Chemical Change. Day 1: 70 minutes; Day 2: minutes
|
|
|
- Neal McCarthy
- 5 years ago
- Views:
Transcription
1 Chemical Change Lesson Concept When one substance interacts with another substance, a chemical change may occur. There are five indicators that a chemical change has occurred: gas production (bubbles), color change, Link Students experienced chemical reactions during the Kitchen Chemistry lesson. The examples of chemical reactions presented here help students understand the indicators of chemical change. Time Day 1: 70 minutes; Day 2: minutes Materials Whole class SBCEO portal: Chemistry in Action: Reactions (VL) Segment 1: evidence of chemical reactions Technology to view video Demonstration materials for chemical reactions, i.e.,15ml of each powder and liquid listed below: Baking soda and vinegar Calcium chloride and water Red cabbage juice and vinegar 3 quart Ziploc bags Matches or lighter Stations: Station #1: iodine and cornstarch in sealed jar or baggie Station #2: glow stick, any color Station #3: ripped paper Station #4: burned paper Station #5: hot cocoa mix and water in sealed jar Station #6: cupcake or muffin Station #7: trail mix in sealed jar Station #8: popcorn in sealed jar 8 trays (I for each station) Labels for each station with letter and substance Science Matters 1
2 Advance Preparation Procedure: Engage Per Group (table groups) Birthday candle Modeling clay Pie plates Individual Chemical Lab worksheet Science Notebook 1. Gather materials. 2. Copy lab papers. 3. Assemble each station tray with materials and labels. Premix any jars. 4. Prepare a tray of materials and chemicals for teacher demonstration. (20 minutes) There are five indicators that a chemical change has occurred: gas production (bubbles), color change, 1. Discuss observations from previous lesson (Kitchen Chemistry) and brainstorm list of chemical change observations i.e., gas production, color change, precipitate production, temperature change, light production, etc. List on the board. 2. Model gas production by combining 15 ml (1 tbsp) baking soda and 15 ml (1 tbsp) vinegar in a baggie and seal. Have students observe the reaction. Discuss outcome. 3. Model color change by combining 15 ml (1 tbsp) iodine and 15 ml (1 tbsp) cornstarch in baggie and seal. Have students observe the reaction. Discuss outcome. 4. Model temperature change by combining 15 ml (1 tbsp) calcium chloride and 15ml (1 tbsp) of water. Allow students to observe the baggie using the sense of touch. Discuss outcome. 5. Review the indicators of a chemical change listed on the board. Ensure that all indicators are listed and students understand that during a chemical change matter (reactants) are rearranged to form new products with new properties. Remind students that in physical change no new products form. 6. Record student ideas on a T-Chart to contrast the indicators of chemical change and physical change. Have students add the T-Chart to their notebook to use as a tool for exploration. Explore (50 minutes) There are five indicators that a chemical change has occurred: gas production (bubbles), color change, Science Matters 2
3 7. Explain to students that they will rotate through 8 stations in small groups. At each station students will record: the name of the substance(s), observation of changes, whether the changes are examples of a chemical change or a physical change, and why. 8. Distribute Chemical Lab worksheet. Have students record their observations on the worksheet. 9. Allow students 3-5 minutes for each station. Explain (30 minutes) During a chemical reaction the atoms in the reactants (matter) rearrange to form products with different properties. 10. Bring students back to whole group. Facilitate a discussion to help students compare their results with others at their table groups. 11. Discuss students results, specifically stations where students were in disagreement about whether there was a chemical or physical change. Ask students to give evidence for reasoning. 12. Show video segment from SBCEO portal: Chemistry in Action: Reactions (VL) Segment 1: evidence of chemical reactions 13. Explain that when something interacts with a substance and causes molecules to break apart and rearrange and bond again, a chemical reaction has taken place. Extend (10 minutes) When something interacts with a substance and causes the molecules to break apart, rearrange, and bond again, a chemical change has taken place. There are five indicators that this has happened: gas is produced, change of color, change in temperature, precipitate is formed, and light is produced. 14. Distribute birthday candles (One per table group). Place birthday candle in a small piece of modeling clay (on top of an aluminum pie plate). Students observe burning candle and note signs of chemical change. Evaluate (5-10 minutes) When something interacts with a substance and causes the molecules to break apart, rearrange, and bond again, a chemical change has taken place. There are five indicators that this has happened: gas is produced, change of color (acids and bases), change in temperature, precipitate is formed, and light is produced. 15. Distribute exit cards. Ask students, What is the difference between a physical change and a chemical change? Ask students to give an example of a physical change and a chemical change. Science Matters 3
4 Name: Chemical Change Lab Station # Substances Observation of changes Identify chemical or physical change Why? Science Matters 4
5 Exit Card Name: Homeroom Teacher: What is the difference between a physical change and a chemical change? Give an example of a physical change: Give an example of a chemical change: Exit Card Name: Homeroom Teacher: What is the difference between a physical change and a chemical change? Give an example of a physical change: Give an example of a chemical change: Science Matters 5
Matter Lesson 2. Learning Goal 3: I can describe the differences between physical and chemical changes of matter.
 Matter Lesson 2 Learning Goal 2: I can describe the differences between intensive physical properties, extensive physical properties, and chemical properties of matter. Learning Goal 3: I can describe
Matter Lesson 2 Learning Goal 2: I can describe the differences between intensive physical properties, extensive physical properties, and chemical properties of matter. Learning Goal 3: I can describe
Energy Transformations Activities Students engage in different activities to observe energy changing form!
 Energy Transformations Activities Students engage in different activities to observe energy changing form! Name: Energy Labs and Activities Activity 1: Static Electricity Glue this side down Procedure:
Energy Transformations Activities Students engage in different activities to observe energy changing form! Name: Energy Labs and Activities Activity 1: Static Electricity Glue this side down Procedure:
5 th GRADE PHYSICAL AND CHEMICAL TESTS
 5 th GRADE PHYSICAL AND CHEMICAL TESTS Summary: Students investigate 5 unknown white powders. They gather clues by observing the physical and chemical changes of the powders. At the end of the activity,
5 th GRADE PHYSICAL AND CHEMICAL TESTS Summary: Students investigate 5 unknown white powders. They gather clues by observing the physical and chemical changes of the powders. At the end of the activity,
Physical & Chemical PROPERTIES
 Name Test Date Hour Group Chem #4 Notebook Properties & Changes LEARNING TARGETS I can give examples of physical and chemical properties. I can give examples of physical and chemical changes. I can identify
Name Test Date Hour Group Chem #4 Notebook Properties & Changes LEARNING TARGETS I can give examples of physical and chemical properties. I can give examples of physical and chemical changes. I can identify
Chapter 6, Lesson 7: Energy Changes in Chemical Reactions
 Chapter 6, Lesson 7: Energy Changes in Chemical Reactions Key Concepts If two substances react and the temperature of the mixture decreases, the reaction is endothermic. If two substances react and the
Chapter 6, Lesson 7: Energy Changes in Chemical Reactions Key Concepts If two substances react and the temperature of the mixture decreases, the reaction is endothermic. If two substances react and the
MiSP CHEMICAL REACTIONS, L3 Teacher Guide. Introduction
 MiSP CHEMICAL REACTIONS, L3 Teacher Guide Introduction This weeklong unit should be included with other chemistry content teaching and learning. It is designed to follow Intermediate Level Science Core
MiSP CHEMICAL REACTIONS, L3 Teacher Guide Introduction This weeklong unit should be included with other chemistry content teaching and learning. It is designed to follow Intermediate Level Science Core
States of Matter in Food
 Science Unit: Lesson 13: Matter States of Matter in Food Summary: Science skills: In this lesson, students explore the concept of state change, using various food and drink, in three activities: (1) Students
Science Unit: Lesson 13: Matter States of Matter in Food Summary: Science skills: In this lesson, students explore the concept of state change, using various food and drink, in three activities: (1) Students
Section 1: What is a Chemical Reaction
 Section 1: What is a Chemical Reaction I can describe and give examples of physical and chemical changes. I can identify reactants and products. I can explain what happens to molecules in chemical reactions
Section 1: What is a Chemical Reaction I can describe and give examples of physical and chemical changes. I can identify reactants and products. I can explain what happens to molecules in chemical reactions
Exploring Acids & Bases
 Food Explorations Lab: Exploring Acids & Bases STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will use cabbage juice indicator to determine if two unknown samples are acids or
Food Explorations Lab: Exploring Acids & Bases STUDENT LAB INVESTIGATIONS Name: Lab Overview In this investigation, you will use cabbage juice indicator to determine if two unknown samples are acids or
Friday, November 2, 2018 GLE/Standard: The fact that atoms are conserved, together
 Mrs. Chausse s Physical Science Gifted Lesson Plan: Unit 7 Chemical Reactions November 1 16, 2018 Thursday, November 1, 2018 Objective: SWBAT balance a chemical equation that satisfies the Law of Conservation
Mrs. Chausse s Physical Science Gifted Lesson Plan: Unit 7 Chemical Reactions November 1 16, 2018 Thursday, November 1, 2018 Objective: SWBAT balance a chemical equation that satisfies the Law of Conservation
The Next Generation Science Standards (NGSS)
 The Next Generation Science Standards (NGSS) CHAPTER 6, LESSON 1: WHAT IS A CHEMICAL REACTION? MS-PS1-2. Analyze and interpret data on the properties of substances before and after the substances interact
The Next Generation Science Standards (NGSS) CHAPTER 6, LESSON 1: WHAT IS A CHEMICAL REACTION? MS-PS1-2. Analyze and interpret data on the properties of substances before and after the substances interact
CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED)
 CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED) Overview: Students investigate chemical reactions using given substances. Students identify chemical reactions in their daily lives. Objectives: The student
CHEMICAL REACTION IN A BAGGY (MODIFIED FOR ADEED) Overview: Students investigate chemical reactions using given substances. Students identify chemical reactions in their daily lives. Objectives: The student
Chemistry Unit Test 1 Review
 Chemistry Unit Test 1 Review Name: S8P1a. Students will distinguish between atoms and molecules. 1. Which of the following particles combine to form molecules? a. Atoms b. Protons c. Electrons d. Compounds
Chemistry Unit Test 1 Review Name: S8P1a. Students will distinguish between atoms and molecules. 1. Which of the following particles combine to form molecules? a. Atoms b. Protons c. Electrons d. Compounds
LESSON 1. Chemical Reactions. Fireflies, also called lightning bugs, are small insects that generate their own light using chemical reactions.
 LESSON 1 Chemical Reactions Fireflies, also called lightning bugs, are small insects that generate their own light using chemical reactions. By the end of this lesson... you will be able to explain ways
LESSON 1 Chemical Reactions Fireflies, also called lightning bugs, are small insects that generate their own light using chemical reactions. By the end of this lesson... you will be able to explain ways
Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from
 Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from http://www.csun.edu/chemteach/activities/beginninganalysis.pdf ] In this lab, you will be observing physical and chemical properties
Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from http://www.csun.edu/chemteach/activities/beginninganalysis.pdf ] In this lab, you will be observing physical and chemical properties
Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from
 Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from http://www.csun.edu/chemteach/activities/beginninganalysis.pdf ] In this lab, you will be observing physical and chemical properties
Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from http://www.csun.edu/chemteach/activities/beginninganalysis.pdf ] In this lab, you will be observing physical and chemical properties
Characteristics of Chemical Change
 Section 2 Characteristics of Chemical Change What Do You See? Learning Outcomes In this section you will Observe several typical examples of evidence that a chemical change is occurring. Make generalizations
Section 2 Characteristics of Chemical Change What Do You See? Learning Outcomes In this section you will Observe several typical examples of evidence that a chemical change is occurring. Make generalizations
Activity Sheet Chapter 6, Lesson 1 What is a Chemical Reaction?
 Activity Sheet Chapter 6, Lesson 1 What is a Chemical Reaction? Name Date DEMNSTRATIN 1. Your teacher lit a candle and told you that this was a chemical reaction. What are the reactants in this chemical
Activity Sheet Chapter 6, Lesson 1 What is a Chemical Reaction? Name Date DEMNSTRATIN 1. Your teacher lit a candle and told you that this was a chemical reaction. What are the reactants in this chemical
Jeopardy. Final Jeopardy. Other. Matter $100 $100 $100 $100 $100 $200 $200 $200 $200 $200 $300 $300 $300 $300 $400 $400 $400 $500 $500 $500 $500 $500
 Jeopardy Matter States of matter Physical changes and chemical changes Physical and chemical properties Other $100 $100 $100 $100 $100 $200 $200 $200 $200 $200 $300 $300 $300 $300 $300 $400 $400 $400 $400
Jeopardy Matter States of matter Physical changes and chemical changes Physical and chemical properties Other $100 $100 $100 $100 $100 $200 $200 $200 $200 $200 $300 $300 $300 $300 $300 $400 $400 $400 $400
What Do You Think? Investigate GOALS
 Activity 2 More Chemical Changes GOALS In this activity you will: Observe several typical examples of evidence that a chemical change is occurring. Make generalizations about the combinations of materials
Activity 2 More Chemical Changes GOALS In this activity you will: Observe several typical examples of evidence that a chemical change is occurring. Make generalizations about the combinations of materials
Grade Six: Earthquakes/Volcanoes Lesson 6.2: Fault Formations
 Lesson Concept Link Time Grade Six: Earthquakes/Volcanoes Lesson 6.2: Fault Formations Forces in the Earth (tension, compression, shearing) cause stress at plate boundaries. Lesson 6.2 builds on the earthquake
Lesson Concept Link Time Grade Six: Earthquakes/Volcanoes Lesson 6.2: Fault Formations Forces in the Earth (tension, compression, shearing) cause stress at plate boundaries. Lesson 6.2 builds on the earthquake
Equilibrium/ Le Chatelier s Principle
 Equilibrium/ Le Chatelier s Principle Modified from an activity originally created by participating teachers 2008 Summer Green Chemistry workshop View video as demonstration guide: https://www.youtube.com/watch?v=fbdyl3hlbui#t=379
Equilibrium/ Le Chatelier s Principle Modified from an activity originally created by participating teachers 2008 Summer Green Chemistry workshop View video as demonstration guide: https://www.youtube.com/watch?v=fbdyl3hlbui#t=379
UC Irvine FOCUS! 5 E Lesson Plan Title: Acid/Base-pH Lab Grade Level and Course: 8 th grade Physical Science, Grades 9-12 Chemistry Materials:
 UC Irvine FOCUS! 5 E Lesson Plan Title: Acid/Base-pH Lab Grade Level and Course: 8 th grade Physical Science, Grades 9-12 Chemistry Materials: Detergent-quart, shampoo- quart Lemon-juice-quart Vinegar
UC Irvine FOCUS! 5 E Lesson Plan Title: Acid/Base-pH Lab Grade Level and Course: 8 th grade Physical Science, Grades 9-12 Chemistry Materials: Detergent-quart, shampoo- quart Lemon-juice-quart Vinegar
Maintaining Mass Created By: Allyn Short
 Maintaining Mass Created By: Allyn Short Focus on Inquiry The student will demonstrate that mass is conserved when substances undergo chemical and/or physical changes through experimentation and evaluation
Maintaining Mass Created By: Allyn Short Focus on Inquiry The student will demonstrate that mass is conserved when substances undergo chemical and/or physical changes through experimentation and evaluation
INTRODUCTION TO LESSON CLUSTER 5
 INTRODUCTION TO LESSON CLUSTER 5 EXPLAINING DISSOLVING A. Lesson Cluster Goals and Lesson Objectives Goals Students should be able to explain dissolving of solids in liquids in terms of molecules. Lesson
INTRODUCTION TO LESSON CLUSTER 5 EXPLAINING DISSOLVING A. Lesson Cluster Goals and Lesson Objectives Goals Students should be able to explain dissolving of solids in liquids in terms of molecules. Lesson
3. Kira makes 93 greeting cards for a craft fair. She sells the cards in packs of 5. How many full packs of greeting cards does Kira make?
 Chapter 4 Test 1. 2. 3. Kira makes 93 greeting cards for a craft fair. She sells the cards in packs of 5. How many full packs of greeting cards does Kira make? packs Work Space: 5. A kennel is moving 160
Chapter 4 Test 1. 2. 3. Kira makes 93 greeting cards for a craft fair. She sells the cards in packs of 5. How many full packs of greeting cards does Kira make? packs Work Space: 5. A kennel is moving 160
Grade Six Plate Tectonics Unit Lesson 6.2: Layers of the Earth
 Grade Six Plate Tectonics Unit Lesson 6.2: Layers of the Earth Lesson Concept Link The Earth has different layers with different densities and temperatures. Direct and Indirect evidence is used to explain
Grade Six Plate Tectonics Unit Lesson 6.2: Layers of the Earth Lesson Concept Link The Earth has different layers with different densities and temperatures. Direct and Indirect evidence is used to explain
Chapter 6, Lesson 1: What is a Chemical Reaction?
 Chapter 6, Lesson 1: What is a Chemical Reaction? Key Concepts: A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. In a chemical reaction,
Chapter 6, Lesson 1: What is a Chemical Reaction? Key Concepts: A physical change, such as a state change or dissolving, does not create a new substance, but a chemical change does. In a chemical reaction,
OBJECTIVES: By the end of class, students will be able to. SWBAT identify and describe physical and chemical changes of matter.
 7 th Grade Science Name: Unit: Matter and Periodic Table Lesson: Matter 4 Chemical and Physical Changes Part 2 Date: Thursday, December 1, 2016 Homeroom: OBJECTIVES: By the end of class, students will
7 th Grade Science Name: Unit: Matter and Periodic Table Lesson: Matter 4 Chemical and Physical Changes Part 2 Date: Thursday, December 1, 2016 Homeroom: OBJECTIVES: By the end of class, students will
Author(s): Tim Bumpus, Stephanie Sanders, Camilo Sarmiento, Daniel Urul. Standards: Next Generation Science Standards (www.nextgenscience.
 Unknown Powders Author(s): Tim Bumpus, Stephanie Sanders, Camilo Sarmiento, Daniel Urul Date Created: February, 2016 Subject: Chemistry Grade Level: Grades 3-6 Standards: Next Generation Science Standards
Unknown Powders Author(s): Tim Bumpus, Stephanie Sanders, Camilo Sarmiento, Daniel Urul Date Created: February, 2016 Subject: Chemistry Grade Level: Grades 3-6 Standards: Next Generation Science Standards
5 th Grade Lesson Plan: Matter and Chemical Reactions
 5 th Grade Lesson Plan: Matter and Chemical Reactions Objective: Teach students that matter is neither created nor destroyed during a chemical reaction, rather, it is transformed. Identify evidence that
5 th Grade Lesson Plan: Matter and Chemical Reactions Objective: Teach students that matter is neither created nor destroyed during a chemical reaction, rather, it is transformed. Identify evidence that
Chemistry: classifying chemical and physical changes in various materials/substances
 Chemistry: classifying chemical and physical changes in various materials/substances Nikki Schilling, Ames, St. Paul Mn Based on original activity from Cool Chemistry Concoctions by Joe Rhatigan and Veronika
Chemistry: classifying chemical and physical changes in various materials/substances Nikki Schilling, Ames, St. Paul Mn Based on original activity from Cool Chemistry Concoctions by Joe Rhatigan and Veronika
Saturday Science Lesson Plan Fall 2008
 Saturday Science Lesson Plan Fall 2008 LEARNING OBJECTIVES STANDARDS 1.1.1 Observe, describe, draw, and sort objects carefully to learn about them. 1.2.6 Describe and compare objects in terms of number,
Saturday Science Lesson Plan Fall 2008 LEARNING OBJECTIVES STANDARDS 1.1.1 Observe, describe, draw, and sort objects carefully to learn about them. 1.2.6 Describe and compare objects in terms of number,
Thermal Energy and Temperature Lab. Experiment Question: How can the difference between thermal energy and temperature be experimentally observed?
 Thermal Energy and Temperature Lab Name 7 th Grade PSI Grade / 20 Experiment Question: How can the difference between thermal energy and temperature be experimentally observed? Hypothesis Starters: 1.
Thermal Energy and Temperature Lab Name 7 th Grade PSI Grade / 20 Experiment Question: How can the difference between thermal energy and temperature be experimentally observed? Hypothesis Starters: 1.
Working with Solutions. (and why that s not always ideal)
 Page 1 of 13 Working with Solutions (and why that s not always ideal) Learning Objectives: Solutions are prepared by dissolving a solute into a solvent A solute is typically a solid, but may also be a
Page 1 of 13 Working with Solutions (and why that s not always ideal) Learning Objectives: Solutions are prepared by dissolving a solute into a solvent A solute is typically a solid, but may also be a
Physical and Chemical Properties and Changes Lab
 Name: Date: Period: Group Members Physical and Chemical Properties and Changes Lab Station 1 Color Station Instruction: Describe the color of the following substances. Substance Color 1. Sulfur 2. Ammonium
Name: Date: Period: Group Members Physical and Chemical Properties and Changes Lab Station 1 Color Station Instruction: Describe the color of the following substances. Substance Color 1. Sulfur 2. Ammonium
Chemical Matrix of Acids and Bases
 Chemical Matrix of Acids and Bases Overview:If you love the idea of mixing up chemicals and dream of having your own mad science lab, this one is for you. You are going to mix up solids and liquids in
Chemical Matrix of Acids and Bases Overview:If you love the idea of mixing up chemicals and dream of having your own mad science lab, this one is for you. You are going to mix up solids and liquids in
Lesson 5: Oxygen, Oxidation, and Combustion
 Lesson 5: Oxidation, and Students are introduced to exothermic reactions that involve oxidation. Main Concept: Oxygen is a highly reactive element involved in chemical reactions that release heat energy.
Lesson 5: Oxidation, and Students are introduced to exothermic reactions that involve oxidation. Main Concept: Oxygen is a highly reactive element involved in chemical reactions that release heat energy.
Chemical Reaction Lab Bagged Chemical Reactions
 Learning Target: The student experiments and determines that the rates of reaction among atoms and molecules depend on the concentration, pressure, and temperature of the reactants and the presence or
Learning Target: The student experiments and determines that the rates of reaction among atoms and molecules depend on the concentration, pressure, and temperature of the reactants and the presence or
Observing Chemical Change - 5.1
 Observing Chemical Change - 5.1 Vocabulary: Physical change - Chemical change - Reactant Product Precipitate Exothermic reaction - Endothermic reaction - Properties of matter: Two kinds of properties of
Observing Chemical Change - 5.1 Vocabulary: Physical change - Chemical change - Reactant Product Precipitate Exothermic reaction - Endothermic reaction - Properties of matter: Two kinds of properties of
Post-Show. Chemistry. Periodic Table of the Elements. After the Show. Traveling Science Shows
 Traveling Science Shows Post-Show Chemistry After the Show We recently presented a Chemistry show at your school, and thought you and your students might like to continue investigating this topic. The
Traveling Science Shows Post-Show Chemistry After the Show We recently presented a Chemistry show at your school, and thought you and your students might like to continue investigating this topic. The
2 nd Quarter Test Review
 2 nd Quarter Test Review 1. Create a Venn diagram and add the following to the Venn diagram: a. Made from just one element b. Made from more than one element c. Forms through a chemical reaction Elements
2 nd Quarter Test Review 1. Create a Venn diagram and add the following to the Venn diagram: a. Made from just one element b. Made from more than one element c. Forms through a chemical reaction Elements
How can you tell rocks on another planet apart?
 How can you tell rocks on another planet apart? Grade Range: K - 6 G.L.E Focus: 1.1.5 Time Budget: 1 hour WASL Vocabulary: Overview: Students learn that scientists send rovers to other planets to learn
How can you tell rocks on another planet apart? Grade Range: K - 6 G.L.E Focus: 1.1.5 Time Budget: 1 hour WASL Vocabulary: Overview: Students learn that scientists send rovers to other planets to learn
8.4 Changes in Matter: Hot Ice and the Carbon Snake. Grade 8 Activity Plan
 8.4 Changes in Matter: Hot Ice and the Carbon Snake Grade 8 Activity Plan 1 Reviews and Updates - Hot Ice and Carbon Snake Activity add by Fola Akpan in July 2017 2 8.4 Changes in Matter: Hot Ice and the
8.4 Changes in Matter: Hot Ice and the Carbon Snake Grade 8 Activity Plan 1 Reviews and Updates - Hot Ice and Carbon Snake Activity add by Fola Akpan in July 2017 2 8.4 Changes in Matter: Hot Ice and the
1 st Semester Exam Study Guide 1.) Which of the following is NOT a compound? Explain why. a. H2O b. O2
 1 st Semester Exam Study Guide 1.) Which of the following is NOT a compound? Explain why. a. H2O b. O2 2.) A chemist has discovered what she thinks is a new molecule. In order for it to be a molecule,
1 st Semester Exam Study Guide 1.) Which of the following is NOT a compound? Explain why. a. H2O b. O2 2.) A chemist has discovered what she thinks is a new molecule. In order for it to be a molecule,
Recognizing Chemical_Reactions By Dr. Kathleen Vandiver
 Recognizing Chemical_Reactions By Dr. Kathleen Vandiver Hello. My name is Dr. Kathy Vandiver. And we're here at the Edgerton Center at MIT, a university in the United States. And the Edgerton Center is
Recognizing Chemical_Reactions By Dr. Kathleen Vandiver Hello. My name is Dr. Kathy Vandiver. And we're here at the Edgerton Center at MIT, a university in the United States. And the Edgerton Center is
3. Watch video All About Solids, Liquids, & Gases. Watch first eight minutes.
 Structure and Transformation of Matter Original (2008) Lesson Plan I can classify matter into different categories. I can describe the differences between solid, liquid, and gas. Lesson 1: Search for Matter
Structure and Transformation of Matter Original (2008) Lesson Plan I can classify matter into different categories. I can describe the differences between solid, liquid, and gas. Lesson 1: Search for Matter
Characteristics & Types of Chemical Reactions
 Characteristics & Types of Chemical Reactions What is a chemical reaction? Another name for a chemical change Bonds break and atoms of one or more substances are rearranged and new bonds form New substance(s)
Characteristics & Types of Chemical Reactions What is a chemical reaction? Another name for a chemical change Bonds break and atoms of one or more substances are rearranged and new bonds form New substance(s)
Chemical reactions are processes in which the atoms of one or more substances are rearranged to form different chemical compounds.
 Chapter 9 Chemical Reactions Notes Chemical Reactions: Chemical reactions are processes in which the atoms of one or more substances are rearranged to form different chemical compounds. How to tell if
Chapter 9 Chemical Reactions Notes Chemical Reactions: Chemical reactions are processes in which the atoms of one or more substances are rearranged to form different chemical compounds. How to tell if
VANDERBILT STUDENT VOLUNTEERS FOR SCIENCE
 VANDERBILT STUDENT VOLUNTEERS FOR SCIENCE http://studentorgs.vanderbilt.edu/vsvs Evidence of a Chemical Reaction Fall 2018 Goal: To show students evidence of a chemical change. Fits TN standards:7ps1.2,
VANDERBILT STUDENT VOLUNTEERS FOR SCIENCE http://studentorgs.vanderbilt.edu/vsvs Evidence of a Chemical Reaction Fall 2018 Goal: To show students evidence of a chemical change. Fits TN standards:7ps1.2,
Unit 6M.3: Changing materials
 Unit 6M.3: Adding materials to water. Chemical reactions Skill you will use: Classifying Observing Predicting By the end of this unit you should: Know that when substances are added to water, some will
Unit 6M.3: Adding materials to water. Chemical reactions Skill you will use: Classifying Observing Predicting By the end of this unit you should: Know that when substances are added to water, some will
CO 2. Lesson 1. Production of a gas
 Lesson 1 Production of a gas T E A C H E R G U I D E CO 2 Lesson summary Students meet volcanologist Victor Helguson, who is studying the gases released by volcanoes in Iceland. Students conduct a chemical
Lesson 1 Production of a gas T E A C H E R G U I D E CO 2 Lesson summary Students meet volcanologist Victor Helguson, who is studying the gases released by volcanoes in Iceland. Students conduct a chemical
PROPERTIES OF MATTER STATION 1. Where did the water droplets on the outside of this cup come from? What phase change is this?
 PROPERTIES OF MATTER STATION 1 Where did the water droplets on the outside of this cup come from? What phase change is this? PROPERTIES OF MATTER STATION 2 Make a copy of this graph on your paper. Label
PROPERTIES OF MATTER STATION 1 Where did the water droplets on the outside of this cup come from? What phase change is this? PROPERTIES OF MATTER STATION 2 Make a copy of this graph on your paper. Label
Bay Area Scientists in Schools Presentation Plan
 Bay Area Scientists in Schools Presentation Plan Lesson Name Presenter(s) Chemical Reactions Mercedes Taylor, Nick Settineri, Jessica Ziegler, Tyler Hurlburt, Parker Deal Grade Level 5 Standards Connection(s)
Bay Area Scientists in Schools Presentation Plan Lesson Name Presenter(s) Chemical Reactions Mercedes Taylor, Nick Settineri, Jessica Ziegler, Tyler Hurlburt, Parker Deal Grade Level 5 Standards Connection(s)
Signs of Chemical Reactions
 Signs of Chemical Reactions Tell the difference between changes which are PHYSICAL and those which involve a CHEMICAL reaction Doing six different experiments and observing the changes which
Signs of Chemical Reactions Tell the difference between changes which are PHYSICAL and those which involve a CHEMICAL reaction Doing six different experiments and observing the changes which
4. Every CHANGE in matter includes a change in, which is conserved in a chemical reaction and. TRANSFORMED from one form to another.
 Part C: Lesson 1.4 - Read pages 20-29 to answer these questions. Add to notes - 1. A physical change alters the form or appearance of matter, such as Change in size, a change in SIZE or. SHAPE shape, or
Part C: Lesson 1.4 - Read pages 20-29 to answer these questions. Add to notes - 1. A physical change alters the form or appearance of matter, such as Change in size, a change in SIZE or. SHAPE shape, or
Student Exploration: Chemical Changes
 Name: Date: Student Exploration: Chemical Changes Vocabulary: acid, base, catalyst, chemical change, coefficient, conservation of matter, decomposition, dissolve, double replacement, endothermic, exothermic,
Name: Date: Student Exploration: Chemical Changes Vocabulary: acid, base, catalyst, chemical change, coefficient, conservation of matter, decomposition, dissolve, double replacement, endothermic, exothermic,
Conserving Water in the Desert
 Conserving Water in the Desert Lesson 2: What s the Matter? Water s Changing States Enduring Understanding Water is the only natural substance that is found in three different physical states - liquid,
Conserving Water in the Desert Lesson 2: What s the Matter? Water s Changing States Enduring Understanding Water is the only natural substance that is found in three different physical states - liquid,
Copyright 2016 Edmentum - All rights reserved.
 Copyright 2016 Edmentum - All rights reserved. SI: Quiz 5 Question #1 Which of the following is true about the mass of the reactants in a chemical reaction? The total mass of the reactants in a chemical
Copyright 2016 Edmentum - All rights reserved. SI: Quiz 5 Question #1 Which of the following is true about the mass of the reactants in a chemical reaction? The total mass of the reactants in a chemical
LEVEL ZERO VOICE CATALYST (10 minutes, individual work): 1. Counting atoms
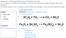 Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
Science Grade 08 Unit 04 Exemplar Lesson 01: Formulas, Equations, and the Conservation of Mass
 Unit: 04 Lesson: 01 Suggested Duration: 15 days Grade 08 Unit 04 Exemplar Lesson 01: Formulas, Equations, and the Conservation of Mass This lesson is one approach to teaching the State Standards associated
Unit: 04 Lesson: 01 Suggested Duration: 15 days Grade 08 Unit 04 Exemplar Lesson 01: Formulas, Equations, and the Conservation of Mass This lesson is one approach to teaching the State Standards associated
Chemical Reactions. Chapter Test A. Multiple Choice. 1 Pearson Education, Inc., or its affiliates. All rights reserved.
 Name Date Class Chemical Reactions Chapter Test A Multiple Choice Write the letter of the correct answer on the line at the left. 1. Chemistry is a. a characteristic of a substance that can be observed
Name Date Class Chemical Reactions Chapter Test A Multiple Choice Write the letter of the correct answer on the line at the left. 1. Chemistry is a. a characteristic of a substance that can be observed
Today is: Monday, October 17th
 Today is: Monday, October 17th Get out your homework! 10/17/2016 #motivationmonday (This is Week 11 Warm Ups!) 1. It s a new quarter, which means a fresh start! What can you do to stay motivated this quarter?
Today is: Monday, October 17th Get out your homework! 10/17/2016 #motivationmonday (This is Week 11 Warm Ups!) 1. It s a new quarter, which means a fresh start! What can you do to stay motivated this quarter?
Chemical Formulas and Equations
 Part I: The Big Picture Chemical Formulas and Equations Reminder: H is the element symbol for Hydrogen. H 2 is the chemical formula for hydrogen. The subscript 2 after the H means that two atoms of hydrogen
Part I: The Big Picture Chemical Formulas and Equations Reminder: H is the element symbol for Hydrogen. H 2 is the chemical formula for hydrogen. The subscript 2 after the H means that two atoms of hydrogen
The grade 5 English science unit, Acids and Bases, meets the academic content standards set in the Korean curriculum, which state students should:
 This area addresses ph among different characteristics of solutions. It will be interesting for students to classify a variety of solutions into acids and bases by using the characteristics of the solutions.
This area addresses ph among different characteristics of solutions. It will be interesting for students to classify a variety of solutions into acids and bases by using the characteristics of the solutions.
composition of matter, and the changes that matter undergoes. Examples of Uses of Chemistry in Everyday Life
 Name Matter and Change: Unit Objective Study Guide Date Due Directions: Write your answers to the following questions in the space provided. For problem solving, all of the work leading up to the final
Name Matter and Change: Unit Objective Study Guide Date Due Directions: Write your answers to the following questions in the space provided. For problem solving, all of the work leading up to the final
Lesson 1: What are chemical changes?
 Lesson 1 Summary Lesson 1: What are chemical changes? Vocabulary Use with pp. 375 377 physical change a change in which the material keeps its identify chemical change a change in which one substance or
Lesson 1 Summary Lesson 1: What are chemical changes? Vocabulary Use with pp. 375 377 physical change a change in which the material keeps its identify chemical change a change in which one substance or
Science in the Kitchen
 Program Support Notes by: Margaret Bishop B.Ed, Dip T Produced by: VEA Pty Ltd Commissioning Editor: Sandra Frerichs B.Ed, M.Ed. Executive Producer: Simon Garner B.Ed, Dip Management Davis Film and Video
Program Support Notes by: Margaret Bishop B.Ed, Dip T Produced by: VEA Pty Ltd Commissioning Editor: Sandra Frerichs B.Ed, M.Ed. Executive Producer: Simon Garner B.Ed, Dip Management Davis Film and Video
Chemical Reactions. Teachers Guide.
 Chemical Reactions. Teachers Guide. Introduction: Almost everything you will ever see in this universe is made of atoms. Each different type of atom is known as a chemical element there are currently 118
Chemical Reactions. Teachers Guide. Introduction: Almost everything you will ever see in this universe is made of atoms. Each different type of atom is known as a chemical element there are currently 118
Photosynthesis-Cellular Respiration Cycle
 Photosynthesis-Cellular Respiration Cycle Lesson Concept Link Photosynthesis and cellular respiration are reverse processes. Plants use photosynthesis to make food and release oxygen and plants and animals
Photosynthesis-Cellular Respiration Cycle Lesson Concept Link Photosynthesis and cellular respiration are reverse processes. Plants use photosynthesis to make food and release oxygen and plants and animals
THIRD GRADE OCEANS 1 WEEK LESSON PLANS AND ACTIVITIES
 THIRD GRADE OCEANS 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF THIRD GRADE WATER WEEK 1. PRE: Comparing the different components of the water cycle. LAB: Contrasting water with hydrogen
THIRD GRADE OCEANS 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF THIRD GRADE WATER WEEK 1. PRE: Comparing the different components of the water cycle. LAB: Contrasting water with hydrogen
4 th Grade Science Unit: Matter Matters Conservation of Matter Unit Snapshot
 4 th Grade Science Unit: Matter Matters Conservation of Matter Unit Snapshot Grade Level: 4 Topic: Electricity, Heat and Matter Duration: 14 days Summary This series of activities is for students to develop
4 th Grade Science Unit: Matter Matters Conservation of Matter Unit Snapshot Grade Level: 4 Topic: Electricity, Heat and Matter Duration: 14 days Summary This series of activities is for students to develop
LESSON 2. Chemical Equations. When copper sulfate reacts with ammonia, a precipitate forms and the solution color changes to deep blue.
 LESSN 2 Chemical Equations When copper sulfate reacts with ammonia, a precipitate forms and the solution color changes to deep blue. By the end of this lesson... you will be able to explain how chemical
LESSN 2 Chemical Equations When copper sulfate reacts with ammonia, a precipitate forms and the solution color changes to deep blue. By the end of this lesson... you will be able to explain how chemical
MATTER. Physical Science 2nd Semester NAME: CLASS PERIOD: TEACHER: HW POINTS EARNED LAB POINTS EARNED. PAGE NUMBERS Learning Targets: Matter 1-2
 MATTER Physical Science 2nd Semester ASSIGNMENT PAGE NUMBERS Learning Targets: Matter 1-2 NAME: CLASS PERIOD: TEACHER: DUE DATE HW POINTS EARNED LAB POINTS EARNED Density Lab 3-4 Density Calculations Worksheet
MATTER Physical Science 2nd Semester ASSIGNMENT PAGE NUMBERS Learning Targets: Matter 1-2 NAME: CLASS PERIOD: TEACHER: DUE DATE HW POINTS EARNED LAB POINTS EARNED Density Lab 3-4 Density Calculations Worksheet
More Chemical Changes
 Activity 2 More Chemical Changes Activity 2 More Chemical Changes GOALS In this activity you will: Observe several typical examples of evidence that a chemical change is occurring. Make generalizations
Activity 2 More Chemical Changes Activity 2 More Chemical Changes GOALS In this activity you will: Observe several typical examples of evidence that a chemical change is occurring. Make generalizations
Stoichiometry Lab Report
 Stoichiometry Lab Report Touche G10 Variables Variable Dependent Controlled: Amount of NaHCO3 Theoretical Yield Vinegar Evaporation Manipulation We measure the remaining NaC 2 H 3 O 2 and compare it with
Stoichiometry Lab Report Touche G10 Variables Variable Dependent Controlled: Amount of NaHCO3 Theoretical Yield Vinegar Evaporation Manipulation We measure the remaining NaC 2 H 3 O 2 and compare it with
C E C U R R I C U L U M I E N S C B L E I T A. i N T E G R A T I N G A R T S i n O N A T I D U C B L I P U. Student Learning Objectives:
 We athering E Q U I T A B L E S C I E N C E C U R R I C U L U M Lesson 1 i N T E G R A T I N G A R T S i n P U B L I C E D U C A T I O N NGSS Science Standard: 4-ESS1-1 Identify evidence from patterns
We athering E Q U I T A B L E S C I E N C E C U R R I C U L U M Lesson 1 i N T E G R A T I N G A R T S i n P U B L I C E D U C A T I O N NGSS Science Standard: 4-ESS1-1 Identify evidence from patterns
Signs of Chemical Reactions
 Signs of Chemical Reactions Tell the difference between changes which are PHYSICAL and those which involve a CHEMICAL reaction Doing six different experiments and observing the changes which
Signs of Chemical Reactions Tell the difference between changes which are PHYSICAL and those which involve a CHEMICAL reaction Doing six different experiments and observing the changes which
The Next Generation Science Standards (NGSS)
 The Next Generation Science Standards (NGSS) CHAPTER 3, LESSON 1: WHAT IS DENSITY? MS-PS1-1. Develop models to describe the atomic composition of simple molecules and extended structures. DISCIPLINARY
The Next Generation Science Standards (NGSS) CHAPTER 3, LESSON 1: WHAT IS DENSITY? MS-PS1-1. Develop models to describe the atomic composition of simple molecules and extended structures. DISCIPLINARY
Determining ph. Standards: 5.e. Students know how to determine whether a solution is acidic, basic, or neutral
 Science Stars: 8 th grade Lesson Plan Determining ph Standards: 5.e. Students know how to determine whether a solution is acidic, basic, or neutral Suggested time: 50 minutes Anticipatory Set (Engage):
Science Stars: 8 th grade Lesson Plan Determining ph Standards: 5.e. Students know how to determine whether a solution is acidic, basic, or neutral Suggested time: 50 minutes Anticipatory Set (Engage):
MONDAY (12/12) TUESDAY (12/13) WEDNESDAY (12/14) THURSDAY (12/15) FRIDAY (12/16) Making Acid Rain (a lab) Quiz
 Homework Activities Name: Date: Period: This week, we will be using our knowledge of acids and bases and studying how acids, specifically acid rain, affect our lives and our environment. We will also end
Homework Activities Name: Date: Period: This week, we will be using our knowledge of acids and bases and studying how acids, specifically acid rain, affect our lives and our environment. We will also end
Physical Changes can be observed without changing the identity of the substance (often states of matter changes).
 Physical Changes can be observed without changing the identity of the substance (often states of matter changes). Remember: States of matter changes are physical changes. The identify of the substance
Physical Changes can be observed without changing the identity of the substance (often states of matter changes). Remember: States of matter changes are physical changes. The identify of the substance
Investigation 4: Fizz Quiz
 5 th Science Notebook Mixtures and Solutions Investigation 4 Investigation 4: Fizz Quiz Name: Big Question: How can matter be changed? Explain. 1 Alignment with New York State Science Standards & Performance
5 th Science Notebook Mixtures and Solutions Investigation 4 Investigation 4: Fizz Quiz Name: Big Question: How can matter be changed? Explain. 1 Alignment with New York State Science Standards & Performance
Observations. Direct Observations Those observations we make ourselves
 Observations Direct Observations Those observations we make ourselves Indirect Observations Those observations that we figure out from direct observations Observing Milk Consumption The cafeteria is considering
Observations Direct Observations Those observations we make ourselves Indirect Observations Those observations that we figure out from direct observations Observing Milk Consumption The cafeteria is considering
Student Notes. Chemical Reactions LINK
 LCPS Core Experience Chemical Reactions Student Notes OBJECTIVES Students will: investigate the relationship between reactants and products. investigate an exothermic reaction. investigate an endothermic
LCPS Core Experience Chemical Reactions Student Notes OBJECTIVES Students will: investigate the relationship between reactants and products. investigate an exothermic reaction. investigate an endothermic
Conservation of Mass in Chemical Reactions Lab. Experiment Question: What happens to the total mass of substances when a chemical reaction occurs?
 Conservation of Mass in Chemical Reactions Lab Name: 5 th Grade PSI Science Score: / 5 Experiment Question: What happens to the total mass of substances when a chemical reaction occurs? Hypothesis Starters:
Conservation of Mass in Chemical Reactions Lab Name: 5 th Grade PSI Science Score: / 5 Experiment Question: What happens to the total mass of substances when a chemical reaction occurs? Hypothesis Starters:
Southern Nevada Regional Professional Development Program. How do rocks react to vinegar?
 3-5 Earth Science How do rocks react to vinegar? Collection of rocks Vinegar Plastic cup for each rock Small samples of calcite 1. Place the sample of calcite into a cup. 2. Pour about 25 ml of vinegar
3-5 Earth Science How do rocks react to vinegar? Collection of rocks Vinegar Plastic cup for each rock Small samples of calcite 1. Place the sample of calcite into a cup. 2. Pour about 25 ml of vinegar
Activity Sheet Chapter 6, Lesson 10 Carbon Dioxide Can Make a Solution Acidic
 Activity Sheet Chapter 6, Lesson 10 Carbon Dioxide Can Make a Solution Acidic Name Date DEMONSTRATION 1. Your teacher blew through a straw into a universal indicator solution until it changed color. Did
Activity Sheet Chapter 6, Lesson 10 Carbon Dioxide Can Make a Solution Acidic Name Date DEMONSTRATION 1. Your teacher blew through a straw into a universal indicator solution until it changed color. Did
Lesson 4. Temperature change
 Lesson 4 Temperature change T E A C H E R G U I D E Lesson summary Students meet scientist Jason Williams, an industrial chemist who designs the materials and processes for making solar cells. He explains
Lesson 4 Temperature change T E A C H E R G U I D E Lesson summary Students meet scientist Jason Williams, an industrial chemist who designs the materials and processes for making solar cells. He explains
How are elements, compounds, and mixtures related?
 Essential Question: How are elements, compounds, and mixtures related? S8P1b. Describe the difference between pure substances (elements and compounds) and mixtures Matter is anything that has mass and
Essential Question: How are elements, compounds, and mixtures related? S8P1b. Describe the difference between pure substances (elements and compounds) and mixtures Matter is anything that has mass and
Grade Six: Plate Tectonics 6.11 Density of Granite and Basalt. Density of basalt and granite affect the formation of landmasses on Earth.
 Grade Six: Plate Tectonics 6.11 Density of Granite and Basalt Lesson Concept Density of basalt and granite affect the formation of landmasses on Earth. Link Mountain formation in Lesson 6.10 is dependent
Grade Six: Plate Tectonics 6.11 Density of Granite and Basalt Lesson Concept Density of basalt and granite affect the formation of landmasses on Earth. Link Mountain formation in Lesson 6.10 is dependent
Chapter 5, Lesson 5 Using Dissolving to Identify an Unknown
 Chapter 5, Lesson 5 Using Dissolving to Identify an Unknown Key Concepts Different substances are made from different atoms, ions, or molecules, which interact with water in different ways. Since dissolving
Chapter 5, Lesson 5 Using Dissolving to Identify an Unknown Key Concepts Different substances are made from different atoms, ions, or molecules, which interact with water in different ways. Since dissolving
The Mystery Reaction A Lesson on Chemical Reactions
 The Impact of Environmental Factors on Changes in ph and Dissolved Oxygen Levels in Pond Water- A Probeware Based Activity The Mystery Reaction Kelly Miller Middle School Washington, District of Columbia
The Impact of Environmental Factors on Changes in ph and Dissolved Oxygen Levels in Pond Water- A Probeware Based Activity The Mystery Reaction Kelly Miller Middle School Washington, District of Columbia
Chemical reactions are processes in which the atoms of one or more substances are rearranged to form different chemical compounds.
 Chapter 11 Chemical Reactions Notes Chemical Reactions: Chemical reactions are processes in which the atoms of one or more substances are rearranged to form different chemical compounds. How to tell if
Chapter 11 Chemical Reactions Notes Chemical Reactions: Chemical reactions are processes in which the atoms of one or more substances are rearranged to form different chemical compounds. How to tell if
Grade 8 Science Unit 2 Test» Form A (Master Copy) Directions: Please choose the best answer choice for each of the following questions.
 Directions: Please choose the best answer choice for each of the following questions. 1. Which of the following could be a molecule BUT not a compound? a pair of atoms of the same element a collection
Directions: Please choose the best answer choice for each of the following questions. 1. Which of the following could be a molecule BUT not a compound? a pair of atoms of the same element a collection
Recommended Resources: The following resources may be useful in teaching this
 Unit C: Plant Physiology Lesson 1: Understanding Plant Physiology Student Learning Objectives: Instruction in this lesson should result in students achieving the following objectives: 1. Describe the process
Unit C: Plant Physiology Lesson 1: Understanding Plant Physiology Student Learning Objectives: Instruction in this lesson should result in students achieving the following objectives: 1. Describe the process
Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic
 Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic Key Concepts Carbon dioxide (CO 2 ) gas dissolved in water can cause water to become acidic. The acidity of water from dissolved CO 2 can
Chapter 6, Lesson 10: Carbon Dioxide Can Make a Solution Acidic Key Concepts Carbon dioxide (CO 2 ) gas dissolved in water can cause water to become acidic. The acidity of water from dissolved CO 2 can
Table of Contents DSM II. Powders and Crystals (Grades 3 5) Place your order by calling us toll-free
 DSM II Powders and Crystals (Grades 3 5) Table of Contents Actual page size: 8.5" x 11" Philosophy and Structure Overview 1 Overview Chart 2 Materials List 3 Schedule of Activities 4 Preparing for the
DSM II Powders and Crystals (Grades 3 5) Table of Contents Actual page size: 8.5" x 11" Philosophy and Structure Overview 1 Overview Chart 2 Materials List 3 Schedule of Activities 4 Preparing for the
Matter (Unitmatter) 1. What is the smallest particle of the element gold (Au) that can still be classified as gold?
 Name: Date: 1. What is the smallest particle of the element gold (Au) that can still be classified as gold? A. atom B. molecule C. neutron D. proton This online assessment item contains material that has
Name: Date: 1. What is the smallest particle of the element gold (Au) that can still be classified as gold? A. atom B. molecule C. neutron D. proton This online assessment item contains material that has
Virtual Library Lesson: Oobleck, Gloop, and Glurch
 Oobleck, Gloop, and Glurch Lesson Overview Throughout this lesson, students will use inquiry skills to identify states of matter, describe physical properties, and modify the recipe to change physical
Oobleck, Gloop, and Glurch Lesson Overview Throughout this lesson, students will use inquiry skills to identify states of matter, describe physical properties, and modify the recipe to change physical
