YEAR 9 - AQA GCSE COMBINED SCIENCE (9-1) Cell Biology
|
|
|
- Tobias Morgan
- 5 years ago
- Views:
Transcription
1 YEAR 9 - AQA GCSE COMBINED SCIENCE (9-1) Cell Biology Cell structure Eukaryotes and prokaryotes Plant and animal cells (eukaryotic cells) have a cell membrane, cytoplasm and genetic material enclosed in a nucleus. Bacterial cells (prokaryotic cells) are much smaller in comparison. They have cytoplasm and a cell membrane surrounded by a cell wall. The genetic material is not enclosed in a nucleus. It is a single DNA loop and there may be one or more small rings of DNA called plasmids. WS 4.4 Use prefixes centi, milli, micro and nano. MS 2a, 2h Demonstrate an understanding of the scale and size of cells and be able to make order of magnitude calculations. Animal and plant cells Students should be able to explain how the main sub-cellular structures, including the nucleus, cell membranes, mitochondria, chloroplasts in plant cells and plasmids in bacterial cells are related to their functions. Most animal cells have the following parts: a nucleus, which controls the activities of the cell cytoplasm, in which most of the chemical reactions take place a cell membrane, which controls the passage of substances into and out of the cell mitochondria, which is where aerobic respiration takes place ribosomes, which are where protein synthesis occurs. In addition to the parts found in animal cells, plant cells often have: chloroplasts, which absorb light to make food by photosynthesis a permanent vacuole filled with cell sap. Plant and algal cells also have a cell wall made of cellulose, which strengthens the cell. Cell specialisation Students should be able to, when provided with appropriate information, explain how the structure of different types of cell relate to their function in a tissue, an organ or organ system, or the whole organism. Cells may be specialised to carry out a particular function: sperm cells, nerve cells and muscle cells in animals root hair cells, xylem and phloem cells in plants. Cell differentiation As an organism develops, cells differentiate to form different types of cells. Most types of animal cell differentiate at an early stage whereas many types of plant cells retain the ability to differentiate throughout life. In mature animals, cell division is mainly restricted to repair and replacement. As a cell differentiates it acquires different sub-cellular structures to enable it to carry out a certain function. It has become a specialised cell. 1
2 Microscopy An electron microscope has much higher magnification and resolving power than a light microscope. This means that it can be used to study cells in much finer detail. (Limited to the differences in magnification and resolution.) This has enabled biologists to see and understand many more sub-cellular structures. WS 1.1 Explain how electron microscopy has increased understanding of subcellular structures. WS 4.4 Use prefixes centi, milli, micro and nano. MS 1a, 1b, 2h, 3b Carry out calculations involving magnification, real size and image size using the formula: magnification = size of image / size of real object Cell division Chromosomes The nucleus of a cell contains chromosomes made of DNA molecules. Each chromosome carries a large number of genes. In body cells the chromosomes are normally found in pairs. Mitosis and the cell cycle Cells divide in a series of stages called the cell cycle. One of these stages is mitosis where the DNA, which has already been copied, divides. During the cell cycle the genetic material is doubled and then divided into two identical cells. Before a cell can divide it needs to grow and increase the number of sub-cellular structures such as ribosomes and mitochondria. The DNA replicates to form two copies of each chromosome. One set of chromosomes is pulled to each end of the cell and the nucleus divides. Finally, the cytoplasm and cell membranes divide to form two identical cells. Knowledge of the stages of mitosis is not required. Cell division by mitosis is important in the growth and development of multicellular organisms. Students should be able to recognise and describe situations in given contexts where mitosis is occurring. WS 1.2 Use models and analogies to develop explanations of how cells divide. Stem cells A stem cell is an undifferentiated cell of an organism which is capable of giving rise to many more cells of the same type, and from which certain other cells can arise from differentiation. Stem cells from human embryos and adult bone marrow can be cloned and made to differentiate into many different types of human cells. Knowledge and understanding of stem cell techniques are not required. Treatment with stem cells may be able to help conditions such as diabetes and paralysis. In therapeutic cloning an embryo is produced with the same genes as the patient. Stem cells from the embryo are not rejected by the patient s body so they may be used for medical treatment. The use of stem cells has potential risks such as transfer of viral infection, and some people have ethical or religious objections. Stem cells from meristems in plants can be used to produce clones of plants quickly and economically. Rare species can be cloned to protect from extinction. Large numbers of identical crop plants with special features such as disease resistance. 2
3 Transport in cells Diffusion Substances may move into and out of cells across the cell membranes via diffusion. Diffusion is the spreading of the particles of any substance in solution, or particles of a gas, resulting in a net movement from an area of higher concentration to an area of lower concentration. Some of the substances transported in and out of cells by diffusion are oxygen and carbon dioxide in gas exchange, and of the waste product urea from cells into the blood plasma for excretion in the kidney. Factors which affect the rate of diffusion are: the difference in concentrations (concentration gradient) the temperature the surface area of the membrane. A single-celled organism has a relatively large surface area to volume ratio. This allows sufficient transport of molecules into and out of the cell to meet the needs of the organism. In multicellular organisms the smaller surface area to volume ratio means surfaces and organ systems are specialised for exchanging materials. This is to allow sufficient molecules to be transported into and out of cells for the organism s needs. The effectiveness of an exchange surface is increased by: having a large surface area a membrane that is thin, to provide a short diffusion path (in animals) having an efficient blood supply (in animals, for gaseous exchange) being ventilated. Students should be able to explain how the small intestine and lungs in mammals, gills in fish, and the roots and leaves in plants, are adapted for exchanging materials. Osmosis Water may move across cell membranes via osmosis. Osmosis is the diffusion of water from a dilute solution to a concentrated solution through a partially permeable membrane. MS 1c, 2b, 4a, 4b, 4c, 4d Calculate percentages, use negative numbers and construct graphs. Active Transport Active transport moves substances from a more dilute solution to a more concentrated solution (against a concentration gradient). This requires energy from respiration. Active transport allows mineral ions to be absorbed into plant root hairs from very dilute solutions in the soil. Plants require ions for healthy growth. Students should be able to link the structure of a root hair cell to its function. (also in ) It also allows sugar molecules to be absorbed from lower concentrations in the gut into the blood which has a higher sugar concentration. Sugar molecules are used for cell respiration. Students should be able to explain the differences between diffusion, osmosis and active transport. WS 1.4 Students should be able to describe how kidney dialysis works. WS 1.5 Use of isotonic drinks and high energy drinks in sport. 3
4 AQA GCSE COMBINED SCIENCE (9-1) Atomic Structure and the Periodic Table Atoms, elements and compounds Atoms, elements and compounds All substances are made of atoms. An atom is the smallest part of an element that can exist. Atoms of each element are represented by a chemical symbol, eg O represents an atom of oxygen, Na represents an atom of sodium. There are about 100 different elements. Elements are shown in the periodic table. Compounds are formed from elements by chemical reactions. Compounds contain two or more elements chemically combined in fixed proportions and can be represented by formulae using the symbols of the atoms from which they were formed. Compounds can only be separated into elements by chemical reactions. Chemical reactions can be represented by word equations or equations using symbols and formulae. Students will be supplied with a periodic table for the exam and should be able to: use the names and symbols of the first 20 elements in the periodic table, the elements in Groups 1 and 7, and other elements in this specification. name compounds of these elements from given formulae or symbol equations. write word equations for the reactions in this specification. write formulae and balanced chemical equations for the reactions in this specification. (HT only) write balanced half equations and ionic equations where appropriate. Mixtures A mixture consists of two or more elements or compounds not chemically combined together. The chemical properties of each substance in the mixture are unchanged. Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography. These physical processes do not involve chemical reactions. WS 2.2 Describe, explain and give examples of the specified processes of separation. WS 2.3 Suggest suitable separation and purification techniques for mixtures when given appropriate information. AT 4 Safe use of a range of equipment to separate chemical mixtures. Scientific models of the atom (common content with physics) New experimental evidence may lead to a scientific model being changed or replaced. Before the discovery of the electron, atoms were thought to be tiny spheres that could not be divided. The discovery of the electron led to the plum pudding model of the atom. The plum pudding model suggested that the atom was a ball of positive charge with negative electrons embedded in it. The results from the Rutherford and Marsden s alpha scattering experiments led to the plum pudding model being replaced by the nuclear model. Niels Bohr adapted the nuclear model by suggesting that electrons orbit the nucleus at specific distances. The theoretical calculations of Bohr agreed with experimental observations. 4
5 Later experiments led to the idea that the positive charge of any nucleus could be subdivided into a whole number of smaller particles, each particle having the same amount of positive charge. The name proton was given to these particles. In 1932, the experimental work of James Chadwick provided the evidence to show the existence of neutrons within the nucleus. WS 1.1 Describe why the new evidence from the scattering experiment led to a change in the atomic model. WS 1.2 Describe the difference between the plum pudding model of the atom and the nuclear model of the atom. Details of experimental work supporting the Bohr model are not required. Details of these experiments are not required. Details of Chadwick s experimental work are not required. Relative electrical charges of subatomic particles (some common content with physics) The relative electrical charges of the particles in atoms are: In an atom, the number of electrons is equal to the number of protons in the nucleus. Atoms have no overall electrical charge (they are neutral). The number of protons in an atom of an element is its atomic number. All atoms of a particular element have the same number of protons. Atoms of different elements have different numbers of protons. WS 1.2 Students should be able to use the atomic model to describe atoms. Size and mass of atoms (some common content with physics) Atoms are very small, having a radius of about 0.1 nm (1 x m). The radius of a nucleus is less than 1/ of that of the atom (about 1 x m). WS 4.3 Use SI units and the prefix nano. MS 1b Recognise expressions in standard form. MS 1d Estimate the size and scale of atoms. The relative masses of protons, neutrons and electrons are: The sum of the protons and neutrons in an atom is its mass number Atoms of the same element can have different numbers of neutrons; these atoms are called isotopes of that element. Atoms can be represented as shown in this example: 5
6 Students should be able to calculate the numbers of protons, neutrons and electrons in an atom or ion, given its atomic number and mass number. Electronic structure The electrons in an atom occupy the lowest available energy levels (innermost available shells). The electronic structure of an atom can be represented by numbers or by a diagram. For example, the electronic structure of sodium is 2,8,1 or showing two electrons in the lowest energy level, eight in the second energy level and one in the third energy level. Students may answer questions in terms of either energy levels or shells. WS 1.2 Students should be able to represent the electronic structures of the first twenty elements of the periodic table in both forms. MS 5b Visualise and represent 2D and 3D forms including two dimensional representations of 3D objects. The periodic table The periodic table The elements in the periodic table are arranged in order of atomic (proton) number and so that elements with similar properties are in columns, known as groups. The table is called a periodic table because similar properties occur at regular intervals. Elements in the same group in the periodic table have the same number of electrons in their outer shell (outer electrons) and this gives them similar chemical properties. WS 1.2 Explain how the position of an element in the periodic table is related to the arrangement of electrons in its atoms and hence to its atomic number. WS 1.2 Predict possible reactions and probable reactivity of elements from their positions in the periodic table. Development of the periodic table Before the discovery of protons, neutrons and electrons, scientists attempted to classify the elements by arranging them in order of their atomic weights. The early periodic tables were incomplete and some elements were placed in inappropriate groups if the strict order of atomic weights was followed. Mendeleev overcame some of the problems by leaving gaps for elements that he thought had not been discovered and in some places changed the order based on atomic weights. Elements with properties predicted by Mendeleev were discovered and filled the gaps. Knowledge of isotopes made it possible to explain why the order based on atomic weights was not always correct. Students should be able to describe these steps in the development of the periodic table. WS 1.1 Explain how testing a prediction can support or refute a new scientific idea. Metals and non-metals Elements that react to form positive ions are metals. Elements that do not form positive ions are non-metals. 6
7 The majority of elements are metals. Metals are found to the left and towards the bottom of the periodic table. Non-metals are found towards the right and top of the periodic table. Explain the differences between metals and non-metals on the basis of their characteristic physical and chemical properties. Links with Group 0, Group 1, Group 7 and Bonding, structure and the properties of matter. Explain how the atomic structure of metals and non-metals relates to their position in the periodic table. Explain how the reactions of elements are related to the arrangement of electrons in their atoms and hence to their atomic number. Group 0 The elements in Group 0 of the periodic table are called the noble gases. They are unreactive and do not easily form molecules because their atoms have stable arrangements of electrons. The noble gases have eight electrons in their outer energy level, except for helium, which has only two electrons. The boiling points of the noble gases increase with increasing relative atomic mass (going down the group). WS 1.2 Explain how properties of the elements in Group 0 depend on the outer shell of electrons of the atoms. WS 1.2 Predict properties from given trends down the group. Group 1 The elements in Group 1 of the periodic table, known as the alkali metals: are metals with low density (the first three elements in the group are less dense than water). react with non-metals to form ionic compounds in which the metal ion carries a charge of +1. The compounds are white solids that dissolve in water to form colourless solutions. react with water, releasing hydrogen. form hydroxides that dissolve in water to give alkaline solutions. In Group 1, the reactivity of the elements increases going down the group. WS 1.2 Explain how properties of the elements in Group 1 depend on the outer shell of electrons of the atoms. WS 1.2 Predict properties from given trends down the group. Group 7 The elements in Group 7 of the periodic table, known as the halogens: are non-metals consist of molecules which are made up of pairs of atoms react with metals to form ionic compounds in which the halide ion carries a charge of 1 form molecular compounds with other non-metallic elements. In Group 7, the further down the group an element is the higher its relative molecular mass, melting point and boiling point. In Group 7, the reactivity of the elements decreases going down the group. A more reactive halogen can displace a less reactive halogen from an aqueous solution of its salt. WS 1.2 Explain how properties of the elements in Group 7 depend on the outer shell of electrons of the atoms. WS 1.2 Predict properties from given trends down the group. A copy of the periodic table is provided in the exam. 7
8 AQA GCSE COMBINED SCIENCE (9-1) Particle model of matter Changes of state and the particle model Density of materials The density of a material is defined by the equation: density = mass density, ρ, in kilograms per metre cubed, kg/m 3 mass, m, in kilograms, kg volume, V, in metres cubed, m 3 volume ρ = m V Students should be able to recall and/or apply this equation to changes where mass is conserved. The particle model can be used to explain the different states of matter differences in density Students should be able to recognise/draw simple diagrams to model the difference between solids, liquids and gases [common content with chemistry]. REQUIRED PRACTICAL Density. Changes of state When substances change state (melt, freeze, boil, evaporate, condense or sublimate), mass is conserved. Students should understand why there is no change in the mass of a substance when it changes state. Changes of state are physical changes: the change does not produce a new substance. If the change is reversed the substance recovers its original properties. Internal energy and energy transfers Internal energy Energy is stored inside a system by the particles (atoms and molecules) that make up the system. This is called internal energy. Internal energy is the total kinetic energy and potential energy of all the particles (atoms and molecules) that make up a system. Heating changes the energy stored within the system by increasing the energy of the particles that make up the system. This either raises the temperature of the system or produces a change of state. Temperature changes in a system and specific heat capacity If the temperature of the system increases: The increase in temperature depends on the mass of the substance heated, the type of material and the energy input to the system. The following equation applies: change in thermal energy = mass x specific heat capacity x temperature change E = m c θ change in thermal energy, E, in joules, J mass, m, in kilograms, kg specific heat capacity, c, in joules per kilogram per degree Celsius, J/kg C temperature change, θ, in degrees Celsius, C. 8
9 The specific heat capacity of a substance is the amount of energy required to raise the temperature of one kilogram of the substance by one degree Celsius. Changes of heat and specific latent heat If a change of state happens: The energy needed for a substance to change state is called latent heat. When a change of state occurs, the energy supplied changes the energy stored (internal energy) but not the temperature. The specific latent heat of a substance is the amount of energy required to change the state of one kilogram of the substance with no change in temperature. The following equation applies: energy for a change of state = mass x specific latent heat energy, E, in joules, J mass, m, in kilograms, kg specific latent heat, L, in joules per kilogram, J/kg Specific latent heat of fusion change of state from solid to liquid Specific latent heat of vaporisation change of state from liquid to vapour E = m L Students should be able to interpret heating and cooling graphs that include changes of state. Particle model and pressure Particle motion in gases The molecules of a gas are in constant random motion. The temperature of the gas is related to the average kinetic energy of the molecules. The higher the temperature the greater the average kinetic energy and so the faster the average speed of the molecules. When the molecules collide with the wall of their container they exert a force on the wall. The total force exerted by all of the molecules inside the container on a unit area of the walls is the gas pressure. Changing the temperature of a gas, held at constant volume, changes the pressure exerted by the gas. WS 1.2 Students should be able to use the particle model to explain the effect of changing temperature on the pressure of a gas held at constant volume. Energy changes in a system, and the ways energy is stored before and after such changes Energy stores and systems A system is an object or group of objects. There are changes in the way energy is stored when a system changes. For example: an object projected upwards a moving object hitting an obstacle an object accelerated by a constant force a vehicle slowing down bringing water to a boil in an electric kettle. Students should be able to: Describe all the changes involved in the way energy is stored when a system changes, for common situations (including the examples above). 9
10 Throughout this Energy topic, calculate the changes in energy involved when a system is changed by: heating, work done by forces, work done when a current flows. Use calculations to show on a common scale how the overall energy in a system is redistributed when the system is changed. Conservation and dissipation of energy Energy transfers in a system Energy can be transferred usefully, stored or dissipated, but cannot be created or destroyed. Whenever there are energy transfers in a system only part of the energy is usefully transferred. The rest of the energy is dissipated so that it is stored in less useful ways. This energy is often described as being wasted. Unwanted energy transfers can be reduced in a number of ways, for example through lubrication and the use of thermal insulation. The higher the thermal conductivity of a material the higher the rate of energy transfer by conduction across the material. Students should be able to: Describe, with examples, where there are energy transfers in a closed system, that there is no net change to the total energy. Describe, with examples, how in all system changes energy is dissipated, so that it is stored in less useful ways. The energy is often described as being wasted. Explain ways of reducing unwanted energy transfers, for example, through lubrication and the use of thermal insulation. Describe how the rate of cooling of a building is affected by the thickness and thermal conductivity of its walls. Students do not need to know the definition of thermal conductivity. Efficiency The energy efficiency for any energy transfer can be calculated using the equation: Efficiency may also be calculated using the equation: Students should be able to: Recall and apply both equations for efficiency. Calculate or use efficiency values as a decimal or as a percentage. (HT only) Describe ways to increase the efficiency of an intended energy transfer. 10
11 National and global energy resources The main energy resources available for use on Earth include: fossil fuels (coal, oil and gas), nuclear fuel, bio-fuel, wind, hydroelectricity, geothermal, the tides, the Sun and water waves. A renewable energy resource is one that is being (or can be) replenished as it is used. The uses of energy resources include: transport, electricity generation and heating. Students should be able to: Describe the main energy sources available. Descriptions of how energy resources are used to generate electricity are not required. Distinguish between energy resources that are renewable and energy resources that are nonrenewable. Compare ways that different energy resources are used, the uses to include transport, electricity generation and heating. Understand why some energy resources are more reliable than others. Describe the environmental impact arising from the use of different energy resources. Explain patterns and trends in the use of energy resources. Consider the environmental issues that may arise from the use of different energy resources. Show that science has the ability to identify environmental issues arising from the use of energy resources but not always the power to deal with the issues because of political, social, ethical or economic considerations. GCSE Combined Science Trilogy Physics Equation Sheet 11
12 12
Plant and animal cells (eukaryotic cells) have a cell membrane, cytoplasm and genetic material enclosed in a nucleus.
 4.1 Cell biology Cells are the basic unit of all forms of life. In this section we explore how structural differences between types of cells enables them to perform specific functions within the organism.
4.1 Cell biology Cells are the basic unit of all forms of life. In this section we explore how structural differences between types of cells enables them to perform specific functions within the organism.
Plant and animal cells (eukaryotic cells) have a cell membrane, cytoplasm and genetic material enclosed in a nucleus.
 4.1 Cell biology Cells are the basic unit of all forms of life. In this section we explore how structural differences between types of cells enables them to perform specific functions within the organism.
4.1 Cell biology Cells are the basic unit of all forms of life. In this section we explore how structural differences between types of cells enables them to perform specific functions within the organism.
How many lessons is it?
 Science Unit Learning Summary Content Eukaryotes and Prokaryotes Cells are the basic unit of all life forms. A eukaryotic cell contains genetic material enclosed within a nucleus. Plant and animal cells
Science Unit Learning Summary Content Eukaryotes and Prokaryotes Cells are the basic unit of all life forms. A eukaryotic cell contains genetic material enclosed within a nucleus. Plant and animal cells
4.1.1 A simple model of the atom, symbols, relative atomic mass, electronic charge and isotopes Atoms, elements and compounds
 4.1 Atomic structure and the periodic table The periodic table provides chemists with a structured organisation of the known chemical elements from which they can make sense of their physical and chemical
4.1 Atomic structure and the periodic table The periodic table provides chemists with a structured organisation of the known chemical elements from which they can make sense of their physical and chemical
4.1.1 A simple model of the atom, symbols, relative atomic mass, electronic charge and isotopes
 4.1 Atomic structure and the periodic table The periodic table provides chemists with a structured organisation of the known chemical elements from which they can make sense of their physical and chemical
4.1 Atomic structure and the periodic table The periodic table provides chemists with a structured organisation of the known chemical elements from which they can make sense of their physical and chemical
Animal Cell Organelles. Plant Cell. Organelle. Cell Wall. Chloroplasts. Vacuole
 Cell Biology Higher Electron vs Light Microscope Light use light and lenses to magnify specimen Electron use a beam of electrons to form an image Electron higher magnification and higher resolution Electron
Cell Biology Higher Electron vs Light Microscope Light use light and lenses to magnify specimen Electron use a beam of electrons to form an image Electron higher magnification and higher resolution Electron
Cell Biology. AQA Biology topic 1
 Cell Biology AQA Biology topic 1 1.1 Cell Structure Plant and Animal cells (eukaryotic cells) Eukaryotic cells have these features: 1) Cytoplasm 2) Genetic material within a nucleus 3) Cell Membrane Typical
Cell Biology AQA Biology topic 1 1.1 Cell Structure Plant and Animal cells (eukaryotic cells) Eukaryotic cells have these features: 1) Cytoplasm 2) Genetic material within a nucleus 3) Cell Membrane Typical
Lymm High School- KS3 Life after levels - Science Y9
 Biology BRONZE SILVER GOLD PLATINUM D and below= GCSE 1,2,3 C= GCSE 4 C/B= GCSE 5,6 A/A*= GCSE 7,8,9 Explain that cells are very Demonstrate an small and a microscope is understanding of the scale needed
Biology BRONZE SILVER GOLD PLATINUM D and below= GCSE 1,2,3 C= GCSE 4 C/B= GCSE 5,6 A/A*= GCSE 7,8,9 Explain that cells are very Demonstrate an small and a microscope is understanding of the scale needed
Foundation Cell Biology
 Foundation Cell Biology Electron vs Light Microscope Light use light and lenses to magnify specimen Electron use a beam of electrons to form an image Electron higher magnification and higher resolution
Foundation Cell Biology Electron vs Light Microscope Light use light and lenses to magnify specimen Electron use a beam of electrons to form an image Electron higher magnification and higher resolution
04/05/2017. Cell Biology. AQA 2016 Syllabus
 Cell Biology AQA 2016 Syllabus 1.1 Cell Structure Plant and Animal cells (eukaryotic cells) Eukaryotic cells have these features: 1) Cytoplasm 2) Genetic material within a nucleus 3) Cell Membrane Typical
Cell Biology AQA 2016 Syllabus 1.1 Cell Structure Plant and Animal cells (eukaryotic cells) Eukaryotic cells have these features: 1) Cytoplasm 2) Genetic material within a nucleus 3) Cell Membrane Typical
Ark Elvin Academy Year 10 Science Study Pack Spring assessment 2018 Cumulative content part 1
 Ark Elvin Academy Year 10 Science Study Pack Spring assessment 2018 Cumulative content part 1 Name Biology Cell Biology: structure and transport Part 1: Glossary 1. active transport the movement of substances
Ark Elvin Academy Year 10 Science Study Pack Spring assessment 2018 Cumulative content part 1 Name Biology Cell Biology: structure and transport Part 1: Glossary 1. active transport the movement of substances
4.1 Energy Energy changes in a system, and the ways energy is stored before and after such changes Energy stores and systems.
 4.1 Energy The concept of energy emerged in the 19th century. The idea was used to explain the work output of steam engines and then generalised to understand other heat engines. It also became a key tool
4.1 Energy The concept of energy emerged in the 19th century. The idea was used to explain the work output of steam engines and then generalised to understand other heat engines. It also became a key tool
Revision Guide: 4.1 Atomic Structure and the Periodic Table
 Revision Guide: 4.1 Atomic Structure and the Periodic Table Atoms, Elements and Compounds Atoms All substances are made of atoms. An atom is the smallest part of an element that can eist. Atoms of each
Revision Guide: 4.1 Atomic Structure and the Periodic Table Atoms, Elements and Compounds Atoms All substances are made of atoms. An atom is the smallest part of an element that can eist. Atoms of each
SCIENCE ROAD TO GOLD. Part 1- Biology Paper 1 Cell Biology Triple Science
 SCIENCE ROAD TO GOLD Part 1- Biology Paper 1 Cell Biology Triple Science 1 Below is a checklist for everything you need to know for this topic 2 A. Cell structure part 1 Eukaryotes, prokaryotes and animal
SCIENCE ROAD TO GOLD Part 1- Biology Paper 1 Cell Biology Triple Science 1 Below is a checklist for everything you need to know for this topic 2 A. Cell structure part 1 Eukaryotes, prokaryotes and animal
Revision Guide: 4.1 Atomic Structure and the Periodic Table
 Revision Guide: 4.1 Atomic Structure and the Periodic Table Atoms, Elements and Compounds Atoms All substances are made of atoms. An atom is the smallest part of an element that can exist. Atoms of each
Revision Guide: 4.1 Atomic Structure and the Periodic Table Atoms, Elements and Compounds Atoms All substances are made of atoms. An atom is the smallest part of an element that can exist. Atoms of each
KnowIT Questions AQA GCSE Cell Biology
 A. Cell structure part 1 Eukaryotes, prokaryotes and animal and plant cells 1. Where is the genetic material in a prokaryotic cell? 2. Where is the genetic material in a eukaryotic cell? 3. Complete the
A. Cell structure part 1 Eukaryotes, prokaryotes and animal and plant cells 1. Where is the genetic material in a prokaryotic cell? 2. Where is the genetic material in a eukaryotic cell? 3. Complete the
GCSE Biology. The PiXL Club Ltd, Company number
 he PiXL Club The PiXL Club The PiXL Club The PiXL Club The PiXL Club The PiXL Club The PiXL Club The PiXL Club The PiXL Club The PiXL Club The PiXL Club The PiXL Club The PiXL Club The PiXL Club he PiXL
he PiXL Club The PiXL Club The PiXL Club The PiXL Club The PiXL Club The PiXL Club The PiXL Club The PiXL Club The PiXL Club The PiXL Club The PiXL Club The PiXL Club The PiXL Club The PiXL Club he PiXL
4.1 Atomic structure and the periodic table. GCSE Chemistry
 4.1 Atomic structure and the periodic table GCSE Chemistry All substances are made of atoms this is cannot be chemically broken down it is the smallest part of an element. Elements are made of only one
4.1 Atomic structure and the periodic table GCSE Chemistry All substances are made of atoms this is cannot be chemically broken down it is the smallest part of an element. Elements are made of only one
AQA GCSE PHYSICS (9-1) Topic 1: Energy
 AQA GCSE PHYSICS (9-1) Topic 1: Energy 4.1.1 Energy changes in a system, and the ways energy is stored before and after such changes 4.1.1.1 Energy stores and systems A system is an object or group of
AQA GCSE PHYSICS (9-1) Topic 1: Energy 4.1.1 Energy changes in a system, and the ways energy is stored before and after such changes 4.1.1.1 Energy stores and systems A system is an object or group of
4.3.1 Changes of state and the particle model Density of materials. ρ = m. Content. Key opportunities for skills development
 4.3 Particle model of matter The particle model is widely used to predict the behaviour of solids, liquids and gases and this has many applications in everyday life. It helps us to explain a wide range
4.3 Particle model of matter The particle model is widely used to predict the behaviour of solids, liquids and gases and this has many applications in everyday life. It helps us to explain a wide range
Biology Combined Chemistry Combined Physics Combined. Content Hours Content Hours Content Hours
 Biology Combined Chemistry Combined Physics Combined Content Hours Content Hours Content Hours Half Term.7.. Communities Competition Ecosystems Interdependence ASSESSED HOMEWORK (Communities).7.. Abiotic
Biology Combined Chemistry Combined Physics Combined Content Hours Content Hours Content Hours Half Term.7.. Communities Competition Ecosystems Interdependence ASSESSED HOMEWORK (Communities).7.. Abiotic
Part 5- Chemistry Paper 1 Atomic Structure Knowledge Questions
 Part 5- Chemistry Paper 1 Atomic Structure Knowledge Questions How bonding and structure are related to the properties of substances A simple model of the atom, symbols, relative atomic mass, electronic
Part 5- Chemistry Paper 1 Atomic Structure Knowledge Questions How bonding and structure are related to the properties of substances A simple model of the atom, symbols, relative atomic mass, electronic
OCR Chemistry Checklist
 Topic 1. Particles Video: The Particle Model Describe the main features of the particle model in terms of states of matter. Explain in terms of the particle model the distinction between physical changes
Topic 1. Particles Video: The Particle Model Describe the main features of the particle model in terms of states of matter. Explain in terms of the particle model the distinction between physical changes
Particles and Periodic Table
 Specification points Year 9 Particles The three states of matter The three states of matter are solid, liquid and gas. In chemical equations, the three states of matter are shown as (s), (l) and (g), with
Specification points Year 9 Particles The three states of matter The three states of matter are solid, liquid and gas. In chemical equations, the three states of matter are shown as (s), (l) and (g), with
Cecil Jones Academy Science Fundamentals Map
 Fundamentals: Year 9 Biology Knowledge Unit 1 Skills B1 Cell Structure and Transport Unit 2 Use the terms 'eukaryotic' and 'prokaryotic' to describe types of cells Describe the features of bacterial (prokaryotic)
Fundamentals: Year 9 Biology Knowledge Unit 1 Skills B1 Cell Structure and Transport Unit 2 Use the terms 'eukaryotic' and 'prokaryotic' to describe types of cells Describe the features of bacterial (prokaryotic)
Reason... (2) Reason... (2) Reason... (2)
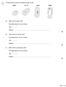 1 The figure below shows four different types of cell. (a) Which cell is a plant cell? Give one reason for your answer. Cell... Reason... (b) Which cell is an animal cell? Give one reason for your answer.
1 The figure below shows four different types of cell. (a) Which cell is a plant cell? Give one reason for your answer. Cell... Reason... (b) Which cell is an animal cell? Give one reason for your answer.
B I O. 1. B I O A N A L Y Z E T H E C E L L A S A L I V I N G S Y S T E M.
 Goal 1 B I O. 1. 1 U N D E R S T A N D T H E R E L A T I O N S H I P B E T W E E N T H E S T R U C T U R E S A N D F U N C T I O N S O F C E L L S A N D T H E I R O R G A N E L L E S. B I O. 1. 2 A N A
Goal 1 B I O. 1. 1 U N D E R S T A N D T H E R E L A T I O N S H I P B E T W E E N T H E S T R U C T U R E S A N D F U N C T I O N S O F C E L L S A N D T H E I R O R G A N E L L E S. B I O. 1. 2 A N A
Science Year 10 Unit 1 Biology
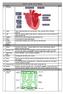 Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
AQA GCSE CHEMISTRY (9-1) Topic 1: Atomic Structure and the Periodic Table
 AQA GCSE CHEMISTRY (9-1) Topic 1: Atomic Structure and the Periodic Table 4.1.1 Atoms, elements and compounds 4.1.1.1 Atoms, elements and compounds All substances are made of atoms. An atom is the smallest
AQA GCSE CHEMISTRY (9-1) Topic 1: Atomic Structure and the Periodic Table 4.1.1 Atoms, elements and compounds 4.1.1.1 Atoms, elements and compounds All substances are made of atoms. An atom is the smallest
Science Home Learning Task. Year 9. GCSE Cell structure and transport
 Science Home Learning Task Year 9 GCSE Cell structure and transport Name Tutor Group Teacher Given out: Monday 23 April Hand in: Monday 30 April Parent/Carer Comment Staff Comment GCSE level Target Investigating
Science Home Learning Task Year 9 GCSE Cell structure and transport Name Tutor Group Teacher Given out: Monday 23 April Hand in: Monday 30 April Parent/Carer Comment Staff Comment GCSE level Target Investigating
Quiz 1: Cells and Cell Structures
 Quiz 1: Cells and Cell Structures 1. Identify the structures in the diagram. (3 marks) 2. List the 3 cell structures not found in animal cells but are found in plants cells. (1 mark) 3. Where is DNA found
Quiz 1: Cells and Cell Structures 1. Identify the structures in the diagram. (3 marks) 2. List the 3 cell structures not found in animal cells but are found in plants cells. (1 mark) 3. Where is DNA found
Edexcel Chemistry Checklist
 Topic 1. Key concepts in chemistry Video: Developing the atomic model Describe how and why the atomic model has changed over time. Describe the difference between the plum-pudding model of the atom and
Topic 1. Key concepts in chemistry Video: Developing the atomic model Describe how and why the atomic model has changed over time. Describe the difference between the plum-pudding model of the atom and
GraspIT AQA GCSE Atomic Structure & the Periodic Table
 A. Atoms, Elements, Compounds and Mixtures part 1 Atoms, Elements, Compounds, Word and Symbol Equations 1. Describe the differences between an element and a compound. (2) element: all atoms same type,
A. Atoms, Elements, Compounds and Mixtures part 1 Atoms, Elements, Compounds, Word and Symbol Equations 1. Describe the differences between an element and a compound. (2) element: all atoms same type,
Physics GCSE (9-1) Energy
 Topic Student Checklist R A G Define a system as an object or group of objects and State examples of changes in the way energy is stored in a system Describe how all the energy changes involved in an energy
Topic Student Checklist R A G Define a system as an object or group of objects and State examples of changes in the way energy is stored in a system Describe how all the energy changes involved in an energy
Personalised Learning Checklists AQA Physics Paper 1
 6.1.1 Energy changes in a system, and the ways energy is stored before and after such changes AQA TRILOGY Physics (8464) from 2016 Topics T6.1. Energy Topic Student Checklist R A G Define a system as an
6.1.1 Energy changes in a system, and the ways energy is stored before and after such changes AQA TRILOGY Physics (8464) from 2016 Topics T6.1. Energy Topic Student Checklist R A G Define a system as an
What is the resultant force acting on the object? BASE jumpers jump from very high buildings and mountains for sport.
 Forces Easier (a) The diagram shows two forces acting on an object. What is the resultant force acting on the object? Tick ( ) one box. 8 N to the right 8 N to the left 4 N to the right 4 N to the left
Forces Easier (a) The diagram shows two forces acting on an object. What is the resultant force acting on the object? Tick ( ) one box. 8 N to the right 8 N to the left 4 N to the right 4 N to the left
GraspIT AQA GCSE Cell Biology
 A. Cell structure part 1 Eukaryotes, prokaryotes and animal and plant cells 1. Describe the similarities and differences between a typical plant and a typical animal cell. (4)...... 2. Ribosomes synthesise
A. Cell structure part 1 Eukaryotes, prokaryotes and animal and plant cells 1. Describe the similarities and differences between a typical plant and a typical animal cell. (4)...... 2. Ribosomes synthesise
What is the role of the nucleus? What is the role of the cytoplasm? What is the role of the mitochondria? What is the role of the cell wall. membrane?
 Page 1 What is the role of the nucleus? What is the role of the cytoplasm? What is the role of the cell membrane? What is the role of the mitochondria? What is the role of ribosomes? What is the role of
Page 1 What is the role of the nucleus? What is the role of the cytoplasm? What is the role of the cell membrane? What is the role of the mitochondria? What is the role of ribosomes? What is the role of
Personalised Learning Checklists AQA Trilogy Chemistry Paper 1
 AQA TRILOGY Chemistry (8464) from 2016 Topics T5.1 Atomic structure and the periodic table State that everything is made of atoms and recall what they are Describe what elements and compounds are State
AQA TRILOGY Chemistry (8464) from 2016 Topics T5.1 Atomic structure and the periodic table State that everything is made of atoms and recall what they are Describe what elements and compounds are State
AQA Chemistry Checklist
 Topic 1. Atomic structure Video: Atoms, elements, compounds, mixtures Use the names and symbols of the first 20 elements in the periodic table, the elements in Groups 1 and 7, and other elements in this
Topic 1. Atomic structure Video: Atoms, elements, compounds, mixtures Use the names and symbols of the first 20 elements in the periodic table, the elements in Groups 1 and 7, and other elements in this
Biology Topics 1 and 2
 Biology Topics and 2 Practise Questions 30 minutes 30 marks These are GCSE questions but very simiilar to the igcse ones. Page of 2 Q. The diagram shows an animal cell. (a) (i) Name structures A and B
Biology Topics and 2 Practise Questions 30 minutes 30 marks These are GCSE questions but very simiilar to the igcse ones. Page of 2 Q. The diagram shows an animal cell. (a) (i) Name structures A and B
AQA (Trilogy) Combined Science GCSE Unit 6.3 Particle Model of Matter
 AQA (Trilogy) Combined Science GCSE Unit 6.3 Particle Model of Matter Test (Levels 4 9) Time allowed: 50 minutes Question Links to Student Progress Sheet Score Total Marks Available Score Estimated Grade
AQA (Trilogy) Combined Science GCSE Unit 6.3 Particle Model of Matter Test (Levels 4 9) Time allowed: 50 minutes Question Links to Student Progress Sheet Score Total Marks Available Score Estimated Grade
- know that in multi-cellular organisms cells are massed together to form tissues, and tissues can be massed together to form organs
 Science Age 12-13 (A) BIOLOGY: ORGANISMS, THEIR BEHAVIOUR AND THE ENVIRONMENT 1. Cells and their functions - know that in multi-cellular organisms cells are massed together to form tissues, and tissues
Science Age 12-13 (A) BIOLOGY: ORGANISMS, THEIR BEHAVIOUR AND THE ENVIRONMENT 1. Cells and their functions - know that in multi-cellular organisms cells are massed together to form tissues, and tissues
Page 2. Q1.The electronic structure of the atoms of five elements are shown in the figure below. The letters are not the symbols of the elements.
 Q1.The electronic structure of the atoms of five elements are shown in the figure below. The letters are not the symbols of the elements. Choose the element to answer the question. Each element can be
Q1.The electronic structure of the atoms of five elements are shown in the figure below. The letters are not the symbols of the elements. Choose the element to answer the question. Each element can be
2012 SECONDARY 1 SCIENCE SCHEME OF WORK
 2012 SECONDARY 1 SCIENCE SCHEME OF WORK Term 1 1 Orientation 2 Introduction to Science Lab Safety 3 Physical Quantities and Units 1. All physical quantities consist of a numerical magnitude and a unit
2012 SECONDARY 1 SCIENCE SCHEME OF WORK Term 1 1 Orientation 2 Introduction to Science Lab Safety 3 Physical Quantities and Units 1. All physical quantities consist of a numerical magnitude and a unit
Topic Student Checklist R A G
 Personalised Learning Checklist AQA TRILOGY Physics (8464) from 2016 Topics T6.1. Energy Topic Student Checklist R A G 6.1.1 Energy changes in a system, and the ways energy is stored before and after such
Personalised Learning Checklist AQA TRILOGY Physics (8464) from 2016 Topics T6.1. Energy Topic Student Checklist R A G 6.1.1 Energy changes in a system, and the ways energy is stored before and after such
Atomic Structure and the Periodic Table. Quick Fire Questions
 Atomic Structure and the Periodic Table Quick Fire Questions This work sheet is fully supported by a video tutorial https://youtu.be/mjlipj_c018 1. What element is represented by W? 2. What element is
Atomic Structure and the Periodic Table Quick Fire Questions This work sheet is fully supported by a video tutorial https://youtu.be/mjlipj_c018 1. What element is represented by W? 2. What element is
CELLS. Structure and Function
 CELLS Structure and Function Cell Structure All plant and animal tissue consist of cells. Cells are microscopic in size. In general, each cell performs all the characteristics of life and, though in reality
CELLS Structure and Function Cell Structure All plant and animal tissue consist of cells. Cells are microscopic in size. In general, each cell performs all the characteristics of life and, though in reality
STUDENT PACKET #1 Student Exploration: Cell Structure
 STUDENT PACKET #1 Student Exploration: Cell Structure Big Idea 14: Organization and Development of Living Organisms SC.6.L.14.1 Describe and identify patterns in the hierarchical organization of organisms
STUDENT PACKET #1 Student Exploration: Cell Structure Big Idea 14: Organization and Development of Living Organisms SC.6.L.14.1 Describe and identify patterns in the hierarchical organization of organisms
Combined Science: Physics Paper 1 Higher. Knowledge Organisers. Physics Paper 1 23 rd May PM 1h 15min. Conservation and Dissipation of Energy P2
 Combined Science: Physics Paper 1 Higher Knowledge Organisers Physics Paper 1 23 rd May PM 1h 15min Topics in the Paper: P1 Conservation and Dissipation of Energy P2 Energy Transfer By Heating P3 P4 P5
Combined Science: Physics Paper 1 Higher Knowledge Organisers Physics Paper 1 23 rd May PM 1h 15min Topics in the Paper: P1 Conservation and Dissipation of Energy P2 Energy Transfer By Heating P3 P4 P5
Changes of State & Particle Model
 Changes of State & Particle Model Question Paper Level GCSE (9-1) Subject Combined Science: Trilogy - Physics Exam Board AQA Topic 6.3 Particle Model of Matter Sub-Topic Changes of State & Particle Model
Changes of State & Particle Model Question Paper Level GCSE (9-1) Subject Combined Science: Trilogy - Physics Exam Board AQA Topic 6.3 Particle Model of Matter Sub-Topic Changes of State & Particle Model
Organelles & Cells Student Edition. A. chromosome B. gene C. mitochondrion D. vacuole
 Name: Date: 1. Which structure is outside the nucleus of a cell and contains DNA? A. chromosome B. gene C. mitochondrion D. vacuole 2. A potato core was placed in a beaker of water as shown in the figure
Name: Date: 1. Which structure is outside the nucleus of a cell and contains DNA? A. chromosome B. gene C. mitochondrion D. vacuole 2. A potato core was placed in a beaker of water as shown in the figure
Biology Paper 1 1hr 15mins 70 marks
 Biology Paper 1 1hr 15mins 70 marks Cell Biology (Yr9) Cell Organisation (Yr9) Infection and Response (Yr10) Bioenergetics (Yr10) Cells Cell Organisation Competition Photosynthesis Microscopy Enzymes Abiotic
Biology Paper 1 1hr 15mins 70 marks Cell Biology (Yr9) Cell Organisation (Yr9) Infection and Response (Yr10) Bioenergetics (Yr10) Cells Cell Organisation Competition Photosynthesis Microscopy Enzymes Abiotic
Unit 2: Cells. Students will understand that the organs in an organism are made of cells that have structures & perform specific life functions
 Unit 2: Cells Students will understand that the organs in an organism are made of cells that have structures & perform specific life functions Vocabulary Cell Chloroplast Tissue Cell wall Organ Lysosome
Unit 2: Cells Students will understand that the organs in an organism are made of cells that have structures & perform specific life functions Vocabulary Cell Chloroplast Tissue Cell wall Organ Lysosome
Cells. A. The iodine diffused into the bag. B. The starch was changed to sugar.
 Name: Date: 1. A student filled a bag of dialysis tubing with a milky-white starch solution and placed the bag in a beaker of iodine-water as shown in the diagram. An hour later, the student observed that
Name: Date: 1. A student filled a bag of dialysis tubing with a milky-white starch solution and placed the bag in a beaker of iodine-water as shown in the diagram. An hour later, the student observed that
Chapter: Cell Processes
 Table of Contents Chapter: Cell Processes Section 1: Chemistry of Life Section 2: Moving Cellular Materials Section 3: Energy for Life 1 Chemistry of Life The Nature of Matter Matter is anything that has
Table of Contents Chapter: Cell Processes Section 1: Chemistry of Life Section 2: Moving Cellular Materials Section 3: Energy for Life 1 Chemistry of Life The Nature of Matter Matter is anything that has
Name # Class Date Regents Review: Cells & Cell Transport
 Name # Class Date Regents Review: Cells & Cell Transport 1. All of the following are true regarding cells except? A) All cells have genetic material B) All cells have cell walls C) All cells have plasma
Name # Class Date Regents Review: Cells & Cell Transport 1. All of the following are true regarding cells except? A) All cells have genetic material B) All cells have cell walls C) All cells have plasma
Chapter 2. The Chemistry of Life
 Chapter 2 The Chemistry of Life Introduction Cells, tissues and organs composed of chemicals Chemical reactions important for function Chemistry is the study of elements, compounds, chemical reactions,
Chapter 2 The Chemistry of Life Introduction Cells, tissues and organs composed of chemicals Chemical reactions important for function Chemistry is the study of elements, compounds, chemical reactions,
Chemistry Paper 1 Revision Knowledge Questions. Topic 1- Atomic Structure and the periodic Table. Topic 2- Bonding, Structure and Properties of Matter
 Chemistry Paper 1 Revision Knowledge Questions Topic 1- Atomic Structure and the periodic Table Topic 2- Bonding, Structure and Properties of Matter Why am I doing this? To answer exam questions you need
Chemistry Paper 1 Revision Knowledge Questions Topic 1- Atomic Structure and the periodic Table Topic 2- Bonding, Structure and Properties of Matter Why am I doing this? To answer exam questions you need
Explain your answer:
 Biology Midterm Exam Review Introduction to Biology and the Scientific Method Name: Date: Hour: 1. Biology is the study of: 2. A living thing is called a(n): 3. All organisms are composed of: 4. The smallest
Biology Midterm Exam Review Introduction to Biology and the Scientific Method Name: Date: Hour: 1. Biology is the study of: 2. A living thing is called a(n): 3. All organisms are composed of: 4. The smallest
Atoms And The Periodic Table
 Tick one box to choose the correct answer 1) What elements are found in the compound water (H 2 O)? Hydrogen and oxygen Helium and oxygen Hydrogen and nitrogen 2) Which of the following is a metal element?
Tick one box to choose the correct answer 1) What elements are found in the compound water (H 2 O)? Hydrogen and oxygen Helium and oxygen Hydrogen and nitrogen 2) Which of the following is a metal element?
Midterm Study Guide Major Concepts
 Midterm Study Guide Name 7 th Grade PSI Major Concepts 1. What is an atom? 2. What is a molecule? 3. What is an element? 4. What is a compound? 5. What are physical properties? Describe a few examples.
Midterm Study Guide Name 7 th Grade PSI Major Concepts 1. What is an atom? 2. What is a molecule? 3. What is an element? 4. What is a compound? 5. What are physical properties? Describe a few examples.
(a) (i) What is represented by... (ii) What is represented by... (2) (b) What is the symbol for lithium?... (1) (Total 3 marks)
 1 The diagram shows the structure of a lithium atom. (a) (i) What is represented by... (ii) What is represented by... (b) What is the symbol for lithium?... (Total 3 marks) 2 (a) Balance these chemical
1 The diagram shows the structure of a lithium atom. (a) (i) What is represented by... (ii) What is represented by... (b) What is the symbol for lithium?... (Total 3 marks) 2 (a) Balance these chemical
B05 comparison of plant and animal cells.notebook. November 22, 2012
 Worksheet:List of Cell Organelles and Functions Instructions: After watching the video, complete the following table http://www.youtube.com/watch?v=o1gqycijata&feature=related Organelle Location Function
Worksheet:List of Cell Organelles and Functions Instructions: After watching the video, complete the following table http://www.youtube.com/watch?v=o1gqycijata&feature=related Organelle Location Function
1.1 Characteristics common to organisms
 Biology Form 3 Page 5 Ms. R. Buttigieg 1.1 Characteristics common to organisms see GCSE Biology pg. 292 Biology is the study of living things. We call living things organisms. Plants and animals are ALL
Biology Form 3 Page 5 Ms. R. Buttigieg 1.1 Characteristics common to organisms see GCSE Biology pg. 292 Biology is the study of living things. We call living things organisms. Plants and animals are ALL
PREFACE O-LEVEL TOPICAL SCIENCE (BIOLOGY)
 PREFACE O-LEVEL TOPICAL SCIENCE (BIOLOGY) provides a thorough revision for students taking the GCE O-Level Science (Biology) Examination. Past examination questions have been carefully classified into
PREFACE O-LEVEL TOPICAL SCIENCE (BIOLOGY) provides a thorough revision for students taking the GCE O-Level Science (Biology) Examination. Past examination questions have been carefully classified into
HASTINGS HIGH SCHOOL
 Subject HASTINGS HIGH SCHOOL YEAR 11 EXAMINATION GUIDE 20167-19 COMBINED SCIENCE TRILOGY Physics Course code AQA GCSE COMBINED SCIENCE TRILOGY 8464 Website address Provisional examination dates http://www.aqa.org.uk/subjects/science/gcse/combined-science-trilogy-
Subject HASTINGS HIGH SCHOOL YEAR 11 EXAMINATION GUIDE 20167-19 COMBINED SCIENCE TRILOGY Physics Course code AQA GCSE COMBINED SCIENCE TRILOGY 8464 Website address Provisional examination dates http://www.aqa.org.uk/subjects/science/gcse/combined-science-trilogy-
Chapter 2: the Structure of the Atom
 Chapter 2: the Structure of the Atom exists in MATTER made of goes through Solid Liquid Gas differs in Melting Freezing Boiling Condensation Sublimation consists of Atoms Arrangement of particles Movement
Chapter 2: the Structure of the Atom exists in MATTER made of goes through Solid Liquid Gas differs in Melting Freezing Boiling Condensation Sublimation consists of Atoms Arrangement of particles Movement
It helps scientists understand the workings of the human body and of other animals and plants
 Science 8 Unit 1 Worksheet Chapter 1 Cells Online resources: Click on Chapter 1 at the site below. http://www.nelson.com/bcscienceprobe8/student/weblinks.html Chapter 1.1 1. Organism is another word used
Science 8 Unit 1 Worksheet Chapter 1 Cells Online resources: Click on Chapter 1 at the site below. http://www.nelson.com/bcscienceprobe8/student/weblinks.html Chapter 1.1 1. Organism is another word used
Paget High School. Preparing for A level Biology
 Paget High School Preparing for A level Biology You will need a copy of the Head Start guide above and use it to make notes on the learning outcomes below. Topic areas Learning outcome Describe with the
Paget High School Preparing for A level Biology You will need a copy of the Head Start guide above and use it to make notes on the learning outcomes below. Topic areas Learning outcome Describe with the
Describe how the inter-conversion of solids, liquids and gases are achieved and recall names used for these inter-conversions
 Understand the arrangements, movements and energy of the particle in each of the 3 states of matter : solid, liquid and gas Describe how the inter-conversion of solids, liquids and gases are achieved and
Understand the arrangements, movements and energy of the particle in each of the 3 states of matter : solid, liquid and gas Describe how the inter-conversion of solids, liquids and gases are achieved and
AQA TRILOGY Chemistry (8464) from 2016 Topics T5.1 Atomic structure and the periodic table (Paper 1) To pic. Student Checklist
 Personalised Learning Checklist AQA TRILOGY Chemistry (8464) from 2016 s T5.1 Atomic structure and the periodic table (Paper 1) State that everything is made of atoms and recall what they are 5.1.1 A simple
Personalised Learning Checklist AQA TRILOGY Chemistry (8464) from 2016 s T5.1 Atomic structure and the periodic table (Paper 1) State that everything is made of atoms and recall what they are 5.1.1 A simple
Physical Science Final Review
 Physical Science Final Review Who is the Father of Atomic Theory? Thomson s Experiment: In Thomson s model of the atom, the negative charges are scattered throughout an atom filled with a positively charged
Physical Science Final Review Who is the Father of Atomic Theory? Thomson s Experiment: In Thomson s model of the atom, the negative charges are scattered throughout an atom filled with a positively charged
1. Cell Theory Organelle containing the genetic information of the cell.
 GLOSSARY MATCHING GAME The words and definitions are all mixed up. Cut out each word and definition and glue the correct matches into your workbook. Word Definition 1. Cell Theory Organelle containing
GLOSSARY MATCHING GAME The words and definitions are all mixed up. Cut out each word and definition and glue the correct matches into your workbook. Word Definition 1. Cell Theory Organelle containing
 http://koning.ecsu.ctstateu.edu/cell/cell.html 4A: Students will compare and contrast prokaryotic and eukaryotic cells Robert Hooke (1665) Used a compound microscope to look at thin slices of cork (oak
http://koning.ecsu.ctstateu.edu/cell/cell.html 4A: Students will compare and contrast prokaryotic and eukaryotic cells Robert Hooke (1665) Used a compound microscope to look at thin slices of cork (oak
Military High School AL- Ain. Grade 10 &11. Biology Sample Questions. Student Name: Computer #:
 Military High School AL- Ain Grade 10 &11 Biology Sample Questions Student Name: Computer #: Chapter 1: Cells In all multiple choice questions, more than answer could be correct Section : 1 What Is a Cell?
Military High School AL- Ain Grade 10 &11 Biology Sample Questions Student Name: Computer #: Chapter 1: Cells In all multiple choice questions, more than answer could be correct Section : 1 What Is a Cell?
Personalised Learning Checklists AQA Chemistry Paper 1
 AQA Chemistry (8462) from 2016 Topics C4.1 Atomic structure and the periodic table State that everything is made of atoms and recall what they are Describe what elements and compounds are State that elements
AQA Chemistry (8462) from 2016 Topics C4.1 Atomic structure and the periodic table State that everything is made of atoms and recall what they are Describe what elements and compounds are State that elements
Education Transformation Office (ETO) 8 th Grade Unit #4 Assessment
 Education Transformation Office (ETO) 8 th Grade Unit #4 Assessment 1. Which of these shows the correct hierarchical sequence? A. organs cells tissues organ systems B. cells tissues organs organ systems
Education Transformation Office (ETO) 8 th Grade Unit #4 Assessment 1. Which of these shows the correct hierarchical sequence? A. organs cells tissues organ systems B. cells tissues organs organ systems
Main Topic Sub-topics Students should be able to R O G
 Second Year Science Syllabus Biology Revision Check-list Where is your learning at? Green: I know it all. Orange: I have some idea check the answers. Red: I need to start studying this section. Main Topic
Second Year Science Syllabus Biology Revision Check-list Where is your learning at? Green: I know it all. Orange: I have some idea check the answers. Red: I need to start studying this section. Main Topic
Additional Science Chemistry
 Additional Science Chemistry C2 Core Questions and Keywords and Definitions Question How did Mendeleev arrange the elements known at the time into a periodic table? How did Mendeleev use his table? Where
Additional Science Chemistry C2 Core Questions and Keywords and Definitions Question How did Mendeleev arrange the elements known at the time into a periodic table? How did Mendeleev use his table? Where
How to use this book. How the book is organised. Answering questions. Learning and using the terminology. Developing skills
 How to use this book Welcome to the beginning of your Human and Social Biology course! We hope that you really enjoy your course, and that this book will help you to understand your work, and to do well
How to use this book Welcome to the beginning of your Human and Social Biology course! We hope that you really enjoy your course, and that this book will help you to understand your work, and to do well
Physical Science Midterm Review
 Chapter 1: Science Skills, pages 2-25 1. What is science? Science is a system of knowledge and the methods you use to find that knowledge. 2. What is the relationship between science and technology? Science
Chapter 1: Science Skills, pages 2-25 1. What is science? Science is a system of knowledge and the methods you use to find that knowledge. 2. What is the relationship between science and technology? Science
CELL PRACTICE TEST
 Name: Date: 1. As a human red blood cell matures, it loses its nucleus. As a result of this loss, a mature red blood cell lacks the ability to (1) take in material from the blood (2) release hormones to
Name: Date: 1. As a human red blood cell matures, it loses its nucleus. As a result of this loss, a mature red blood cell lacks the ability to (1) take in material from the blood (2) release hormones to
Assessment Schedule 2017 Biology: Demonstrate understanding of life processes at the cellular level (91156)
 NCEA Level 2 Biology (91156) 2017 page 1 of 6 Assessment Schedule 2017 Biology: Demonstrate understanding of life processes at the cellular level (91156) Assessment Criteria Achievement Achievement with
NCEA Level 2 Biology (91156) 2017 page 1 of 6 Assessment Schedule 2017 Biology: Demonstrate understanding of life processes at the cellular level (91156) Assessment Criteria Achievement Achievement with
CORE CONCEPTS & TERMINOLOGY FALL 2010
 CORE CONCEPTS & TERMINOLOGY FALL 2010 The following concepts and terms will be covered by all BIO 120 lecture instructors. Presentation of additional concepts is left to the discretion of the individual
CORE CONCEPTS & TERMINOLOGY FALL 2010 The following concepts and terms will be covered by all BIO 120 lecture instructors. Presentation of additional concepts is left to the discretion of the individual
Cell Structure, Function & Ultrastructure
 Cell Structure, Function & Ultrastructure Learning Objectives 2.1.2 Components of the cell as seen under the light microscope and their functions. Cell Structure and Function 1. Plant cells: cell wall,
Cell Structure, Function & Ultrastructure Learning Objectives 2.1.2 Components of the cell as seen under the light microscope and their functions. Cell Structure and Function 1. Plant cells: cell wall,
Cell Organelles. 2. Cells are the basic unit of organization in an organism Cells tissues organ organ system organism
 Cell Organelles What are some of the differences you see between these two cells? A. Cell Theory 1. All organisms are made up of one or more cells 2. Cells are the basic unit of organization in an organism
Cell Organelles What are some of the differences you see between these two cells? A. Cell Theory 1. All organisms are made up of one or more cells 2. Cells are the basic unit of organization in an organism
Review Chemistry Paper 1
 Atomic Structure Topic Define an atom and element. Use scientific conventions to identify chemical symbols Identify elements by chemical symbols Define compound Use chemical formulae to show different
Atomic Structure Topic Define an atom and element. Use scientific conventions to identify chemical symbols Identify elements by chemical symbols Define compound Use chemical formulae to show different
Name Period Date Physical Science 2nd Semester Final Exam Study Guide ( )
 Name Period Date Physical Science 2nd Semester Final Exam Study Guide (2012-2013) 1. Physical Science Basics a. What tool(s) would you want to use to find the: i. Mass of an object? Basic SI Unit? ii.
Name Period Date Physical Science 2nd Semester Final Exam Study Guide (2012-2013) 1. Physical Science Basics a. What tool(s) would you want to use to find the: i. Mass of an object? Basic SI Unit? ii.
CHAPTER 3 ATOMS ATOMS MATTER 10/17/2016. Matter- Anything that takes up space (volume) and has mass. Atom- basic unit of matter.
 CHAPTER 3 MATTER Matter- Anything that takes up space (volume) and has mass. Matter Combining Matter States of Matter Atom- basic unit of matter. Subatomic particles- protons, neutrons, and electrons.
CHAPTER 3 MATTER Matter- Anything that takes up space (volume) and has mass. Matter Combining Matter States of Matter Atom- basic unit of matter. Subatomic particles- protons, neutrons, and electrons.
Chapter Two (Chemistry of Life)
 1 Chapter Two (Chemistry of Life) SECTION ONE: THE COMPOSITION OF MATTER MATTER Everything in the universe is made of matter. Matter is anything that occupies space and has mass. Mass is the quantity of
1 Chapter Two (Chemistry of Life) SECTION ONE: THE COMPOSITION OF MATTER MATTER Everything in the universe is made of matter. Matter is anything that occupies space and has mass. Mass is the quantity of
Biology Midterm Test Review
 Biology Midterm Test Review Levels of Organization 1. Put these levels of organization in order from simplest to most complex (smallest to largest): cell, community, atom, organism, biosphere, organ system,
Biology Midterm Test Review Levels of Organization 1. Put these levels of organization in order from simplest to most complex (smallest to largest): cell, community, atom, organism, biosphere, organ system,
Semester II Final Exam Study Questions Answer Key
 Semester II Final Exam Study Questions Answer Key Unit 5: Matter Standards: Standard 1: Structure and Properties of Matter All matter is made up of atoms. Its structure is made up of repeating patterns
Semester II Final Exam Study Questions Answer Key Unit 5: Matter Standards: Standard 1: Structure and Properties of Matter All matter is made up of atoms. Its structure is made up of repeating patterns
Queen s Park High School Key Stage 3 Assessment Science - Earth IA6 target.
 Year 7 Enquiry Skills Queen s Park High School Key Stage 3 Assessment Science - Earth IA6 target. EARTH STRUCTURE AND UNIVERSE Draw simple graphs outlining information about planets Construct models of
Year 7 Enquiry Skills Queen s Park High School Key Stage 3 Assessment Science - Earth IA6 target. EARTH STRUCTURE AND UNIVERSE Draw simple graphs outlining information about planets Construct models of
Year 8 Chemistry Knowledge Organiser Topic 1: Periodic Table
 KPI 1.1: Identify, with reasons, differences between atoms, elements and compounds Key Terms Element Mixture Compound Elements Definitions A substance that contains only one type of atom A substance that
KPI 1.1: Identify, with reasons, differences between atoms, elements and compounds Key Terms Element Mixture Compound Elements Definitions A substance that contains only one type of atom A substance that
PLC Booklet for. Paper 1
 PLC Booklet for Combined Science Paper 1 This personalised learning checklist (PLC) booklet has been put together to support your preparation for Biology Paper 1, Chemistry Paper 1 and Physics Paper1.
PLC Booklet for Combined Science Paper 1 This personalised learning checklist (PLC) booklet has been put together to support your preparation for Biology Paper 1, Chemistry Paper 1 and Physics Paper1.
Honors Biology-CW/HW Cell Biology 2018
 Class: Date: Honors Biology-CW/HW Cell Biology 2018 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Hooke s discovery of cells was made observing a. living
Class: Date: Honors Biology-CW/HW Cell Biology 2018 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Hooke s discovery of cells was made observing a. living
The diagram below represents levels of organization within a cell of a multicellular organism.
 STATION 1 1. Unlike prokaryotic cells, eukaryotic cells have the capacity to a. assemble into multicellular organisms b. establish symbiotic relationships with other organisms c. obtain energy from the
STATION 1 1. Unlike prokaryotic cells, eukaryotic cells have the capacity to a. assemble into multicellular organisms b. establish symbiotic relationships with other organisms c. obtain energy from the
Year 7 Science Booklet Name:
 Year 7 Science Booklet Name: Acids and Alkalis Use a dictionary or internet to look up the key words and write a definition. Acid Alkali Litmus Fizz Neutral Reactant Product Irritant Harmful indicator
Year 7 Science Booklet Name: Acids and Alkalis Use a dictionary or internet to look up the key words and write a definition. Acid Alkali Litmus Fizz Neutral Reactant Product Irritant Harmful indicator
OCR Chemistry Checklist
 Topic 1. Particles Video: The Particle Model Describe the main features of the particle model in terms of states of matter. Explain in terms of the particle model the distinction between physical changes
Topic 1. Particles Video: The Particle Model Describe the main features of the particle model in terms of states of matter. Explain in terms of the particle model the distinction between physical changes
