- electrolytes: substances that dissolve in water to form charge-carrying solutions
|
|
|
- Arron Kelley
- 5 years ago
- Views:
Transcription
1 111 Electrolytes and Ionic Theory - electrolytes: substances that dissolve in water to form charge-carrying solutions * Electrolytes form ions in solution - (ions that are mobile are able to carry charge!). These IONS can interact with one another and undergo certain kinds of chemistry! IONIC THEORY - the idea that certain compounds DISSOCIATE in water to form free IONS Strong vs weak? - If an electrolyte COMPLETELY IONIZES in water, it's said to be STRONG - If an electrolyte only PARTIALLY IONIZES in water, it's said to be WEAK - Both kinds of electrolyte undergo similar kinds of chemistry.
2 112 What kinds of compounds are electrolytes? MOLECULAR COMPOUNDS - Most molecular compounds are NONELECTROLYTES - they don't ionize in water -ACIDS and BASES will ionize in water. Most of these are WEAK ELECTROLYTES, but there are a few STRONG ACIDS and STRONG BASES. IONIC COMPOUNDS - SOLUBLE ionic compounds are STRONG ELECTROLYTES - they completely ionize in qater. - Not all ionic compounds are water soluble, however!
3 113 - What good is ionic theory? - provides an easy-to-understand MECHANISM for certain kinds of chemical reactions. - "Exchange" reactions. (a.k.a "double replacement" reactions) These free ions mix and can interact with each other! "ion soup"! Insoluble AgCl falls out of solution as it is formed - "precipitation"
4 114 Looking a bit more closely... The nitrate and sodium ions do not really participate in this reaction. They start and end in exactly the same state. We call them "SPECTATOR IONS". "Molecular equation" "Net ionic equation" (The net ionic equation shows only ions and substances that change during the course of the reaction!) - The net ionic equation tells us that any source of aqueous silver and chloride ions will exhibit this same chemistry, not just silver nitrate and sodium chloride!
5 115 A bit more about molecular, ionic, and net ionic equations - molecular equations: Represent all substances (even ionic substances) as if they were molecules. Include spectator ions, and do not show charges on ions. Traditional chemical equations. - ionic equations: Show all free ions - including spectators - in a chemical reaction. Molecules and WEAK electrolytes are shown as molecules. STRONG electrolytes (like HCl) are shown as ions. Ions that are part of undissolved ionic compounds are shown as molecules. - NET ionic equation: An ionic equation that leaves out spectator ions. Intended to show only things that actually change in a reaction. * You can get from the complete ionic equation to the net ionic equation by crossing out the spectator ions on both sides.
6 116 "Undissolved ionic compounds": How can I tell if an ionic compound dissolves in water? - consult experimental data: "solubility rules"! A few of the "rules"... - Compounds that contain a Group IA cation (or ammonium) are soluble - Nitrates and acetates are soluble - Carbonates, phosphates, and hydroxides tend to be insoluble... or see the web site for a solubility chart. #8 - hydroxides generally insoiluble, except Group IA, ammonium, calcium strontium, barium Conclusion: iron(iii) hydroxide is insoluble. #2 - acetates are soluble, no common exceptions. Conclusion: calcium acetate is soluble. #3 - Iodides usually dissolve, exceptions are silver, mercury, lead Conclusion: silver(i) iodide is INSOLUBLE #5 - Most carbonates are insoluble Conclusion - barium carbonate is insoluble.
7 117 Exchange Chemistry - Three kinds of exchange chemistry. PRECIPITATION ACID/BASE or NEUTRALIZATION GAS FORMATION (formation of unstable molecules) SOME (but not all) reactions that form gases are examples of exchange chemistry. Just because you mix together two ionic compounds does NOT mean that a reaction will occur. You need a DRIVING FORCE for a reaction.
8 118 PRECIPITATION REACTIONS - driving force is the formation of an insoluble ionic compound. ions: The formation of SOLID MAGNESIUM PHOSPHATE drives this reaction! When you're trying to complete a precipitation reaction: Write the IONS that form when the reactants are dissolved. Make NEW compounds by pairing up cations with anions. Don't forget that the positive and negative charges must balance each other out! Use the solubility rules to determine the PHASE of each new compound - solid or aqueous. Balance the overall equation.
9 119 NO REACTION! ions: "exchange"... dissolves in water... dissolves in water So, no solid forms here. All possible combinations of these four ions result in compounds that dissolve readily in water. NO CHANGE, therefore NO DRIVING FORCE, and NO REACTION We will learn about other driving forces than the formation of solid, but these driving forces do not apply to this reaction
10 120 ACID/BASE REACTIONS (also called NEUTRALIZATION REACTIONS) - There are several stable molecules that may be formed in double replacement reactions, but the most common is WATER! - Double replacement reactions that form water are also called "neutralizations" acid base salt ionic compound * To make water ( ), you need a source of hydrogen ion ( ) and hydroxide ion ( )... assumes you're reacting STRONG acid with STRONG base! This is the NET IONIC EQUATION for many neutralizations
11 121 ACIDS - compounds that release hydrogen ion (H ), when dissolved in water. Properties of acids: - Corrosive: React with most metals to give off hydrogen gas - Cause chemical burns on contact - Taste sour (like citrus - citric acid!) - Changes litmus indicator to RED BASES - Substances that release hydroxide ion (OH ) when dissolved in water Properties of bases: - Caustic: Attack and dissolve organic matter (think lye, which is NaOH) - Cause skin/eye damage on contact - Taste bitter - changes litmus indicator to BLUE Due to the dissolving action of base on your skin, bases will feel "slippery". The base ITSELF is not particularly slippery, but what's left of your skin IS!
"Undissolved ionic compounds":
 115 "Undissolved ionic compounds": How can I tell if an ionic compound dissolves in water? - consult experimental data: "solubility rules"! A few of the "rules"... - Compounds that contain a Group IA cation
115 "Undissolved ionic compounds": How can I tell if an ionic compound dissolves in water? - consult experimental data: "solubility rules"! A few of the "rules"... - Compounds that contain a Group IA cation
97 MOLECULAR AND IONIC EQUATIONS
 97 MOLECULAR AND IONIC EQUATIONS - Ions that show up IN THE SAME FORM on the left and right sides of a chemical equation are called SPECTATOR IONS. If we rewrite an ionic equation to leave out the spectator
97 MOLECULAR AND IONIC EQUATIONS - Ions that show up IN THE SAME FORM on the left and right sides of a chemical equation are called SPECTATOR IONS. If we rewrite an ionic equation to leave out the spectator
acrylonitrile Calculate how many grams of acrylonitrile could be obtained from 651 kg of propylene, assuming there is excess NO present.
 propylene acrylonitrile Calculate how many grams of acrylonitrile could be obtained from 651 kg of propylene, assuming there is excess NO present. 1 - Change the mass of propylene to moles propylene (formula
propylene acrylonitrile Calculate how many grams of acrylonitrile could be obtained from 651 kg of propylene, assuming there is excess NO present. 1 - Change the mass of propylene to moles propylene (formula
- Double replacement reactions that form water are also called "neutralizations"
 119 ACID/BASE REACTIONS (also called NEUTRALIZATION REACTIONS) - There are several stable molecules that may be formed in double replacement reactions, but the most common is WATER! - Double replacement
119 ACID/BASE REACTIONS (also called NEUTRALIZATION REACTIONS) - There are several stable molecules that may be formed in double replacement reactions, but the most common is WATER! - Double replacement
How many milliliters of 6.00M hydrochloric acid is needed to completely react with 25.0 g of sodium carbonate?
 102 Example: How many milliliters of 6.00M hydrochloric acid is needed to completely react with 25.0 g of sodium carbonate? 1) Convert 25.0 grams sodium carbonate to moles. Use FORMULA WEIGHT. 2) Convert
102 Example: How many milliliters of 6.00M hydrochloric acid is needed to completely react with 25.0 g of sodium carbonate? 1) Convert 25.0 grams sodium carbonate to moles. Use FORMULA WEIGHT. 2) Convert
- Double replacement reactions that form water are also called "neutralizations"
 101 ACID/BASE REACTIONS (also called NEUTRALIZATION REACTIONS) - There are several stable molecules that may be formed in double replacement reactions, but the most common is WATER! - Double replacement
101 ACID/BASE REACTIONS (also called NEUTRALIZATION REACTIONS) - There are several stable molecules that may be formed in double replacement reactions, but the most common is WATER! - Double replacement
Solubility Rules and Net Ionic Equations
 Solubility Rules and Net Ionic Equations Why? Solubility of a salt depends upon the type of ions in the salt. Some salts are soluble in water and others are not. When two soluble salts are mixed together
Solubility Rules and Net Ionic Equations Why? Solubility of a salt depends upon the type of ions in the salt. Some salts are soluble in water and others are not. When two soluble salts are mixed together
Unit 12: Acids & Bases. Aim: What are the definitions and properties of an acid and a base? Properties of an Acid. Taste Sour.
 Unit 12: Acids & Bases Aim: What are the definitions and properties of an acid and a base? Mar 23 12:08 PM Properties of an Acid 3. Are electrolytes. (Dissociate and conduct electricity when aq) 2. Turns
Unit 12: Acids & Bases Aim: What are the definitions and properties of an acid and a base? Mar 23 12:08 PM Properties of an Acid 3. Are electrolytes. (Dissociate and conduct electricity when aq) 2. Turns
Net Ionic Equations. Making Sense of Chemical Reactions
 Making Sense of Chemical Reactions Now that you have mastered writing balanced chemical equations it is time to take a deeper look at what is really taking place chemically in each reaction. There are
Making Sense of Chemical Reactions Now that you have mastered writing balanced chemical equations it is time to take a deeper look at what is really taking place chemically in each reaction. There are
Name CHEMISTRY / / Oxide Reactions & Net Ionic Reactions
 Name CHEMISTRY / / Oxide Reactions & Net Ionic Reactions The first type of reactions we will look at today are reactions between an oxide (a compound with oxygen as its anion) and water. There are two
Name CHEMISTRY / / Oxide Reactions & Net Ionic Reactions The first type of reactions we will look at today are reactions between an oxide (a compound with oxygen as its anion) and water. There are two
Acids Bases and Salts Acid
 Acids Bases and Salts Acid ph less than 7.0 Sour taste Electrolyte Names of Acids Binary acids Contain only 2 elements Begin with hydro; end with ic Ternary acids Ex: H 2 S = hydrosulfuric Contain a polyatomic
Acids Bases and Salts Acid ph less than 7.0 Sour taste Electrolyte Names of Acids Binary acids Contain only 2 elements Begin with hydro; end with ic Ternary acids Ex: H 2 S = hydrosulfuric Contain a polyatomic
Reactions in Aqueous Solutions
 Copyright 2004 by houghton Mifflin Company. Reactions in Aqueous Solutions Chapter 7 All rights reserved. 1 7.1 Predicting if a Rxn Will Occur When chemicals are mixed and one of these driving forces can
Copyright 2004 by houghton Mifflin Company. Reactions in Aqueous Solutions Chapter 7 All rights reserved. 1 7.1 Predicting if a Rxn Will Occur When chemicals are mixed and one of these driving forces can
NET IONIC EQUATIONS. Electrolyte Behavior
 NET IONIC EQUATIONS Net ionic equations are useful in that they show only those chemical species directly participating in a chemical reaction. They are thus simpler than the overall equation, and help
NET IONIC EQUATIONS Net ionic equations are useful in that they show only those chemical species directly participating in a chemical reaction. They are thus simpler than the overall equation, and help
CHM Electrolytes and the Ionic Theory (r14) Charles Taylor 1/5
 CHM 110 - Electrolytes and the Ionic Theory (r14) - 2014 Charles Taylor 1/5 Introduction In 1884, Arrhenius proposed that some substances broke up when dissolved in water to form freely moving ions. We've
CHM 110 - Electrolytes and the Ionic Theory (r14) - 2014 Charles Taylor 1/5 Introduction In 1884, Arrhenius proposed that some substances broke up when dissolved in water to form freely moving ions. We've
Chemical Change. Section 9.1. Chapter 9. Electrolytes and Solution Conductivity. Goal 1. Electrical Conductivity
 Chapter 9 Chemical Change Section 9.1 Electrolytes and Solution Conductivity Goal 1 Electrical Conductivity Distinguish among strong electrolytes, weak electrolytes, and nonelectrolytes. Strong Electrolyte:
Chapter 9 Chemical Change Section 9.1 Electrolytes and Solution Conductivity Goal 1 Electrical Conductivity Distinguish among strong electrolytes, weak electrolytes, and nonelectrolytes. Strong Electrolyte:
Chapter 3: Solution Chemistry (For best results when printing these notes, use the pdf version of this file)
 Chapter 3: Solution Chemistry (For best results when printing these notes, use the pdf version of this file) Section 3.1: Solubility Rules (For Ionic Compounds in Water) Section 3.1.1: Introduction Solubility
Chapter 3: Solution Chemistry (For best results when printing these notes, use the pdf version of this file) Section 3.1: Solubility Rules (For Ionic Compounds in Water) Section 3.1.1: Introduction Solubility
11.3 Reactions in Aqueous Solution. Chapter 11 Chemical Reactions Reactions in Aqueous Solution
 Chapter 11 Chemical Reactions 11.1 Describing Chemical Reactions 11.2 Types of Chemical Reactions 11.3 Reactions in Aqueous Solution 1 CHEMISTRY & YOU How did soda straws get into limestone caves? These
Chapter 11 Chemical Reactions 11.1 Describing Chemical Reactions 11.2 Types of Chemical Reactions 11.3 Reactions in Aqueous Solution 1 CHEMISTRY & YOU How did soda straws get into limestone caves? These
2. What characteristic of water makes it the universal solvent? Nonpolar large molecules long-chain hydrocarbon molecules polar
 PS Chemistry Chapter 22 & 23 Review Test Date Chapter 22 Suggestions for Studying Section 1 Know that a solution is made up of a solute and solvent. Be able to provide an example of a solute and a solvent.
PS Chemistry Chapter 22 & 23 Review Test Date Chapter 22 Suggestions for Studying Section 1 Know that a solution is made up of a solute and solvent. Be able to provide an example of a solute and a solvent.
CHEMICAL REACTIONS. There are three ways we write chemical equations. 1. Molecular Equations 2. Full Ionic Equations 3. Net Ionic Equations
 CHEMICAL REACTIONS Reactants: Zn + I 2 Product: Zn I 2 Unit 2 Chemical Reactions The unit 2 exam will cover material from multiple chapters. You are responsible for the following from your text on exam
CHEMICAL REACTIONS Reactants: Zn + I 2 Product: Zn I 2 Unit 2 Chemical Reactions The unit 2 exam will cover material from multiple chapters. You are responsible for the following from your text on exam
Net Ionic Reactions. The reaction between strong acids and strong bases is one example:
 Net Ionic Reactions Model 1 Net Ionic Reactions. Net ionic reactions are frequently used when strong electrolytes react in solution to form nonelectrolytes or weak electrolytes. These equations let you
Net Ionic Reactions Model 1 Net Ionic Reactions. Net ionic reactions are frequently used when strong electrolytes react in solution to form nonelectrolytes or weak electrolytes. These equations let you
INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin
 Lecture INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Acids and Bases Properties of Acids An acid is any substance that releases hydrogen ions, H +, into water.
Lecture INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Acids and Bases Properties of Acids An acid is any substance that releases hydrogen ions, H +, into water.
Solubility Reactions. objectives
 Solubility Reactions objectives (#4 2) How do chemicals undergo a solubility reaction? (#4 2a) A student shall be able to identify if a reaction is a solubility reaction? (#4 2b) Students should be able
Solubility Reactions objectives (#4 2) How do chemicals undergo a solubility reaction? (#4 2a) A student shall be able to identify if a reaction is a solubility reaction? (#4 2b) Students should be able
insoluble partial very soluble (< 0.1 g/100ml) solubility (> 1 g/100ml) Factors Affecting Solubility in Water
 Aqueous Solutions Solubility is a relative term since all solutes will have some solubility in water. Insoluble substances simply have extremely low solubility. The solubility rules are a general set of
Aqueous Solutions Solubility is a relative term since all solutes will have some solubility in water. Insoluble substances simply have extremely low solubility. The solubility rules are a general set of
chapter 14: ions in aqueous solutions
 chapter 14: ions in aqueous solutions Dissociation When a compound that is made of ions dissolves in water, the ions separate from one another. This is called dissociation. NaCl(s) Na + (aq) + Cl - (aq)
chapter 14: ions in aqueous solutions Dissociation When a compound that is made of ions dissolves in water, the ions separate from one another. This is called dissociation. NaCl(s) Na + (aq) + Cl - (aq)
ed. Brad Collins Aqueous Chemistry Chapter 5 Some images copyright The McGraw-Hill Companies, Inc. Sunday, August 18, 13
 ed. Brad Collins Aqueous Chemistry Chapter 5 Some images copyright The McGraw-Hill Companies, Inc. A solution is a homogenous mixture of 2 or more substances at the molecular level The solute(s) is(are)
ed. Brad Collins Aqueous Chemistry Chapter 5 Some images copyright The McGraw-Hill Companies, Inc. A solution is a homogenous mixture of 2 or more substances at the molecular level The solute(s) is(are)
A reaction in which a solid forms is called a precipitation reaction. Solid = precipitate
 Chapter 7 Reactions in Aqueous Solutions 1 Section 7.1 Predicting Whether a Reaction Will Occur Four Driving Forces Favor Chemical Change 1. Formation of a solid 2. Formation of water 3. Transfer of electrons
Chapter 7 Reactions in Aqueous Solutions 1 Section 7.1 Predicting Whether a Reaction Will Occur Four Driving Forces Favor Chemical Change 1. Formation of a solid 2. Formation of water 3. Transfer of electrons
Part 01 - Notes: Reactions & Classification
 Objectives: Identify, define, and explain: combination reaction, synthesis reaction, decomposition reaction, single replacement reaction, double replacement reaction, combustion reaction, rapid oxidation,
Objectives: Identify, define, and explain: combination reaction, synthesis reaction, decomposition reaction, single replacement reaction, double replacement reaction, combustion reaction, rapid oxidation,
Name HONORS CHEMISTRY / / Oxide Reactions & Net Ionic Reactions
 Name HONORS CHEMISTRY / / Oxide Reactions & Net Ionic Reactions The first type of reactions we will look at today are reactions between an oxide (a compound with oxygen as its anion) and water. There are
Name HONORS CHEMISTRY / / Oxide Reactions & Net Ionic Reactions The first type of reactions we will look at today are reactions between an oxide (a compound with oxygen as its anion) and water. There are
DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME
 DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The name of an ionic compound is made of the names of the CATION and ANION in the compound. - To get the FORMULA, you must figure out the SMALLEST
DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The name of an ionic compound is made of the names of the CATION and ANION in the compound. - To get the FORMULA, you must figure out the SMALLEST
Unit 5 Lesson 2 Acids, Bases, and Salts. Copyright Houghton Mifflin Harcourt Publishing Company
 Donations Accepted What are acids and bases? Acids and bases are chemicals that increase the number of ions present in a water solution when they dissolve. Lemon juice and vinegar both contain acid. Shampoo
Donations Accepted What are acids and bases? Acids and bases are chemicals that increase the number of ions present in a water solution when they dissolve. Lemon juice and vinegar both contain acid. Shampoo
Chapter 4: Chemical Quantities and Aqueous Reactions
 Chapter 4: Chemical Quantities and Aqueous Reactions C (s) + O 2 (g) CO 2 (g) CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 0 (g) 2 C 8 H 18 (g) + 25 O 2 (g) 16 CO 2 (g) + 18 H 2 0 (g) Stoichiometry Calculations
Chapter 4: Chemical Quantities and Aqueous Reactions C (s) + O 2 (g) CO 2 (g) CH 4 (g) + 2 O 2 (g) CO 2 (g) + 2 H 2 0 (g) 2 C 8 H 18 (g) + 25 O 2 (g) 16 CO 2 (g) + 18 H 2 0 (g) Stoichiometry Calculations
Session 8: LECTURE OUTLINE (SECTIONS I1 I4 pp F61 F67)
 Session 8: LECTURE OUTLINE (SECTIONS I1 I4 pp F61 F67) I. Elecrolytes a. Soluble substances b. Insoluble substances c. Electrolytes d. Non-Electrolytes e. Ions and electrical conductivity f. Strong and
Session 8: LECTURE OUTLINE (SECTIONS I1 I4 pp F61 F67) I. Elecrolytes a. Soluble substances b. Insoluble substances c. Electrolytes d. Non-Electrolytes e. Ions and electrical conductivity f. Strong and
Chapter 14: Acids and Bases
 Chapter 14: Acids and Bases Properties of Acids and Bases What is an acid? Some examples of common items containing acids: Vinegar contains acetic acid; lemons and citrus fruits contain citric acid; many
Chapter 14: Acids and Bases Properties of Acids and Bases What is an acid? Some examples of common items containing acids: Vinegar contains acetic acid; lemons and citrus fruits contain citric acid; many
Identification of White Powders
 CSI Chemistry Activity 4 Identification of White Powders GOALS In this activity you will: Create and use a flowchart to identify an unknown entity. Identify an unknown ionic compound based on an understanding
CSI Chemistry Activity 4 Identification of White Powders GOALS In this activity you will: Create and use a flowchart to identify an unknown entity. Identify an unknown ionic compound based on an understanding
Double replacement reactions
 1. Learn to predict Double replacement reaction If and when a reaction occurs, what are the products? 2. Learn to write Double replacement reaction: (i) Balanced chemical reaction (ii) Net ionicreaction
1. Learn to predict Double replacement reaction If and when a reaction occurs, what are the products? 2. Learn to write Double replacement reaction: (i) Balanced chemical reaction (ii) Net ionicreaction
Experiment 8 - Double Displacement Reactions
 Experiment 8 - Double Displacement Reactions A double displacement reaction involves two ionic compounds that are dissolved in water. In a double displacement reaction, it appears as though the ions are
Experiment 8 - Double Displacement Reactions A double displacement reaction involves two ionic compounds that are dissolved in water. In a double displacement reaction, it appears as though the ions are
Chapter 4 Reactions in Aqueous Solution
 Chapter 4 Reactions in Aqueous Solution Homework Chapter 4 11, 15, 21, 23, 27, 29, 35, 41, 45, 47, 51, 55, 57, 61, 63, 73, 75, 81, 85 1 2 Chapter Objectives Solution To understand the nature of ionic substances
Chapter 4 Reactions in Aqueous Solution Homework Chapter 4 11, 15, 21, 23, 27, 29, 35, 41, 45, 47, 51, 55, 57, 61, 63, 73, 75, 81, 85 1 2 Chapter Objectives Solution To understand the nature of ionic substances
I. Properties of Aqueous Solutions A) Electrolytes and Non-Electrolytes B) Predicting Solubility* II. Reactions of Ionic Compounds in Solution*
 Chapter 5 Reactions in Aqueous Solutions Titrations Kick Acid!!! 1 I. Properties of Aqueous Solutions A) Electrolytes and Non-Electrolytes B) Predicting Solubility* II. Reactions of Ionic Compounds in
Chapter 5 Reactions in Aqueous Solutions Titrations Kick Acid!!! 1 I. Properties of Aqueous Solutions A) Electrolytes and Non-Electrolytes B) Predicting Solubility* II. Reactions of Ionic Compounds in
Reactions in Aqueous Solutions
 Reactions in Aqueous Solutions 1 Chapter 4 General Properties of Aqueous Solutions (4.1) Precipitation Reactions (4.2) Acid-Base Reactions (4.3) Oxidation-Reduction Reactions (4.4) Concentration of Solutions
Reactions in Aqueous Solutions 1 Chapter 4 General Properties of Aqueous Solutions (4.1) Precipitation Reactions (4.2) Acid-Base Reactions (4.3) Oxidation-Reduction Reactions (4.4) Concentration of Solutions
You try: 2) HC 7H 6O 2 3) N 2O 5. 5) HClO 4. 7) Rb 2C 2O 4 8) H 3PO 4 9) AgI 10) Sr(OH) 2. What kind of compound is it? NON ELECTROLYTE (NE)
 Solubility: Solubility is the measure of how much of a solute will dissolve in a solvent. In general chemistry, we usually talk about water as the solvent, so we are talking about what compounds will dissolve
Solubility: Solubility is the measure of how much of a solute will dissolve in a solvent. In general chemistry, we usually talk about water as the solvent, so we are talking about what compounds will dissolve
Reactions in Aqueous Solution
 1 Reactions in Aqueous Solution Chapter 4 For test 3: Sections 3.7 and 4.1 to 4.5 Copyright The McGrawHill Companies, Inc. Permission required for reproduction or display. 2 A solution is a homogenous
1 Reactions in Aqueous Solution Chapter 4 For test 3: Sections 3.7 and 4.1 to 4.5 Copyright The McGrawHill Companies, Inc. Permission required for reproduction or display. 2 A solution is a homogenous
- Some MOLECULES can gain or lose electrons to form CATIONS or ANIONS. These are called POLYATOMIC IONS
 63 POLYATOMIC IONS - Some MOLECULES can gain or lose electrons to form CATIONS or ANIONS. These are called POLYATOMIC IONS - Polyatomic ions form ionic compounds in the same way that single-element ions
63 POLYATOMIC IONS - Some MOLECULES can gain or lose electrons to form CATIONS or ANIONS. These are called POLYATOMIC IONS - Polyatomic ions form ionic compounds in the same way that single-element ions
SCHOOL YEAR CH- 13 IONS IN AQUEOUS SOLUTIONS AND COLLIGATIVE PROPERTIES SUBJECT: CHEMISTRY GRADE : 11 TEST A
 SCHOOL YEAR 2017-18 NAME: CH- 13 IONS IN AQUEOUS SOLUTIONS AND COLLIGATIVE PROPERTIES SUBJECT: CHEMISTRY GRADE : 11 TEST A Choose the best answer from the options that follow each question. 1. A solute
SCHOOL YEAR 2017-18 NAME: CH- 13 IONS IN AQUEOUS SOLUTIONS AND COLLIGATIVE PROPERTIES SUBJECT: CHEMISTRY GRADE : 11 TEST A Choose the best answer from the options that follow each question. 1. A solute
Chapter 4: Phenomena. Electrolytes. Electrolytes. Electrolytes. Chapter 4 Types of Chemical Reactions and Solution Stoichiometry.
 Chapter 4: Phenomena Phenomena: Many different reactions are known to occur. Scientists wondered if these reactions could be separated into groups based on their properties. Look at the reactions below
Chapter 4: Phenomena Phenomena: Many different reactions are known to occur. Scientists wondered if these reactions could be separated into groups based on their properties. Look at the reactions below
Chapter 4: Phenomena. Electrolytes. Electrolytes. Electrolytes. Chapter 4 Types of Chemical Reactions and Solution Stoichiometry
 Chapter 4: Phenomena Phenomena: Many different reactions are known to occur. Scientists wondered if these reactions could be separated into groups based on their properties. Look at the reactions below
Chapter 4: Phenomena Phenomena: Many different reactions are known to occur. Scientists wondered if these reactions could be separated into groups based on their properties. Look at the reactions below
Chapter 4: Phenomena. (aq)+ 4H + (aq)+ 2e - Chapter 4: Types of Chemical Reactions and Solution Stoichiometry
 Chapter 4: Phenomena Phenomena: Many different reactions are known to occur. Scientists wondered if these reactions could be separated into groups based on their properties. Look at the reactions below
Chapter 4: Phenomena Phenomena: Many different reactions are known to occur. Scientists wondered if these reactions could be separated into groups based on their properties. Look at the reactions below
- Use the STEM NAME of the element, then add "-ide" suffix
 65 ANIONS 2 kinds Main-group nonmetals - Use the STEM NAME of the element, then add "-ide" suffix N : "nitride" ion P : "phosphide ion" O : "oxide ion" F : "fluoride ion" Polyatomic ions - Memorize list.(see
65 ANIONS 2 kinds Main-group nonmetals - Use the STEM NAME of the element, then add "-ide" suffix N : "nitride" ion P : "phosphide ion" O : "oxide ion" F : "fluoride ion" Polyatomic ions - Memorize list.(see
NET IONIC REACTIONS in AQUEOUS SOLUTIONS AB + CD AD + CB
 NET IONIC REACTIONS in AQUEOUS SOLUTIONS Double replacements are among the most common of the simple chemical reactions. Consider the hypothetical reaction: AB + CD AD + CB where AB exists as A + and B
NET IONIC REACTIONS in AQUEOUS SOLUTIONS Double replacements are among the most common of the simple chemical reactions. Consider the hypothetical reaction: AB + CD AD + CB where AB exists as A + and B
9/24/09 Chem 111 Experiment #7 Solutions and Reactions Brown, LeMay, and Bursten Chapter
 Chem 111 Experiment #7 Solutions and Reactions Brown, LeMay, and Bursten Chapter 4.1-4.4 KEY VOCABULARY: 1. Ionic compound a compound composed of cations (+) and anions (-). Many ionic compounds dissociate
Chem 111 Experiment #7 Solutions and Reactions Brown, LeMay, and Bursten Chapter 4.1-4.4 KEY VOCABULARY: 1. Ionic compound a compound composed of cations (+) and anions (-). Many ionic compounds dissociate
Reactions in Aqueous Solution
 Reading Assignments: Reactions in Aqueous Solution Chapter 4 Chapter 4 in R. Chang, Chemistry, 9 th Ed., McGraw-Hill, 2006. or previous editions. Or related topics in other textbooks. Consultation outside
Reading Assignments: Reactions in Aqueous Solution Chapter 4 Chapter 4 in R. Chang, Chemistry, 9 th Ed., McGraw-Hill, 2006. or previous editions. Or related topics in other textbooks. Consultation outside
AP Chemistry Honors Unit Chemistry #4 2 Unit 3. Types of Chemical Reactions & Solution Stoichiometry
 HO AP Chemistry Honors Unit Chemistry #4 2 Unit 3 Chapter 4 Zumdahl & Zumdahl Types of Chemical Reactions & Solution Stoichiometry Students should be able to:! Predict to some extent whether a substance
HO AP Chemistry Honors Unit Chemistry #4 2 Unit 3 Chapter 4 Zumdahl & Zumdahl Types of Chemical Reactions & Solution Stoichiometry Students should be able to:! Predict to some extent whether a substance
Unit 3 - Solubility of Ionic Substances. 1. How to use the Solubility Table to develop a scheme for identification of an unknown ion in a solution.
 In Tutorial 3 you will be shown: 1. How to use the Solubility Table to develop a scheme for identification of an unknown ion in a solution. 2. How to use the Solubility Table to outline a procedure to
In Tutorial 3 you will be shown: 1. How to use the Solubility Table to develop a scheme for identification of an unknown ion in a solution. 2. How to use the Solubility Table to outline a procedure to
Chemical Reactions and Equations
 Chemical Reactions and Equations 5-1 5.1 What is a Chemical Reaction? A chemical reaction is a chemical change. A chemical reaction occurs when one or more substances is converted into one or more new
Chemical Reactions and Equations 5-1 5.1 What is a Chemical Reaction? A chemical reaction is a chemical change. A chemical reaction occurs when one or more substances is converted into one or more new
CHEMICAL REACTIONS. The process by which one or more substances are changed into one or more different substances
 CHEMICAL REACTIONS The process by which one or more substances are changed into one or more different substances Equations Reactions are represented by a chemical equation Reactants Products Must have
CHEMICAL REACTIONS The process by which one or more substances are changed into one or more different substances Equations Reactions are represented by a chemical equation Reactants Products Must have
3/27/2015. So the question that arises is, how can you tell the difference between an ionic solution and a solution containing a molecular acid?
 A neat thing about chemistry is that there are exceptions to most rules. Previously, we learned that ionic compounds form electrolytic solutions but molecular compounds do not form electrolytic solutions.
A neat thing about chemistry is that there are exceptions to most rules. Previously, we learned that ionic compounds form electrolytic solutions but molecular compounds do not form electrolytic solutions.
Experiment Six Precipitation Reactions
 Experiment Six Precipitation Reactions Objective Identify the ions present in various aqueous solutions. Systematically combine solutions and identify the reactions that form precipitates and gases. Write
Experiment Six Precipitation Reactions Objective Identify the ions present in various aqueous solutions. Systematically combine solutions and identify the reactions that form precipitates and gases. Write
Chapter 4; Reactions in Aqueous Solutions. Chapter 4; Reactions in Aqueous Solutions. V. Molarity VI. Acid-Base Titrations VII. Dilution of Solutions
 Chapter 4; Reactions in Aqueous Solutions I. Electrolytes vs. NonElectrolytes II. Precipitation Reaction a) Solubility Rules III. Reactions of Acids a) Neutralization b) Acid and Carbonate c) Acid and
Chapter 4; Reactions in Aqueous Solutions I. Electrolytes vs. NonElectrolytes II. Precipitation Reaction a) Solubility Rules III. Reactions of Acids a) Neutralization b) Acid and Carbonate c) Acid and
Chapter 6. Types of Chemical Reactions and Solution Stoichiometry
 Chapter 6 Types of Chemical Reactions and Solution Stoichiometry Chapter 6 Table of Contents (6.1) (6.2) (6.3) (6.4) (6.5) (6.6) (6.7) (6.8) Water, the common solvent The nature of aqueous solutions: Strong
Chapter 6 Types of Chemical Reactions and Solution Stoichiometry Chapter 6 Table of Contents (6.1) (6.2) (6.3) (6.4) (6.5) (6.6) (6.7) (6.8) Water, the common solvent The nature of aqueous solutions: Strong
CH 4 AP. Reactions in Aqueous Solutions
 CH 4 AP Reactions in Aqueous Solutions Water Aqueous means dissolved in H 2 O Moderates the Earth s temperature because of high specific heat H-bonds cause strong cohesive and adhesive properties Polar,
CH 4 AP Reactions in Aqueous Solutions Water Aqueous means dissolved in H 2 O Moderates the Earth s temperature because of high specific heat H-bonds cause strong cohesive and adhesive properties Polar,
- Use the STEM NAME of the element, then add "-ide" suffix
 65 ANIONS 2 kinds Main-group nonmetals - Use the STEM NAME of the element, then add "-ide" suffix N : "nitride" ion P : "phosphide ion" O : "oxide ion" F : "fluoride ion" Polyatomic ions - Memorize list.(see
65 ANIONS 2 kinds Main-group nonmetals - Use the STEM NAME of the element, then add "-ide" suffix N : "nitride" ion P : "phosphide ion" O : "oxide ion" F : "fluoride ion" Polyatomic ions - Memorize list.(see
Announcements. There are 3-classes of chemical reactions that occur in aqueous solution.
 Announcements Exam 1 Results: Mean: 71% Range: 39.5%-93.5% Median: 72% Other Bio-LS Class Mean 72% Please read Chapter 4 and complete problems. Please see me for help. There are 3-classes of chemical reactions
Announcements Exam 1 Results: Mean: 71% Range: 39.5%-93.5% Median: 72% Other Bio-LS Class Mean 72% Please read Chapter 4 and complete problems. Please see me for help. There are 3-classes of chemical reactions
The Chemistry of Acids and Bases
 The Chemistry of Acids and Bases 1 Acid and Bases 2 Acid and Bases 3 Acid and Bases 4 Acids 5 Have a sour taste. Vinegar is a solution of acetic acid. Citrus fruits contain citric acid. React with certain
The Chemistry of Acids and Bases 1 Acid and Bases 2 Acid and Bases 3 Acid and Bases 4 Acids 5 Have a sour taste. Vinegar is a solution of acetic acid. Citrus fruits contain citric acid. React with certain
Acids and Bases. April 10, Honors Acid and Bases Intro.notebook. Q: What does it mean for a reaction not to go to completion or equilibrium?
 Acids and Bases Unit objectives Q: What makes a solution acidic or basic? Q: What about an acid/base makes it acidic/basic? Q: How does and acid/base produce H+/OH In other words: What are the ways in
Acids and Bases Unit objectives Q: What makes a solution acidic or basic? Q: What about an acid/base makes it acidic/basic? Q: How does and acid/base produce H+/OH In other words: What are the ways in
Chemical Equations. Chemical Reactions. The Hindenburg Reaction 5/25/11
 Chemical Reactions CHM 1032C Chemical Equations Chemical change involves a reorganization of the atoms in one or more substances. The Hindenburg Reaction Reactants are on left, products to the right. Arrow
Chemical Reactions CHM 1032C Chemical Equations Chemical change involves a reorganization of the atoms in one or more substances. The Hindenburg Reaction Reactants are on left, products to the right. Arrow
NAMING IONIC COMPOUNDS
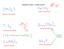 NAMING IONIC COMPOUNDS iron(ii) carbonate ammonium sulfide titanium (IV) sulfide barium phosphate Spelling matters! calcium nitrate sodium phosphide DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE
NAMING IONIC COMPOUNDS iron(ii) carbonate ammonium sulfide titanium (IV) sulfide barium phosphate Spelling matters! calcium nitrate sodium phosphide DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE
We CAN have molecular solutions (ex. sugar in water) but we will be only working with ionic solutions for this unit.
 Solubility Equilibrium The Basics (should be mostly review) Solubility is defined as the maximum amount of a substance which can be dissolved in a given solute at a given temperature. The solubility of
Solubility Equilibrium The Basics (should be mostly review) Solubility is defined as the maximum amount of a substance which can be dissolved in a given solute at a given temperature. The solubility of
Classifying Substances
 Classifying Substances There are other ways you can use to classify compounds. For example, you can classify some compounds as acids or bases. You are very familiar with acids and bases because you see
Classifying Substances There are other ways you can use to classify compounds. For example, you can classify some compounds as acids or bases. You are very familiar with acids and bases because you see
DOUBLE DISPLACEMENT REACTIONS. Double your pleasure, double your fun
 DOUBLE DISPLACEMENT REACTIONS Double your pleasure, double your fun Industrial processes produce unwanted by-products. Dissolved toxic metal ions-copper, mercury, and cadmium-are common leftovers in the
DOUBLE DISPLACEMENT REACTIONS Double your pleasure, double your fun Industrial processes produce unwanted by-products. Dissolved toxic metal ions-copper, mercury, and cadmium-are common leftovers in the
ie) HCl (aq) H + (aq) + Cl - (aq) *Like all equations, dissociation equations are written in balanced form
 Acids and Bases Acids - substances which dissolve in water to form H + ions in solution ie) HCl (aq) H + (aq) + Cl - (aq) *Like all equations, dissociation equations are written in balanced form a) contain
Acids and Bases Acids - substances which dissolve in water to form H + ions in solution ie) HCl (aq) H + (aq) + Cl - (aq) *Like all equations, dissociation equations are written in balanced form a) contain
Chapter 4 Notes Types of Chemical Reactions and Solutions Stoichiometry A Summary
 Chapter 4 Notes Types of Chemical Reactions and Solutions Stoichiometry A Summary 4.1 Water, the Common Solvent A. Structure of water 1. Oxygen s electronegativity is high (3.5) and hydrogen s is low (2.1)
Chapter 4 Notes Types of Chemical Reactions and Solutions Stoichiometry A Summary 4.1 Water, the Common Solvent A. Structure of water 1. Oxygen s electronegativity is high (3.5) and hydrogen s is low (2.1)
ELECTROLYTES & NEUTRALIZATION
 ELECTROLYTES & NEUTRALIZATION BUT FIRST LET S REVIEW IONS AND BONDING What is the Lewis dot diagram for Magnesium? Mg 2 2- S What is the Lewis dot diagram for Sulfur? How would these 2 elements bond? What
ELECTROLYTES & NEUTRALIZATION BUT FIRST LET S REVIEW IONS AND BONDING What is the Lewis dot diagram for Magnesium? Mg 2 2- S What is the Lewis dot diagram for Sulfur? How would these 2 elements bond? What
Combustion Reactions (another example of redox) Combustion
 Combustion Reactions (another example of redox) Combustion is the burning of a fuel by rapid oxidation with oxygen in air. - General reaction for carbon based fuels is: Balancing Combustion Reactions Step
Combustion Reactions (another example of redox) Combustion is the burning of a fuel by rapid oxidation with oxygen in air. - General reaction for carbon based fuels is: Balancing Combustion Reactions Step
Lecture 4 :Aqueous Solutions
 LOGO Lecture 4 :Aqueous Solutions International University of Sarajevo Chemistry - SPRING 2014 Course lecturer : Jasmin Šutković 11 th March 2014 Contents International University of Sarajevo 1. Aqueous
LOGO Lecture 4 :Aqueous Solutions International University of Sarajevo Chemistry - SPRING 2014 Course lecturer : Jasmin Šutković 11 th March 2014 Contents International University of Sarajevo 1. Aqueous
Solutions Solubility. Chapter 14
 Copyright 2004 by Houghton Mifflin Company. Solutions Chapter 14 All rights reserved. 1 Solutions Solutions are homogeneous mixtures Solvent substance present in the largest amount Solute is the dissolved
Copyright 2004 by Houghton Mifflin Company. Solutions Chapter 14 All rights reserved. 1 Solutions Solutions are homogeneous mixtures Solvent substance present in the largest amount Solute is the dissolved
15 Acids, Bases, and Salts. Lemons and limes are examples of foods that contain acidic solutions.
 15 Acids, Bases, and Salts Lemons and limes are examples of foods that contain acidic solutions. Chapter Outline 15.1 Acids and Bases 15.2 Reactions of Acids and Bases 15.3 Salts 15.4 Electrolytes and
15 Acids, Bases, and Salts Lemons and limes are examples of foods that contain acidic solutions. Chapter Outline 15.1 Acids and Bases 15.2 Reactions of Acids and Bases 15.3 Salts 15.4 Electrolytes and
Quick Review. - Chemical equations - Types of chemical reactions - Balancing chemical equations - Stoichiometry - Limiting reactant/reagent
 Quick Review - Chemical equations - Types of chemical reactions - Balancing chemical equations - Stoichiometry - Limiting reactant/reagent Water H 2 O Is water an ionic or a covalent compound? Covalent,
Quick Review - Chemical equations - Types of chemical reactions - Balancing chemical equations - Stoichiometry - Limiting reactant/reagent Water H 2 O Is water an ionic or a covalent compound? Covalent,
EXPERIMENT A5: TYPES OF REACTIONS. Learning Outcomes. Introduction. Upon completion of this lab, the student will be able to:
 1 Learning Outcomes EXPERIMENT A5: TYPES OF REACTIONS Upon completion of this lab, the student will be able to: 1) Examine different types of chemical reactions. 2) Express chemical equations in molecular,
1 Learning Outcomes EXPERIMENT A5: TYPES OF REACTIONS Upon completion of this lab, the student will be able to: 1) Examine different types of chemical reactions. 2) Express chemical equations in molecular,
Name Honors Chemistry / /
 Name Honors Chemistry / / Redox Reactions Rules for Assigning Oxidation Numbers Oxidation state of: Charge Examples Neutral monoatomic or molecular elements 0 Na(s), Cl 2 (g), S 8 (s), O 2 (g), Hg(l) Fluorine
Name Honors Chemistry / / Redox Reactions Rules for Assigning Oxidation Numbers Oxidation state of: Charge Examples Neutral monoatomic or molecular elements 0 Na(s), Cl 2 (g), S 8 (s), O 2 (g), Hg(l) Fluorine
Ch 7 Chemical Reactions Study Guide Accelerated Chemistry SCANTRON
 Ch 7 Chemical Reactions Study Guide Accelerated Chemistry SCANTRON Name /80 TRUE/FALSE. Write 'T' if the statement is true and 'F' if the statement is false. Correct the False statments by changing the
Ch 7 Chemical Reactions Study Guide Accelerated Chemistry SCANTRON Name /80 TRUE/FALSE. Write 'T' if the statement is true and 'F' if the statement is false. Correct the False statments by changing the
Understand what acids and alkalis are, and where they are found.
 Lesson Aims- Understand what acids and alkalis are, and where they are found. Test a range of household products with litmus indicator to see whether they are acidic or alkaline. Found in citrus fruit
Lesson Aims- Understand what acids and alkalis are, and where they are found. Test a range of household products with litmus indicator to see whether they are acidic or alkaline. Found in citrus fruit
AP Chemistry Unit #4. Types of Chemical Reactions & Solution Stoichiometry
 AP Chemistry Unit #4 Chapter 4 Zumdahl & Zumdahl Types of Chemical Reactions & Solution Stoichiometry Students should be able to: Predict to some extent whether a substance will be a strong electrolyte,
AP Chemistry Unit #4 Chapter 4 Zumdahl & Zumdahl Types of Chemical Reactions & Solution Stoichiometry Students should be able to: Predict to some extent whether a substance will be a strong electrolyte,
Properties of Compounds
 Chapter 6. Properties of Compounds Comparing properties of elements and compounds Compounds are formed when elements combine together in fixed proportions. The compound formed will often have properties
Chapter 6. Properties of Compounds Comparing properties of elements and compounds Compounds are formed when elements combine together in fixed proportions. The compound formed will often have properties
Acids and Bases. Acids
 1 Acids and Bases Acids Although some acids can burn and are dangerous to handle, most acids in foods are safe to eat. What acids have in common, however, is that they contain at least one hydrogen atom
1 Acids and Bases Acids Although some acids can burn and are dangerous to handle, most acids in foods are safe to eat. What acids have in common, however, is that they contain at least one hydrogen atom
CHAPTER 11: CHEMICAL REACTIONS. Mrs. Brayfield
 CHAPTER 11: CHEMICAL REACTIONS Mrs. Brayfield WRITING EQUATIONS Write the chemical equation for the following: Magnesium metal reacts with hydrochloric acid to form aqueous magnesium chloride and hydrogen
CHAPTER 11: CHEMICAL REACTIONS Mrs. Brayfield WRITING EQUATIONS Write the chemical equation for the following: Magnesium metal reacts with hydrochloric acid to form aqueous magnesium chloride and hydrogen
CHEM 103 Acid-Base Reactions
 CHEM 103 Acid-Base Reactions Lecture Notes March 2, 2006 Prof. Sevian 1 Chem 103 Please sit with your groups today. We will be doing a group problem at the end of class. 2 2005 H. Sevian 1 Agenda Recap:
CHEM 103 Acid-Base Reactions Lecture Notes March 2, 2006 Prof. Sevian 1 Chem 103 Please sit with your groups today. We will be doing a group problem at the end of class. 2 2005 H. Sevian 1 Agenda Recap:
Acids and Bases. Chapters 20 and 21
 Acids and Bases Chapters 20 and 21 Acid and Bases Have a sour taste. Vinegar is a solution of acetic acid. Citrus fruits contain citric acid. React with certain metals to produce hydrogen gas. React with
Acids and Bases Chapters 20 and 21 Acid and Bases Have a sour taste. Vinegar is a solution of acetic acid. Citrus fruits contain citric acid. React with certain metals to produce hydrogen gas. React with
CHEMICAL EQUATIONS WHAT BALANCING AN EQUATION MEANS
 17 CHEMICAL EQUATIONS WHAT BALANCING AN EQUATION MEANS WHAT IS A CHEMICAL EQUATION? A chemical equation is a way of representing a chemical reaction in symbolic form. For example, when hydrochloric acid
17 CHEMICAL EQUATIONS WHAT BALANCING AN EQUATION MEANS WHAT IS A CHEMICAL EQUATION? A chemical equation is a way of representing a chemical reaction in symbolic form. For example, when hydrochloric acid
Chapter 12: Chemical Equilibrium The Extent of Chemical Reactions
 Chapter 12: Chemical Equilibrium The Extent of Chemical Reactions When a system reaches equilibrium, the [products] and [reactants] remain constant. A + B C + D [5M] [2M] [3M] [1.5M] Rate fwd = Rate rev
Chapter 12: Chemical Equilibrium The Extent of Chemical Reactions When a system reaches equilibrium, the [products] and [reactants] remain constant. A + B C + D [5M] [2M] [3M] [1.5M] Rate fwd = Rate rev
Chapter Outline. Ch 8: Aqueous Solutions: Chemistry of the Hydrosphere. H 2 S + Cu 2+ CuS(s) + 2H + (Fe, Ni, Mn also) HS O 2 HSO 4
 Ch 8: Aqueous Solutions: Chemistry of the Hydrosphere H 2 S + Cu 2+ CuS(s) + 2H + (Fe, Ni, Mn also) HS - + 2 O 2 HSO 4 - + energy (supports life) Figure taken from Principles of Biochemistry, 2nd Ed. By
Ch 8: Aqueous Solutions: Chemistry of the Hydrosphere H 2 S + Cu 2+ CuS(s) + 2H + (Fe, Ni, Mn also) HS - + 2 O 2 HSO 4 - + energy (supports life) Figure taken from Principles of Biochemistry, 2nd Ed. By
Chapter 4. Chemical Quantities and Aqueous Reactions
 Lecture Presentation Chapter 4 Chemical Quantities and Aqueous Reactions Reaction Stoichiometry: How Much Carbon Dioxide? The balanced chemical equations for fossilfuel combustion reactions provide the
Lecture Presentation Chapter 4 Chemical Quantities and Aqueous Reactions Reaction Stoichiometry: How Much Carbon Dioxide? The balanced chemical equations for fossilfuel combustion reactions provide the
(A) Composition (B) Decomposition (C) Single replacement (D) Double replacement: Acid-base (E) Combustion
 AP Chemistry - Problem Drill 08: Chemical Reactions No. 1 of 10 1. What type is the following reaction: H 2 CO 3 (aq) + Ca(OH) 2 (aq) CaCO 3 (aq) + 2 H 2 O (l)? (A) Composition (B) Decomposition (C) Single
AP Chemistry - Problem Drill 08: Chemical Reactions No. 1 of 10 1. What type is the following reaction: H 2 CO 3 (aq) + Ca(OH) 2 (aq) CaCO 3 (aq) + 2 H 2 O (l)? (A) Composition (B) Decomposition (C) Single
Solutions & Solubility: Net Ionic Equations (9.1 in MHR Chemistry 11)
 Solutions & Solubility: Net Ionic Equations (9.1 in MHR Chemistry 11) 1 Solubility vs. Temperature 2 Solubility Table Anions SOLUBILITY Table 8.3 page 363 in MHR Cl Br I S OH SO CO 3 PO 3 SO 3 C 2 H 3
Solutions & Solubility: Net Ionic Equations (9.1 in MHR Chemistry 11) 1 Solubility vs. Temperature 2 Solubility Table Anions SOLUBILITY Table 8.3 page 363 in MHR Cl Br I S OH SO CO 3 PO 3 SO 3 C 2 H 3
Unit 11: Equilibrium / Acids & Bases Text Questions from Corwin
 Unit 11: Equilibrium / Acids & Bases Name: KEY Text Questions from Corwin 16.3 1. How can a reversible reaction proceed? spontaneously in both the forward and reverse directions 2. When is a reversible
Unit 11: Equilibrium / Acids & Bases Name: KEY Text Questions from Corwin 16.3 1. How can a reversible reaction proceed? spontaneously in both the forward and reverse directions 2. When is a reversible
CHAPTER 4 TYPES OF CHEMICAL REACTIONS & SOLUTION STOICHIOMETRY
 Advanced Chemistry Name Hour Advanced Chemistry Approximate Timeline Students are expected to keep up with class work when absent. CHAPTER 4 TYPES OF CHEMICAL REACTIONS & SOLUTION STOICHIOMETRY Day Plans
Advanced Chemistry Name Hour Advanced Chemistry Approximate Timeline Students are expected to keep up with class work when absent. CHAPTER 4 TYPES OF CHEMICAL REACTIONS & SOLUTION STOICHIOMETRY Day Plans
Chapter 4: Types of Chemical Reactions and Solution Stoichiometry
 Chapter 4: Types of Chemical Reactions and Solution Stoichiometry 4.1 Water, the Common Solvent 4.2 The Nature of Aqueous Solutions: Strong and Weak Electrolytes 4.3 The Composition of Solutions (MOLARITY!)
Chapter 4: Types of Chemical Reactions and Solution Stoichiometry 4.1 Water, the Common Solvent 4.2 The Nature of Aqueous Solutions: Strong and Weak Electrolytes 4.3 The Composition of Solutions (MOLARITY!)
Various Types of Reactions
 Various Types of Reactions Matthew Park Outline: 1. Synthesis / Replacement / Decomposition Reactions 2. Precipitation Reactions 3. Acid-Base Reactions 4. Summary: Metathesis Reactions NOTE: Not all of
Various Types of Reactions Matthew Park Outline: 1. Synthesis / Replacement / Decomposition Reactions 2. Precipitation Reactions 3. Acid-Base Reactions 4. Summary: Metathesis Reactions NOTE: Not all of
Lesson #7: Introduction to Acids and Bases
 Lesson #7: Introduction to Acids and Bases Acid (As Defined by Arrhenius) Properties of Acids In 1887 Swedish Chemist Svante Arrhenius defined an Acid as: A Compound (Ionic or Molecular) that dissociates
Lesson #7: Introduction to Acids and Bases Acid (As Defined by Arrhenius) Properties of Acids In 1887 Swedish Chemist Svante Arrhenius defined an Acid as: A Compound (Ionic or Molecular) that dissociates
I. What Are the Properties of Acids?
 Chapter 6.3 DESCRIBING ACIDS AND BASES I. What Are the Properties of Acids? I. An Acid reacts with metals, reacts wth carbonates, tastes sour, and turns blue litmus paper red. A. REACTIONS WITH METALS
Chapter 6.3 DESCRIBING ACIDS AND BASES I. What Are the Properties of Acids? I. An Acid reacts with metals, reacts wth carbonates, tastes sour, and turns blue litmus paper red. A. REACTIONS WITH METALS
Reactions in Aqueous Solutions
 Reactions in Aqueous Solutions Chapter 4 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. A solution is a homogenous mixture of 2 or more substances. The solute
Reactions in Aqueous Solutions Chapter 4 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. A solution is a homogenous mixture of 2 or more substances. The solute
What Do You Think? Investigate GOALS
 Cool Chemistry Show Activity 4 Chemical Equations GOALS In this activity you will: Represent chemical changes using word equations and chemical equations. Distinguish between different classes of chemical
Cool Chemistry Show Activity 4 Chemical Equations GOALS In this activity you will: Represent chemical changes using word equations and chemical equations. Distinguish between different classes of chemical
