Supporting Information
|
|
|
- Cori Owens
- 5 years ago
- Views:
Transcription
1 Supporting Information An acid-free conversion of cellulose to -hydroxymethyl-furfural catalyzed by hot Seawater Xiangcheng Li, Yayun Zhang, Qineng Xia, Xiaohui Liu, Kaihao Peng, Sihai Yang, * Yanqin Wang * Shanghai Key Laboratory of Functional Materials Chemistry, Research Institute of Industrial Catalysis, School of Chemistry and Molecular Engineering, East China University of Science and Technology, 130 Meilong Road, Shanghai, 0037, P. R. China. Key Laboratory of Low-grade Energy Utilization Technologies and Systems, Ministry of Education, College of Power Engineering, Chongqing University, Chongqing , China. Bioproducts, Sciences and Engineering Laboratory, Department of Biological Systems Engineering, Washington State University, Richland, WA , USA. School of Chemistry, University of Manchester, Manchester, M13 9PL, UK. * Correspondence s:sihai.yang@manchester.ac.uk; wangyanqin@ecust.edu.cn. S1
2 Lignin hydrodeoxygenation and details of alkylcyclohexanes analysis. The hydrodeoxygenation of lignin was conducted in a 0 ml Teflon-lined stainless-steel autoclave. In a typical run, lignin residue (0.10 g), %Pt/NbP 4 (0.0 g) and cyclohexane (10 ml) were charged in the reactor, which was then sealed, purged three times with and charged to an initial pressure of 3.0 MPa with. The reactor was then slowly heated to 0 C with a magnetic stirring speed of 700 rpm and held at this temperature for 0 h. After the reaction, the reactor was cooled down quickly. The amounts of alkylcyclohexanes were analysed by gas chromatography (GC) and GC-mass spectroscopy (GC-MS) on an Agilent 7890B gas chromatograph with flame ionisation detector (FID) and an Agilent 7890A GC-MS instrument, both equipped with P- capillary columns. Tridecane was added as an internal standard. The mole yield of C 7 ~C 9 cycloalkanes was defined as follows: mole of C 7 ~C 9 cycloalkanes produced (µmol/g lignin) Mole yield of C 7 ~C 9 cycloalkanes= mole of C 7 ~C 9 hydrocarbons obtained by NB method (µmol/g lignin) The mass yield of liquid alkanes was defined as follows: mass of alkylcyclohexanes Mass yield of alkylcyclohexanes = mass of lignin residues Analysis of lignin monomers by alkaline nitrobenzene oxidation method (NB). The analysis of birch lignin monomers was conducted by alkaline nitrobenzene oxidation method (NB) according to literature. 1 In a typical reaction, birch lignin (40 mg) was mixed with nitrobenzene (0.4 ml) and M Na (7 ml) and reacted at 170 C for h. Afterwards, the reactor was cooled in ice-water and 1 ml of freshly prepared ethyl vanillin (3-ethoxy-4-hydroxybenzaldehyde, EV) ( µmol/ml) in 0.1 M Na solution was added to the reaction mixture as an internal standard. The mixture was transferred to a 100-mL separation funnel and washed three times with 1 ml of dichloromethane. The remaining aqueous layer was acidified with M, until the p was below 3.0 and extracted twice with 0 ml of dichloromethane and 0 ml of diethyl ether. The combined organic layer was S
3 washed with deionised water (0 ml) and dried over Na S 4. After filtration, the filtrate was collected in a 100-mL pear-shaped flask and dried under reduced pressure. For the TMS (trimethylsilyl) derivatisation step, NB-products were washed with pyridine (3 00 µl) into a GC vial and BSTFA (10 µl) was added. The mixture was heated to 0 C for 30 min. The silylated NB-products were analyzed by GC-MS (Agilent 7890A GC-MS) equipped with ap- capillary column (30 m 0 µm) to identify the products by the comparison with the peak retention time and mass spectra of the authentic compounds. The identified products were quantified by GC-FID (Agilent 7890B) using the same column. Initial column temperature: 10 C (held for 10 min), raised at C/min to 80 C (held for 0 min). Intensity micro-crystalline cellulose ball-milled cellulose Theta/degree Figure S1. XRD patterns of the micro-crystalline and ball-milled cellulose. S3
4 Table S1. Analysis of the components of seawater a. Sea water (original, ca. 3wt% salts) Sea water (concentrated, ca. 1wt% salts) b Sea water (concentrated, ca. 30wt% salts) b Ions Percentage of simulated Percentage of Percentage of seawater (g/kg) simulated seawater simulated seawater (g/kg) (g/kg) Na S Mg K Ca Total a the components of seawater were analysed by ion chromatography (ICS1100). b As the salts concentration increases, the Ca +, S 4 - are gradually salted out in the form of CaS 4. S4
5 Table S. Yields of MF and furfural obtained from microcrystalline cellulose and various sources of lignocellulosic biomass in the TF/seawater system. Entry Sample Glucan (wt%) Xylan (wt%) The Molar Yield (%) The Mass Yield (%) MF Furfural MF Furfural Total 1 a Microcrystalline cellulose b Cornstalk b Pine b Birch b Poplar a Reaction condition: wt% cellulose, 0. M N, 1 ml concentrated seawater (ca. 30wt% salts), 6 ml TF, 00 C, 6 h; b Reaction condition: wt% biomass, 0. M N, 1 ml concentrated seawater (ca. 30wt% salts), 6 ml TF, 00 C, h. The compositions of lignocellulosic biomass were adapted from ref. according to the procedures of the Van Soest method. S
6 Conversion/Selectivity Cellulose conversion; Glucose selectivity; Fructose selectivity; MF selectivity 0 wt% 10wt% Cellulose concentration 0wt% Figure S. Influence of the cellulose concentration on the products distribution of cellulose conversion in the TF/seawater system. Reaction condition: 0.M N, 1 ml concentrated seawater (ca. 30wt% salts), 6mL TF, 00 C, 6 h. S6
7 (A) Weight (%) (B) Weight (%) TGA umins (cellulose) 449. DTA Temperature ( o C) Lignin residue 493 TGA DTA 36wt% Temperature ( o C) eat Flow Endo Down (mw) eat Flow Endo Down (mw) Figure S3. Thermal analysis of (A) humins (cellulose) and (B) lignin residue in air. The TGA curve shows that one weight loss region is observed and centered at 449. C for humins sample. When temperature reached to 470 C, almost all humins was pyrolyzed. While the lignin residue pryolysis was focused at C with the maximum weight loss rate at 493 C, and ca. 36 wt% may be considered the lignin content of lignin residue based on the weight loss after 470 C. S7
8 Table S3. Summary of product yields from direct hydrodeoxygenation of lignin residues over the Pt/NbP 4 catalyst. a Substrate Products distribution (wt%) C 7 ~C 9 cycloalkanes thers b Total mass Yield (%) Birch a Reaction conditions: lignin 0.1 g, catalyst 0. g, cyclohexane 1 ml, 0 C, MPa, 0 h. The metal loading in catalyst was wt%. b S8
9 Table S4. Representative GC-MS spectra of liquid products obtained from birch lignin conversion for 0 h over the Pt/NbP 4 catalyst. GC Spectra No. Ret. time (min) Compound No. Ret. time (min) Compound C 13 6 (Standard) S9
10 µmol monomers/g lignin C7~C9 cycloalkanes S G 0 NB D Figure S4. Monomer analysis of lignin residues. NB refers to the nitrobenzene oxidation method; D refers to direct hydrodeoxygenation over Pt/NbP 4 ; S refers to syringy units; G refers to guaiacyl units. The corresponding carbon yield of C 7 ~C 9 hydrocarbons is ca. 89.3% based on lignin monomers. Conversion/Selectivity Cellulose conversion; Glucose selectivity; Fructose selectivity; MF selectivity Reaction time (h) Figure S. Influence of reaction time on the products distribution of cellulose conversion in the TF/Na- system. Reaction condition: wt% cellulose, 30wt% Na, 0. M N, 1mL, 6mL TF, 00 C. S10
11 Table S. Products distribution of cellulose conversion catalyzed by a range of salts from seawater. Entry Salt Conv. S MF Y MF Y glucose Y fructose Y LA Y humins 1 Blank a Blank Na K Mg > Ca > b Mg b Ca Na S Reaction condition: wt% cellulose, 30wt% salts, 0.M N, 1mL, 6mL TF, 00 C, h. a reaction time : 6 h. b reaction time : 40 min. S11
12 Table S6. Products distribution of cellulose conversion catalyzed by a range of neutral chloride-, bromide-based salts, N 4 and. Entry Salt Conv. S MF Y MF Y glucose Y fructose Y LA Y humins 1 Blank Li > Na > K > LiBr > NaBr > KBr > a N 4 > b > Reaction condition: wt% cellulose, 30wt% salts, 0.M N, 1mL, 6mL TF, 00 C, 8 h. a reaction time : h. b catalyst: wt%, reaction time : h. S1
13 Table S7. Influence of temperature on the products distribution of cellulose conversion in the TF/Na- system. Entry Temperture / C Conv. S MF Y MF Y glucose Y fructose Y LA Y humins 1 a a a > a > Reaction condition: wt% cellulose, 30wt% Na, 0. M N, 1mL, 6mL TF, 8 h. a reaction without the addition of Na. Conversion/Selectivity Cellulose conversion; Glucose selectivity; Fructose selectivity; MF selectivity 0 Blank wt% 10wt% Na 0wt% 30wt% Figure S6. Influence of Na concentration on the products distribution from cellulose conversion in the TF/Na- system. Reaction condition: wt% cellulose, 0. M N, 1mL, 6mL TF, 00 C, 8 h. S13
14 Conformational 3 3 Ring opening 4 transition 6 - transfer R pathway 1 1-I1 1-I 1-I3 1-I4 ff Ring opening R pathway -I1 -I I3 Conformational 3 transition ff Ring opening R pathway transfer I1 3-I 3-I I4 4 6 Conformational transition ff 4 6 with Ring opening transfer R pathway 1 cl-1-i cl-1-i3 cl-1-i Ring opening R pathway cl--i cl--i1 3 Conformational 3 4 transition cl-1-i4 ff 3 4 Conformational 3 transition cl--i3 ff Ring opening R cl-3-i1 pathway cl-3-i 6 transfer cl-3-i cl-3-i4 6 Conformational transition ff Scheme S1. Reaction pathways of glucose conversion to fructose with and without -. nly one - was added here to present condition with chloride ions. Inserted figures presents reaction free energy change of each step. In aqueous condition without chloride ion (Figure a), the protonation of glucose tends to locate at and the analysis of its geometry (I1) shows a relatively large difference in the distance between C1 and (from Å to 1.9 Å before and after protonation respectively). Meanwhile, the hydroxyl group () is nearly completely formed according to the bond length of 0.983Å. The cationic fragment maintains its stability by developing the hydrogen bond between and 6. While the slight geometrical perturbations is found in the bond length of C1-1, which indicates the C1 site remains sp 3 -hybridized. Whereas, the subsequent ring opening occurs through isomerisation with rotation of group. This configuration change is accompanied by the development of sp character on C1 atom, evidenced from the shortening of the C1-1 bond by 0.09Å (from Å to 1.61Å) and S14
15 approaching of three angles ( θ CC11, θ 1C11, θ CC11) towards the value of 10 o. The C1 atom is located within the C11 plane, further supporting its sp character. Thermodynamic analysis indicates this ring opening step is slightly unfavourable ( G = 3.7kJ/mol). The formed hydrogen bonds ( 3, 3 4, and 6) also contribute to the stability of cationic fragment (I in Figure a). A thermodynamic favorable step (I TS1 I3) then occurs that the hydrogen atom transfers from C to C1 with G = -7.1kJ/mol, which echoes 1,-hydride shift mechanism proposed in our experimental results of glucose conversion using isotope labelling method (Figure 6), moreover, a considerable kinetic isotopic effect is observed that almost a two-fold decrease in the initial reaction rate (k /k D = 1.87, Figure S7) happened due to the deuterium substitution at the C- position, revealing a considerable kinetic isotopic effect, further confirming that the 1,-hydride shift of glucose isomerisation is a rate-determining step. The activation free energy for this elemental reaction is only 6.4 kj/mol, which is also in accordance with 8 kj/mol under the gas phase in the previous theoretical study. 3 The following conformational transition from I3 to I4 is highly preferred due to the sharp drop of G (-6.7kJ/mol), where the group approaches toward C and the distance between and C is shorten to 1.69Å. Finally, the deprotonation of I4 intermediate leads to closure of the furanose ring and generation of fructose (ff), where G is only 18.9 kj/mol revealing tinily unfavorable forward reaction tendency. The ring opening and deprotonation reactions restrict the whole isomerisation of glucose converting to fructose and thus causing a relatively slow reaction rate of glucose decomposition and low fructose selectivity, which agree with corresponding experimental phenomenon mentioned above. Interestingly, chloride ions play a pivotal role in this reaction pathway when - is added to the solvent and the reaction process is described in Scheme S1(a). The average length of hydroxyl bonds of cl-r is elongated by 0.0Å and the hydrogen bonds disappear due to the chelation between chloride ions and glucose, which contributes to accelerating reactivity of reactant. The protonation at of cl-r here with assistance of chloride ions resembles that observed in the absence of -. The Gibbs free energy change, however, decreases to -8.9kJ/mol, which presents much more thermodynamic favorable trend. Similarly, the ring opening step is also greatly promoted with G =9.7kJ/mol and therefore increases the rate of glucose isomerisation. In addition, a slight positive effect ( G changes from -7.1 to -1.7 kj/mol) occurs in the following hydrogen transfer reaction in terms of thermodynamic analysis, which matches very well with the corresponding kinetic study that the activation energy decreases from 6.4 kj/mol to 46.3 kj/mol without and with - respectively. Although the subsequent conformational transition experiences some negative perturbation of S1
16 G, this elemental reaction maintains occurring spontaneously. Most important, the deprotonation prior to forming fructose is accelerated dramatically with G = kj/mol and the hydrogen is released via forming the molecule as studied in previous theoretical work. 4 In the Figure b, the free energy changes along the reaction processes of glucose conversion to fructose are compared together with corresponding configurations in the absence and presence of -. Thermodynamic and kinetic analysis both show the promotion induced by introducing this halogen ion to the reaction system and therefore the MF yield from glucose is enhanced as observed in our experiments. Table S8. Changes of Gibbs free energy of the protonation of glucose and fructose at different sites (kj mol -1 ) Glucose Fructose / -6.4 S16
17 ring opening 4 4 transfer conformational transition cl-r cl-i1 - cl-i cl-i3 cl-i (a) 1 cl-ff (cl-ff) cl-i1 cl-i cl-i3 cl-i4 1 MF cl-i cl-i cl-i cl-i Scheme S. Reaction pathways of glucose to fructose (a) and fructose converts to MF (b) in the presence of chloride ion. (b) The mechanism of MF formation from fructose The reaction of fructose also comes with protonation first under the condition with the Brønsted catalyst in hot water as shown in Figure a. Protonation of the tertiary group is preferred with a free energy change of -1.6 kj/mol (see Table S6 for other protonation reactions). Analysis of geometry shows a large distance between C and (1.61Å) together with generation of a new -, which indicates that a water molecule is almost formed and tends to depart from the carbohydrate (f-i1 in Figure a). Intermediate f-i is then formed through dehydration of f-i1, which is highly preferred because of the large reduction of its free energy. Meanwhile, the development of sp character on the C atom occurs with the water molecule escaping, due to the shortening of C- bond and bond angles relevant to C getting to the value of 10 o. This geometrical perturbations contribute to the stability of cationic configuration with fructofuranose ring (f-i). Whereas, the subsequent deprotonation experiences the slight unfavorable change that hydrogen atom S17
18 escapes from C1 site and C1=C bond formed, where G is 6.7 kj/mol and therefore it is less thermodynamically favorable. Further protonation at the 3 group and the following dehydration are both thermodynamically preferred to produce intermediates f-i4 and f-i, respectively. C1=1 bond is then formed by deprotonation at 1 position to form the intermediate structure (f-i6). Similarly, the protonation at 4, followed by dehydration and deprotonation leads to the production of MF and the thermodynamic analysis also supports these three consecutive reactions. Adding chloride ions in the system of fructose conversion to MF, similarly, brings chelation between chloride ions and hydroxyl groups of fructose in the initial stage (eg.cl-ff in Scheme S (b)). f-i1 in Figure a and cl-i1 in Scheme S(b) present I1 in the absence and presence of -, respectively, Figure c presents the free energy changes along the reaction pathway. Protonation is promoted through the whole pathway with the assistance of -, namely, reactions of cl-ff to cl-i1, cl-i3 to cl-i4 and cl-i6 to cl-i7. In addition, deprotonation is also vastly accelerated in the view of thermodynamic and kinetic analysis. Specifically, the free energy change of reaction I to I3 reduces from 6.7 to -7. kj/mol and the activation energy decreases from 9. to 33.8kJ/mol without and with chloride ions, respectively. ther two deprotonations (cl-i to cl-i6 and cl-i8 to MF) have similar trends of relative energy changes. The reason of these positive influence can be interpreted by strong interaction force between hydrogen atom and chloride ion, which leads to a higher rate of protonation and deprotonation. owever, water molecule elimination from the carbohydrate slows down with a slight increase of the free energy after halogen presenting in the reactions. Based on the aforementioned thermodynamic and kinetic analysis, it can be concluded that adding chloride ions conduces to the protonation and deprotonation to a large extend and therefore promotes the conversion from fructose to MF, which is in excellent agreement with our experiment results. Compared with the process of glucose converting to fructose, it indicates that the completion of reaction degree is larger in MF generation because of lower free energy change, namely, more MF is generated than fructose. But the reaction rate is higher in forming fructose due to lower activation energy in the pathways of fructose formations, presenting shorter reaction time, in the absence of chloride ion. While with the assistance of -, both the completion of reaction degree and reaction rate are higher in the pathway of fructose conversion to MF because of lower Gibbs free energy change and lower activation energy, indicating more MF is formed faster than fructose generation. These analyses are highly consistent with the trends of lines in Figure 3 describing yields of fructose and MF and corresponding reaction time. S18
19 Glucose conversion (%) Glucose-D Unlabeled glucose k /k D = Time/min Figure S7. Profile of glucose isomerization with labeled and unlabeled glucose at 10 C using Na as the catalyst. Reaction condition: 1wt% glucose, 30wt% Na, 0. M N, 10 ml. References (1) Shuai, L.; Amiri, M. T.; Questell-Santiago, Y. M.; éroguel, F.; Li, Y. D.; Kim,.; Meilan, R.; Chapple, C.; Ralph, J.; Luterbacher. J. S. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 016, 34, (DI: /science.aaf7810) () Li, C. Z.; Zheng, M. Y.; Wang, A. Q.; Zhang, T. ne-pot catalytic hydrocracking of raw woody biomass into chemicals over supported carbide catalysts: simultaneous conversion of cellulose, hemicellulose and lignin. Energy Environ. Sci. 01,, (DI: /C1EE0684D) (3) Yang, G.; Pidko, E. A.; ensen, E. J. M. Mechanism of Brønsted acid-catalyzed conversion of carbohydrates. J. Catal. 01, 9, (DI: /j.jcat ) (4) Pidko, E. A.; Degirmenci, V.; ensen, E. J. M. n the mechanism of Lewis acid catalyzed glucose transformations in ionic liquids. ChemCatChem. 01, 4, (DI: /cctc ) S19
Supporting Information
 Supporting Information Robinson Annulation-directed Synthesis of Jet Fuel Ranged Alkylcyclohexanes from Biomass-derived Chemicals Yaxuan Jing, Qineng Xia, Junjian Xie, Xiaohui Liu, Yong Guo, Ji-jun Zou,
Supporting Information Robinson Annulation-directed Synthesis of Jet Fuel Ranged Alkylcyclohexanes from Biomass-derived Chemicals Yaxuan Jing, Qineng Xia, Junjian Xie, Xiaohui Liu, Yong Guo, Ji-jun Zou,
Supplementary Information for Efficient catalytic conversion of fructose into hydroxymethylfurfural by a novel carbon based solid acid
 Supplementary Information for Efficient catalytic conversion of fructose into hydroxymethylfurfural by a novel carbon based solid acid Jianjian Wang, Wenjie Xu, Jiawen Ren*, Xiaohui Liu, Guanzhong Lu,
Supplementary Information for Efficient catalytic conversion of fructose into hydroxymethylfurfural by a novel carbon based solid acid Jianjian Wang, Wenjie Xu, Jiawen Ren*, Xiaohui Liu, Guanzhong Lu,
Synthesis of jet fuel range cycloalkanes with diacetone alcohol. from lignocellulose
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Supporting Information Synthesis of jet fuel range cycloalkanes with diacetone alcohol from
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Supporting Information Synthesis of jet fuel range cycloalkanes with diacetone alcohol from
4023 Synthesis of cyclopentanone-2-carboxylic acid ethyl ester from adipic acid diethyl ester
 NP 4023 Synthesis of cyclopentanone-2-carboxylic acid ethyl ester from adipic acid diethyl ester NaEt C 10 H 18 4 Na C 2 H 6 C 8 H 12 3 (202.2) (23.0) (46.1) (156.2) Classification Reaction types and substance
NP 4023 Synthesis of cyclopentanone-2-carboxylic acid ethyl ester from adipic acid diethyl ester NaEt C 10 H 18 4 Na C 2 H 6 C 8 H 12 3 (202.2) (23.0) (46.1) (156.2) Classification Reaction types and substance
Supporting Information
 Supporting Information Protonated Titanate Nanotubes as Solid Acid Catalyst Masaaki Kitano, Kiyotaka Nakajima, Junko N. Kondo, Shigenobu Hayashi, and Michikazu Hara *,, П Materials and Structures Laboratory,
Supporting Information Protonated Titanate Nanotubes as Solid Acid Catalyst Masaaki Kitano, Kiyotaka Nakajima, Junko N. Kondo, Shigenobu Hayashi, and Michikazu Hara *,, П Materials and Structures Laboratory,
Mechanism and Kinetics of the Synthesis of 1,7-Dibromoheptane
 Asian Journal of Chemistry Vol. 22, No. 9 (2010), 6945-6954 Mechanism and Kinetics of the Synthesis of 1,7-Dibromoheptane HUA LI*, JIANG ZHU and JUAN LIU School of Chemical and Energy Engineering, Zhengzhou
Asian Journal of Chemistry Vol. 22, No. 9 (2010), 6945-6954 Mechanism and Kinetics of the Synthesis of 1,7-Dibromoheptane HUA LI*, JIANG ZHU and JUAN LIU School of Chemical and Energy Engineering, Zhengzhou
10/26/2010. An Example of a Polar Reaction: Addition of H 2 O to Ethylene. to Ethylene
 6.5 An Example of a Polar Reaction: Addition of H 2 O to Ethylene Addition of water to ethylene Typical polar process Acid catalyzed addition reaction (Electophilic addition reaction) Polar Reaction All
6.5 An Example of a Polar Reaction: Addition of H 2 O to Ethylene Addition of water to ethylene Typical polar process Acid catalyzed addition reaction (Electophilic addition reaction) Polar Reaction All
Supporting information for Mesoporous Nitrogen-Doped Carbons with High Nitrogen Content and
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2015 Supporting information for Mesoporous Nitrogen-Doped Carbons with High Nitrogen Content
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2015 Supporting information for Mesoporous Nitrogen-Doped Carbons with High Nitrogen Content
9. Hydroboration-Oxidation of Alkenes
 9. ydroboration-xidation of Alkenes A. Introduction 1. ydroboration-xidation of Alkenes Alkenes can be oxidized to alcohols using a two-step method of hydroboration followed by oxidation. The first step
9. ydroboration-xidation of Alkenes A. Introduction 1. ydroboration-xidation of Alkenes Alkenes can be oxidized to alcohols using a two-step method of hydroboration followed by oxidation. The first step
Study on the Selective Hydrogenation of Nitroaromatics to N-aryl hydroxylamines using a Supported Pt nanoparticle Catalyst
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 204 Supporting Information Study on the Selective Hydrogenation of Nitroaromatics
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 204 Supporting Information Study on the Selective Hydrogenation of Nitroaromatics
MODEL QUESTION PAPER FOR SUMMATIVE ASSESSMENT CHEMISTRY PART-A
 MODEL QUESTION PAPER FOR SUMMATIVE ASSESSMENT CHEMISTRY Time: 3 Hours Max Marks: 70 INSTRUCTIONS: i) The question paper has five parts A.B.C.D and E. All the parts are compulsory. Write balanced chemical
MODEL QUESTION PAPER FOR SUMMATIVE ASSESSMENT CHEMISTRY Time: 3 Hours Max Marks: 70 INSTRUCTIONS: i) The question paper has five parts A.B.C.D and E. All the parts are compulsory. Write balanced chemical
ACID-CATALYZED DEHYDRATION OF 2-METHYLCYCLOHEXANOL. Douglas G. Balmer. (T.A. Mike Hall) Dr. Dailey
 ACID-CATALYZED DEYDRATIN F 2-METYLCYCLEXANL Douglas G. Balmer (T.A. Mike all) Dr. Dailey Submitted 8 August 2007 Balmer 1 Introduction: The purpose of this experiment is to acid-catalyze the dehydration
ACID-CATALYZED DEYDRATIN F 2-METYLCYCLEXANL Douglas G. Balmer (T.A. Mike all) Dr. Dailey Submitted 8 August 2007 Balmer 1 Introduction: The purpose of this experiment is to acid-catalyze the dehydration
Synthesis of renewable diesel with hydroxyacetone and 2-methyl-furan
 Supporting Information Synthesis of renewable diesel with hydroxyacetone and 2-methyl-furan Guangyi Li, a,b Ning Li, a Shanshan Li, a,b Aiqin Wang, a Yu Cong, a Xiaodong Wang a and Tao Zhang a * a State
Supporting Information Synthesis of renewable diesel with hydroxyacetone and 2-methyl-furan Guangyi Li, a,b Ning Li, a Shanshan Li, a,b Aiqin Wang, a Yu Cong, a Xiaodong Wang a and Tao Zhang a * a State
Supporting Information
 Supporting Information Highly Cross-Linked Imidazolium Salts Entrapped Magnetic Particles Preparation and Applications Paola Agrigento, a Matthias Josef Beier, b Jesper T. N. Knijnenburg, c Alfons Baiker
Supporting Information Highly Cross-Linked Imidazolium Salts Entrapped Magnetic Particles Preparation and Applications Paola Agrigento, a Matthias Josef Beier, b Jesper T. N. Knijnenburg, c Alfons Baiker
Supplementary Material. Direct methane conversion to methanol by ionic liquids dissolved platinum catalysts
 Supplementary Material Direct methane conversion to methanol by ionic liquids dissolved platinum catalysts Jihong Cheng, Zaiwei Li, Mark Haught, Yongchun Tang * Power, Environmental, and Energy Research
Supplementary Material Direct methane conversion to methanol by ionic liquids dissolved platinum catalysts Jihong Cheng, Zaiwei Li, Mark Haught, Yongchun Tang * Power, Environmental, and Energy Research
Structure and Preparation of Alkenes: Elimination Reactions
 Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
Supplementary information for:
 Supplementary information for: Solvent dispersible nanoplatinum-carbon nanotube hybrids for application in homogeneous catalysis Yuhong Chen, Xueyan Zhang and Somenath Mitra* Department of Chemistry and
Supplementary information for: Solvent dispersible nanoplatinum-carbon nanotube hybrids for application in homogeneous catalysis Yuhong Chen, Xueyan Zhang and Somenath Mitra* Department of Chemistry and
The Synthesis of Triphenylmethano. will synthesize Triphenylmethanol, a white crystalline aromatic
 HEM 333L rganic hemistry Laboratory Revision 2.0 The Synthesis of Triphenylmethano ol In this laboratory exercise we will synthesize Triphenylmethanol, a white crystalline aromatic compound. Triphenylmethanol
HEM 333L rganic hemistry Laboratory Revision 2.0 The Synthesis of Triphenylmethano ol In this laboratory exercise we will synthesize Triphenylmethanol, a white crystalline aromatic compound. Triphenylmethanol
BIOCHEMISTRY NOTES - UNIT 2-
 BIOCHEMISTRY NOTES - UNIT 2- ATOMS - the basic unit of matter. Contains subatomic particles o (+ charge) o (no charge/neutral) o (- charge) Protons and neutrons have about the same mass. Electrons are
BIOCHEMISTRY NOTES - UNIT 2- ATOMS - the basic unit of matter. Contains subatomic particles o (+ charge) o (no charge/neutral) o (- charge) Protons and neutrons have about the same mass. Electrons are
Alcohols, Ethers, & Epoxides
 Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) Multi-scale promoting effects
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) Multi-scale promoting effects
A Tunable Process: Catalytic Transformation of Renewable Furfural with. Aliphatic Alcohols in the Presence of Molecular Oxygen. Supporting Information
 Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 A Tunable Process: Catalytic Transformation of Renewable Furfural with Aliphatic
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 A Tunable Process: Catalytic Transformation of Renewable Furfural with Aliphatic
A green and efficient oxidation of alcohols by supported gold. conditions
 A green and efficient oxidation of alcohols by supported gold catalysts using aqueous H 2 O 2 under organic solvent-free conditions Ji Ni, Wen-Jian Yu, Lin He, Hao sun, Yong Cao,* He-Yong He, and Kang-Nian
A green and efficient oxidation of alcohols by supported gold catalysts using aqueous H 2 O 2 under organic solvent-free conditions Ji Ni, Wen-Jian Yu, Lin He, Hao sun, Yong Cao,* He-Yong He, and Kang-Nian
Chemistry 283g- Experiment 4
 EXPEIMENT 4: Alkenes: Preparations and eactions elevant sections in the text: Fox & Whitesell, 3 rd Ed. Elimination eactions of Alcohols: pg. 426-428, 431-432 Electrophilic Addition to Alkenes: pg. 484-488,
EXPEIMENT 4: Alkenes: Preparations and eactions elevant sections in the text: Fox & Whitesell, 3 rd Ed. Elimination eactions of Alcohols: pg. 426-428, 431-432 Electrophilic Addition to Alkenes: pg. 484-488,
Mechanistic Insights into Metal-Lewis Acid Mediated Catalytic Transfer. Hydrogenation of Furfural to 2-Methylfuran
 Supporting information for Mechanistic Insights into Metal-Lewis Acid Mediated Catalytic Transfer Hydrogenation of Furfural to 2-Methylfuran Matthew J. Gilkey, Paraskevi Panagiotopoulou, Alexander V. Mironenko,
Supporting information for Mechanistic Insights into Metal-Lewis Acid Mediated Catalytic Transfer Hydrogenation of Furfural to 2-Methylfuran Matthew J. Gilkey, Paraskevi Panagiotopoulou, Alexander V. Mironenko,
Experiment 1: Preparation of Vanillyl Alcohol
 Experiment 1: Preparation of Vanillyl Alcohol INTRDUCTIN A common method for preparing alcohols is the reduction of aldehydes to form primary alcohols [equation (1)] or of ketones to produce secondary
Experiment 1: Preparation of Vanillyl Alcohol INTRDUCTIN A common method for preparing alcohols is the reduction of aldehydes to form primary alcohols [equation (1)] or of ketones to produce secondary
Chemistry Higher level Paper 1
 M15/4/EMI/PM/ENG/TZ1/XX hemistry igher level Paper 1 Thursday 14 May 2015 (afternoon) 1 hour Instructions to candidates Do not open this examination paper until instructed to do so. Answer all the questions.
M15/4/EMI/PM/ENG/TZ1/XX hemistry igher level Paper 1 Thursday 14 May 2015 (afternoon) 1 hour Instructions to candidates Do not open this examination paper until instructed to do so. Answer all the questions.
Atoms And The Periodic Table
 Tick one box to choose the correct answer 1) What elements are found in the compound water (H 2 O)? Hydrogen and oxygen Helium and oxygen Hydrogen and nitrogen 2) Which of the following is a metal element?
Tick one box to choose the correct answer 1) What elements are found in the compound water (H 2 O)? Hydrogen and oxygen Helium and oxygen Hydrogen and nitrogen 2) Which of the following is a metal element?
Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c,
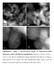 Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c, 2 nm; d, 2 nm. The low magnification images demonstrate
Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c, 2 nm; d, 2 nm. The low magnification images demonstrate
A thermally remendable epoxy resin
 Supplementary Information A thermally remendable epoxy resin Qiao Tian a, Yan Chao Yuan a, Min Zhi Rong *b, Ming Qiu Zhang b a Key Laboratory for Polymeric Composite and Functional Materials of Ministry
Supplementary Information A thermally remendable epoxy resin Qiao Tian a, Yan Chao Yuan a, Min Zhi Rong *b, Ming Qiu Zhang b a Key Laboratory for Polymeric Composite and Functional Materials of Ministry
*Correspondence to:
 Supporting Information for Carbonate-promoted hydrogenation of carbon dioxide to multi-carbon carboxylates Aanindeeta Banerjee 1 and Matthew W. Kanan 1 * 1 Department of Chemistry, Stanford University,
Supporting Information for Carbonate-promoted hydrogenation of carbon dioxide to multi-carbon carboxylates Aanindeeta Banerjee 1 and Matthew W. Kanan 1 * 1 Department of Chemistry, Stanford University,
Selective Degradation of Wood Lignin Over Noble Metal
 Selective Degradation of Wood Lignin Over Noble Metal Catalysts in a Two-step Process Ning Yan, a, b Chen Zhao, a Paul J. Dyson, b Chen Wang, a Ling-tao Liu, a Yuan Kou a * Email: yuankou@pku.edu.cn a
Selective Degradation of Wood Lignin Over Noble Metal Catalysts in a Two-step Process Ning Yan, a, b Chen Zhao, a Paul J. Dyson, b Chen Wang, a Ling-tao Liu, a Yuan Kou a * Email: yuankou@pku.edu.cn a
Production of 5-Hydroxymethylfurfural from Glucose Using a Combination of Lewis and
 Supporting Information Production of 5-ydroxymethylfurfural from Glucose Using a Combination of Lewis and Brønsted Acid Catalysts in Water in a Biphasic Reactor with an Alkylphenol Solvent Yomaira J. Pagán-Torres,
Supporting Information Production of 5-ydroxymethylfurfural from Glucose Using a Combination of Lewis and Brønsted Acid Catalysts in Water in a Biphasic Reactor with an Alkylphenol Solvent Yomaira J. Pagán-Torres,
Supporting Information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 ucleophilic addition of amines, alcohols, and thiophenol with epoxide/olefin using highly efficient
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 ucleophilic addition of amines, alcohols, and thiophenol with epoxide/olefin using highly efficient
Chemistry. Atomic and Molecular Structure
 Chemistry Atomic and Molecular Structure 1. The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates
Chemistry Atomic and Molecular Structure 1. The periodic table displays the elements in increasing atomic number and shows how periodicity of the physical and chemical properties of the elements relates
Biology Slide 1 of 34
 Biology 1 of 34 2 4 Chemical Reactions and Enzymes 2 of 34 2 4 Chemical Reactions and Enzymes Chemical Reactions Chemical Reactions A chemical reaction is a process that changes one set of chemicals into
Biology 1 of 34 2 4 Chemical Reactions and Enzymes 2 of 34 2 4 Chemical Reactions and Enzymes Chemical Reactions Chemical Reactions A chemical reaction is a process that changes one set of chemicals into
cp final review part 2
 Name: Class: Date: cp final review part 2 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Standard conditions when working with gases are
Name: Class: Date: cp final review part 2 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Standard conditions when working with gases are
Supporting Information
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2019 AROMATICS FROM LIGNIN THROUGH ULTRAFAST REACTIONS IN WATER. Nerea Abad-Fernández a, Eduardo
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2019 AROMATICS FROM LIGNIN THROUGH ULTRAFAST REACTIONS IN WATER. Nerea Abad-Fernández a, Eduardo
Contents. 1 Matter: Its Properties and Measurement 1. 2 Atoms and the Atomic Theory Chemical Compounds Chemical Reactions 111
 Ed: Pls provide art About the Authors Preface xvii xvi 1 Matter: Its Properties and Measurement 1 1-1 The Scientific Method 2 1-2 Properties of Matter 4 1-3 Classification of Matter 5 1-4 Measurement of
Ed: Pls provide art About the Authors Preface xvii xvi 1 Matter: Its Properties and Measurement 1 1-1 The Scientific Method 2 1-2 Properties of Matter 4 1-3 Classification of Matter 5 1-4 Measurement of
Bimetallic ruthenium-copper nanoparticles embedded in. mesoporous carbon as an effective hydrogenation catalyst
 Supporting Information Bimetallic ruthenium-copper nanoparticles embedded in mesoporous carbon as an effective hydrogenation catalyst Jiajia Liu, *a Li Li Zhang, b Jiatao Zhang, a Tao Liu, c and X. S.
Supporting Information Bimetallic ruthenium-copper nanoparticles embedded in mesoporous carbon as an effective hydrogenation catalyst Jiajia Liu, *a Li Li Zhang, b Jiatao Zhang, a Tao Liu, c and X. S.
CHEM 344 Fall 2015 Final Exam (100 pts)
 CHEM 344 Fall 2015 Final Exam (100 pts) Name: TA Name: DO NOT REMOVE ANY PAGES FROM THIS EXAM PACKET. Have a swell winter break. Directions for drawing molecules, reactions, and electron-pushing mechanisms:
CHEM 344 Fall 2015 Final Exam (100 pts) Name: TA Name: DO NOT REMOVE ANY PAGES FROM THIS EXAM PACKET. Have a swell winter break. Directions for drawing molecules, reactions, and electron-pushing mechanisms:
Course Goals for CHEM 202
 Course Goals for CHEM 202 Students will use their understanding of chemical bonding and energetics to predict and explain changes in enthalpy, entropy, and free energy for a variety of processes and reactions.
Course Goals for CHEM 202 Students will use their understanding of chemical bonding and energetics to predict and explain changes in enthalpy, entropy, and free energy for a variety of processes and reactions.
SIR MICHELANGELO REFALO
 SIR MICELANGELO REFALO SIXT FORM alf-yearly Exam 2014 Name: CEMISTRY ADV 1 ST 3 hrs ANSWER ANY 7 QUESTIONS. All questions carry equal marks. You are reminded of the importance of clear presentation in
SIR MICELANGELO REFALO SIXT FORM alf-yearly Exam 2014 Name: CEMISTRY ADV 1 ST 3 hrs ANSWER ANY 7 QUESTIONS. All questions carry equal marks. You are reminded of the importance of clear presentation in
Unit 9a: Kinetics and Energy Changes
 Unit 9a: Kinetics and Energy Changes Student Name: Key Class Period: Website upload 2015 Page 1 of 43 Unit 9a (Kinetics & Energy Changes) Key Page intentionally blank Website upload 2015 Page 2 of 43 Unit
Unit 9a: Kinetics and Energy Changes Student Name: Key Class Period: Website upload 2015 Page 1 of 43 Unit 9a (Kinetics & Energy Changes) Key Page intentionally blank Website upload 2015 Page 2 of 43 Unit
One-Pot Conversion of Methane to Light Olefins or Higher Hydrocarbons through H-SAPO-34 Catalyzed in-situ Halogenation
 S1 Supporting Information One-Pot Conversion of Methane to Light Olefins or Higher Hydrocarbons through H-SAPO-34 Catalyzed in-situ Halogenation Patrice T. D. Batamack, Thomas Mathew, G. K. Surya Prakash*
S1 Supporting Information One-Pot Conversion of Methane to Light Olefins or Higher Hydrocarbons through H-SAPO-34 Catalyzed in-situ Halogenation Patrice T. D. Batamack, Thomas Mathew, G. K. Surya Prakash*
Experiment : Reduction of Ethyl Acetoacetate
 Experiment 7-2007: eduction of Ethyl Acetoacetate EXPEIMENT 7: eduction of Carbonyl Compounds: Achiral and Chiral eduction elevant sections in the text: Fox & Whitesell, 3 rd Ed. Chapter 12, pg.572-584.
Experiment 7-2007: eduction of Ethyl Acetoacetate EXPEIMENT 7: eduction of Carbonyl Compounds: Achiral and Chiral eduction elevant sections in the text: Fox & Whitesell, 3 rd Ed. Chapter 12, pg.572-584.
Final Exam Review-Honors Name Period
 Final Exam Review-Honors Name Period This is not a fully comprehensive review packet. This packet is especially lacking practice of explanation type questions!!! You should study all previous review sheets
Final Exam Review-Honors Name Period This is not a fully comprehensive review packet. This packet is especially lacking practice of explanation type questions!!! You should study all previous review sheets
ph = -log[h+], [H+] = 10-pH ph + poh = 14
![ph = -log[h+], [H+] = 10-pH ph + poh = 14 ph = -log[h+], [H+] = 10-pH ph + poh = 14](/thumbs/89/98654987.jpg) You may remove this page. ph = -log[h+], [H+] = 10-pH McVc = MdVd ph + poh = 14 NA = 6.02 x 1023 mol-1 JBA 2017 Chemistry Exam 3 Name: Score: /100 = /80 Multiple choice questions are worth two points each.
You may remove this page. ph = -log[h+], [H+] = 10-pH McVc = MdVd ph + poh = 14 NA = 6.02 x 1023 mol-1 JBA 2017 Chemistry Exam 3 Name: Score: /100 = /80 Multiple choice questions are worth two points each.
CHEMISTRY HIGHER LEVEL
 *P15* PRE-LEAVING CERTIFICATE EXAMINATION, 2008 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions from Section A All questions carry
*P15* PRE-LEAVING CERTIFICATE EXAMINATION, 2008 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions from Section A All questions carry
Supplementary Information. Synthesis and Characterization of Fibrous Silica ZSM-5 for Cumene Hydrocracking
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2016 1 Supplementary Information Synthesis and Characterization of Fibrous Silica
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2016 1 Supplementary Information Synthesis and Characterization of Fibrous Silica
Expt 10: Friedel-Crafts Alkylation of p-xylene
 Expt 10: Friedel-Crafts Alkylation of p-xylene INTRODUCTION The Friedel-Crafts alkylation reaction is one of the most useful methods for adding alkyl substituents to an aromatic ring. Mechanistically,
Expt 10: Friedel-Crafts Alkylation of p-xylene INTRODUCTION The Friedel-Crafts alkylation reaction is one of the most useful methods for adding alkyl substituents to an aromatic ring. Mechanistically,
Chemistry FINAL: CONTENT Review Packet
 Chemistry FINAL: CONTENT Review Packet Name: Period: Date: Classification of Matter & Chemical/ Physical Changes 1. are substances that are made up of two or more elements which are chemically combined
Chemistry FINAL: CONTENT Review Packet Name: Period: Date: Classification of Matter & Chemical/ Physical Changes 1. are substances that are made up of two or more elements which are chemically combined
THE CHEMISTRY OF LIFE
 THE CHEMISTRY OF LIFE ATOMS All living things are made up of matter Atoms are the smallest unit of matter Made up of 3 subatomic particles: 1. Protons- positively charged, found in the nucleus, has mass
THE CHEMISTRY OF LIFE ATOMS All living things are made up of matter Atoms are the smallest unit of matter Made up of 3 subatomic particles: 1. Protons- positively charged, found in the nucleus, has mass
Supporting Information
 Supporting Information Nb 2 5 nh 2 as a heterogeneous catalyst with water-tolerant Lewis acid sites Kiyotaka Nakajima, Yusuke Baba, Ryouhei Noma, Masaaki Kitano, Junko N. Kondo, Shigenobu Hayashi, П,*
Supporting Information Nb 2 5 nh 2 as a heterogeneous catalyst with water-tolerant Lewis acid sites Kiyotaka Nakajima, Yusuke Baba, Ryouhei Noma, Masaaki Kitano, Junko N. Kondo, Shigenobu Hayashi, П,*
REALLY, REALLY STRONG BASES. DO NOT FORGET THIS!!!!!
 CHEM 345 Problem Set 4 Key Grignard (RMgX) Problem Set You will be using Grignard reagents throughout this course to make carbon-carbon bonds. To use them effectively, it will require some knowledge from
CHEM 345 Problem Set 4 Key Grignard (RMgX) Problem Set You will be using Grignard reagents throughout this course to make carbon-carbon bonds. To use them effectively, it will require some knowledge from
Chapter 27: Gas Chromatography
 Chapter 27: Gas Chromatography Gas Chromatography Mobile phase (carrier gas): gas (He, N 2, H 2 ) - do not interact with analytes - only transport the analyte through the column Analyte: volatile liquid
Chapter 27: Gas Chromatography Gas Chromatography Mobile phase (carrier gas): gas (He, N 2, H 2 ) - do not interact with analytes - only transport the analyte through the column Analyte: volatile liquid
4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY
 4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY During the early 1800's, a group of compounds of natural origin became collectively known as aromatic compounds. As several of these compounds
4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY During the early 1800's, a group of compounds of natural origin became collectively known as aromatic compounds. As several of these compounds
2 4 Chemical Reactions and Enzymes Slide 1 of 34
 2 4 Chemical Reactions and Enzymes 1 of 34 Chemical Reactions Chemical Reactions A chemical reaction is a process that changes one set of chemicals into another set of chemicals. Some chemical reactions
2 4 Chemical Reactions and Enzymes 1 of 34 Chemical Reactions Chemical Reactions A chemical reaction is a process that changes one set of chemicals into another set of chemicals. Some chemical reactions
Unit 7 Kinetics and Thermodynamics
 17.1 The Flow of Energy Heat and Work Unit 7 Kinetics and Thermodynamics I. Energy Transformations A. Temperature 1. A measure of the average kinetic energy of the particles in a sample of matter B. Heat
17.1 The Flow of Energy Heat and Work Unit 7 Kinetics and Thermodynamics I. Energy Transformations A. Temperature 1. A measure of the average kinetic energy of the particles in a sample of matter B. Heat
Edexcel Chemistry Checklist
 Topic 1. Key concepts in chemistry Video: Developing the atomic model Describe how and why the atomic model has changed over time. Describe the difference between the plum-pudding model of the atom and
Topic 1. Key concepts in chemistry Video: Developing the atomic model Describe how and why the atomic model has changed over time. Describe the difference between the plum-pudding model of the atom and
An Overview of Organic Reactions. Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants:
 An Overview of Organic Reactions Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants: 1. Addition (forward) Gain of atoms across a bond Example:
An Overview of Organic Reactions Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants: 1. Addition (forward) Gain of atoms across a bond Example:
media), except those of aluminum and calcium
 1- Aspirin occurs as white crystals or as a white crystalline powder. 2- It is slightly soluble in water (1:300), soluble in alcohol (1 :5), chloroform (1:17) & ether (1:15). It dissolves easily in glycerin.
1- Aspirin occurs as white crystals or as a white crystalline powder. 2- It is slightly soluble in water (1:300), soluble in alcohol (1 :5), chloroform (1:17) & ether (1:15). It dissolves easily in glycerin.
Supporting Information
 Supporting Information MgFeCe ternary layered double hydroxide as highly efficient and recyclable heterogeneous base catalyst for synthesis of dimethyl carbonate by transesterification Nayana T. Nivangune
Supporting Information MgFeCe ternary layered double hydroxide as highly efficient and recyclable heterogeneous base catalyst for synthesis of dimethyl carbonate by transesterification Nayana T. Nivangune
Experiment 11: Dehydration of Cyclohexanol
 Experiment 11: Dehydration of yclohexanol INTRODUTION In this experiment, cyclohexanol is dehydrated by aqueous sulfuric acid to produce cyclohexene as the sole product [equation (1)], and no rearrangement
Experiment 11: Dehydration of yclohexanol INTRODUTION In this experiment, cyclohexanol is dehydrated by aqueous sulfuric acid to produce cyclohexene as the sole product [equation (1)], and no rearrangement
Supporting Information
 Supporting Information Ag.1 Pd.9 /rgo: An Efficient Catalyst for Hydrogen Generation from Formic Acid/Sodium Formate Yun Ping, Jun-Min Yan*, Zhi-Li Wang, Hong-Li Wang, Qing Jiang Key Laboratory of Automobile
Supporting Information Ag.1 Pd.9 /rgo: An Efficient Catalyst for Hydrogen Generation from Formic Acid/Sodium Formate Yun Ping, Jun-Min Yan*, Zhi-Li Wang, Hong-Li Wang, Qing Jiang Key Laboratory of Automobile
Name: Score: /100. Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each
 Name: Score: /100 Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each 1. Which of the following contains the greatest number of moles of O? A) 2.3 mol H 2 O
Name: Score: /100 Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each 1. Which of the following contains the greatest number of moles of O? A) 2.3 mol H 2 O
Preparation and Characterization of Double Metal Cyanide Complex Catalysts
 Molecules 2003, 8, 67-73 molecules ISSN 1420-3049 http://www.mdpi.org Preparation and Characterization of Double Metal Cyanide Complex Catalysts Hanxia Liu 1, Xikui Wang 1, *, Yao Gu 2 and Weilin Guo 1
Molecules 2003, 8, 67-73 molecules ISSN 1420-3049 http://www.mdpi.org Preparation and Characterization of Double Metal Cyanide Complex Catalysts Hanxia Liu 1, Xikui Wang 1, *, Yao Gu 2 and Weilin Guo 1
1.4 Energetics. N Goalby chemrevise.org 1. Standard Enthalpy Change of Formation. Standard Enthalpy Change of Combustion
 1.4 Energetics Definition: Enthalpy change is the amount of heat energy taken in or given out during any change in a system provided the pressure is constant. In an exothermic change energy is transferred
1.4 Energetics Definition: Enthalpy change is the amount of heat energy taken in or given out during any change in a system provided the pressure is constant. In an exothermic change energy is transferred
2017 Reaction of cinnamic acid chloride with ammonia to cinnamic acid amide
 217 Reaction of cinnamic acid chloride with ammonia to cinnamic acid amide O O Cl NH 3 NH 2 C 9 H 7 ClO (166.6) (17.) C 9 H 9 NO (147.2) Classification Reaction types and substance classes reaction of
217 Reaction of cinnamic acid chloride with ammonia to cinnamic acid amide O O Cl NH 3 NH 2 C 9 H 7 ClO (166.6) (17.) C 9 H 9 NO (147.2) Classification Reaction types and substance classes reaction of
SELECTIVE OXIDATION OF TOLUENE TO BENZALDEHYDE USING Cu/Sn/Br CATALYST SYSTEM
 Int. J. Chem. Sci.: 9(2), 211, 545-552 ISSN 972-768X www.sadgurupublications.com SELECTIVE OXIDATION OF TOLUENE TO BENZALDEHYDE USING Cu/Sn/Br CATALYST SYSTEM KALPENDRA RAJURKAR *, NILESH KULKARNI, VILAS
Int. J. Chem. Sci.: 9(2), 211, 545-552 ISSN 972-768X www.sadgurupublications.com SELECTIVE OXIDATION OF TOLUENE TO BENZALDEHYDE USING Cu/Sn/Br CATALYST SYSTEM KALPENDRA RAJURKAR *, NILESH KULKARNI, VILAS
10.5 Catalytic reactions Catalyzed reactions. Out-class extensive reading: Levine, p Catalysis Enzyme catalysis
 10.5 Catalytic reactions Catalyzed reactions Out-class extensive reading: Levine, p.577 17.16 Catalysis 17.17 Enzyme catalysis 5.1 Catalysts and catalysis Catalyst A substance of small amount that can
10.5 Catalytic reactions Catalyzed reactions Out-class extensive reading: Levine, p.577 17.16 Catalysis 17.17 Enzyme catalysis 5.1 Catalysts and catalysis Catalyst A substance of small amount that can
Contents. Efficient synthesis of 5-(chloromethyl)furfural (CMF) from high fructose corn syrup (HFCS) using continuous flow processing
 Electronic Supplementary Material (ESI) for Reaction Chemistry & Engineering. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information (ESI) Efficient synthesis of 5-(chloromethyl)furfural
Electronic Supplementary Material (ESI) for Reaction Chemistry & Engineering. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information (ESI) Efficient synthesis of 5-(chloromethyl)furfural
SAMPLE FINAL 3150:110
 SAMPLE FINAL 3150:110 Print out a copy. Answer the questions on your own. Check the answers in GOBC Ans.pdf. Good luck! 1. Given the following: Element Electronegativity 2.1 Li 1.0 C 2.5 F 4.0 Al 1.5 2.8
SAMPLE FINAL 3150:110 Print out a copy. Answer the questions on your own. Check the answers in GOBC Ans.pdf. Good luck! 1. Given the following: Element Electronegativity 2.1 Li 1.0 C 2.5 F 4.0 Al 1.5 2.8
Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers
 Chapter Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers Ch 1-Structure and bonding Ch 2-Polar covalent bonds: Acids and bases McMurry, J. (2004) Organic Chemistry 6 th Edition
Chapter Organic Chemistry 112 A B C - Syllabus Addendum for Prospective Teachers Ch 1-Structure and bonding Ch 2-Polar covalent bonds: Acids and bases McMurry, J. (2004) Organic Chemistry 6 th Edition
For the entire exam, solutions are aqueous and T = 25 C unless stated otherwise. Questions 1 15 cover material from Exam 1.
 For the entire exam, solutions are aqueous and T = 25 C unless stated otherwise. Questions 1 15 cover material from Exam 1. 1. What state of matter is described as follows? On the molecular level, the
For the entire exam, solutions are aqueous and T = 25 C unless stated otherwise. Questions 1 15 cover material from Exam 1. 1. What state of matter is described as follows? On the molecular level, the
Expt 9: The Aldol Condensation
 Expt 9: The Aldol Condensation INTRDUCTIN Reactions that form carbon-carbon bonds are particularly important in organic chemistry as they allow the synthesis of more complex structures from simpler molecules.
Expt 9: The Aldol Condensation INTRDUCTIN Reactions that form carbon-carbon bonds are particularly important in organic chemistry as they allow the synthesis of more complex structures from simpler molecules.
Gummy Bear Demonstration:
 Name: Unit 8: Chemical Kinetics Date: Regents Chemistry Aim: _ Do Now: a) Using your glossary, define chemical kinetics: b) Sort the phrases on the SmartBoard into the two columns below. Endothermic Rxns
Name: Unit 8: Chemical Kinetics Date: Regents Chemistry Aim: _ Do Now: a) Using your glossary, define chemical kinetics: b) Sort the phrases on the SmartBoard into the two columns below. Endothermic Rxns
Chemistry 283g Experiment 4
 Chemistry 283g xperiment 4 XPRIMNT 4: lectrophilic Aromatic Substitution: A Friedel-Craft Acylation Reaction Relevant sections in the text: Fox & Whitesell, 3 rd d. Chapter 11, especially pg. 524-526,
Chemistry 283g xperiment 4 XPRIMNT 4: lectrophilic Aromatic Substitution: A Friedel-Craft Acylation Reaction Relevant sections in the text: Fox & Whitesell, 3 rd d. Chapter 11, especially pg. 524-526,
GCSE CHEMISTRY REVISION LIST
 GCSE CHEMISTRY REVISION LIST OCR Gateway Chemistry (J248) from 2016 Topic C1: Particles C1.1 Describe the main features of the particle model in terms of states of matter and change of state Explain, in
GCSE CHEMISTRY REVISION LIST OCR Gateway Chemistry (J248) from 2016 Topic C1: Particles C1.1 Describe the main features of the particle model in terms of states of matter and change of state Explain, in
AQA Chemistry Checklist
 Topic 1. Atomic structure Video: Atoms, elements, compounds, mixtures Use the names and symbols of the first 20 elements in the periodic table, the elements in Groups 1 and 7, and other elements in this
Topic 1. Atomic structure Video: Atoms, elements, compounds, mixtures Use the names and symbols of the first 20 elements in the periodic table, the elements in Groups 1 and 7, and other elements in this
Heterogeneously catalyzed selective aerobic oxidative cross-coupling of terminal alkynes and amides with simple copper(ii) hydroxide
 Electronic Supplementary Information (ESI) for Heterogeneously catalyzed selective aerobic oxidative cross-coupling of terminal alkynes and amides with simple copper(ii) hydroxide Xiongjie Jin, Kazuya
Electronic Supplementary Information (ESI) for Heterogeneously catalyzed selective aerobic oxidative cross-coupling of terminal alkynes and amides with simple copper(ii) hydroxide Xiongjie Jin, Kazuya
The effect of phase transition of methanol on the reaction rate in the alkylation of hydroquinone
 Korean J. Chem. Eng., 26(3), 649-653 (2009) SHORT COMMUNICATION The effect of phase transition of methanol on the reaction rate in the alkylation of hydroquinone Jung Je Park*, Soo Chool Lee*, Sang Sung
Korean J. Chem. Eng., 26(3), 649-653 (2009) SHORT COMMUNICATION The effect of phase transition of methanol on the reaction rate in the alkylation of hydroquinone Jung Je Park*, Soo Chool Lee*, Sang Sung
Supplementary data Methanolysis of Ammonia Borane by Shape-Controlled Mesoporous Copper Nanostructures for Hydrogen Generation
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2014 Supplementary data Methanolysis of Ammonia Borane by Shape-Controlled Mesoporous Copper
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2014 Supplementary data Methanolysis of Ammonia Borane by Shape-Controlled Mesoporous Copper
IB Topics 5 & 15 Multiple Choice Practice
 IB Topics 5 & 15 Multiple Choice Practice 1. Which statement is correct for this reaction? Fe 2O 3 (s) + 3CO (g) 2Fe (s) + 3CO 2 (g) ΔH = 26.6 kj 13.3 kj are released for every mole of Fe produced. 26.6
IB Topics 5 & 15 Multiple Choice Practice 1. Which statement is correct for this reaction? Fe 2O 3 (s) + 3CO (g) 2Fe (s) + 3CO 2 (g) ΔH = 26.6 kj 13.3 kj are released for every mole of Fe produced. 26.6
Supporting information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry C. This journal is The Royal Society of Chemistry 2015 Supporting information Porosity induced emission: exploring color-controllable
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry C. This journal is The Royal Society of Chemistry 2015 Supporting information Porosity induced emission: exploring color-controllable
OCR Chemistry Checklist
 Topic 1. Particles Video: The Particle Model Describe the main features of the particle model in terms of states of matter. Explain in terms of the particle model the distinction between physical changes
Topic 1. Particles Video: The Particle Model Describe the main features of the particle model in terms of states of matter. Explain in terms of the particle model the distinction between physical changes
Selective aerobic oxidation of biomass-derived HMF to 2,5- diformylfuran using a MOF-derived magnetic hollow Fe-Co
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Selective aerobic oxidation of biomass-derived HMF to 2,5- diformylfuran using a MOF-derived
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Selective aerobic oxidation of biomass-derived HMF to 2,5- diformylfuran using a MOF-derived
CHAPTER 17 REVIEW. Reaction Kinetics. Answer the following questions in the space provided. Energy B A. Course of reaction
 CHAPTER 17 REVIEW Reaction Kinetics SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Refer to the energy diagram below to answer the following questions. D Energy C d c d
CHAPTER 17 REVIEW Reaction Kinetics SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Refer to the energy diagram below to answer the following questions. D Energy C d c d
Synthesis of 2 ) Structures by Small Molecule-Assisted Nucleation for Plasmon-Enhanced Photocatalytic Activity
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Synthesis of Au@UiO-66(NH 2 ) Structures by Small Molecule-Assisted
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Synthesis of Au@UiO-66(NH 2 ) Structures by Small Molecule-Assisted
Advanced Subsidiary Unit 1: The Core Principles of Chemistry
 Write your name here Surname Other names Pearson Edexcel GE entre Number hemistry Advanced Subsidiary Unit 1: The ore Principles of hemistry andidate Number Friday 22 May 2015 Morning Time: 1 hour 30 minutes
Write your name here Surname Other names Pearson Edexcel GE entre Number hemistry Advanced Subsidiary Unit 1: The ore Principles of hemistry andidate Number Friday 22 May 2015 Morning Time: 1 hour 30 minutes
Unit 7 Practice Test. Matching
 Unit 7 Practice Test Matching Match each item with the correct statement below. a. positron d. transuranium element b. alpha particle e. gamma radiation c. beta particle f. transmutation 1. particle of
Unit 7 Practice Test Matching Match each item with the correct statement below. a. positron d. transuranium element b. alpha particle e. gamma radiation c. beta particle f. transmutation 1. particle of
1. Four consecutive processes are given:
 Instruction for the items 1_30: The following tasks include a question and four suggest answers, only one of which is correct. Find the cells which correspond to the answers chosen by you on the answer
Instruction for the items 1_30: The following tasks include a question and four suggest answers, only one of which is correct. Find the cells which correspond to the answers chosen by you on the answer
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/NCHEM.2417 Conversion of alkanes to linear alkylsilanes using an iridium-iron-catalysed tandem dehydrogenation-isomerisation-hydrosilylation Xiangqing Jia 1 and Zheng Huang 1 * 1 State Key
DOI: 10.1038/NCHEM.2417 Conversion of alkanes to linear alkylsilanes using an iridium-iron-catalysed tandem dehydrogenation-isomerisation-hydrosilylation Xiangqing Jia 1 and Zheng Huang 1 * 1 State Key
Saturday Study Session 1 3 rd Class Student Handout Thermochemistry
 Saturday Study Session 1 3 rd Class Student Handout Thermochemistry Multiple Choice Identify the choice that best completes the statement or answers the question. 1. C 2 H 4 (g) + 3 O 2 (g) 2 CO 2 (g)
Saturday Study Session 1 3 rd Class Student Handout Thermochemistry Multiple Choice Identify the choice that best completes the statement or answers the question. 1. C 2 H 4 (g) + 3 O 2 (g) 2 CO 2 (g)
Supplementary Information. Rational Design of Soluble and Clickable Polymers Prepared by. Conventional Free Radical Polymerization of
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2017 Supplementary Information Rational Design of Soluble and Clickable Polymers Prepared by
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2017 Supplementary Information Rational Design of Soluble and Clickable Polymers Prepared by
Supporting Information
 Supporting Information Bamboo-Like Carbon Nanotube/Fe 3 C Nanoparticle Hybrids and Their Highly Efficient Catalysis for Oxygen Reduction Wenxiu Yang a,b, Xiangjian Liu a,b, Xiaoyu Yue a,b, Jianbo Jia,
Supporting Information Bamboo-Like Carbon Nanotube/Fe 3 C Nanoparticle Hybrids and Their Highly Efficient Catalysis for Oxygen Reduction Wenxiu Yang a,b, Xiangjian Liu a,b, Xiaoyu Yue a,b, Jianbo Jia,
Urchin-like Ni-P microstructures: A facile synthesis, properties. and application in the fast removal of heavy-metal ions
 SUPPORTING INFORMATION Urchin-like Ni-P microstructures: A facile synthesis, properties and application in the fast removal of heavy-metal ions Yonghong Ni *a, Kai Mi a, Chao Cheng a, Jun Xia a, Xiang
SUPPORTING INFORMATION Urchin-like Ni-P microstructures: A facile synthesis, properties and application in the fast removal of heavy-metal ions Yonghong Ni *a, Kai Mi a, Chao Cheng a, Jun Xia a, Xiang
Topic 1: Quantitative chemistry
 covered by A-Level Chemistry products Topic 1: Quantitative chemistry 1.1 The mole concept and Avogadro s constant 1.1.1 Apply the mole concept to substances. Moles and Formulae 1.1.2 Determine the number
covered by A-Level Chemistry products Topic 1: Quantitative chemistry 1.1 The mole concept and Avogadro s constant 1.1.1 Apply the mole concept to substances. Moles and Formulae 1.1.2 Determine the number
Ethers & Epoxides. Chapter 5. Dr. Seham ALTERARY. Chem 340-2nd semester
 Ethers & Epoxides Chapter 5 Dr. Seham ALTERARY Chapter s out line Ethers Definition; General formula; Classification and Types Nomenclature - Common Names. - IUPAC Naming. Physical Properties General methods
Ethers & Epoxides Chapter 5 Dr. Seham ALTERARY Chapter s out line Ethers Definition; General formula; Classification and Types Nomenclature - Common Names. - IUPAC Naming. Physical Properties General methods
Q1. (a) State what is meant by the term activation energy of a reaction. (1)
 Q1. (a) State what is meant by the term activation energy of a reaction. (c) State in general terms how a catalyst increases the rate of a chemical reaction. The curve below shows the Maxwell Boltzmann
Q1. (a) State what is meant by the term activation energy of a reaction. (c) State in general terms how a catalyst increases the rate of a chemical reaction. The curve below shows the Maxwell Boltzmann
