RINCIPLE OF COLORIMETER AND SPECTOPHOTOMETER AND VARIOUS TYPE OF ANALYSER USED IN CLINICAL BIOCHEMISTRY
|
|
|
- Avis Franklin
- 5 years ago
- Views:
Transcription
1 RINCIPLE OF COLORIMETER AND SPECTOPHOTOMETER AND VARIOUS TYPE OF ANALYSER USED IN CLINICAL BIOCHEMISTRY
2 COLORIMETER What is colorimeter? Colorimetry. Principle of colorimeter. Beer's and Lambert's law. Components of colorimeter. Functions of components. Advantages and Disadvantages of single cell photometry.
3 What is colorimeter? meter is a instrument used for the measurement of ed substance in solution. nstrument is operative in the visible range of the magnetic spectrum.
4 COLORIMETRY It is a most common analytical technique used in biochemical estimation in clinical laboratory. It involves the quantitative estimation of colour. A substrate must be estimated colorimetrically, must be coloured or it should be capable of forming chromogens (coloured complexes) through the addition of reagents.
5 Coloured substance absorb light in relation to their colour density. The colour density will be proportional to the concentration of coloured substance. The instruments used in this method are called colorimeter or photometer.
6 PRINCIPLE OF COLORIMETER When a monochromatic light passes through a coloured solution, some specific wavelength of light is absorbed which is related to colour density. The amount of light absorbed or transmitted by a colour solution is accordance with two law, i.e. Beer s law and Lambert s law.
7 COMPONENT OF COLORIMETER Light source Slit Monochromator(filter) Cuvette Photocell Galvanometer
8
9
10 FUNCTION OF EACH COMPONANT Light source Two kinds of lamp:- 1.Halogen Deuterium :- for measurement in the ultraviolet range nm. 2.Tungsten lamp:- for measurement in the visible nm and near-infrared ranges.
11 MONOCHROMATOR(FILTER) : FILTER: Used for selecting the monochromatic light. Filters will absorb light of unwanted wavelength and allow only monochromatic light to pass through. Three Types: 1.Prism 2.Grating
12 PRISM When light travels from one medium to another medium, it is refracted and enters in the new medium at a different angle.
13 Prism wavelength spectrum
14 GLASS FILTER: Glass filters are selectively transmit particular range of wavelength. light in
15 GRATINGS : GRAPHITE Light (Tungsten light) is reflected on graphite. This graft separate light in different wave length. By rotation of slit, desirable wave length of light come out from slit. And Beam of that wave length is generated. Desired wavelength selected by the adjustment of an exit slit.
16 CUVETTE (Sample cell ): As per lambert beer's law path length is fixed to 1 cm. Sample cell has 1 cm diameter. A container that contains a sample is usually called cell.
17 THREE TYPES OF CELL:1.Glass 340nm wavelength of light absorbed in glass cell. cheap
18 2. Quartz It allows passage both type of light, ultraviolet & visible ranges. So used for measurement of both ranges. costly. 3.Plastic cuvette Shorter Life Span Easily get Scratches Low Cost
19 PHOTOCELL (PHOTODETECTOR) These are the devices to measure the intensity of light by converting light energy in to electric energy. They are made up of light sensitive material such as selenium. GALVANOMETER Readout device. A galvanometer is used to detect and measure electrical current produced by the photodetector.
20 ADVANTAGE: It is very easy to operate. DISADVANTAGES: Less sensitive. Limited range of filters are available. If the light source is not stable,there is a possibility of errors due to a change from the initial light intensity during a measurement. The manual operation are limited.
21 Spectrophotometer
22
23 Principle The working of colorimeter & Spectrometers is based on Beer's & Lambert's law. Beer's Law:-It states that the optical density of a solution is directly proportional to the concentration of the solution. Lambert's law:-it states that the optical density of a coloured solution is directly proportional to the path of light.
24 According to Beer's & Lambert's law where, T=10-kcL, T=transmittance K=Constant characteristic of the solution C=concentration of the coloured solution L=Path of light through the coloured solution O.D.=2 log T%
25 Differences: Colorimeter & Spectrophotometer Colorimeter Limited for the visible portion of spectrum (visible light) Cheap Two digit reading after desimal point. Less sensitive Glass are used. Spectrophotometer Ultra violet & infrared region also visible e.g.340nm Very costly Four digits reading after desimal point. More sensitive Prism are used.
26 Glass cuvette or test tube is used for reading which absorb 340nm light. Tungsten lamps are used. Can t use specific filter. Can t do kinetic method. Quartz cuvette is used which does not absorb 340nm light. Halogen lamps are used. Can use specific filter. Can do kinetic method.
27 Auto analyzers are mainly two types: Semi Auto Analyzer Fully Auto Analyzer
28 Semi Auto Analyzer Example : ERBA CHEM 5- PLUS Advantage : Displaying the test results Printing & memorizing these results Graphs of all linear & nonlinear reactions. Disadvantage : initial stage of Analysis are performed manually Pipetting of reagent Pipetting of specimen Mixing & incubation. This instrument require minimum 500 microliters of reagent for test. Manual L-J chart to draw
29
30
31
32 Fully Auto Analyzer The auto analyzer perform all the function of semi auto analyzer. 1.Automatic dispensing of reagent (by reagent probe). 2.Automatic dispensing of samples. 3.Automatic mixing of reaction mixtures. 4.Incubating of reacting mixture.
33 Advantages: Many samples with different parameter can analyzed at time. Good precision Less reagent required. Less sample required. Less man power required. Maintain the temperature For Sample & For Reagent For incubation period. Can stored result in memory. It have facility to accommodate various samples, standards, calibrations & Q.C. Sera. Automated L-J Chart is visible Programmable wash cycles between samples & tests for minimum carry over. Auto dilution is also possible
34 Two types of fully auto analyzer:batch analyzer Random analyzer
35 Random Access analyzer - Perform Any number of Parameter from any number of sample. - More sample in the short period of time. Facility of continuous loading of sample Facility of stat analysis - Urgent sample. Facility of autodilution. Plotting of daily & monthly Q.C. Chart (L-Jchart). Capability to perform a test with 2 to 3 reagents. Some of the analyzer has separate assembly to wash cuvette so very less chance of contamination. Example : ERBA XL 640
COLORIMETER AND LAMBERT S-BEER S LAW. Shingala vaishali Sandha prafulla Tiwari Kuldeep
 COLORIMETER AND LAMBERT S-BEER S LAW Shingala vaishali Sandha prafulla Tiwari Kuldeep TOPIC What is colorimeter? Use of colorimeter. Component & It s function. Function of colorimeter. The principle of
COLORIMETER AND LAMBERT S-BEER S LAW Shingala vaishali Sandha prafulla Tiwari Kuldeep TOPIC What is colorimeter? Use of colorimeter. Component & It s function. Function of colorimeter. The principle of
Spectroscopy. Page 1 of 8 L.Pillay (2012)
 Spectroscopy Electromagnetic radiation is widely used in analytical chemistry. The identification and quantification of samples using electromagnetic radiation (light) is called spectroscopy. Light has
Spectroscopy Electromagnetic radiation is widely used in analytical chemistry. The identification and quantification of samples using electromagnetic radiation (light) is called spectroscopy. Light has
Spectrophotometry. Introduction
 Spectrophotometry Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. The basic principle
Spectrophotometry Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. The basic principle
Investigating Transition Metal Complexes
 Exercise 4 Investigating Transition Metal Complexes 4 Introduction Colour is a well known property of the transition metals. The colour produced as parts of the visible spectrum are due to electron transitions
Exercise 4 Investigating Transition Metal Complexes 4 Introduction Colour is a well known property of the transition metals. The colour produced as parts of the visible spectrum are due to electron transitions
Compact Knowledge: Absorbance Spectrophotometry. Flexible. Reliable. Personal.
 L A B O R A T O R Y C O M P E T E N C E Compact Knowledge: Absorbance Spectrophotometry Flexible. Reliable. Personal. The interaction of light with molecules is an essential and well accepted technique
L A B O R A T O R Y C O M P E T E N C E Compact Knowledge: Absorbance Spectrophotometry Flexible. Reliable. Personal. The interaction of light with molecules is an essential and well accepted technique
Spectrophotometry. Dr. Shareef SHAIK ASST. PROFESSOR Pharmacology
 Spectrophotometry Dr. Shareef SHAIK ASST. PROFESSOR Pharmacology Content Introduction Beer-Lambert law Instrument Applications Introduction 3 Body fluids such as blood, csf and urine contain organic and
Spectrophotometry Dr. Shareef SHAIK ASST. PROFESSOR Pharmacology Content Introduction Beer-Lambert law Instrument Applications Introduction 3 Body fluids such as blood, csf and urine contain organic and
Course: M.Sc (Chemistry) Analytical Chemistry Unit: III
 Course: M.Sc (Chemistry) Analytical Chemistry Unit: III Syllabus: Principle of spectrophotometry Types of spectrophotometer Applications - Dissociation constants of an indicator simultaneous spectrophotometric
Course: M.Sc (Chemistry) Analytical Chemistry Unit: III Syllabus: Principle of spectrophotometry Types of spectrophotometer Applications - Dissociation constants of an indicator simultaneous spectrophotometric
Beer's Law and Data Analysis *
 OpenStax-CNX module: m15131 1 Beer's Law and Data Analysis * Mary McHale This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 2.0 1 Beer's Law and Data Analysis
OpenStax-CNX module: m15131 1 Beer's Law and Data Analysis * Mary McHale This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 2.0 1 Beer's Law and Data Analysis
MOLEBIO LAB #4: Using a Spectrophotometer
 Introduction: Spectrophotometry MOLEBIO LAB #4: Using a Spectrophotometer Many kinds of molecules interact with or absorb specific types of radiant energy in a predictable fashion. For example, when while
Introduction: Spectrophotometry MOLEBIO LAB #4: Using a Spectrophotometer Many kinds of molecules interact with or absorb specific types of radiant energy in a predictable fashion. For example, when while
Basic Instrumentation. Learning Objectives:
 Proteomics Basic Instrumentation Basic Instrumentation Handling instruments plays an important role in laboratory working on a daily basis. The use of most instruments is almost inevitable and hence a
Proteomics Basic Instrumentation Basic Instrumentation Handling instruments plays an important role in laboratory working on a daily basis. The use of most instruments is almost inevitable and hence a
JAPAN QUALITY. Automated Clinical Analyzer
 JAPAN QUALITY Automated Clinical Analyzer Higher test performance and operability with new functions The clinical analyzer in the next stage 1 The best to the requirements for functionality and usability
JAPAN QUALITY Automated Clinical Analyzer Higher test performance and operability with new functions The clinical analyzer in the next stage 1 The best to the requirements for functionality and usability
SPECTROPHOTOMETERS. Visible and UV-Visible
 Visible and UV-Visible The Manifacturer ONDA Spectrophotometers are manifactured by a company with over than ten years of experience in development of UV/Vis instruments, Single beam and Double beam, with
Visible and UV-Visible The Manifacturer ONDA Spectrophotometers are manifactured by a company with over than ten years of experience in development of UV/Vis instruments, Single beam and Double beam, with
JAPAN QUALITY. Automated Clinical Analyzer
 JAPAN QUALITY Automated Clinical Analyzer Higher test performance and operability with new functions The clinical analyzer in the next stage 1 The best to the requirements for functionality and usability
JAPAN QUALITY Automated Clinical Analyzer Higher test performance and operability with new functions The clinical analyzer in the next stage 1 The best to the requirements for functionality and usability
Questions on Instrumental Methods of Analysis
 Questions on Instrumental Methods of Analysis 1. Which one of the following techniques can be used for the detection in a liquid chromatograph? a. Ultraviolet absorbance or refractive index measurement.
Questions on Instrumental Methods of Analysis 1. Which one of the following techniques can be used for the detection in a liquid chromatograph? a. Ultraviolet absorbance or refractive index measurement.
Automated Clinical Analyzer
 Automated Clinical Analyzer Improved ease of operation and better test efficiency, and provides the optimal solution for routine operations, STAT operation and a broad range of clinical analysis requirements.
Automated Clinical Analyzer Improved ease of operation and better test efficiency, and provides the optimal solution for routine operations, STAT operation and a broad range of clinical analysis requirements.
Determining the Concentration of a Solution: Beer s Law
 Determining the Concentration of a Solution: Beer s Law The primary objective of this experiment is to determine the concentration of an unknown cobalt (II) chloride solution. You will use a Vernier SpectroVis
Determining the Concentration of a Solution: Beer s Law The primary objective of this experiment is to determine the concentration of an unknown cobalt (II) chloride solution. You will use a Vernier SpectroVis
Physics 30: Chapter 5 Exam Wave Nature of Light
 Physics 30: Chapter 5 Exam Wave Nature of Light Name: Date: Mark: /33 Numeric Response. Place your answers to the numeric response questions, with units, in the blanks at the side of the page. (1 mark
Physics 30: Chapter 5 Exam Wave Nature of Light Name: Date: Mark: /33 Numeric Response. Place your answers to the numeric response questions, with units, in the blanks at the side of the page. (1 mark
GENERAL PHARMACOPOEIA MONOGRAPH
 MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION GENERAL PHARMACOPOEIA MONOGRAPH Spectrophotometry in the ultraviolet GPM.1.2.1.1.0003.15 and visible spectral regions Replaces the SPRF X GPM, SPRF XI GPM,
MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION GENERAL PHARMACOPOEIA MONOGRAPH Spectrophotometry in the ultraviolet GPM.1.2.1.1.0003.15 and visible spectral regions Replaces the SPRF X GPM, SPRF XI GPM,
Beer's- Lambert Law and Standard Curves. BCH 312 [Practical]
![Beer's- Lambert Law and Standard Curves. BCH 312 [Practical] Beer's- Lambert Law and Standard Curves. BCH 312 [Practical]](/thumbs/78/77692425.jpg) Beer's- Lambert Law and Standard Curves BCH 312 [Practical] Spectrophotometer: Spectrophotometer can be used to measure the amount of light absorbed or transmitted by a solution. It consist of two parts:
Beer's- Lambert Law and Standard Curves BCH 312 [Practical] Spectrophotometer: Spectrophotometer can be used to measure the amount of light absorbed or transmitted by a solution. It consist of two parts:
Chemistry 141 Laboratory Spectrometric Determination of Iron Concentration Lab Lecture Notes 8/29/2011 Dr. Abrash
 Chemistry 141 Laboratory Spectrometric Determination of Iron Concentration Lab Lecture Notes 8/29/2011 Dr. Abrash What is the purpose of this experiment? We re going to learn a way to quantify the amount
Chemistry 141 Laboratory Spectrometric Determination of Iron Concentration Lab Lecture Notes 8/29/2011 Dr. Abrash What is the purpose of this experiment? We re going to learn a way to quantify the amount
Improved Process Measurement & Control. OD 600 vs. AU Measurements in Fermentation Cell Density
 Finesse, LLC 3350 Scott Boulevard #1 Santa Clara, CA 95054 9351 Irvine Boulevard Irvine, CA 92618 800-598-9515 www.finesse-inc.com P01 OD 600 vs. AU Measurements in Fermentation Cell Density This technical
Finesse, LLC 3350 Scott Boulevard #1 Santa Clara, CA 95054 9351 Irvine Boulevard Irvine, CA 92618 800-598-9515 www.finesse-inc.com P01 OD 600 vs. AU Measurements in Fermentation Cell Density This technical
Absorption photometry
 The light Absorption photometry Szilvia Barkó University of Pécs, Faculty of Medicines, Dept. Biophysics February 2011 Transversal wave E Electromagnetic wave electric gradient vector wavelength The dual
The light Absorption photometry Szilvia Barkó University of Pécs, Faculty of Medicines, Dept. Biophysics February 2011 Transversal wave E Electromagnetic wave electric gradient vector wavelength The dual
Outline of Recombinant DNA technology. Application of UV spectroscopy in recombinant DNA technology
 NIKHIL.K.POTDUKHE Outline of UV spectrophotometer Outline of Recombinant DNA technology Application of UV spectroscopy in recombinant DNA technology References Lambert law: When a beam of light is allowed
NIKHIL.K.POTDUKHE Outline of UV spectrophotometer Outline of Recombinant DNA technology Application of UV spectroscopy in recombinant DNA technology References Lambert law: When a beam of light is allowed
CHAPTER 1 SPECTROPHOTOMETRY
 CHAPTER 1 SPECTROPHOTOMETRY PHOTOMETRIC ANALYSIS. This method of analysis is one of the most useful assay techniques in biochemistry. Comparison is made between the amount of light absorbed by the unknown
CHAPTER 1 SPECTROPHOTOMETRY PHOTOMETRIC ANALYSIS. This method of analysis is one of the most useful assay techniques in biochemistry. Comparison is made between the amount of light absorbed by the unknown
UNIT I COLORIMETER AND SPECTROPHOTOMETERS PART A
 UNIT I COLORIMETER AND SPECTROPHOTOMETERS PART A 1. List any four elements used in spectrophotometers. 1.Radiant source 2.wavelength selector 3.photodetector 4.sample 2. What is meant by flame emission
UNIT I COLORIMETER AND SPECTROPHOTOMETERS PART A 1. List any four elements used in spectrophotometers. 1.Radiant source 2.wavelength selector 3.photodetector 4.sample 2. What is meant by flame emission
Construction of Absorption Filter for colorimetry and its performance characteristics
 Construction of Absorption Filter for colorimetry and its performance characteristics Author(s): Adeeyinwo C. E. and Adedeji A. L. Vol. 18, No. 2 (2007-05 - 2007-08) Biomedical Research 2007; 18 (2): 93-96
Construction of Absorption Filter for colorimetry and its performance characteristics Author(s): Adeeyinwo C. E. and Adedeji A. L. Vol. 18, No. 2 (2007-05 - 2007-08) Biomedical Research 2007; 18 (2): 93-96
Design and Development of a Smartphone Based Visible Spectrophotometer for Analytical Applications
 Design and Development of a Smartphone Based Visible Spectrophotometer for Analytical Applications Bedanta Kr. Deka, D. Thakuria, H. Bora and S. Banerjee # Department of Physicis, B. Borooah College, Ulubari,
Design and Development of a Smartphone Based Visible Spectrophotometer for Analytical Applications Bedanta Kr. Deka, D. Thakuria, H. Bora and S. Banerjee # Department of Physicis, B. Borooah College, Ulubari,
Fig. 8.1 illustrates the three measurements. air medium A. ray 1. air medium A. ray 2. air medium A. ray 3. Fig For Examiner s Use
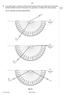 9 9 9 14 8 In an optics lesson, a Physics student traces the paths of three s of light near the boundary between medium A and. The student uses a protractor to measure the various angles. Fig. 8.1 illustrates
9 9 9 14 8 In an optics lesson, a Physics student traces the paths of three s of light near the boundary between medium A and. The student uses a protractor to measure the various angles. Fig. 8.1 illustrates
Determining the Concentration of a Solution: Beer s Law
 Determining the Concentration of a Solution: Beer s Law LabQuest 11 The primary objective of this experiment is to determine the concentration of an unknown nickel (II) sulfate solution. You will be using
Determining the Concentration of a Solution: Beer s Law LabQuest 11 The primary objective of this experiment is to determine the concentration of an unknown nickel (II) sulfate solution. You will be using
SPECTROMETER / COLORIMETER - flame
 INSTRUCTION SHEET SPECTROMETER / COLORIMETER - flame Cat: CH3792-001 Flame emission and Atomic Absorption DESCRIPTION: The IEC Flame & AA Spectrometer is used for the teaching of analytical chemistry.
INSTRUCTION SHEET SPECTROMETER / COLORIMETER - flame Cat: CH3792-001 Flame emission and Atomic Absorption DESCRIPTION: The IEC Flame & AA Spectrometer is used for the teaching of analytical chemistry.
EXPERIMENT #3 A Beer's Law Study
 OBJECTVES: EXPERMENT #3 A Beer's Law Study To operate a Spectronic 20 To convert from percent transmission to absorbance units To plot absorbance versus wavelength and find max To plot absorbance versus
OBJECTVES: EXPERMENT #3 A Beer's Law Study To operate a Spectronic 20 To convert from percent transmission to absorbance units To plot absorbance versus wavelength and find max To plot absorbance versus
Introduction. The amount of radiation absorbed may be measured in a number of ways: Transmittance, T = P / P 0 % Transmittance, %T = 100 T
 Introduction Many compounds absorb ultraviolet (UV) or visible (Vis.) light. The diagram below shows a beam of monochromatic radiation of radiant power P 0, directed at a sample solution. Absorption takes
Introduction Many compounds absorb ultraviolet (UV) or visible (Vis.) light. The diagram below shows a beam of monochromatic radiation of radiant power P 0, directed at a sample solution. Absorption takes
2001 Spectrometers. Instrument Machinery. Movies from this presentation can be access at
 2001 Spectrometers Instrument Machinery Movies from this presentation can be access at http://www.shsu.edu/~chm_tgc/sounds/sound.html Chp20: 1 Optical Instruments Instrument Components Components of various
2001 Spectrometers Instrument Machinery Movies from this presentation can be access at http://www.shsu.edu/~chm_tgc/sounds/sound.html Chp20: 1 Optical Instruments Instrument Components Components of various
Principles of Biomedical Systems & Devices
 Principles of Biomedical Systems & Devices Lecture 17 Clinical Systems Today Week in PBS&D Clinical Laboratory Instrumentation Spectrophotometry Autoanalyzers Chromatology - Gas Chromatgraphs (Mass) Spectroscopy
Principles of Biomedical Systems & Devices Lecture 17 Clinical Systems Today Week in PBS&D Clinical Laboratory Instrumentation Spectrophotometry Autoanalyzers Chromatology - Gas Chromatgraphs (Mass) Spectroscopy
Characterization of Group (II-VI) Semiconductor Nanoparticles by UV-visible Spectroscopy *
 OpenStax-CNX module: m34601 1 Characterization of Group 12-16 (II-VI) Semiconductor Nanoparticles by UV-visible Spectroscopy * Sravani Gullapalli Andrew R. Barron This work is produced by OpenStax-CNX
OpenStax-CNX module: m34601 1 Characterization of Group 12-16 (II-VI) Semiconductor Nanoparticles by UV-visible Spectroscopy * Sravani Gullapalli Andrew R. Barron This work is produced by OpenStax-CNX
Colorimetric analysis of aspirin content in a commercial tablet
 Colorimetric analysis of aspirin content in a commercial tablet v010214 Objective In this lab, you will prepare standard solutions, and use Beer s Law to construct a calibration curve. You will determine
Colorimetric analysis of aspirin content in a commercial tablet v010214 Objective In this lab, you will prepare standard solutions, and use Beer s Law to construct a calibration curve. You will determine
ASTORIA PACIFIC. Automated Wet-Chemistry Analyzers and Diagnostic Reagents. rapid-t
 ASTORIA PACIFIC Automated Wet-Chemistry Analyzers and Diagnostic Reagents rapid-t rapid-t System ASTORIA-PACIFIC PROUDLY OFFERS OUR rapid-t SYSTEM: Durable. Accurate. Precise. Economical. Open software
ASTORIA PACIFIC Automated Wet-Chemistry Analyzers and Diagnostic Reagents rapid-t rapid-t System ASTORIA-PACIFIC PROUDLY OFFERS OUR rapid-t SYSTEM: Durable. Accurate. Precise. Economical. Open software
Chapter 13 An Introduction to Ultraviolet/Visible Molecular Absorption Spectrometry
 Chapter 13 An Introduction to Ultraviolet/Visible Molecular Absorption Spectrometry 13A Measurement Of Transmittance and Absorbance Absorption measurements based upon ultraviolet and visible radiation
Chapter 13 An Introduction to Ultraviolet/Visible Molecular Absorption Spectrometry 13A Measurement Of Transmittance and Absorbance Absorption measurements based upon ultraviolet and visible radiation
UV-Vis optical fiber assisted spectroscopy in thin films and solutions
 UV-Vis optical fiber assisted spectroscopy in thin films and solutions Description UV-Visible absorption and transmission spectra provide fundamental information for all experiments related to the attenuation
UV-Vis optical fiber assisted spectroscopy in thin films and solutions Description UV-Visible absorption and transmission spectra provide fundamental information for all experiments related to the attenuation
Chromatography & instrumentation in Organic Chemistry
 Chromatography & instrumentation in Organic Chemistry What is Chromatography? Chromatography is a technique for separating mixtures into their components in order to analyze, identify, purify, and/or quantify
Chromatography & instrumentation in Organic Chemistry What is Chromatography? Chromatography is a technique for separating mixtures into their components in order to analyze, identify, purify, and/or quantify
Spectroscopy: Introduction. Required reading Chapter 18 (pages ) Chapter 20 (pages )
 Spectroscopy: Introduction Required reading Chapter 18 (pages 378-397) Chapter 20 (pages 424-449) Spectrophotometry is any procedure that uses light to measure chemical concentrations Properties of Light
Spectroscopy: Introduction Required reading Chapter 18 (pages 378-397) Chapter 20 (pages 424-449) Spectrophotometry is any procedure that uses light to measure chemical concentrations Properties of Light
25 Instruments for Optical Spectrometry
 25 Instruments for Optical Spectrometry 25A INSTRUMENT COMPONENTS (1) source of radiant energy (2) wavelength selector (3) sample container (4) detector (5) signal processor and readout (a) (b) (c) Fig.
25 Instruments for Optical Spectrometry 25A INSTRUMENT COMPONENTS (1) source of radiant energy (2) wavelength selector (3) sample container (4) detector (5) signal processor and readout (a) (b) (c) Fig.
Analytical Technologies in Biotechnology Prof. Dr. Ashwani K Sharma Department of Biotechnology Indian Institute of Technology, Roorkee
 Analytical Technologies in Biotechnology Prof. Dr. Ashwani K Sharma Department of Biotechnology Indian Institute of Technology, Roorkee Module - 6 Spectroscopic Techniques Lecture - 2 UV-Visible Spectroscopy
Analytical Technologies in Biotechnology Prof. Dr. Ashwani K Sharma Department of Biotechnology Indian Institute of Technology, Roorkee Module - 6 Spectroscopic Techniques Lecture - 2 UV-Visible Spectroscopy
SPECTROPHOTOMETRY/BEER S LAW LECTURE HONORS CHEMISTRY NAME
 SPECTROPHOTOMETRY/BEER S LAW LECTURE HONORS CHEMISTRY NAME Overview: Spectroscopy will be a tool that you will use as you continue in your chemistry, biology and physics courses. Background: We will begin
SPECTROPHOTOMETRY/BEER S LAW LECTURE HONORS CHEMISTRY NAME Overview: Spectroscopy will be a tool that you will use as you continue in your chemistry, biology and physics courses. Background: We will begin
Course Details. Analytical Techniques Based on Optical Spectroscopy. Course Details. Textbook. SCCH 211: Analytical Chemistry I
 SCCH 211: Analytical Chemistry I Analytical Techniques Based on Optical Spectroscopy Course Details September 22 October 10 September 22 November 7 November 17 December 1 Topic Period Introduction to Spectrometric
SCCH 211: Analytical Chemistry I Analytical Techniques Based on Optical Spectroscopy Course Details September 22 October 10 September 22 November 7 November 17 December 1 Topic Period Introduction to Spectrometric
INSTRUCTION MANUAL ANALOG COLORIMETER
 INSTRUCTION MANUAL ANALOG COLORIMETER QUICK CHECK/OPERATION 1. OPEN SHIPPING BOX - TAKE OUT INSTRUMENT 2. ANY DAMAGE? - REPORT TO FREIGHT COMPANY - CALL DISTRIBUTOR/FACTORY 3. SET WAVELENGTH DIAL TO CORRECT
INSTRUCTION MANUAL ANALOG COLORIMETER QUICK CHECK/OPERATION 1. OPEN SHIPPING BOX - TAKE OUT INSTRUMENT 2. ANY DAMAGE? - REPORT TO FREIGHT COMPANY - CALL DISTRIBUTOR/FACTORY 3. SET WAVELENGTH DIAL TO CORRECT
Obviously, in this kind of instrument, samples must be. preselected according to the tests required (for example,
 Automatic Chemistry Vol. 10, No. 4 (October-December 1988), pp. 167-170 Selectivity and random-access in automatic analysers Pierangelo Bonini, Ferruccio Ceriotti Istituto Scientifico San Raffaele, Via
Automatic Chemistry Vol. 10, No. 4 (October-December 1988), pp. 167-170 Selectivity and random-access in automatic analysers Pierangelo Bonini, Ferruccio Ceriotti Istituto Scientifico San Raffaele, Via
High Volume Modular Analyzer For Drug Testing and General Chemistries
 High Volume Modular Analyzer For Drug Testing and General Chemistries High Volume Modular Analyzer For Drug Testing and General Chemistries Large Capacity Sample Loader and Track Auto-track loading for
High Volume Modular Analyzer For Drug Testing and General Chemistries High Volume Modular Analyzer For Drug Testing and General Chemistries Large Capacity Sample Loader and Track Auto-track loading for
C101-E100. Talk Letter. Vol.1 October 2008
 C101-E100 UV Talk Letter Vol.1 October 2008 UV Talk Letter UV Talk Letter On the Occasion of Publication Vol.1 October 2008 2 Thank you for your continued support of our products. Following on from the
C101-E100 UV Talk Letter Vol.1 October 2008 UV Talk Letter UV Talk Letter On the Occasion of Publication Vol.1 October 2008 2 Thank you for your continued support of our products. Following on from the
Chemical Equilibrium: Finding a Constant, Kc
 Chemical Equilibrium: Finding a Constant, Kc Experiment 20 The purpose of this lab is to experimentally determine the equilibrium constant, K c, for the following chemical reaction: Fe 3+ (aq) + SCN -
Chemical Equilibrium: Finding a Constant, Kc Experiment 20 The purpose of this lab is to experimentally determine the equilibrium constant, K c, for the following chemical reaction: Fe 3+ (aq) + SCN -
MEASUREMENT: PART II
 1 MEASUREMENT: PART II Copyright: Department of Chemistry, University of Idaho, Moscow, ID 83844-2343, 2013. INTRODUCTION Read and/or review Section 1.7 and Figure 7.5 in your textbook. The first part
1 MEASUREMENT: PART II Copyright: Department of Chemistry, University of Idaho, Moscow, ID 83844-2343, 2013. INTRODUCTION Read and/or review Section 1.7 and Figure 7.5 in your textbook. The first part
9/28/10. Visible and Ultraviolet Molecular Spectroscopy - (S-H-C Chapters 13-14) Valence Electronic Structure. n σ* transitions
 Visible and Ultraviolet Molecular Spectroscopy - (S-H-C Chapters 13-14) Electromagnetic Spectrum - Molecular transitions Widely used in chemistry. Perhaps the most widely used in Biological Chemistry.
Visible and Ultraviolet Molecular Spectroscopy - (S-H-C Chapters 13-14) Electromagnetic Spectrum - Molecular transitions Widely used in chemistry. Perhaps the most widely used in Biological Chemistry.
Clinical Chemistry (CHE221) Professor Hicks Week 1. Statistics Made Slightly Less Boring and Introduction to Spectrophotometry. Accuracy vs Precision
 Clinical Chemistry (CHE221) Professor Hicks Week 1 Statistics Made Slightly Less Boring and Introduction to Spectrophotometry 3 Accuracy vs Precision Precision is the consistency of a measurement made
Clinical Chemistry (CHE221) Professor Hicks Week 1 Statistics Made Slightly Less Boring and Introduction to Spectrophotometry 3 Accuracy vs Precision Precision is the consistency of a measurement made
Preparation of Standard Curves. Principle
 Preparation of Standard urves Principle Many laboratory tests require the measurement of concentration be evaluated or read in a photometer (colorimeter or spectrophotometer). Since these instruments are
Preparation of Standard urves Principle Many laboratory tests require the measurement of concentration be evaluated or read in a photometer (colorimeter or spectrophotometer). Since these instruments are
Spectrometer User s Guide
 Spectrometer User s Guide (Order Codes: V-SPEC, SPRT-VIS, SP-VIS, SP-UV-VIS, ESRT-VIS) The spectrometer is a portable light spectrophotometer, combining a spectrometer and a light source/cuvette holder.
Spectrometer User s Guide (Order Codes: V-SPEC, SPRT-VIS, SP-VIS, SP-UV-VIS, ESRT-VIS) The spectrometer is a portable light spectrophotometer, combining a spectrometer and a light source/cuvette holder.
NOTE: The color of the actual product may differ from the color pictured in this catalog due to printing limitation.
 NOTE: The color of the actual product may differ from the color pictured in this catalog due to printing limitation. SCANNING UV VISIBLE SPECTROPHOTOMETER Features SCANNING UV VISIBLE SPECTROPHOTOMETER
NOTE: The color of the actual product may differ from the color pictured in this catalog due to printing limitation. SCANNING UV VISIBLE SPECTROPHOTOMETER Features SCANNING UV VISIBLE SPECTROPHOTOMETER
Because light behaves like a wave, we can describe it in one of two ways by its wavelength or by its frequency.
 Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Light We can use different terms to describe light: Color Wavelength Frequency Light is composed of electromagnetic waves that travel through some medium. The properties of the medium determine how light
Experiment 11 Beer s Law
 Experiment 11 Beer s Law OUTCOMES After completing this experiment, the student should be able to: determine the wavelength (color) of maximum absorbance for a solution. examine the relationship between
Experiment 11 Beer s Law OUTCOMES After completing this experiment, the student should be able to: determine the wavelength (color) of maximum absorbance for a solution. examine the relationship between
L-malic acid measurement in wines using the SpectraMax Plus 384 Microplate Reader
 APPLICATION NOTE L-malic acid measurement in wines using the SpectraMax Plus 384 Microplate Reader Introduction Analysis of malic acid, residual sugar, volatile acidity and ammonia is very important for
APPLICATION NOTE L-malic acid measurement in wines using the SpectraMax Plus 384 Microplate Reader Introduction Analysis of malic acid, residual sugar, volatile acidity and ammonia is very important for
Experiment 11 Beer s Law
 Experiment 11 Beer s Law OUTCOMES After completing this experiment, the student should be able to: determine the wavelength (color) of maximum absorbance for a solution. examine the relationship between
Experiment 11 Beer s Law OUTCOMES After completing this experiment, the student should be able to: determine the wavelength (color) of maximum absorbance for a solution. examine the relationship between
Colorimetry Extinction coefficient ( ) Lambda max ( max) Qualitative vs. quantitative analysis
 Lab Week 2/3 - Spectrophotometry Purpose: Introduce students to the use of spectrophotometry for qualitative (what is it) and quantitative (how much is there of it) analysis of biological samples and molecules.
Lab Week 2/3 - Spectrophotometry Purpose: Introduce students to the use of spectrophotometry for qualitative (what is it) and quantitative (how much is there of it) analysis of biological samples and molecules.
1901 Application of Spectrophotometry
 1901 Application of Spectrophotometry Chemical Analysis Problem: 1 Application of Spectroscopy Organic Compounds Organic compounds with single bonds absorb in the UV region because electrons from single
1901 Application of Spectrophotometry Chemical Analysis Problem: 1 Application of Spectroscopy Organic Compounds Organic compounds with single bonds absorb in the UV region because electrons from single
FLAME PHOTOMETRY AIM INTRODUCTION
 FLAME PHOTOMETRY AIM INTRODUCTION Atomic spectroscopy is based on the absorption, emission or fluorescence process of light by atoms or elementary ions. Information for atomic scale is obtained in two
FLAME PHOTOMETRY AIM INTRODUCTION Atomic spectroscopy is based on the absorption, emission or fluorescence process of light by atoms or elementary ions. Information for atomic scale is obtained in two
UNIT 2 UV-VISIBLE SPECTROMETRY
 Molecular Spectroscopic Methods-I UNIT 2 UV-VISIBLE SPECTROMETRY Structure 2.1 Introduction Objectives 2.2 Origin and Characteristics of UV-VIS Spectrum Origin of UV-VIS spectrum Characteristics of UV-VIS
Molecular Spectroscopic Methods-I UNIT 2 UV-VISIBLE SPECTROMETRY Structure 2.1 Introduction Objectives 2.2 Origin and Characteristics of UV-VIS Spectrum Origin of UV-VIS spectrum Characteristics of UV-VIS
INTRODUCTION The fundamental law of spectrophotometry is known as the Beer-Lambert Law or Beer s Law. It may be stated as: log(po/p) = A
 S2. INTRODUCTION TO ULTRA-VIOLET / VISIBLE SPECTROSCOPY AIM 1. To become familiar with the operation of a conventional scanning ultra-violet spectrophotometer 2. To determine suitable cells and solvents
S2. INTRODUCTION TO ULTRA-VIOLET / VISIBLE SPECTROSCOPY AIM 1. To become familiar with the operation of a conventional scanning ultra-violet spectrophotometer 2. To determine suitable cells and solvents
Instrumental Technique: Cuvette. Md Rabiul Islam
 Instrumental Technique: Cuvette Md Rabiul Islam 16-7-2016 What is cuvette? A cuvette is a small tube of circular or square cross section, sealed at one end, made of plastic, glass, or fused quartz (for
Instrumental Technique: Cuvette Md Rabiul Islam 16-7-2016 What is cuvette? A cuvette is a small tube of circular or square cross section, sealed at one end, made of plastic, glass, or fused quartz (for
Experiment#1 Beer s Law: Absorption Spectroscopy of Cobalt(II)
 : Absorption Spectroscopy of Cobalt(II) OBJECTIVES In successfully completing this lab you will: prepare a stock solution using a volumetric flask; use a UV/Visible spectrometer to measure an absorption
: Absorption Spectroscopy of Cobalt(II) OBJECTIVES In successfully completing this lab you will: prepare a stock solution using a volumetric flask; use a UV/Visible spectrometer to measure an absorption
Identification Of The Common Laboratory Glassware, Pipettes And Equipment. BCH 312 [Practical]
![Identification Of The Common Laboratory Glassware, Pipettes And Equipment. BCH 312 [Practical] Identification Of The Common Laboratory Glassware, Pipettes And Equipment. BCH 312 [Practical]](/thumbs/75/71986713.jpg) Identification Of The Common Laboratory Glassware, Pipettes And Equipment BCH 312 [Practical] (1) Identification of the common laboratory glassware : Conical flasks and beakers: Graduated cylinders Volumetric
Identification Of The Common Laboratory Glassware, Pipettes And Equipment BCH 312 [Practical] (1) Identification of the common laboratory glassware : Conical flasks and beakers: Graduated cylinders Volumetric
Microplate Spectrophotometer
 Microplate Spectrophotometer Features Benefits Applications Bali, October 1999 Optical System Xenon Flashing Lamp Order Sorting Filters Monochromator Data Light collection Light Transfer Why are these
Microplate Spectrophotometer Features Benefits Applications Bali, October 1999 Optical System Xenon Flashing Lamp Order Sorting Filters Monochromator Data Light collection Light Transfer Why are these
Instructor s Advance Preparation
 INSTRUCTOR'S MANUAL Instructor s Advance Preparation This protocol is designed for 80 workstations of 4 students. Each group will prepare a set of standards, a blank, and 2 milk samples (can be a blind
INSTRUCTOR'S MANUAL Instructor s Advance Preparation This protocol is designed for 80 workstations of 4 students. Each group will prepare a set of standards, a blank, and 2 milk samples (can be a blind
AIM To verify Beer - Lambert s law and to determine the dissociation constant (Ka) of methyl red, Spectrophotometrically.
 C 141(Expt. No. ) NAME : ROLL No. : SIGNATURE : BATCH : DATE : VERIFICATION OF BEER - LAMBERT S LAW & DETERMINATION OF DISSOCIATION CONSTANT (Ka) OF METHYLRED, SPECTROPHOTOMETRICALLY AIM To verify Beer
C 141(Expt. No. ) NAME : ROLL No. : SIGNATURE : BATCH : DATE : VERIFICATION OF BEER - LAMBERT S LAW & DETERMINATION OF DISSOCIATION CONSTANT (Ka) OF METHYLRED, SPECTROPHOTOMETRICALLY AIM To verify Beer
Pre-lab Quiz/PHYS 224. Your name Lab section
 Pre-lab Quiz/PHYS 224 THE DIFFRACTION GRATING AND THE OPTICAL SPECTRUM Your name Lab section 1. What are the goals of this experiment? 2. If the period of a diffraction grating is d = 1,000 nm, where the
Pre-lab Quiz/PHYS 224 THE DIFFRACTION GRATING AND THE OPTICAL SPECTRUM Your name Lab section 1. What are the goals of this experiment? 2. If the period of a diffraction grating is d = 1,000 nm, where the
CLINICAL CHEMISTRY ANALYSER. The economical solution for all your needs GIVING YOU PEACE OF MIND
 CLINICAL CHEMISTRY ANALYSER The economical solution for all your needs GIVING YOU PEACE OF MIND Proven and modern technology meeting all your needs: reliability, flexibility, convenience and economy. The
CLINICAL CHEMISTRY ANALYSER The economical solution for all your needs GIVING YOU PEACE OF MIND Proven and modern technology meeting all your needs: reliability, flexibility, convenience and economy. The
1 WHAT IS SPECTROSCOPY?
 1 WHAT IS SPECTROSCOPY? 1.1 The Nature Of Electromagnetic Radiation Anyone who has been sunburnt will know that light packs a punch: in scientific terms, it contains considerable amounts of energy. All
1 WHAT IS SPECTROSCOPY? 1.1 The Nature Of Electromagnetic Radiation Anyone who has been sunburnt will know that light packs a punch: in scientific terms, it contains considerable amounts of energy. All
09/05/40 MOLECULAR ABSORPTION METHODS
 MOLECULAR ABSORPTION METHODS Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of wavelength ( absorption spectrum ), due to its interaction
MOLECULAR ABSORPTION METHODS Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of wavelength ( absorption spectrum ), due to its interaction
Statistical Thermodynamics Approach to Urinary Analysis Employing Micro-Canonical Ensemble
 Statistical Thermodynamics Approach to Urinary Analysis Employing Micro-Canonical Ensemble * Chukwuneke J. L., Achebe C.H., Okolie P. C. and Okonkwo U. C. Mechanical Engineering Department, NnamdiAzikiwe
Statistical Thermodynamics Approach to Urinary Analysis Employing Micro-Canonical Ensemble * Chukwuneke J. L., Achebe C.H., Okolie P. C. and Okonkwo U. C. Mechanical Engineering Department, NnamdiAzikiwe
Spectrophotometric Determination of Iron
 Spectrophotometric Determination of Iron INTRODUCTION Many investigations of chemical species involve the interaction between light and matter. One class of these investigations, called absorbance spectrophotometry,
Spectrophotometric Determination of Iron INTRODUCTION Many investigations of chemical species involve the interaction between light and matter. One class of these investigations, called absorbance spectrophotometry,
Bio 120 Lab 5: Quantitative Analysis
 Bio 120 Lab 5: Quantitative Analysis Remember from the measurement lab, concentration is the amount of a solute, also called an analyte, in a given amount of solution. We need a way to measure the concentration
Bio 120 Lab 5: Quantitative Analysis Remember from the measurement lab, concentration is the amount of a solute, also called an analyte, in a given amount of solution. We need a way to measure the concentration
PRELIMINARY ACTIVITY FOR
 PRELIMINARY ACTIVITY FOR Beer s Law Investigations Guided Inquiry Version Experiment 11 The primary objective of this Preliminary Activity is to determine the concentration of an unknown copper (II) sulfate
PRELIMINARY ACTIVITY FOR Beer s Law Investigations Guided Inquiry Version Experiment 11 The primary objective of this Preliminary Activity is to determine the concentration of an unknown copper (II) sulfate
Dissolution Automation: Basic Questionnaire.
 Dissolution Automation: Basic Questionnaire. In order to quote the most suitable automated Dissolution System we would like you to answer this questionnaire: 1. Do you want to work : a) On line (on line
Dissolution Automation: Basic Questionnaire. In order to quote the most suitable automated Dissolution System we would like you to answer this questionnaire: 1. Do you want to work : a) On line (on line
Spectroscopy Meditsiiniline keemia/medical chemistry LOKT Spectroscopy
 Meditsiiniline keemia/medical chemistry LOKT.00.009 Spectroscopy 04.09.12 http://tera.chem.ut.ee/~koit/arstpr/spe_en.pdf 1 ntroduction Spectroscopy is a general term for methods that investigate interactions
Meditsiiniline keemia/medical chemistry LOKT.00.009 Spectroscopy 04.09.12 http://tera.chem.ut.ee/~koit/arstpr/spe_en.pdf 1 ntroduction Spectroscopy is a general term for methods that investigate interactions
The ROXI Colorimeter & Fluorimeter. Laboratory Application I. Colorimetric measurements via Beer s Law.
 The ROXI Colorimeter & Fluorimeter. Laboratory Application I. Colorimetric measurements via Beer s Law. Required Supplies & Costs: RGB LED; $1.95 Light Sensors; $3.95 ea 3-way switch; $6.54 3 ohm resistor;
The ROXI Colorimeter & Fluorimeter. Laboratory Application I. Colorimetric measurements via Beer s Law. Required Supplies & Costs: RGB LED; $1.95 Light Sensors; $3.95 ea 3-way switch; $6.54 3 ohm resistor;
Chemical Equilibrium: Finding a Constant, Kc
 Chemical Equilibrium: Finding a Constant, Kc Experiment 20 The purpose of this lab is to experimentally determine the equilibrium constant, K c, for the following chemical reaction: Fe 3+ (aq) + SCN (aq)
Chemical Equilibrium: Finding a Constant, Kc Experiment 20 The purpose of this lab is to experimentally determine the equilibrium constant, K c, for the following chemical reaction: Fe 3+ (aq) + SCN (aq)
Determining the Concentration of a Solution: Beer s Law
 Determining the Concentration of a Solution: Beer s Law Vernier Spectrometer 1 The primary objective of this experiment is to determine the concentration of an unknown copper (II) sulfate solution. You
Determining the Concentration of a Solution: Beer s Law Vernier Spectrometer 1 The primary objective of this experiment is to determine the concentration of an unknown copper (II) sulfate solution. You
C101-E111. Talk Letter. Vol.2 February 2009
 C101-E111 UV Talk Letter Vol.2 February 2009 UV Talk Letter UV Talk Letter The Structure of a Spectrophotometer Vol.2 February 2009 1.The Measurement Principle Used by a Spectrophotometer The basic measurement
C101-E111 UV Talk Letter Vol.2 February 2009 UV Talk Letter UV Talk Letter The Structure of a Spectrophotometer Vol.2 February 2009 1.The Measurement Principle Used by a Spectrophotometer The basic measurement
SPECTROPHOTOMETRY AND SPECTROMETRY - CONCEPT AND APPLICATIONS
 SPECTROPHOTOMETRY AND SPECTROMETRY - CONCEPT AND APPLICATIONS Renjini A 1, Dani Dileep 2 1 Assistant Professor, Department of ECE, Rajadhani Institute of Engineering and Technology, Kerala, India 2 PG
SPECTROPHOTOMETRY AND SPECTROMETRY - CONCEPT AND APPLICATIONS Renjini A 1, Dani Dileep 2 1 Assistant Professor, Department of ECE, Rajadhani Institute of Engineering and Technology, Kerala, India 2 PG
BIOLIGHT STUDIO IN ROUTINE UV/VIS SPECTROSCOPY
 BIOLIGHT STUDIO IN ROUTINE UV/VIS SPECTROSCOPY UV/Vis Spectroscopy is a technique that is widely used to characterize, identify and quantify chemical compounds in all fields of analytical chemistry. The
BIOLIGHT STUDIO IN ROUTINE UV/VIS SPECTROSCOPY UV/Vis Spectroscopy is a technique that is widely used to characterize, identify and quantify chemical compounds in all fields of analytical chemistry. The
Rydberg constant from atomic spectra of gases
 Page 1 of 8 Rydberg constant from atomic spectra of gases Objective - Calibrating a prism spectrometer to convert the scale readings in wavelengths of spectral lines. - Observing the Balmer series of atomic
Page 1 of 8 Rydberg constant from atomic spectra of gases Objective - Calibrating a prism spectrometer to convert the scale readings in wavelengths of spectral lines. - Observing the Balmer series of atomic
levels. The signal is either absorbance vibrational and rotational energy levels or percent transmittance of the analyte
 1 In this chapter, absorption by molecules, rather than atoms, is considered. Absorption in the ultraviolet and visible regions occurs due to electronic transitions from the ground state to excited state.
1 In this chapter, absorption by molecules, rather than atoms, is considered. Absorption in the ultraviolet and visible regions occurs due to electronic transitions from the ground state to excited state.
INSTRUCTION MANUAL. Lowry Assay Kit. Kit for Protein Quantification. (Cat. No )
 INSTRUCTION MANUAL Lowry Assay Kit Kit for Protein Quantification (Cat. No. 39236) SERVA Electrophoresis GmbH Carl-Benz-Str. 7 D-69115 Heidelberg Phone +49-6221-138400, Fax +49-6221-1384010 e-mail: info@serva.de
INSTRUCTION MANUAL Lowry Assay Kit Kit for Protein Quantification (Cat. No. 39236) SERVA Electrophoresis GmbH Carl-Benz-Str. 7 D-69115 Heidelberg Phone +49-6221-138400, Fax +49-6221-1384010 e-mail: info@serva.de
PROCEEDINGS OF SPIE. Shedding the light on spectrophotometry: the SpecUP educational spectrophotometer
 PROCEEDINGS OF SPIE SPIEDigitalLibrary.org/conference-proceedings-of-spie Shedding the light on spectrophotometry: the SpecUP educational spectrophotometer Johan A. Nöthling, Patricia B. C. Forbes Johan
PROCEEDINGS OF SPIE SPIEDigitalLibrary.org/conference-proceedings-of-spie Shedding the light on spectrophotometry: the SpecUP educational spectrophotometer Johan A. Nöthling, Patricia B. C. Forbes Johan
World Journal of Pharmaceutical Research SJIF Impact Factor 8.074
 SJIF Impact Factor 8.074 Volume 7, Issue 11, 1170-1180. Review Article ISSN 2277 7105 DEVELOPMENT AND OPTIMIZATION OF UV-VIS SPECTROSCOPY - A REVIEW Govinda Verma* and Dr. Manish Mishra Shri Guru Ram Rai
SJIF Impact Factor 8.074 Volume 7, Issue 11, 1170-1180. Review Article ISSN 2277 7105 DEVELOPMENT AND OPTIMIZATION OF UV-VIS SPECTROSCOPY - A REVIEW Govinda Verma* and Dr. Manish Mishra Shri Guru Ram Rai
Ocean Optics Red Tide UV-VIS Spectrometer (Order Code: SPRT-UV-VIS)
 Ocean Optics Red Tide UV-VIS Spectrometer (Order Code: SPRT-UV-VIS) The UV-VIS spectrometer is a portable ultraviolet light and visible light spectrophotometer, combining a spectrometer and a light source/cuvette
Ocean Optics Red Tide UV-VIS Spectrometer (Order Code: SPRT-UV-VIS) The UV-VIS spectrometer is a portable ultraviolet light and visible light spectrophotometer, combining a spectrometer and a light source/cuvette
Chem 310 rd. 3 Homework Set Answers
 -1- Chem 310 rd 3 Homework Set Answers 1. A double line labeled S 0 represents the _ground electronic_ state and the _ground vibrational_ state of a molecule in an excitation state diagram. Light absorption
-1- Chem 310 rd 3 Homework Set Answers 1. A double line labeled S 0 represents the _ground electronic_ state and the _ground vibrational_ state of a molecule in an excitation state diagram. Light absorption
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur
 VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
Rate law Determination of the Crystal Violet Reaction Using the Isolation Method
 Rate law Determination of the Crystal Violet Reaction Using the Isolation Method Introduction A common challenge in chemical kinetics is to determine the rate law for a reaction with multiple reactants.
Rate law Determination of the Crystal Violet Reaction Using the Isolation Method Introduction A common challenge in chemical kinetics is to determine the rate law for a reaction with multiple reactants.
S2. INTRODUCTION TO ULTRA-VIOLET / VISIBLE SPECTROSCOPY
 S2. INTRODUCTION TO ULTRA-VIOLET / VISIBLE SPECTROSCOPY PURPOSE 1. To become familiar with the operation of a conventional scanning ultra-violet spectrophotometer 2. To determine suitable cells and solvents
S2. INTRODUCTION TO ULTRA-VIOLET / VISIBLE SPECTROSCOPY PURPOSE 1. To become familiar with the operation of a conventional scanning ultra-violet spectrophotometer 2. To determine suitable cells and solvents
Experiment 2: The Beer-Lambert Law for Thiocyanatoiron (III)
 Chem 1B Dr. White 11 Experiment 2: The Beer-Lambert Law for Thiocyanatoiron (III) Objectives To use spectroscopy to relate the absorbance of a colored solution to its concentration. To prepare a Beer s
Chem 1B Dr. White 11 Experiment 2: The Beer-Lambert Law for Thiocyanatoiron (III) Objectives To use spectroscopy to relate the absorbance of a colored solution to its concentration. To prepare a Beer s
Reference literature. (See: CHEM 2470 notes, Module 8 Textbook 6th ed., Chapters )
 September 17, 2018 Reference literature (See: CHEM 2470 notes, Module 8 Textbook 6th ed., Chapters 13-14 ) Reference.: https://slideplayer.com/slide/8354408/ Spectroscopy Usual Wavelength Type of Quantum
September 17, 2018 Reference literature (See: CHEM 2470 notes, Module 8 Textbook 6th ed., Chapters 13-14 ) Reference.: https://slideplayer.com/slide/8354408/ Spectroscopy Usual Wavelength Type of Quantum
