EMBO. Reggie-1/flotillin-2 promotes secretion of the long-range signalling forms of Wingless and Hedgehog in Drosophila EMBO. open
|
|
|
- Rosalind Willis
- 6 years ago
- Views:
Transcription
1 (2008), 1 13 Some Rights Reserved /08 Reggie-1/flotillin-2 promotes secretion of the long-range signalling forms of Wingless and Hedgehog in Drosophila THE EMBO JOURNAL EMBO open This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits distribution, and reproduction in any medium, provided the original author and source are credited. This license does not permit commercial exploitation or the creation of derivative works without specific permission. Vladimir L Katanaev 1, *, Gonzalo P Solis 2, George Hausmann 3, Silke Buestorf 1, Natalya Katanayeva 1, Yvonne Schrock 2, Claudia AO Stuermer 2 and Konrad Basler 3 1 Department of Biology, TransRegio-SFB11, University of Konstanz, Konstanz, Germany, 2 Developmental Neurobiology, Department of Biology, University of Konstanz, Konstanz, Germany and 3 Institut für Molekularbiologie, Universität Zürich, Zürich, Switzerland The lipid-modified morphogens Wnt and Hedgehog diffuse poorly in isolation yet can spread over long distances in vivo, predicting existence of two distinct forms of these mophogens. The first is poorly mobile and activates short-range target genes. The second is specifically packed for efficient spreading to induce long-range targets. Subcellular mechanisms involved in the discriminative secretion of these two forms remain elusive. Wnt and Hedgehog can associate with membrane microdomains, but the function of this association was unknown. Here we show that a major protein component of membrane microdomains, reggie-1/flotillin-2, plays important roles in secretion and spreading of Wnt and Hedgehog in Drosophila. Reggie-1 loss-of-function results in reduced spreading of the morphogens, while its overexpression stimulates secretion of Wnt and Hedgehog and expands their diffusion. The resulting changes in the morphogen gradients differently affect the short- and long-range targets. In its action reggie-1 appears specific for Wnt and Hedgehog. These data suggest that reggie-1 is an important component of the Wnt and Hedgehog secretion pathway dedicated to formation of the mobile pool of these morphogens. advance online publication, 24 January 2008; doi: /sj.emboj Subject Categories: membranes & transport; development Keywords: hedgehog; morphogen; reggie/flotillin; secretion; wingless Introduction *Corresponding author. Department of Biology, TransRegio SFB11, University of Konstanz, Universitätstrasse 10, Box M643, Konstanz 78457, Germany. Tel.: þ ; Fax: þ ; vladimir.katanaev@uni-konstanz.de Received: 1 July 2007; accepted: 19 December 2007 Morphogens are molecules spreading from the region of production and inducing tissue patterning in a concentration-dependent manner (Lawrence, 2001; Lander, 2007). Different threshold levels of the morphogen concentrations exist, which through appropriate levels of receptor activation are translated into transcriptional induction of different target genes. Examples of morphogens include the families of Wnt, Hh (Hedgehog) and TGFbeta-secreted proteins, which can bear multiple post-translational modifications. Wnt proteins are doubly N-glycosylated (Tanaka et al, 2002) and bear palmitate (Willert et al, 2003) and palmitoylate (Takada et al, 2006) groups. Hh is palmitoylated and cholesterol-modified (Porter et al, 1996; Pepinsky et al, 1998). These modifications are necessary for the proper secretion and activity of the morphogens (Miura and Treisman, 2006), and require specific mechanisms for Wnt and Hh secretion and spreading. Multipass transmembrane proteins Wntless/Evi and Dispatched are indispensable for secretion of Wnt and Hh, respectively (Burke et al, 1999; Banziger et al, 2006; Bartscherer et al, 2006). Due to the hydrophobic nature of the modifications, secreted Wnt and Hh are not freely diffusible in their isolated form. In aqueous solutions, Wnt proteins aggregate and precipitate (Willert et al, 2003). In vivo, Wnt and Hh bind to the cell surface and extracellular matrix, which strongly reduces their diffusion properties (Papkoff and Schryver, 1990; Lee et al, 1994). How is effective long-range diffusion of Wnt and Hh achieved in vivo? It has been speculated that two routes for their secretion exist (Coudreuse and Korswagen, 2006; Hausmann et al, 2007). The first would involve a default secretion pathway, releasing monomeric or low-degree oligomeric forms of the morphogens, whose diffusion would be very limited. Accumulation of these forms close to the source of production turns on short-range target genes. The second route, poorly understood, is predicted to produce the morphogens specifically packed for efficient spreading permitting induction of the long-range targets. Multiple ways to pack morphogens for long-range diffusion may exist. In Drosophila, Wnt and Hh can associate with fat body-derived lipoprotein particles, promoting spreading and activation of long-range target genes (Panakova et al, 2005). In cell culture, mammalian Hh can form highly diffusive, biologically active aggregates of a high molecular weight (Zeng et al, 2001). It remains elusive what kind of intracellular mechanism can direct the morphogens into the long diffusion rangespecific secretion, as opposed to the default secretion route. Wnt and Hh can associate with the detergent-resistant membrane (DRM) microdomains (Rietveld et al, 1999; Zhai et al, 2004), which has been proposed important for Hh packing into high-order aggregates (Zeng et al, 2001); lipid modifications of Wnt and Hh are required for the DRM association. 1
2 Membrane microdomains are involved in a variety of cellular functions, for example, being platforms for specific signal transduction cascades, as well as for particular types of intracellular trafficking (Pike, 2004; Le Roy and Wrana, 2005). Different types of microdomains can be described by their major resident proteins, such as annexin, reggie/ flotillin, or caveolin (absent in Drosophila) (Le Roy and Wrana, 2005). In this article, we show that a major component of the membrane microdomains, reggie-1/flotillin-2 (Langhorst et al, 2005; Stuermer and Plattner, 2005), plays important roles in Wnt and Hh secretion in Drosophila. Overexpression of reggie-1 leads to increased secretion and enhanced spreading of these morphogens. Loss of function of reggie-1 results in the reciprocal reduction in the morphogen gradient. Our results suggest that reggie-1 is an integral part of the intracellular trafficking events directing Wnt and Hh morphogens along the secretion route destined for the long-range spreading. Results Overexpression of reggie-1 in Drosophila wing results in loss of a subset of Wnt-signalling responses Two reggie isoforms (reggie-1/flotillin-2 and reggie-2/flotillin- 1) exist in mammals and fruit flies (Hoehne et al, 2005; Langhorst et al, 2005). Co-overexpression of both isoforms in Drosophila results in massive morphological defects, whereas reggie-2 alone produces no phenotypes (Hoehne et al, 2005). To investigate whether reggies affect Wg (Wingless, Drosophila Wnt-1) signal transduction, we repeated the overexpression in a more controlled manner using the MS1096- Gal4, engrailed-gal4 (en-gal4), dpp-gal4 or tubulin-gal4 (tub-gal4) lines. As we obtained identical initial results by expression of reggie-1 alone or coexpression of reggie-1 þ 2, experiments presented in this paper describe expression of reggie-1 alone. Adult wings lost wing margin structures in the regions of reggie-1 overexpression (Figure 1B and C). Since such phenotypes can arise from defective Wg signalling, we analysed the expression of Wg target genes in wing imaginal discs of late third instar larvae. We found downregulation of the short-range Wg target genes Cut and Senseless (Sens) in the reggie-1 overexpression domain (Figure 1D and E; Figure 2C; Figure 1F shows wild-type Sens expression). Another readout for high Wg signalling is the zone of nonproliferating cells (ZNC) forming close to the source of Wg synthesis (Johnston and Edgar, 1998; Figure 1F). The ZNC was eliminated in the region of reggie-1 overexpression (Figure 1G). Similar effect can be obtained by expression of Axin (an inhibitor of Wg signalling) or cyclin E (which forces exit from the cell-cycle arrest; Johnston and Edgar, 1998). However, unlike Axin and reggie-1, cyclin E was unable to downregulate Sens (Supplementary Figure S1). Thus, analysis of responses to high levels of Wg signalling revealed that overexpression of reggie-1 reduced Wg signalling. Loss of Wg signalling can result in misexpression of the hinge marker Homothorax in the wing pouch (Azpiazu and Morata, 2000). Overexpression of reggie-1 reduced the size of the pouch (but not hinge) domain in an autonomous manner (Figure 1C; also compare Figure 2A and B). As Wg signalling controls growth (Day and Lawrence, 2000; Johnston and Gallant, 2002), this reduction in pouch size could be attributed to a defective Wg signalling. However, pouch Figure 1 Overexpression of reggie-1 leads to loss of a subset of Wg responses. (A) Wild-type adult wing. (B, C) Adult wings overexpressing reggie-1 by en-gal4 (B) or MS1096-Gal4 (C) lose wing margin (arrows); inserts show where these lines drive expression. Panel B also shows an Hh phenotype: broadening between veins 3 and 4 (yellow bar). (D H) Wing imaginal discs of late third instar larvae. Ventral is up, posterior is right. White arrows indicate the A/P border; en-gal4 expresses to the right from the arrows, marked by GFP. en-gal4-driven reggie-1 downregulates Wg short-range targets Cut (D 0 ) and Sens (E 0 ), but not the long-range target Dll (H 0 ). Wild-type Sens is shown in panel F. The ZNC visualized by the gap in BrdU staining (F 0 ) is autonomously eliminated by reggie-1 (G 0 ). 2
3 Figure 2 Overexpression of reggie-1 leads to enhanced spreading of Wg. (A) Wild-type wing disc expressing GFP in the posterior compartment showing normal Wg and Dll staining. (B D) Overexpression of reggie-1 by en-gal4 (B, D) or dpp-gal4 (C) results in broadening of the Wg diffusion domain (B 0,C 0 ), loss of Sens expression (C 00 ) and vast increase in the extracellular Wg staining (D 0 ). White parentheses in panel C mark dpp-gal4 expression zone. (B 000 ) shows a high-power magnification of panel B 0 ; the green line demarcates reggie-1 overexpression. Arrowheads mark Wg puncta appearing far from the production zone. expression of Homothorax was not induced by reggie-1 (Supplementary Figure S2). Furthermore, expression of a long-range Wg target Distalless (Dll) was not reduced by reggie-1 overexpression (Figure 1H), and in fact was often enhanced (Supplementary Figure S3; Figure 2A 000 shows wildtype Dll expression). Thus, only short-range Wg targets were downregulated by reggie-1. And among those, Sens in the reggie-1-overexpressing domain could be rescued in the vicinity of the wild-type region (Supplementary Figure S4). These observations suggested that reggie-1 was not affecting Wg signal transduction per se, but Wg gradient formation. Overexpression of reggie-1 expands the Wg diffusion gradient In wild-type discs, the majority of anti-wg staining is localized close to the stripe of Wg-producing cells, and the concentration of Wg rapidly decays away from the source of production (Figure 2A). Overexpression of reggie-1 by various drivers resulted in erosion of the Wg gradient (Figure 2B and C): Wg could be seen spreading far into the disc at the expense of reduced levels at the source of Wg production. High magnification revealed far-spreading punctate anti-wg staining (Figure 2B 000 ); the number of Wg puncta and their apparent size were also increased upon reggie-1 overexpression. We also found a dramatic autonomous increase in extracellular Wg in the reggie-1 overexpression domain (Figure 2D; Supplementary Figure S5). Wg puncta were not seen without cell permeabilization and thus likely reflected Wg endocytosed by the receiving cells. Thus, reggie-1 activated Wg secretion and spreading. Erosion of the Wg gradient resulted in reduction of the 3
4 short-range targets requiring high levels of Wg, whereas longrange targets were normal or even upregulated. Reggie-1 changes Wg properties in the Wg-producing and not -responding cells To analyse whether reggie-1 acted in the Wg-producing or -receiving cells, we restricted reggie-1 overexpression to the domain of Wg production by two independent means. First, we used the wg-gal4 line to overexpress reggie-1 only in the endogenous Wg-producing stripe. Remarkably, this reproduced many phenotypes of reggie-1 overexpression in a broader domain: notched adult wings, abnormal Sens expression and reduced wing pouch size (Figure 3G, J and K). Most importantly, Wg gradient also became abnormal: a more diffuse pattern of Wg was seen both looking at total and extracellular anti-wg staining (Figure 3H and L). Interestingly, the total anti-wg signal was eroded at the apical level (Figure 3C and I), but unaffected basolaterally (Supplementary Figure S6), indicating that reggie-1 stimulated the apical secretion of Wg, which is the normal way of Wg secretion in polarized cells (Simmonds et al, 2001; Pfeiffer et al, 2002). Second, we expressed Wg in somatic clones using the tub-gal4 line, either alone or together with reggie-1; in this way we could also exclude the possibility that reggie-1 was indirectly affecting Wg production. Expression of Wg alone in this manner resulted in ectopic activation of the short-range target Sens (Figure 3O). Further, it resulted in downregulation of the endogenous Wg production at the dorso-ventral border (Figure 3N), a phenomenon known to arise from strong overactivation of Wg signalling (Rulifson et al, 1996). Remarkably, coexpression of reggie-1 with Wg drastically reduced the capacity of this ectopic Wg to induce ectopic Sens or downregulate endogenous Wg (Figure 3S and T). In contrast, the long-range target Dll was upregulated in both conditions (Supplementary Figure S7). We also Figure 3 Overexpression of reggie-1 affects Wg-producing cells. (A F) Wild-type (wg-gal4) adult wing (A) and wing disc (B, D F). (G L) wg- Gal4; UAS-reggie-1 adult wing (G) and wing disc (H, J L). Reggie-1 overexpressed by wg-gal4 loses wing margin structures (red arrows in panel G), reduces Sens expression and wing pouch size (J, K) and broadens the Wg gradient (H) as compared with wild-type discs (B). Wg gradient is eroded at the apical level: panels C and I show representative pixel intensity scans of wild-type and UAS-reggie-1 discs; the dorso-ventral border is at position 25 mm. Panels F and L show extracellular anti-wg stainings oriented at 901 in relation with other panels. (M P) Multiple small tub- Gal4; UAS-Wg clones (marked by loss of GFP) induce a reduction in the endogenous Wg production (yellow arrow in panel N) and ectopic Sens induction (white arrows in panel O). (R U) Multiple small clones coexpressing Wg and reggie-1 produce more diffusive Wg (compare the diffuse anti-wg staining in panel S with localized staining in panel N), incapable to reduce endogenous Wg (S) or induce ectopic Sens (T). 4
5 observed enhanced Wg diffusion from the clones coexpressing Wg and reggie-1 (Figure 3). Thus, overexpression of reggie-1 changes the way Wgproducing cells release Wg. This change apparently involves higher secretion and produces a more mobile form of Wg, affecting gradient formation. Loss of reggie-1 reduces Wg spreading Reggie-1 loss-of-function flies are viable and fertile and do not show obvious morphological defects (Hoehne et al, 2005). This might be due to redundancy with other reggie-like proteins (Langhorst et al, 2005) or compensatory pathways. However, more acute loss of reggie-1 function by expression of a UAS-RNAi construct against reggie-1 by apterous- Gal4 (ap-gal4) or en-gal4 resulted in aberrant wings (Supplementary Figure S8). As reggie-1 overexpression broadened the Wg gradient, we expected the reciprocal phenotypes upon reggie-1 loss of function. Indeed, we found that the gradient of Wg protein, both total and extracellular, was shortened in reggie-1 / wing discs (Figure 4A D), but that wg transcription was unchanged (Figure 4E and F). We found a similar reduction in the Wg gradient when reggie-1 / somatic clones were induced and intersected the Wg production stripe (Figure 4L). High magnification revealed a strong decrease in the amount of Wg puncta emanating from the Wg-producing cells of the reggie-1 / clones (Figure 4L 000 ). Similar decrease in Wg diffusion and puncta formation could be seen by reggie-1- RNAi expression by en-gal4 (Figure 4M and N) and ap-gal4 (Supplementary Figure S9). Counting Wg puncta in the RNAiexpressing and wild-type halves of same discs revealed a twofold decrease in Wg puncta formation upon reggie-1 loss of function; the apparent size of Wg puncta was also reduced (Figure 4N and O). We next analysed expression of Wg target genes in reggie- 1 / discs. The domain of the long-range target Dll was narrowed approximately twofold and its intensity reduced in discs deficient for reggie-1 (Figure 4I and J; Supplementary Figure S10); quantification of eight reggie-1 / and wild-type discs stained together revealed a threefold decrease in the Dll expression levels in reggie-1 / (Figure 4K). A decrease in Dll expression was also seen when reggie-1 / clones were induced; this decrease could be non-autonomous resulting from reduced Wg spreading from the reggie-1 / tissue within the Wg-producing stripe (Figure 4L). Narrowing of the Dll expression domain was also seen in the region of reggie-1- RNAi expression (Supplementary Figure S9). In contrast, reggie-1 / discs had normal expression of the short-range target Sens (Figure 4G and H; Supplementary Figure S10); reggie-1 RNAi also did not prevent Sens expression (Supplementary Figure S9). As a result, wing margin structures were normal in reggie-1 / wings and wings expressing reggie-1-rnai (see Supplementary Figure S8). Interestingly, in discs homozygous mutant for reggie-1 and heterozygous for a mutation in the wg gene, a slight but significant narrowing of the zone of Sens expression could be seen (Supplementary Figure S11). Thus, reggie-1 is required for the proper Wg gradient formation. The range of Wg spreading and the number of Wg puncta is significantly decreased upon loss of reggie-1, resulting in a strong decrease in expression of the long-range Wg target. In contrast, short-range targets are not affected by loss of reggie-1, unless a concomitant reduction in Wg levels is performed. Reggie-1 increases Wg and Hh secretion in tissue culture and markedly stimulates Wg endocytosis by co-cultured cells We next switched to a cell culture to address molecular mechanisms of reggie-1 action. In Drosophila cell lines co-overexpressing reggie-1 and Wg, reggie-1 localized to the plasma membrane as well as to intracellular compartments; the Wg signal was mostly intracellular (Supplementary Figure S12). We could observe a limited colocalization of Wg and reggie-1, both intracellularly and at the plasma membrane (Supplementary Figure S12). This limited colocalization might reflect a specific portion of Wg secreted through a reggie-1-dependent pathway. However, by Western blots we did not see a significant increase in Wg secretion upon transfection of Wg-producing S2 cells with reggie-1 (Figure 5A, lanes 5 and 6). Wg secreted by cultured cells can stay attached to the cell membranes and is released by heparan sulphate treatment (Reichsman et al, 1996). Heparan extraction produced similar amounts of Wg from control and reggie-1-transfected cells (Figure 5A, lanes 3 and 4). Wg-signalling activity was also not changed by reggie-1 (Supplementary Figure S13). Western blot analysis is insufficiently sensitive to see small differences. Thus, we used a Wg-Renilla luciferase (Rluc) fusion protein to quantify secretion more accurately. We also prepared an Hh-Rluc fusion to monitor whether secretion of Hh might be affected by reggie-1. When we examined the ratio of Rluc activity in media to that in the cells, we observed a statistically significant enhancement of the morphogen secretion by S2R þ cells upon reggie-1 transfection (Figure 5B): Wg levels in the medium increased by approximately 25% and levels of Hh by approximately 50%. However, no significant effect on morphogen secretion was seen by treating the cells with dsrna against reggie-1 (8977 and 10379% of control for Wg and Hh, respectively), indicating that amounts of reggie-1 in S2R þ cells were too low or unimportant for the net morphogen secretion. In contrast, the dominant-negative reggie-1 construct (see Materials and methods) significantly decreased Wg secretion by approximately 20%, potentially affecting other proteins of the reggie-like family (Langhorst et al, 2005), but did not have a significant effect on Hh secretion (Figure 5B). In contrast to Wg and Hh, release of a secreted form of luciferase was not affected by reggie-1 transfection (Figure 5B), demonstrating that reggie-1 did not non-specifically stimulate exocytosis. We wondered whether the effects of reggie-1 on morphogen secretion could be masked by re-uptake by the cultured cells. Indeed, immunostaining Wg-secreting cells revealed higher intracellular Wg levels upon reggie-1 transfection (not shown). This could be explained by enhanced retention/re-uptake of Wg by the reggie-1-transfected cells. Alternatively, reggie-1 overexpression in the Wg-producing cell could change the properties of this Wg, such that it became more uptakable by surrounding cells regardless of their own reggie-1 levels. To discriminate between these possibilities, we separately transfected S2 cells with Wg plus reggie-1 (or Wg plus control) and DsRed or EGFP (for independent labelling) and 5
6 Figure 4 Loss of reggie-1 results in shortening of the Wg gradient and target gene expression. reggie-1-mutant discs show more narrow total (B) and extracellular (D) Wg gradient formation than wild-type discs (A, C) stained in parallel, but wg transcription (E, F) and Sens expression (G, H) are unchanged. In contrast, reggie-1 / discs have narrowed Dll expression domains (marked by dotted lines in I, J) and an overall threefold reduction in Dll expression levels (K, data shown as mean7s.e.m., n ¼ 8 discs); Student s t-test was used to determine statistical significance. (L) reggie-1 / somatic clones (marked by loss of GFP) intersecting the Wg-producing stripe reduce Wg spreading (L 00 ) and Dll expression (L 0 ); the number of Wg particles emanating from the reggie-1 / Wg-producing cells (demarcated by white lines) is reduced (L 000 ). (M O) Wg spreading and the number and size of Wg particles are reduced in the domain expressing reggie-1 RNAi (marked by GFP); (N, N 0 ) high-magnification of the wild-type and RNAi-expressing halves of same disc. Wg particles outside the Wg-producing stripe were counted separately in the wild-type and RNAi-expressing halves of identical size of the wing pouch (O); data are shown as mean7s.e.m., n ¼ 10 discs; paired t-test was used to determine statistical significance. 6
7 Figure 5 Cell culture experiments analysing the effects of reggie-1 on Wg and Hh secretion. (A) Effects of overexpression of reggie-1 on Wg secretion measured by Western blots. Serum albumin is shown as the loading control for the medium samples. (B) Effects of overexpression of reggie-1 or a reggie dominant-negative construct on secretion of Wg, Hh or secreted luciferase (sluc) measured by enzymatic assay. Bars represent means7s.e.m. from 12 experiments. P-values from Student s t-test are shown; ns means non-significant (P40.05). (C) GFP-Wg media from panel A were subjected to sucrose-density ultracentrifugation. Thirteen fractions were collected (numbers and sucrose density in alternating fractions are shown on top). (D, E) S2 cells transfected with Wg together with reggie-1 (D) or EGFP (E) were co-cultured with cells independently transfected with DsRed. Accumulation of the anti-wg signal in DsRed-cells is much higher if Wg was produced by reggie-1- transfected cells. (F, G) Pulse chase Texas-red dextran colocalization assays. S2 cells transfected with Wg together with reggie-1 (F) or empty vector (G) were co-cultured with cells independently transfected with EGFP. Accumulation of Wg in dextran-positive early endosomes is much higher if Wg was produced by reggie-1-transfected cells (arrowheads in panel F). Note that unlike in panels D and E where identical confocal settings were used to record anti-wg staining, the settings in panels F and G were independently optimized for highest resolution. (H) Reggie-1 does not stimulate Wg endocytosis cell-autonomously: S2 cells were transfected with Wg plus reggie-1-egfp and co-cultured with cells independently transfected with reggie-1-dsred. Incorporation of Wg into the Wg-receiving cells was the same whether they overexpressed reggie-1-dsred or not (white arrows). co-cultured these lines. We then analysed whether the cocultured cells could uptake Wg differently depending on its source. We found a dramatically higher uptake of Wg by DsRed cells when Wg was provided by the reggie-1-overexpressing cells (Figure 5D and E). To prove that this intracellular Wg staining resulted from endocytosis, we performed pulse chase assays with fluorescent dextran beads and found that Wg provided by reggie-1-expressing cells colocalized with dextran in early endocytic compartments (Figure 5F) whose particulate appearance was reminiscent of the punctate staining seen in wing discs. In contrast, Wg obtained from control-transfected cells was poorly endocytosed by the co-cultured cells (Figure 5G). We could also demonstrate that reggie-1 overexpression did not increase Wg uptake cell-autonomously: overexpression of reggie-1 in the cells co-cultured with Wg-producing cells did not increase Wg uptake by these co-cultured cells (Figure 5H). Thus, reggie-1 overexpression was changing the properties of Wg in the Wg-producing cells, such that this Wg became markedly more internalizable by the co-cultured cells. This conclusion agrees well with our in vivo observations. Such increased uptake of Wg might be solely due to enhanced secretion of Wg by reggie-1-overexpressing cells, or also due to a molecular change in the form Wg is secreted. For example, reggie-1 overexpression could enhance Wg packing into lipoprotein particles. To investigate this possibility, we performed sucrose-density ultracentrifugation of the Wg medium from reggie-1- or control-transfected cells (Figure 5C). We found that Wg had identical migration in sucrose gradients in both samples and peaked at the density of approximately 1.24 g/ml; identical results were obtained using GFP-Wg and non-tagged Wg. No signal was detected at the level of lipoprotein particles, which migrate close to the top of the gradient (Panakova et al, 2005), despite the fact that the cells were cultured in the presence of bovine serum which contains lipoprotein particles of various sorts (Chapman, 1986). However, it is possible that Drosophila Wg can principally not pack into serum lipoprotein particles. 7
8 The high density of the Wg signal probably reflects Wg multimers; it is heavier than exosomes, which have the density of approximately 1.13 g/ml (Fevrier and Raposo, 2004). Thus, in the cell culture we find an effect of reggie-1 on Wg and Hh secretion. Wg produced by reggie-1-transfected cells is more prone to uptake by surrounding cells; the resulting Wg accumulation in early endosomes is similar to the punctate Wg localization stimulated by reggie-1 in vivo. The nature of Wg produced by cultured cells is probably multimers, reminiscent of the form mammalian Hh is produced (Zeng et al, 2001). Hh diffusion and target gene expression is affected by reggie-1, whereas Dpp and GFP-GPI are not The cell culture experiments demonstrate that reggie-1 affects secretion of Wg and Hh, but not luciferase, whereas in vivo reggie-1 controls Wg secretion and spreading. We next investigated whether reggie-1 had a similar effect on the spreading of Hh, Dpp and GPI-linked GFP (Greco et al, 2001) in wing discs. Whereas Hh was affected by reggie-1 (see below), spreading properties of the Dpp morphogen or GFP-GPI were not changed upon reggie-1 overexpression (Supplementary Figures S14 and S15), arguing that reggie-1 was specifically acting on the Wg and Hh morphogens. Figure 6 Modulations in reggie-1 levels affect Hh secretion, gradient formation and target gene expression. (A, B) Wild-type (A) and ap-gal4; UAS-reggie-1 (B) discs stained for Ci, Ptc and Hh; ap-gal4 drives expression below the white dotted line. Overexpression of reggie-1 results in a dramatic enhancement of Hh spreading into the anterior domain (white arrow (B 00 )) and broadening of the Ptc expression (white arrows (B 0 )). High magnification (A 0000, B 0000 ) shows four anti-ci staining zones, from right to left: (1) no staining in the posterior compartment, (2) low staining representing Ci* (between the two green lines), (3) high staining of Ci-155 (between the green and blue lines) and (4) lower staining in the rest of the anterior representing Ci-75 (left from the blue line). Overexpression of reggie-1 strongly broadens the Ci* zone (B 0000 ). (C H) Wild-type (E, F), hh-gal4; UAS-reggie-1 (C, D) and reggie-1 / (G, H) discs stained for Ci (C, E, G), Col (C 0,E 0,G 0 ), and extracellular Hh (D, F, H). (D 0,F 0, H 0 ) are higher magnifications of panels D, F and H; the red arrows show the range of Hh diffusion into the anterior compartment (beginning of the arrows demarcates the A/P border). (C, C 00,E,E 00,G,G 00 ) White bars mark the Ci* staining; red bars in (C 0,E 0,G 0 ) mark Col expression. 8
9 Hh is produced by the posterior wing cells and infiltrates the anterior compartment. Cubitus interruptus (Ci) is a crucial transducer of Hh signalling and exists in multiple forms. Depending on the concentration of Hh, Ci*, Ci-155 and Ci-75 forms (listed in the order of high-to-low Hh signalling) can be identified (Methot and Basler, 1999; Figure 6A 0000 ). Short-range (like patched, Ptc) and long-range (like dpp) Hh target genes exist, as well as intermediate targets like collier (Col) whose expression overlaps with Ptc and dpp (Vervoort et al, 1999). We used staining for Hh target genes and the signalling intermediate Ci to monitor Hh signalling levels in response to reggie-1 overexpression or loss of function. We first overexpressed reggie-1 with ap-gal4, which allowed us to compare the reggie-1-overexpressing dorsal half with the wild-type ventral half within same discs. We found dramatic Hh phenotypes: massive infiltration of Hh into the dorsal anterior domain (Figure 6B 00 ), and as a result strong thickening of the high-hh response region expressing Ptc and Ci* (Figure 6B 0 and B 0000 ). The zone of Ci-155 was shifted to the left from the A/P border, but not broadened (compare Figure 6A 0000 and B 0000 ). To narrow the effects of reggie-1 to the Hh-producing cells, we overexpressed reggie-1 using hh-gal4 and found similar effects: the domain of Ci* was expanded, as well as the zone of Col expression (Figure 6C and E). In adult wings, the outcome was the broadening of the region between veins 3 and 4 (shown in Figure 1B for overexpression of reggie-1 by en-gal4, another posterior expression line). Reggie-1 also induced a dramatic upregulation of extracellular Hh (Figure 6F and D) and enhanced spreading of extracellular Hh into the anterior domain (Figure 6D 0 and F 0 ). Thus, similar to our findings concerning the Wg gradient formation, we find that overexpression of reggie-1 increases Hh secretion and spreading, changing the pattern of expression of Hh targets. We next investigated Hh diffusion and signalling in reggie-1 loss-of-function. There was a slight reduction in the extracellular Hh in reggie-1 / (Figure 6H) as compared with wild-type discs (Figure 6F) stained in parallel. The A/P border, normally showing a diffuse pattern of extracellular Hh reflecting the diffusion of Hh into the anterior domain (Figure 6F and F 0 ), became considerably sharper in reggie-1 / wing discs (Figure 6H and H 0 ). Thus, spreading properties of Hh produced by the reggie-1 / tissue were reduced. Consequently, the zone of highest Hh signalling represented by formation of Ci* was narrowed (Figure 6G and G 00 ). Col was not significantly affected (Figure 6G 0 ). Reggie-1 can affect either short-range or long-range morphogen targets depending on the amount of the morphogen Morphogen gradient formation can be modelled by a diffusion equation pffiffi v w CðxÞ ¼ pffiffiffiffiffiffiffiffiffi e x kd ð1þ 2a 2 D k where C(x) is morphogen concentration at distance x from the production zone, v is effective morphogen secretion rate, w is width of the production zone, a is cell diameter, k is degradation rate and D is effective morphogen diffusion coefficient (Kicheva et al, 2007; Lander, 2007). The wildtype Wg gradient in Drosophila wing disc can be modelled using the experimentally determined parameters (Kicheva et al, 2007); the threshold Wg concentrations for shortand long-range targets can be roughly set based on the known sizes of Sens and Dll expression domains (Figure 7A and B). We can now simulate the effects of reggie-1 on Wg by varying the diffusion coefficient D: a fivefold decrease in D could faithfully model the Wg phenotypes of reggie-1 loss of function, whereas a fivefold increase in D could simulate the Wg phenotypes of reggie-1 overexpression (Figure 7A and B). Our experiments clearly show an important role of reggie-1 for formation of the Wg and Hh gradients. However, the functional consequences of changes in reggie-1 levels differed between the two morphogens. For example, overexpression of reggie-1 led to reduction of the Wg short-range targets but expanded the Hh short-range targets. We hypothesized that some of these differences were due to different production levels of Wg and Hh: Wg is produced by a narrow stripe of cells along the dorso-ventral boundary, whereas Hh is produced by the whole posterior half of the disc. Indeed, modelling predicts that broadening of the Wg production zone threefold, with a subsequent fivefold increase in the diffusion coefficient D, will result not in a reduction, but broadening of the short-range targets (Figure 7C); a reciprocal narrowing of the Hh production zone with a concomitant increase of the diffusion coefficient predicts a Wg-like behaviour and reduction in the short-range targets. To test these predictions, we induced Wg production in the broad zone of the dpp-gal4 line. This massive misexpression of Wg induced ectopic Sens (Figure 7D) and Dll expression (not shown). We next co-overexpressed reggie-1 and Wg by dpp-gal4. As expected from the earlier experiments, this resulted in broader diffusion of the ectopic Wg (Figure 7E); further, a more punctate Wg staining was seen (Figure 7E 00 and D 00 ). As predicted, this broadly produced Wg co-overexpressed with reggie-1 was now fully capable of inducing the short-range target Sens, in a domain broader than when induced by Wg alone (Figure 7E 0 and D 0 ). Thus, provided that Wg is in excess, its enhanced mobility does not lead to loss of the short-range targets. In the case of Hh, we performed the reciprocal experiment reducing Hh amounts. Previous overexpression of reggie-1 by hh-gal4 was performed at 171C and diminished the Hh-producing domain (see Figure 6C). At 251C reggie-1 overexpression was higher and the posterior domain became even smaller (Figure 7F). Hh spread deeper into the anterior compartment of such discs (Figure 7G and H). Importantly, we found that expression of the intermediate target Col was dramatically narrowed in hh-gal4; UAS-reggie- 1 discs grown at 251C, while the domain of the long-range target Ci-155 was significantly broadened (Figure 7F 0 and F 00 ). These changes in expression of short-range versus long-range targets are very similar to what we see when endogenous Wg targets are analysed upon overexpression of reggie-1 (see Figure 1). Thus, the differential activities of reggie-1 on short-range versus long-range targets of Wg and Hh are determined by the properties of these morphogens, such as the broadness of their production zone. Reggie-1 controls spreading efficiency, but not formation of a particular signalling form of the morphogens, which would be able to induce only longrange targets and not short-range targets. 9
10 Figure 7 Modulations in reggie-1 levels differently affect short-range and long-range targets of Wg and Hh depending on the morphogen production quantities. (A C) Modelling Wg gradients using Equation 1. The parameters were as follows: v ¼ 18.7 molecules/s cell; w ¼ 6 mm; a ¼ 3 mm; D ¼ 0.05 mm 2 /s and k ¼ s 1 ( wild-type gradient in panels A and B). D was 0.01 mm 2 /s in gradient in reggie-1 / (A) and 0.25 mm 2 /s in gradient in UAS-reggie-1 (B, C); w was 18 mm in broad production zone gradients (C). Yellow and blue arrows at the y-axis indicate the arbitrarily set [Wg] threshold levels for the expression of the short- and long-range targets respectively. The zone of expression of these targets is indicated by yellow and blue rectangles. Changes in the broadness of the target expression domains in panels A and B are indicated by grey arrows. (D, E) Ectopic Wg expressed by dpp-gal4 (marked by UAS-GFP) together with UAS-reggie-1 spreads further away from the production zone (E), shows a more punctate staining (E 00 ) and induces ectopic Sens, and is a broader domain (E 0 ) than ectopic Wg expressed without reggie-1 (D D 00 ). GFP staining in (D 0,E 0 ) is in blue pseudocolour for a better visualization. (F) Expression of reggie-1 by hh-gal4 at 251C strongly reduces the size of posterior compartment and changes Hh responses: Hh now induces narrow Col (arrows in F 00 ) but broad Ci-155 (brackets in (F 0 )) zones. (G, H) High magnification shows enhanced diffusion of Hh into the anterior compartment upon reggie-1 overexpression (G) as compared with wild-type discs (H). The A/P border is shown with a thick yellow line and the range of Hh infiltration is marked with a thin yellow line. Discussion With their dual lipid modifications, Wnt and Hh morphogens would be predicted to travel poorly through the extracellular space. Yet they are able to act over long distances, suggesting that specific mechanisms facilitating their spreading must exist, as well as alternative secretion pathways in the morphogen-producing cells, resulting in release of the poorly diffusive and the more mobile pools of the morphogens (Panakova et al, 2005; Coudreuse and Korswagen, 2006; Hausmann et al, 2007). However, it is unclear how morphogen secretion can be directed for one or the other of the alternative secretion routes. Here we show that reggie-1/flotillin-2 plays an important role in the morphogen-producing cells, promoting secretion and long-range spreading of Wg and Hh in Drosophila wing disc and cell culture. Reggie-1 appears to be specific for these lipid-modified morphogens, as it neither affected spreading of Dpp and GPI-linked GFP in wing discs, nor secretion of an unrelated protein (luciferase) in cultured cells. The net secretion of Wg and Hh can be stimulated by reggie-1 in vivo and in cell culture, whereas changes in the morphogen gradient shapes cannot be explained solely by changes in the morphogen secretion rates. For example, overexpression of reggie-1 erodes the Wg gradient, resulting in a loss of the short-range but not long-range targets, whereas loss of reggie-1 makes the gradient steeper, narrowing the domain of expression of the long-range targets, but not affecting the short-range target genes. From Equation 1 it becomes clear that an increase in the secretion rate can only shift the gradient curve on Figure 7B to the right, to the same extent as broadening of the morphogen production zone achieves (Figure 7C), but cannot erode the gradient. Modelling shows that only an increase in the diffusion coefficient D can erode the gradient such that short-range targets are lost whereas long-range are not, and only a 10
11 decrease in D can make the gradient steeper such that the long-range targets are narrowed whereas the short-range are unaffected (Figure 7A and B). Thus, reggie-1 does change the way Wg and Hh morphogens are released, promoting their packing for the long-range spreading. The Wg phenotypes we observe in discs can be modelled in silico by changing the effective diffusion coefficient D fivefold (up for reggie-1 overexpression and down for loss-of-function), giving an idea how significantly reggie-1 changes spreading properties of the morphogens. We predict that reggie-1 stands on one of the two alternative secretion pathways, and thus overexpression of reggie-1 also results in enhanced net secretion. Thus, reggie-1 becomes the first protein identified, which is specifically required within the morphogen-producing cell for secretion of the long-range-spreading forms of Wg and Hh. Both in vivo and in vitro, reggie-1 affects the number and intensity of intracellular Wg puncta, which in the cell culture colocalize with dextran beads and can be identified as endosomes. Enhanced endocytosis would be expected to impede morphogen spreading through the tissue, rather than promote it. However, we think that the enhanced endocytosis of Wg we see upon reggie-1 overexpression (or reduced upon reggie-1 loss-of-function), is a secondary effect of the reggie-1 action on morphogen secretion and spreading. Indeed, increased secretion of Wg by the producing cells should already be sufficient to see more Wg endocytosed by the Wg-receiving cells. Moreover, enhanced spreading of the morphogen through the tissue due to increased mobility would allow detect Wg-containing endosomes in Wg-receiving cells further away from the source of production. Thus, the number and brightness of the Wg-containing intracellular puncta is rather a consequence and readout of Wg secretion and spreading under the control of reggie-1. It is not clear whether reggie-1 promotes packing of Wg and Hh into lipoprotein particles (Panakova et al, 2005) or high-order multimers (Zeng et al, 2001), or into another yet unidentified form promoting efficient spreading. Our experiments in vitro suggest that Wg is multimerized by cultured cells. In wing discs, loss of reggie-1 produces stronger phenotypes on Wg long-range target activation than the previously reported loss of lipoprotein particles: a threefold reduction in Dll expression is observed in reggie-1 / discs (Figure 4K), compared with a slight decrease in the lipoprotein-rnai animals (Panakova et al, 2005). However, additional experiments are required to determine whether the S2 cells-derived Wg could associate with lipoprotein particles of Drosophila (rather than serum) origin, and whether in vivo effects of reggie-1 are lipoprotein-dependent or not. It is also possible that the reggie-dependent pathway is redundant with another way of long-range morphogen spreading, and removal of both might produce additive effects. It is also possible that the reggie-dependent pathway may utilize additional reggie-related proteins. Reggie-2 is unstable in the absence of reggie-1 (Hoehne et al, 2005; Solis et al, 2007) and reggie-2 overexpression or loss-of-function produces no phenotypes ((Hoehne et al, 2005) and this work). However, a certain redundancy with other members of the SPFH (Stomatin/Prohibitin/Flotilin/HflK/C) protein family (Tavernarakis et al, 1999) might be expected (Langhorst et al, 2005). Eleven Drosophila genes, including reggie-1 and -2, are listed in InterPro as encoding proteins with the SPFH domain (also known as Band 7 domain); most of these genes are poorly studied and described in the Flybase as components of the cytoskeleton based on electronic annotation. Reggie-1 and possibly other members of the SPFH family function as protein platforms for organizing specific types of membrane microdomains (lipid rafts) (Langhorst et al, 2005; Stuermer and Plattner, 2005). Based on our observations, we can speculate that association of Wg and Hh with reggie-1- based membrane microdomains is crucial for the efficient formation of the mobile forms of these morphogens. We could observe partial colocalization of Wg and reggie-1 in cultured cells, but the exact identification of the intracellular compartment(s), where reggie-1-dependent secretion of Wg and Hh occurs, requires additional investigation. In conclusion, we have uncovered an important function of the membrane microdomain-scaffolding protein reggie-1 in secretion and gradient formation of the lipid-modified Wg and Hh morphogens in Drosophila. Reggie-1 is the first protein identified, which is specifically required in cis for the long-range-directed spreading of these morphogens. This illustrates that in the morphogen-producing cell, multiple secretion pathways and ways of morphogen packing exist, allowing proper activation of the short- and long-range target genes. Materials and methods Genetics The Drosophila lines used are as follows: UAS-reggie-1, UAS-reggie-2 and reggie-1 / (Flo-2 KG00210 ) (Hoehne et al, 2005); en-gal4, UAS- GFP-GPI (Greco et al, 2001); hh-gal4 (Tanimoto et al, 2000); wg-gal4 (ND382) (Gerlitz et al, 2002); tub4y þ GFP4Gal4 (gift from G Struhl); UAS-ArmDRGS (Willert et al, 1999); UAS-cyclinE (Johnston and Edgar, 1998); UAS-GFP-Dpp (Entchev et al, 2000); UAS-RNAireggie-1 and UAS-RNAi-reggie-2 (Dietzl et al, 2007). MS1096-Gal4; ap-gal4; dpp-gal4; wg-lacz (wg en11 ) were from Bloomington Stock Center. Mitotic clones were induced by the Flp-mediated recombination (Golic and Lindquist, 1989) between reggie-1, frt19a and ubi-gfp, frt19a chromosomes using hsp70-flp (Struhl and Basler, 1993). Heat-shock (371C) was applied for 1 h, h after egg laying for reggie-1 / clones and for 30 min, h after egg laying for tub4gal4 clones. Flies were kept at 251C; crosses for Figure 6 were performed at 171C. Histology Wing discs from late third instar larvae were fixed in 3.7% formaline, permeabilized in 0.5% NP-40 and immunostained in 0.2% Tween 20 in PBS, followed by confocal microscopy. For extracellular staining, permabilization was omitted; discs were incubated 30 min on ice with a triple antibody concentration before fixation. BrdU staining was performed as described in Johnston and Edgar (1998). Antibodies used are as follows: guinea pig anti-sens 1:1000 (Nolo et al, 2000) and anti-hth 1:1000 (Abu-Shaar et al, 1999); rat anti- BrdU 1:200 (Serotec) and anti-ci 1:20 (Motzny and Holmgren, 1995); rabbit anti-dll 1:100 (gift from R Mann), anti-reggie-1 (R722) 1:100 (Hoehne et al, 2005) and anti-hh (NHhI) 1:1000 (Takei et al, 2004); mouse anti-b-gal 1:100 (Promega) and anti-col 1:50 (gift from A Vincent and M Crozatier). Mouse antibodies against Cut (1:30), Wg (1:50) and Ptc (1:10) were from DSHB. FITC-, Cy3- and Cy5-labeled (Jackson ImmunoResearch) and Alexa Fluor s 405 (Invitrogen) secondary antibodies were used. reggie-1 / and en-gal4, UAS-GFP larvae were mixed and their discs stained together for precise comparison of wild-type and reggie-1 / phenotypes; the same procedure was followed for UASreggie-1 and wild-type discs; GFP and anti-reggie-1 were used to distinguish the genotypes. 11
12 Molecular cloning The following constructs were cloned into pac5.1/v5-his (Invitrogen): reggie-1 EGFP: RT PCR product (primers CGAATTCATGGGCAA CATACACACGACGGGTCCC, CACCGGTGCCTTGGCACCCGGTATC TTGGACAGA) from total S2 cell RNA was cloned into pcrii- TOPO and then pegfp-n1; reggie-1 untagged cloned directly from pcrii-topo above; reggie-1-dominant negative EGFP: was created analogously to the one in rat (Langhorst et al, 2006) and included aa ; EGFP and DsRed-monomer cdnas (BD Biosciences); reggie-1-dsred-monomer fusion; Rluc fusion: was generated replacing the GFP-coding sequence of Wg-GFP with the Rluc coding sequence; a secreted form of luciferase (sluc): was generated by fusing the signal peptide of haemagglutinin (MAIIYLILLFTAVRG) to the firefly luciferase coding sequence. dsrna targeting the coding sequence reggie-1 was generated using the Ambion Megascript kit and primers containing a T7 polymerase-binding site plus the following sequence-specific primer sequences: ACGCTTACAGTAGAAGAGG, CTTGGCCTGCGCAAGG GTCTGG; CAACGATGTGACTCGCTTGG, GAGTACATTTAGCTACACG TTCG; CAAGAAGCGCACGATTGTGG, GTATCTTGGACAGAACCTT GG. dsrna treatment was performed as in Worby et al (2001) and induced loss of anti-reggie-1 staining in reggie-1-transfected cells. Cell culture S2, S2R þ, S2-GFP-Wg (Piddini et al, 2005) and Kc167 cells were grown at room temperature in Schneider s medium with 10% foetal bovine serum, 1% glutamine and 1% penicillin streptomycin, and transfected using CellFECTIN (all from Invitrogen). S2-GFP-Wg cells were transfected with reggie-1 EGFP or EGFP for 24 h, washed 3 PBS and the expression of GFP-Wg was induced by 0.5 mm CuSO 4 for 24 h. For heparan extraction, the cells were washed 3 with PBS and treated with 10 mg/ml of each heparan and chondroitin sulphate A (Sigma) in serum-free medium for 2 h. For sucrose-density ultracentrifugation, 200 ml of media were overlaid on top of 150 ml of 25% sucrose overlaid on top of 45% sucrose over 80% sucrose (all in PBS), and centrifugation was performed at g 48 h 41C in a swing-out rotor. 50-ml volume fractions were collected and sucrose density was determined by refractometry. Western blot was performed using mouse anti-wg (DSHB) at 1:500. For the activity assays 48 h post-transfection, cells in the conditioned medium were mixed with S2 cell line stably transfected with DFz2, LEF and a LEF-luciferase reporter (Schweizer and Varmus, 2003), and cultured for additional 24 h before harvesting and measuring luciferase values using the Dual Luciferase Assay System (Promega). For Hh secretion assays, the UAS-Hh-Rluc construct was cotransfected with Ac-Gal4 (Ma et al, 2002). The control sluc construct was cotransfected with the Wg- or Hh-Rluc fusions. The levels of Rluc and sluc in the supernatant and cell lysates at 48 h were measured. The sluc/rluc ratios were determined to normalize for transfection efficiency. For studies of Wg uptake, S2 cells were separately cotransfected with Wg plus reggie-1-egfp or Wg plus EGFP, and another cell population was transfected with DsRed. Three hours post-transfection, the cells were washed 3 with PBS, co-cultured for 24 h and prepared for immunofluorescence. For endocytosis assays following Entchev et al (2000), S2 cells were separately cotransfected with Wg plus reggie-1 or Wg plus empty vector, and another cell population was transfected with EGFP. Three hours post-transfection, the cells were washed 3 with PBS and co-cultured for 24 h. Then, cells were incubated on ice with 0.5 mm Texas-red dextran (lysine fixable, M r 3000; Molecular Probes) in the medium for 10 min (pulse) and washed 3 with cold PBS. This was followed by immediate fixation or incubation with fresh medium at 261C for 20 min (chase) to visualize early endocytic compartments with subsequent fixation. For colocalization studies, S2R þ cells were cotransfected with Wg and reggie-1-egfp for 24 h, adhered to Alcian blue-coated coverslips, fixed and immunostained. Supplementary data Supplementary data are available at Online ( Acknowledgements We thank the researchers listed in the Materials and methods section for sharing fly strains, antibodies and cell lines. The work was supported by the Deutsche Forschungsgemeinschaft (SFB-TR11) to V.L.K. and C.A.O.S., and Swiss National Science Foundation to K.B. References Abu-Shaar M, Ryoo HD, Mann RS (1999) Control of the nuclear localization of Extradenticle by competing nuclear import and export signals. Genes Dev 13: Azpiazu N, Morata G (2000) Function and regulation of homothorax in the wing imaginal disc of Drosophila. Development 127: Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K (2006) Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell 125: Bartscherer K, Pelte N, Ingelfinger D, Boutros M (2006) Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell 125: Burke R, Nellen D, Bellotto M, Hafen E, Senti KA, Dickson BJ, Basler K (1999) Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell 99: Chapman MJ (1986) Comparative analysis of mammalian plasma lipoproteins. Methods Enzymol 128: Coudreuse D, Korswagen HC (2006) The making of Wnt: new insights into Wnt maturation, sorting and secretion. Development 134: 3 12 Day SJ, Lawrence PA (2000) Measuring dimensions: the regulation of size and shape. Development 127: Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ (2007) A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: Entchev EV, Schwabedissen A, Gonzalez-Gaitan M (2000) Gradient formation of the TGF-beta homolog Dpp. Cell 103: Fevrier B, Raposo G (2004) Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol 16: Gerlitz O, Nellen D, Ottiger M, Basler K (2002) A screen for genes expressed in Drosophila imaginal discs. Int J Dev Biol 46: Golic KG, Lindquist S (1989) The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell 59: Greco V, Hannus M, Eaton S (2001) Argosomes: a potential vehicle for the spread of morphogens through epithelia. Cell 106: Hausmann G, Banziger C, Basler K (2007) Helping Wingless take flight: how WNT proteins are secreted. Nat Rev Mol Cell Biol 8: Hoehne M, de Couet HG, Stuermer CA, Fischbach KF (2005) Lossand gain-of-function analysis of the lipid raft proteins reggie/ flotillin in Drosophila: they are posttranslationally regulated, and misexpression interferes with wing and eye development. Mol Cell Neurosci 30: Johnston LA, Edgar BA (1998) Wingless and Notch regulate cellcycle arrest in the developing Drosophila wing. Nature 394: Johnston LA, Gallant P (2002) Control of growth and organ size in Drosophila. Bioessays 24:
purpose of this Chapter is to highlight some problems that will likely provide new
 119 Chapter 6 Future Directions Besides our contributions discussed in previous chapters to the problem of developmental pattern formation, this work has also brought new questions that remain unanswered.
119 Chapter 6 Future Directions Besides our contributions discussed in previous chapters to the problem of developmental pattern formation, this work has also brought new questions that remain unanswered.
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
Segment boundary formation in Drosophila embryos
 Segment boundary formation in Drosophila embryos Development 130, August 2003 Camilla W. Larsen, Elizabeth Hirst, Cyrille Alexandre and Jean Paul Vincent 1. Introduction: - Segment boundary formation:
Segment boundary formation in Drosophila embryos Development 130, August 2003 Camilla W. Larsen, Elizabeth Hirst, Cyrille Alexandre and Jean Paul Vincent 1. Introduction: - Segment boundary formation:
Pattern formation: Wingless on the move Robert Howes and Sarah Bray
 R222 Dispatch Pattern formation: Wingless on the move Robert Howes and Sarah Bray Wingless is a key morphogen in Drosophila. Although it is evident that Wingless acts at a distance from its site of synthesis,
R222 Dispatch Pattern formation: Wingless on the move Robert Howes and Sarah Bray Wingless is a key morphogen in Drosophila. Although it is evident that Wingless acts at a distance from its site of synthesis,
Chapter 4 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays.
 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Supplementary Information
 Supplementary Information Supplementary Figure 1. JAK/STAT in early wing development (a-f) Wing primordia of second instar larvae of the indicated genotypes labeled to visualize expression of upd mrna
Supplementary Information Supplementary Figure 1. JAK/STAT in early wing development (a-f) Wing primordia of second instar larvae of the indicated genotypes labeled to visualize expression of upd mrna
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
Endocytosis of Hedgehog through Dispatched Regulates Long-Range Signaling
 Article Endocytosis of Hedgehog through Dispatched Regulates Long-Range Signaling Graphical Abstract Authors Gisela D Angelo, Tamás Matusek, Sandrine Pizette, Pascal P. Thérond Correspondence dangelo@unice.fr
Article Endocytosis of Hedgehog through Dispatched Regulates Long-Range Signaling Graphical Abstract Authors Gisela D Angelo, Tamás Matusek, Sandrine Pizette, Pascal P. Thérond Correspondence dangelo@unice.fr
Midterm 1. Average score: 74.4 Median score: 77
 Midterm 1 Average score: 74.4 Median score: 77 NAME: TA (circle one) Jody Westbrook or Jessica Piel Section (circle one) Tue Wed Thur MCB 141 First Midterm Feb. 21, 2008 Only answer 4 of these 5 problems.
Midterm 1 Average score: 74.4 Median score: 77 NAME: TA (circle one) Jody Westbrook or Jessica Piel Section (circle one) Tue Wed Thur MCB 141 First Midterm Feb. 21, 2008 Only answer 4 of these 5 problems.
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins
 13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
Nature Neuroscience: doi: /nn.2662
 Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
Formation of the Long Range Dpp Morphogen Gradient
 Gerald Schwank 1, Sascha Dalessi 2,3, Schu-Fee Yang 1, Ryohei Yagi 1, Aitana Morton de Lachapelle 2,3, Markus Affolter 4, Sven Bergmann 2,3, Konrad Basler 1 * 1 Institute of Molecular Life Sciences, University
Gerald Schwank 1, Sascha Dalessi 2,3, Schu-Fee Yang 1, Ryohei Yagi 1, Aitana Morton de Lachapelle 2,3, Markus Affolter 4, Sven Bergmann 2,3, Konrad Basler 1 * 1 Institute of Molecular Life Sciences, University
Supplementary Materials for
 www.sciencemag.org/cgi/content/full/science.1244624/dc1 Supplementary Materials for Cytoneme-Mediated Contact-Dependent Transport of the Drosophila Decapentaplegic Signaling Protein Sougata Roy, Hai Huang,
www.sciencemag.org/cgi/content/full/science.1244624/dc1 Supplementary Materials for Cytoneme-Mediated Contact-Dependent Transport of the Drosophila Decapentaplegic Signaling Protein Sougata Roy, Hai Huang,
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles
 Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Establishing positional information through gradient dynamics
 Extra view Fly 4:4, 273-277; October/November/December 2010; 2010 Landes Bioscience Extra view Establishing positional information through gradient dynamics A lesson from the Hedgehog signaling pathway
Extra view Fly 4:4, 273-277; October/November/December 2010; 2010 Landes Bioscience Extra view Establishing positional information through gradient dynamics A lesson from the Hedgehog signaling pathway
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila
 Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
Chapter 11. Development: Differentiation and Determination
 KAP Biology Dept Kenyon College Differential gene expression and development Mechanisms of cellular determination Induction Pattern formation Chapter 11. Development: Differentiation and Determination
KAP Biology Dept Kenyon College Differential gene expression and development Mechanisms of cellular determination Induction Pattern formation Chapter 11. Development: Differentiation and Determination
Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice Supplementary Figure 2.
 Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice. Percentage of marginal zone B cells in the spleen of wild-type mice (+/+), mice homozygous for cpm or pri
Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice. Percentage of marginal zone B cells in the spleen of wild-type mice (+/+), mice homozygous for cpm or pri
Developmental genetics: finding the genes that regulate development
 Developmental Biology BY1101 P. Murphy Lecture 9 Developmental genetics: finding the genes that regulate development Introduction The application of genetic analysis and DNA technology to the study of
Developmental Biology BY1101 P. Murphy Lecture 9 Developmental genetics: finding the genes that regulate development Introduction The application of genetic analysis and DNA technology to the study of
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2215 Figure S1 Number of egfp-vps4a bursts versus cellular expression levels. The total number of egfp-vps4a bursts, counted at the end of each movie (frame 2000, after 1h 28 min) are plotted
DOI: 10.1038/ncb2215 Figure S1 Number of egfp-vps4a bursts versus cellular expression levels. The total number of egfp-vps4a bursts, counted at the end of each movie (frame 2000, after 1h 28 min) are plotted
Morphogens in biological development: Drosophila example
 LSM5194 Morphogens in biological development: Drosophila example Lecture 29 The concept of morphogen gradients The concept of morphogens was proposed by L. Wolpert as a part of the positional information
LSM5194 Morphogens in biological development: Drosophila example Lecture 29 The concept of morphogen gradients The concept of morphogens was proposed by L. Wolpert as a part of the positional information
THE Hedgehog (Hh) signaling pathway regulates
 Copyright Ó 2006 by the Genetics Society of America DOI: 10.1534/genetics.106.061036 The Contributions of Protein Kinase A and Smoothened Phosphorylation to Hedgehog Signal Transduction in Drosophila melanogaster
Copyright Ó 2006 by the Genetics Society of America DOI: 10.1534/genetics.106.061036 The Contributions of Protein Kinase A and Smoothened Phosphorylation to Hedgehog Signal Transduction in Drosophila melanogaster
Shavenbaby Couples Patterning to Epidermal Cell Shape Control. Chanut-Delalande H, Fernandes I, Roch F, Payre F, Plaza S (2006) PLoS Biol 4(9): e290
 Shavenbaby Couples Patterning to Epidermal Cell Shape Control. Chanut-Delalande H, Fernandes I, Roch F, Payre F, Plaza S (2006) PLoS Biol 4(9): e290 Question (from Introduction): How does svb control the
Shavenbaby Couples Patterning to Epidermal Cell Shape Control. Chanut-Delalande H, Fernandes I, Roch F, Payre F, Plaza S (2006) PLoS Biol 4(9): e290 Question (from Introduction): How does svb control the
Drosophila Life Cycle
 Drosophila Life Cycle 1 Early Drosophila Cleavage Nuclei migrate to periphery after 10 nuclear divisions. Cellularization occurs when plasma membrane folds in to divide nuclei into cells. Drosophila Superficial
Drosophila Life Cycle 1 Early Drosophila Cleavage Nuclei migrate to periphery after 10 nuclear divisions. Cellularization occurs when plasma membrane folds in to divide nuclei into cells. Drosophila Superficial
AP Biology Gene Regulation and Development Review
 AP Biology Gene Regulation and Development Review 1. What does the regulatory gene code for? 2. Is the repressor by default active/inactive? 3. What changes the repressor activity? 4. What does repressor
AP Biology Gene Regulation and Development Review 1. What does the regulatory gene code for? 2. Is the repressor by default active/inactive? 3. What changes the repressor activity? 4. What does repressor
7.06 Problem Set #4, Spring 2005
 7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
INVESTIGATING THE ROLE OF WNTLESS (WLS) IN WINGLESS (WG) GRADIENT FORMATION. Babak Basiri
 INVESTIGATING THE ROLE OF WNTLESS (WLS) IN WINGLESS (WG) GRADIENT FORMATION by Babak Basiri A thesis submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements for
INVESTIGATING THE ROLE OF WNTLESS (WLS) IN WINGLESS (WG) GRADIENT FORMATION by Babak Basiri A thesis submitted to the Faculty of the University of Delaware in partial fulfillment of the requirements for
Why Flies? stages of embryogenesis. The Fly in History
 The Fly in History 1859 Darwin 1866 Mendel c. 1890 Driesch, Roux (experimental embryology) 1900 rediscovery of Mendel (birth of genetics) 1910 first mutant (white) (Morgan) 1913 first genetic map (Sturtevant
The Fly in History 1859 Darwin 1866 Mendel c. 1890 Driesch, Roux (experimental embryology) 1900 rediscovery of Mendel (birth of genetics) 1910 first mutant (white) (Morgan) 1913 first genetic map (Sturtevant
Development of Drosophila
 Development of Drosophila Hand-out CBT Chapter 2 Wolpert, 5 th edition March 2018 Introduction 6. Introduction Drosophila melanogaster, the fruit fly, is found in all warm countries. In cooler regions,
Development of Drosophila Hand-out CBT Chapter 2 Wolpert, 5 th edition March 2018 Introduction 6. Introduction Drosophila melanogaster, the fruit fly, is found in all warm countries. In cooler regions,
BILD7: Problem Set. 2. What did Chargaff discover and why was this important?
 BILD7: Problem Set 1. What is the general structure of DNA? 2. What did Chargaff discover and why was this important? 3. What was the major contribution of Rosalind Franklin? 4. How did solving the structure
BILD7: Problem Set 1. What is the general structure of DNA? 2. What did Chargaff discover and why was this important? 3. What was the major contribution of Rosalind Franklin? 4. How did solving the structure
Developmental roles of heparan sulfate proteoglycans in Drosophila
 Glycoconjugate Journal 19, 363 368, 2003 C 2003 Kluwer Academic Publishers. Manufactured in The Netherlands. Developmental roles of heparan sulfate proteoglycans in Drosophila Xinhua Lin 1 and Norbert
Glycoconjugate Journal 19, 363 368, 2003 C 2003 Kluwer Academic Publishers. Manufactured in The Netherlands. Developmental roles of heparan sulfate proteoglycans in Drosophila Xinhua Lin 1 and Norbert
Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila
 Development 126, 5441-5452 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV7755 5441 Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila Chiann-mun Chen
Development 126, 5441-5452 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV7755 5441 Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila Chiann-mun Chen
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
SUPPLEMENTARY FIGURES AND TABLES AND THEIR LEGENDS. Transient infection of the zebrafish notochord triggers chronic inflammation
 SUPPLEMENTARY FIGURES AND TABLES AND THEIR LEGENDS Transient infection of the zebrafish notochord triggers chronic inflammation Mai Nguyen-Chi 1,2,5, Quang Tien Phan 1,2,5, Catherine Gonzalez 1,2, Jean-François
SUPPLEMENTARY FIGURES AND TABLES AND THEIR LEGENDS Transient infection of the zebrafish notochord triggers chronic inflammation Mai Nguyen-Chi 1,2,5, Quang Tien Phan 1,2,5, Catherine Gonzalez 1,2, Jean-François
targets. clustering show that different complex pathway
 Supplementary Figure 1. CLICR allows clustering and activation of cytoplasmic protein targets. (a, b) Upon light activation, the Cry2 (red) and LRP6c (green) components co-cluster due to the heterodimeric
Supplementary Figure 1. CLICR allows clustering and activation of cytoplasmic protein targets. (a, b) Upon light activation, the Cry2 (red) and LRP6c (green) components co-cluster due to the heterodimeric
Hes5. Hes Relative mrna levels
 A a Absolute mrna levels 6 5 4 3 2 1 hjag1 EP cdl1 EP Jag1 E2+1 b d m Hey1 c e EP Hey1 Dl1 E2+1 f h m Hey1 g i EP Hey1 1 a Hes5 Hes5 a Hes5 Hes5 B a b EP e 1.4 1.2 EP GFP GFP E2+1 c m Hey1 d Hey1 Relative
A a Absolute mrna levels 6 5 4 3 2 1 hjag1 EP cdl1 EP Jag1 E2+1 b d m Hey1 c e EP Hey1 Dl1 E2+1 f h m Hey1 g i EP Hey1 1 a Hes5 Hes5 a Hes5 Hes5 B a b EP e 1.4 1.2 EP GFP GFP E2+1 c m Hey1 d Hey1 Relative
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila November 6, 2007 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Developmental Biology Biology 4361 Axis Specification in Drosophila November 6, 2007 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc
 OPTIMIZATION OF IMMUNOBLOT PROTOCOL 121 Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc Jacqueline Bjornton and John Wheeler Faculty Sponsor: Anne
OPTIMIZATION OF IMMUNOBLOT PROTOCOL 121 Optimization of Immunoblot Protocol for Use with a Yeast Strain Containing the CDC7 Gene Tagged with myc Jacqueline Bjornton and John Wheeler Faculty Sponsor: Anne
The Wingless morphogen gradient is established by the cooperative action of Frizzled and Heparan Sulfate Proteoglycan receptors
 Developmental Biology 276 (2004) 89 100 www.elsevier.com/locate/ydbio The Wingless morphogen gradient is established by the cooperative action of Frizzled and Heparan Sulfate Proteoglycan receptors Gyeong-Hun
Developmental Biology 276 (2004) 89 100 www.elsevier.com/locate/ydbio The Wingless morphogen gradient is established by the cooperative action of Frizzled and Heparan Sulfate Proteoglycan receptors Gyeong-Hun
Two Frizzled Planar Cell Polarity Signals in the Drosophila Wing Are Differentially Organized by the Fat/Dachsous Pathway
 Two Frizzled Planar Cell Polarity Signals in the Drosophila Wing Are Differentially Organized by the Fat/Dachsous Pathway Justin Hogan, Meagan Valentine, Chris Cox, Kristy Doyle, Simon Collier* Department
Two Frizzled Planar Cell Polarity Signals in the Drosophila Wing Are Differentially Organized by the Fat/Dachsous Pathway Justin Hogan, Meagan Valentine, Chris Cox, Kristy Doyle, Simon Collier* Department
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila November 2, 2006 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Developmental Biology Biology 4361 Axis Specification in Drosophila November 2, 2006 Axis Specification in Drosophila Fertilization Superficial cleavage Gastrulation Drosophila body plan Oocyte formation
Robustness of Tissue Patterns*
 MCBU Project II - 2014 Robustness of Tissue Patterns* June, 2014 Frederic Y.M. Wan Mathematics University of California, Irvine Supported by: NIH Grants R01-GM67247 P50-GM66051 Biological Patterning The
MCBU Project II - 2014 Robustness of Tissue Patterns* June, 2014 Frederic Y.M. Wan Mathematics University of California, Irvine Supported by: NIH Grants R01-GM67247 P50-GM66051 Biological Patterning The
Reading: Chapter 5, pp ; Reference chapter D, pp Problem set F
 Mosaic Analysis Reading: Chapter 5, pp140-141; Reference chapter D, pp820-823 Problem set F Twin spots in Drosophila Although segregation and recombination in mitosis do not occur at the same frequency
Mosaic Analysis Reading: Chapter 5, pp140-141; Reference chapter D, pp820-823 Problem set F Twin spots in Drosophila Although segregation and recombination in mitosis do not occur at the same frequency
Cell Biology Review. The key components of cells that concern us are as follows: 1. Nucleus
 Cell Biology Review Development involves the collective behavior and activities of cells, working together in a coordinated manner to construct an organism. As such, the regulation of development is intimately
Cell Biology Review Development involves the collective behavior and activities of cells, working together in a coordinated manner to construct an organism. As such, the regulation of development is intimately
Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1
 Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1 F/F ;mir-17~92 F/F (Pkd1-miR-17~92KO) mice. (A) Q-PCR
Supplementary Figure 1: To test the role of mir-17~92 in orthologous genetic model of ADPKD, we generated Ksp/Cre;Pkd1 F/F (Pkd1-KO) and Ksp/Cre;Pkd1 F/F ;mir-17~92 F/F (Pkd1-miR-17~92KO) mice. (A) Q-PCR
Supplementary Figure 1: Mechanism of Lbx2 action on the Wnt/ -catenin signalling pathway. (a) The Wnt/ -catenin signalling pathway and its
 Supplementary Figure 1: Mechanism of Lbx2 action on the Wnt/ -catenin signalling pathway. (a) The Wnt/ -catenin signalling pathway and its transcriptional activity in wild-type embryo. A gradient of canonical
Supplementary Figure 1: Mechanism of Lbx2 action on the Wnt/ -catenin signalling pathway. (a) The Wnt/ -catenin signalling pathway and its transcriptional activity in wild-type embryo. A gradient of canonical
SUPPLEMENTARY INFORMATION
 GP2 Type I-piliated bacteria FAE M cell M cell pocket idc T cell mdc Generation of antigenspecific T cells Induction of antigen-specific mucosal immune response Supplementary Figure 1 Schematic diagram
GP2 Type I-piliated bacteria FAE M cell M cell pocket idc T cell mdc Generation of antigenspecific T cells Induction of antigen-specific mucosal immune response Supplementary Figure 1 Schematic diagram
Developmental Biology
 Developmental Biology 334 (2009) 161 173 Contents lists available at ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Wingless signaling and the control of cell
Developmental Biology 334 (2009) 161 173 Contents lists available at ScienceDirect Developmental Biology journal homepage: www.elsevier.com/developmentalbiology Wingless signaling and the control of cell
Supplementary Figure 1.
 Supplementary Figure 1. Characterisation of IHG-1 overexpressing and knockdown cell lines. (A) Total cellular RNA was prepared from HeLa cells stably overexpressing IHG-1 or mts-ihg-1. IHG-1 mrna was quantified
Supplementary Figure 1. Characterisation of IHG-1 overexpressing and knockdown cell lines. (A) Total cellular RNA was prepared from HeLa cells stably overexpressing IHG-1 or mts-ihg-1. IHG-1 mrna was quantified
Lecture 7. Development of the Fruit Fly Drosophila
 BIOLOGY 205/SECTION 7 DEVELOPMENT- LILJEGREN Lecture 7 Development of the Fruit Fly Drosophila 1. The fruit fly- a highly successful, specialized organism a. Quick life cycle includes three larval stages
BIOLOGY 205/SECTION 7 DEVELOPMENT- LILJEGREN Lecture 7 Development of the Fruit Fly Drosophila 1. The fruit fly- a highly successful, specialized organism a. Quick life cycle includes three larval stages
Drosophila glypicans control the cell-to-cell movement of Hedgehog
 601 Drosophila glypicans control the cell-to-cell movement of Hedgehog by a dynamin-independent process Chun Han, Tatyana Y. Belenkaya*, Bei Wang* and Xinhua Lin Division of Developmental Biology, Children
601 Drosophila glypicans control the cell-to-cell movement of Hedgehog by a dynamin-independent process Chun Han, Tatyana Y. Belenkaya*, Bei Wang* and Xinhua Lin Division of Developmental Biology, Children
The Imperatives of Context and Contour for Morphogen Dispersion
 2252 Biophysical Journal Volume 103 December 2012 2252 2256 The Imperatives of Context and Contour for Morphogen Dispersion Thomas B. Kornberg* Cardiovascular Research Institute, University of California,
2252 Biophysical Journal Volume 103 December 2012 2252 2256 The Imperatives of Context and Contour for Morphogen Dispersion Thomas B. Kornberg* Cardiovascular Research Institute, University of California,
16 The Cell Cycle. Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization
 The Cell Cycle 16 The Cell Cycle Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization Introduction Self-reproduction is perhaps
The Cell Cycle 16 The Cell Cycle Chapter Outline The Eukaryotic Cell Cycle Regulators of Cell Cycle Progression The Events of M Phase Meiosis and Fertilization Introduction Self-reproduction is perhaps
Nature Biotechnology: doi: /nbt Supplementary Figure 1. Overexpression of YFP::GPR-1 in the germline.
 Supplementary Figure 1 Overexpression of YFP::GPR-1 in the germline. The pie-1 promoter and 3 utr were used to express yfp::gpr-1 in the germline. Expression levels from the yfp::gpr-1(cai 1.0)-expressing
Supplementary Figure 1 Overexpression of YFP::GPR-1 in the germline. The pie-1 promoter and 3 utr were used to express yfp::gpr-1 in the germline. Expression levels from the yfp::gpr-1(cai 1.0)-expressing
Cell-Cell Communication in Development
 Biology 4361 - Developmental Biology Cell-Cell Communication in Development October 2, 2007 Cell-Cell Communication - Topics Induction and competence Paracrine factors inducer molecules Signal transduction
Biology 4361 - Developmental Biology Cell-Cell Communication in Development October 2, 2007 Cell-Cell Communication - Topics Induction and competence Paracrine factors inducer molecules Signal transduction
downstream (0.8 kb) homologous sequences to the genomic locus of DIC. A DIC mutant strain (ro- 6
 A B C D ts Figure S1 Generation of DIC- mcherry expressing N.crassa strain. A. N. crassa colony morphology. When a cot1 (top, left panel) strain is grown at permissive temperature (25 C), it exhibits straight
A B C D ts Figure S1 Generation of DIC- mcherry expressing N.crassa strain. A. N. crassa colony morphology. When a cot1 (top, left panel) strain is grown at permissive temperature (25 C), it exhibits straight
Supplemental material
 Supplemental material THE JOURNAL OF CELL BIOLOGY Mourier et al., http://www.jcb.org/cgi/content/full/jcb.201411100/dc1 Figure S1. Size and mitochondrial content in Mfn1 and Mfn2 knockout hearts. (A) Body
Supplemental material THE JOURNAL OF CELL BIOLOGY Mourier et al., http://www.jcb.org/cgi/content/full/jcb.201411100/dc1 Figure S1. Size and mitochondrial content in Mfn1 and Mfn2 knockout hearts. (A) Body
Dpp Signaling Activity Requires Pentagone to Scale with Tissue Size in the Growing Drosophila Wing Imaginal Disc
 Dpp Signaling Activity Requires Pentagone to Scale with Tissue Size in the Growing Drosophila Wing Imaginal Disc Fisun Hamaratoglu 1. *, Aitana Morton de Lachapelle 2,3., George Pyrowolakis 4,5, Sven Bergmann
Dpp Signaling Activity Requires Pentagone to Scale with Tissue Size in the Growing Drosophila Wing Imaginal Disc Fisun Hamaratoglu 1. *, Aitana Morton de Lachapelle 2,3., George Pyrowolakis 4,5, Sven Bergmann
7.06 Problem Set
 7.06 Problem Set 5 -- 2006 1. In the first half of the course, we encountered many examples of proteins that entered the nucleus in response to the activation of a cell-signaling pathway. One example of
7.06 Problem Set 5 -- 2006 1. In the first half of the course, we encountered many examples of proteins that entered the nucleus in response to the activation of a cell-signaling pathway. One example of
SUPPLEMENTARY INFORMATION
 med!1,2 Wild-type (N2) end!3 elt!2 5 1 15 Time (minutes) 5 1 15 Time (minutes) med!1,2 end!3 5 1 15 Time (minutes) elt!2 5 1 15 Time (minutes) Supplementary Figure 1: Number of med-1,2, end-3, end-1 and
med!1,2 Wild-type (N2) end!3 elt!2 5 1 15 Time (minutes) 5 1 15 Time (minutes) med!1,2 end!3 5 1 15 Time (minutes) elt!2 5 1 15 Time (minutes) Supplementary Figure 1: Number of med-1,2, end-3, end-1 and
Honors Biology Reading Guide Chapter 11
 Honors Biology Reading Guide Chapter 11 v Promoter a specific nucleotide sequence in DNA located near the start of a gene that is the binding site for RNA polymerase and the place where transcription begins
Honors Biology Reading Guide Chapter 11 v Promoter a specific nucleotide sequence in DNA located near the start of a gene that is the binding site for RNA polymerase and the place where transcription begins
Supplementary Figure 1. Real time in vivo imaging of SG secretion. (a) SGs from Drosophila third instar larvae that express Sgs3-GFP (green) and
 Supplementary Figure 1. Real time in vivo imaging of SG secretion. (a) SGs from Drosophila third instar larvae that express Sgs3-GFP (green) and Lifeact-Ruby (red) were imaged in vivo to visualize secretion
Supplementary Figure 1. Real time in vivo imaging of SG secretion. (a) SGs from Drosophila third instar larvae that express Sgs3-GFP (green) and Lifeact-Ruby (red) were imaged in vivo to visualize secretion
The Wnt Wingless (Wg) pathway regulates development
 Published Online: 10 April, 2006 Supp Info: http://doi.org/10.1083/jcb.200510123 Downloaded from jcb.rupress.org on August 25, 2018 JCB: ARTICLE Internalization is required for proper Wingless signaling
Published Online: 10 April, 2006 Supp Info: http://doi.org/10.1083/jcb.200510123 Downloaded from jcb.rupress.org on August 25, 2018 JCB: ARTICLE Internalization is required for proper Wingless signaling
TGF-β/BMP superfamily members, Gbb-60A and Dpp, cooperate to provide pattern information and establish cell identity in the Drosophila wing
 Development 125, 2723-2734 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV5189 2723 TGF-β/BMP superfamily members, Gbb-60A and Dpp, cooperate to provide pattern information and
Development 125, 2723-2734 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV5189 2723 TGF-β/BMP superfamily members, Gbb-60A and Dpp, cooperate to provide pattern information and
Heather Currinn, Benjamin Guscott, Zita Balklava, Alice Rothnie and Thomas Wassmer*
 Online Resources APP controls the formation of PI(3,5)P 2 vesicles through its binding of the PIKfyve complex. Cellular and Molecular Life Sciences Heather Currinn, Benjamin Guscott, Zita Balklava, Alice
Online Resources APP controls the formation of PI(3,5)P 2 vesicles through its binding of the PIKfyve complex. Cellular and Molecular Life Sciences Heather Currinn, Benjamin Guscott, Zita Balklava, Alice
Exam 1 ID#: October 4, 2007
 Biology 4361 Name: KEY Exam 1 ID#: October 4, 2007 Multiple choice (one point each) (1-25) 1. The process of cells forming tissues and organs is called a. morphogenesis. b. differentiation. c. allometry.
Biology 4361 Name: KEY Exam 1 ID#: October 4, 2007 Multiple choice (one point each) (1-25) 1. The process of cells forming tissues and organs is called a. morphogenesis. b. differentiation. c. allometry.
AP Biology Unit 6 Practice Test 1. A group of cells is assayed for DNA content immediately following mitosis and is found to have an average of 8
 AP Biology Unit 6 Practice Test Name: 1. A group of cells is assayed for DNA content immediately following mitosis and is found to have an average of 8 picograms of DNA per nucleus. How many picograms
AP Biology Unit 6 Practice Test Name: 1. A group of cells is assayed for DNA content immediately following mitosis and is found to have an average of 8 picograms of DNA per nucleus. How many picograms
MOLECULAR CONTROL OF EMBRYONIC PATTERN FORMATION
 MOLECULAR CONTROL OF EMBRYONIC PATTERN FORMATION Drosophila is the best understood of all developmental systems, especially at the genetic level, and although it is an invertebrate it has had an enormous
MOLECULAR CONTROL OF EMBRYONIC PATTERN FORMATION Drosophila is the best understood of all developmental systems, especially at the genetic level, and although it is an invertebrate it has had an enormous
Unicellular: Cells change function in response to a temporal plan, such as the cell cycle.
 Spatial organization is a key difference between unicellular organisms and metazoans Unicellular: Cells change function in response to a temporal plan, such as the cell cycle. Cells differentiate as a
Spatial organization is a key difference between unicellular organisms and metazoans Unicellular: Cells change function in response to a temporal plan, such as the cell cycle. Cells differentiate as a
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11419 Supplementary Figure 1 Schematic representation of innate immune signaling pathways induced by intracellular Salmonella in cultured macrophages. a, During the infection Salmonella
doi:10.1038/nature11419 Supplementary Figure 1 Schematic representation of innate immune signaling pathways induced by intracellular Salmonella in cultured macrophages. a, During the infection Salmonella
Regulation and signaling. Overview. Control of gene expression. Cells need to regulate the amounts of different proteins they express, depending on
 Regulation and signaling Overview Cells need to regulate the amounts of different proteins they express, depending on cell development (skin vs liver cell) cell stage environmental conditions (food, temperature,
Regulation and signaling Overview Cells need to regulate the amounts of different proteins they express, depending on cell development (skin vs liver cell) cell stage environmental conditions (food, temperature,
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1 Sns and Duf co-localise in embryonic nephrocytes a-c, Wild-type stage 11 (a),14 (b) and 16 (c) embryos stained with anti-duf (green) and anti-sns (red). Higher magnification images
Supplementary Figure 1 Sns and Duf co-localise in embryonic nephrocytes a-c, Wild-type stage 11 (a),14 (b) and 16 (c) embryos stained with anti-duf (green) and anti-sns (red). Higher magnification images
Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering
 Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering by Valverde et. Al Published in Science 2004 Presented by Boyana Grigorova CBMG 688R Feb. 12, 2007 Circadian Rhythms: The Clock Within
Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering by Valverde et. Al Published in Science 2004 Presented by Boyana Grigorova CBMG 688R Feb. 12, 2007 Circadian Rhythms: The Clock Within
4) Please cite Dagda et al J Biol Chem 284: , for any publications or presentations resulting from use or modification of the macro.
 Supplement Figure S1. Algorithmic quantification of mitochondrial morphology in SH- SY5Y cells treated with known fission/fusion mediators. Parental SH-SY5Y cells were transiently transfected with an empty
Supplement Figure S1. Algorithmic quantification of mitochondrial morphology in SH- SY5Y cells treated with known fission/fusion mediators. Parental SH-SY5Y cells were transiently transfected with an empty
On the Mechanism of Wing Size Determination in Fly Development
 On the Mechanism of Wing Size Determination in Fly Development PNAS Paper Authors: Lars Hufnagel, Aurelio A. Teleman, Herve Rouault, Stephen M. Cohen, and Boris I. Shraiman Group Members: Rebekah Starks,
On the Mechanism of Wing Size Determination in Fly Development PNAS Paper Authors: Lars Hufnagel, Aurelio A. Teleman, Herve Rouault, Stephen M. Cohen, and Boris I. Shraiman Group Members: Rebekah Starks,
Drosophila wing. Temporal regulation of Apterous activity during development of the. Marco Milán and Stephen M. Cohen* SUMMARY
 Development 127, 3069-3078 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV2547 3069 Temporal regulation of Apterous activity during development of the Drosophila wing Marco Milán
Development 127, 3069-3078 (2000) Printed in Great Britain The Company of Biologists Limited 2000 DEV2547 3069 Temporal regulation of Apterous activity during development of the Drosophila wing Marco Milán
Richik N. Ghosh, Linnette Grove, and Oleg Lapets ASSAY and Drug Development Technologies 2004, 2:
 1 3/1/2005 A Quantitative Cell-Based High-Content Screening Assay for the Epidermal Growth Factor Receptor-Specific Activation of Mitogen-Activated Protein Kinase Richik N. Ghosh, Linnette Grove, and Oleg
1 3/1/2005 A Quantitative Cell-Based High-Content Screening Assay for the Epidermal Growth Factor Receptor-Specific Activation of Mitogen-Activated Protein Kinase Richik N. Ghosh, Linnette Grove, and Oleg
23-. Shoot and root development depend on ratio of IAA/CK
 Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
Cell Cell Communication in Development
 Biology 4361 Developmental Biology Cell Cell Communication in Development June 25, 2008 Cell Cell Communication Concepts Cells in developing organisms develop in the context of their environment, including
Biology 4361 Developmental Biology Cell Cell Communication in Development June 25, 2008 Cell Cell Communication Concepts Cells in developing organisms develop in the context of their environment, including
SUPPLEMENTARY INFORMATION
 Supplementary Discussion Rationale for using maternal ythdf2 -/- mutants as study subject To study the genetic basis of the embryonic developmental delay that we observed, we crossed fish with different
Supplementary Discussion Rationale for using maternal ythdf2 -/- mutants as study subject To study the genetic basis of the embryonic developmental delay that we observed, we crossed fish with different
Axis Specification in Drosophila
 Developmental Biology Biology 4361 Axis Specification in Drosophila July 9, 2008 Drosophila Development Overview Fertilization Cleavage Gastrulation Drosophila body plan Oocyte formation Genetic control
Developmental Biology Biology 4361 Axis Specification in Drosophila July 9, 2008 Drosophila Development Overview Fertilization Cleavage Gastrulation Drosophila body plan Oocyte formation Genetic control
Lesson Overview. Gene Regulation and Expression. Lesson Overview Gene Regulation and Expression
 13.4 Gene Regulation and Expression THINK ABOUT IT Think of a library filled with how-to books. Would you ever need to use all of those books at the same time? Of course not. Now picture a tiny bacterium
13.4 Gene Regulation and Expression THINK ABOUT IT Think of a library filled with how-to books. Would you ever need to use all of those books at the same time? Of course not. Now picture a tiny bacterium
Importance of Protein sorting. A clue from plastid development
 Importance of Protein sorting Cell organization depend on sorting proteins to their right destination. Cell functions depend on sorting proteins to their right destination. Examples: A. Energy production
Importance of Protein sorting Cell organization depend on sorting proteins to their right destination. Cell functions depend on sorting proteins to their right destination. Examples: A. Energy production
T H E J O U R N A L O F C E L L B I O L O G Y
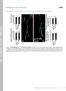 Supplemental material Kasprowicz et al., http://www.jcb.org/cgi/content/full/jcb.201310090/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. NMJ morphology of shi 12-12B ; shi-4c not treated
Supplemental material Kasprowicz et al., http://www.jcb.org/cgi/content/full/jcb.201310090/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. NMJ morphology of shi 12-12B ; shi-4c not treated
The neuron as a secretory cell
 The neuron as a secretory cell EXOCYTOSIS ENDOCYTOSIS The secretory pathway. Transport and sorting of proteins in the secretory pathway occur as they pass through the Golgi complex before reaching the
The neuron as a secretory cell EXOCYTOSIS ENDOCYTOSIS The secretory pathway. Transport and sorting of proteins in the secretory pathway occur as they pass through the Golgi complex before reaching the
Axis determination in flies. Sem 9.3.B.5 Animal Science
 Axis determination in flies Sem 9.3.B.5 Animal Science All embryos are in lateral view (anterior to the left). Endoderm, midgut; mesoderm; central nervous system; foregut, hindgut and pole cells in yellow.
Axis determination in flies Sem 9.3.B.5 Animal Science All embryos are in lateral view (anterior to the left). Endoderm, midgut; mesoderm; central nervous system; foregut, hindgut and pole cells in yellow.
JAK/STAT controls organ size and fate specification by regulating morphogen production and signalling
 Received 18 May 2016 Accepted 2 ov 2017 Published 3 Jan 2017 JAK/STAT controls organ size and fate specification by regulating morphogen production and signalling Carles Recasens-Alvarez 1, *, Ana Ferreira
Received 18 May 2016 Accepted 2 ov 2017 Published 3 Jan 2017 JAK/STAT controls organ size and fate specification by regulating morphogen production and signalling Carles Recasens-Alvarez 1, *, Ana Ferreira
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1121356/dc1 Supporting Online Material for Polar PIN Localization Directs Auxin Flow in Plants Justyna Wiśniewska, Jian Xu, Daniela Seifertová, Philip B. Brewer, Kamil
www.sciencemag.org/cgi/content/full/1121356/dc1 Supporting Online Material for Polar PIN Localization Directs Auxin Flow in Plants Justyna Wiśniewska, Jian Xu, Daniela Seifertová, Philip B. Brewer, Kamil
Supplementary Information
 Supplementary Information MAP2/Hoechst Hyp.-AP ph 6.5 Hyp.-SD ph 7.2 Norm.-SD ph 7.2 Supplementary Figure 1. Mitochondrial elongation in cortical neurons by acidosis. Representative images of neuronal
Supplementary Information MAP2/Hoechst Hyp.-AP ph 6.5 Hyp.-SD ph 7.2 Norm.-SD ph 7.2 Supplementary Figure 1. Mitochondrial elongation in cortical neurons by acidosis. Representative images of neuronal
Compartments and the control of growth in the Drosophila wing imaginal disc
 Access Development the most First recent posted version epress online at online on http://dev.biologists.org/lookup/doi/10.1242/dev.02618 11 October publication 2006 as date 10.1242/dev.02618 11 October
Access Development the most First recent posted version epress online at online on http://dev.biologists.org/lookup/doi/10.1242/dev.02618 11 October publication 2006 as date 10.1242/dev.02618 11 October
Argosomes: A Potential Vehicle for the Spread of Morphogens through Epithelia
 Cell, Vol. 106, 633 645, September 7, 2001, Copyright 2001 by Cell Press Argosomes: A Potential Vehicle for the Spread of Morphogens through Epithelia Valentina Greco, Michael Hannus, and Suzanne Eaton
Cell, Vol. 106, 633 645, September 7, 2001, Copyright 2001 by Cell Press Argosomes: A Potential Vehicle for the Spread of Morphogens through Epithelia Valentina Greco, Michael Hannus, and Suzanne Eaton
Supplementary Figures
 Supplementary Figures Supplementary Fig. S1: Normal development and organization of the embryonic ventral nerve cord in Platynereis. (A) Life cycle of Platynereis dumerilii. (B-F) Axonal scaffolds and
Supplementary Figures Supplementary Fig. S1: Normal development and organization of the embryonic ventral nerve cord in Platynereis. (A) Life cycle of Platynereis dumerilii. (B-F) Axonal scaffolds and
Chapter 18 Lecture. Concepts of Genetics. Tenth Edition. Developmental Genetics
 Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Cells to Tissues. Peter Takizawa Department of Cell Biology
 Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
Cells to Tissues Peter Takizawa Department of Cell Biology From one cell to ensembles of cells. Multicellular organisms require individual cells to work together in functional groups. This means cells
A re-evaluation of the contributions of Apterous and Notch to the dorsoventral lineage restriction boundary in the Drosophila wing
 Development 130, 553-562 2003 The Company of Biologists Ltd doi:10.1242/dev.00276 553 A re-evaluation of the contributions of Apterous and Notch to the dorsoventral lineage restriction boundary in the
Development 130, 553-562 2003 The Company of Biologists Ltd doi:10.1242/dev.00276 553 A re-evaluation of the contributions of Apterous and Notch to the dorsoventral lineage restriction boundary in the
Specificities of Dishevelled
 Casein Kinase Iε Modulates the Signaling Specificities of Dishevelled Feng Cong, Liang Schweizer and Harold Varmus Mol. Cell. Biol. 2004, 24(5):2000. DOI: 10.1128/MCB.24.5.2000-2011.2004. REFERENCES CONTENT
Casein Kinase Iε Modulates the Signaling Specificities of Dishevelled Feng Cong, Liang Schweizer and Harold Varmus Mol. Cell. Biol. 2004, 24(5):2000. DOI: 10.1128/MCB.24.5.2000-2011.2004. REFERENCES CONTENT
Morphogen gradient interpretation by a regulated trafficking step during ligand receptor transduction
 Morphogen gradient interpretation by a regulated trafficking step during ligand receptor transduction Jerome Jullien and John Gurdon 1 Wellcome Trust/Cancer Research UK Gurdon Institute, University of
Morphogen gradient interpretation by a regulated trafficking step during ligand receptor transduction Jerome Jullien and John Gurdon 1 Wellcome Trust/Cancer Research UK Gurdon Institute, University of
Drosophila IAP1-mediated ubiquitylation controls activation of the initiator caspase DRONC independent of protein degradation
 University of Massachusetts Medical School escholarship@umms Molecular, Cell and Cancer Biology Publications Molecular, Cell and Cancer Biology 9-1-2011 Drosophila IAP1-mediated ubiquitylation controls
University of Massachusetts Medical School escholarship@umms Molecular, Cell and Cancer Biology Publications Molecular, Cell and Cancer Biology 9-1-2011 Drosophila IAP1-mediated ubiquitylation controls
