UNIT 8: CHEMICAL FORMULAS AND NOMENCLATURE CHEMISTRY 215, DUFFEY
|
|
|
- Reynard Shaw
- 5 years ago
- Views:
Transcription
1 UNIT 8: CHEMICAL FORMULAS AND NOMENCLATURE CHEMISTRY 215, DUFFEY BIG IDEAS o 7.1 Ion formation - o 7.2 Ionic bonding - o 7.4 Metallic bonding - o 8.1 & 8.2 Covalent bonding - CHEMICAL BONDING o The purpose of bonding in any form is to Which elements on the periodic table are already stable? Why? o Any atom with a is stable, it is to be more stable. Atoms generally achieve this by (ionic) or (covalent) electrons CHEMICAL FORMULAS o A chemists shorthand way of indicating Shows which elements are Subscripts indicate the relative or actual number of unit or molecule o Formula Unit ( ) Repeat to form a o Molecule ( ) Exist as, no of each element in a formula O = C = O IONIC BONDS o Transfer of from cation to anion The oppositely charged electrons have an (+/-) that forms the bond. o Ionic compounds are formed. Binary Ionic Compounds Contain only two different Generally form between a Other Ionic Compounds Contain more than A metal or nonmetal with a (or polyatomics) between them
2 Duffey, Chem 215, Unit 8 Notes, p. 2 IONIC COMPOUND FORMATION o Formation occurs in a way that the compound is (carries no overall ) Example a compound formed from and To form a compound that is you need: o Ca 2+ for every F 1- [ ] So the formula for the neutral compound is The simplest method for this is to Example - and o Take the from the Cu and give it to as a o Take the from the S and give it to as a FORMULA: (then reduce if needed) EMPIRICAL FORMULAS o Ionic compounds consist of so they ONLY have empirical formulas Empirical Formula a chemical formula that shows the rather than the actual number of atoms in the substance This means that two different empirical formula since it is not the Example o Ethyne is o Benzene is WRITING FORMULAS FROM NAMES o Polyatomic Ions compounds can have the formula for the substance empirical formula empirical formula If you need more than one of a polyatomic ion you will need to use Example: Aluminum Carbonate BE ESPECIALLY CAREFUL WITH THESE IONS: THESE ARE THE MOST COMMON MISTAKES MADE WITH FORGETTING PARENTHESIS!!! o Empirical Formulas See the Writing Formulas From Names Reference sheet o Ionic Formula Writing Examples: YOU TRY Write ionic formulas for: Lithium Chloride Sodium Fluoride Calcium Bromide Nickel (II) Acetate Magnesium Phosphate Calcium Cyanide Ammonium Carbonate Potassium Sulfite Copper (II) Hydroxide Mercury (I) Bromide Barium Sulfate Manganese (II) Sulfide Zinc Nitrate Magnesium Bisulfate
3 PROPERTIES OF IONIC COMPOUNDS o Ions are packed in a regular and repulsion between the ions. Duffey, Chem 215, Unit 8 Notes, p. 3 that balances the forces of attraction o Electrical Conductivity Ionic compounds conduct as solids, but do if they are or Conductivity depends on available o An ionic compound whose solution ( called an o A compound whose solution does conduct is a o High Melting Point/High Boiling Point Due to relatively ) conducts is Lattice Energy is the energy required to the solid compound into o Crystals with Transition Metals are often The colors are also seen when they are in o Ionic Crystals are hard, rigid, and brittle as They can be METALLIC BONDS o Metals can form bonds with the same principles as Lattices are based on o Sea of Electrons Metals in the lattice do not,, or their electrons Instead the overlap to form a The outer electrons are then by an specific atom and can move easily between This is called Metallic bond: o PROPERTIES OF METALS Melting and boiling points vary greatly In general they have Malleability, Ductility, and Durability, can be drawn into a or hammered into a Thermal and Electrical Conductivity (electrons are already moving around) Hardness and Strength Transition metals actually use some of their and create stronger bonds due to more electrons. Alkali metals have delocalized e -, so they are
4 o Metal Alloys It is relatively to introduce other elements into Alloy a mixture of elements that has a o Duffey, Chem 215, Unit 8 Notes, p. 4 Properties of Alloys o from the elements they contain o Specific combinations are used to achieve the Added elements like also contribute COVALENT BONDS o Form by the Generally form between A is formed The shared electrons are considered to be part of the o Example: Diatomic Molecules F 2 H 2 N 2 Br 2 O 2 Cl 2 I 2 Memory Trick o COVALENT BOND FORMULA WRITING See the Writing Formulas From Names Reference sheet o Covalent Formula Practice: Sulfur dioxide Dinitrogen trioxide Diphosphorus pentoxide Tetracarbon decahydride Nitrogen monoxide o Molecular Formulas (in general) Represent compounds that exist in (covalent only!) Subscripts tell the of elements/atoms Subscripts show a simple whole number ratio o Then the molecular formula is the same as the Or the ratio between subscripts may be of the empirical ratio Examples: Empirical formula: H 2 O CO 2 CH 4 C 2 H 6 C 4 H 10 o Multiple Bonds Bond strength is dependent on bond As the number of increases, the bond length are the longest bonds (share pair of e - ) o WEAKEST
5 (multiple bonds continued) o BONDS AND ENERGY ACIDS o An acid is a are shorter (share pair of e - ) o INTERMEDIATE Duffey, Chem 215, Unit 8 Notes, p. 5 are the shortest (share pair of e - ) o STRONGEST Bonding involves energy changes: Bond BREAKING is always Bond MAKING is always An endothermic reaction Occurs when a greater amount of energy is to BREAK the bonds than is when new bonds form. An exothermic reaction Occurs when more energy is as new bonds are FORMED than is to break the original bonds. bonded molecular compound that contains one or more that can form cations. When added to the hydrogen(s) are This makes hydronium ions ( ) and the ph The closer the ph is to 0 or 1 the more the solution o Acid Nomenclature and Formulas Binary Acids have at least hydrogen and an anion that ends in Acid name in the form Hydro (root) ic Acid o Example: Oxyacids (contain anions with oxygen in them) Still have at least one o If the anion ends in Acid name in the form (root) ic Acid Example: o If the anion ends in o Acid Formula Writing Practice Hydrochloric Acid Permanganic Acid Hydronitric Acid Carbonic Acid Hypochlorous Acid Acid name in the form (root) ous Acid Example:
Naming and Formula Writing
 + Naming and Formula Writing + Chemical Formulas Shows the kind and number of atoms in the smallest piece of a substance Use subscripts to show the number of atoms per element Molecular formula- number
+ Naming and Formula Writing + Chemical Formulas Shows the kind and number of atoms in the smallest piece of a substance Use subscripts to show the number of atoms per element Molecular formula- number
UNIT 5.1. Types of bonds
 UNIT 5.1 Types of bonds REVIEW OF VALENCE ELECTRONS Valence electrons are electrons in the outmost shell (energy level). They are the electrons available for bonding. Group 1 (alkali metals) have 1 valence
UNIT 5.1 Types of bonds REVIEW OF VALENCE ELECTRONS Valence electrons are electrons in the outmost shell (energy level). They are the electrons available for bonding. Group 1 (alkali metals) have 1 valence
Chapter 6. Naming Compounds Writing Formulas
 Chapter 6 Naming Compounds Writing Formulas Systematic Naming There are too many compounds to remember the names of them all. Compound is made of two or more elements. Put together atoms. Name should tell
Chapter 6 Naming Compounds Writing Formulas Systematic Naming There are too many compounds to remember the names of them all. Compound is made of two or more elements. Put together atoms. Name should tell
Chapter 5. Naming Compounds Writing Formulas
 Chapter 5 Naming Compounds Writing Formulas Systematic Naming There are too many compounds to remember the names of them all. Compound is made of two or more elements. Put together atoms. Name should tell
Chapter 5 Naming Compounds Writing Formulas Systematic Naming There are too many compounds to remember the names of them all. Compound is made of two or more elements. Put together atoms. Name should tell
Ionic Compounds and Metals
 Ionic Compounds and Metals Chapter 7 Ch. 7.1 Chemical bond Cation Anion Vocabulary Ch. 7.2 Ionic bond Ionic compound Binary compound Crystal lattice Electrolyte Lattice energy 2 Objectives Define a chemical
Ionic Compounds and Metals Chapter 7 Ch. 7.1 Chemical bond Cation Anion Vocabulary Ch. 7.2 Ionic bond Ionic compound Binary compound Crystal lattice Electrolyte Lattice energy 2 Objectives Define a chemical
Formation of Ions. Ions formed when atoms gain or lose valence e - to achieve a stable octet
 Ionic Bonding Formation of Ions Ions formed when atoms gain or lose valence e - to achieve a stable octet Cation Positively charged ion Forms when atom loses electrons Anion negatively charged ion Forms
Ionic Bonding Formation of Ions Ions formed when atoms gain or lose valence e - to achieve a stable octet Cation Positively charged ion Forms when atom loses electrons Anion negatively charged ion Forms
Ionic and Metallic Bonding
 Unit 5: Ionic and Metallic Bonding H 2 O Valence Electrons are? The electrons responsible for the chemical properties of atoms, and are those in the outer energy level. Valence electrons - The s and p
Unit 5: Ionic and Metallic Bonding H 2 O Valence Electrons are? The electrons responsible for the chemical properties of atoms, and are those in the outer energy level. Valence electrons - The s and p
Chemical Bonding. Comparison of Properties Ionic Compounds Covalent Compounds Metals
 Chemical Bonding Comparison of Properties Ionic Compounds Covalent Compounds Metals Essential Questions Why/How do atoms combine with one another to form the vast array of chemical substances that exist?
Chemical Bonding Comparison of Properties Ionic Compounds Covalent Compounds Metals Essential Questions Why/How do atoms combine with one another to form the vast array of chemical substances that exist?
Part 01 - Notes: Reactions & Classification
 Objectives: Identify, define, and explain: combination reaction, synthesis reaction, decomposition reaction, single replacement reaction, double replacement reaction, combustion reaction, rapid oxidation,
Objectives: Identify, define, and explain: combination reaction, synthesis reaction, decomposition reaction, single replacement reaction, double replacement reaction, combustion reaction, rapid oxidation,
ELECTRONS. Construct your own electron dot diagram Choose one element & drag the correct number of VALENCE Br electrons around it.
 Ch. 6 - Chemical Bonds Chemical reactivity depends on electron configuration. Remember the Stable Octet rule: when the highest energy level occupied is filled with electrons (8 electrons for most atoms),
Ch. 6 - Chemical Bonds Chemical reactivity depends on electron configuration. Remember the Stable Octet rule: when the highest energy level occupied is filled with electrons (8 electrons for most atoms),
Ch.2: Atoms, Molecules, and Ions
 Ch.2: Atoms, Molecules, and Ions Naming Recall Ionic Bond = electrostatic attraction due to the transfer of vse - s between a metal and nonmetal Covalent Bond = sharing of valence electrons between nonmetals
Ch.2: Atoms, Molecules, and Ions Naming Recall Ionic Bond = electrostatic attraction due to the transfer of vse - s between a metal and nonmetal Covalent Bond = sharing of valence electrons between nonmetals
IONIC BONDS & IONIC FORMULAS
 IONIC BONDS & IONIC FORMULAS BONDING CHEMICAL BONDING Chemical bond an attraction between 2 atoms involving their valence electrons Ionic bond -chemical bond resulting from the electrostatic attraction
IONIC BONDS & IONIC FORMULAS BONDING CHEMICAL BONDING Chemical bond an attraction between 2 atoms involving their valence electrons Ionic bond -chemical bond resulting from the electrostatic attraction
Chemical Nomenclature
 Elements Chemical Nomenclature Elements are said with just their name Mg = magnesium Ca = calcium How to write and say chemical formulas Compounds Most elements are not found separately but combined in
Elements Chemical Nomenclature Elements are said with just their name Mg = magnesium Ca = calcium How to write and say chemical formulas Compounds Most elements are not found separately but combined in
Chapter 3 - Molecules, Compounds and Chemical Equations
 Chapter 3 - Molecules, Compounds and Chemical Equations Section 3.2 two general types of bonding between atoms found in compounds, ionic and covalent ionic bonds result when electrons have been transferred
Chapter 3 - Molecules, Compounds and Chemical Equations Section 3.2 two general types of bonding between atoms found in compounds, ionic and covalent ionic bonds result when electrons have been transferred
Chemical Formulas and Chemical Nomenclature. Mr. Matthew Totaro Legacy High School Honors Chemistry
 Chemical Formulas and Chemical Nomenclature Mr. Matthew Totaro Legacy High School Honors Chemistry 1 Molecular View of Elements and Compounds 2 Atomic Elements Atomic Elements = elements whose smallest
Chemical Formulas and Chemical Nomenclature Mr. Matthew Totaro Legacy High School Honors Chemistry 1 Molecular View of Elements and Compounds 2 Atomic Elements Atomic Elements = elements whose smallest
Big Idea: Matter & Atoms
 Big Idea: Matter & Atoms Naming Ionic Compounds Naming Covalent Compounds Naming Acids Naming Hydrates The cation (positive ion) is written first Takes the same name as the element if only forms one charge
Big Idea: Matter & Atoms Naming Ionic Compounds Naming Covalent Compounds Naming Acids Naming Hydrates The cation (positive ion) is written first Takes the same name as the element if only forms one charge
H 2 O. Chapter 9 Chemical Names and Formulas
 H 2 O Chapter 9 Chemical Names and Formulas Section 9.1 Naming Ions OBJECTIVES: Identify the charges on monatomic ions by using the periodic table, and name the ions. Section 9.1 Naming Ions OBJECTIVES:
H 2 O Chapter 9 Chemical Names and Formulas Section 9.1 Naming Ions OBJECTIVES: Identify the charges on monatomic ions by using the periodic table, and name the ions. Section 9.1 Naming Ions OBJECTIVES:
Bonding and Nomenclature notes.notebook
 Chemical Bonding & Nomenclature Objectives: Distinguish between covalent and ionic bonding Explain the process of bonding Name ionic and covalent compounds and acids Write chemical formulas for ionic and
Chemical Bonding & Nomenclature Objectives: Distinguish between covalent and ionic bonding Explain the process of bonding Name ionic and covalent compounds and acids Write chemical formulas for ionic and
Unit 5: Bonding Part 2 (Covalent Bonds/Bond & Molecular Polarity/IMF)
 Unit 5: Bonding Part 2 (Covalent Bonds/Bond & Molecular ity/imf) The following pages are practice questions for this unit, and will be submitted for homework! You must complete: Ionic vs. Covalent Properties
Unit 5: Bonding Part 2 (Covalent Bonds/Bond & Molecular ity/imf) The following pages are practice questions for this unit, and will be submitted for homework! You must complete: Ionic vs. Covalent Properties
Chapter 7. Ionic Compounds and Metals
 Chapter 7 Ionic Compounds and Metals Periodic Trends Metals O Hate electrons O Give electrons away. O Have a low ionization energy. O Ions are always postive. O Cations (meow) Non-Metals O Love electrons
Chapter 7 Ionic Compounds and Metals Periodic Trends Metals O Hate electrons O Give electrons away. O Have a low ionization energy. O Ions are always postive. O Cations (meow) Non-Metals O Love electrons
Experiment #4. Chemical Nomenclature
 Experiment #4. Chemical Nomenclature Many everyday and historically important chemical compounds have common names. For example, water is the common name for H 2 O, baking soda is the common name for NaHCO
Experiment #4. Chemical Nomenclature Many everyday and historically important chemical compounds have common names. For example, water is the common name for H 2 O, baking soda is the common name for NaHCO
CHEM 1105 S10 January 21, 2014
 CHEM 1105 S10 January 21, 2014 Chapter 3: Compounds and Formulas Today: Types of compounds: Ionic vs. covalent Naming ionic compounds Naming binary covalent compounds (two elements only) Ionic Bonding
CHEM 1105 S10 January 21, 2014 Chapter 3: Compounds and Formulas Today: Types of compounds: Ionic vs. covalent Naming ionic compounds Naming binary covalent compounds (two elements only) Ionic Bonding
IB Chemistry. Chapter 4.1
 IB Chemistry Chapter 4.1 Chemical Bonds Atoms or ions that are strongly attached to one another Chemical bonds will form if potential energy decreases (becomes more stable) 2 Valence Electrons Valence
IB Chemistry Chapter 4.1 Chemical Bonds Atoms or ions that are strongly attached to one another Chemical bonds will form if potential energy decreases (becomes more stable) 2 Valence Electrons Valence
Name HONORS CHEMISTRY / / Oxide Reactions & Net Ionic Reactions
 Name HONORS CHEMISTRY / / Oxide Reactions & Net Ionic Reactions The first type of reactions we will look at today are reactions between an oxide (a compound with oxygen as its anion) and water. There are
Name HONORS CHEMISTRY / / Oxide Reactions & Net Ionic Reactions The first type of reactions we will look at today are reactions between an oxide (a compound with oxygen as its anion) and water. There are
Compounds form when elements, or electrons Ionic Compound: when one element and another element o Formed between a and a
 UNIT 5: IONIC COMPOUNDS NOTES ***Refer to Ion Charge Chart and Periodic Table for Reference Review Compounds Simple Ions All atoms want to have a full outer set of valence electrons this makes them Octet
UNIT 5: IONIC COMPOUNDS NOTES ***Refer to Ion Charge Chart and Periodic Table for Reference Review Compounds Simple Ions All atoms want to have a full outer set of valence electrons this makes them Octet
Name: Period: Score: Everything About Chemical Formulas
 Name: Period: Score: Everything About Formulas Compounds have unique names that identify them for us when we study chemical properties and changes. Chemists have devised a shorthand way of representing
Name: Period: Score: Everything About Formulas Compounds have unique names that identify them for us when we study chemical properties and changes. Chemists have devised a shorthand way of representing
Types of bonding: OVERVIEW
 1 of 43 Boardworks Ltd 2009 Types of bonding: OVERVIEW 2 of 43 Boardworks Ltd 2009 There are three types of bond that can occur between atoms: an ionic bond occurs between a metal and non-metal atom (e.g.
1 of 43 Boardworks Ltd 2009 Types of bonding: OVERVIEW 2 of 43 Boardworks Ltd 2009 There are three types of bond that can occur between atoms: an ionic bond occurs between a metal and non-metal atom (e.g.
Chemistry Chapter 2. Atoms, Molecules, and Ions Section Periodic Table Ions Chemical Bonds Nomenclature
 Chemistry Chapter 2 Atoms, Molecules, and Ions Section 2 2.6-2.8 Periodic Table Ions Chemical Bonds Nomenclature Organization of the Periodic Table Columns, Groups or Families elements in the same vertical
Chemistry Chapter 2 Atoms, Molecules, and Ions Section 2 2.6-2.8 Periodic Table Ions Chemical Bonds Nomenclature Organization of the Periodic Table Columns, Groups or Families elements in the same vertical
WRITING FORMULAS: MOLAR MASS, %COMPOSITION, EMPIRICAL AND MOLECULAR FORMULAS
 WRITING FORMULAS: MOLAR MASS, %COMPOSITION, EMPIRICAL AND MOLECULAR FORMULAS REVIEW: Polyatomic ions, writing names from formulas, oxidation number rules I. WRITING FORMULAS FROM NAMES: A. Rules: 1. Know
WRITING FORMULAS: MOLAR MASS, %COMPOSITION, EMPIRICAL AND MOLECULAR FORMULAS REVIEW: Polyatomic ions, writing names from formulas, oxidation number rules I. WRITING FORMULAS FROM NAMES: A. Rules: 1. Know
Ionic, Covalent, Metallic
 Ionic, Covalent, Metallic Physical Properties of Types of Compounds IONIC COVALENT METALLIC Attractive/force strength Melting/Boiling point Strong Weak Varies High Low Varies Vapor pressure Low High Varies
Ionic, Covalent, Metallic Physical Properties of Types of Compounds IONIC COVALENT METALLIC Attractive/force strength Melting/Boiling point Strong Weak Varies High Low Varies Vapor pressure Low High Varies
Ionic bonds occur between a metal and a nonmetal. Covalent bonds occur between two or more nonmetals. Metallic bonds occur between metal atoms only.
 Ionic bonds occur between a metal and a nonmetal. Covalent bonds occur between two or more nonmetals. Metallic bonds occur between metal atoms only. Using chemical equations to show ionization: Na Na +
Ionic bonds occur between a metal and a nonmetal. Covalent bonds occur between two or more nonmetals. Metallic bonds occur between metal atoms only. Using chemical equations to show ionization: Na Na +
Nomenclature (Naming Compounds) and Chemical Formulas
 Nomenclature (Naming Compounds) and Chemical Formulas 1 Ions formed from a single atom Monatomic Ions Charges are determined by whether ion has lost electrons (+) or gained electrons (-) Symbols are written
Nomenclature (Naming Compounds) and Chemical Formulas 1 Ions formed from a single atom Monatomic Ions Charges are determined by whether ion has lost electrons (+) or gained electrons (-) Symbols are written
Chapter 7: Ionic Compounds and Metals
 Chapter 7: Ionic Compounds and Metals Section 7.1 Section 7.2 Section 7.3 Section 7.4 Ion Formation Ionic Bonds and Ionic Compounds Names and Formulas for Ionic Compounds Metallic Bonds and the Properties
Chapter 7: Ionic Compounds and Metals Section 7.1 Section 7.2 Section 7.3 Section 7.4 Ion Formation Ionic Bonds and Ionic Compounds Names and Formulas for Ionic Compounds Metallic Bonds and the Properties
1/28/13. Naming and Writing Formulas > for Ionic Compounds
 chemistry 1 of 29 Naming and Writing Formulas A recipe is a formula for the sauce a complete list of ingredients and their proportions. Chemistry also uses formulas. Once you know the rules, you can write
chemistry 1 of 29 Naming and Writing Formulas A recipe is a formula for the sauce a complete list of ingredients and their proportions. Chemistry also uses formulas. Once you know the rules, you can write
Chapter 3 Molecules, Compounds, and Chemical Equations
 Chapter 3 Molecules, Compounds, and Chemical Equations Molecular View of Elements and Compounds 2 How do atom join together to form a compound? compounds are made of atoms held together by chemical bonds
Chapter 3 Molecules, Compounds, and Chemical Equations Molecular View of Elements and Compounds 2 How do atom join together to form a compound? compounds are made of atoms held together by chemical bonds
Names and Formulas of Compounds. J. Venables
 Names and Formulas of Compounds Chemistry 2 Honors J. Venables Northwestern High School Formation of Compounds When an atom or molecule loses electrons, it becomes positively charged. For example, when
Names and Formulas of Compounds Chemistry 2 Honors J. Venables Northwestern High School Formation of Compounds When an atom or molecule loses electrons, it becomes positively charged. For example, when
Name: Hour: Unit 2 Periodic Table Nomenclature. Notepack Chapters 5 and 6
 Name: Hour: Unit 2 Periodic Table Nomenclature Notepack Chapters 5 and 6 1 Periodic Table & Nomenclature Chapter 5 Part One: Review of Atomic Structure (Pages 107-121) A. Define atom 1. proton - 2. neutron-
Name: Hour: Unit 2 Periodic Table Nomenclature Notepack Chapters 5 and 6 1 Periodic Table & Nomenclature Chapter 5 Part One: Review of Atomic Structure (Pages 107-121) A. Define atom 1. proton - 2. neutron-
Chapter 5 BONDING AND MOLECULES
 Chapter 5 BONDING AND MOLECULES How Do Atoms Combine to Form Compounds? (5.1) Chemical bonds: a force of attraction between atoms or ions. Octet Rule: atoms tend to gain, lose, or share electrons in order
Chapter 5 BONDING AND MOLECULES How Do Atoms Combine to Form Compounds? (5.1) Chemical bonds: a force of attraction between atoms or ions. Octet Rule: atoms tend to gain, lose, or share electrons in order
CHEM 103 Naming Compounds
 CHEM 103 Naming Compounds Lecture Notes February 9, 2006 Prof. Sevian Chem 103 Please sit with your groups today. We will be doing a group problem at the end of class. 2 2005 H. Sevian 1 Agenda How we
CHEM 103 Naming Compounds Lecture Notes February 9, 2006 Prof. Sevian Chem 103 Please sit with your groups today. We will be doing a group problem at the end of class. 2 2005 H. Sevian 1 Agenda How we
NAMING IONIC COMPOUNDS. ammonium sulfide. iron(ii) carbonate. barium phosphate. titanium(iv) sulfide. Spelling matters!
 67 NAMING IONIC COMPOUNDS ammonium sulfide iron(ii) carbonate titanium(iv) sulfide barium phosphate Spelling matters! barium phosphide 68 DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The
67 NAMING IONIC COMPOUNDS ammonium sulfide iron(ii) carbonate titanium(iv) sulfide barium phosphate Spelling matters! barium phosphide 68 DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The
Unit 4. Bonding and Nomenclature
 Unit 4 Bonding and Nomenclature A. Vocabulary Chemical Bond attractive force between atoms or ions that binds them together as a unit bonds form in order to decrease potential energy (PE) increase stability
Unit 4 Bonding and Nomenclature A. Vocabulary Chemical Bond attractive force between atoms or ions that binds them together as a unit bonds form in order to decrease potential energy (PE) increase stability
Science 1206 Ch. 3 - Chemical names, formulas and equations
 Science 1206 Ch. 3 - Chemical names, formulas and equations 3.1 - Ionic and molecular compounds (pp. 98-107) Compounds A compound is a pure substance made of a combination of elements. The elements are
Science 1206 Ch. 3 - Chemical names, formulas and equations 3.1 - Ionic and molecular compounds (pp. 98-107) Compounds A compound is a pure substance made of a combination of elements. The elements are
Chemistry 1-2E Semester I Study Guide
 Chemistry 1-2E Semester I Study Guide Name Hour Chapter 1 1. Define the following terms. Matter Mass Law of Conservation of Mass 2. Define and give 2 examples of the following: Pure substance Element Compound
Chemistry 1-2E Semester I Study Guide Name Hour Chapter 1 1. Define the following terms. Matter Mass Law of Conservation of Mass 2. Define and give 2 examples of the following: Pure substance Element Compound
NOMENCLATURE AND WRITING FORMULAS
 NOMENCLATURE AND WRITING FORMULAS PART I--FORMULAS AND NOMENCLATURE OF IONIC COMPOUND Composed of Cations and Anions. Types of Cations (positive ions): A. Metals lose electrons to form positive ions. These
NOMENCLATURE AND WRITING FORMULAS PART I--FORMULAS AND NOMENCLATURE OF IONIC COMPOUND Composed of Cations and Anions. Types of Cations (positive ions): A. Metals lose electrons to form positive ions. These
7 Ionic Compounds and Metals
 Date Class 7 Ionic Compounds and Metals Section 7.1 Ion Formation In your textbook, read about chemical bonds and formation of ions. Use each of the terms below just once to complete the passage. chemical
Date Class 7 Ionic Compounds and Metals Section 7.1 Ion Formation In your textbook, read about chemical bonds and formation of ions. Use each of the terms below just once to complete the passage. chemical
- Use the STEM NAME of the element, then add "-ide" suffix
 65 ANIONS 2 kinds Main-group nonmetals - Use the STEM NAME of the element, then add "-ide" suffix N : "nitride" ion P : "phosphide ion" O : "oxide ion" F : "fluoride ion" Polyatomic ions - Memorize list.(see
65 ANIONS 2 kinds Main-group nonmetals - Use the STEM NAME of the element, then add "-ide" suffix N : "nitride" ion P : "phosphide ion" O : "oxide ion" F : "fluoride ion" Polyatomic ions - Memorize list.(see
CHAPTER 8 Ionic and Metallic Bonds
 CHAPTER 8 Ionic and Metallic Bonds Shows the kind of atoms and number of atoms in a compound. MgCl 2 NaCl CaCO 3 Al 2 O 3 Ca 3 (PO 4 ) 2 Chemical Formulas Al: Cl: counting atoms AlCl 3 Pb: N: O: Pb(NO
CHAPTER 8 Ionic and Metallic Bonds Shows the kind of atoms and number of atoms in a compound. MgCl 2 NaCl CaCO 3 Al 2 O 3 Ca 3 (PO 4 ) 2 Chemical Formulas Al: Cl: counting atoms AlCl 3 Pb: N: O: Pb(NO
- Some MOLECULES can gain or lose electrons to form CATIONS or ANIONS. These are called POLYATOMIC IONS
 63 POLYATOMIC IONS - Some MOLECULES can gain or lose electrons to form CATIONS or ANIONS. These are called POLYATOMIC IONS - Polyatomic ions form ionic compounds in the same way that single-element ions
63 POLYATOMIC IONS - Some MOLECULES can gain or lose electrons to form CATIONS or ANIONS. These are called POLYATOMIC IONS - Polyatomic ions form ionic compounds in the same way that single-element ions
Atoms and Bonding. Chapter 18 Physical Science
 Atoms and Bonding Chapter 18 Physical Science 2017-2018 Atoms and Bonding: Chemical Bonding The combining of atoms of elements to form new substances. Bonding of atoms determine a compound s properties.
Atoms and Bonding Chapter 18 Physical Science 2017-2018 Atoms and Bonding: Chemical Bonding The combining of atoms of elements to form new substances. Bonding of atoms determine a compound s properties.
World of Chemistry Notes for Students [Chapter 4, page 1] Chapter 4 Nomenclature
![World of Chemistry Notes for Students [Chapter 4, page 1] Chapter 4 Nomenclature World of Chemistry Notes for Students [Chapter 4, page 1] Chapter 4 Nomenclature](/thumbs/78/77609914.jpg) World of Chemistry Notes for Students [Chapter 4, page 1] Chapter 4 Nomenclature 1) The Periodic Table Review from Chapter The elements are arranged in rows and columns on the Periodic Table according
World of Chemistry Notes for Students [Chapter 4, page 1] Chapter 4 Nomenclature 1) The Periodic Table Review from Chapter The elements are arranged in rows and columns on the Periodic Table according
Nomenclature of inorganic compounds. = naming non carbon (mostly) compounds. Some definitions:
 1 Chemistry 047 Inorganic Nomenclature Nomenclature of inorganic compounds = naming non carbon (mostly) compounds Some definitions: Nomenclature = system used by chemists to name and identify compounds
1 Chemistry 047 Inorganic Nomenclature Nomenclature of inorganic compounds = naming non carbon (mostly) compounds Some definitions: Nomenclature = system used by chemists to name and identify compounds
Identify the reaction type, predict the products, and balance the equations. If it is a special decomposition or synthesis, identify which kind.
 Identify the reaction type, predict the products, and balance the equations. If it is a special decomposition or synthesis, identify which kind. 1. calcium + oxygen 2. cupric carbonate 3. aluminum + hydrochloric
Identify the reaction type, predict the products, and balance the equations. If it is a special decomposition or synthesis, identify which kind. 1. calcium + oxygen 2. cupric carbonate 3. aluminum + hydrochloric
Chemical Nomenclature
 Chemical Nomenclature Learn names you will Review: Valence electrons (the outer most electrons) are responsible for the interaction between atoms when forming chemical compounds. Another way to say that
Chemical Nomenclature Learn names you will Review: Valence electrons (the outer most electrons) are responsible for the interaction between atoms when forming chemical compounds. Another way to say that
DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME
 DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The name of an ionic compound is made of the names of the CATION and ANION in the compound. - To get the FORMULA, you must figure out the SMALLEST
DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The name of an ionic compound is made of the names of the CATION and ANION in the compound. - To get the FORMULA, you must figure out the SMALLEST
Molecules Compounds chemical bonds
 Chemical Bonds Molecules are made up of more than one atom. Compounds are made up of more than one element. When atoms combine to make molecules, they form chemical bonds. Types of Bonds: Ionic Bonds Covalent
Chemical Bonds Molecules are made up of more than one atom. Compounds are made up of more than one element. When atoms combine to make molecules, they form chemical bonds. Types of Bonds: Ionic Bonds Covalent
Name CHEMISTRY / / Oxide Reactions & Net Ionic Reactions
 Name CHEMISTRY / / Oxide Reactions & Net Ionic Reactions The first type of reactions we will look at today are reactions between an oxide (a compound with oxygen as its anion) and water. There are two
Name CHEMISTRY / / Oxide Reactions & Net Ionic Reactions The first type of reactions we will look at today are reactions between an oxide (a compound with oxygen as its anion) and water. There are two
Bonding: Part Two. Three types of bonds: Ionic Bond. transfer valence e - Metallic bond. (NaCl) (Fe) mobile valence e - Covalent bond
 Bonding: Part Two Three types of bonds: Ionic Bond transfer valence e - Metallic bond mobile valence e - Covalent bond (NaCl) (Fe) shared valence e - (H 2 O) 1 Single Covalent Bond H + H H H H-atoms H
Bonding: Part Two Three types of bonds: Ionic Bond transfer valence e - Metallic bond mobile valence e - Covalent bond (NaCl) (Fe) shared valence e - (H 2 O) 1 Single Covalent Bond H + H H H H-atoms H
O ( ) are only used with polyatomic ions and only when there is more than one of any group. a. Examples: HNO 3. ) is incorrect; Al 2 ) 3 (SO 4
 HONORS CHEMISTRY - CHAPTER 9 CHEMICAL NAMES AND FORMULAS NOMENCLATURE PACKET - V16 NAME: DATE: PAGE: I. Writing formulas of ionic compounds when given the component parts. 1. Many compounds are composed
HONORS CHEMISTRY - CHAPTER 9 CHEMICAL NAMES AND FORMULAS NOMENCLATURE PACKET - V16 NAME: DATE: PAGE: I. Writing formulas of ionic compounds when given the component parts. 1. Many compounds are composed
Unit 1 Physical Science: Chemical Reactions
 Unit 1 Physical Science: Chemical Reactions The physical sciences are concerned with the study of inanimate natural objects. Chemistry is the study of matter, its properties, how and why substances combine
Unit 1 Physical Science: Chemical Reactions The physical sciences are concerned with the study of inanimate natural objects. Chemistry is the study of matter, its properties, how and why substances combine
Physical Science Study Guide
 Name: Class: Date: Physical Science Study Guide Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Mendeleev arranged the known chemical elements in a table
Name: Class: Date: Physical Science Study Guide Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Mendeleev arranged the known chemical elements in a table
Bonding: Part Two. Three types of bonds: Ionic Bond. transfer valence e - Metallic bond. (NaCl) (Fe) mobile valence e - Covalent bond
 Bonding: Part Two Three types of bonds: Ionic Bond transfer valence e - Metallic bond mobile valence e - Covalent bond (NaCl) (Fe) shared valence e - (H 2 O) 1 Single Covalent Bond H + H H H H-atoms H
Bonding: Part Two Three types of bonds: Ionic Bond transfer valence e - Metallic bond mobile valence e - Covalent bond (NaCl) (Fe) shared valence e - (H 2 O) 1 Single Covalent Bond H + H H H H-atoms H
Chapter 7 & 8 Nomenclature Notes/Study Guide. Properties of ionic bonds & compounds. Section 7-2
 Objectives Properties of ionic bonds & compounds Section 72 Define chemical bond. Describe formation of ionic bonds structure of ionic compounds. Generalize of ionic bonds based on Main Idea of ionic compounds
Objectives Properties of ionic bonds & compounds Section 72 Define chemical bond. Describe formation of ionic bonds structure of ionic compounds. Generalize of ionic bonds based on Main Idea of ionic compounds
2 Ionic and Covalent Bonding
 CHAPTER 6 2 Ionic and Covalent Bonding SECTION The Structure of Matter KEY IDEAS As you read this section, keep these questions in mind: Why do atoms form bonds? How do ionic bonds and covalent bonds differ?
CHAPTER 6 2 Ionic and Covalent Bonding SECTION The Structure of Matter KEY IDEAS As you read this section, keep these questions in mind: Why do atoms form bonds? How do ionic bonds and covalent bonds differ?
Naming Simple Compounds
 Naming Simple Compounds Ionic Compounds Ionic compounds consist of positive and negative ions. have attractions called ionic bonds between positively and negatively charged ions. have high melting and
Naming Simple Compounds Ionic Compounds Ionic compounds consist of positive and negative ions. have attractions called ionic bonds between positively and negatively charged ions. have high melting and
Cartoon courtesy of NearingZero.net. Unit 3: Chemical Bonding and Molecular Structure
 Cartoon courtesy of NearingZero.net Unit 3: Chemical Bonding and Molecular Structure Bonds Forces that hold groups of atoms together and make them function as a unit. Ionic bonds transfer of electrons
Cartoon courtesy of NearingZero.net Unit 3: Chemical Bonding and Molecular Structure Bonds Forces that hold groups of atoms together and make them function as a unit. Ionic bonds transfer of electrons
Topic 5: The Language of Chemistry
 Topic 5: The Language of Chemistry Chemical Formulas & Chemical Compounds (Chapter 7 in Modern Chemistry) A Chemical Formula Recall that a chemical formula indicates the relative number of atoms of each
Topic 5: The Language of Chemistry Chemical Formulas & Chemical Compounds (Chapter 7 in Modern Chemistry) A Chemical Formula Recall that a chemical formula indicates the relative number of atoms of each
lost, gained or shared chemical bonds symbols subscripts NaCl, H O, CaCO, CO
 Topics Chemical Bonds Force that holds atom together Stability in Bonding Valence Electrons Reactivity Types of Bonds Ionic, Metallic, Covalent, Hydrogen bond Writing Formulas and Naming Compounds Binary
Topics Chemical Bonds Force that holds atom together Stability in Bonding Valence Electrons Reactivity Types of Bonds Ionic, Metallic, Covalent, Hydrogen bond Writing Formulas and Naming Compounds Binary
Ionic Bonding (Ch.7) Covalent Bonding (Ch.8) Metallic Bonding
 Unit 3: Chemical Bonding Outline Ionic Bonding (Ch.7) Valence electrons Positive and negative ions and transition metal ions Ionic bonding: Charge on compounds Ionic compounds characteristics Writing ionic
Unit 3: Chemical Bonding Outline Ionic Bonding (Ch.7) Valence electrons Positive and negative ions and transition metal ions Ionic bonding: Charge on compounds Ionic compounds characteristics Writing ionic
Description Computer Bonding. Late Lab Stamp (this stamp means you are not qualified to do lab and test corrections) Name: Period:
 Chemistry: Hood River Valley High School Unit 4 Note Pack and Goals Name: Period: Unit 4 Metals, Non-Metals, Metalloids, Ions, and Ionic compounds. Unit Goals- As you work through this unit, you should
Chemistry: Hood River Valley High School Unit 4 Note Pack and Goals Name: Period: Unit 4 Metals, Non-Metals, Metalloids, Ions, and Ionic compounds. Unit Goals- As you work through this unit, you should
What are the rules for writing and naming stable ionic formulas?
 1 1. Define electronegativity. a measure of the ability of an atom in a chemical compound to attract electrons. 2. On the periodic table, where are the LEAST/MOST electronegative elements found? Least-Bottom
1 1. Define electronegativity. a measure of the ability of an atom in a chemical compound to attract electrons. 2. On the periodic table, where are the LEAST/MOST electronegative elements found? Least-Bottom
Introduction To Nomenclature. based on procedures created by IUPAC which stands for the International Union of Pure and Applied Chemistry
 Introduction To Nomenclature the skill of determining the name and/or chemical formula of a compound based on procedures created by IUPAC which stands for the International Union of Pure and Applied Chemistry
Introduction To Nomenclature the skill of determining the name and/or chemical formula of a compound based on procedures created by IUPAC which stands for the International Union of Pure and Applied Chemistry
Naming Inorganic Compounds. common names systematic names
 Naming Inorganic Compounds common names systematic names Molecular Common Systematic Formula name name AgCl Lunar caustic Silver chloride H 2 SO 4 Oil of vitriol Sulfuric acid MgSO 4 Epsom salts Magnesium
Naming Inorganic Compounds common names systematic names Molecular Common Systematic Formula name name AgCl Lunar caustic Silver chloride H 2 SO 4 Oil of vitriol Sulfuric acid MgSO 4 Epsom salts Magnesium
Nomenclature. Naming Compounds
 Nomenclature Naming Compounds Ionic Compounds Metal bonding with non-metal One atom gains electrons, one atom loses electrons Exist as ions with full highest energy levels. Are held together in a giant
Nomenclature Naming Compounds Ionic Compounds Metal bonding with non-metal One atom gains electrons, one atom loses electrons Exist as ions with full highest energy levels. Are held together in a giant
CHEMICAL BONDING IONIC BONDS COVALENT BONDS HYDROGEN BONDS METALLIC BONDS
 CHEMICAL BONDING IONIC BONDS COVALENT BONDS HYDROGEN BONDS METALLIC BONDS IONIC BONDING When an atom of a nonmetal takes one or more electrons from an atom of a metal so both atoms end up with eight valence
CHEMICAL BONDING IONIC BONDS COVALENT BONDS HYDROGEN BONDS METALLIC BONDS IONIC BONDING When an atom of a nonmetal takes one or more electrons from an atom of a metal so both atoms end up with eight valence
The Structure of Matter:
 The Structure of Matter: How atoms form compounds and Chemical Bonding This information is found in Chapter 6 Sections 1 & 3. 1 Compounds Are formed when two or more elements combine (or compounds combine)
The Structure of Matter: How atoms form compounds and Chemical Bonding This information is found in Chapter 6 Sections 1 & 3. 1 Compounds Are formed when two or more elements combine (or compounds combine)
NAMING IONIC COMPOUNDS
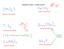 NAMING IONIC COMPOUNDS iron(ii) carbonate ammonium sulfide titanium (IV) sulfide barium phosphate Spelling matters! calcium nitrate sodium phosphide DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE
NAMING IONIC COMPOUNDS iron(ii) carbonate ammonium sulfide titanium (IV) sulfide barium phosphate Spelling matters! calcium nitrate sodium phosphide DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE
Chemical Bonding and Naming Compounds. Ionic. Acid. Base. Oct 4 7:40 PM
 Chemical Bonding and Naming Compounds (Chapter 9) Types of Bonds Ionic Bonds Molecular Bonds Types of Compounds Ionic Molecular Acid Base Chemical Bonding Atoms will bond together using their valence electrons.
Chemical Bonding and Naming Compounds (Chapter 9) Types of Bonds Ionic Bonds Molecular Bonds Types of Compounds Ionic Molecular Acid Base Chemical Bonding Atoms will bond together using their valence electrons.
Chemical Bonding. Chemical Bonds. Metals, Ions, or Molecules. All Matter Exists as Atoms,
 Chemical Bonding Valence electrons (the outer most electrons) are responsible for the interaction between atoms when forming chemical compounds. Another way to say that is that valence electrons are the
Chemical Bonding Valence electrons (the outer most electrons) are responsible for the interaction between atoms when forming chemical compounds. Another way to say that is that valence electrons are the
Chemical Bonds I. Why Atoms Combine
 Chemical Bonds I. Why Atoms Combine Chemical Formula Chemical Bond Stability What is a compound? What does the word compound mean in this sentence? I have a compound fracture in my leg. What is a compound?
Chemical Bonds I. Why Atoms Combine Chemical Formula Chemical Bond Stability What is a compound? What does the word compound mean in this sentence? I have a compound fracture in my leg. What is a compound?
Chapter 6 and 15 Ionic Compounds
 Chapter 6 and 15 Ionic Compounds Chapter 6 Ionic compounds 6.3, 6.4 6.1: Intro to Chemical Bonding A chemical bond is a mutual electrical attraction between the nuclei and valence electrons of different
Chapter 6 and 15 Ionic Compounds Chapter 6 Ionic compounds 6.3, 6.4 6.1: Intro to Chemical Bonding A chemical bond is a mutual electrical attraction between the nuclei and valence electrons of different
Why does an element want to bond?
 Why does an element want to bond? State 3 differences between ionic vs. covalent compounds What is a chemical formula? It indicates the relative number of atoms of each kind in an ionic compound. Ex Al
Why does an element want to bond? State 3 differences between ionic vs. covalent compounds What is a chemical formula? It indicates the relative number of atoms of each kind in an ionic compound. Ex Al
Nomenclature Naming Ionic Compounds Worksheet #1
 Naming Ionic Compounds Worksheet #1 In forming ionic compounds with non-metals, the transition metals often exhibit more than one valence. For example, in the reaction between iron and chlorine, two products
Naming Ionic Compounds Worksheet #1 In forming ionic compounds with non-metals, the transition metals often exhibit more than one valence. For example, in the reaction between iron and chlorine, two products
Chemistry Day 32. Monday, November 26 th Tuesday, November 27 th, 2018
 Chemistry Day 32 Monday, November 26 th Tuesday, November 27 th, 2018 Do-Now: Unit Exam Day Do-Now 1. Write down today s FLT 2. The element in group 7A & period 4 is. 3. The are in group 2A and have valence
Chemistry Day 32 Monday, November 26 th Tuesday, November 27 th, 2018 Do-Now: Unit Exam Day Do-Now 1. Write down today s FLT 2. The element in group 7A & period 4 is. 3. The are in group 2A and have valence
Ions and Ionic Compounds
 Ions and Ionic Compounds Elements combine in a specific ratio to form compounds. Compounds can be categorized as ionic or covalent depending on the type of bond present within the compound. Ionic compounds
Ions and Ionic Compounds Elements combine in a specific ratio to form compounds. Compounds can be categorized as ionic or covalent depending on the type of bond present within the compound. Ionic compounds
Experiment #4. Elements and Compounds.
 Experiment #4. Elements and Compounds. Goals To practice naming and classifying elements and compounds Background Properties of Elements Elements on the periodic table can be classified as metals, nonmetals
Experiment #4. Elements and Compounds. Goals To practice naming and classifying elements and compounds Background Properties of Elements Elements on the periodic table can be classified as metals, nonmetals
Chemistry Study Guide
 Chemistry Study Guide Marking Period 3 Exam Week of 3/21/17 Study Guide due - When studying for this test, use your do nows, notes, homework, class handouts, and your textbook. Vocabulary Chapter 7 Anion
Chemistry Study Guide Marking Period 3 Exam Week of 3/21/17 Study Guide due - When studying for this test, use your do nows, notes, homework, class handouts, and your textbook. Vocabulary Chapter 7 Anion
Nomenclature. Ex. For sodium the oxidation number is +1. For oxygen the oxidation number is -2.
 Nomenclature 1. BONDING CAPACITY (VALENCE) The number of bonds an atom can make. For a Cation, the bonding capacity is the number of electrons lost to become stable. For an Anion, the bonding capacity
Nomenclature 1. BONDING CAPACITY (VALENCE) The number of bonds an atom can make. For a Cation, the bonding capacity is the number of electrons lost to become stable. For an Anion, the bonding capacity
Formula of a Compound
 Name Formula of a Compound 1. Useful only if it correctly represents the substance. 2. The composition is determined in chemical analysis. 3. The formula then is derived by atomic theory and chemical bonding
Name Formula of a Compound 1. Useful only if it correctly represents the substance. 2. The composition is determined in chemical analysis. 3. The formula then is derived by atomic theory and chemical bonding
9/19/07. Chemistry 6A Fall 2007 Dr. J. A. Mack. Molar Masses. Avagagro s s Number. Avogadro s Number and the Mole
 Chemistry 6A Fall 007 Dr. J. A. Mack Avogadro s Number and the Mole The concept of a mole is defined so that we may equate the amount of matter (mass) to the number of particles (mole). The Standard is
Chemistry 6A Fall 007 Dr. J. A. Mack Avogadro s Number and the Mole The concept of a mole is defined so that we may equate the amount of matter (mass) to the number of particles (mole). The Standard is
4.0-Ionic Compounds Unit
 4.0-Ionic Compounds Unit Objectives: --Given formula, determine if compound is ionic, molecular, or an acid. --Given name, write formula --Given formula, write name --Understand how ionic compounds form
4.0-Ionic Compounds Unit Objectives: --Given formula, determine if compound is ionic, molecular, or an acid. --Given name, write formula --Given formula, write name --Understand how ionic compounds form
Naming and Counting Atoms and Molecules. Chemistry--Unit 2
 Naming and Counting Atoms and Molecules Chemistry--Unit 2 Masses of 22.4 L O 2, N 2, & HCl N 2 28 g O 2 32 g HCl 36.5 g Observation: At 1 atmosphere pressure and 0 o C, 22.4 L of 3 different gases have
Naming and Counting Atoms and Molecules Chemistry--Unit 2 Masses of 22.4 L O 2, N 2, & HCl N 2 28 g O 2 32 g HCl 36.5 g Observation: At 1 atmosphere pressure and 0 o C, 22.4 L of 3 different gases have
The chemical formulas of most of the elements are simply their elemental symbol:
 Chemical Formulas A chemical formula gives the numbers and types of atoms that are found in a substance. When the substance is a discrete molecule, then the chemical formula is also its molecular formula.
Chemical Formulas A chemical formula gives the numbers and types of atoms that are found in a substance. When the substance is a discrete molecule, then the chemical formula is also its molecular formula.
Chemical Nomenclature
 Chemical Nomenclature! The first names for chemicals were common names: Sugar, quicklime, Epsom salts, milk of magnesia, gypsom, laughing gas Simple, but not practical, the tell us little about the chemicals
Chemical Nomenclature! The first names for chemicals were common names: Sugar, quicklime, Epsom salts, milk of magnesia, gypsom, laughing gas Simple, but not practical, the tell us little about the chemicals
Chemistry 51 Chapter 5 OCTET RULE & IONS
 OCTET RULE & IONS Most elements, except noble gases, combine to form compounds. Compounds are the result of the formation of chemical bonds between two or more different elements. In the formation of a
OCTET RULE & IONS Most elements, except noble gases, combine to form compounds. Compounds are the result of the formation of chemical bonds between two or more different elements. In the formation of a
Occurs when electrons are transferred electrostatic attractions (btw positive & negative atoms)
 Ionic Bonding Ionic Bonding Occurs when electrons are transferred from one atom to another, forming two ions The ions stay together because of electrostatic attractions (btw positive & negative atoms)
Ionic Bonding Ionic Bonding Occurs when electrons are transferred from one atom to another, forming two ions The ions stay together because of electrostatic attractions (btw positive & negative atoms)
For a quick and enjoyable introduction to Covalent vs Ionic Bonding watch this video:
 Covalent Bonding Covalent Bonding is the result of sharing of electron pairs between 2 nonmetal atoms Caution: sharing can be complicated Recall the Octet Rule: Atoms tend to gain, lose or share valence
Covalent Bonding Covalent Bonding is the result of sharing of electron pairs between 2 nonmetal atoms Caution: sharing can be complicated Recall the Octet Rule: Atoms tend to gain, lose or share valence
CHEM 103 Naming Compounds
 CHEM 103 Naming Compounds Lecture Notes February 7, 2006 Prof. Sevian 1 Agenda How we name compounds depends on what kind of compounds they are Ionic compounds Molecular compounds Acids are molecular compounds
CHEM 103 Naming Compounds Lecture Notes February 7, 2006 Prof. Sevian 1 Agenda How we name compounds depends on what kind of compounds they are Ionic compounds Molecular compounds Acids are molecular compounds
What holds a compound together? How can the structure of chemical compounds be shown? What determines the properties of a compounds?
 The Structure of Matter Section 6.1: Compounds and Molecules What holds a compound together? How can the structure of chemical compounds be shown? What determines the properties of a compounds? Types of
The Structure of Matter Section 6.1: Compounds and Molecules What holds a compound together? How can the structure of chemical compounds be shown? What determines the properties of a compounds? Types of
Unit 7. Bonds and Naming
 Unit 7 Bonds and Naming I. Ionic Bonds Positive ion is attracted to a negative ion; usually a metal & a nonmetal Ionic compound: a substance that has ionic bonds Cation: positive ion Anion: negative ion
Unit 7 Bonds and Naming I. Ionic Bonds Positive ion is attracted to a negative ion; usually a metal & a nonmetal Ionic compound: a substance that has ionic bonds Cation: positive ion Anion: negative ion
Chapter 1 Section 1- Pages 4-7: Electrons and Chemical Bonding COMBINING ATOMS THROUGH CHEMICAL BONDING
 Study Guide Chapter 1 and 2 Interactions of Matter Chapter 1 Section 1- Pages 4-7: Electrons and Chemical Bonding COMBINING ATOMS THROUGH CHEMICAL BONDING 1. Which of these substances is a combination
Study Guide Chapter 1 and 2 Interactions of Matter Chapter 1 Section 1- Pages 4-7: Electrons and Chemical Bonding COMBINING ATOMS THROUGH CHEMICAL BONDING 1. Which of these substances is a combination
