H H H OH OH H OH H O 1 CH 2 OH
|
|
|
- Barbra Campbell
- 5 years ago
- Views:
Transcription
1
2 Name 215 F07-Exam No. 3 Page 2 I. (29 points) Streptomycetes are soil-dwelling, filamentous, Gram-positive saprophytic bacteria. They are responsible for over 50% of the known microbial metabolites, including many antibiotics used in human and veterinary medicine. In an article published earlier this year, a group of scientists from Czech Republic reported the isolation of a number of extracellular carbohydrate metabolites from Streptomyces coelicolor A3(2) [J. Nat. Prod. 2007, 70, 768]. The structures of some of these carbohydrates are shown below. 2 C 2 C C 2 1 C C 2 Answer the following questions about the structures of these carbohydrates 1 3 and their hydrolysis products. (1) For each of these three carbohydrates, answer if it is a reducing sugar. Circle yes if it is a reducing sugar and, no if not a reducing sugar. For 1: For 2: For 3: yes; no 2 yes; no yes; no 2 2 (2) ow many of the sugar units of trisaccharide 1 are D sugars? Circle one that applies () Is the glycosidic bond in glycoside 2! or "? Circle one that applies.! " 3 (6) Draw in the box below a Fischer projection formula for the carbohydrate product, obtainable upon acid hydrolysis of glycoside 2, in an open-chain form. (3) Is the sugar unit of glycoside 2 D sugar? Circle one that applies. yes; no 3 (5) Is the stereochemistry of the anomeric in furanose 3! or "? Circle one that applies.! " 3 (7) Draw in the box below a Fischer projection formula for furanose 3 in an open-chain form. 6 5
3 Name 215 F07-Exam No. 3 Page 3 II. (17 points) Cyclopentanoid 5 shown below has been proposed to be biosynthesized from the C- oxidized D-glucose via the ene-diol intermediate under acid-catalyzed conditions (Tetrahedron Lett. 2002, 3, 51). Provide in the small box below a structure for the ene-diol and propose in the box below a step-by-step, curved-arrow reaction mechanism for this transformation from to 5. Use + B- and B: for the acid catalyst and its conjugate base, respectively. You do not need to explain the stereochemistry at the newly created chiral centers in 5. acid catalyst 5 B "ene-diol" intermediate 3 5 III. (8 points) Provide in the boxes below the structures of the reaction products. anomeric mixture 3 C 3 C 3 C C 3 C 3 C p-ts or BF 3 (C 2 C 3 ) 2 (catalytic) PhS 1. NaC 3 (catalytic) 3 C 2. Na (excess) PhC 2 Br!-anomer; C S (excess) TF + 3 CC(=)
4 Name 215 F07-Exam No. 3 Page IV (8 points) Treatment of keto-diol 6 with p-ts results in the highly diastereoselective formation of spiroketal 7 (Angew. Chem. Int. Ed. 2007, 6, 766). Draw the stereostructure of the major product 7 by completing the remaining structure in the box. 3 C 3 C p-ts (catalytic) benzene 3 C 3 C C 3 C 7 5 The term used to rationalize the fact that the above stereoisomer is the more stable diastereomer is: 3 V. (16 points) Methyl α-d-glucofuranoside can readily be converted into methyl α-d-glucopyranoside under acidic conditions. Draw in the box below a step-by-step, curved-arrow mechanism for this reaction. Use + B and B: for the acid catalyst and its conjugate base, respectively. C 2 B C 3 methyl!-d-glucofuranoside C 2 C 3 methyl!-d-glucopyranoside 16
5 Name 215 F07-Exam No. 3 Page 5 VI (1 points) Draw in the box below a step-by-step, curved-arrow mechanism for the reaction shown below including the aqueous acid workup step (Synthesis 2007, 3185). ( - C 2 C 3 ) and its conjugate acid, respectively. Use B - and B- for the base 3 CC 2 N C 2 C 3 1.NaC 2 C 3 (1 mol equiv) C 2 C workup (protonation) 3 CC 2 N + C 2 C 3 3 CC 2 B N C 2 C 3 3 CC 2 N 3 + workup 1 VII. (12 points) Complete the following reaction schemes by providing in the boxes the structures of the corresponding products for each of the following reaction schemes. (1) [Synthesis 2007, 3185] 3 CC 2 N 3 +! 1. LiAl (excess) TF 2. aqueous workup C 7 11 N 2+ C2 C 3 + C 2 C 7 15 N (2) [Tetrahedron Lett. 2007, 8, 761] Li N C Si(C 2 C 3 ) workup + TF C N + (C 3 )C 3
6 Name 215 F07-Exam No. 3 Page 6 VIII. (16 points) Complete the following reaction schemes by providing in the boxes the structures of the corresponding products. (1) 3 C C 3 C 3 1. NaC 3 (1 mol equiv) benzene 2. Cl/ 3 + (protonation) + C 3 3 +! + C 3 + C 2 (2) [see: rg. Lett. 2007, 9, 1879] lithium enolate Cu Li TF -78 C + Cu(C 2 C 2 C 2 C 3 ) + enantiomer BrC 2 Ph + LiBr + enantiomer
7
8
9
O H HO H. !-D-galactopyranose
 ame Key W06-Exam o. Page I. ( points) A disaccharide is cleaved by a β-glycosidase, an enzyme that specifically hydrolyzes a β- glycosidic linkage. When the disaccharide is treated with excess dimethyl
ame Key W06-Exam o. Page I. ( points) A disaccharide is cleaved by a β-glycosidase, an enzyme that specifically hydrolyzes a β- glycosidic linkage. When the disaccharide is treated with excess dimethyl
(4) (5) (6) a. b. H. explanation: explanation:
 ame Key 15 W11-Exam o. Page I. (15 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that is
ame Key 15 W11-Exam o. Page I. (15 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that is
H CH 2 -OH (4) H b. (5) H H. (6) a. b.
 ame Key 15 F010-Exam o. Page I. (15 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that
ame Key 15 F010-Exam o. Page I. (15 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that
Structures in equilibrium at point A: Structures in equilibrium at point B: (ii) Structure at the isoelectric point: O O
 ame 21 F10-Final Exam Page 2 I. (42 points) (1) (16 points) The titration curve for L-lysine is shown below. Provide (i) the main structures in equilibrium at each of points and B indicated below and (ii)
ame 21 F10-Final Exam Page 2 I. (42 points) (1) (16 points) The titration curve for L-lysine is shown below. Provide (i) the main structures in equilibrium at each of points and B indicated below and (ii)
(5) a. b. (6) N H CH 3 N H O H O (7) CH 3 O (8) OCH 3 2. explanation:
 ame Key 15 W1-Exam o. Page I. (16 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that is
ame Key 15 W1-Exam o. Page I. (16 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that is
H CH 2 -OH (4) H b. H H (5) (6) a. b.
 ame 215 F010-Exam o. 2 Page 2 I. (15 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that
ame 215 F010-Exam o. 2 Page 2 I. (15 points) For each of the following pairs of compounds, predict which compound is more acidic. Compare the two underlined s for each pair and circle the compound that
Structures in equilibrium at point A: Structures in equilibrium at point B: (ii) Structure at the isoelectric point:
 ame 21 F10-Final Exam Page 2 I. (42 points) (1) (16 points) The titration curve for L-lysine is shown below. Provide (i) the main structures in equilibrium at each of points A and B indicated below and
ame 21 F10-Final Exam Page 2 I. (42 points) (1) (16 points) The titration curve for L-lysine is shown below. Provide (i) the main structures in equilibrium at each of points A and B indicated below and
Structure at the isoelectric point: The predominant form of this tripeptide at ph 1 has a net charge of: (circle one)
 ame Key 21 F07-Final exam Page 2 I. (1 points) Using the pka information given on page 12, match the following isoelectric point (pi) values (3.08, 6.00, and 10.76) to their corresponding amino acids by
ame Key 21 F07-Final exam Page 2 I. (1 points) Using the pka information given on page 12, match the following isoelectric point (pi) values (3.08, 6.00, and 10.76) to their corresponding amino acids by
Importance of Carbohydrates
 Chapter 25 Importance of Carbohydrates Distributed widely in nature Key intermediates of metabolism (sugars) Structural components of plants (cellulose) Central to materials of industrial products: paper,
Chapter 25 Importance of Carbohydrates Distributed widely in nature Key intermediates of metabolism (sugars) Structural components of plants (cellulose) Central to materials of industrial products: paper,
C C H 2. O p-tsoh (catalytic) H 3 O +! O
 ame Key 15 W06-Exam o. Page I. ( points) Pheromones are those chemicals that play a role in communication between individual animals or insects. Structure 1 is the pheromone of the Japanese peach moth
ame Key 15 W06-Exam o. Page I. ( points) Pheromones are those chemicals that play a role in communication between individual animals or insects. Structure 1 is the pheromone of the Japanese peach moth
CHEM 203. Final Exam December 12, This a closed-notes, closed-book exam. You may use your set of molecular models. This test contains 11 pages
 CEM 203 Final Exam December 12, 2012 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test contains 11 pages Time: 2h 30 min 1. / 20 2. / 24 3. / 26 4. / 30
CEM 203 Final Exam December 12, 2012 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test contains 11 pages Time: 2h 30 min 1. / 20 2. / 24 3. / 26 4. / 30
Final Exam Version 1. Chemistry 140C. Fall Good Luck! Dec 5, :30 am 2:30 pm This exam accounts for 50% of the final grade.
 Chemistry 140C Final Exam Version 1 Fall 2006 Dec 5, 2006 11:30 am 2:30 pm This exam accounts for 50% of the final grade. Mark your final answer clearly. Completely erase irrelevant information! Exams
Chemistry 140C Final Exam Version 1 Fall 2006 Dec 5, 2006 11:30 am 2:30 pm This exam accounts for 50% of the final grade. Mark your final answer clearly. Completely erase irrelevant information! Exams
Chem 215 F11 Notes Dr. Masato Koreeda - Page 1 of 18. Date: November 9, 2011
 Chem F Notes Dr. Masato Koreeda - Page of 8. Date: November 9, 0 Chapters.8; -,,, and 7: Carbohydrates Part I Carbohydrate nomenclature: http://www.chem.qmul.ac.uk/iupac/carb/ Carbohydrates: Polyhydroxylated
Chem F Notes Dr. Masato Koreeda - Page of 8. Date: November 9, 0 Chapters.8; -,,, and 7: Carbohydrates Part I Carbohydrate nomenclature: http://www.chem.qmul.ac.uk/iupac/carb/ Carbohydrates: Polyhydroxylated
CHEM 203. Final Exam December 18, This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 18, 2013 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
CEM 203 Final Exam December 18, 2013 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
Midterm Exam 2. Chem 3B, Fall 2016 Thursday, Nov. 3, :00 9:00 pm. Name
 Midterm Exam 2 Chem 3B, Fall 2016 Thursday, Nov. 3, 2016 7:00 9:00 pm Name Student ID (Note: write the last 3 digits of your SID in the top right corner of each page) You have 120 minutes to complete this
Midterm Exam 2 Chem 3B, Fall 2016 Thursday, Nov. 3, 2016 7:00 9:00 pm Name Student ID (Note: write the last 3 digits of your SID in the top right corner of each page) You have 120 minutes to complete this
Chemistry 233 Exam 3. The Periodic Table
 Name: Last First MI Chemistry 233 Exam 3 Fall 2017 Dr. J. sbourn Instructions: The first 8 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
Name: Last First MI Chemistry 233 Exam 3 Fall 2017 Dr. J. sbourn Instructions: The first 8 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
R or S? oxidation #: hybridization:
 Remember Lone pair-assisted ionization. ame 15 F07-Exam o. 1 Page I. (0 points) The following compounds are those used in our study on the enzymatic transformation of cholesterol to pregnenolone. 1) Designate
Remember Lone pair-assisted ionization. ame 15 F07-Exam o. 1 Page I. (0 points) The following compounds are those used in our study on the enzymatic transformation of cholesterol to pregnenolone. 1) Designate
CEM 252, Fall 2018 Midterm Exam 3, Version A Wednesday, 14 November, 2018, 6:00 PM Room 138, Chemistry Name (print) Signature Student # Key
 CEM 252, Fall 2018 Midterm Exam 3, Version A Wednesday, 14 November, 2018, 6:00 PM Room 138, Chemistry Name (print) Signature Student # Key Version Section Number (-2 pts if you don t know this) A Feed
CEM 252, Fall 2018 Midterm Exam 3, Version A Wednesday, 14 November, 2018, 6:00 PM Room 138, Chemistry Name (print) Signature Student # Key Version Section Number (-2 pts if you don t know this) A Feed
R or S? 2) oxidation numbers of the designated carbon atoms: note: An oxidation number must have a sign. F 3 C OCH 3 oxidation #: OH.
 ame Key 5 0-Exam o. Page. ( points) The following compounds are those used in our study on the mechanism of the chemical carcinogenesis of benzo[a]pyrene. ) esignate in each of the boxes below the stereochemistry
ame Key 5 0-Exam o. Page. ( points) The following compounds are those used in our study on the mechanism of the chemical carcinogenesis of benzo[a]pyrene. ) esignate in each of the boxes below the stereochemistry
CEM 252, Fall 2018 Midterm Exam 3, Version B Wednesday, 14 November, 2018, 6:00 PM Room 138, Chemistry
 CEM 252, Fall 2018 Midterm Exam 3, Version B Wednesday, 14 November, 2018, 6:00 PM Room 138, Chemistry Name (print) KEY VERSIN B Signature Student # Section Number (-2 pts if you don t know this) Feed
CEM 252, Fall 2018 Midterm Exam 3, Version B Wednesday, 14 November, 2018, 6:00 PM Room 138, Chemistry Name (print) KEY VERSIN B Signature Student # Section Number (-2 pts if you don t know this) Feed
Final Exam. Chem 3B, Fall 2016 Monday, Dec 12, pm. Name Answer Key. Student ID. If you are making up an incomplete, list the semester here:
 Final Exam Chem 3B, Fall 2016 Monday, Dec 12, 2016 3-6 pm ame Answer Key Student ID If you are making up an incomplete, list the semester here: You have 180 minutes to complete this exam. Please provide
Final Exam Chem 3B, Fall 2016 Monday, Dec 12, 2016 3-6 pm ame Answer Key Student ID If you are making up an incomplete, list the semester here: You have 180 minutes to complete this exam. Please provide
Chemistry 233 Exam 3 (Green) The Periodic Table
 Name: Last First MI Chemistry Exam (Green) Summer 7 Dr. J. sbourn Instructions: The first questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
Name: Last First MI Chemistry Exam (Green) Summer 7 Dr. J. sbourn Instructions: The first questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
11/14/2016. NaBH 4. Superimposable on mirror image. (achiral) D-ribose. NaBH 4 NaBH 4. Not superimposable on mirror image.
 There are multiple ways to relate the structures of ketoses to aldoses. Reduction of a ketose with NaB 4 forms a new stereocenter and thus forms a mixture of two alditols. An aldopentose forms a chiral
There are multiple ways to relate the structures of ketoses to aldoses. Reduction of a ketose with NaB 4 forms a new stereocenter and thus forms a mixture of two alditols. An aldopentose forms a chiral
(R)-(+)-thalidomide. optical rotation = -63 2% H 2 SO 4 /H 2 O room temp. (S)-(-)-thalidomide
 I. (22 points) (a) ame Page 1 F.0.21e3p1 In the mid/late 190s, mainly in Europe, a racemic mixture of thalidomide (see below) was prescribed to alleviate "morning sickness" in first trimester pregnant
I. (22 points) (a) ame Page 1 F.0.21e3p1 In the mid/late 190s, mainly in Europe, a racemic mixture of thalidomide (see below) was prescribed to alleviate "morning sickness" in first trimester pregnant
II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction
 P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
P. Wipf - Chem 2320 1 3/20/2006 II. Special Topics IIA. Enolate Chemistry & the Aldol Reaction Boger Notes: p. 147-206 (Chapter VIII) Carey/Sundberg: B p. 57-95 (Chapter B 2.1) Problem of the Day: Wang,
California State Polytechnic University, Pomona. Exam Points 1. Nomenclature (1) 25
 hem 316 Final Spring, 2004 Beauchamp ame: Topic Total Points Exam Points 1. omenclature (1) 25 redit Tautomers (acidic or base conditions) 20 3. Reactions Page (10 x 3 = 30) 30 4. Aromatic Mechanism and
hem 316 Final Spring, 2004 Beauchamp ame: Topic Total Points Exam Points 1. omenclature (1) 25 redit Tautomers (acidic or base conditions) 20 3. Reactions Page (10 x 3 = 30) 30 4. Aromatic Mechanism and
Loudon Chapter 24 Review: Carbohydrates CHEM 3331, Jacquie Richardson, Fall Page 1
 CEM 3331, Jacquie Richardson, Fall 2010 - Page 1 This chapter is about carbohydrates molecules with the general formula of C n ( 2 ) n, or in other words C n 2n n. This is a very common formula for sugars
CEM 3331, Jacquie Richardson, Fall 2010 - Page 1 This chapter is about carbohydrates molecules with the general formula of C n ( 2 ) n, or in other words C n 2n n. This is a very common formula for sugars
216 S10-Exam #1 Page 2. Name
 216 S10-Exam #1 Page 2. Name I. (3 points) Arrange the following four compounds in order of their R f values when analyzed by thinlayer chromatography (TLC) on silica gel-coated plates using C 2 Cl 2 as
216 S10-Exam #1 Page 2. Name I. (3 points) Arrange the following four compounds in order of their R f values when analyzed by thinlayer chromatography (TLC) on silica gel-coated plates using C 2 Cl 2 as
CHEM 203. Final Exam December 16, This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 16, 2014 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
CEM 203 Final Exam December 16, 2014 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
ORGANIC - BROWN 8E CH CARBOHYDRATES.
 !! www.clutchprep.com CONCEPT: INTRODUCTION TO CARBOHYDRATE MONOSACCHARIDES Sugars or saccharides are also referred to as carbo-hydrates, implying that carbon has been combined with Monosaccharides are
!! www.clutchprep.com CONCEPT: INTRODUCTION TO CARBOHYDRATE MONOSACCHARIDES Sugars or saccharides are also referred to as carbo-hydrates, implying that carbon has been combined with Monosaccharides are
Chem 332, Exam 4. Spring Provide reagents for the following transformations (2 pts each) NaOH , KOH. 4) H2/Pd NO 2. 1) AlCl 3 O 2) H 2 NNH 2
 AME 1. Provide reagents for the following transformations (2 pts each) 2 a 2 1) Al 3 2) 2 2, K h! 1) 3, 2) LDA 3) + 4) 2/Pd C 2 AME 2a Circle the correct product (no mechanisms or partial credit). 3 pts
AME 1. Provide reagents for the following transformations (2 pts each) 2 a 2 1) Al 3 2) 2 2, K h! 1) 3, 2) LDA 3) + 4) 2/Pd C 2 AME 2a Circle the correct product (no mechanisms or partial credit). 3 pts
CHEM 203. Final Exam December 18, 2013 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Your name: Final Exam December 18, 2013 ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3.
CEM 203 Your name: Final Exam December 18, 2013 ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3.
CHAPTER 23 HW: ENOLS + ENOLATES
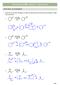 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CHEM 203. Final Exam December 15, 2010 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
Chem 232. Problem Set 9. Question 1. D. J. Wardrop
 Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 9 Question 1. a. Rank in order of increasing number of chirality centers (1 = fewest chirality centers; 5 = most chirality). 3 C C 3 b. Rank in order
Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 9 Question 1. a. Rank in order of increasing number of chirality centers (1 = fewest chirality centers; 5 = most chirality). 3 C C 3 b. Rank in order
R or S? 2) oxidation numbers of the designated carbon atoms: note: An oxidation number must have a sign. F 3 C OCH 3 oxidation #: OH.
 ame 5 F0-Exam o. Page I. ( points) The following compounds are those used in our study on the mechanism of the chemical carcinogenesis of benzo[a]pyrene. ) Designate in each of the boxes below the stereochemistry
ame 5 F0-Exam o. Page I. ( points) The following compounds are those used in our study on the mechanism of the chemical carcinogenesis of benzo[a]pyrene. ) Designate in each of the boxes below the stereochemistry
CHEMISTRY Section A3. Final Exam - December 16, Dr. John C. Vederas. 200 Points - 3 Hours II 32 TURN IN THIS BOOKLET WITH ANSWER SHEET
 CEMISTRY 261 - Section A3 Final Exam - December 16, 2014 - Dr. John C. Vederas 200 Points - 3 ours Part Points PRINT LAST NAME: I 75 II 32 TURN IN TIS BKLET WIT ANSWER SEET III 70 IV 23 PUT ALL ANSWERS
CEMISTRY 261 - Section A3 Final Exam - December 16, 2014 - Dr. John C. Vederas 200 Points - 3 ours Part Points PRINT LAST NAME: I 75 II 32 TURN IN TIS BKLET WIT ANSWER SEET III 70 IV 23 PUT ALL ANSWERS
ORGANIC - BRUICE 8E CH THE ORGANIC CHEMISTRY OF CARBOHYDRATES
 !! www.clutchprep.com CONCEPT: INTRODUCTION TO CARBOHYDRATE MONOSACCHARIDES Sugars or saccharides are also referred to as carbo-hydrates, implying that carbon has been combined with Monosaccharides are
!! www.clutchprep.com CONCEPT: INTRODUCTION TO CARBOHYDRATE MONOSACCHARIDES Sugars or saccharides are also referred to as carbo-hydrates, implying that carbon has been combined with Monosaccharides are
Midterm Exam #1 /310 CHEM 6352 Fall 2012
 Midterm Exam #1 /310 CEM 6352 Fall 2012 ( %) Name ct 5 th, 2012 18:30-21:00 You may NT use any references or aids to complete the following with the exception of a chemical model set and the scrap paper
Midterm Exam #1 /310 CEM 6352 Fall 2012 ( %) Name ct 5 th, 2012 18:30-21:00 You may NT use any references or aids to complete the following with the exception of a chemical model set and the scrap paper
Midterm #2 Chem 3A - Fall 2013 Nov. 12, :00 8:45 pm. Name SID
 Midterm #2 Chem 3A - Fall 2013 Nov. 12, 2013 7:00 8:45 pm Name SID Including the title page, there should be 6 total questions spread over 8 pages (printed on both sides). Please provide all answers in
Midterm #2 Chem 3A - Fall 2013 Nov. 12, 2013 7:00 8:45 pm Name SID Including the title page, there should be 6 total questions spread over 8 pages (printed on both sides). Please provide all answers in
Suggested solutions for Chapter 41
 s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
CHAPTER 24 HW: CARBONYL CONDENSATIONS
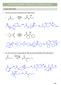 CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
Initials: 1. Chem 633: Advanced Organic Chemistry 2011 Final Exam
 Initials: 1 ame: Chem 633: Advanced rganic Chemistry 2011 Final Exam Please answer the following questions clearly and concisely. In general, use pictures and less than 10 words in your answers. Write
Initials: 1 ame: Chem 633: Advanced rganic Chemistry 2011 Final Exam Please answer the following questions clearly and concisely. In general, use pictures and less than 10 words in your answers. Write
CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTION AND/OR GSI IF YOU ARE IN THE LABORATORY COURSE:
 CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN THE LABRATRY CURSE: You will have 75 minutes in which to work. BE NEAT! Non-legible structure
CHEMISTRY 112A FALL 2015 EXAM 1 SEPTEMBER 27, 2016 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN THE LABRATRY CURSE: You will have 75 minutes in which to work. BE NEAT! Non-legible structure
Final Exam Professor R. Hoenigman
 I pledge to uphold the CU onor Code: CEM 3311-200 Fall 2006 Final Exam Professor. oenigman Average Score = 145 igh Score = 235 Low Score = 27 Signature Name (printed) Last four digits of your student ID
I pledge to uphold the CU onor Code: CEM 3311-200 Fall 2006 Final Exam Professor. oenigman Average Score = 145 igh Score = 235 Low Score = 27 Signature Name (printed) Last four digits of your student ID
CHEM 330. Final Exam December 11, This a closed-notes, closed-book exam. The use of molecular models is allowed. This exam contains 12 pages
 CEM 330 Final Exam December 11, 2007 Your name: This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4. / 40
CEM 330 Final Exam December 11, 2007 Your name: This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4. / 40
Organic Chemistry I (Chem340), Spring Final Exam
 rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
ORGANIC - CLUTCH CH CARBOHYDRATES.
 !! www.clutchprep.com CONCEPT: INTRODUCTION TO CARBOHYDRATE MONOSACCHARIDES Sugars or saccharides are also referred to as carbo-hydrates, implying that carbon has been combined with Monosaccharides are
!! www.clutchprep.com CONCEPT: INTRODUCTION TO CARBOHYDRATE MONOSACCHARIDES Sugars or saccharides are also referred to as carbo-hydrates, implying that carbon has been combined with Monosaccharides are
Chemistry 118 C Spring 2011 Second Midterm Fri. May 20 th, 2011 Instructor: Lievens. Name: Last First MI. Student ID. # T.A.
 Chemistry 118 C pring 2011 econd Midterm Fri. May 20 th, 2011 Instructor: Lievens This exam contains seven (7) pages and eight (8) problems. Please make sure that your copy contains all seven pages. If
Chemistry 118 C pring 2011 econd Midterm Fri. May 20 th, 2011 Instructor: Lievens This exam contains seven (7) pages and eight (8) problems. Please make sure that your copy contains all seven pages. If
As time allows, additional practice questions for Exam 2 follow. This is an incomplete collection. Content & emphasis will vary.
 Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
Massachusetts Institute of Technology. Chemistry 5.43 February 28 th, Exam #1. Question 1a /4 points Question 2b /10 points
 Massachusetts Institute of Technology Chemistry 5.43 February 28 th, 2007 Professor M. Movassaghi Exam #1 Question 1a /4 points Question 2b /10 points Question 1b /2 points Question 2c /6 points Question
Massachusetts Institute of Technology Chemistry 5.43 February 28 th, 2007 Professor M. Movassaghi Exam #1 Question 1a /4 points Question 2b /10 points Question 1b /2 points Question 2c /6 points Question
Name: CHEM 633/634 Problem Set 1: Review Due Tues, Aug 29, 2017 (First Lecture!)
 ame: CEM 633/634 Problem Set 1: Review Due Tues, Aug 29, 2017 (First Lecture!) Please print this problem set. Answers must be in the spaces or boxes provided to receive full credit. You may work in groups,
ame: CEM 633/634 Problem Set 1: Review Due Tues, Aug 29, 2017 (First Lecture!) Please print this problem set. Answers must be in the spaces or boxes provided to receive full credit. You may work in groups,
Chem 112A: Final Exam
 Chem 112A: Final Exam December 15th, 2010 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Chem 112A: Final Exam December 15th, 2010 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
a) Write the mechanism of Friedel-Crafts alkylation of ethyl benzene to give 1,4- diethylbenzene. Show all arrow pushing.
 a) Write the mechanism of Friedel-Crafts alkylation of ethyl benzene to give 1,4- diethylbenzene. Show all arrow pushing. Br AlBr 3 b) Using resonance and inductive effects, explain why an ethyl group
a) Write the mechanism of Friedel-Crafts alkylation of ethyl benzene to give 1,4- diethylbenzene. Show all arrow pushing. Br AlBr 3 b) Using resonance and inductive effects, explain why an ethyl group
Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections
 Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections Fischer Projections are 2-dimensional representations of 3-dimensional
Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections Fischer Projections are 2-dimensional representations of 3-dimensional
Synthetic Organic Chemistry Final Exam Resit (6KM33) Thursday, 26th June, 2014, 9:00 12:00 (3 h)
 Synthetic rganic Chemistry Final Exam Resit (6KM33) Thursday, 26th June, 2014, 9:00 12:00 (3 h) This is an open-book written exam. The course textbooks (Warren & Wyatt, 2 nd Ed.) with personal notes and
Synthetic rganic Chemistry Final Exam Resit (6KM33) Thursday, 26th June, 2014, 9:00 12:00 (3 h) This is an open-book written exam. The course textbooks (Warren & Wyatt, 2 nd Ed.) with personal notes and
(CHE 325) Organic Chemistry II Spring 2011 EXAM #4
 (CE 325) rganic Chemistry II Spring 2011 EXAM #4 ame: KEY ID#: Check your exam to be sure it is complete. There are eight questions in this exam. It is worth 100 points. To receive full credit for your
(CE 325) rganic Chemistry II Spring 2011 EXAM #4 ame: KEY ID#: Check your exam to be sure it is complete. There are eight questions in this exam. It is worth 100 points. To receive full credit for your
ORGANIC - EGE 5E EXTRA: CARBOHYDRATES.
 !! www.clutchprep.com CONCEPT: INTRODUCTION TO CARBOHYDRATE MONOSACCHARIDES Sugars or saccharides are also referred to as carbo-hydrates, implying that carbon has been combined with Monosaccharides are
!! www.clutchprep.com CONCEPT: INTRODUCTION TO CARBOHYDRATE MONOSACCHARIDES Sugars or saccharides are also referred to as carbo-hydrates, implying that carbon has been combined with Monosaccharides are
(2) After dissolving a solid in a solvent at high temperature, the solution is not filtered.
 Name Key 216 W13-Exam No. 1 Page 2 I. (10 points) The goal of recrystallization is to obtain purified material with a maximized recovery. For each of the following cases, indicate as to which of the two
Name Key 216 W13-Exam No. 1 Page 2 I. (10 points) The goal of recrystallization is to obtain purified material with a maximized recovery. For each of the following cases, indicate as to which of the two
Lecture Notes Chem 51B S. King I. Conjugation
 Lecture Notes Chem 51B S. King Chapter 16 Conjugation, Resonance, and Dienes I. Conjugation Conjugation occurs whenever p-orbitals can overlap on three or more adjacent atoms. Conjugated systems are more
Lecture Notes Chem 51B S. King Chapter 16 Conjugation, Resonance, and Dienes I. Conjugation Conjugation occurs whenever p-orbitals can overlap on three or more adjacent atoms. Conjugated systems are more
Exam 4. Name CHEM 210. PBr 3. HBr. 1) NaH 2) Br H 2 SO 4. 1) NaOCH 3 2) dil. H 3 O + O CH 3 OH, H 2 SO 4. 1) LDA, -78 o C.
 Exam 4 Name CEM 210 1. (48 pts) Complete the equations for the following reactions. Show all organic products if two or more products form, indicate the major product. Indicate proper stereochemistry where
Exam 4 Name CEM 210 1. (48 pts) Complete the equations for the following reactions. Show all organic products if two or more products form, indicate the major product. Indicate proper stereochemistry where
Faculty of Science Mid-Term Examination II. Chem 222/234 Introductory Organic Chemistry I
 Student Name: Student ID #: Faculty of Science Mid-Term Examination II Chem 222/234 Introductory rganic Chemistry I Examiner: Professor James L. Gleason Tuesday, November 10, 2009 Associate Examiner: Professor
Student Name: Student ID #: Faculty of Science Mid-Term Examination II Chem 222/234 Introductory rganic Chemistry I Examiner: Professor James L. Gleason Tuesday, November 10, 2009 Associate Examiner: Professor
Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron sheet.
 Test 3 Chemistry 2321 April 25, 2003 McMurry, Chapters 9-11 103 Total Points Name Please Print Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron
Test 3 Chemistry 2321 April 25, 2003 McMurry, Chapters 9-11 103 Total Points Name Please Print Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron
Practice Problems December 4, 2000
 Practice Problems December, 000 ) Propose sequences of reactions that could accomplish each of the following transformations. Starting from N a) C Starting from Starting from c) ) Propose detailed, stepwise
Practice Problems December, 000 ) Propose sequences of reactions that could accomplish each of the following transformations. Starting from N a) C Starting from Starting from c) ) Propose detailed, stepwise
Homework - Review of Chem 2310
 omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
Chapter 24 Carbohydrates
 hapter arbohydrates Review of oncepts Fill in the blanks below. To verify that your answers are correct, look in your textbook at the end of hapter. Each of the sentences below appears verbatim in the
hapter arbohydrates Review of oncepts Fill in the blanks below. To verify that your answers are correct, look in your textbook at the end of hapter. Each of the sentences below appears verbatim in the
Total synthesis of Spongistatin
 Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
CHEMISTRY 332 FINAL EXAM. December 14, I. (18 points) II. (48 points) III. (50 points) IV. (40 points) V. (26 points) VI.
 Seat No. Name (please print and circle your last name) CEMISTRY 332 FINAL EXAM December 14, 2005 I. (18 points) II. (48 points) III. (50 points) IV. (40 points) V. (26 points) VI. (18 points) TTAL (200
Seat No. Name (please print and circle your last name) CEMISTRY 332 FINAL EXAM December 14, 2005 I. (18 points) II. (48 points) III. (50 points) IV. (40 points) V. (26 points) VI. (18 points) TTAL (200
CHE 232 Organic Chemistry II Exam 4 Name: KEY
 CE 232 rganic Chemistry II Exam 4 ame: KEY Student number: Before you begin this exam: First: You are allowed to have a simple model set at your seat. Please put away all other materials. Second: Place
CE 232 rganic Chemistry II Exam 4 ame: KEY Student number: Before you begin this exam: First: You are allowed to have a simple model set at your seat. Please put away all other materials. Second: Place
Final Exam /415 ( CHEM 6352 Fall %) Name
 Final Exam /415 ( CEM 6352 Fall 2011 %) Name Dec. 15 th, 2011 8:00-12:00 You may NT use any references or aids to complete the following with the exception of a chemical model set and the scrap paper that
Final Exam /415 ( CEM 6352 Fall 2011 %) Name Dec. 15 th, 2011 8:00-12:00 You may NT use any references or aids to complete the following with the exception of a chemical model set and the scrap paper that
Electrophilic Carbenes
 Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
HO C. Explain briefly (in one or two short sentences) the meaning of the following basic stereochemical terms.
 Chem 232 D. J. Wardrop wardropd@uic.edu Problem et 3 Answers Question 1. Four compounds, each having the molecular formula C 3 5, have the I spectra summarized below. What are their structures? a. ne sharp
Chem 232 D. J. Wardrop wardropd@uic.edu Problem et 3 Answers Question 1. Four compounds, each having the molecular formula C 3 5, have the I spectra summarized below. What are their structures? a. ne sharp
Chem 263 Nov 19, Cl 2
 Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
CHE 322 Summer 2012 Final Exam Form Choose the lowest unoccupied molecular orbital (LUMO) of the pentadienyl cation.
 Multiple Choice Questions: 100 points 1. Choose the lowest unoccupied molecular orbital (LUMO) of the pentadienyl cation. 2. Select the possible reactants of the following reaction. A i B ii C iii D i
Multiple Choice Questions: 100 points 1. Choose the lowest unoccupied molecular orbital (LUMO) of the pentadienyl cation. 2. Select the possible reactants of the following reaction. A i B ii C iii D i
Chemistry 39. Final Exam
 Chemistry 39 Final Exam Tuesday, May 7, 2005 ame (printed) 11:10 am S.S. # Signature Please show your picture I.D. to a proctor when you hand in your exam. TTAL 1 There are 25 questions on this exam. Check
Chemistry 39 Final Exam Tuesday, May 7, 2005 ame (printed) 11:10 am S.S. # Signature Please show your picture I.D. to a proctor when you hand in your exam. TTAL 1 There are 25 questions on this exam. Check
1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. KH (1 equiv.) + KCl THF. + HBr.
 1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. K (1 equiv.) TF K 3 2 2 3 enantiomer While writing the mechanism, justify both the regiochemistry the relative
1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. K (1 equiv.) TF K 3 2 2 3 enantiomer While writing the mechanism, justify both the regiochemistry the relative
Organocatalytic Umpolung via N- Heterocyclic Carbenes. Qinghe Liu Hu Group Meeting August 20 th 2015
 rganocatalytic Umpolung via N- Heterocyclic Carbenes Qinghe Liu Hu Group Meeting August 20 th 2015 Contents Part 1: Introduction Part 2: N-Heterocyclic carbene-catalyzed umpolung: classical umpolung, conjugated
rganocatalytic Umpolung via N- Heterocyclic Carbenes Qinghe Liu Hu Group Meeting August 20 th 2015 Contents Part 1: Introduction Part 2: N-Heterocyclic carbene-catalyzed umpolung: classical umpolung, conjugated
Chem 312 SS05 Page 1 of 6 Kantorowski. 17 August 2005 Name:
 Chem 32 SS05 Page of 6 Kantorowski Exam III Key 7 August 2005 Name: This exam contains 5 pages of questions confirm this once you begin. You will have 50 minutes An abbreviated Periodic Table can be found
Chem 32 SS05 Page of 6 Kantorowski Exam III Key 7 August 2005 Name: This exam contains 5 pages of questions confirm this once you begin. You will have 50 minutes An abbreviated Periodic Table can be found
CHEMISTRY MIDTERM # 2 November 02, The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck!
 CEMISTRY 314-01 MIDTERM # 2 November 02, 2009 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (8 pts) Mark as true (T) or false (F) the following
CEMISTRY 314-01 MIDTERM # 2 November 02, 2009 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (8 pts) Mark as true (T) or false (F) the following
Problems Points Credit
 hem 201 Final Fall, 2012 Beauchamp Name Problems Points redit 1. Functional Group Nomenclature (1 large structure) (R/S and E/Z too) 30 2. Types of Isomers, Degrees of Unsaturation 25 3. yclohexane onformations,
hem 201 Final Fall, 2012 Beauchamp Name Problems Points redit 1. Functional Group Nomenclature (1 large structure) (R/S and E/Z too) 30 2. Types of Isomers, Degrees of Unsaturation 25 3. yclohexane onformations,
Spring 2017 Organisk kemi I Eszter Borbas. Homework /05/03
 Homework 5 2017/05/03 1) A PhD student carries out the following reaction: a) Identify the nucleophile and the electrophile! See scheme b) Draw the mechanism of the reaction! c) What type of reaction is
Homework 5 2017/05/03 1) A PhD student carries out the following reaction: a) Identify the nucleophile and the electrophile! See scheme b) Draw the mechanism of the reaction! c) What type of reaction is
CHEMISTRY 112A FALL 2014 FINAL EXAM DECEMBER 17, 2014 NAME- WRITE BIG STUDENT ID: SECTION AND/OR GSI IF YOU ARE IN THE LABORATORY COURSE:
 CEMISTRY 112A FALL 2014 FINAL EXAM DECEMBER 17, 2014 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN TE LABRATRY CURSE: You will have 2 hours 50 minutes in which to work. BE NEAT! Non-legible
CEMISTRY 112A FALL 2014 FINAL EXAM DECEMBER 17, 2014 NAME- WRITE BIG STUDENT ID: SECTIN AND/R GSI IF YU ARE IN TE LABRATRY CURSE: You will have 2 hours 50 minutes in which to work. BE NEAT! Non-legible
Chem 112A: Final Exam
 Chem 112A: Final Exam December 14th, 2011 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Chem 112A: Final Exam December 14th, 2011 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Midterm Exam 1. Chem 3B, Spring 2016 Monday, February 29, :00 9:00 pm. Name. Student ID
 Midterm Exam 1 Chem 3B, Spring 2016 Monday, February 29, 2016 7:00 9:00 pm Name Student ID You have 120 minutes to complete this exam. Please provide all answers in the space provided. Work drawn in the
Midterm Exam 1 Chem 3B, Spring 2016 Monday, February 29, 2016 7:00 9:00 pm Name Student ID You have 120 minutes to complete this exam. Please provide all answers in the space provided. Work drawn in the
HO HO. 1) BH 3 HCl. + enantiomer either one may be drawn 4. + enantiomer either one may be drawn 4 1) O 3
 . (0 points) Page 1 A. Reaction analysis: provide the requested information in each of the following chemical transformations. how all missing starting materials and products unless otherwise indicated,
. (0 points) Page 1 A. Reaction analysis: provide the requested information in each of the following chemical transformations. how all missing starting materials and products unless otherwise indicated,
Dr. Steven Pedersen April 11, Chemistry 3B. Midterm 2. Student name: Answer Key Student signature: Lab section number (if you are in 3BL):
 Dr. Steven Pedersen April 11, 2011 Chemistry 3B Midterm 2 Student name: Answer Key Student signature: Lab section number (if you are in 3BL): Problem 1 (21 pts) Problem 2(a-c) (21 pts) Problem 2(d-g) (18
Dr. Steven Pedersen April 11, 2011 Chemistry 3B Midterm 2 Student name: Answer Key Student signature: Lab section number (if you are in 3BL): Problem 1 (21 pts) Problem 2(a-c) (21 pts) Problem 2(d-g) (18
Chemistry 234 Exam 3. The Periodic Table
 ame: Last First MI Chemistry 234 Exam 3 Fall 2017 Dr. J. sbourn Instructions: The first 24 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
ame: Last First MI Chemistry 234 Exam 3 Fall 2017 Dr. J. sbourn Instructions: The first 24 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron
EXAM #3 EXTRA PROBLEMS
 Massachusetts Institute of Technology 5.13, Fall 2006 Dr. Kimberly L. Berkowski rganic chemistry II EXAM #3 EXTRA PRBLEMS What to expect on Exam #3: 1. ~1 Labeling experiment 2. ~2 chanisms 3. ~2 Syntheses
Massachusetts Institute of Technology 5.13, Fall 2006 Dr. Kimberly L. Berkowski rganic chemistry II EXAM #3 EXTRA PRBLEMS What to expect on Exam #3: 1. ~1 Labeling experiment 2. ~2 chanisms 3. ~2 Syntheses
CHAPTER 20 HW: CARBONYL REDOX RXNS
 APTER 20 W: ARBNYL REDX RXNS YDRIDE REATIN MEANISM 1. Give the curved arrow mechanism for each reaction. LiAl 4 NaB 4 3 2 YDRIDE REATINS 2. Give the major organic product(s) for each reaction. Indicate
APTER 20 W: ARBNYL REDX RXNS YDRIDE REATIN MEANISM 1. Give the curved arrow mechanism for each reaction. LiAl 4 NaB 4 3 2 YDRIDE REATINS 2. Give the major organic product(s) for each reaction. Indicate
UCSC, Binder (First and Last) CHEM 8A, Organic Chemistry I Summer 18 FINAL EXAM (400 points) 1 (60)
 UCSC, Binder Name (First and Last) CEM 8A, rganic Chemistry I Summer 18 FINAL EXAM (400 points) 1 (60) 2 (65) 3 (60) 4 (70) 5 (75) 6 (70) Total (400) % In each of the following problems, use your knowledge
UCSC, Binder Name (First and Last) CEM 8A, rganic Chemistry I Summer 18 FINAL EXAM (400 points) 1 (60) 2 (65) 3 (60) 4 (70) 5 (75) 6 (70) Total (400) % In each of the following problems, use your knowledge
CEM 852 Final Exam. May 5, 2011
 CEM 852 Final Exam May 5, 2011 This exam consists of 8 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 150. In answering your questions,
CEM 852 Final Exam May 5, 2011 This exam consists of 8 pages. Please make certain that your exam has all of the necessary pages. Total points possible for this exam are 150. In answering your questions,
Lecture 15. More Carbonyl Chemistry. Alcohols React with Aldehydes and Ketones in two steps first O R'OH, H + OR" 2R"OH R + H 2 O OR" 3/8/16
 Lecture 15 More Carbonyl Chemistry R" R C + R' 2R" R C R" R' + 2 March 8, 2016 Alcohols React with Aldehydes and Ketones in two steps first R', + R R 1 emiacetal reacts further in acid to yield an acetal
Lecture 15 More Carbonyl Chemistry R" R C + R' 2R" R C R" R' + 2 March 8, 2016 Alcohols React with Aldehydes and Ketones in two steps first R', + R R 1 emiacetal reacts further in acid to yield an acetal
7 Steroids and sugars
 7 Steroids and sugars Purpose In this chapter you widen your scope on stereochemistry from that of relatively simple compounds to that of more complex and naturally occurring compounds. ne stereochemically
7 Steroids and sugars Purpose In this chapter you widen your scope on stereochemistry from that of relatively simple compounds to that of more complex and naturally occurring compounds. ne stereochemically
1. methyl 2. methylene 3. methine 4. primary 5. secondary 6. tertiary 7. quarternary 8. isopropyl
 hem 201 Sample Midterm Beauchamp Exams are designed so that no one question will make or break you. The best strategy is to work steadily, starting with those problems you understand best. Make sure you
hem 201 Sample Midterm Beauchamp Exams are designed so that no one question will make or break you. The best strategy is to work steadily, starting with those problems you understand best. Make sure you
Loudon Chapter 24 Review: Carbohydrates Jacquie Richardson, CU Boulder Last updated 4/26/2018
 This chapter is about carbohydrates molecules with the general formula of C n( 2O) n, or in other words C n 2nO n. This is a very common formula for sugars and many other natural products. The structure
This chapter is about carbohydrates molecules with the general formula of C n( 2O) n, or in other words C n 2nO n. This is a very common formula for sugars and many other natural products. The structure
First 2-Hour Exam. By printing your name below, you pledge that
 Chem 3331 Fall 2004 Exam # 1 1 Name First 2-our Exam By printing your name below, you pledge that "n my honor, as a University of Colorado at Boulder student, I have neither given nor received unauthorized
Chem 3331 Fall 2004 Exam # 1 1 Name First 2-our Exam By printing your name below, you pledge that "n my honor, as a University of Colorado at Boulder student, I have neither given nor received unauthorized
"Pip tazo" is a nickname given to a popular combination of antibiotics prescribed for a variety of infections. A.
 ame I. (6 points) Page 1 "Pip tazo" is a nickname given to a popular combination of antibiotics prescribed for a variety of infections. (a) ircle all the tetrahedral (chiral) stereocenters in both piperacillin
ame I. (6 points) Page 1 "Pip tazo" is a nickname given to a popular combination of antibiotics prescribed for a variety of infections. (a) ircle all the tetrahedral (chiral) stereocenters in both piperacillin
1. (6 points) Provide IUPAC accepted names for the following compounds. 2. (6 points) Provide a structure for the following compounds.
 Chem52 omework Set 1 This homework set is similar to a Chem 51 final exam. Please provide answers in the spaces provided or, preferably, on attached sheets. Point values are listed only to give you the
Chem52 omework Set 1 This homework set is similar to a Chem 51 final exam. Please provide answers in the spaces provided or, preferably, on attached sheets. Point values are listed only to give you the
MgBr 17 H 16 N 2 O 3 HO N. no partial. 4 each; can be either order -2 if steps unnumbered 1) CH 3 OH/TsOH. 1) NaH 2) CH 3 I
 I. (43 points) ame Page 1.05.215EP1 omplete the following reaction schemes as necessary. Sequential experimental steps should be numbered appropriately! omplete structures should be shown. (a) J 2005 70
I. (43 points) ame Page 1.05.215EP1 omplete the following reaction schemes as necessary. Sequential experimental steps should be numbered appropriately! omplete structures should be shown. (a) J 2005 70
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
