Molecular Phylogeny of Noctilucoid Dinoflagellates (Noctilucales, Dinophyceae)
|
|
|
- Barry Rogers
- 6 years ago
- Views:
Transcription
1 Protist, Vol. ], ]]] ]]], ]] ]]]] Published online date 26 February 2010 ORIGINAL PAPER Molecular Phylogeny of Noctilucoid Dinoflagellates (Noctilucales, Dinophyceae) Fernando Gómez a,1, David Moreira b, and Purificación López-García b a Marine Microbial Ecology Group, Université Pierre et Marie Curie, CNRS UMR 7093, Laboratoire d Océanographie de Villefranche, Station Zoologique, BP 28, Villefranche-sur-Mer, France b Unité d Ecologie, Systématique et Evolution, CNRS UMR 8079, Université Paris-Sud, Bâtiment 360, Orsay Cedex, France Submitted September 2, 2009; Accepted December 13, 2009 Monitoring Editor: Michael Melkonian The order Noctilucales or class Noctiluciphyceae encompasses three families of aberrant dinoflagellates (Noctilucaceae, Leptodiscaceae and Kofoidiniaceae) that, at least in some life stages, lack typical dinoflagellate characters such as the ribbon-like transversal flagellum or condensed chromosomes. Noctiluca scintillans, the first dinoflagellate to be described, has been intensively investigated. However, its phylogenetic position based on the small subunit ribosomal DNA (SSU rdna) sequence is unstable and controversial. Noctiluca has been placed either as an early diverging lineage that diverged after Oxyrrhis and before the dinokaryotes -core dinoflagellates- or as a recent lineage branching from unarmoured dinoflagellates in the order Gymnodiniales. So far, the lack of other noctilucoid sequences has hampered the elucidation of their phylogenetic relationships to other dinoflagellates. Furthermore, even the monophyly of the noctilucoids remained uncertain. We have determined SSU rrna gene sequences for Kofoidiniaceae, those of the type Spatulodinium (=Gymnodinium) pseudonoctiluca and another Spatulodinium species, as well as of two species of Kofoidinium, and the first gene sequence of Leptodiscaceae, that of Abedinium (=Leptophyllus) dasypus. These taxa were collected from their type localities, the English Channel and the NW Mediterranean Sea, respectively. Phylogenetic analyses place the Noctilucales as a monophyletic group at a basal position close to parasites of the Marine Alveolate Group I (MAGI) and the Syndiniales (MAGII), before the core of dinokaryotic dinoflagellates, although with moderate support. & 2010 Elsevier Ltd. All rights reserved. Key words: alveolate evolution; Dinoflagellata; Gymnodinium lebourae; Kofoidinium; Spatulodinium; SSU rdna phylogeny. Introduction Noctiluca scintillans (Macartney) Kofoid is the first known dinoflagellate (Baker 1753; Slabber 1771). Initially classified as a jelly fish, Haeckel (1873) proposed that Noctiluca Suriray ex Lamarck should be included in the Cystoflagellata within 1 Corresponding author; fax þ fernando.gomez@fitoplancton.com (F. Gómez). the dinoflagellates. Based on detailed observations of the trophonts, Kofoid (1920) homologized Abbreviations: bp, base pairs; BV, bootstrap value; DAPI, 4,6-diamidino-2-phenylindole; MAGI, Marine Alveolate Group I; PCR, Polymerase chain reaction; SSU, small subunit; rdna, ribosomal deoxyribonucleic acid & 2010 Elsevier Ltd. All rights reserved. doi: /j.protis
2 2 F. Gómez et al. the structures recognised in Noctiluca with the sulcus, cingulum, longitudinal and transverse flagella of the dinoflagellates, and since then, Noctiluca was classified within dinoflagellates as the type of the order Noctilucales. In current taxonomic schemes, dinoflagellates (Dinoflagellata) are divided into two subdivisions: Dinokaryota and Syndinea (Fensome et al. 1993). Syndinea comprises one single order, Syndiniales, of exclusively non-photosynthetic parasitic species that, among other features, lack most characters defining the typical nucleus of the Dinokaryota. The syndinean nucleus contains a low number (usually four to ten) of non-fibrillar and V-shaped chromosomes (Cachon 1964; Hollande 1974). The chromatin of Dinokaryota has fibrillar chromosomes (20-300) condensed throughout the cell cycle (Pfiester 1984; Rizzo 2003). Likewise, the diploid Noctiluca trophonts do not retain characters shared by typical dinoflagellates, such as a transverse flagellum and condensed chromosomes. In contrast, the haploid zoospores maintain primitive dinoflagellate-like characteristics including two grooves, slightly differentiated flagella with different lengths and paraxial rod, and condensed chromosomes (Afzelius 1963; Fukuda and Endoh 2006; Höhfeld and Melkonian 1995; Melkonian and Höhfeld 1988; Soyer 1969, 1970, 1972; Zingmark 1970). Zingmark (1970) considered Noctiluca not to be a true dinoflagellate because its vegetative nucleus is of conventional eukaryotic type and not dinokaryotic. However, Fensome et al. (1993), based on the dinokaryotic nature of the gamete nucleus and the intergradational nature with other Noctilucales (such as Kofoidinium Pavillard), placed the noctilucoids in the class Noctiluciphyceae within the subdivision Dinokaryota (Fensome et al. 1993). In the 1990s, the phylogenetic position of Noctiluca was re-evaluated using molecular information. The first phylogenetic analyses based on the large subunit (LSU) and small subunit (SSU) rdna sequences suggested that Noctiluca represented a basal lineage within the dinoflagellates (Lenaers et al. 1991; Saunders et al. 1997). Recent studies based on other markers (light-emitting enzyme luciferase gene, editing density of mitochondrial cox1 mrna and Spliced-Leader RNA trans-splicing groups, b-tubulin and heat shock protein 90 -Hsp90-) also suggested that Noctiluca might belong to an early diverging dinoflagellate lineage that diverged after that leading to Oxyrrhis Dujardin and before the core dinoflagellates, although the corresponding phylogenies were most often poorly supported (Fukuda and Endoh 2008; Liu and Hastings 2007; Zhang and Lin 2008). Similarly, in the SSU rdna phylogeny, the most extended marker for protist phylogenetic and diversity studies, the placement of Noctiluca was unstable, its position likely influenced by the number of nucleotides used in the alignment (Saldarriaga et al. 2004). An analysis using diverse alveolate SSU rdna sequences as outgroup suggested that Noctiluca occupies a basal position among dinoflagellates, while another analysis rooted using sequences only from Perkinsus Levine and Syndiniales placed Noctiluca within one of the clades of the order Gymnodiniales (Saldarriaga et al. 2004). The SSU rdna phylogeny turned out to be too weak to place Noctiluca with confidence. Since the enrichment of taxonomic sampling is well known to improve the resolution of phylogenetic analyses, the sequencing of SSU rdna from other noctilucoids might help to resolve the phylogenetic position of this group within the dinoflagellates. In addition to Noctiluca, which is easily accessible from cultures and widespread in eutrophic coastal waters, other noctilucoids have a predominantly tropical to warm-temperate oceanic distribution (Cachon and Cachon 1967, 1969; Gómez and Furuya 2005, 2007). Most of the Noctilucales have been described from the NW Mediterranean and knowledge on their morphology and life cycle is almost completely restricted to the studies by Cachon and Cachon (1967, 1969). More than 100 nucleotide sequences are publicly available for N. scintillans, but sequences from other noctilucoid protists are lacking. Spatulodinium pseudonoctiluca (Pouchet) Cachon et Cachon, the type of the family Kofoidiniaceae, was first named Gymnodinium pseudonoctiluca due to the intermediate morphological characters between Gymnodinium Stein and Noctiluca. Pouchet (1885) already noted that members of this taxon undergo a complex ontogenetic process from gymnodinioid or amphidinioid immature stages to tentacle-bearing noctilucoid trophonts and he showed that G. pseudonoctiluca is a single species with two different morphotypes. The immature morphotype has two differentiated flagella, including the ribbon-like transverse flagellum, and the cingulum and the sulcus, whereas the mature morphotype, which also keeps these dinoflagellate morphological features, has radiating contractile fibrils from the perinuclear region and a tentacle that resembles the one of Noctiluca. Other authors described the immature stages of Spatulodinium J. Cachon et M. Cachon as separate species (i.e., Gymnodinium conicum
3 Molecular Phylogeny of Noctilucoid Dinoflagellates 3 Kofoid et Swezy, G. fulgens Kofoid et Swezy, and G. lebouriae Pavillard). Pouchet s observations were confirmed by Cachon and Cachon (1967) and more recent studies (Gómez and Souissi 2007; Konovalova and Selina 2002). Nevertheless, G. lebouriae and S. pseudonoctiluca often appear as a separate species in the literature (Hansen and Larsen 1992; Hoppenrath 2004). The family Leptodiscaceae is the least known among the Noctilucales due to their delicacy and warm-water oceanic distribution. Their cell bodies are strongly antero-posteriorly flattened with a bilateral symmetry or with equatorial wing-like expansions, being able to contract suddenly (Cachon and Cachon 1967; Cachon and Cachon-Enjumet 1964, 1966). The present study provides the first SSU rrna gene sequences for representatives of the families Kofoidiniaceae and Leptodiscaceae. This allows us to test the monophyly of the noctilucoid dinoflagellates and to study the placement of Noctiluca in the SSU rdna phylogeny, namely, to test whether the noctilucoid protists are an early diverging lineage or a recent lineage derived from typical dinokaryotes phylogenetically related to Gymnodiniales. Results Observations of Spatulodinium Despite one year of intensive sampling in the coast of Marseille, NW Mediterranean Sea, from October 2007 to September 2008, the records of Spatulodinium were very scarce. We observed only eight specimens on May 10-11th, which corresponded to Gymnodinium lebouriae, stage D in the life cycle of S. pseudonoctiluca according to Cachon and Cachon (1967) (Fig. 1A-D). The sampling continued from October 2008 to July 2009 in the port of Banyuls sur Mer, NW Mediterranean Sea. The first specimens of G. lebouriae appeared on April 29th and the records were numerous throughout May (Fig. 1E- H). One specimen collected on June 11th showed the shape of G. lebouriae and the tentacle of the mature stage (Fig. 1I-J). No specimens of the tentacle-bearing mature stage were observed in the coasts of Marseille or Banyuls sur Mer, and only one specimen was observed in summer from Lugol-fixed samples collected from the shallow coastal Berre Lagoon, near Marseille ( N, E). In contrast, there were numerous mature specimens of Spatulodinium with different morphologies in Lugol-fixed samples collected in open waters during the BOUM cruise. Some specimens showed an extremely long tentacle and a cell body that clearly differed from the type species (Fig. 1R-S). We succeeded in determining the sequence of one specimen of Spatulodinium (120 mm in diameter) that was highly distorted, but recognizable by its tentacle (Fig. 1Q). We also observed and isolated for single-cell PCR four specimens of the tentacle-bearing Spatulodinium and six specimens of G. lebouriae (Fig. 1K-P) from samples collected in early summer at Wimereux, coastal English Channel. At this location, the stage known as Gymnodinium lebouriae co-occurred with the tentacle-bearing specimens in nearly all the samples. The immature specimens, G. lebouriae, from the Mediterranean and Atlantic waters showed a subovate cell shape ( mm long, mm wide) with an anterior cingulum that resembled a large pigmented Amphidinium-like cell. The hyposome showed several longitudinal grooves and the sulcus. The longitudinal flagellum was clearly visible and a ribbon-like flagellum was inserted in the cingulum (Fig. 1A-B). The specimens showed numerous dispersed small globular chloroplasts. All the specimens showed chlorophyll a under blue light Figure 1. Light micrographs of Spatulodinium and Kofoidinium. A-L. Immature specimens of S. pseudonoctiluca (Gymnodinium lebouriae) from Marseille, collected on May 2008 (A-D), from Banyuls sur Mer, collected on 24 April and 23 May 2009 (E-J) and from Wimereux, NE English Channel, collected on 6-8 July 2008 (K-L). Gymnodinium lebouriae with a tentacle from Banyuls sur Mer (I-J). Arrows in Figures A-B indicate the longitudinal flagellum. Figures F and J illustrate the red fluorescence of the chlorophyll a illuminated with UV light of the specimens on the left adjacent micrographs. H. Note the nucleus stained with DAPI. M-P. Mature specimens from Wimereux collected on 6-8 July Arrows in Figures O-P indicate the shell or coque. Q. Ethanol-fixed Spatulodinium sp. from the open Gulf of Lions. R-S. Lugolfixed unidentified Spatulodinium with a long tentacle. T. Lugol-fixed specimen of Kofoidinium sp. U-Y. Kofoidinium pavillardii. U-V. Two frames of the movement onto the shell. X-Y. Detail of the shell of the same specimen. Z. Immature stage of Kofoidinum. AA. Ethanol fixed Kofoidinium cf. pavillardii from the open Gulf of Lions. Ci=cingulum; LF=longitudinal flagellum; Nu=nucleus; Te=tentacle; TF=transversal flagellum; Sh=shell; Su=sulcus. Scale bar=50 mm.
4 4 F. Gómez et al.
5 Molecular Phylogeny of Noctilucoid Dinoflagellates 5 epifluorescence microscopy (Fig. 1F, J). The nucleus appeared as a pale area in the posterior hyposome (Fig. 1A, C), as confirmed by DAPI staining (Fig. 1H). The mature specimens of S. pseudonoctiluca showed a rotund flattened hyposome ( mm in diameter) with a moveable tentacle (Fig. 1M-P). From the episome emerged a highly transparent extracellular hemispherical dome, known as shell or coque according to Cachon and Cachon (1967), which easily detached during the manipulation. The diameter of the shell was slightly similar to that of the cell (Fig. 1O-P). Observations of Kofoidinium Several live specimens of Kofoidinium pavillardii J. Cachon et M. Cachon were observed from the port of Banyuls sur Mer. The cell (350 mm in diameter) rotated on the shell attached by two hook-like anchorages (Fig. 1U-V, see video 1 as supplementary material watch?v=e-labkdiibe). During the manipulation, the specimen releases the shell, which has a slightly ellipsoidal shape ( mm diameter) (Fig. 1W-Y). These mature specimens coincided with numerous immature stages, the morphology of which resembled the stage Gymnodinium lebouriae of Spatulodinium, but they were larger (200 mm long), non-pigmented, lacking the longitudinal grooves and the structure of fibrils was more marked (Fig. 1Z). We successfully obtained the SSU rdna sequence from one small specimen (40 mm in diameter) ascribed to genus Kofoidinium (this specimen was not photographed due to a camera failure). It did not correspond to any of the three larger described species and it may likely go unnoticed due to its small size. The specimen had the pointed extension that arises from the episome towards the ventral side of the cell. This pointed extension was flexible and tended to be in a different plane from the flattened hyposome. This species was also observed in Lugol-fixed samples from Mediterranean open waters (Fig. 1T) and illustrated as Kofoidinium sp. in the figures 7-9 by Gómez and Furuya (2007) from samples of the Pacific Ocean. From the ethanol-fixed samples of the Mediterranean Sea open waters, we successfully obtained the SSU rdna sequence from a large specimen of Kofoidinium (400 mm in diameter) that was highly distorted. In the replicate Lugol-fixed sample, numerous specimens of K. pavillardii appeared. Based on this feature, general morphology and size and despite the cell was highly distorted (Fig. 1AA), we ascribed this sequence to K. cf. pavillardii. Observations of Abedinium dasypus The specimens of the family Leptodiscaceae were hyaline and extremely fragile, disintegrating very easily. In the coast of Marseille, several specimens of Scaphodinium mirabile Margalef and Abedinium dasypus (J. Cachon et Cachon-Enjumet) Loeblich Jr. et A.R. Loeblich III were observed along the autumn of 2008, but they were dead and highly deteriorated. Only three specimens of the leaf-shaped A. dasypus (200 mm in length, 80 mm in width) were observed alive. In their anterior part, they showed a tentacle with an orange-pigmented distal extreme (Fig. 2A-F). The tentacle oscillated from one side to the other as a tactile organ while the cell moved forward (Fig. 2A-D). At the other extremity, a long flagellum served to regulate the forward movement. About ten transparent filaments were visible in a radial orientation to the periphery of the nucleus (Fig. 2A, E). These filaments are considered to be ejectile bodies based on the observations by Cachon and Cachon-Enjumet (1964). The specimens were able to contract suddenly when meeting an obstacle or if the surrounding water was disturbed (Fig. 2F). They were then able to roll up immediately and the tentacle folded from the base against its superior face. Relaxation was reached soon after. We were unable to determine the SSU rdna sequences of these specimens from the coast of Marseille, but we successfully obtained those from two single specimens of A. dasypus collected in October 2008 from the port of Banyuls sur Mer (Table 1, Fig. 2G). Molecular Phylogeny We obtained SSU rdna sequences of representatives of the families Kofoidiniaceae and Leptodiscaceae, which were determined after PCR amplification from cells identified, photographed and collected under the microscope (Table 1). We carried out phylogenetic analyses including the new sequences and a variety of dinoflagellate sequences, using perkinsozoan sequences as outgroups. Since the Noctilucales possess quite divergent SSU rdna sequences, we did not include the sequences of several extremely divergent alveolates, in particular Oxyrrhis and ellobiopsid species, to decrease the probability of phylogenetic artefacts, especially long branch attraction. In our SSU rdna phylogeny, the new
6 6 F. Gómez et al. Figure 2. Abedinium dasypus from Marseille, collected on 17 December A-D. Different positions of the distal part of the tentacle. E. Detail of the ejectile bodies. F. Contracted specimen. G. Abedinium dasypus from Banyuls sur Mer, collected on 31 October EB=ejectile body; LF=longitudinal flagellum; Nu=nucleus; Te=tentacle. Scale bar=50 mm. Table 1. List of new SSU rdna sequences of Noctilucales. Taxa Spatulodinium pseudonoctiluca f. Gymnodinium lebouriae FG126 Spatulodinium pseudonoctiluca f. Gymnodinium lebouriae FG127 Spatulodinium pseudonoctiluca FG200 GenBank No. Sequence length (bp) GC content (%) Geographical origin (date) Figure GU Marseille (10 May 2008) Fig. 1B GU Marseille (11 May 2008) Fig. 1C GU Wimereux (7 Jul 2008) Fig. 1M Spatulodinium sp. FG541 GU open Gulf of Lions (14 Jul 2008) Fig. 1Q Kofoidinium sp. FG256 GU Banyuls (13 Oct 2008) Kofoidinium cf. pavillardii GU Open Gulf of Lions Fig. 1AA FG540 (14 Jul 2008) Abedinium dasypus FG257 Abedinium dasypus FG239 GU Banyuls (31 Oct 2008) Fig. 2G GU Banyuls (7 Oct 2008)
7 Molecular Phylogeny of Noctilucoid Dinoflagellates 7 Figure 3. Maximum likelihood phylogenetic tree of alveolate SSU rdna sequences, based on 1,194 aligned positions. Names in bold represent sequences obtained in this study. Numbers at the nodes are bootstrap proportions (values under 50% are omitted). For the nodes concerning the position of the Noctilucales, Bayesian posterior probability values are also indicated. Accession numbers are provided in brackets. The scale bar represents the number of substitutions for a unit branch length. The epithet of Polykrikos herdmaniae misspelled as herdmanae is corrected according to ICBN: Art ; Recom. 60C.1., Art
8 8 F. Gómez et al. sequences, together with those of Noctiluca scintillans, branched with a moderate support (bootstrap values -BV- between 61 and 64%) between the Syndiniales (Marine Alveolate Group II) and the core dinoflagellates (Fig. 3). The monophyly of the Noctilucales was poorly supported (BV of 59%). They appeared subdivided into three well-supported clades, one for the sequences of leptodiscaceans (Abedinium dasypus), the second for the Kofoidinium sequences and the third for the rest of Noctilucales (Noctiluca and Spatulodinium). Our study confirmed using molecular data that Gymnodinium lebouriae is a life stage of S. pseudonoctiluca. In fact, the sequences of G. lebouriae from the coastal Mediterranean Sea (Fig. 1A-D) and the tentacle-bearing S. pseudonoctiluca from the coastal Atlantic Ocean (Fig. 1M-P) were identical. Although the genus Spatulodinium is considered as monotypic, our molecular data evidenced that there is at least a second species in open waters within the genus (Spatulodinium sp. FG541, Fig. 1Q). The genera Spatulodinium and Kofoidinium share the lateral flattening and the shell, whereas the sac-like Noctiluca and Spatulodinium share the occurrence of a tentacle. In our SSU rdna phylogeny, Noctiluca branched among the species of the genera Spatulodinium (Fig. 3). Therefore, our results do not support the split of the Noctilucales into the families Noctilucaceae and Kofoidiniaceae, especially in what refers to the conservation of Noctiluca as the only representative of its own family. Discussion Based on SSU rdna phylogenetic analyses rooted using sequences from Perkinsus and Syndiniales as outgroup, Saldarriaga et al. (2004) found that Noctiluca branched close to the Amphidinium Clapar ede et Lachmann species within one of the clades of the order Gymnodiniales, suggesting a common gymnodinioid ancestor for the entire group. Saldarriaga et al. (2004, p. 97) reported that the basal position of Noctiluca within the dinokaryotic dinoflagellates should be reexamined: the two main arguments for proposing such a basal position have been shown to be either very weak (SSU-based phylogenetic analyses), or probably wrong (the ostensible presence of histones in the nuclei of feeding stages). They also reported that the immature stages of noctilucoids (i.e., Spatulodinium) are practically indistinguishable from a number of Gymnodiniales, especially Amphidinium. According to Saldarriaga et al. (2004), Noctiluca shares with members of the Gymnodinium sensu stricto group two rare morphological features: 1) peculiar chambers (ampullae) in which the nuclear pores are situated, observed in the trophont of Noctiluca (Afzelius 1963; Soyer 1969) and in other noctilucoids, such as Petalodinium (Gómez and Furuya 2005), but also in several representatives of the Gymnodinium sensu stricto group (Daugbjerg et al. 2000; Hansen and Moestrup 2005; Hoppenrath and Leander 2007); and 2) the absence of a transverse striated flagellar root in Gymnodinium and in the zoospores of Noctiluca, otherwise a feature typical of most dinoflagellates (Hansen et al. 2000). An additional feature that may suggest a relationship between noctilucoids and the Gymnodiniales is the presence of ejectile bodies. In fact, dinoflagellate representatives of the Gymnodinium sensu stricto group, like Polykrikos Bütschli and Nematodinium Kofoid et Swezy, possess ejectile bodies, such as nematocysts, used for prey capture (Greuet 1987; Hoppenrath and Leander 2007), and Cachon and Cachon-Enjumet (1964) observed that stressed specimens of Abedinium dasypus are able to project 200 mmlong trichocysts whose function may be convergent with the ejectile bodies of Polykrikos. However, ejectile bodies or extrusomes used for food capture or escape from predation are known in other alveolates groups such as the ciliates (Wessenberg and Antipa 1970). Moreover, despite all those putative common characters relating Noctilucales and Gymnodiniales, our SSU rdna phylogeny does not reveal a close phylogenetic relation between both groups (Fig. 3). This was also the case in phylogenetic trees constructed using the relatively short-branching Abedinium sequences as the only representatives of the Noctilucales, which still retrieved a basal emergence for these species, although poorly supported (data not shown). In our SSU rdna phylogeny, the Noctilucales emerge as a clade of free-living species in a basal position close to a variety of lineages of parasitic alveolates (perkinsoids, Marine Alveolate Group I and Syndiniales-MAGII) lacking chloroplasts and as sister to a large clade containing the nonphotosynthetic parasite Haplozoon Dogiel and the rest of dinokaryotes. In contrast, some typical dinokaryotes with a parasitic lifestyle possess plastids, e.g. Blastodinium Chatton and Dissodinium Klebs (Chatton 1920; Gómez et al. 2009a).
9 Molecular Phylogeny of Noctilucoid Dinoflagellates 9 Though the Noctilucales, including Spatulodinium, have been traditionally considered as exclusively heterotrophic (Larsen and Sournia 1991), we show in this study the occurrence of chloroplasts in S. pseudonoctiluca using epiflourescence microscopy (Fig. 1F, J). Chloroplasts are present in other species of Spatulodinium as illustrated in Gómez and Furuya (2007). Pomatodinium J. Cachon et Cachon-Enjumet may contain zooxanthellae according to Cachon and Cachon-Enjumet (1966). In the tropical to sub-tropical areas of Southeast Asia a green form of Noctiluca harbours thousands of free-swimming cells of the chlorophyte Pedinomonas noctilucae (Subrahmanyan) Sweeney inside the cell (Sweeney 1976). Hence, the Noctilucales appear to behave like other dinoflagellates in their ability to incorporate, replace or loose chloroplasts, a common phenomenon in the dinoflagellate core (Saldarriaga et al. 2001; Shalchian-Tabrizi et al. 2006) that contrasts with the rare occurrence of chloroplasts in other alveolate groups (Moore et al. 2008). Studies on the characteristics of the Spatulodinium plastids (pigmentation, ultrastructure and molecular sequences) will be necessary to determine whether their chloroplasts are keptoplastids (Koike et al. 2005) or if they derive from ancient endosymbiosis as in other dinoflagellate families. Most published phylogenies have placed Noctiluca as an early diverging lineage within dinoflagellates, branching after Oxyrrhis and before the core dinoflagellates (Cavalier-Smith and Chao 2004; Fukuda and Endoh 2008; Gómez et al. 2009b; Lenaers et al. 1991; Liu and Hastings 2007; Moore et al. 2008; Saldarriaga et al. 2001; Saunders et al. 1997; Shalchian-Tabrizi et al. 2006; Zhang and Lin 2008). In the present SSU rdna phylogeny, with more representatives, the Noctilucales appear to maintain their basal position (Fig. 3). Several morphological and ultrastructural features apparently agree with the placement of Noctiluca and the other noctilucoids as early diverging dinoflagellates, between the dinoflagellate core and the clades of Marine Alveolate Group I (Duboscquella Chatton and Ichthyodinium Hollande et Cachon) and Group II-Syndiniales (Amoebophrya Koeppen). The process of cell division in Marine Alveolate Groups I and II is very fast and distinctive (Cachon 1964; Harada et al. 2007). Duboscquella shows no evidence of condensed interphase chromosomes at any stage of its life cycle (Harada et al. 2007) and, during sporogenesis, a long, rosary-like chain of transparent sporocytes forms (Cachon 1964; Harada et al. 2007). Likewise, during sporogenesis, Kofoidinium sporocytes distribute helicoidally in a way that recalls that of Syndiniales such as Amoebophrya (Cachon and Cachon 1967). In Spatulodinium, Gómez and Souissi (2007) illustrated the formation of peculiar sporocyte clusters that are unknown in typical dinoflagellates. Cachon and Cachon (1967, p. 28) also observed that the division of the leptodiscacean Leptodiscus Hertwig was similar to that of Syndiniales as illustrated in Cachon (1964). Cachon and Cachon (1969, p. 15) illustrated a large (700 mm long) multiflagellate and multinucleate spororont of the leptodiscacean Scaphodinium mirabile. This resembles the multiflagellate stage that is released at the end of the intracellular phase of Amoebophrya. The vermiform stage differentiates and breaks apart releasing the new infective dinospores (Cachon and Cachon 1987, p. 586). All these observations converge to suggest that the sporogenesis in Noctilucales involves a helicoidal disposition of the sporocytes or vermiform stages, which resembles more the one of Syndiniales than that of typical dinokaryotic dinoflagellates. A peculiar characteristic of several noctilucoid genera, such as Noctiluca, Spatulodinium and Abedinium, is the presence of a highly moveable ventral tentacle, which is missing in typical dinoflagellates and other alveolates. The tentacle of Noctiluca moves slowly and shows a contraction-relaxation cycle helping in food capture and used only to convey the prey to the cytostome located at its base (Soyer 1970). It does not play any role in keeping the cell in suspension, as it appears to be the case also in Spatulodinium, which has a tentacle involved in the concentration of food particles (Cachon and Cachon 1967). Our study illustrates for the first time an extremely long tentacle in Spatulodinium specimens, suggesting that in oceanic conditions it may have other functions (Fig. 1R). The ventral tentacle of Noctiluca is striated, whereas there is not striation in Spatulodinium. The tentacle of Leptophyllus differs from those of Noctiluca and Spatulodinium. The cell moves the tentacle in all directions while it advances forward, like a tactile structure. The extreme of the tentacle has an intense orange pigmentation that suggests a putative photoreceptor function (Fig. 2A-D). It could be speculated that the extremity of the tentacle provides information to trigger the ejectile bodies for prey capture, but evidence for this potential role is missing at present. In contrast with all these noctilucoids, no tentacle has been reported in the three known species of Kofoidinium. However, in this study we have illustrated a species, likely
10 10 F. Gómez et al. unnoticed until now due to its delicacy and small size, showing a short pointed appendix that may be homologous to an atrophied tentacle (Fig. 1T). Another undescribed kofoidiniacean has two tentacles (Gómez and Furuya 2007). In addition to the morphological features mentioned above regarding sporogenesis and distinctive tentacles in several genera, all Noctilucales have in common cytoplasmic strands that extend radially from the nucleus to the membrane. They are involved in the locomotion, short-time shape changes and the complex ontogenetic process of these organisms (Figs 1 and 2; see v=wdhj6iailis). While these general traits can be useful for the diagnosis of the group, the cell shape has little taxonomic value. For instance, in our SSU rdna phylogeny, the sac-like Noctiluca species appeared among the laterally flattened Spatulodinium species. Furthermore, the cell morphology changes along the life cycle and very different shapes can be unambiguously attributed to the same species using molecular data. Such was the case of Gymnodinium lebouriae, unambiguously shown to be a life stage of S. pseudonoctiluca. From an evolutionary point of view, although our study suggests a basal position for the Noctilucales after the emergence of several parasitic alveolate lineages, this position is only weakly supported. Similarly, the monophyly of the otherwise three well-supported noctilucoid clades is weakly supported itself. Future analyses of additional phylogenetic markers and an extended taxon sampling should help to clarify their actual phylogenetic position. Methods Collection of organisms: Mediterranean specimens were collected by slowly filtering surface seawater taken from the end of the pier (depth 3 m) of the Station Marine d Endoume, Marseille ( N, E) from October 2007 to September A strainer with netting of 20, 40 or 60-mm mesh-size was used to collect the organisms and the filtered volume varied between 10 and 100 liters, according to the concentration of particles. The concentrated sample was examined in Utermöhl chambers at 100 magnification with a Nikon inverted microscope (Nikon Eclipse TE200) and was photographed at 200 or 400 magnification with a digital camera (Nikon Coolpix E995). Sampling continued from October 2008 to May 2009 in the surface waters of the port (depth of 2 m) of Banyuls sur Mer, France ( N, E). The samples were prepared with the same procedure described above. The specimens were observed with an Olympus inverted microscope (Olympus IX51) and photographed with an Olympus DP71 digital camera. Some specimens were stained with 4,6-diamidino-2-phenylindole (DAPI, Sigma) that specifically binds to double-stranded DNA, and when excited with ultraviolet (UV) light the DAPI-DNA complex fluoresces as bright blue. In addition, open-water samples were collected from the Gulf of Lions, NW Mediterranean Sea ( N, E) on July 14th 2008 within the context of the BOUM (Biogeochemistry from the Oligotrophic to the Ultra-oligotrophic Mediterranean) cruise. Ten liters were collected from the surface with a bucket and filtered by using a strainer of 20-mm netting aperture. The retained material was fixed with ethanol. Several replicate samples of 0.5 l were fixed with acid Lugol s solution. At the laboratory, the ethanol sample was examined following the procedure described above. In contrast to the live specimens, the ethanol-fixed samples showed a precipitate of elongate crystals and the specimens appeared highly distorted and non-pigmented. When compared with the Lugol-fixed sample, all the unarmoured dinoflagellates disappear in the ethanol samples, except for a few of the larger forms of Noctilucales. The type locality of Spatulodinium pseudonoctiluca is the English Channel, NE Atlantic Ocean (Pouchet 1885). A previous study based on Lugol-fixed plankton collected from an 8-year time-series in the NE English Channel, revealed a higher abundance of Spatulodinium in early summer (Gómez and Souissi 2007). The Atlantic specimens were collected with the method described above from the surface of a large tidal pool (30 m diameter, 1 m maximal depth) at the beach of Wimereux, France ( N, E) from 5-8 July The concentrate was scanned in settling chambers at 100 magnification with an inverted fluorescence microscope (Olympus IX71) and was photographed at 200 or 400 magnification with a digital camera (Olympus DP70-BSW). In all cases, each specimen was micropipetted individually with a fine capillary into a clean chamber and washed several times in serial drops of 0.2-mm filtered and sterilized seawater (live specimens from coastal waters) or ethanol (ethanol pre-fixed specimens from open waters). Finally, the specimen was deposited into a 0.2 ml Eppendorf tube filled with several drops of 100% ethanol. The sample was kept at room temperature and in darkness until the molecular analysis could be performed. PCR amplification of small subunit rrna genes (SSU rdnas) and sequencing: The specimens fixed in ethanol were centrifuged gently for 5 min at 3,000 rpm. Ethanol was then evaporated in a vacuum desiccator and single cells were resuspended directly in 25 ml of Ex TaKaRa (TaKaRa, distributed by Lonza Cia., Levallois-Perret, France) PCR reactions were done in a volume of 30 to 50 ml reaction mix containing pmol of the eukaryotic-specific SSU rdna primers EK-42F (5 0 -CTCAARGAYTAAGCCATGCA-3 0 ) and EK- 1520R (5 0 -CYGCAGGTTCACCTAC-3 0 ). The PCR reactions were performed under the following conditions: 2 min denaturation at 94 1C; 10 cycles of touch-down PCR (denaturation at 94 1C for 15 s; a 30 s annealing step at decreasing temperature from 65 down to 55 1C employing a 1 1C decrease with each cycle-, extension at 72 1C for 2 min); 20 additional cycles at 55 1C annealing temperature; and a final elongation step of 7 min at 72 1C. A nested PCR reaction was then carried out using 2-5 ml of the first PCR reaction in a GoTaq (Promega, Lyon, France) polymerase reaction mix containing the eukaryotic-specific primers EK-82F (5 0 - GAAACTGCGAATGGCTC-3 0 ) and EK-1498R (5 0 -CACCTACG- GAAACCTTGTTA-3 0 ) and similar PCR conditions as described above. A third, semi-nested, PCR was carried out using the dinoflagellate specific primer DIN464F (5 0 -TAACAATACAGGG- CATCCAT-3 0 ). Negative controls without template DNA were used at all amplification steps. Amplicons of the expected size (1,200 bp) were then sequenced bidirectionally using primers DIN464F and EK-1498R (Cogenics, Meylan, France).
11 Molecular Phylogeny of Noctilucoid Dinoflagellates 11 Phylogenetic analyses: The new sequences were aligned to a large multiple sequence alignment containing 1,100 publicly available complete or nearly complete (41,300 bp) dinoflagellate SSU rdna sequences using the profile alignment option of MUSCLE 3.7 (Edgar 2004). The resulting alignment was manually inspected using the program ED of the MUST package (Philippe 1993). Ambiguously aligned regions and gaps were excluded in phylogenetic analyses. Preliminary phylogenetic trees with all sequences were constructed using the Neighbour Joining (NJ) method (Saitou and Nei 1987) implemented in the MUST package (Philippe 1993). These trees allowed identifying the closest relatives of our sequences, which were selected, together with a sample of other dinoflagellate species, to carry out more computationally-intensive Maximum Likelihood (ML) and Bayesian Inference (BI) analyses. ML analyses were done with the program TREEFINDER (Jobb et al. 2004) applying a GTR þ G þ I model of nucleotide substitution, taking into account a proportion of invariable sites, and a G-shaped distribution of substitution rates with four rate categories. Bootstrap values were calculated using 1,000 pseudoreplicates with the same substitution model. The BI analyses were carried out with the program PHYLOBAYES applying a GTR þ CAT Bayesian mixture model (Lartillot and Philippe 2004), with two independent runs and 1,000,000 generations per run. After checking convergence (maximum difference between all bipartitions o0.01) and eliminating the first 1,500 trees (burn-in), a consensus tree was constructed sampling every 100 trees. Sequences were deposited in GenBank with the following accession numbers: GU GU (see also Table 1). The phylogenetic position of the noctilucoid dinoflagellates was analyzed by means of a global alignment of 103 taxa representing sequences of alveolates: perkinsoids (Perkinsus), Marine Alveolate Group I (Duboscquella, Ichthyodinium), syndineans / Alveolate Group II (Amoebophrya), environmental sequences and representative species of the dinokaryotes, with the perkinsoid sequences used as outgroup. Acknowledgements This is a contribution to the project DIVERPLAN- MED supported by a post-doctoral grant to F.G. of the Ministerio Español de Educación y Ciencia # D.M. and P.L.G. acknowledge financial support from the French CNRS and the ANR Biodiversity program (ANR BDIV Aquaparadox). We thank S. Souissi for laboratory facilities at the Station Marine de Wimereux, France, and A. Nowaczyk for the offshore ethanol samples. This is a contribution of the BOUM (Biogeochemistry from the Oligotrophic to the Ultraoligotrophic Mediterranean) project of the French national LEFE-CYBER program and of the European IP SESAME. Appendix A. Supplementary material Supplementary data associated with this article can be found in the online version at doi: / j.protis References Afzelius BA (1963) The nucleus of Noctiluca scintillans. Aspects of nucleocytoplasmic exchanges and the formation of nuclear membranes. J Cell Biol 19: Baker M (1753) Employment for the Microscope. Dodsley Cachon J (1964) Contribution a l étude des Péridiniens parasites. Cytologie, cycles évolutifs. Ann Sci Nat 12 (Ser VI)1 158 Cachon J, Cachon M (1967) Contribution a l étude des Noctilucidae Saville-Kent, I. Les Kofoidininae Cachon J. et M. Évolution, morphologique et systématique. Protistologica 3: Cachon J, Cachon M (1969) Contribution a l étude des Noctilucidae Saville-Kent. Évolution, morphologique, cytologie, systématique. II. Les Leptodiscinae Cachon J. et M. Protistologica 5:11 33 Cachon J, Cachon M (1987) Parasitic Dinoflagellates. In Taylor FJR (ed) The Biology of Dinoflagellates. Botanical Monographs, Vol. 21. Blackwell, Oxford, pp Cachon J, Cachon-Enjumet M (1964) Leptospathium navicula nov. gen., nov. sp. et Leptophyllus dasypus nov. gen. sp., Péridiniens Noctilucidae (Hertwig) du plancton néritique de Villefranche-sur-Mer. Bull Inst Océanogr Monaco 62 (1292) 1 12 Cachon J, Cachon-Enjumet M (1966) Pomatodinium impatiens nov. gen. nov. sp. péridiniens Noctilucidae Kent. Protistologica 2:23 30 Cavalier-Smith T, Chao EE (2004) Protalveolate phylogeny and systematics and the origins of Sporozoa and dinoflagellates (phylum Myzozoa nom. nov.). Eur J Protistol 40: Chatton E (1920) Les Péridiniens parasites. Morphologie, reproduction, éthologie. Arch Zool Exp Gén 59:1 475 Daugbjerg N, Hansen G, Larsen J, Moestrup Ø (2000) Phylogeny of some of the major genera of dinoflagellates based on ultrastructure and partial LSU rdna sequence data, including the erection of three new genera of unarmoured dinoflagellates. Phycologia 39: Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: Fensome RA, Taylor FJR, Norris G, Sarjeant WAS, Wharton DI, Williams GL (1993) A Classification of Living and Fossil Dinoflagellates. Micropaleontology Special Publication Number 7, American Museum of Natural History. Sheridan Press, Hanover, USA Fukuda Y, Endoh H (2006) New details from the complete life cycle of the red-tide dinoflagellate Noctiluca scintillans (Ehrenberg) McCartney. Eur J Protistol 42: Fukuda Y, Endoh H (2008) Phylogenetic analyses of the dinoflagellate Noctiluca scintillans based on b-tubulin and Hsp90 genes. Eur J Protistol 44:27 33 Gómez F, Furuya K (2005) Leptodiscaceans (Noctilucales, Dinophyceae) from the Pacific Ocean: First records of Petalodinium and Leptodiscus beyond the Mediterranean Sea. Eur J Protistol 41:
12 12 F. Gómez et al. Gómez F, Furuya K (2007) Kofoidinium, Spatulodinium and other kofoidiniaceans (Noctilucales, Dinophyceae) in the Pacific Ocean. Eur J Protistol 43: Gómez F, Souissi S (2007) The distribution and life cycle of the dinoflagellate Spatulodinium pseudonoctiluca (Dinophyceae, Noctilucales) in the northeastern English Channel. C R Biologies 330: Gómez F, Moreira D, López-García P(2009a) Life cycle and molecular phylogeny of the dinoflagellates Chytriodinium and Dissodinium, ectoparasites of copepod eggs. Eur J Protistol 45: Gómez F, López-García P, Nowaczyk A, Moreira D (2009b) The crustacean parasites Ellobiopsis Caullery, 1910 and Thalassomyces Niezabitowski, 1913 form a monophyletic divergent clade within the Alveolata. Syst Parasitol 74:65 74 Greuet C (1987) Complex Organelles. In Taylor FJR (ed) The Biology of Dinoflagellates. Botanical Monographs, Vol. 21. Blackwell, Oxford, pp Haeckel E (1873) Natürliche Schöpfungsgeschichte. Reimer, Berlin Hansen G, Larsen J (1992) Dinoflagellater i Danske Farvande. In Thomsen HA (ed) Plankton i de Indre Danske Farvande, 11. Havforskning fra Miljøstyrelsen, Copenhagen, pp Hansen G, Moestrup Ø (2005) Flagellar apparatus and nuclear chambers of the green dinoflagellate Gymnodinium chlorophorum. Phycol Res 53: Hansen G, Moestrup Ø, Roberts KR (2000) Light and electron microscopical observations on the type species of Gymnodinium, G. fuscum (Dinophyceae). Phycologia 39: Harada A, Ohtsuka S, Horiguchi T (2007) Species of the parasitic genus Duboscquella are members of the enigmatic Marine Alveolate Group I. Protist 158: Höhfeld I, Melkonian M (1995) Ultrastructure of the flagellar apparatus of Noctiluca miliaris Suriray swarmers (Dinophyceae). Phycologia 34: Hollande A (1974) Étude comparée de la mitose syndinienne, de celle des péridiniens libres et des hypermastigines; infrastructure et cycle évolutif des syndinides parasites de radiolaires. Protistologica 10: Hoppenrath M (2004) A revised checklist of planktonic diatoms and dinoflagellates from Helgoland (North Sea, German Bight). Helgoland Mar Res 58: Hoppenrath M, Leander BS (2007) Morphology and phylogeny of the pseudocolonial dinoflagellates Polykrikos lebourae and Polykrikos herdmanae n. sp. Protist 158: Jobb G, von Haeseler A, Strimmer K (2004) TREEFINDER: A powerful graphical analysis environment for molecular phylogenetics. BMC Evol Biol 4:18 Kofoid CA (1920) A new morphological interpretation of the structure of Noctiluca, and its bearing on the status of the Cystoflagellata (Haeckel). Univ Calif Publ Zool 19: Koike K, Sekiguchi H, Kobiyama A, Takishita K, Kawachi M, Koike K, Ogata T (2005) A novel type of kleptoplastidy in Dinophysis (Dinophyceae): presence of haptophyte-type plastid in Dinophysis mitra. Protist 156: Konovalova GV, Selina MS (2002) Life cycle of Spatulodinium pseudonoctiluca (Dinophyta) from the Sea of Japan. Bot Zh 87:38 43 Larsen J, Sournia A (1991) The Diversity of Heterotrophic Dinoflagellates. In Larsen J, Patterson DJ (eds) The Biology of Free-living Heterotrophic Flagellates. Systematics Association Spec. Vol 45, Clarendon Press, Oxford, pp Lartillot N, Philippe H (2004) A Bayesian mixture model for across-site heterogeneities in the amino-acid replacement process. Mol Biol Evol 21: Lenaers G, Scholin C, Bhaud Y, Saint-Hilaire D (1991) A molecular phylogeny of dinoflagellate protists (Pyrrhophyta) inferred from the sequence of 24S rrna divergent domain D1 and D8. J Mol Evol 32:53 63 Liu L, Hastings JW (2007) Two different domains of the luciferase gene in the heterotrophic dinoflagellate Noctiluca scintillans occur as two separate genes in photosynthetic species. Proc Natl Acad Sci USA 104: Melkonian M, Höhfeld I (1988) Amphiesmal ultrastructure in Noctiluca miliaris Suriray (Dinophyceae). Helgol Meeresunters 42: Moore R, Oborník M, Janouskovec J, Chrudimsky T, Vancová M, Green DH, Wright SW et al (2008) A photosynthetic alveolate closely related to apicomplexan parasites. Nature 451: Pfiester AL (1984) Dinoflagellate Nuclei. In Spector DL (ed) Dinoflagellates. Academic Press, Orlando, pp Philippe H (1993) MUST, a computer package of management utilities for sequences and trees. Nucleic Acids Res 21: Pouchet G (1885) Nouvelle contribution a lhistoire des Péridiniens marins. J Anat Physiol 21:28 88 Rizzo PJ (2003) Those amazing dinoflagellate chromosomes. Cell Res 13: Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4: Saldarriaga JF, Taylor FJR, Keeling PJ, Cavalier-Smith T (2001) Dinoflagellate nuclear SSU rrna phylogeny suggests multiple plastid losses and replacements. J Mol Evol 53: Saldarriaga JF, Taylor FJR, Cavalier-Smith T, Menden- Deuer S, Keeling PJ (2004) Molecular data and the evolutionary history of dinoflagellates. Eur J Protistol 40: Saunders GW, Hill DR, Sexton JP, Andersen RA (1997) Small-subunit Ribosomal RNA Sequences from Selected Dinoflagellates: Testing Classical Evolutionary Hypotheses with Molecular Systematic Methods. In Bhattacharya D (ed) Origins of Algae and their Plastids. Springer, Wien, New York, pp Shalchian-Tabrizi K, Minge MA, Cavalier-Smith T, Nedreklepp JM, Klaveness D, Jakobsen KS (2006) Combined heat shock protein 90 and ribosomal RNA sequence phylogeny supports multiple replacements of dinoflagellate plastids. J Eukaryot Microbiol 53:
13 Molecular Phylogeny of Noctilucoid Dinoflagellates 13 Slabber M. (1771) Natuurkundige Verlustigingen behelzende microscopise Waarneemingen van In- en Uit-landse Water- en Land-Dieren, J. Bosch, Haarlem Soyer MO (1969) L enveloppe nucléaire chez Noctiluca miliaris S. (i.e. scintillans) (Dinoflagellata). I. Quelques données sur son ultrastructure et son évolution au cours de la sporogenese. J Microsc 8: Soyer MO (1970) Les ultrastructures liées aux fonctions de relation chez Noctiluca miliaris S. (Dinoflagellata). Z Zellforsch 104:29 55 Soyer MO (1972) Les ultrastructures nucléaires de la Noctiluque (Dinoflagellé libre) au cours de la sporogenese. Chromosoma 39: Sweeney BM (1976) Pedinomonas noctilucae (Prasinophyceae), the flagellate symbiotic in Noctiluca (Dinophyceae) in Southeast Asia. J Phycol 12: Wessenberg H, Antipa G (1970) Capture and ingestion of Paramecium by Didinium nasutum. J Protozool 17: Zhang H, Lin S (2008) mrna editing and spliced-leader RNA trans-splicing groups Oxyrrhis, Noctiluca, Heterocapsa, and Amphidinium as basal lineages of dinoflagellates. J Phycol 44: Zingmark RG (1970) Sexual reproduction in the dinoflagellate Noctiluca miliaris Suriray. J Phycol 6:
THE warnowiids are the only known unicellular organisms
 J. Eukaryot. Microbiol., 56(5), 2009 pp. 440 445 r 2009 The Author(s) Journal compilation r 2009 by the International Society of Protistologists DOI: 10.1111/j.1550-7408.2009.00420.x Molecular Phylogeny
J. Eukaryot. Microbiol., 56(5), 2009 pp. 440 445 r 2009 The Author(s) Journal compilation r 2009 by the International Society of Protistologists DOI: 10.1111/j.1550-7408.2009.00420.x Molecular Phylogeny
GÓMEZ ET AL.---MOLECULAR PHYLOGENY OF OCELLOID-BEARING DINOFLAGELLATES
 GÓMEZ ET AL.---MOLECULAR PHYLOGENY OF OCELLOID-BEARING DINOFLAGELLATES Molecular Phylogeny of the Ocelloid-bearing Dinoflagellates Erythropsidinium and Warnowia (Warnowiaceae, Dinophyceae) FERNANDO GÓMEZ,
GÓMEZ ET AL.---MOLECULAR PHYLOGENY OF OCELLOID-BEARING DINOFLAGELLATES Molecular Phylogeny of the Ocelloid-bearing Dinoflagellates Erythropsidinium and Warnowia (Warnowiaceae, Dinophyceae) FERNANDO GÓMEZ,
Importance of Protists
 Protists Protists The kingdom Protista is a very diverse kingdom. Eukaryotes that are not classified as fungi, plants, or animals are classified as protists. However, even though they are officially in
Protists Protists The kingdom Protista is a very diverse kingdom. Eukaryotes that are not classified as fungi, plants, or animals are classified as protists. However, even though they are officially in
BIOLOGY - CLUTCH CH.29 - PROTISTS.
 !! www.clutchprep.com Eukrayotic cells are large, have a nucleus, contain membrane-bound organelles, and use a cytoskeleton The nucleus is the synapomorphy that unifies eukaryotes Endosymbiotic theory
!! www.clutchprep.com Eukrayotic cells are large, have a nucleus, contain membrane-bound organelles, and use a cytoskeleton The nucleus is the synapomorphy that unifies eukaryotes Endosymbiotic theory
Culture of green Noctiluca under laboratory conditions: I. Feeding behavior and sexual reproduction
 Culture of green Noctiluca under laboratory conditions: I. Feeding behavior and sexual reproduction Thaithaworn Lirdwitayaprasit Department of Marine Science, Faculty of Science, Chulalongkorn University,
Culture of green Noctiluca under laboratory conditions: I. Feeding behavior and sexual reproduction Thaithaworn Lirdwitayaprasit Department of Marine Science, Faculty of Science, Chulalongkorn University,
Fig. 16. Majority rule consensus tree depicting phylogenetic relationships inferred among 74 species of heterokont algae. Note that A.
 Plate 1 Figs. 1-8. Light microscopic images of Anthophysa vegetans colonies and individual motile cells. Figs 1-5. Stalked (arrow; Figs 1,2) or unstalked (Figs 3-5) colonies consisting of ca. 10-20 spherical
Plate 1 Figs. 1-8. Light microscopic images of Anthophysa vegetans colonies and individual motile cells. Figs 1-5. Stalked (arrow; Figs 1,2) or unstalked (Figs 3-5) colonies consisting of ca. 10-20 spherical
Received 21 January 2009, revised 11 February 2009, accepted 16 February 2009.
 Communications The first recorded bloom of Pseudochattonella farcimen (Dictyochophyceae, Heterokonta),(Riisberg I.,2008)intheGulfof Gdańsk* OCEANOLOGIA, 51(1), 2009. pp. 139 143. C 2009,byInstituteof Oceanology
Communications The first recorded bloom of Pseudochattonella farcimen (Dictyochophyceae, Heterokonta),(Riisberg I.,2008)intheGulfof Gdańsk* OCEANOLOGIA, 51(1), 2009. pp. 139 143. C 2009,byInstituteof Oceanology
Re-classification of Pheopolykrikos hartmannii as Polykrikos (Dinophyceae) based partly on the ultrastructure of complex extrusomes
 European Journal of Protistology 46 (2010) 29 37 European Journal of PROTISTOLOGY www.elsevier.de/ejop Re-classification of Pheopolykrikos hartmannii as Polykrikos (Dinophyceae) based partly on the ultrastructure
European Journal of Protistology 46 (2010) 29 37 European Journal of PROTISTOLOGY www.elsevier.de/ejop Re-classification of Pheopolykrikos hartmannii as Polykrikos (Dinophyceae) based partly on the ultrastructure
Prasinophyaceae Evolutionary Relict s Class of Algae
 Prasinophyaceae Evolutionary Relict s Class of Algae Teena Agrawal* School of Applied Science, Banasthali University, Rajasthan, India Review Article Received: 18/10/2017 Accepted: 22/10/2017 Published:
Prasinophyaceae Evolutionary Relict s Class of Algae Teena Agrawal* School of Applied Science, Banasthali University, Rajasthan, India Review Article Received: 18/10/2017 Accepted: 22/10/2017 Published:
Single-cell genomics applied to the picobiliphytes using next-generation sequencing
 Department of Ecology, Evolution and Natural Resources and Institute of Marine and Coastal Sciences Rutgers University, NJ 08901 Single-cell genomics applied to the picobiliphytes using next-generation
Department of Ecology, Evolution and Natural Resources and Institute of Marine and Coastal Sciences Rutgers University, NJ 08901 Single-cell genomics applied to the picobiliphytes using next-generation
Chapter 26: Phylogeny and the Tree of Life Phylogenies Show Evolutionary Relationships
 Chapter 26: Phylogeny and the Tree of Life You Must Know The taxonomic categories and how they indicate relatedness. How systematics is used to develop phylogenetic trees. How to construct a phylogenetic
Chapter 26: Phylogeny and the Tree of Life You Must Know The taxonomic categories and how they indicate relatedness. How systematics is used to develop phylogenetic trees. How to construct a phylogenetic
29/11/2012. Characteristics. Protist Diversity. Characteristics. Kingdom Protista. Examples of Plant-like Protists
 Kingdom Protista Learning Outcome B1 Characteristics Appeared in the fossil record 1.5 billion years ago have an evolutionary advancement over bacteria, because they have a membranebound nucleus. also
Kingdom Protista Learning Outcome B1 Characteristics Appeared in the fossil record 1.5 billion years ago have an evolutionary advancement over bacteria, because they have a membranebound nucleus. also
Protists The Simplest Eukaryotes. Chapter 22 Part 1
 Protists The Simplest Eukaryotes Chapter 22 Part 1 Impacts, Issues The Malaria Menace Plasmodium, a single-celled protist, causes malaria but also manipulates its mosquito and human hosts to maximize its
Protists The Simplest Eukaryotes Chapter 22 Part 1 Impacts, Issues The Malaria Menace Plasmodium, a single-celled protist, causes malaria but also manipulates its mosquito and human hosts to maximize its
There are two commonly accepted theories for how eukaryotic cells evolved: infolding and endosymbiosis. Infolding
 Protists Protists The kingdom Protista is a very diverse kingdom. Eukaryotes that are not classified as fungi, plants, or animals are classified as protists. However, even though they are officially in
Protists Protists The kingdom Protista is a very diverse kingdom. Eukaryotes that are not classified as fungi, plants, or animals are classified as protists. However, even though they are officially in
Taxonomy. Content. How to determine & classify a species. Phylogeny and evolution
 Taxonomy Content Why Taxonomy? How to determine & classify a species Domains versus Kingdoms Phylogeny and evolution Why Taxonomy? Classification Arrangement in groups or taxa (taxon = group) Nomenclature
Taxonomy Content Why Taxonomy? How to determine & classify a species Domains versus Kingdoms Phylogeny and evolution Why Taxonomy? Classification Arrangement in groups or taxa (taxon = group) Nomenclature
Kingdom Protista. The following organisms will be examined in the lab today: Volvox, Oedogonium, Spirogyra, Ulva
 Kingdom Protista I. Introduction The protists are a diverse group of organisms. In the past they have been classified as fungi, plants and animals. They can be green, autotrophs or nongreen heterotrophs.
Kingdom Protista I. Introduction The protists are a diverse group of organisms. In the past they have been classified as fungi, plants and animals. They can be green, autotrophs or nongreen heterotrophs.
Protists. Protists. Protist Feeding Strategies. Protist Body Plans. Endosymbiosis. Protist Reproduction 3/3/2011. Eukaryotes Not a monophyletic group
 Protists Protists Eukaryotes Not a monophyletic group Paraphyletic March 3 rd, 2011 Still use the term protist All eukaryotes except Plants, Fungi, Animals Most unicellular Some colonial Some multicelled
Protists Protists Eukaryotes Not a monophyletic group Paraphyletic March 3 rd, 2011 Still use the term protist All eukaryotes except Plants, Fungi, Animals Most unicellular Some colonial Some multicelled
8/23/2014. Introduction to Animal Diversity
 Introduction to Animal Diversity Chapter 32 Objectives List the characteristics that combine to define animals Summarize key events of the Paleozoic, Mesozoic, and Cenozoic eras Distinguish between the
Introduction to Animal Diversity Chapter 32 Objectives List the characteristics that combine to define animals Summarize key events of the Paleozoic, Mesozoic, and Cenozoic eras Distinguish between the
Mitosis vs Meiosis. Mitosis and Meiosis -- Internet Tutorial
 Mitosis and Meiosis -- Internet Tutorial In this internet lesson, you will review the steps of mitosis and meiosis and view video simulations of cell division. Mitosis: An Interactive Animation (http://www.cellsalive.com/mitosis.htm)
Mitosis and Meiosis -- Internet Tutorial In this internet lesson, you will review the steps of mitosis and meiosis and view video simulations of cell division. Mitosis: An Interactive Animation (http://www.cellsalive.com/mitosis.htm)
Biology 1 Notebook. Review Answers Pages 17 -?
 Biology 1 Notebook Review Answers Pages 17 -? The History of Cell Studies 1. Robert Hook (1665) used a microscope to examine a thin slice of cork. The little boxes he observed reminded him of the small
Biology 1 Notebook Review Answers Pages 17 -? The History of Cell Studies 1. Robert Hook (1665) used a microscope to examine a thin slice of cork. The little boxes he observed reminded him of the small
8/23/2014. Phylogeny and the Tree of Life
 Phylogeny and the Tree of Life Chapter 26 Objectives Explain the following characteristics of the Linnaean system of classification: a. binomial nomenclature b. hierarchical classification List the major
Phylogeny and the Tree of Life Chapter 26 Objectives Explain the following characteristics of the Linnaean system of classification: a. binomial nomenclature b. hierarchical classification List the major
BINF6201/8201. Molecular phylogenetic methods
 BINF60/80 Molecular phylogenetic methods 0-7-06 Phylogenetics Ø According to the evolutionary theory, all life forms on this planet are related to one another by descent. Ø Traditionally, phylogenetics
BINF60/80 Molecular phylogenetic methods 0-7-06 Phylogenetics Ø According to the evolutionary theory, all life forms on this planet are related to one another by descent. Ø Traditionally, phylogenetics
INTRODUCTION prokaryotic eukaryotic pigments
 INTRODUCTION This exercise is intended for you to get familiar and comfortable with using a microscope as well as identifying common microbial groups. Thus, we will observe representatives of all microbes
INTRODUCTION This exercise is intended for you to get familiar and comfortable with using a microscope as well as identifying common microbial groups. Thus, we will observe representatives of all microbes
Amoeba hunts and kills paramecia and stentor. Eukaryotic photosynthetic cells
 Amoeba hunts and kills paramecia and stentor Eukaryotic photosynthetic cells 1 Eukaryotic organelles are odd in many ways Organelles: membrane bound compartments in a cell Nucleus, chloroplasts, and mitochondria
Amoeba hunts and kills paramecia and stentor Eukaryotic photosynthetic cells 1 Eukaryotic organelles are odd in many ways Organelles: membrane bound compartments in a cell Nucleus, chloroplasts, and mitochondria
SPECIATION. REPRODUCTIVE BARRIERS PREZYGOTIC: Barriers that prevent fertilization. Habitat isolation Populations can t get together
 SPECIATION Origin of new species=speciation -Process by which one species splits into two or more species, accounts for both the unity and diversity of life SPECIES BIOLOGICAL CONCEPT Population or groups
SPECIATION Origin of new species=speciation -Process by which one species splits into two or more species, accounts for both the unity and diversity of life SPECIES BIOLOGICAL CONCEPT Population or groups
Eukaryotic photosynthetic cells
 Amoeba hunts and kills paramecia and stentor Eukaryotic photosynthetic cells Eukaryotic organelles are odd in many ways Organelles: membrane bound compartments in a cell Nucleus, chloroplasts, and mitochondria
Amoeba hunts and kills paramecia and stentor Eukaryotic photosynthetic cells Eukaryotic organelles are odd in many ways Organelles: membrane bound compartments in a cell Nucleus, chloroplasts, and mitochondria
Warm-Up Pairs Discuss the diagram What Where Which Why
 Warm-Up In Pairs Discuss the diagram What is it? Where does it come from? Which parts can you label? (in pencil) Why do you think you will learn about it? 5 m Eukaryote: Organelles, Structure and Function
Warm-Up In Pairs Discuss the diagram What is it? Where does it come from? Which parts can you label? (in pencil) Why do you think you will learn about it? 5 m Eukaryote: Organelles, Structure and Function
Protists 9/11/2017. Endosymbiosis
 Protists Chapter 28 Most eukaryotes are single-celled organisms Protists are eukaryotes Eukaryotic cells have organelles and are more complex than prokaryotic cells Most protists are unicellular, but there
Protists Chapter 28 Most eukaryotes are single-celled organisms Protists are eukaryotes Eukaryotic cells have organelles and are more complex than prokaryotic cells Most protists are unicellular, but there
Chapter 19: Taxonomy, Systematics, and Phylogeny
 Chapter 19: Taxonomy, Systematics, and Phylogeny AP Curriculum Alignment Chapter 19 expands on the topics of phylogenies and cladograms, which are important to Big Idea 1. In order for students to understand
Chapter 19: Taxonomy, Systematics, and Phylogeny AP Curriculum Alignment Chapter 19 expands on the topics of phylogenies and cladograms, which are important to Big Idea 1. In order for students to understand
10/1/2014. Chapter Explain why the cell is considered to be the basic unit of life.
 Chapter 4 PSAT $ by October by October 11 Test 3- Tuesday October 14 over Chapter 4 and 5 DFA- Monday October 20 over everything covered so far (Chapters 1-5) Review on Thursday and Friday before 1. Explain
Chapter 4 PSAT $ by October by October 11 Test 3- Tuesday October 14 over Chapter 4 and 5 DFA- Monday October 20 over everything covered so far (Chapters 1-5) Review on Thursday and Friday before 1. Explain
The Microbial World. Chapter 5
 The Microbial World Chapter 5 Viruses Non-cellular infectious agents that have two basic characteristics: Not capable of reproduction without a host cell Structure: Nucleic acid core- can be DNA or RNA
The Microbial World Chapter 5 Viruses Non-cellular infectious agents that have two basic characteristics: Not capable of reproduction without a host cell Structure: Nucleic acid core- can be DNA or RNA
AP Biology. Biology is the only subject in which multiplication is the same thing as division. The Cell Cycle: Cell Growth, Cell Division
 QuickTime and and a TIFF TIFF (Uncompressed) decompressor are are needed needed to to see see this this picture. picture. Biology is the only subject in which multiplication is the same thing as division
QuickTime and and a TIFF TIFF (Uncompressed) decompressor are are needed needed to to see see this this picture. picture. Biology is the only subject in which multiplication is the same thing as division
Eucaryotic Cell Structure and Function
 Chapter 4 Part II Eucaryotic Cell Structure and Function The Nucleus and Cell Division! Constant feature in eukaryotic cells! Place where the cell s genetic information and its control center Nuclear Structure!
Chapter 4 Part II Eucaryotic Cell Structure and Function The Nucleus and Cell Division! Constant feature in eukaryotic cells! Place where the cell s genetic information and its control center Nuclear Structure!
CHAPTER 10 Taxonomy and Phylogeny of Animals
 CHAPTER 10 Taxonomy and Phylogeny of Animals 10-1 10-2 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Linnaeus and Taxonomy More than 1.5 million species of
CHAPTER 10 Taxonomy and Phylogeny of Animals 10-1 10-2 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Linnaeus and Taxonomy More than 1.5 million species of
Chapter 6: A Tour of the Cell
 Chapter 6: A Tour of the Cell 1. The study of cells has been limited by their small size, and so they were not seen and described until 1665, when Robert Hooke first looked at dead cells from an oak tree.
Chapter 6: A Tour of the Cell 1. The study of cells has been limited by their small size, and so they were not seen and described until 1665, when Robert Hooke first looked at dead cells from an oak tree.
What Are the Protists?
 Protists 1 What Are the Protists? 2 Protists are all the eukaryotes that are not fungi, plants, or animals. Protists are a paraphyletic group. Protists exhibit wide variation in morphology, size, and nutritional
Protists 1 What Are the Protists? 2 Protists are all the eukaryotes that are not fungi, plants, or animals. Protists are a paraphyletic group. Protists exhibit wide variation in morphology, size, and nutritional
Protists: Algae Lecture 5 Spring 2014
 Protists: Algae Lecture 5 Spring 2014 Meet the algae 1 Protist Phylogeny Algae - Not monophyletic What unites them as a group? Range from unicellular to multicellular From phytoplankton to kelp forests
Protists: Algae Lecture 5 Spring 2014 Meet the algae 1 Protist Phylogeny Algae - Not monophyletic What unites them as a group? Range from unicellular to multicellular From phytoplankton to kelp forests
This is a repository copy of Microbiology: Mind the gaps in cellular evolution.
 This is a repository copy of Microbiology: Mind the gaps in cellular evolution. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/114978/ Version: Accepted Version Article:
This is a repository copy of Microbiology: Mind the gaps in cellular evolution. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/114978/ Version: Accepted Version Article:
Protists: Algae Lecture 5 Spring Protist Phylogeny. Meet the algae. Primary & Secondary Endosymbiosis. Endosymbiosis. Secondary Endosymbiosis
 Meet the algae Protists: Algae Lecture 5 Spring 2014 Protist Phylogeny 1 Primary & Secondary Endosymbiosis 2 Algae - Not monophyletic What unites them as a group? Range from unicellular to multicellular
Meet the algae Protists: Algae Lecture 5 Spring 2014 Protist Phylogeny 1 Primary & Secondary Endosymbiosis 2 Algae - Not monophyletic What unites them as a group? Range from unicellular to multicellular
InDel 3-5. InDel 8-9. InDel 3-5. InDel 8-9. InDel InDel 8-9
 Lecture 5 Alignment I. Introduction. For sequence data, the process of generating an alignment establishes positional homologies; that is, alignment provides the identification of homologous phylogenetic
Lecture 5 Alignment I. Introduction. For sequence data, the process of generating an alignment establishes positional homologies; that is, alignment provides the identification of homologous phylogenetic
Classification, Phylogeny yand Evolutionary History
 Classification, Phylogeny yand Evolutionary History The diversity of life is great. To communicate about it, there must be a scheme for organization. There are many species that would be difficult to organize
Classification, Phylogeny yand Evolutionary History The diversity of life is great. To communicate about it, there must be a scheme for organization. There are many species that would be difficult to organize
Microbial Taxonomy. Microbes usually have few distinguishing properties that relate them, so a hierarchical taxonomy mainly has not been possible.
 Microbial Taxonomy Traditional taxonomy or the classification through identification and nomenclature of microbes, both "prokaryote" and eukaryote, has been in a mess we were stuck with it for traditional
Microbial Taxonomy Traditional taxonomy or the classification through identification and nomenclature of microbes, both "prokaryote" and eukaryote, has been in a mess we were stuck with it for traditional
Phylogeny 9/8/2014. Evolutionary Relationships. Data Supporting Phylogeny. Chapter 26
 Phylogeny Chapter 26 Taxonomy Taxonomy: ordered division of organisms into categories based on a set of characteristics used to assess similarities and differences Carolus Linnaeus developed binomial nomenclature,
Phylogeny Chapter 26 Taxonomy Taxonomy: ordered division of organisms into categories based on a set of characteristics used to assess similarities and differences Carolus Linnaeus developed binomial nomenclature,
PHYLOGENY AND SYSTEMATICS
 AP BIOLOGY EVOLUTION/HEREDITY UNIT Unit 1 Part 11 Chapter 26 Activity #15 NAME DATE PERIOD PHYLOGENY AND SYSTEMATICS PHYLOGENY Evolutionary history of species or group of related species SYSTEMATICS Study
AP BIOLOGY EVOLUTION/HEREDITY UNIT Unit 1 Part 11 Chapter 26 Activity #15 NAME DATE PERIOD PHYLOGENY AND SYSTEMATICS PHYLOGENY Evolutionary history of species or group of related species SYSTEMATICS Study
Chapter 26 Phylogeny and the Tree of Life
 Chapter 26 Phylogeny and the Tree of Life Biologists estimate that there are about 5 to 100 million species of organisms living on Earth today. Evidence from morphological, biochemical, and gene sequence
Chapter 26 Phylogeny and the Tree of Life Biologists estimate that there are about 5 to 100 million species of organisms living on Earth today. Evidence from morphological, biochemical, and gene sequence
Kingdom Protista. The world of Protists: Animal-like Protists Plant-like Protists Fungus-like Protists
 Kingdom Protista The world of Protists: Animal-like Protists Plant-like Protists Fungus-like Protists DOMAIN EUKARYA PROTISTS KINGDOM PROTISTA Any eukaryote that is not classified as a fungus, plant, or
Kingdom Protista The world of Protists: Animal-like Protists Plant-like Protists Fungus-like Protists DOMAIN EUKARYA PROTISTS KINGDOM PROTISTA Any eukaryote that is not classified as a fungus, plant, or
Chapter 6 A Tour of the Cell
 Chapter 6 A Tour of the Cell The cell is the basic unit of life Although cells differ substantially from one another, they all share certain characteristics that reflect a common ancestry and remind us
Chapter 6 A Tour of the Cell The cell is the basic unit of life Although cells differ substantially from one another, they all share certain characteristics that reflect a common ancestry and remind us
Lecture 11 Friday, October 21, 2011
 Lecture 11 Friday, October 21, 2011 Phylogenetic tree (phylogeny) Darwin and classification: In the Origin, Darwin said that descent from a common ancestral species could explain why the Linnaean system
Lecture 11 Friday, October 21, 2011 Phylogenetic tree (phylogeny) Darwin and classification: In the Origin, Darwin said that descent from a common ancestral species could explain why the Linnaean system
Chapter 22: Protists
 Chapter 22: Protists Protists Protistans are Unlike Prokaryotes Have a nucleus and organelles Have proteins associated with DNA Use microtubules in a cytoskeleton, spindle apparatus, and cilia and flagella
Chapter 22: Protists Protists Protistans are Unlike Prokaryotes Have a nucleus and organelles Have proteins associated with DNA Use microtubules in a cytoskeleton, spindle apparatus, and cilia and flagella
Outline. v Definition and major characteristics of animals v Dividing animals into groups based on: v Animal Phylogeny
 BIOSC 041 Overview of Animal Diversity: Animal Body Plans Reference: Chapter 32 Outline v Definition and major characteristics of animals v Dividing animals into groups based on: Body symmetry Tissues
BIOSC 041 Overview of Animal Diversity: Animal Body Plans Reference: Chapter 32 Outline v Definition and major characteristics of animals v Dividing animals into groups based on: Body symmetry Tissues
Microbial Taxonomy and the Evolution of Diversity
 19 Microbial Taxonomy and the Evolution of Diversity Copyright McGraw-Hill Global Education Holdings, LLC. Permission required for reproduction or display. 1 Taxonomy Introduction to Microbial Taxonomy
19 Microbial Taxonomy and the Evolution of Diversity Copyright McGraw-Hill Global Education Holdings, LLC. Permission required for reproduction or display. 1 Taxonomy Introduction to Microbial Taxonomy
FLOW: Amigos de Bolsa Chica Citizen Science Program. Plankton Collection and Identification Report
 FLOW: Amigos de Bolsa Chica Citizen Science Program Plankton Collection and Identification Report Date: 05/03/13 Time: 10:30 AM Collectors: Judy H., Dennis P., Nicole G., Joana T. Tide: ebb (going out)
FLOW: Amigos de Bolsa Chica Citizen Science Program Plankton Collection and Identification Report Date: 05/03/13 Time: 10:30 AM Collectors: Judy H., Dennis P., Nicole G., Joana T. Tide: ebb (going out)
Overview of Cells. Prokaryotes vs Eukaryotes The Cell Organelles The Endosymbiotic Theory
 Overview of Cells Prokaryotes vs Eukaryotes The Cell Organelles The Endosymbiotic Theory Prokaryotic Cells Archaea Bacteria Come in many different shapes and sizes.5 µm 2 µm, up to 60 µm long Have large
Overview of Cells Prokaryotes vs Eukaryotes The Cell Organelles The Endosymbiotic Theory Prokaryotic Cells Archaea Bacteria Come in many different shapes and sizes.5 µm 2 µm, up to 60 µm long Have large
Assessing an Unknown Evolutionary Process: Effect of Increasing Site- Specific Knowledge Through Taxon Addition
 Assessing an Unknown Evolutionary Process: Effect of Increasing Site- Specific Knowledge Through Taxon Addition David D. Pollock* and William J. Bruno* *Theoretical Biology and Biophysics, Los Alamos National
Assessing an Unknown Evolutionary Process: Effect of Increasing Site- Specific Knowledge Through Taxon Addition David D. Pollock* and William J. Bruno* *Theoretical Biology and Biophysics, Los Alamos National
Topic 17 Introduction to Domain Eukarya - Organisms with nucleated cells
 Topic 17 Introduction to Domain Eukarya - Organisms with nucleated cells Domain Eukarya. Eukaryotes have nucleated cells. Endosymbiosis has played an important role in the evolution of the group. Both
Topic 17 Introduction to Domain Eukarya - Organisms with nucleated cells Domain Eukarya. Eukaryotes have nucleated cells. Endosymbiosis has played an important role in the evolution of the group. Both
The Cell Notes 1 of 11
 The Cell The basic unit of structure and function in living things The smallest units in living things The smallest units in living things that show the characteristics of life Organisms can be made of
The Cell The basic unit of structure and function in living things The smallest units in living things The smallest units in living things that show the characteristics of life Organisms can be made of
CLASSIFICATION. Why Classify? 2/18/2013. History of Taxonomy Biodiversity: variety of organisms at all levels from populations to ecosystems.
 Why Classify? Classification has been around ever since people paid attention to organisms. CLASSIFICATION One primeval system was based on harmful and non-harmful organisms. Life is easier when we organize
Why Classify? Classification has been around ever since people paid attention to organisms. CLASSIFICATION One primeval system was based on harmful and non-harmful organisms. Life is easier when we organize
C3020 Molecular Evolution. Exercises #3: Phylogenetics
 C3020 Molecular Evolution Exercises #3: Phylogenetics Consider the following sequences for five taxa 1-5 and the known outgroup O, which has the ancestral states (note that sequence 3 has changed from
C3020 Molecular Evolution Exercises #3: Phylogenetics Consider the following sequences for five taxa 1-5 and the known outgroup O, which has the ancestral states (note that sequence 3 has changed from
9/11/18. Molecular and Cellular Biology. 3. The Cell From Genes to Proteins. key processes
 Molecular and Cellular Biology Animal Cell ((eukaryotic cell) -----> compare with prokaryotic cell) ENDOPLASMIC RETICULUM (ER) Rough ER Smooth ER Flagellum Nuclear envelope Nucleolus NUCLEUS Chromatin
Molecular and Cellular Biology Animal Cell ((eukaryotic cell) -----> compare with prokaryotic cell) ENDOPLASMIC RETICULUM (ER) Rough ER Smooth ER Flagellum Nuclear envelope Nucleolus NUCLEUS Chromatin
CHARACTERISTICS OF LIFE ORGANIZATION OF LIFE CELL THEORY TIMELINE
 CHARACTERISTICS OF LIFE 1. composed of cells either uni/multi 2. reproduce sexual and/or asexual 3. contain DNA in cells 4. grow and develop 5. use material/energy in metabolic reactions 6. respond to
CHARACTERISTICS OF LIFE 1. composed of cells either uni/multi 2. reproduce sexual and/or asexual 3. contain DNA in cells 4. grow and develop 5. use material/energy in metabolic reactions 6. respond to
CELL TYPE. Unit #4: Cell Structure & Func2on. Classifica(on, Endosymbiosis, Cell Type, Cell Organelles
 Unit #4: Cell Structure & Func2on Classifica(on, Endosymbiosis, Cell Type, Cell Organelles How are prokaryo(c cells and eukaryo(c cells similar? different? CELL TYPE Cell Theory Many scientists were involved
Unit #4: Cell Structure & Func2on Classifica(on, Endosymbiosis, Cell Type, Cell Organelles How are prokaryo(c cells and eukaryo(c cells similar? different? CELL TYPE Cell Theory Many scientists were involved
Notes - Microbiology Protista
 Notes - Microbiology Protista Part 1 Animal like Protists - Kingdom Protista is a very diverse group of organisms. There are over 115 000 different kinds, with traits that fit with fungi, plants, and animals.
Notes - Microbiology Protista Part 1 Animal like Protists - Kingdom Protista is a very diverse group of organisms. There are over 115 000 different kinds, with traits that fit with fungi, plants, and animals.
METHODS OF CLASSIFYING INTO A CERTAIN KINGDOM: 1. prokaryote OR eukaryote 2. single OR multi celled 3. autotroph OR heterotroph
 CH. 22 PROTISTS METHODS OF CLASSIFYING INTO A CERTAIN KINGDOM: 1. prokaryote OR eukaryote 2. single OR multi celled 3. autotroph OR heterotroph 6 Kingdoms 1. Eubacteria prokaryotes; single cell; heterotroph
CH. 22 PROTISTS METHODS OF CLASSIFYING INTO A CERTAIN KINGDOM: 1. prokaryote OR eukaryote 2. single OR multi celled 3. autotroph OR heterotroph 6 Kingdoms 1. Eubacteria prokaryotes; single cell; heterotroph
Protists. There are NO typical protists. Protist General Characteristics - usually single cell - eukaryotic - paraphyletic group
 There are NO typical protists. Protist General Characteristics - usually single cell - eukaryotic - paraphyletic group Traditional Classification There are three divisions of the Kingdom Protista: Protozoa,
There are NO typical protists. Protist General Characteristics - usually single cell - eukaryotic - paraphyletic group Traditional Classification There are three divisions of the Kingdom Protista: Protozoa,
Apicoplast. Apicoplast - history. Treatments and New drug targets
 Treatments and New drug targets What is the apicoplast? Where does it come from? How are proteins targeted to the organelle? How does the organelle replicate? What is the function of the organelle? - history
Treatments and New drug targets What is the apicoplast? Where does it come from? How are proteins targeted to the organelle? How does the organelle replicate? What is the function of the organelle? - history
19.1 Diversity of Protists. KEY CONCEPT Kingdom Protista is the most diverse of all the kingdoms.
 19.1 Diversity of Protists KEY CONCEPT Kingdom Protista is the most diverse of all the kingdoms. 19.1 Diversity of Protists Protists can be animal-like, plantlike, or funguslike. Protists are eukaryotes
19.1 Diversity of Protists KEY CONCEPT Kingdom Protista is the most diverse of all the kingdoms. 19.1 Diversity of Protists Protists can be animal-like, plantlike, or funguslike. Protists are eukaryotes
Supplementary Information
 Supplementary Information Supplementary Figure 1. Schematic pipeline for single-cell genome assembly, cleaning and annotation. a. The assembly process was optimized to account for multiple cells putatively
Supplementary Information Supplementary Figure 1. Schematic pipeline for single-cell genome assembly, cleaning and annotation. a. The assembly process was optimized to account for multiple cells putatively
122-Biology Guide-5thPass 12/06/14. Topic 1 An overview of the topic
 Topic 1 http://bioichiban.blogspot.com Cellular Functions 1.1 The eukaryotic cell* An overview of the topic Key idea 1: Cell Organelles Key idea 2: Plasma Membrane Key idea 3: Transport Across Membrane
Topic 1 http://bioichiban.blogspot.com Cellular Functions 1.1 The eukaryotic cell* An overview of the topic Key idea 1: Cell Organelles Key idea 2: Plasma Membrane Key idea 3: Transport Across Membrane
Basic Structure of a Cell
 Basic Structure of a Cell Prokaryotic Cells No nucleus Archaea & Eubacteria One circular chromosome Extremely small Eukaryotic Cells Has a nucleus!!! Membrane-bound organelles Plants, Animals, Fungi, &
Basic Structure of a Cell Prokaryotic Cells No nucleus Archaea & Eubacteria One circular chromosome Extremely small Eukaryotic Cells Has a nucleus!!! Membrane-bound organelles Plants, Animals, Fungi, &
Lecture #9-2/8 Dr. Kopeny
 Lecture #9-2/8 Dr. Kopeny Protistans, Part 1 Lecture VIII Protistans Lecture Themes structure and function; recurring evolutionary themes and unifying features the origin of mitochondria and chloroplasts
Lecture #9-2/8 Dr. Kopeny Protistans, Part 1 Lecture VIII Protistans Lecture Themes structure and function; recurring evolutionary themes and unifying features the origin of mitochondria and chloroplasts
FLOW: Amigos de Bolsa Chica Citizen Science Program. Plankton Collection and Identification Report
 FLOW: Amigos de Bolsa Chica Citizen Science Program Plankton Collection and Identification Report Date: 04/26/13 Time: 2:35 PM Collectors: Judy H., Chuck D., Belen C., Joana T. (analysis also performed
FLOW: Amigos de Bolsa Chica Citizen Science Program Plankton Collection and Identification Report Date: 04/26/13 Time: 2:35 PM Collectors: Judy H., Chuck D., Belen C., Joana T. (analysis also performed
Viruses. Viruses. Chapter 5. Prokaryotes. Prokaryotes. Prokaryotes
 Viruses Chapter 5 The Microbial World Non-cellular infectious agents that have two basic characteristics: Not capable of reproduction without a host cell Structure: Nucleic acid core- can be DNA or RNA
Viruses Chapter 5 The Microbial World Non-cellular infectious agents that have two basic characteristics: Not capable of reproduction without a host cell Structure: Nucleic acid core- can be DNA or RNA
Amira A. AL-Hosary PhD of infectious diseases Department of Animal Medicine (Infectious Diseases) Faculty of Veterinary Medicine Assiut
 Amira A. AL-Hosary PhD of infectious diseases Department of Animal Medicine (Infectious Diseases) Faculty of Veterinary Medicine Assiut University-Egypt Phylogenetic analysis Phylogenetic Basics: Biological
Amira A. AL-Hosary PhD of infectious diseases Department of Animal Medicine (Infectious Diseases) Faculty of Veterinary Medicine Assiut University-Egypt Phylogenetic analysis Phylogenetic Basics: Biological
Protists: Molds Lecture 3 Spring 2014
 Meet the Protists 1 Protists: Molds Lecture 3 Spring 2014 Domain Eukarya What unites them as a group? The Origin of Eukaryotic Cells Evolution of the endomembrane system Which organelles are included in
Meet the Protists 1 Protists: Molds Lecture 3 Spring 2014 Domain Eukarya What unites them as a group? The Origin of Eukaryotic Cells Evolution of the endomembrane system Which organelles are included in
Protists: Molds Lecture 3 Spring 2014
 Protists: Molds Lecture 3 Spring 2014 Meet the Protists 1 Domain Eukarya What unites them as a group? The Origin of Eukaryotic Cells 2 Evolution of the endomembrane system Which organelles are included
Protists: Molds Lecture 3 Spring 2014 Meet the Protists 1 Domain Eukarya What unites them as a group? The Origin of Eukaryotic Cells 2 Evolution of the endomembrane system Which organelles are included
Multiple Choice Review- Eukaryotic Gene Expression
 Multiple Choice Review- Eukaryotic Gene Expression 1. Which of the following is the Central Dogma of cell biology? a. DNA Nucleic Acid Protein Amino Acid b. Prokaryote Bacteria - Eukaryote c. Atom Molecule
Multiple Choice Review- Eukaryotic Gene Expression 1. Which of the following is the Central Dogma of cell biology? a. DNA Nucleic Acid Protein Amino Acid b. Prokaryote Bacteria - Eukaryote c. Atom Molecule
Surface morphology of the marine parasite Haplozoon axiothellae Siebert (Dinoflagellata)
 Europ. J. Protistol. 38, 287 297 (2002) Urban & Fischer Verlag http://www.urbanfischer.de/journals/ejp Surface morphology of the marine parasite Haplozoon axiothellae Siebert (Dinoflagellata) Brian S.
Europ. J. Protistol. 38, 287 297 (2002) Urban & Fischer Verlag http://www.urbanfischer.de/journals/ejp Surface morphology of the marine parasite Haplozoon axiothellae Siebert (Dinoflagellata) Brian S.
Autotrophs capture the light energy from sunlight and convert it to chemical energy they use for food.
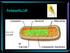 Prokaryotic Cell Eukaryotic Cell Autotrophs capture the light energy from sunlight and convert it to chemical energy they use for food. Heterotrophs must get energy by eating autotrophs or other heterotrophs.
Prokaryotic Cell Eukaryotic Cell Autotrophs capture the light energy from sunlight and convert it to chemical energy they use for food. Heterotrophs must get energy by eating autotrophs or other heterotrophs.
Chapter 4 Active Reading Guide A Tour of the Cell
 Name: AP Biology Mr. Croft Chapter 4 Active Reading Guide A Tour of the Cell Section 1 1. The study of cells has been limited by their small size, and so they were not seen and described until 1665, when
Name: AP Biology Mr. Croft Chapter 4 Active Reading Guide A Tour of the Cell Section 1 1. The study of cells has been limited by their small size, and so they were not seen and described until 1665, when
Modern Evolutionary Classification. Section 18-2 pgs
 Modern Evolutionary Classification Section 18-2 pgs 451-455 Modern Evolutionary Classification In a sense, organisms determine who belongs to their species by choosing with whom they will mate. Taxonomic
Modern Evolutionary Classification Section 18-2 pgs 451-455 Modern Evolutionary Classification In a sense, organisms determine who belongs to their species by choosing with whom they will mate. Taxonomic
Post-doc fellowships to non-eu researchers FINAL REPORT. Home Institute: Centro de Investigaciones Marinas, Universidad de La Habana, CUBA
 Recipient: Maickel Armenteros Almanza. Post-doc fellowships to non-eu researchers FINAL REPORT Home Institute: Centro de Investigaciones Marinas, Universidad de La Habana, CUBA Promoter: Prof. Dr. Wilfrida
Recipient: Maickel Armenteros Almanza. Post-doc fellowships to non-eu researchers FINAL REPORT Home Institute: Centro de Investigaciones Marinas, Universidad de La Habana, CUBA Promoter: Prof. Dr. Wilfrida
Biology I Fall Semester Exam Review 2014
 Biology I Fall Semester Exam Review 2014 Biomolecules and Enzymes (Chapter 2) 8 questions Macromolecules, Biomolecules, Organic Compunds Elements *From the Periodic Table of Elements Subunits Monomers,
Biology I Fall Semester Exam Review 2014 Biomolecules and Enzymes (Chapter 2) 8 questions Macromolecules, Biomolecules, Organic Compunds Elements *From the Periodic Table of Elements Subunits Monomers,
Biosc 41 9/10 Announcements
 Biosc 41 9/10 Announcements v Genetics review: group problem sets Groups of 3-4 Correct answer presented to class = 2 pts extra credit Incorrect attempt = 1 pt extra credit v Lecture: Animal Body Plans
Biosc 41 9/10 Announcements v Genetics review: group problem sets Groups of 3-4 Correct answer presented to class = 2 pts extra credit Incorrect attempt = 1 pt extra credit v Lecture: Animal Body Plans
2.1 CELL STRUCTURE. The cell is the smallest unit of living organisms that shows the characteristics of life.
 2.1.1 Microscopy The cell is the smallest unit of living organisms that shows the characteristics of life. A general introduction to the microscope. The light microscope All cells are microscopic which
2.1.1 Microscopy The cell is the smallest unit of living organisms that shows the characteristics of life. A general introduction to the microscope. The light microscope All cells are microscopic which
Human biology Laboratory. Cell division. Lecturer Maysam A Mezher
 Human biology Laboratory Cell division Lecturer Maysam A Mezher CHROMOSOME STRUCTURE 1. During nuclear division, the DNA (as chromatin) in a Eukaryotic cell's nucleus is coiled into very tight compact
Human biology Laboratory Cell division Lecturer Maysam A Mezher CHROMOSOME STRUCTURE 1. During nuclear division, the DNA (as chromatin) in a Eukaryotic cell's nucleus is coiled into very tight compact
The Phylogenetic Reconstruction of the Grass Family (Poaceae) Using matk Gene Sequences
 The Phylogenetic Reconstruction of the Grass Family (Poaceae) Using matk Gene Sequences by Hongping Liang Dissertation submitted to the Faculty of the Virginia Polytechnic Institute and State University
The Phylogenetic Reconstruction of the Grass Family (Poaceae) Using matk Gene Sequences by Hongping Liang Dissertation submitted to the Faculty of the Virginia Polytechnic Institute and State University
UoN, CAS, DBSC BIOL102 lecture notes by: Dr. Mustafa A. Mansi. The Phylogenetic Systematics (Phylogeny and Systematics)
 - Phylogeny? - Systematics? The Phylogenetic Systematics (Phylogeny and Systematics) - Phylogenetic systematics? Connection between phylogeny and classification. - Phylogenetic systematics informs the
- Phylogeny? - Systematics? The Phylogenetic Systematics (Phylogeny and Systematics) - Phylogenetic systematics? Connection between phylogeny and classification. - Phylogenetic systematics informs the
PROTISTS James Bier
 PROTISTS 2013-2015 James Bier Objectives 1. List the characteristics shared among the protists. 2. Describe secondary endosymbiosis and the evidence for this hypothesis. 3. List the five major taxa of
PROTISTS 2013-2015 James Bier Objectives 1. List the characteristics shared among the protists. 2. Describe secondary endosymbiosis and the evidence for this hypothesis. 3. List the five major taxa of
Number of questions TEK (Learning Target) Biomolecules & Enzymes
 Unit Biomolecules & Enzymes Number of questions TEK (Learning Target) on Exam 8 questions 9A I can compare and contrast the structure and function of biomolecules. 9C I know the role of enzymes and how
Unit Biomolecules & Enzymes Number of questions TEK (Learning Target) on Exam 8 questions 9A I can compare and contrast the structure and function of biomolecules. 9C I know the role of enzymes and how
Dr. Amira A. AL-Hosary
 Phylogenetic analysis Amira A. AL-Hosary PhD of infectious diseases Department of Animal Medicine (Infectious Diseases) Faculty of Veterinary Medicine Assiut University-Egypt Phylogenetic Basics: Biological
Phylogenetic analysis Amira A. AL-Hosary PhD of infectious diseases Department of Animal Medicine (Infectious Diseases) Faculty of Veterinary Medicine Assiut University-Egypt Phylogenetic Basics: Biological
ULTRASTRUCTURAL FEATURES OF THE BASAL DINOFLAGELLATE Ellobiopsis chattoni (ELLOBIOPSIDAE, ALVEOLATA), PARASITE OF COPEPODS
 CICIMAR Oceánides 29(2): 1-10 (2014) ULTRASTRUCTURAL FEATURES OF THE BASAL DINOFLAGELLATE Ellobiopsis chattoni (ELLOBIOPSIDAE, ALVEOLATA), PARASITE OF COPEPODS Gómez, F. 1 & T. Horiguchi 2 1 Laboratory
CICIMAR Oceánides 29(2): 1-10 (2014) ULTRASTRUCTURAL FEATURES OF THE BASAL DINOFLAGELLATE Ellobiopsis chattoni (ELLOBIOPSIDAE, ALVEOLATA), PARASITE OF COPEPODS Gómez, F. 1 & T. Horiguchi 2 1 Laboratory
7-2 Eukaryotic Cell Structure
 1 of 49 Comparing the Cell to a Factory Eukaryotic Cell Structures Structures within a eukaryotic cell that perform important cellular functions are known as organelles. Cell biologists divide the eukaryotic
1 of 49 Comparing the Cell to a Factory Eukaryotic Cell Structures Structures within a eukaryotic cell that perform important cellular functions are known as organelles. Cell biologists divide the eukaryotic
Protists are in the Eukaryote Domain
 Protista Protists are in the Eukaryote Domain All protists are eukaryotic (cells with a nucleus) Euglena Paramecium Amoeba Protists are really just all of the Eukaryotes that don t fit into the Animal,
Protista Protists are in the Eukaryote Domain All protists are eukaryotic (cells with a nucleus) Euglena Paramecium Amoeba Protists are really just all of the Eukaryotes that don t fit into the Animal,
Chapters 25 and 26. Searching for Homology. Phylogeny
 Chapters 25 and 26 The Origin of Life as we know it. Phylogeny traces evolutionary history of taxa Systematics- analyzes relationships (modern and past) of organisms Figure 25.1 A gallery of fossils The
Chapters 25 and 26 The Origin of Life as we know it. Phylogeny traces evolutionary history of taxa Systematics- analyzes relationships (modern and past) of organisms Figure 25.1 A gallery of fossils The
v Scientists have identified 1.3 million living species of animals v The definition of an animal
 Biosc 41 9/10 Announcements BIOSC 041 v Genetics review: group problem sets Groups of 3-4 Correct answer presented to class = 2 pts extra credit Incorrect attempt = 1 pt extra credit v Lecture: Animal
Biosc 41 9/10 Announcements BIOSC 041 v Genetics review: group problem sets Groups of 3-4 Correct answer presented to class = 2 pts extra credit Incorrect attempt = 1 pt extra credit v Lecture: Animal
Microbes usually have few distinguishing properties that relate them, so a hierarchical taxonomy mainly has not been possible.
 Microbial Taxonomy Traditional taxonomy or the classification through identification and nomenclature of microbes, both "prokaryote" and eukaryote, has been in a mess we were stuck with it for traditional
Microbial Taxonomy Traditional taxonomy or the classification through identification and nomenclature of microbes, both "prokaryote" and eukaryote, has been in a mess we were stuck with it for traditional
Microbial Taxonomy. Slowly evolving molecules (e.g., rrna) used for large-scale structure; "fast- clock" molecules for fine-structure.
 Microbial Taxonomy Traditional taxonomy or the classification through identification and nomenclature of microbes, both "prokaryote" and eukaryote, has been in a mess we were stuck with it for traditional
Microbial Taxonomy Traditional taxonomy or the classification through identification and nomenclature of microbes, both "prokaryote" and eukaryote, has been in a mess we were stuck with it for traditional
Protist Classification the Saga Continues
 Protist Classification the Saga Continues Learning Objectives Explain what a protist is. Describe how protists are related to other eukaryotes. What Are Protists? Photosynthetic Motile Unicellular Multicellular
Protist Classification the Saga Continues Learning Objectives Explain what a protist is. Describe how protists are related to other eukaryotes. What Are Protists? Photosynthetic Motile Unicellular Multicellular
Kingdom Protista. Lab Exercise 20. Introduction. Contents. Objectives
 Lab Exercise Kingdom Protista Contents Objectives 1 Introduction 1 Activity.1 Animal-like Protists 2 Activity.2 Fungal-like Protists 3 Activity.3 Plant-like Protists 3 Resutls Section 5 Introduction This
Lab Exercise Kingdom Protista Contents Objectives 1 Introduction 1 Activity.1 Animal-like Protists 2 Activity.2 Fungal-like Protists 3 Activity.3 Plant-like Protists 3 Resutls Section 5 Introduction This
