Electrochemistry and Concentration Effects on Electrode Potentials Prelab
 Electrochemistry and Concentration Effects on Electrode Potentials Prelab 1. What is the purpose of this experiment? 2. a. Calculate the standard cell potential of a cell constructed from Mg 2+ /Mg and
Electrochemistry and Concentration Effects on Electrode Potentials Prelab 1. What is the purpose of this experiment? 2. a. Calculate the standard cell potential of a cell constructed from Mg 2+ /Mg and
Experiment 21. Voltaic Cells
 Experiment 21 Voltaic Cells INTRODUCTION: A voltaic cell is a specially prepared system in which an oxidation-reduction reaction occurs spontaneously. The oxidation and reduction half-reactions are separated
Experiment 21 Voltaic Cells INTRODUCTION: A voltaic cell is a specially prepared system in which an oxidation-reduction reaction occurs spontaneously. The oxidation and reduction half-reactions are separated
Section A: Summary Notes
 ELECTROCHEMICAL CELLS 25 AUGUST 2015 Section A: Summary Notes Important definitions: Oxidation: the loss of electrons by a substance during a chemical reaction Reduction: the gain of electrons by a substance
ELECTROCHEMICAL CELLS 25 AUGUST 2015 Section A: Summary Notes Important definitions: Oxidation: the loss of electrons by a substance during a chemical reaction Reduction: the gain of electrons by a substance
Electrochemistry: Voltaic Cells
 Name: Band: Date: An Overview and Review Electrochemistry: Voltaic Cells Electrochemistry is the field of chemistry that focuses on reactions involving electrical energy. All electrochemical reactions
Name: Band: Date: An Overview and Review Electrochemistry: Voltaic Cells Electrochemistry is the field of chemistry that focuses on reactions involving electrical energy. All electrochemical reactions
Electrochemistry Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry
 2012 Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry Electricity from Chemistry Many chemical reactions involve the transfer of electrons between atoms or ions electron transfer
2012 Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry Electricity from Chemistry Many chemical reactions involve the transfer of electrons between atoms or ions electron transfer
Chapter 20. Electrochemistry. Chapter 20 Problems. Electrochemistry 7/3/2012. Problems 15, 17, 19, 23, 27, 29, 33, 39, 59
 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 20 John D. Bookstaver St. Charles Community College Cottleville, MO Chapter 20 Problems
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 20 John D. Bookstaver St. Charles Community College Cottleville, MO Chapter 20 Problems
Determination of an Electrochemical Series
 In electrochemistry, a voltaic cell is a specially prepared system in which an oxidation-reduction reaction occurs spontaneously. This spontaneous reaction produces an easily measured electrical potential
In electrochemistry, a voltaic cell is a specially prepared system in which an oxidation-reduction reaction occurs spontaneously. This spontaneous reaction produces an easily measured electrical potential
Electrochemistry. The study of the interchange of chemical and electrical energy.
 Electrochemistry The study of the interchange of chemical and electrical energy. Oxidation-reduction (redox) reaction: involves a transfer of electrons from the reducing agent to the oxidizing agent. oxidation:
Electrochemistry The study of the interchange of chemical and electrical energy. Oxidation-reduction (redox) reaction: involves a transfer of electrons from the reducing agent to the oxidizing agent. oxidation:
THE IRON(III) THIOCYANATE REACTION SYSTEM
 Experiment 7 THE IRON(III) THIOCYANATE REACTION SYSTEM Prepared by Ross S. Nord, Chemistry Department, Eastern Michigan University PURPOSE To investigate a novel reaction system by utilizing a spectrophotometer.
Experiment 7 THE IRON(III) THIOCYANATE REACTION SYSTEM Prepared by Ross S. Nord, Chemistry Department, Eastern Michigan University PURPOSE To investigate a novel reaction system by utilizing a spectrophotometer.
ELECTROCHEMICAL CELLS
 ELECTROCHEMICAL CELLS Electrochemistry 1. Redox reactions involve the transfer of electrons from one reactant to another 2. Electric current is a flow of electrons in a circuit Many reduction-oxidation
ELECTROCHEMICAL CELLS Electrochemistry 1. Redox reactions involve the transfer of electrons from one reactant to another 2. Electric current is a flow of electrons in a circuit Many reduction-oxidation
Oxidation-Reduction Review. Electrochemistry. Oxidation-Reduction Reactions. Oxidation-Reduction Reactions. Sample Problem.
 1 Electrochemistry Oxidation-Reduction Review Topics Covered Oxidation-reduction reactions Balancing oxidationreduction equations Voltaic cells Cell EMF Spontaneity of redox reactions Batteries Electrolysis
1 Electrochemistry Oxidation-Reduction Review Topics Covered Oxidation-reduction reactions Balancing oxidationreduction equations Voltaic cells Cell EMF Spontaneity of redox reactions Batteries Electrolysis
Electrochemistry. Pre-Lab Assignment. Purpose. Background. Experiment 12
 Experiment 12 Electrochemistry Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. The questions should be answered
Experiment 12 Electrochemistry Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. The questions should be answered
THE TEMPERATURE DEPENDENCE OF THE EQUILIBRIUM CONSTANT
 Experiment 7B THE TEMPERATURE DEPENDENCE OF THE EQUILIBRIUM CONSTANT Prepared by Ross S. Nord, Chemistry Department, Eastern Michigan University PURPOSE To investigate the relationship between the equilibrium
Experiment 7B THE TEMPERATURE DEPENDENCE OF THE EQUILIBRIUM CONSTANT Prepared by Ross S. Nord, Chemistry Department, Eastern Michigan University PURPOSE To investigate the relationship between the equilibrium
Unit 13 Redox Reactions & Electrochemistry Ch. 19 & 20 of your book.
 Unit 13 Redox Reactions & Electrochemistry Ch. 19 & 20 of your book. Early Booklet E.C.: + 2 Unit 13 Hwk. Pts.: / 32 Unit 13 Lab Pts.: / 32 Late, Incomplete, No Work, No Units Fees? Y / N Learning Targets
Unit 13 Redox Reactions & Electrochemistry Ch. 19 & 20 of your book. Early Booklet E.C.: + 2 Unit 13 Hwk. Pts.: / 32 Unit 13 Lab Pts.: / 32 Late, Incomplete, No Work, No Units Fees? Y / N Learning Targets
Chapter 18. Electrochemistry
 Chapter 18 Electrochemistry Section 17.1 Spontaneous Processes and Entropy Section 17.1 http://www.bozemanscience.com/ap-chemistry/ Spontaneous Processes and Entropy Section 17.1 Spontaneous Processes
Chapter 18 Electrochemistry Section 17.1 Spontaneous Processes and Entropy Section 17.1 http://www.bozemanscience.com/ap-chemistry/ Spontaneous Processes and Entropy Section 17.1 Spontaneous Processes
KINETICS: INITIAL RATES
 Experiment 6B KINETICS: INITIAL RATES Prepared by Ross S. Nord, Stephen E. Schullery, and Masanobu M. Yamauchi, Eastern Michigan University PURPOSE Learn how to measure initial rates. Determine the order
Experiment 6B KINETICS: INITIAL RATES Prepared by Ross S. Nord, Stephen E. Schullery, and Masanobu M. Yamauchi, Eastern Michigan University PURPOSE Learn how to measure initial rates. Determine the order
Electrochemical Cells: Virtual Lab
 Electrochemical Cells: Virtual Lab Electrochemical cells involve the transfer of electrons from one species to another. In these chemical systems, the species that loses electrons is said to be oxidized
Electrochemical Cells: Virtual Lab Electrochemical cells involve the transfer of electrons from one species to another. In these chemical systems, the species that loses electrons is said to be oxidized
ELECTROCHEMICAL CELLS NAME ROW PD
 4-26-12 NAME ROW PD (1) Which statement describes the redox reaction that occurs when an object is electroplated? The diagram below shows the electrolysis of fused KCl. A) It is spontaneous and requires
4-26-12 NAME ROW PD (1) Which statement describes the redox reaction that occurs when an object is electroplated? The diagram below shows the electrolysis of fused KCl. A) It is spontaneous and requires
Batteries. How does a battery (voltaic cell) work? Time Passes
 Why? Batteries How does a battery (voltaic cell) work? When we use portable devices like MP3 players and cell phones we need a ready source of electricity to provide a flow of electrons. Batteries are
Why? Batteries How does a battery (voltaic cell) work? When we use portable devices like MP3 players and cell phones we need a ready source of electricity to provide a flow of electrons. Batteries are
Electrochemical Cells
 reactions involve the processes of oxidation and reduction. In this three-part lab, these reactions are studied by with sugars, fats, and proteins that provide energy for life to the corrosion of metals,
reactions involve the processes of oxidation and reduction. In this three-part lab, these reactions are studied by with sugars, fats, and proteins that provide energy for life to the corrosion of metals,
Electrochemical Cells
 Chem 100 Section Experiment 13 Name Partner s Name Electrochemical Cells Introduction This experiment is designed to accompany Chapter 8 in Chemistry in Context 5th Ed. You will construct several simple
Chem 100 Section Experiment 13 Name Partner s Name Electrochemical Cells Introduction This experiment is designed to accompany Chapter 8 in Chemistry in Context 5th Ed. You will construct several simple
What Do You Think? Investigate GOALS. Part A: Solutions That Conduct Electricity
 Chemical Dominoes Activity 6 Electrochemical Cells GALS In this activity you will: Determine if a substance will conduct electricity when dissolved in water. Construct a galvanic cell and explain the function
Chemical Dominoes Activity 6 Electrochemical Cells GALS In this activity you will: Determine if a substance will conduct electricity when dissolved in water. Construct a galvanic cell and explain the function
Chemistry 102 Chapter 19 OXIDATION-REDUCTION REACTIONS
 OXIDATION-REDUCTION REACTIONS Some of the most important reaction in chemistry are oxidation-reduction (redox) reactions. In these reactions, electrons transfer from one reactant to the other. The rusting
OXIDATION-REDUCTION REACTIONS Some of the most important reaction in chemistry are oxidation-reduction (redox) reactions. In these reactions, electrons transfer from one reactant to the other. The rusting
Chapter 20 Electrochemistry
 Chapter 20 Electrochemistry Learning goals and key skills: Identify oxidation, reduction, oxidizing agent, and reducing agent in a chemical equation Complete and balance redox equations using the method
Chapter 20 Electrochemistry Learning goals and key skills: Identify oxidation, reduction, oxidizing agent, and reducing agent in a chemical equation Complete and balance redox equations using the method
Chapter Nineteen. Electrochemistry
 Chapter Nineteen Electrochemistry 1 Electrochemistry The study of chemical reactions through electrical circuits. Monitor redox reactions by controlling electron transfer REDOX: Shorthand for REDuction-OXidation
Chapter Nineteen Electrochemistry 1 Electrochemistry The study of chemical reactions through electrical circuits. Monitor redox reactions by controlling electron transfer REDOX: Shorthand for REDuction-OXidation
General Chemistry I. Dr. PHAN TẠI HUÂN Faculty of Food Science and Technology Nong Lam University
 General Chemistry I Dr. PHAN TẠI HUÂN Faculty of Food Science and Technology Nong Lam University Module 7: Oxidation-reduction reactions and transformation of chemical energy Oxidation-reduction reactions
General Chemistry I Dr. PHAN TẠI HUÂN Faculty of Food Science and Technology Nong Lam University Module 7: Oxidation-reduction reactions and transformation of chemical energy Oxidation-reduction reactions
Ch 20 Electrochemistry: the study of the relationships between electricity and chemical reactions.
 Ch 20 Electrochemistry: the study of the relationships between electricity and chemical reactions. In electrochemical reactions, electrons are transferred from one species to another. Learning goals and
Ch 20 Electrochemistry: the study of the relationships between electricity and chemical reactions. In electrochemical reactions, electrons are transferred from one species to another. Learning goals and
If a piece of magnesium is placed in an aqueous solution of copper (II) sulfate, the magnesium displaces the copper in a single displacement reaction.
 5.3 REDOX Reactions Half-reactions from Full Redox Equations If a piece of magnesium is placed in an aqueous solution of copper (II) sulfate, the magnesium displaces the copper in a single displacement
5.3 REDOX Reactions Half-reactions from Full Redox Equations If a piece of magnesium is placed in an aqueous solution of copper (II) sulfate, the magnesium displaces the copper in a single displacement
Electrochemistry. Chapter 19. Concept Check Concept Check Solution. Solution
 Chapter 19 Electrochemistry Concept Check 19.1 If you were to construct a wet cell and decided to replace the salt bridge with a piece of copper wire, would the cell produce sustainable current? Explain
Chapter 19 Electrochemistry Concept Check 19.1 If you were to construct a wet cell and decided to replace the salt bridge with a piece of copper wire, would the cell produce sustainable current? Explain
Name AP CHEM / / Collected Essays Chapter 17
 Name AP CHEM / / Collected Essays Chapter 17 1980 - #2 M(s) + Cu 2+ (aq) M 2+ (aq) + Cu(s) For the reaction above, E = 0.740 volt at 25 C. (a) Determine the standard electrode potential for the reaction
Name AP CHEM / / Collected Essays Chapter 17 1980 - #2 M(s) + Cu 2+ (aq) M 2+ (aq) + Cu(s) For the reaction above, E = 0.740 volt at 25 C. (a) Determine the standard electrode potential for the reaction
Guide for Reading. Vocabulary. reduction potential reduction potential. standard cell potential standard hydrogen electrode.
 21.2 Half-Cells and Cell Potentials Connecting to Your World Batteries provide current to power lights and many kinds of electronic devices such as the personal digital assistant shown here. One type of
21.2 Half-Cells and Cell Potentials Connecting to Your World Batteries provide current to power lights and many kinds of electronic devices such as the personal digital assistant shown here. One type of
Electrochemical Cells
 Electrochemical Cells There are two types: Galvanic and Electrolytic Galvanic Cell: a cell in which a is used to produce electrical energy, i.e., Chemical energy is transformed into Electrical energy.
Electrochemical Cells There are two types: Galvanic and Electrolytic Galvanic Cell: a cell in which a is used to produce electrical energy, i.e., Chemical energy is transformed into Electrical energy.
Redox Reactions and Electrochemistry
 Redox Reactions and Electrochemistry Redox Reactions and Electrochemistry Redox Reactions (19.1) Galvanic Cells (19.2) Standard Reduction Potentials (19.3) Thermodynamics of Redox Reactions (19.4) The
Redox Reactions and Electrochemistry Redox Reactions and Electrochemistry Redox Reactions (19.1) Galvanic Cells (19.2) Standard Reduction Potentials (19.3) Thermodynamics of Redox Reactions (19.4) The
Chemistry 213. Electrochemistry I
 1 Chemistry 213 Electrochemistry I Electrochemical Cells Objective Oxidation/reduction reactions find their most important use in the construction of voltaic cells (chemical batteries). In this experiment,
1 Chemistry 213 Electrochemistry I Electrochemical Cells Objective Oxidation/reduction reactions find their most important use in the construction of voltaic cells (chemical batteries). In this experiment,
Introduction. can be rewritten as follows: Oxidation reaction. H2 2H + +2e. Reduction reaction: F2+2e 2F. Overall Reaction H2+F2 2H + +2F
 Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called electricity, which can be generated by movements of electrons from one element to
Electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is called electricity, which can be generated by movements of electrons from one element to
What is a Voltaic Cell? Voltaic Cells a.k.a. Electrochemical cells. May 25, Voltaic Cells 2018.notebook
 What is a? s a.k.a. Electrochemical cells Aim: To analyze the process of a spontaneous chemical reaction that produces electricity. Voltaic cell: an electrochemical cell where chemical energy is spontaneously
What is a? s a.k.a. Electrochemical cells Aim: To analyze the process of a spontaneous chemical reaction that produces electricity. Voltaic cell: an electrochemical cell where chemical energy is spontaneously
Electrochemistry Pulling the Plug on the Power Grid
 Electrochemistry 18.1 Pulling the Plug on the Power Grid 18.3 Voltaic (or Galvanic) Cells: Generating Electricity from Spontaneous Chemical Reactions 18.4 Standard Electrode Potentials 18.7 Batteries:
Electrochemistry 18.1 Pulling the Plug on the Power Grid 18.3 Voltaic (or Galvanic) Cells: Generating Electricity from Spontaneous Chemical Reactions 18.4 Standard Electrode Potentials 18.7 Batteries:
N Goalby chemrevise.org
 Redox Equilibria Electrochemical cells This type of cell can be called a Voltaic cell or Galvanic cell. Voltaic cells convert energy from spontaneous, exothermic chemical processes to electrical energy.
Redox Equilibria Electrochemical cells This type of cell can be called a Voltaic cell or Galvanic cell. Voltaic cells convert energy from spontaneous, exothermic chemical processes to electrical energy.
BATTERIES AND ELECTROLYTIC CELLS. Practical Electrochemistry
 BATTERIES AND ELECTROLYTIC CELLS Practical Electrochemistry How Batteries Work One of the most practical applications of spontaneous redox reactions is making batteries. In a battery, a spontaneous electron
BATTERIES AND ELECTROLYTIC CELLS Practical Electrochemistry How Batteries Work One of the most practical applications of spontaneous redox reactions is making batteries. In a battery, a spontaneous electron
Chemistry 132 NT. Electrochemistry. Oxidation-Reduction Reactions
 Chemistry 132 NT If you ever catch on fire, try to avoid seeing yourself in the mirror, because I bet that s what really throws you into a panic. Jack Handey 1 Chem 132 NT Electrochemistry Module 1 HalfReactions
Chemistry 132 NT If you ever catch on fire, try to avoid seeing yourself in the mirror, because I bet that s what really throws you into a panic. Jack Handey 1 Chem 132 NT Electrochemistry Module 1 HalfReactions
Electrochemistry. Review oxidation reactions and how to assign oxidation numbers (Ch 4 Chemical Reactions).
 Electrochemistry Oxidation-Reduction: Review oxidation reactions and how to assign oxidation numbers (Ch 4 Chemical Reactions). Half Reactions Method for Balancing Redox Equations: Acidic solutions: 1.
Electrochemistry Oxidation-Reduction: Review oxidation reactions and how to assign oxidation numbers (Ch 4 Chemical Reactions). Half Reactions Method for Balancing Redox Equations: Acidic solutions: 1.
ELECTROCHEMISTRY. Oxidation/Reduction
 ELECTROCHEMISTRY Electrochemistry involves the relationship between electrical energy and chemical energy. OXIDATION-REDUCTION REACTIONS SPONTANEOUS REACTIONS Examples: voltaic cells, batteries. NON-SPONTANEOUS
ELECTROCHEMISTRY Electrochemistry involves the relationship between electrical energy and chemical energy. OXIDATION-REDUCTION REACTIONS SPONTANEOUS REACTIONS Examples: voltaic cells, batteries. NON-SPONTANEOUS
1.11 Redox Equilibria
 1.11 Redox Equilibria Electrochemical cells Electron flow A cell has two half cells. The two half cells have to be connected with a salt bridge. Simple half cells will consist of a metal (acts an electrode)
1.11 Redox Equilibria Electrochemical cells Electron flow A cell has two half cells. The two half cells have to be connected with a salt bridge. Simple half cells will consist of a metal (acts an electrode)
Chemistry 213. Electrochemistry
 Chemistry 213 Electrochemistry Part A: Electrochemical Cells Objective Oxidation/reduction reactions find their most important use in the construction of voltaic cells (chemical batteries). In this experiment,
Chemistry 213 Electrochemistry Part A: Electrochemical Cells Objective Oxidation/reduction reactions find their most important use in the construction of voltaic cells (chemical batteries). In this experiment,
Chapter 19: Electrochemistry
 Chapter 19: Electrochemistry Overview of the Chapter review oxidation-reduction chemistry basics galvanic cells spontaneous chemical reaction generates a voltage set-up of galvanic cell & identification
Chapter 19: Electrochemistry Overview of the Chapter review oxidation-reduction chemistry basics galvanic cells spontaneous chemical reaction generates a voltage set-up of galvanic cell & identification
Zn + Cr 3+ Zn 2+ + Cr. 9. neutrons remain the same: C. remains the same. Redox/Electrochemistry Regents Unit Review. ANSWERS
 Redox/Electrochemistry Regents Unit Review. ANSWERS 1. ½ red = Cr 3+ + 3e Cr 2. ½ ox = Zn Zn +2 + 2e 3. Balanced = 3Zn + 2Cr 3+ 3Zn +2 + 2Cr 4. Zn loses electrons, 2Cr 3+ gains electrons Zn + Cr 3+ Zn
Redox/Electrochemistry Regents Unit Review. ANSWERS 1. ½ red = Cr 3+ + 3e Cr 2. ½ ox = Zn Zn +2 + 2e 3. Balanced = 3Zn + 2Cr 3+ 3Zn +2 + 2Cr 4. Zn loses electrons, 2Cr 3+ gains electrons Zn + Cr 3+ Zn
Oxidation (oxidized): the loss of one or more electrons. Reduction (reduced): the gain of one or more electrons
 1 of 13 interesting links: Battery Chemistry Tutorial at http://www.powerstream.com/batteryfaq.html Duracell Procell: Battery Chemistry at http://www.duracell.com/procell/chemistries /default.asp I. Oxidation
1 of 13 interesting links: Battery Chemistry Tutorial at http://www.powerstream.com/batteryfaq.html Duracell Procell: Battery Chemistry at http://www.duracell.com/procell/chemistries /default.asp I. Oxidation
AP CHEMISTRY NOTES 12-1 ELECTROCHEMISTRY: ELECTROCHEMICAL CELLS
 AP CHEMISTRY NOTES 12-1 ELECTROCHEMISTRY: ELECTROCHEMICAL CELLS Review: OXIDATION-REDUCTION REACTIONS the changes that occur when electrons are transferred between reactants (also known as a redox reaction)
AP CHEMISTRY NOTES 12-1 ELECTROCHEMISTRY: ELECTROCHEMICAL CELLS Review: OXIDATION-REDUCTION REACTIONS the changes that occur when electrons are transferred between reactants (also known as a redox reaction)
Lecture Presentation. Chapter 18. Electrochemistry. Sherril Soman Grand Valley State University Pearson Education, Inc.
 Lecture Presentation Chapter 18 Electrochemistry Sherril Soman Grand Valley State University Harnessing the Power in Nature The goal of scientific research is to understand nature. Once we understand the
Lecture Presentation Chapter 18 Electrochemistry Sherril Soman Grand Valley State University Harnessing the Power in Nature The goal of scientific research is to understand nature. Once we understand the
CHAPTER 5 REVIEW. C. CO 2 D. Fe 2 O 3. A. Fe B. CO
 CHAPTER 5 REVIEW 1. The following represents the process used to produce iron from iron III oxide: Fe 2 O 3 + 3CO 2Fe + 3CO 2 What is the reducing agent in this process? A. Fe B. CO C. CO 2 D. Fe 2 O 3
CHAPTER 5 REVIEW 1. The following represents the process used to produce iron from iron III oxide: Fe 2 O 3 + 3CO 2Fe + 3CO 2 What is the reducing agent in this process? A. Fe B. CO C. CO 2 D. Fe 2 O 3
5.7 Galvanic Cells. Electrochemical Gizmos
 5.7 Galvanic Cells Have you ever accidentally bitten into a piece of aluminum foil? If you have silver amalgam fillings, you may have experienced a bit of a jolt (Figure 1). The aluminium, in contact with
5.7 Galvanic Cells Have you ever accidentally bitten into a piece of aluminum foil? If you have silver amalgam fillings, you may have experienced a bit of a jolt (Figure 1). The aluminium, in contact with
Electrochemistry. (Hebden Unit 5 ) Electrochemistry Hebden Unit 5
 (Hebden Unit 5 ) is the study of the interchange of chemical energy and electrical energy. 2 1 We will cover the following topics: Review oxidation states and assigning oxidation numbers Redox Half-reactions
(Hebden Unit 5 ) is the study of the interchange of chemical energy and electrical energy. 2 1 We will cover the following topics: Review oxidation states and assigning oxidation numbers Redox Half-reactions
12.05 Galvanic Cells. Zn(s) + 2 Ag + (aq) Zn 2+ (aq) + 2 Ag(s) Ni(s) + Pb 2+ (aq) «Ni 2+ (aq) + Pb(s)
 12.05 Galvanic Cells 1. In an operating voltaic cell, reduction occurs A) at the anode B) at the cathode C) in the salt bridge D) in the wire 2. Which process occurs in an operating voltaic cell? A) Electrical
12.05 Galvanic Cells 1. In an operating voltaic cell, reduction occurs A) at the anode B) at the cathode C) in the salt bridge D) in the wire 2. Which process occurs in an operating voltaic cell? A) Electrical
CHEM 112 Final Exam (New Material) Practice Test Solutions
 CHEM 112 Final Exam (New Material) Practice Test Solutions 1D Another electrolysis problem. This time we re solving for mass, which almost always means solving for number of moles and then converting to
CHEM 112 Final Exam (New Material) Practice Test Solutions 1D Another electrolysis problem. This time we re solving for mass, which almost always means solving for number of moles and then converting to
Zn+2 (aq) + Cu (s) Oxidation: An atom, ion, or molecule releases electrons and is oxidized. The oxidation number of the atom oxidized increases.
 Oxidation-Reduction Page 1 The transfer of an electron from one compound to another results in the oxidation of the electron donor and the reduction of the electron acceptor. Loss of electrons (oxidation)
Oxidation-Reduction Page 1 The transfer of an electron from one compound to another results in the oxidation of the electron donor and the reduction of the electron acceptor. Loss of electrons (oxidation)
 Electrochem 1 Electrochemistry Some Key Topics Conduction metallic electrolytic Electrolysis effect and stoichiometry Galvanic cell Electrolytic cell Electromotive Force (potential in volts) Electrode
Electrochem 1 Electrochemistry Some Key Topics Conduction metallic electrolytic Electrolysis effect and stoichiometry Galvanic cell Electrolytic cell Electromotive Force (potential in volts) Electrode
ELECTROCHEMISTRY OXIDATION-REDUCTION
 ELECTROCHEMISTRY Electrochemistry involves the relationship between electrical energy and chemical energy. OXIDATION-REDUCTION REACTIONS SPONTANEOUS REACTIONS Can extract electrical energy from these.
ELECTROCHEMISTRY Electrochemistry involves the relationship between electrical energy and chemical energy. OXIDATION-REDUCTION REACTIONS SPONTANEOUS REACTIONS Can extract electrical energy from these.
Lecture Presentation. Chapter 20. Electrochemistry. James F. Kirby Quinnipiac University Hamden, CT Pearson Education, Inc.
 Lecture Presentation Chapter 20 James F. Kirby Quinnipiac University Hamden, CT is the study of the relationships between electricity and chemical reactions. It includes the study of both spontaneous and
Lecture Presentation Chapter 20 James F. Kirby Quinnipiac University Hamden, CT is the study of the relationships between electricity and chemical reactions. It includes the study of both spontaneous and
Electrochemistry. Electrochemical Process. The Galvanic Cell or Voltaic Cell
 Electrochemistry Electrochemical Process The conversion of chemical energy into electrical energy and the conversion of electrical energy into chemical energy are electrochemical process. Recall that an
Electrochemistry Electrochemical Process The conversion of chemical energy into electrical energy and the conversion of electrical energy into chemical energy are electrochemical process. Recall that an
OXIDATION-REDUCTIONS REACTIONS. Chapter 19 (From next years new book)
 OXIDATION-REDUCTIONS REACTIONS Chapter 19 (From next years new book) ELECTROCHEMICAL REACTIONS: What are electrochemical reactions? Electrons are transferred from one species to another ACTIVATING PRIOR
OXIDATION-REDUCTIONS REACTIONS Chapter 19 (From next years new book) ELECTROCHEMICAL REACTIONS: What are electrochemical reactions? Electrons are transferred from one species to another ACTIVATING PRIOR
Review: Balancing Redox Reactions. Review: Balancing Redox Reactions
 Review: Balancing Redox Reactions Determine which species is oxidized and which species is reduced Oxidation corresponds to an increase in the oxidation number of an element Reduction corresponds to a
Review: Balancing Redox Reactions Determine which species is oxidized and which species is reduced Oxidation corresponds to an increase in the oxidation number of an element Reduction corresponds to a
Unit 12 Redox and Electrochemistry
 Unit 12 Redox and Electrochemistry Review of Terminology for Redox Reactions OXIDATION loss of electron(s) by a species; increase in oxidation number. REDUCTION gain of electron(s); decrease in oxidation
Unit 12 Redox and Electrochemistry Review of Terminology for Redox Reactions OXIDATION loss of electron(s) by a species; increase in oxidation number. REDUCTION gain of electron(s); decrease in oxidation
EXPERIMENT 29 VOLTAIC CELLS
 Introduction: EXPERIMENT 29 VOLTAIC CELLS When a strip of zinc is placed in a solution containing copper ions, a spontaneous reaction takes place. The zinc atoms lose electrons and become zinc ions. The
Introduction: EXPERIMENT 29 VOLTAIC CELLS When a strip of zinc is placed in a solution containing copper ions, a spontaneous reaction takes place. The zinc atoms lose electrons and become zinc ions. The
9.1 Introduction to Oxidation and Reduction
 9.1 Introduction to Oxidation and Reduction 9.1.1 - Define oxidation and reduction in terms of electron loss and gain Oxidation The loss of electrons from a substance. This may happen through the gain
9.1 Introduction to Oxidation and Reduction 9.1.1 - Define oxidation and reduction in terms of electron loss and gain Oxidation The loss of electrons from a substance. This may happen through the gain
Chapter 18 Electrochemistry
 Chapter 18 Electrochemistry Definition The study of the interchange of chemical and electrical energy in oxidation-reduction (redox) reactions This interchange can occur in both directions: 1. Conversion
Chapter 18 Electrochemistry Definition The study of the interchange of chemical and electrical energy in oxidation-reduction (redox) reactions This interchange can occur in both directions: 1. Conversion
Electrochemistry. To use principles of electrochemistry to understand the properties of electrochemical cells and electrolysis.
 Electrochemistry Objectives: To use principles of electrochemistry to understand the properties of electrochemical cells and electrolysis. Background: Part I: Galvanic Cells: A Galvanic cell is a device
Electrochemistry Objectives: To use principles of electrochemistry to understand the properties of electrochemical cells and electrolysis. Background: Part I: Galvanic Cells: A Galvanic cell is a device
 http://redoxanswers.weebly.com REDOX LESSON LEARNING GOALS http://redoxanswers.weebly.com Lesson 1: Introduction to Redox Relate to examples of oxidation-reduction reactions in the real-world. Understand
http://redoxanswers.weebly.com REDOX LESSON LEARNING GOALS http://redoxanswers.weebly.com Lesson 1: Introduction to Redox Relate to examples of oxidation-reduction reactions in the real-world. Understand
Electrochemistry C020. Electrochemistry is the study of the interconversion of electrical and chemical energy
 Electrochemistry C020 Electrochemistry is the study of the interconversion of electrical and chemical energy Using chemistry to generate electricity involves using a Voltaic Cell or Galvanic Cell (battery)
Electrochemistry C020 Electrochemistry is the study of the interconversion of electrical and chemical energy Using chemistry to generate electricity involves using a Voltaic Cell or Galvanic Cell (battery)
Chapter 20. Electrochemistry Recommendation: Review Sec. 4.4 (oxidation-reduction reactions) in your textbook
 Chapter 20. Electrochemistry Recommendation: Review Sec. 4.4 (oxidation-reduction reactions) in your textbook 20.1 Oxidation-Reduction Reactions Oxidation-reduction reactions = chemical reactions in which
Chapter 20. Electrochemistry Recommendation: Review Sec. 4.4 (oxidation-reduction reactions) in your textbook 20.1 Oxidation-Reduction Reactions Oxidation-reduction reactions = chemical reactions in which
AP Questions: Electrochemistry
 AP Questions: Electrochemistry I 2 + 2 S 2O 2-3 2 I - + S 4O 2-6 How many moles of I 2 was produced during the electrolysis? The hydrogen gas produced at the cathode during the electrolysis was collected
AP Questions: Electrochemistry I 2 + 2 S 2O 2-3 2 I - + S 4O 2-6 How many moles of I 2 was produced during the electrolysis? The hydrogen gas produced at the cathode during the electrolysis was collected
Redox Reactions. CH102, Lab 11 Goals : Safety : Waste :
 Redox Reactions CH102, Lab 11 Goals : Safety : Waste : To explore the relative oxidizing and reducing strength of metals. To measure electrochemical potentials and use the reduction potentials to rank
Redox Reactions CH102, Lab 11 Goals : Safety : Waste : To explore the relative oxidizing and reducing strength of metals. To measure electrochemical potentials and use the reduction potentials to rank
Redox and Electrochemistry
 Redox and Electrochemistry 1 Electrochemistry in Action! 2 Rules for Assigning Oxidation Numbers The oxidation number of any uncombined element is 0. The oxidation number of a monatomic ion equals the
Redox and Electrochemistry 1 Electrochemistry in Action! 2 Rules for Assigning Oxidation Numbers The oxidation number of any uncombined element is 0. The oxidation number of a monatomic ion equals the
Chapter 20. Electrochemistry
 Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 20 John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall,
Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 20 John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall,
Electrochem: It s Got Potential!
 Electrochem: It s Got Potential! Presented by: Denise DeMartino Westlake High School, Eanes ISD Pre-AP, AP, and Advanced Placement are registered trademarks of the College Board, which was not involved
Electrochem: It s Got Potential! Presented by: Denise DeMartino Westlake High School, Eanes ISD Pre-AP, AP, and Advanced Placement are registered trademarks of the College Board, which was not involved
Chapter 20. Electrochemistry
 Chapter 20. Electrochemistry 20.1 OxidationReduction Reactions Oxidationreduction reactions = chemical reactions in which the oxidation state of one or more substance changes (redox reactions). Recall:
Chapter 20. Electrochemistry 20.1 OxidationReduction Reactions Oxidationreduction reactions = chemical reactions in which the oxidation state of one or more substance changes (redox reactions). Recall:
Chapter 17. Electrochemistry
 Chapter 17 Electrochemistry Contents Galvanic cells Standard reduction potentials Cell potential, electrical work, and free energy Dependence of cell potential on concentration Batteries Corrosion Electrolysis
Chapter 17 Electrochemistry Contents Galvanic cells Standard reduction potentials Cell potential, electrical work, and free energy Dependence of cell potential on concentration Batteries Corrosion Electrolysis
